Abstract
Despite the potential of rodent models of maternal immune activation (MIA) to identify new biomarkers and therapeutic interventions for a range of psychiatric disorders, current approaches using these models ignore two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal viral infection and (ii) susceptible pregnancies can lead to different combinations of phenotypes in offspring. Here, we report two new sources of variability—the baseline immunoreactivity (BIR) of isogenic females prior to pregnancy and differences in immune responses in C57BL/6 dams across vendors—that contribute to resilience and susceptibility to distinct combinations of behavioral and biological outcomes in offspring. Similar to the variable effects of human maternal infection, MIA in mice does not cause disease-related phenotypes in all pregnancies and a combination of poly(I:C) dose and BIR predicts susceptibility and resilience of pregnancies to aberrant repetitive behaviors and alterations in striatal protein levels in offspring. Even more surprising is that the intermediate levels of BIR and poly(I:C) dose are most detrimental to offspring, with higher BIR and poly(I:C) doses conferring resilience to measured phenotypes in offspring. Importantly, we identify the BIR of female mice as a biomarker before pregnancy that predicts which dams will be most at risk as well as biomarkers in the brains of newborn offspring that correlate with changes in repetitive behaviors. Together, our results highlight considerations for optimizing MIA protocols to enhance rigor and reproducibility and reveal new factors that drive susceptibility of some pregnancies and resilience of others to MIA-induced abnormalities in offspring.
Introduction
Epidemiological evidence links maternal infection to several neuropsychiatric disorders including autism (ASD) and schizophrenia (SZ) (Patterson, 2009). Animal models of maternal immune activation (MIA) support this link, as mid-gestational injection of the viral mimic, poly(I:C), induces neuropathology and aberrant behaviors in domains shared by these diseases (Bauman et al., 2014; Estes and McAllister, 2015, 2016; Meyer and Feldon, 2010). Thus, the poly(I:C) mouse model provides an opportunity to identify molecular targets that mediate the effects of MIA on brain development that could eventually lead to earlier diagnosis and treatment of brain disease in humans. Despite this promise, though, little is understood about two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal viral infection and (ii) susceptible pregnancies can lead to distinct combinations of phenotypes in offspring.
Recently, attention in the MIA field has started to shift toward studying resilience and susceptibility in large part because of recurring issues of reproducibility stemming from heterogeneous and sometimes opposing findings in MIA offspring (Estes and McAllister, 2016; Harvey and Boksa, 2012; Kentner et al., 2019; Meyer, 2019; Smolders et al., 2015). For MIA models, one of the most fundamental variables is the immunogenicity of the compounds used to induce MIA. The poly(I:C) model was originally designed to cause elevations in maternal serum interleukin-6 (IL-6) comparable to those induced by influenza (Meyer et al., 2006; Smith et al., 2007). Elevation in maternal IL-6 is necessary and sufficient for inducing a range of disease-relevant phenotypes in MIA offspring (Smith et al., 2007). However, many laboratories using this model do not measure maternal serum IL-6, and those that do report widely varying levels that have been steadily decreasing over time even when using the same dose, timing, delivery route, and source of poly(I:C) (Kentner et al., 2019). For example, initial studies that established the mouse MIA model using mid-gestational intraperitoneal injection of poly(I:C) reported 10,000-20,000pg/ml IL-6 in maternal serum 3hr post-injection, while a recent report found only a 350pg/ml increase using an identical MIA induction protocol (Choi et al., 2016; Kowash et al., 2019; Smith et al., 2007). It has also been reported that the immunogenicity of poly(I:C) differs between vendors and between lots from the same vendor, with inconsistent molecular weight ranges of the compounds and varying amounts of endotoxin contamination contributing to variable outcomes (Careaga et al., 2018; Harvey and Boksa, 2012; Kowash et al., 2019; Mueller et al., 2019).
Current recommendations for dealing with the inconsistency of the immunogen in MIA models include optimizing the poly(I:C) dose for each new lot. However, the guidelines for finding the optimal dose are unclear since almost any dose of poly(I:C) will increase maternal IL-6 levels above those in saline-injected controls. For an environmental model such as MIA to have construct validity for ASD and SZ, it stands to reason that a quantifiable threshold of MIA that causes disease-relevant phenotypes must be established, similar to the threshold for maternal infection in humans linked to ASD and SZ (Atladottir et al., 2010; Careaga et al., 2017; Connor et al., 2012; Harvey and Boksa, 2012). Here, we identify several novel sources of variability in generating MIA models that allow us to study susceptibility and resilience to the same dose of a single lot of poly(I:C). Using this approach, we have identified biomarkers in female mice before pregnancy that predict which dams will be most at risk and biomarkers in the brains of newborn offspring that correlate with changes in repetitive behaviors. Our results reveal that maternal factors, even before pregnancy, strongly influence how exposure of isogenic mice to the same gestational risk factor leads to divergent outcomes in offspring.
Materials and Methods
Animal Care and Use.
All studies were conducted with approved protocols from the University of California Davis Animal Care and Use Committee, in compliance with NIH guidelines. Virgin C57BL/6N mice were purchased from Charles River (CR; Kingston, NY), Taconic (TAC; Hudson, NY) and C57BL/6J mice from Jackson (JAX; Sacramento, CA). Mice for MIA experiments were bred in house and maintained on a 12:12h light:dark cycle at 21 ± 1°C with food and water ad libitum. All mice were housed in Techniplast Sealsafe individually ventilated cages (IVC) with corncob bedding. Vivarium-specific environmental factors such as cage system (Mueller et al., 2018), bedding (Trainor et al., 2013), temperature and humidity (Hylander et al., 2017), technician sex (Sorge et al., 2014), and microbiota (Kim et al., 2017) exposure may all contribute to MIA susceptibility and outcomes (Kentner et al., 2019).
Baseline immunoreactivity measurement.
At 7 weeks of age, virgin female mice were injected intraperitoneally (IP) with 4.0-4.4 mg/ml of high molecular weight (HMW) poly(I:C) dsRNA (InvivoGen, San Diego, CA. Cat# tlrl-pic). At 2.5hrs or 4hrs post-injection, whole blood from individual animals was taken via tail snip and spun at 14,000 rpm at 4°C for 8min. Serum was isolated and IL-6 levels were measured using a Bio-Rad Luminex system (Bio-Rad, Laboratories, Hercules, CA) according to the manufacturer’s protocol. The baseline immunoreactivity (BIR) of the dams was assessed by their relative serum interleukin-6 (IL-6) levels, and those levels were used to divide the dams into 3 groups (low, medium, and high) following priming and prior to breeding. All injections were performed by female handlers. Please note that this poly(I:C) compound is distinct from the compound used for MIA; we do not know if using the same compound for BIR assessment and MIA will provide the same predictive power as described in our study.
Maternal Immune Activation.
As previously described (Garay et al., 2013), pregnant mice at gestational day GD12.5 were injected intraperitoneally (IP) with γ-irradiated poly(I:C) dsRNA from Sigma Aldrich (St. Louis, MO, cat #P0913) at various doses spanning 20-40 mg/kg or with vehicle control (sterile 0.9% saline) injected at 5μl/gram of body weight. At time of injection, the male was separated from the dam. Serum was isolated from blood via tail snip for IL-6 levels at either 2.5 or 4hr post injection and serum was isolated from trunk blood 48hr after injection for IL-17a protein levels. Temperature, weight and sickness behavior were observed at injection, 2.5-hour bleed and 24hr post injection times. Sickness behavior was measured as an index of maternal inflammation and scored using a subjective scale (from 1 to 3) of increasing activity ranging from little to no movement in response to being handled (1) to normal resistance to capture and restraint of the dam (3). Experiments were performed on female mice before pregnancy, in pregnant dams, or in newborn or young adult offspring as indicated for each assay.
Immunoblotting.
Western blot analysis was used to investigate protein levels of STAT3, MEF2A, and tyrosine hydroxylase (TH) from the total homogenate of striatal (Str) brain samples. Striata from PO male MIA offspring from the 3 BIR groups and controls were dissected in HBSS, frozen in liquid nitrogen, and stored at −80°C. Samples were disrupted using a probe sonicator (Qsonica Sonicator Q500) with an amplitude of 20% for 5sec in 2X Laemmli buffer, then denatured at 85°C for 5min. Lysates were centrifuged at 16,000g for 10min at room temperature. The supernatant was collected and stored at −80°C. Total protein content was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher), using bovine serum albumin as the calibration standard. Dithiothreitol (Sigma D9779-10G) was then added as a reducing agent to the samples at a final concentration of 100mM, and heated at 85°C for 2min before loading onto a gel. Equal amounts of protein (5ug/lane) were run under reducing conditions on 7.5% TGX gels (Bio-Rad) and electrophoretically transferred onto PVDF membranes (Bio-Rad 162-0177). Membranes were blocked with Odyssey blocking buffer (TBS; Li-Cor) and incubated with: i) monoclonal anti-STAT3 (12640S, 1:1,000; Cell Signaling); ii) monoclonal anti-MEF2A (ab76063, 1:1,000; AbCam); and (iii) polyclonal anti-TH (P40101-150, 1:1,000; Pel-Freez). After washing 3 times with TBS + 0.05% Tween20, membranes were incubated for 45min with fluorescent-tagged secondary antibodies (925-32213 and 925-68072, 1:15,000; Li-Cor). After 4 additional washes in TBS/Tween20, bands were visualized at 680nm and 800nm using the Odyssey CLx imaging system (Li-Cor). Results were standardized using β-tubulin, detected using anti-β-tubulin (Millipore MAB3408; 1:2,000). Band intensities were measured using Image Studio software (Li-Cor)
Flow cytometry.
Splenocytes were isolated from MIA or control dams 48h after injection. For intracellular cytokine assessment, mononuclear cells (1x106 cells/mL) were cultured for 6 h with or without phorbol 12-myristate 13-acetate (PMA, 50ng/mL; Sigma), ionomycin (50ng/mL; Sigma), and GolgiStop (BD) in T cell media: RPMI 1640 (Invitrogen) supplemented with 10% (v/v) heat-inactivated FBS (Hyclone), and 2mM glutamine. Intracellular cytokine staining was performed according to the manufacturer’s protocol (Cytofix/Cytoperm buffer set from BD with PE-Cy7-conjugated anti-CD4 (RM4-5), eFIuor 450-conjugated anti-TCR-β (H57-597), PE-conjugated anti-IL-17a (eBio17B7), FITC-conjugated anti-IFN-γ (XMG1.2), and fixable viability dye eFIuro 780). For transcription factor assessment, isolated mononuclear cells were stained according to the manufacturer’s protocol (Fix/perm buffer set from eBiosciences with PE-Cy7-conjugated anti-CD4 (RM4-5), eFluor 450-conjugated anti-TCR-β (H57-597), PerCP-Cy5.5-conjugated anti-T-bet (4B10), AF488-conjugated anti-Gata3 (TWAJ), PE-conjugated anti-Foxp3 (FJK-16s), APC-conjugated anti-RORγt (B2D)). All antibodies were purchased from eBiosciences except for anti-IFN-γ, which was purchased from Biolegend. LSRFortessa (BD) and FlowJo software (Tree Star) were used for flow cytometry and analysis. Live, CD4+TCRβ+ cells were assessed for cytokine and transcription factor expression. Frontal cortex (FC) was dissected at P0 from the MIA and control offspring and acutely dissociated in papain. Dissociated cells were stained for APC-conjugated anti-ACSA-2 (IH3-18A3), anti-O4 (O4), and anti-CD11b (M1/70.15.11.5) (Miltenyi Biotec), the anti-MHCI subtype H2-Kb-PE (AF6-88.5) (BD) and a viability marker (Far Red Dead Cell Stain from Invitrogen) and then fixed in 1% PFA. Samples were run on a FACSCalibur (BD) flow cytometer and analyzed by Flowjo (Treestar). Data were gated on live, ACSA−O4−CD11b− cells and assessed for sMHCI by anti-H2-Kb-PE fluorescence intensity.
Neuronal culture.
Neurons from postnatal day 0-1 (P0-1) male mouse frontal cortex (FC) were dissociated with papain and plated at a density of 50K/cm2 on poly-L-lysine coated glass coverslips (as previously described (Elmer et al., 2013) and maintained in GS21 media (MTI-Global).
Immunocytochemistry (ICC).
Cells from FC of newborn offspring were fixed at 8 days in vitro (DIV) in 4% paraformaldehyde, 4% sucrose in PBS at 4°C for 10min. Neurons were permeabilized with 0.25% Triton X-100 for 5min, blocked with 10% BSA, and then incubated with primary and secondary antibodies diluted in 3% BSA. Primary antibodies used were: PSD-95 (K28/43, 1:1000; NeuroMab) and VAMP2 (104-202, 1:1000, Synaptic Systems). Secondary antibodies used were AF-488 and Cy3 conjugated anti-rabbit and anti-mouse (1:400, Invitrogen).
Microscopy and image analysis.
Imaging of neurons was performed using a Nikon C2+ (software v4.5) laser scanning confocal microscope with a 60X PlanApo oil immersion objective (1.4 NA). Laser power and PMT levels were held constant between groups within each experiment. 16-bit grayscale images for each channel were acquired sequentially and kalman-averaged over two scans at 2.5X artificial zoom. Synapses were defined as sites of at least 1 pixel of overlap between PSD-95 and VAMP2 puncta, identified using custom software (Ly et al., 2018). All images were prepared in Adobe Photoshop and Illustrator.
Behavioral testing.
P60-80 male and female offspring from the poly(I:C)-injected mothers (MIA offspring) were compared to offspring from saline-injected dams (controls). Mice were handled for three days prior to behavioral testing by male and female handlers. For handling, mice were acclimated in the testing room for 1hr prior to being gently handled for 3min followed by return to the home cage. For the self-grooming assay, the testing room was dimly lit (15-20 lux) and mice were acclimated for 1h. Each mouse was placed individually into an empty standard mouse cage (36×15×13cm) for 20min and behavior was video recorded. Videos were scored offline. After a 10min habituation period in the test cage, the behavior of each mouse was scored with a stopwatch for 10min for cumulative time spent grooming all body regions and for frequency of rearing events. All animals tested are included in the data shown; no outliers were removed.
qPCR for Segmented Filamentous Bacteria (SFB) detection.
Bacterial genomic DNA was extracted from frozen fecal samples (within an experiment the samples were treated identically) following manufacturer’s directions using QIAamp Fast DNA Stool Mini kit (Qiagen, Hilden, Germany). Samples were quantified with a Real-Time PCR System (CFX connect Real-Time PCR, Bio-Rad) using fluorescent SYBR Green technology (Bio-Rad). PCR was performed with primers targeting 16s rRNA genes of SFB and eubacteria (EUB), results were quantified using the standard curve method. Levels of SBF DNA were normalized to EUB DNA and results presented as relative fold change compared to Taconic animals. The primer sequences for detection of SFB and EUB are as follows: SFB 5’- GACGCTGAGGCATGAGAGCAT -3’ and 5’- GACGGCACGGATTGTTATTCA- 3’; EUB 5’- ACTCCTACGGGAGGCAGCAGT- 3’ and 5’- ATTACCGCGGCTGCTGGC -3’.
Data Analysis.
For all experiments, data was collected from at least two (usually three) separate cohorts of animals and is presented as the mean ± SEM. Results were analyzed (Graphpad Prism v.7) by Student’s t-test, one-way ANOVA or two-way ANOVA, followed where appropriate by Tukey’s honestly significant difference post hoc test. For all behavior tests, results were analyzed using nested one-way ANOVA, mixed model approach, followed by Tukey’s post hoc test. For all graphs, significance was defined as: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. When normalized, data were plotted relative to the appropriate, prep-matched control. Data were plotted in Graphpad Prism (v.7) and exported to Adobe Illustrator for figure preparation.
Results
Variability in the immunogenicity of different forms and lots of poly(I:C)
Consistent with recent reports (Kowash et al., 2019; Mueller et al., 2019), we found that the potency of most of the available lots of the Sigma potassium salt poly(I:C) used in the original MIA models (Smith et al., 2007) had dramatically decreased, eliciting an IL-6 response in pregnant mice ranging from 0-2% of the originally reported MIA values. Like other labs, we found that this reduced potency appears to be caused by variability in the poly(I:C) itself because there is wide variation in dsRNA concentration and molecular weight (MW) across manufacturers, lots, and even bottles from the same lot (Careaga et al., 2017; Harvey and Boksa, 2012; Kowash et al., 2019; Mueller et al., 2019). This variability contributes to heterogeneous maternal immune responses (Careaga et al., 2017) since poly(I:C) initiates viral responses in a dsRNA length-dependent manner (Kato et al., 2008; Smith et al., 2007; Zuckerman et al., 2003). In our laboratory, Sigma pure poly(I:C) showed the least variability between lots in inducing MIA (data not shown) and thus, is likely to result in less immunogen-induced variability in the model.
In order to identify the effective dose of our specific lot of pure poly(I:C), multiple doses were tested in pregnant C57BL/6 mice (Charles River; CR). Enhanced sickness behavior was observed in all pregnant mice injected with 20, 30, and 40mg/kg of poly(I:C) at 4hr post-injection (Figure 1A) but, in contrast to previous reports (Smith et al. 2007), dams showed a dose-dependent decrease in temperature at this time-point, with only the 30mg/kg group reaching significance (Figure 1B). Importantly, no dose of poly(I:C) elicited the IL-6 post-injection response of 10,000pg/ml from maternal serum sampled at 4hr post-injection reported in the original study establishing the MIA mouse model (Smith et al., 2007). However, when sampling at the apex of the IL-6 response at 2.5hr post-injection, dams receiving 30 or 40 mg/kg of poly(I:C) met this IL-6 induction threshold and lost weight by 24-hour post-injection in response to immune challenge (Figure 1C–D). In contrast, dams treated with the standard dose (20 mg/kg) fell below this IL-6 threshold on average, even though their IL-6 levels were statistically significantly higher than controls (Figure 1C). The 20 mg/kg dams also trended toward weight gain rather than loss (Figure 1D) and did not show changes in temperature (Figure 1B). Finally, the likelihood of litter resorption during pregnancy was also dependent on the poly(I:C) dose (Figure 1E). As in our previous report (Elmer et al., 2013), neuronal synapse density was significantly decreased in cultures from MIA offspring from dams treated with all doses of poly(I:C) (Figure 1F–G), but levels of major histocompatibility complex I (MHCI) levels were significantly increased only by a 30 mg/kg dose (Figure 1H). Together, our results show that the MIA model is not all-or-nothing in that a threshold maternal IL-6 response has to be crossed to induce any and all phenotypes in offspring. Instead, there may be differing thresholds for specific phenotypes. Thus, the effective dose of poly(I:C) should be determined for each new lot of poly(I:C) when generating the MIA model in order to maximize reproducibility in the specific outcomes of interest.
Figure 1. Measures of sickness behavior, litter loss, maternal IL-6, and offspring neuronal sMHCI are predictive of a disease-relevant dose of poly(I:C).
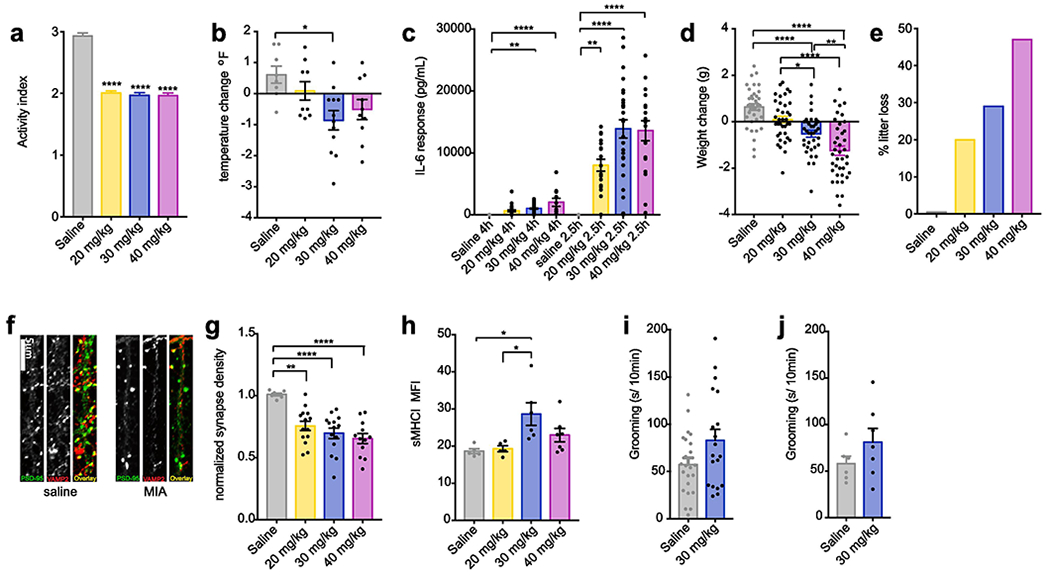
(A) Sickness behavior in pregnant female Charles River C57BL/6 mice at gestational day (GD) 12.5 was observed 4 hr post-injection with all 3 poly(I:C) doses—20, 30, and 40mg/kg (F3,184 = 102.3, P < 0.0001). (B) However, body temperature of GD12.5 dams 4hr post-injection was decreased at 30 and 40mg/kg, but not 20mg/kg, compared to saline, with only the 30mg/kg dose reaching significance (F3,35 = 4.289, P < 0.05). (C) Although all 3 doses caused significant elevations in maternal serum IL-6 at 2.5 and 4 hr post-injection, IL-6 levels were much higher at 2.5hr than at 4hr post-injection and only the 30 and 40mg/kg doses reached the 10,000 pg/ml IL-6 MIA threshold (F3,35 = 25.54, P < 0.0001). (D) The 30 and 40, but not 20, mg/kg doses caused significant weight loss in dams 24hr after poly(I:C) injection compared to saline (F7,187 = 26.93, P < 0.0001). (E) Poly(I:C) caused litter loss in a dose-dependent manner. For the pregnancies that resulted in pups, there was no change in litter size (not shown). (F) Representative images of glutamatergic synapses on dendrites cultured from the frontal cortex (FC) of newborn offspring of saline-injected (saline) or poly(I:C)-injected (MIA) mothers. Neurons were immunostained at 8 DIV for excitatory synapse density using antibodies against PSD-95 (green) and VAMP2 (red). Scale bar = 5 μm. (G) All doses of poly(I:C) were associated with a significant decrease in synapse density (SD) (F3,43 = 11.01, P < 0.0001). Values were normalized to saline control (n ≥ 7 litters). (H) Surface MHCI (H2-Kb) was significantly increased on acutely dissociated neurons from FC of P0 offspring following MIA elicited by 30, but not 20 or 40, mg/kg poly(I:C), as assessed using flow cytometry (n ≥ 5 mice per group, 2 experiments) (F3,19 = 5.156, P < 0.01). (I-J) The effects of poly(I:C) dose on male offspring grooming (secs/10 min) behavior were assessed between P60-80. (I) Although there was a strong trend toward increased grooming in the MIA offspring from the 30 mg/kg group, the results were not significant due to the high variance (P = 0.06) (n ≥ 19 mice per group from at least 6 litters, 3 experiments). (J) The lack of significance and high variance remained after accounting for litter effects by averaging the grooming behavior of individuals within each litter (P = 0.22) (n ≥ 6 litters, 3 experiments). Bars represent mean ± s.e.m *p < 0.05, **p < 0.01, ***p < 0.001.
Baseline immunoreactivity in dams prior to pregnancy predicts susceptibility or resilience of offspring to MIA-induced phenotypes.
MIA offspring display a range of aberrant behaviors with relevance for neurodevelopmental disorders, including perseverative behaviors (Malkova et al., 2012; Ronovsky et al., 2015; Schwartzer et al., 2013). However, even using the optimized lot and most effective 30 mg/kg dose of pure poly(I:C), the behavioral outcomes for grooming for young adult male MIA offspring were so variable that there was only a non-statistically significant trend towards increased repetitive grooming (Figure 1I), which was also evident when we accounted for litter effects (Figure 1J) (Lazic, 2010). Previous reports suggest that the degree of MIA is associated with MIA outcomes (Bronson et al., 2011; Garbett et al., 2012; Shi et al., 2003). Thus, individual differences in immune system response to poly(I:C) could lead to heterogeneity in MIA outcomes. Because the variance of the immune response of pregnant mice to poly(I:C) was also large (Figure 1C), we hypothesized that individual differences in the baseline immunoreactivity (BIR) of the dams could account for the heterogeneity in MIA outcomes in offspring.
To test the baseline immunoreactivity (BIR) of our female breeding stock (C57BL/6N mice from Charles River; CR), 7-8 week-old virgin females were injected with a low dose of poly(I:C) and serum IL-6 levels were measured 2.5hr post-injection. Although there must be additional accompanying differences in immune cell and cytokine responses to the injected poly(I:C), we defined BIR in this study by the single measure of serum IL-6. Surprisingly, there is a wide range of BIR in cohorts of mice delivered together from CR (Figure 2A), despite the fact that they are isogenic, the same age, and exposed to similar environmental factors as litter/cage-mates. These mice were partitioned into three BIR groups based on the serum IL-6 responses across the cohort—low (first quartile), medium (second and third quartile), and high (fourth quartile) responders (Figure 2B). Importantly, these BIR injections before pregnancy did not alter the peak or time-course of IL-6 responses to subsequent poly(I:C) injections during pregnancy (Figure 2C). Finally, because BIR responses in individual virgin female mice to two poly(I:C) injections spaced 2 weeks apart were similar in magnitude and did not change the BIR group categorization, the BIR of females appears to be a stable trait (Figure 2D).
Figure 2. Isogenic C57BL/6 female mice exhibit a wide range of baseline immunoreactivity (BIR) before pregnancy.
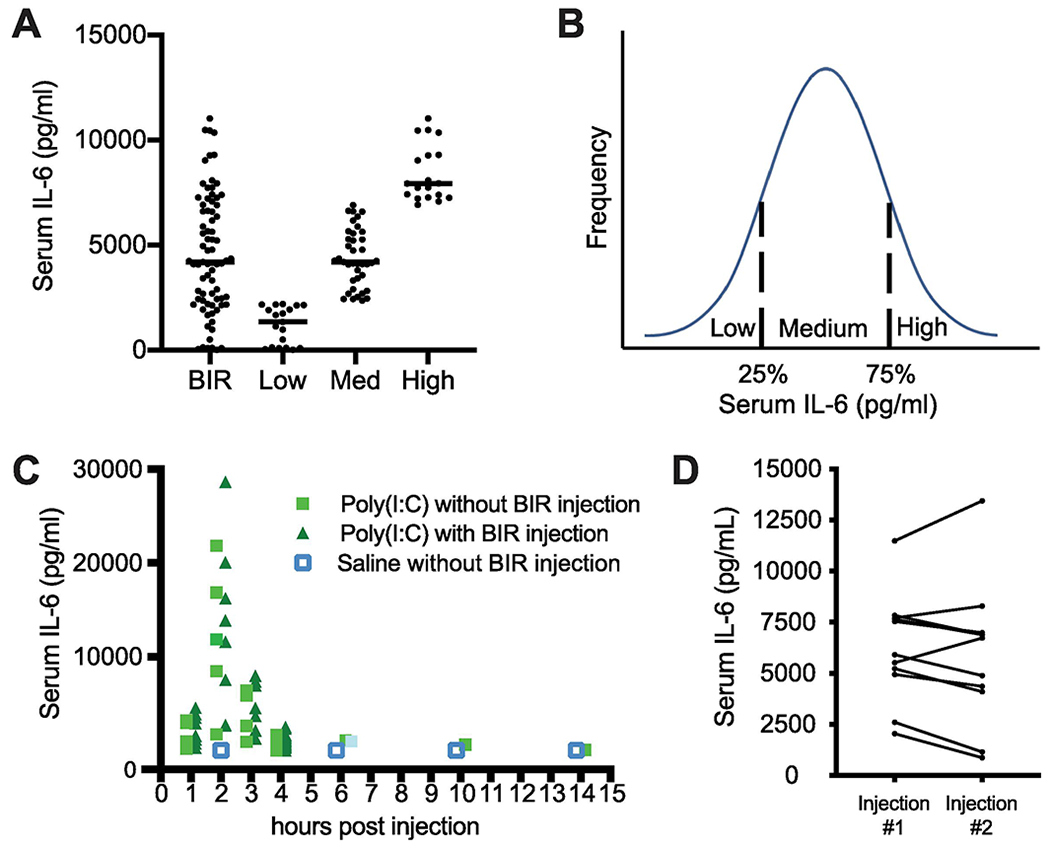
(A) 8 week-old virgin females were injected with low dose (~4 mg/ml) poly(I:C) and assessed for serum IL-6 by tail bleed 2.5hr post-injection, injected intraperitoneally (IP) with 4.0-4.4 mg/ml of high molecular weight (HMW) poly(I:C) dsRNA The BIR of ~80 virgin female CR mice is surprisingly variable, ranging from 0-12,000pg/ml when measured at 2.5h post- injection. (B) These mice can be divided into 3 groups of low, medium, and high responders. The frequency distribution of dams’ BIR defined by their serum IL-6 response to a low test dose was used to define low, medium, and high responders by quartile. (C) Testing for BIR using a low dose of poly(I:C) prior to breeding does not change the maternal response to poly(I:C) at GD12.5. Dams were tail-bled at multiple time points following poly(I:C) or saline injection at GD 12.5 and isolated serum was analyzed by Luminex for IL-6. Maternal IL-6 peaked between 2 and 2.5hr post-injection for all dams. The time-course of maternal IL-6 in unprimed mice (green squares) is the same as in primed mice tested for BIR (triangles) and both are much greater than the responses in saline-injected mice (blue open squares). Data points for each condition are shifted slightly at each time-point for better visualization. Saline injection at GD 12.5 did not alter maternal serum IL-6 at any time-point measured (n > 6). (D) BIR to low dose poly(I:C) is a stable trait in virgin female mice. One week after the first low dose injection to determine BIR, the same females were injected again with the same low dose of poly(I:C) and assessed for serum IL-6. All animals remained within their initial ‘low’, ‘medium,’ and ‘high’ BIR designation from the first to the second injection of low dose poly(I:C).
Importantly, the BIR of the female mice prior to mating and the poly(I:C) dose interact to dictate the susceptibility and resilience of subsequent pregnancies to the effects of MIA on repetitive behaviors in young adult offspring. Mice from the 3 BIR groups were bred and injected i.p. at GD12.5 with 20, 30, or 40 mg/kg of the same lot of Sigma pure poly(I:C). Repetitive behaviors, a robust phenotype in the MIA model, were then measured using a self-grooming assay. Interestingly, young adult male MIA offspring show increases in grooming in a manner dependent on both poly(I:C) dose and the BIR of the mother prior to pregnancy (Figure 3). Grouping young adult male MIA offspring based upon BIR unmasked a significant increase in grooming in MIA offspring from low and medium BIR groups (Figure 3A) exposed to the most effective dose (Figure 1) of 30 mg/kg pure poly(I:C). Unexpectedly, the offspring from dams from the high BIR group exposed to the same 30 mg/kg dose of poly(I:C) did not show any change in grooming compared to controls (Figure 3A). Changes in grooming were also not present at the typically used dose of 20 mg/kg (Figure 3B), suggesting that this dose does not cross the threshold for inducing enough MIA to alter repetitive behaviors in offspring, even though it elevates maternal IL-6 well above control levels. Although we expected to see greater changes in grooming in offspring given the higher 40 mg/kg poly(I:C) dose, grooming was only elevated in offspring from dams with low BIR at 40 mg/kg (Figure 3C), but not in the groups from higher BIR dams exposed to the same higher dose of poly(I:C). Nevertheless, together, these data indicate the surprising result that it is the intermediate levels of BIR and poly(I:C) dose that enhance susceptibility of offspring to repetitive grooming, while lower and higher combinations are protective against these aberrant behaviors.
Figure 3. MIA alters behavior in C57BL/6 CR male offspring in a dose and BIR-dependent manner.
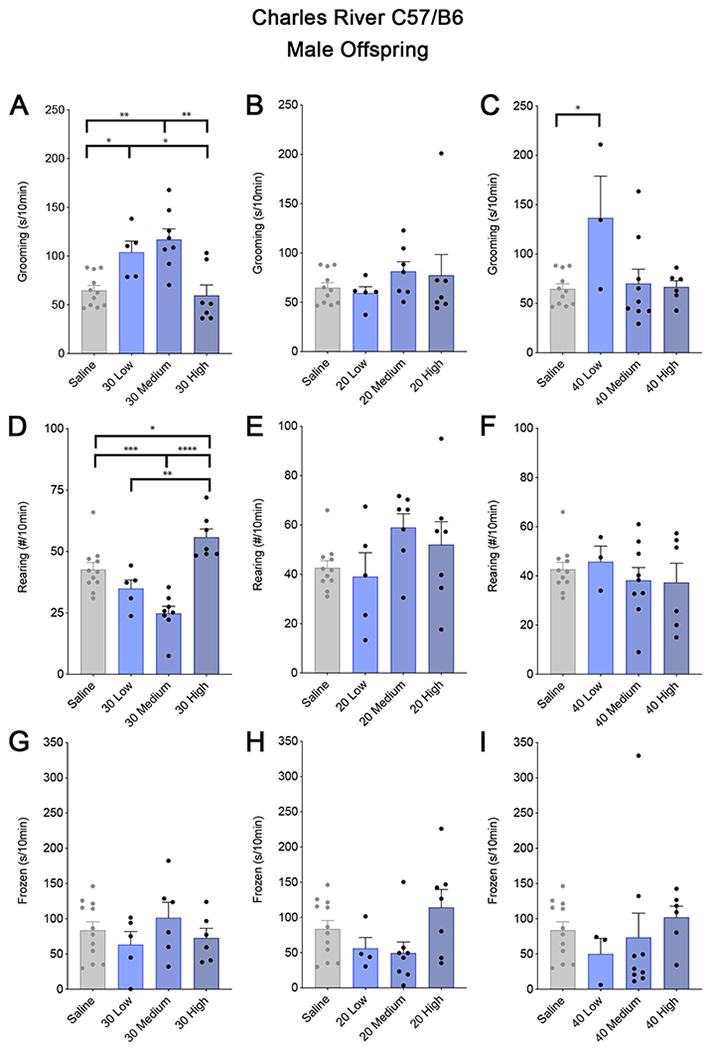
Grouping litters by BIR of the dam revealed BIR-dependent changes in grooming (A-C) and rearing (D-F), but not freezing (G-I) behavior of young adult male offspring (P60-P80) following MIA. The lowest, 20mg/kg dose of poly(I:C) did not alter any of the behaviors tested in offspring, regardless of the dam’s BIR (B, E, H). However, self-grooming was elevated in offspring from dams injected with 30 and 40 mg/kg poly(I:C), in a BIR-dependent manner. In the 30mg/kg group (A), the time spent grooming was significantly increased in the low and medium BIR groups (Nested one-way ANOVA; F3,27 = 8.775; Low: P = 0.0427; Medium: P = 0.0062), but there was no difference from controls in the high BIR group (P = 0.9568). (C) Offspring from low BIR dams injected with a higher, 40mg/kg dose also showed increased grooming, but there was no change in grooming in the medium or high BIR groups at this higher dose (Nested one-way ANOVA; F3,25 = 2.862, Low: P = 0.0442; Medium: P = 0.9859; High: P > 0.9999). (D) Offspring from dams injected with 30mg/kg poly(I:C) also exhibited changes in rearing. Offspring from medium BIR dams showed a significant decrease in frequency, while those from high BIR dams showed the opposite behavior—a significant increase compared to controls (F3,15 = 9.407, Medium: P < 0.001; High: P = 0.0117). Low BIR MIA offspring were not different from controls (P = 0.4910). There were also no changes in rearing at lower (20mg/kg) or higher (40mg/kg) doses of poly(I:C) (E,F). 2-6 pups per litter were assessed for behaviors and their responses were averaged into a single value per litter. It is important to note that the variability in control offspring likely contributes to the lack of significant effects in some of these behaviors. Bars represent mean of litter values for 3-12 litters, as indicated ± SEM. Significance was determined using a nested 1-way ANOVA followed by Tukey’s test for multiple comparisons.
A different pattern across BIR groups was evident for abnormalities in rearing, an exploratory behavior in rodents indicative of anxiety (Borta and Schwarting, 2005; Gao et al., 2011). Medium and high BIR MIA offspring exposed to the 30 mg/kg dose showed a significant difference in rearing compared to control offspring, but the direction of change was opposite in these groups—decreased in the medium and elevated in the high BIR MIA offspring (Figure 3D,). There were no significant differences in either behavior in MIA offspring from any BIR group injected with 20 or 40 mg/kg poly(I:C) (Figure 3E–F) and there were no statistically significant differences in freezing across any of the groups tested (Figure 3G–H). Finally, female offspring from the same litters appear mostly resilient to the effects of MIA in inducing perseverative behaviors (Figure 4), with the exception that 30 mg/kg poly(I:C) dramatically decreased rearing specifically in the medium BIR group (Figure 4D) with no change in grooming or freezing (Figure 4A–C,G–I). For some of these behavioral measures, the variability in control offspring is high, especially for females. For both males and females, this variability is likely influenced by inherent differences in each animal’s environment, especially differences in within-cage rank which are associated with many variables including access to food, microbiome composition, stress, and injury due to fighting (Varholick et al., 2019). Interestingly, the relative effectiveness of the intermediate 30 mg/kg dose of poly(I:C) in causing aberrant behaviors in offspring parallels the dose-specific changes in maternal temperature and weight loss and offspring MHCI levels (Figure 1). These data suggest that a threshold of MIA must be crossed for specific behavioral phenotypes in offspring to be robust and that the level and type of immune activation above this threshold can dictate resilience or susceptibility and lead to distinct combinations of aberrant phenotypes in offspring, reminiscent of the wide range of outcomes in humans exposed to maternal infection (Estes and McAllister, 2015, 2016).
Figure 4. Female C57BL/6 CR offspring are less affected by MIA than their male littermates.
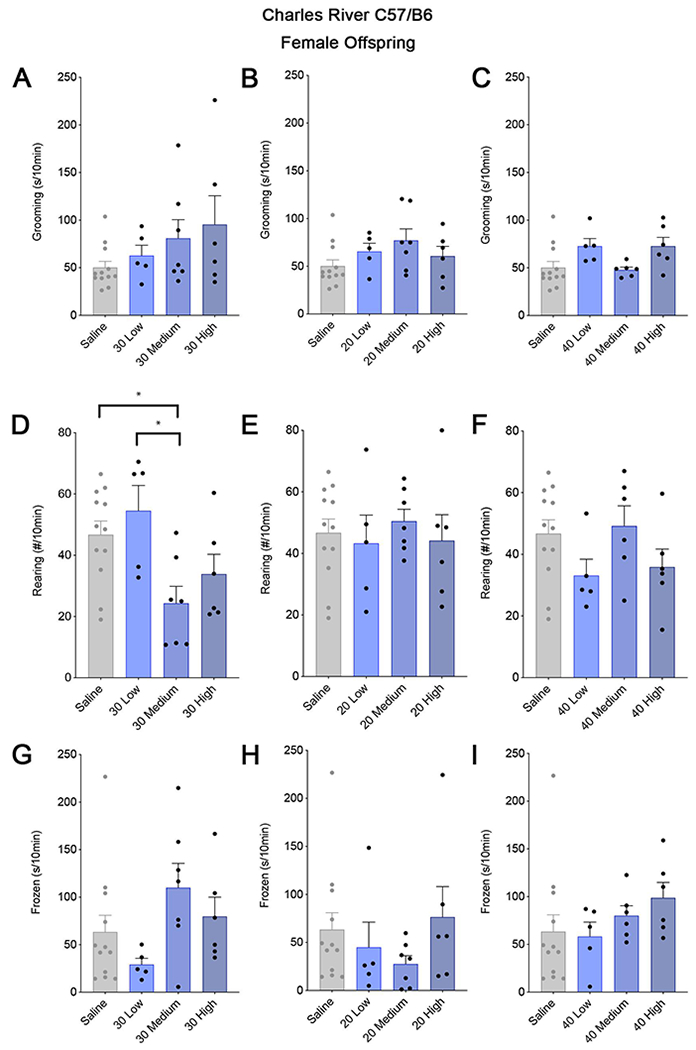
The young adult female littermates of the males shown in Figure 3 were mostly unaffected by MIA, regardless of BIR group. In contrast to male offspring, there was no difference in grooming behavior (A-C) in any of the female offspring from dams injected with any of the three poly(I:C) doses. (D) The only significant behavioral difference in the female offspring was a decrease in rearing in the offspring from dams with medium BIR injected with 30mg/kg poly(I:C) (Nested one-way ANOVA; F3,26 = 4.623, P = 0.0305), similar to the changes found in their male littermates (Figure 3D). Female offspring from dams injected with the same dose, but with low or high BIR, and from dams injected with lower (20mg/kg) (E) or higher doses (40mg/kg poly(I:C) (F), were no different from control offspring (Nested one-way ANOVA; 20mg/kg; F3,26 = 0.1937, Low: P = 0.9849; Medium: P = 0.9693; High: P = 0.9953; 30mg/kg, F3,26 = 4.623, Low: P = 0.8567; High: P = 0.3447; 40mg/kg; F3,25 = 2.239, Low: P = 0.3767; Medium: P = 0.9421, High: P = 0.3248). As in the males, MIA does not alter freezing in offspring, although there were stronger trends toward changes in the females than in the males (G-I). 2-6 pups per litter were assessed for behaviors and their responses were averaged into a single value per litter. It is important to note that the variability in control offspring likely contributes to the lack of significant effects in some of these behaviors. Bars represent mean of litter values for 3-12 litters, as indicated ± SEM. Significance was determined using a nested 1-way ANOVA followed by Tukey’s test for multiple comparisons.
A significant advance for the field would be identification of biomarkers with high predictive value for specific MIA outcomes in offspring. We identified three potential biomarkers in the striatum, a brain region involved in regulating perseverative behaviors (Kalueff et al., 2016), that correlate with enhanced repetitive behaviors in offspring. MEF2A—a transcription factor that mediates synaptic deficits caused by MIA (Elmer et al., 2013), STAT3—a major signaling protein downstream of cytokines, and tyrosine hydroxylase (TH)—an enzyme involved in the synthesis of dopamine—were increased selectively in newborn offspring from medium BIR MIA dams (Figure 5A–D). Thus, levels of these proteins in the striatum of offspring may act as biomarkers for aberrant repetitive behaviors in mice and can be used to determine the effective dose of a new lot of poly(I:C) when access to behavioral or synaptic assays is limited.
Figure 5. Levels of dopaminergic and immune proteins are altered in the striatum of newborn offspring in a BIR-dependent manner that correlates with repetitive behavioral deficits.
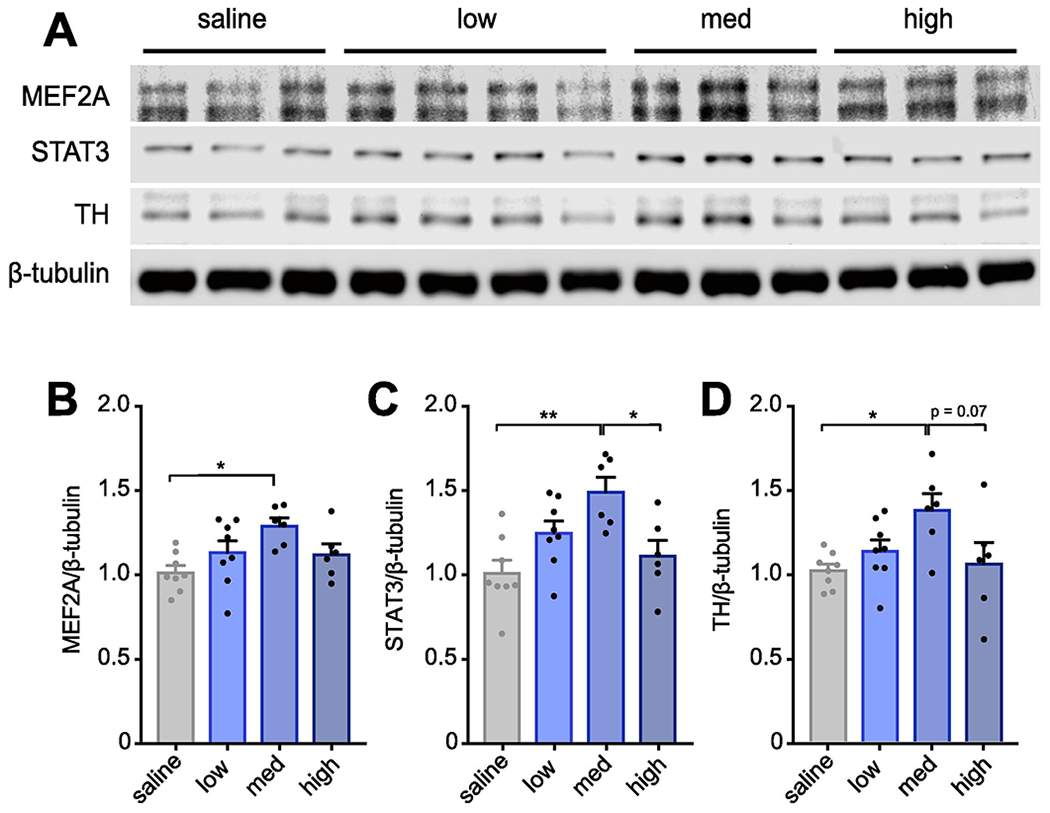
(A) Representative western blot showing increased MEF2, STAT3, and TH protein from striatum of MIA offspring compared to saline control offspring in a manner dependent on BIR of the dams. Each column is from one animal. (B-D) Densitometry shows increased levels of MEF2A (B), STAT3 (C), and TH (D) protein in MIA offspring relative to saline when normalized to β-tubulin loading controls (MEF2A: F3,24 = 3.968, P < 0.05; STAT3: F3,24 = 6.401, P < 0.01; TH: F3,24 = 3.668, P < 0.05). Data were averaged from two males per litter and 6-7 litters per BIR group; each point indicates the litter average value normalized to controls. Bars represent mean ± s.e.m *p < 0.05, **p < 0.01, ***p < 0.001.
MIA engages distinct molecular pathways in C57BL/6 mice from different vendors.
Surprisingly, the source of mice of the same strain also plays a role in determining whether and how offspring will be affected. The MIA model was historically generated with C57BL/6 mice sourced from Charles River (CR) or Jackson (JAX) (Estes and McAllister, 2017). However, it has recently been shown that a maternal microbiome component (SFB), found in C57BL/6 mice sourced from Taconic (TAC), but not JAX, is necessary for the generation of MIA outcomes (Kim et al., 2017). In these MIA TAC mice, increased maternal IL-6 acts to stimulate the secretion of IL-17a from maternal T helper (Th) 17 cells, which is necessary and sufficient to cause aberrant behaviors in offspring (Choi et al., 2016). While this study did not find MIA phenotypes in offspring from SFB-negative JAX dams, mice from JAX have been used successfully as MIA models in previous reports although the levels of SFB were not reported in those papers (Connor et al., 2012; Giovanoli et al., 2015; Malkova et al., 2012; Money et al., 2017; Smith et al., 2007). In order to determine whether SFB and IL-17a are associated with the generation of MIA outcomes, we first confirmed that C57BL/6 mice purchased from TAC harbor SFB while those from JAX do not and we found that CR mice have levels of SFB comparable to TAC mice (Figure 6A). While baseline IL-17a levels were not significantly different in saline-injected CR and TAC dams, only TAC dams showed an increase in IL-17a 48hr after poly(I:C) injection (Figure 6B). In response to immune challenge, splenic maternal TH17 cells increased significantly in TAC, but not CR dams (Figure 6C). In contrast to CR dams, TAC dams displayed no fetal loss in response to poly(I:C) (data not shown) providing further support for the conclusion that cytokine changes caused by MIA that contribute to fetal loss may be distinct between C57BL/6 mice sourced from CR vs. TAC. Additionally, while CR dams lost weight 24hr following poly(I:C) injection, TAC dams generally showed no weight loss or trended towards weight gain (Figure 6D). Although future studies are needed to explore the role for IL-17 and Th17 cells in MIA in CR dams, these surprising differences in maternal response to immune challenge between the same strain of mice sourced from different vendors may also contribute to differences in MIA outcomes.
Figure 6. C57BL/6 mice from different sources differ in their responses to MIA both during pregnancy and in the brains of offspring.
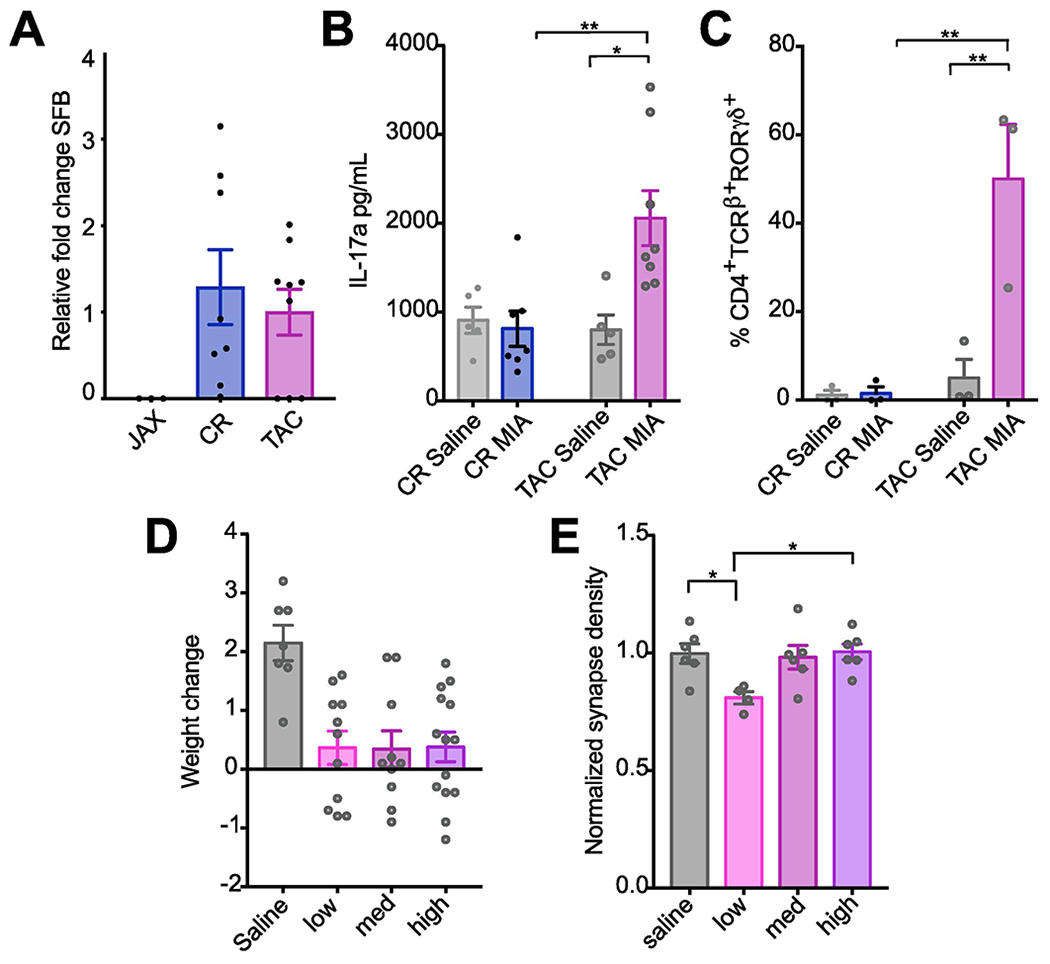
(A) Mice sourced from CR and TAC harbor segmented filamentous bacteria (SFB), while mice sourced from JAX do not. SFB expression was detected using qPCR from the fecal samples from CR and TAC mice. Consistent with previous reports, we did not detect SFB in the fecal samples of mice sourced from JAX. Bars represent mean ± SEM, n > 3 mice per condition. (B) Poly(I:C) injection at GD 12.5 caused dramatic elevations in serum concentrations of maternal IL-17a at GD14.5 in TAC, but not in CR, dams (significant interaction condition x source; F1,21 = 7.164, P < 0.05; n ≥ 5 mice per condition, > 2 experiments). (C) TAC but not CR dams show a significant increase in splenic TH17 cells 48hr following poly(I:C) injection (F1,8 = 11.48, P < 0.01). Splenic CD4+TCRβ+ T cells stained intracellularly for RORγt from GD14.5 dams injected with poly(I:C) or saline at GD12.5 were detected using flow cytometry. (D) TAC dams do not lose weight in any BIR MIA group 24hr following poly(I:C) injection. (E) Neurons from neonatal low BIR MIA offspring from TAC dams showed a significant decrease in synapse density (SD) (F3,18 = 4.014, P < 0.05) whereas there was MIA-induced no change in synapse density in neurons from offspring from med and high BIR dams. Values were normalized to saline control (n ≥ 4 litters). Bars represent mean ± s.e.m *p < 0.05, **p < 0.01, ***p < 0.001.
To test the effects of vendor on vulnerability of C57BL/6 mice to MIA insult using the same lot of pure Sigma poly(I:C) at the most effective dose of 30 mg/kg, we performed the same synaptic and behavioral assays on MIA and control offspring from dams sourced from TAC. In contrast to results from CR mice in which all poly(I:C) doses caused substantial defects in synapse formation in cortical cultures (Figure 1), there was only a slight but significant decrease in synapse formation in TAC MIA offspring from low BIR (but not medium or high) dams (Figure 6E). MIA also induced perseverative behaviors in TAC MIA offspring. Baseline grooming, rearing, and freezing behavior was similar between control offspring from CR and TAC dams (Figures 3, 7). Similar to MIA CR offspring, MIA TAC offspring from low and medium BIR dams showed increased self-grooming (Figure 7A), while offspring exposed to the same poly(I:C) dose but from high BIR dams were not affected. When comparing grooming behavior from MIA offspring we found a significant interaction between source and BIR and a larger effect size in time spent self-grooming in MIA offspring from low BIR dams when animals were sourced from TAC (source x BIR: F3,51 = 3.81, P<0.05; post hoc TAC low>CR low; P<0.01). Rearing behavior was also altered in TAC MIA offspring (Figure 7B). When comparing rearing behaviors of MIA offspring we found a significant interaction between source and BIR (F3,51 = 6.445, P<0.001), where in contrast to CR MIA offspring, rearing was decreased in TAC MIA offspring from all BIR dams. As with CR offspring, there was no change in freezing behavior in male offspring and no change in any of the behaviors tested in female offspring (Figure 7C–F). Together, these results suggest that the source of C57BL/6 mice dictates both the nature of the maternal immune response following MIA, including source-dependent changes in Th17 cells and IL-17 levels, as well as the susceptibility and resilience of offspring to both neuropathology and repetitive behaviors.
Figure. 7. MIA causes elevated grooming and reduced rearing in a BIR-dependent manner in male, but not female, C57BL/6 adult offspring from Taconic.
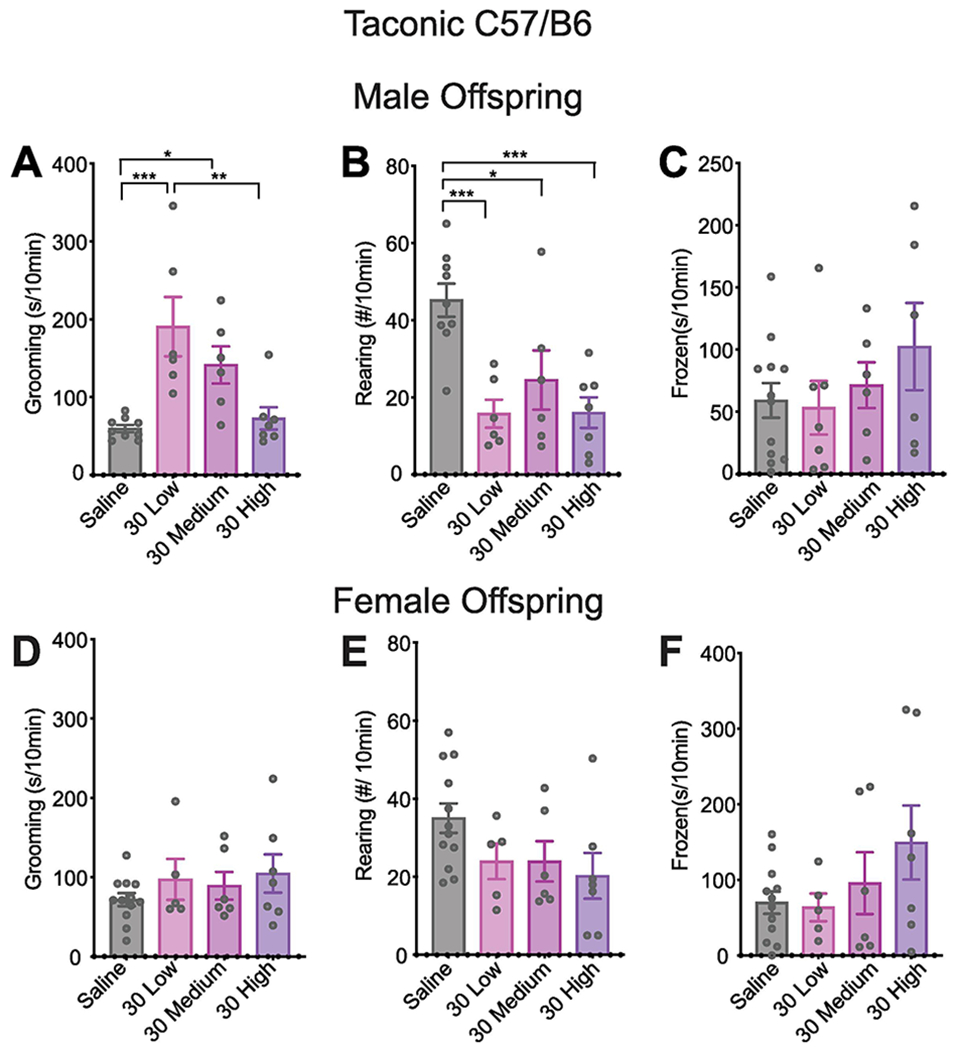
Young adult male (P60-P80) MIA offspring from TAC dams treated with a 30 mg/kg dose of poly(I:C) were assessed for (A) grooming, (B) rearing, and (C) freezing behaviors. Similar to male mice from CR (Figure 3), MIA TAC male offspring showed elevated grooming in low and medium BIR groups, but not the high BIR group (F3,24 = 8.781, low: P < 0.001; medium: P = 0.0393, high: P = 0.9520). However, the effect size for time spent self-grooming in MIA offspring from low BIR dams was larger when animals were sourced from TAC compared to CR (source x BIR: F3,51 = 3.81, P < 0.05; post hoc TAC low > CR low; P < 0.01). (B) Rearing was decreased in all BIR MIA groups in TAC mice (F3,24 = 8.764, low: P = 0.0016; medium: P = 0.0370; high: P = 0.0012), in contrast to the more complex effects of MIA on rearing in CR mice shown in Figure 3. (C) MIA did not alter freezing in male offspring. In contrast to the males, the female offspring exhibited no behavioral changes in (D) grooming, (E) rearing, or (F) freezing. 2-6 pups per litter were assessed for behaviors and their responses were averaged into a single value per litter. It is important to note that the variability in control offspring likely contributes to the lack of significant effects in some of these behaviors. Bars represent mean of litter values for 3-12 litters, as indicated ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Significance was determined using a nested 1-way ANOVA followed by Tukey’s test for multiple comparisons.
Discussion
Despite the potential of rodent models of maternal immune activation (MIA) to identify new biomarkers and therapeutic interventions for a range of psychiatric disorders, current approaches using these models ignore two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal viral infection and (ii) susceptible pregnancies can lead to different combinations of phenotypes in offspring. Here, we report two new sources of variability—the baseline immunoreactivity (BIR) of females prior to pregnancy and differences in immune responses in C57BL/6 dams across vendors—that contribute to resilience and susceptibility to distinct combinations of outcomes in offspring. MIA in mice does not cause disease-related phenotypes in offspring from every pregnancy, even in response to the same lot and poly(I:C) dose, but the combination of dose and BIR predicts susceptibility and resilience to MIA-induced phenotypes in offspring. Importantly, we identify the BIR of female mice as a biomarker before pregnancy that predicts which dams will be most at risk as well as biomarkers in the brains of newborn offspring that correlate with changes in repetitive behaviors. Together, our results identify an approach for optimizing MIA protocols to enhance rigor and reproducibility and reveal new factors that drive susceptibility of some pregnancies and resilience of others to specific combinations of MIA-induced abnormalities in offspring.
Issues of reproducibility of the MIA model are currently a significant obstacle for the field and hamper the use of this important translational model for pre-clinical research (Careaga et al., 2017; Estes and McAllister, 2016; Kentner et al., 2019). Recent reports have demonstrated a lack of efficacy of particular sources and lots of poly(I:C) to induce sufficient MIA (Kowash et al., 2019; Mueller et al., 2019), as well as a link between the magnitude of MIA and outcomes in offspring (Harvey and Boksa, 2012; Meyer, 2014; Mueller et al., 2019; Smolders et al., 2015). Our results extend those findings by identifying a threshold of maternal IL-6 and specific maternal sickness behaviors as well as changes in synapse formation and protein levels in cortex and striatum of offspring, that can be used as biomarkers to find an effective concentration of any lot of poly(I:C) to induce neuropathology and changes in behaviors in offspring. Because poly(I:C) immunogenicity varies so much between forms and lots, we recommend that laboratories use the pure form of poly(I:C) to minimize within-lot variability and that they optimize the effective dose of poly(I:C) for each new lot in order to elicit a comparable level of MIA across lots for their experiments. Reporting measures of maternal cytokines are essential for any study using this model to allow for comparison across studies and maternal serum IL-6, while not the only important cytokine for MIA induction, is the cytokine that most labs use. Maternal levels of serum IL-6 of ~10,000pg/ml on average measured at 2.5hr post-injection from a specific lot and dose of poly(I:C) are a reliable threshold for induction of MIA leading to reproducible changes in repetitive behaviors in offspring. Importantly, this MIA threshold is well above smaller magnitude changes that are statistically significantly elevated compared to saline-injected mice but that have no effect on, at least, repetitive behaviors. Future studies are needed to determine the specific MIA thresholds, and the accompanying nature of the immune responses, for other kinds of behavioral and neuropathological phenotypes. Because the optimization process is costly, labs might consider purchasing a large amount of a single lot of poly(I:C) to maximize the investment in establishing parameters for reproducible results. Finally, although it is well known that MIA phenotypes differ between mouse strains, our results indicate that the source of the mice is also a critical consideration for establishing the model since MIA activates distinct immune responses in C57BL/6 mice from different vendors.
It is generally assumed that isogenic mice will exhibit similar immune responses to stimuli, so we were surprised to find such a wide range of immune responses in female mice both before and during pregnancy to the same lot and dose of poly(I:C). Why some female mice have high BIR whereas other have low BIR is completely unknown, but BIR must be an innate trait since it is stable over time and since mice bred in-house exhibit a similar BIR range (not shown). The cause could be epigenetic, being set soon after birth, or it could be environmental, being set by stress hormones that are well known to potently affect immune function (Howerton and Bale, 2012; Jasarevic and Bale, 2019; Zhang and Cao, 2019). It is important to note that we have defined BIR using only a single measure—serum IL-6 measured 2.5hr following poly(I:C) injection. Our results do not indicate that this cytokine mediates any of the effect of BIR in setting risk of subsequent pregnancies to MIA, necessarily, but rather we have shown that IL-6 levels simply correlate with outcomes during later pregnancies. Future work is warranted to fully characterize the immune cell types and responses that must comprise BIR, in addition to IL-6 measured in our study.
One of the most striking results from our study is that the intermediate levels of BIR and poly(I:C) dose are most detrimental to offspring, with higher BIR and poly(I:C) doses conferring resilience at least to the behaviors and striatal proteins measured in our study. This is surprising since it has been reported that the magnitude of MIA correlates with the severity of outcomes in offspring (Harvey and Boksa, 2012; Meyer, 2014; Mueller et al., 2019; Smolders et al., 2015) and it has been assumed that higher doses of poly(I:C) would lead to more severe consequences in offspring. In contrast, we found that offspring from dams with the same BIR but exposed to a higher dose of poly(I:C)—i.e. medium BIR, 40 mg/kg poly(I:C)—are resilient to the MIA-induced increase in self-grooming caused at lower poly(I:C) doses (30 mg/kg). Moreover, offspring from dams exposed to the same 30 mg/kg dose of poly(I:C) exhibit different combinations of aberrant behaviors, depending on the BIR of the dam. Offspring from low BIR dams show elevated grooming with no change in rearing, those from medium BIR dams show elevated grooming and decreased rearing, while those from high BIR dams show normal grooming and elevated rearing. A more extensive battery of behavioral tests is required to determine whether offspring from higher doses are truly resilient and to determine if BIR causes distinct subsets of behavioral aberrations across multiple domains in offspring exposed to the same poly(I:C) does in utero. Nevertheless, our results already imply that there must be a difference in the maternal immune response in dams exposed to higher poly(I:C) doses that is protective, at least for self-grooming and corresponding changes in striatal proteins. This could be the result of context and dose-dependent properties of maternal cytokines like IL-6, which promote inflammation at intermediate levels and suppress it at higher levels to limit tissue damage (Lauder et al., 2013; Xing et al., 1998). Future studies will be important to identify the differences in nature and kinetics of the full maternal immune response that dictate susceptibility and resilience to MIA.
Even when controlling for the dose, delivery route, and MW of poly(I:C), MIA leads to a wide range of outcomes in offspring. Rather than being a problem for the model, this variability strengthens the construct and face validity of the model since one of the most important aspects of maternal viral infection in humans is the variability in outcomes and the fact that many pregnancies do not result in disease in offspring. Although variable outcomes in humans to disease risk factors are typically attributed to genetic differences, we found that even for female mice of the same genetic background the BIR before pregnancy may be a critical factor dictating susceptibility and resilience to MIA. The sources of variability described here are essential clues for identifying the factors that drive susceptibility of some pregnancies and resilience of others to MIA-induced abnormalities in offspring. Studying the circuit and molecular mechanisms underlying these distinct phenotypes caused by the same environmental insult could help to uncover factors that protect against, or increase susceptibility, to specific MIA outcomes and that can be targeted in the future to prevent the effects of MIA in causing neuropathology and behavioral abnormalities in offspring.
Supplementary Material
Supplementary Table 1. MIA model reporting details. Details are provided for the MIA model as recommended in Kentner et al. 2019, including the ARRIVE reporting guideline and recommendation, as well as the MIA model specific reporting recommendation.
Supplementary Figure 1. Nested Graphs for Male CR Behaviors. Graphs showing all data points for all individual C57B/6 male mice purchased from Charles River (CR) in each litter averaged for the data points shown in Figure 3. Results are shown for repetitive grooming (A-C), rearing (D-F), and freezing (G-I) for offspring from dams injected with (A, D, G) 20 mg/kg, (B, E, H) 30 mg/kg, and (C, F, I) 40 mg/kg ploy(I:C).
Supplementary Figure 2. Nested Graphs for Female CR Behaviors. Graphs showing all data points for all individual C57B/6 female mice purchased from Charles River (CR) in each litter averaged for the data points shown in Figure 4. Results are shown for repetitive grooming (A-C), rearing (D-F), and freezing (G-I) for offspring from dams injected with (A, D, G) 20 mg/kg, (B, E, H) 30 mg/kg, and (C, F, I) 40 mg/kg ploy(I:C).
Supplementary Figure 3. Nested Graphs for TAC Behaviors. Graphs showing all data points for all individual C57B/6 male (A-C) and female (D-F) mice purchased from Taconic (TAC) in each litter averaged for the data points shown in Figure 7. Results are shown for repetitive grooming (A, D), rearing (B, E), and freezing (C, F) for offspring from dams injected with 30 mg/kg ploy(I:C).
Acknowledgments:
We wish to thank Dr. David C. Katz for consultation on statistics and editing the manuscript. This work was supported by the Stanley & Jacqueline Schilling Neuroscience Postdoctoral Research Fellowship (M.L.E.), Dennis Weatherstone Predoctoral Fellowships from Autism Speaks (#7825 M.L.E.), the Letty and James Callinan and Cathy and Andrew Moley Fellowship from the ARCS Foundation (M.L.E.), a Dissertation Year Fellowship from the University of California Office of the President (M.L.E.), the Daniel T. O’Connor, MD Memorial Research Grant Scholar Award (J.P.A.), the Eugene Cota- Robles Fellowship (K.P.), T32-MH112507 (K.P.), P50-MH106438-01 (C.S.C), the University of California Davis Research Investments in Science and Engineering Program (A.K.M.), the MIND Institute IDDRC grant (U54HD079125), and by a grant from the Simons Foundation (SFARI #321998, A.K.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures/Conflicts: The authors declare no competing financial interests.
References
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET, 2010. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH, 2014. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry 75, 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borta A, Schwarting RK, 2005. Post-trial treatment with the nicotinic agonist metanicotine: Differential effects in Wistar rats with high versus low rearing activity. Pharmacol Biochem Behav 80, 541–548. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM, 2011. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res 220, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Murai T, Bauman MD, 2017. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry 81, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Taylor SL, Chang C, Chiang A, Ku KM, Berman RF, Van de Water JA, Bauman MD, 2018. Variability in PolyIC induced immune response: Implications for preclinical maternal immune activation models. J Neuroimmunol 323, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR, 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S, 2012. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res 140, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer BM, Estes ML, Barrow SL, McAllister AK, 2013. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 13791–13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK, 2015. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK, 2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK, 2017. Brain, Immunity, Gut: “BIG” Links between Pregnancy and Autism. Immunity 47, 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Schneider EH, Dimitrov EL, Haun F, Pham TM, Mohammed AH, Usdin TB, Murphy PM, 2011. Reduced fear memory and anxiety-like behavior in mice lacking formylpeptide receptor 1. Behav Genet 41, 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K, 2012. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2, e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Notter T, Richetto J, Labouesse MA, Vuillermot S, Riva MA, Meyer U, 2015. Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. J Neuroinflammation 12, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey L, Boksa P, 2012. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology 62, 1767–1776. [DOI] [PubMed] [Google Scholar]
- Howerton CL, Bale TL, 2012. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav 62, 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander BL, Eng JW, Repasky EA, 2017. The Impact of Housing Temperature-Induced Chronic Stress on Preclinical Mouse Tumor Models and Therapeutic Responses: An Important Role for the Nervous System. Adv Exp Med Biol 1036, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Bale TL, 2019. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front Neuroendocrinol, 100797. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC, 2016. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S, 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 205, 1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD, 2019. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 44, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR, 2017. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowash HM, Potter HG, Edye ME, Prinssen EP, Bandinelli S, Neill JC, Hager R, Glazier JD, 2019. Poly(I:C) source, molecular weight and endotoxin contamination affect dam and prenatal outcomes, implications for models of maternal immune activation. Brain Behav Immun 82, 160–166. [DOI] [PubMed] [Google Scholar]
- Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, Ondondo B, Taylor PR, Clement M, Fielding C, Godkin AJ, Jones SA, Gallimore AM, 2013. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol 43, 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, 2010. The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis? BMC Neurosci 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE, 2018. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH, 2012. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, 2014. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75, 307–315. [DOI] [PubMed] [Google Scholar]
- Meyer U, 2019. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, 2010. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 90, 285–326. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J, 2006. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26, 4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KM, Barke TL, Serezani A, Gannon M, Garbett KA, Aronoff DM, Mirnics K, 2017. Gestational diabetes exacerbates maternal immune activation effects in the developing brain. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller FS, Polesel M, Richetto J, Meyer U, Weber-Stadlbauer U, 2018. Mouse models of maternal immune activation: Mind your caging system! Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Mueller FS, Richetto J, Hayes LN, Zambon A, Pollak DD, Sawa A, Meyer U, Weber-Stadlbauer U, 2019. Influence of poly(I:C) variability on thermoregulation, immune responses and pregnancy outcomes in mouse models of maternal immune activation. Brain Behav Immun 80, 406–418. [DOI] [PubMed] [Google Scholar]
- Patterson PH, 2009. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204, 313–321. [DOI] [PubMed] [Google Scholar]
- Ronovsky M, Berger S, Molz B, Berger A, Pollak DD, 2015. Animal Models of Maternal Immune Activation in Depression Research. Curr Neuropharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P, 2013. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry 3, e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH, 2003. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH, 2007. Maternal immune activation alters fetal brain development through interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders S, Smolders SM, Swinnen N, Gartner A, Rigo JM, Legendre P, Brone B, 2015. Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo. Front Cell Neurosci 9, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS, 2014. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11, 629–632. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ, 2013. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav 63, 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varholick JA, Pontiggia A, Murphy E, Daniele V, Palme R, Voelkl B, Wurbel H, Bailoo JD, 2019. Social dominance hierarchy type and rank contribute to phenotypic variation within cages of laboratory mice. Sci Rep 9, 13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK, 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao X, 2019. Epigenetic regulation of the innate immune response to infection. Nat Rev Immunol 19, 417–432. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I, 2003. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 28, 1778–1789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. MIA model reporting details. Details are provided for the MIA model as recommended in Kentner et al. 2019, including the ARRIVE reporting guideline and recommendation, as well as the MIA model specific reporting recommendation.
Supplementary Figure 1. Nested Graphs for Male CR Behaviors. Graphs showing all data points for all individual C57B/6 male mice purchased from Charles River (CR) in each litter averaged for the data points shown in Figure 3. Results are shown for repetitive grooming (A-C), rearing (D-F), and freezing (G-I) for offspring from dams injected with (A, D, G) 20 mg/kg, (B, E, H) 30 mg/kg, and (C, F, I) 40 mg/kg ploy(I:C).
Supplementary Figure 2. Nested Graphs for Female CR Behaviors. Graphs showing all data points for all individual C57B/6 female mice purchased from Charles River (CR) in each litter averaged for the data points shown in Figure 4. Results are shown for repetitive grooming (A-C), rearing (D-F), and freezing (G-I) for offspring from dams injected with (A, D, G) 20 mg/kg, (B, E, H) 30 mg/kg, and (C, F, I) 40 mg/kg ploy(I:C).
Supplementary Figure 3. Nested Graphs for TAC Behaviors. Graphs showing all data points for all individual C57B/6 male (A-C) and female (D-F) mice purchased from Taconic (TAC) in each litter averaged for the data points shown in Figure 7. Results are shown for repetitive grooming (A, D), rearing (B, E), and freezing (C, F) for offspring from dams injected with 30 mg/kg ploy(I:C).


