RNA modifications affects cellular homeostasis, tissue development, and disease initiation/progression by altering mRNA splicing, stability, and translation. Adenosine-to-inosine (A-to-I) RNA editing has captured the interests of cardiovascular researchers as this modification is significantly elevated in patients with congenital heart defects and atherosclerosis1. However, its role in cardiomyocyte development and function remains largely unknown.
Site-specific deamination of A-to-I is catalyzed by ADAR (adenosine deaminases acting on RNA) editing enzymes. In mammals, three distinct ADAR genes have been identified. ADAR1 and ADAR2 possess catalytic activity, whereas ADAR3 does not. Genetic ablation of Adar1 or Adar2 correspondingly result in embryonic or postnatal lethality in mice. Adar1 null mice exhibit normal development up to E10.5, but display enhanced cellular apoptosis in multiple tissues, including the heart, and late-stage embryonic death2. To follow up on this earlier study, we genetically deleted Adar1 in developing cardiomyocytes by crossing Adar1 floxed mice with Nkx2.5-Cre mice (Figure 1A&B). No surviving Adar1 animals were observed at birth (postnatal day 1), suggesting cardiomyocyte ADAR1 editing activity is essential for early embryonic development.
Figure 1: Adar1 in embryonic cardiac growth and development.
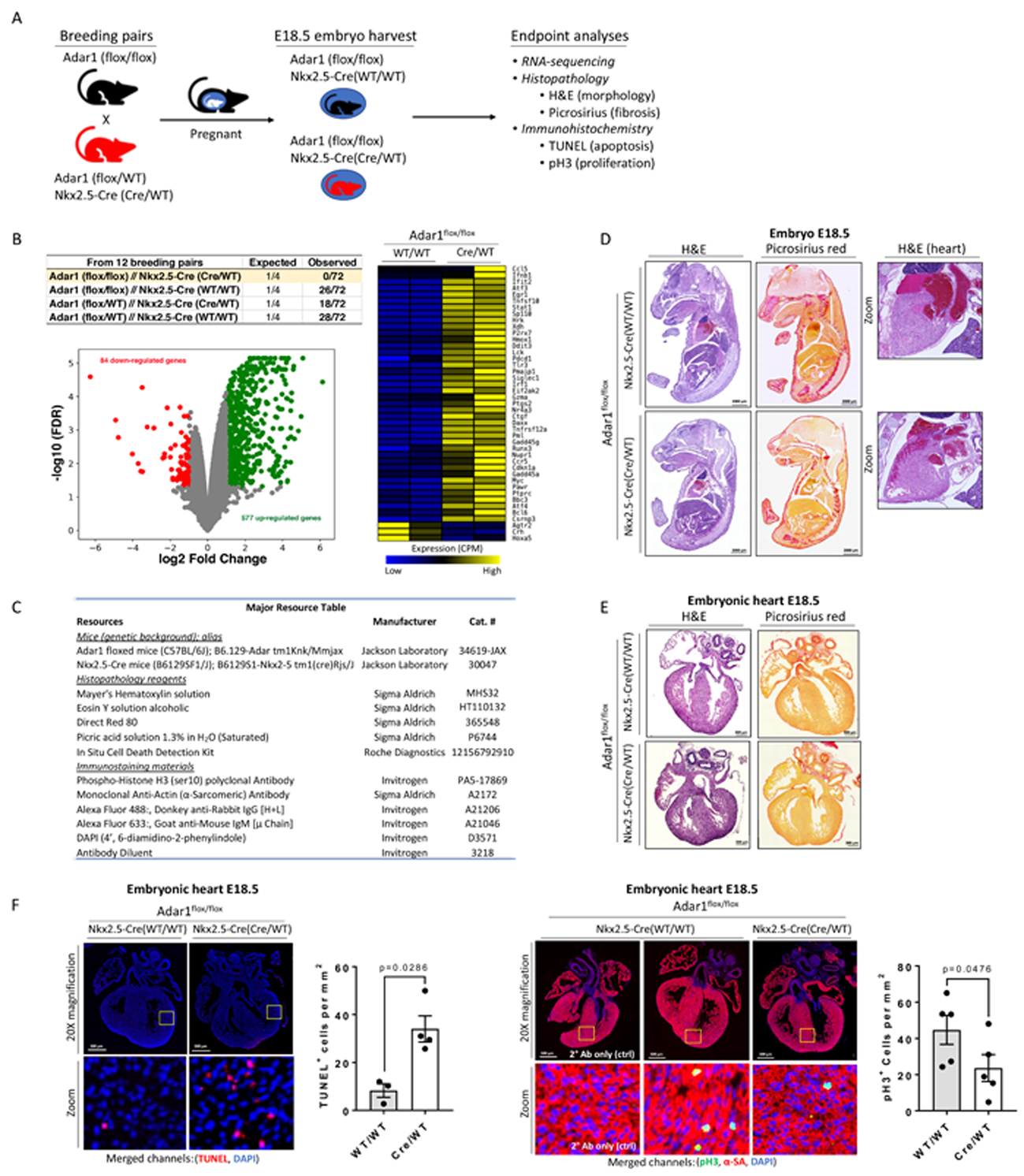
(A) Genetic crosses used in this study. (B) Table details genotypes of 3-week-old pups. Volcano plot depicts RNA-sequencing results of E18.5 hearts (GSE141345) from Adar1-ablated and wild-type littermate controls (N=2 embryos). Heatmap illustrates differentially-regulated genes under the GO category of cell death. (C) Resource table. (D) E18.5 embryos. Scale=2000 μm. N=3 embryos. (E) E18.5 embryonic hearts (N=2~3 embryos). Scale=300 μm. (F) Immunostaining for TUNEL+ or phospho-histone H3+ cells. Scale=500 μm. Graphs depict the total number of TUNEL+ (WT/WT, N=3; Cre/WT, N=4; Mann-Whitney Test)- or pH3+ (WT/WT, N=5; Cre/WT, N=5; Mann-Whitney Test)-nucleated cells per mm2 with mean ± SEM. Animal experiments were approved by the Animal Care and Use Committee of University of Louisville. Histological analyses were carried out in a blinded manner. Both sexes were used in analyses.
RNA-sequencing results identified pronounced alterations in Adar1 knockout embryos compared to wild-type controls (Figure 1B). Consistent with previous reports2, we detected up-regulation of apoptotic genes (Figure 1B). Immunostaining of E18.5 embryos displayed reduced trabecular compaction without evidence of pathological fibrosis (Figure 1 D–E). TUNEL staining identified a significant number of apoptotic cells (Figure 1F), implicating enhanced cell death as a potential contributor to this development defect.
A previous study shows increased expression of adar1 mRNA and enhanced nucleocytoplasmic adar1 protein shuttling in regenerating newt hearts following cardiac injury3. Although no causal relationship between Adar1 expression and cardiomyocyte proliferation was established, we postulated that proliferation of fetal mammalian cardiomyocytes during embryogenesis requires Adar1. Supporting this hypothesis, significant reductions in cardiomyocyte proliferation was observed in Adar1-ablated cardiomyocytes compared to controls (Figure 1G), suggesting that Adar1 inactivation affects both cardiomyocyte survival and proliferation during development.
In summary, Adar1 is essential for embryonic cardiomyocyte survival and proliferation. The limitations to this study include: 1) the increased rate of cellular apoptosis may underly perceived reductions in cell proliferation in Adar1-ablated hearts; 2) as Nkx2.5 is expressed in other cells (endothelium) and tissue types (tongue, thymus, and spleen) during development, additional Nkx2.5-derived cell types/tissues may be impacted by Adar1 ablation and could contribute to embryonic lethality here. However, a recent study ablating Adar1 in adult murine hearts displayed lethality due to increased myocyte apoptosis, which is congruent with our observations in embryonic hearts.4 Further studies are needed to gain a more in-depth understanding of RNA editing regulatory mechanisms and their role in cardiomyocyte biology and disease processes.
SOURCES OF FUNDING
NIH-R01-HL141081 (JBM); NIH-P30-GM127607 (SU).
Footnotes
DISCLOSURES
None.
This manuscript was sent to Chinmay Trivedi, Internal Consulting Editor, for review by expert referees, editorial decision, and final disposition.
REFERENCES
- 1.Uchida S and Jones SP. RNA Editing: Unexplored Opportunities in the Cardiovascular System. Circ Res. 2018;122:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ and Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–61. [DOI] [PubMed] [Google Scholar]
- 3.Witman NM, Behm M, Ohman M and Morrison JI. ADAR-related activation of adenosine-to-inosine RNA editing during regeneration. Stem Cells Dev. 2013;22:2254–67. [DOI] [PubMed] [Google Scholar]
- 4.El Azzouzi H, Vilaca AP, Feyen DAM, Gommans WM, de Weger RA, Doevendans PAF and Sluijter JPG. Cardiomyocyte Specific Deletion of ADAR1 Causes Severe Cardiac Dysfunction and Increased Lethality. Front Cardiovasc Med. 2020;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]


