Abstract
We explore the application of a wide range of sensory stimulation technologies to the area of sleep and dream engineering. We begin by emphasizing the causal role of the body in dream generation, and describe a circuitry between the sleeping body and the dreaming mind. We suggest that nearly any sensory stimuli has potential for modulating experience in sleep. Considering other areas that might afford tools for engineering sensory content in simulated worlds, we turn to Virtual Reality (VR). We outline a collection of relevant VR technologies, including devices engineered to stimulate haptic, temperature, vestibular, olfactory, and auditory sensations. We believe these technologies, which have been developed for high mobility and low cost, can be translated to the field of dream engineering. We close by discussing possible future directions in this field and the ethics of a world in which targeted dream direction and sleep manipulation are feasible.
Keywords: Dreaming, Human Computer interaction, Haptic devices, Virtual reality, Simulation, Sleep
1. Introduction
Dreaming is often considered a subjective experience generated by the mind and brain, while cut off from the body and the external environment (Brueckner, 1986). In philosophy, this view is referred to as brain in a vat consciousness, whereby the brain generates experience even in the absence of physical input or outward control. One of the logical consequences of any brain in a vat theory is that bodiless brains attached to reality simulators would still have the same experience we are having right now. This position is juxtaposed by the richly embodied and immersive experiences brought about in dreams, where we feel our dreamt bodies and engage with our imagined environments. Proponents of neurocognitive theories of dreaming suggest that such experiences are akin to simulations of the waking world (Foulkes, 1985; Revonsuo, 2000; Tart, 1987; see Nielsen, 2010 for a review). They are created from memory – recent and remote – and come alive through sensorimotor, limbic, and default-mode cortical activity. In Hobson’s theory of protoconsciousness (Hobson, 2009; Hobson, Hong, & Friston, 2014), the REM state is viewed as a virtual reality (VR) generator used by the brain to instantiate world interactions and to build predictions of space and time.
Yet dreamed experience does not occur in isolation from the sleeping body. Dreaming shows one-to-one correspondences – termed isomorphisms – between the experiences of the dreaming body and the ‘real’ sleeping body. Bodily sources of dream imagery include sensory incorporation, muscular twitches, and other isomorphisms between eye movements, respiration, motor circuitry, and heart rate (Dement & Wolpert, 1958). Thus, Windt (2018) proposes that dreams are “phenomenally-functionally embodied simulations” Phenomenal embodiment refers to the subjective experience of being-in-a-world, a key in simulation views of dreaming. Functional embodiment refers to “the causal relationship between phenomenal embodiment on the one hand and sensory inputs and motor outputs on the level of the physical body on the other hand.” In other words, the dreaming brain is not in a vat; it is in a circuit with the sleeping body.
Framing dreams as functionally embodied opens up an avenue to manipulating the dreaming mind: the body as a permeable barrier that can be used to interface with the virtual world of dreams. Parallel work in Human-Computer Interaction (HCI) has utilized the body as an interface to increase the immersion of virtual environments by engineering multi-modal devices that can simulate haptic sensations such as touch, temperature, and inertial forces as well as audio-visual or olfactory sensations. We believe these systems are prime candidates to influence dreams on demand and in ambulatory settings. In this review, we present a model of dreaming as being in circuit with the sleeping body and adapt HCI approaches to dream engineering. We discuss a set of technologies to influence sleep for memory enhancement, creativity, emotion regulation and physical rehabilitation. Possible future directions in dream engineering solutions and ethical considerations will be discussed.
1.1. Isomorphisms in the dreaming and sleeping body
The discovery of the “rapid, jerky, binocularly symmetrical” eye movements of REM sleep prompted a surge in scientific studies of dreaming, after 74% of REM awakenings were associated with recall of complex, visual and emotion-filled dreams (Aserinsky & Kleitman, 1953). Rapid eye movements thus became the first objective physiological correlate of conscious experience during sleep. It was later suggested that rapid eye movements correspond with visual scanning of the dreamscape—known as the scanning hypothesis (Dement & Kleitman, 1957a, 1957b). Herman et al. (1984) supported this claim, finding that eye movements produced just before waking matched visual descriptions of dream imagery, although other findings are inconsistent (Jacobs, Feldman, & Bender, 1972; Moskowitz & Berger, 1969). In studies of lucid dreaming, where the dreamer is aware that they are dreaming and able to control their dreaming body to some extent, further evidence supports an isomorphism between dreamed and actual eye movements. Researchers (Hearne, 1978; LaBerge, 1980; LaBerge, Nagel, Dement, & Zarcone, 1981) demonstrated that lucid dreamers were able to signal using deliberate eye movements, such as looking to the left and right, which corresponded with left-right eye movements recorded by an electrooculogram (EOG). The body could, with verifiable physiological signals, bridge dream states with the waking world.
Since then, lucid dreaming and other dream phenomena such as somnambulism and REM behavior disorder (RDB) have revealed further correspondences between the sleeping and the dreaming body. For instance, lucid dreamers can induce muscular twitches in the actual forearm through dream movements (LaBerge et al., 1981) and can actively influence respiration, abdominal and thoracic muscle movements (Oudiette et al., 2018). The authors of the latter study suggest that even in nonlucid dreams the content of dream experience may physically manifest in the sleeping body, which may in part explain the erratic respiration, changes in heart rate, and muscular activity exhibited particularly during REM sleep. For example, nightmares are associated with elevated heart rate (Fisher, Byrne, Edwards, & Kahn, 1970) and increased eye movements and respiration rate (‘high anxiety dreams’; Goodenough, Witkin, Koulack, & Cohen, 1975), which suggests the body is physically experiencing a nightmare as long as the mind is. This view has substantial implications for Posttraumatic Stress Disorder (PTSD) where patients re-experience a trauma during nightmares (Van der Kolk, 1994), leading authors to claim that ‘dreams themselves could be traumatic for the dreamer’ (Campbell & Germain, 2016). Here, we expand on the argument for a stronger role of dreaming in sleeping physiology than has previously been acknowledged and, subsequently, explore the functional implications of this claim.
1.2. Sources of dreaming
1.2.1. Bottom-up bodily sensations
During REM sleep, extensive sensorimotor cortical activity seems to underlie the vivid embodied imagery of dreaming. Siclari et al. (2017) showed that dream content corresponds with activation in appropriate perceptual areas of the brain; for instance perception of faces in dreams is predicted by increased activation in the fusiform face area, similar to in wakefulness. And, functional magnetic resonance imaging (fMRI) of lucid dreams showed that dreamed hand movements correspond to activity in the contralateral sensorimotor cortex (Dresler et al., 2011), and manifest as muscular twitches in the forearm (LaBerge et al., 1981). The activation-synthesis hypothesis of dreaming proposed that sensorimotor cortical activity is generated by brainstem signals during REM sleep and that muscular twitches are simply a by-product of incomplete motor blockade (Hobson & McCarley, 1997), thus reducing the dreaming circuitry to a brain in a vat simulation. However, Blumberg (2010) claims that sensory feedback from muscular twitches produces substantial activation of the somatosensory cortex during REM sleep. Key characteristics include that twitches occur in discrete, repetitive patterns throughout the body against a backdrop of muscle atonia (Robinson, Blumberg, Lane, & Kreber, 2000) and cause amplified and repetitive patterns of activation in the somatosensory cortex. Critically, this feedback completes the circuitry between the dreaming brain and the sleeping body and suggests twitches may be an active bodily source of dream generation.
Perhaps the most common example of dreaming being influenced by muscular twitches occurs during sleep onset when the experience of hypnic jerks can be associated with vivid imagery of falling (Nielsen & Zadra, 2005); a similar interpretation could be provided for “exploding head syndrome” in that twitches of the middle ear muscle (the “hearing” correlate to eye movements) might suddenly be amplified as the body falls into quiescence. In the case of RBD, violent dreams seem to be enacted in often repetitive violent movements, such as kicking or punching (Valli et al., 2015). Blumberg and Plumeau (2016) suggest the loss of muscle atonia in RBD leads to excessive twitching, and the resulting sensory feedback gives rise to vivid and violent dreams. It is otherwise difficult to determine why suddenly, at the onset of RBD, patients would develop such violent dreams. Finally, a recent study showed that using transcranial direct current stimulation to inhibit sensorimotor cortex activity specifically decreased the presence of repetitive actions in REM sleep dreams (Noreika et al., 2020). The authors interpret these findings as evidence that the sensorimotor cortex is causal in the generation of dream movement. However, the specificity of inhibition to repetitive, but not other movement types, could reflect inhibited sensory feedback from repetitive twitches.
Beyond muscular twitches, other internal body states may also play a role in dreaming cognition. For instance, changes in breathing, heartrate, metabolism or circadian rhythm all correspond with changes in the quality of subjective experience in waking (see Critchley & Garfinkel, 2018 for a review). Recent evidence that physiological signals influence dream content is shown in Perogamvros et al. (2019), who found increased heartbeat-evoked potentials during REM sleep in nightmare sufferers. The heartbeat-evoked potential represents cortical response to the heartbeat and is an index of interoception, i.e. the sense of internal body states. Other general support includes evidence that bodily states such as fever (Schredl & Erlacher, 2020), hunger and thirst (O’Nell, 1965) have been associated with changes in dream quality. Recently, Rozen and Soffer-Dudek (2018) found that occurrence of teeth dreams (one of the most common dream themes reported by 39% of the population; Yu, 2010), correlated with the experience of dental irritation, but not with other measures of sleep disturbances or psychological distress.
In sum, we claim that cortical processing of bodily sensation continues during sleep and influences dream generation. Of course, sensation alone does not explain dreaming. A fascinating video of an RBD patient ‘smoking’ his pulse oximeter shows how sensation is interpreted by the dreaming mind: from the perception of pressure around the finger, this particular individual with a history of smoking dreams of enjoying a cigarette (video can be found in Oudiette et al., 2009). The dream emerges in a co-creative manner: real body sensation contributes to dream generation just as individual experience shapes dream narrative, which manifests in further body sensation, and so on, completing the dreaming circuitry. We further explore the role of individual experience in shaping dream imagery in the next section.
1.2.2. Top-down sources of dreaming
Neurocognitive accounts of dreaming agree that memory shapes the content of dream experience in a top-down manner. Dreams are affected by everything from basic perceptual and semantic knowledge, to emotional and autobiographical memory, and socio-cultural and evolutionary biases. Patterns of memory activation studied in waking cognition likewise influence the process of dream generation, including the tendency to better recall recent memories (recency effect) and associative priming of conceptually related memories. Nielsen (2017) examined such patterns in microdreams, also called hypnagogic images-brief dream snippets occurring at sleep onset. Despite lasting only seconds, microdreams reveal a process of sense making that may permeate all dream generation. In microdream examples, the mind seems to instantaneously integrate real perceptions with recent experiences and associated memories into a cohesive image.
“[Image:] A heavy door made of wood suddenly swings open to the R and slams against the corner of a counter top. [Reality:] The conference speaker made a thudding sound by hitting the microphone…A slide on the screen…depicted a closed, large, brown wooden door.” (example of a microdream, Nielsen, 2017)
In this example, the image of a door slamming captures the real sound of a microphone thudding. The image is primed by the recent memory of a door depicted on a slide, and related semantic and episodic knowledge that doors make a sudden noise when slammed. Similar sense making is evident in studies of lucid dreams, where audio or visual stimuli become contextualized in the dream environment (LaBerge & Levitan, 1995). For instance, when presented with flashing LED lights one lucid dreamer reports, “I could tell when the red light came on because it got hot and the sun got brighter” (Carr, Konkoly, Mallett, Edwards, Appel, & Blagrove, 2020). In another example, an audio cue was contextualized in the following dream: “I was shopping in a supermarket… I could hear the beeping and it was like I was getting loads of messages on my phone telling me what to buy … things like, ‘buy some biscuits’. ” To make sense of audio or visual stimulation, the dream relies on knowledge of how such perceptions have arisen in similar environments in the waking world.
Thus, a major contributor to sense making lies in interpreting current experience by drawing on similar experiences from the past. Researchers have even explored the temporal patterns of memory incorporation into dreaming. For instance, microdreams often incorporate at least one recent memory trace, but then link it with more distant past experiences and general semantic knowledge (Stenstrom, Fox, Solomonova, & Nielsen, 2012). Solomonova, Stenstrom, Paquette, and Nielsen (2015) assessed the experience of spending a night in the sleep laboratory as a memory likely to be processed in dreams. One participant reported in the morning: “I wake up and get out of the laboratory bedroom. [It] is exactly the same as I saw it yesterday…somebody is taking the electrodes off my head.” The frequent incorporation of memory from the prior day was coined the “day residue” by Freud (1965). But memories also show delayed incorporation into dreaming, particularly after 7–9 days (the “dream-lag” effect; Nielsen & Powell, 1992). For instance, in Solomonova et al. (2015) one participant reported the following, seven days after sleeping in the laboratory: “I am being admitted to a hospital because I am unable to remember my dreams…A team of doctors stands over the gurney telling me that I must be hospitalized.” Hartmann (1996) proposed that recent experiences become connected over time with more distant associated memories through dreaming, and suggests emotion is a major driving force behind these associative connections. In this way, dream images connect and embody the mutual feelings of experiences. Along these lines, evidence shows that dreamers rely on “feelings of knowing” in order to recognize dream images and characters despite varied and changing appearances (Skrzypińska & Slodka, 2014), suggesting the feeling provides meaning to associatively constructed images, composites of faces and places from memory.
Another example of the sense making process can be observed in the case of sleep paralysis. Episodes of sleep paralysis involve temporary inability to speak or move while falling asleep or upon waking. These episodes are often associated with a sense of pressure on the chest and the “felt presence” of an entity in the room, typically threatening in nature (Solomonova, Frantova, & Nielsen, 2011). The sense of paralysis and pressure on the chest can be neatly explained by the muscular atonia and shallow breathing pattern characteristic of REM sleep (Cheyne, Newby-Clark, & Rueffer, 1999; Cheyne, Rueffer, & Newby-Clark, 1999). Yet, the felt presence takes forms that make sense according to cultural norms: demons, devils, witches, ghosts or aliens (Olunu et al., 2018; Solomonova, 2018). Commonly cited is “the Old Hag”, a figure from Newfoundland folklore of a wretched travelling spirit who sits on the chest of the sleeper (Hufford, 1982). In the United States, felt presence provides one explanation for alien abduction accounts (McNally & Clancy, 2005) and in Egypt, the “Jinn”, a supernatural demon from Islamic mythology appears (Jalal, Simons-Rudolph, Jalal, & Hinton, 2014), whereas Chinese adolescents interpret their experience as ghost oppression (Ma, Wu, & Pi, 2014).
While sleep paralysis is a parasomnia, and therefore may not be representative of dreaming, it demonstrates how cultural biases can shape individual imagery. Other empirical work supports that dream content can be influenced by individual current concerns, a history of adversity or trauma, or childhood attachment style, among others (for review see Domhoff, 2017), although a review is beyond the scope of this paper. In general, dreaming draws on numerous past experiences to create psychologically meaningful imagery, including recent to remote experiences, emotional to episodic memories, and cultural to evolutionary biases. And the complete dreaming circuitry allows a full being-in-the-world, in which each idiosyncratic body—musculature, cardiovascular and sensory systems—interacts with each individual world—social, emotional, conceptual, and more. Given the idiosyncratic nature of dream generation, we focus on manipulating simple attributes of sleep and dreaming rather than attempting to control entire dream narratives (see Section 2.1). Therefore, we now review research on sleep neurophysiology and associated dream content to reveal functional targets of dream engineering.
1.3. Functional stimulation of sleep and dreams
1.3.1. Functional targets of dream engineering
Dream content consistently differs between the various sleep stages, as numerous laboratory studies have shown using polysomnography (PSG), in which sleep stages are classified according to patterns of electrical activity in the brain (Berry, Brooks, Gamaldo, Harding, Marcus, & Vaughn, 2012). Dreaming occurs initially in the form of brief hypnagogic imagery at sleep onset, in non-REM stage 1 sleep (NREM1 or N1). This transitional phase is characterized by a loss of EEG alpha activity and prominent theta activity (3–7 Hz; Berry et al., 2012). Recent experiences and even experimental tasks are frequently incorporated into N1 imagery (e.g., Tetris, Stickgold, Malia, Maguire, Roddenberry, & O’Connor, 2000; alpine racer game, Wamsley, Tucker, Payne, Benavides, & Stickgold, 2010) and incorporation of a learning task into N1 imagery has been correlated with improved performance (Wamsley, Perry, Djonlagic, Reaven, & Stickgold, 2010). N1 images are also described as surreal and have been associated with creativity and insight (McKellar, 1995).
NREM stage 2 (N2), which follows N1 sleep, is characterized by K-complexes (high-voltage slow waves) which are frequently coupled with sleep spindles (bursts of 12–15 Hz oscillations; Berry et al., 2012). Dream reports collected from N2 are longer than those from N1, and often contain reference to thought Speth and Speth (2018). Functionally, N2 sleep and particularly sleep spindles have been linked to declarative memory consolidation (Ruch et al., 2012; Tucker et al., 2006), and sleep spindle density correlates with dream recall (Nielsen et al., 2017). NREM stage 3 (N3), also known as slow wave sleep, is characterized by prominent delta waves (0.5–4 Hz; Berry et al., 2012). N3 is often termed deep sleep and is thought to be most important for homeostatic processes (Tononi & Cirelli, 2003, 2006); dream recall frequency is lowest from N3, with dream reports that are short and phenomenally minimal (Cavallero, Cicogna, Natale, Occhionero, & Zito, 1992). Recent findings suggest that delta activity over parietal areas is a predictor of dreamless sleep (Siclari et al., 2017); whereas fast-wave activity in the same areas, most typical of REM sleep, is a predictor of dream recall.
Finally, REM sleep is characterized by higher frequency activity similar to N1 or wakefulness, with particularly increased theta oscillations (Berry et al., 2012). REM sleep dreaming provides a rich “virtual laboratory for the rehearsal of embodied cognition” (Speth & Speth, 2018). Procedural memory benefits from REM sleep (Smith, 2001), with some evidence that motor practice during lucid dreaming corresponds with improved performance after sleep (Schädlich, Erlacher, & Schredl, 2017). REM sleep is also functionally involved in emotion regulation (Gujar, McDonald, Nishida, & Walker, 2010; Van Der Helm et al., 2011) although nightmares, intense negative dreams, can interfere with this function and cause distress in waking life (Kramer, 1991; Levin & Nielsen, 2009; Nielsen & Carr, 2017). Positive dreams may have the inverse effect, associated with improved sleep quality and subsequent mood (Carr & Nielsen, 2017). Finally, REM sleep is linked to increased insight and creativity (Cai, Mednick, Harrison, Kanady, & Mednick, 2009; Carr & Nielsen, 2015; Stickgold, Scott, Rittenhouse, & Hobson, 1999; Walker, Liston, Hobson, & Stickgold, 2002), with imagery described as hyperassociative and metaphorical, likened to a form of creative expression (Hartmann & Kunzendorf, 2013).
Overall, dream engineering techniques are informed by sleep stage neurophysiology and corresponding dream quality. Functional targets of dream engineering include improving sleep quality, enhancing memory consolidation, ameliorating emotion regulation, inspiring creativity, and augmenting motor learning. To date, several techniques have been developed in sleep laboratories to reach these aims, which we overview below.
1.3.2. Stimulation techniques for dream engineering
Sensory manipulation has been used for millennia to influence dream content. The earliest reference is inscribed on the Chester Beatty papyri, found in Upper Egypt and authored c.1350 BCE. It describes a method of drawing on the hand and covering the hand and neck in black cloth prior to sleep in order to invoke the wisdom of Besa, a dwarf deity (Nielsen, 2012). Fasting has been used to trigger vivid dreams in many cultures from Egyptians to indigenous peoples of North America, and modern cultures believe spicy or dairy foods trigger vivid dreams (Nielsen & Powell, 2015). Experimentally, Dement and Wolpert (1958) collected 15 REM dream reports from individuals deprived of fluids for 24 h prior to sleeping in the laboratory, and five dreams contained thirst-related content. Pre-sleep priming with a visual stimulus is likewise effective; Goodenough et al. (1975) show that watching a stressful film prior to sleep increased dream negativity, whereas use of visual inverting prisms led to more active and vivid dreams (Corsi-Cabrera et al., 1986). Pre-sleep rehearsal of current concerns, as opposed to exposure to stimuli, also has some efficacy in incubating dreams. Barrett (1993) asked college students to think about a personally relevant problem for 15 min before sleep, and in response, 49% of reported dreams were rated as relevant to the problem, with 34% of them containing a solution. Saredi, Baylor, Meier, and Strauch (1997) similarly found that thinking of a question related to a current problem prior to sleep increased the likelihood that dream content reflected the problem. Finally, in Imagery Rehearsal Therapy, visualizing a positive ending to a nightmare prior to sleep leads to resolution of nightmares within sleep (Krakow & Zadra, 2010).
In the absence of pre-sleep manipulation, stimulation applied only during sleep can direct dream content in various ways. A spray of water on the skin (Dement & Wolpert, 1958), application of a pressure cuff to a specific limb (Nielsen, 1993), or application of electrical pulses to cause muscle contractions in a sleeper (Koulack, 1969) have each been shown to affect dream features, increasing vividness or movement sensations in dreams. In Nielsen (1993), pressure application to the leg resulted in visual-kinesthetic synesthesia, direct incorporation of pressure and squeezing sensations, and increased bodily bizarreness in dreams. Schredl et al. (2009) show that pleasant scent increases dream positivity whereas unpleasant scent increases negativity. Stimulation can also influence neural oscillations during sleep (Hennevin, Huetz, & Edeline, 2007; Stickgold, 2001). As research reveals the functional roles of specific sleep oscillations, e.g., sleep spindles being critical for memory consolidation, these rhythms become targets for entrainment. Playing bursts of oscillating white noise during N2 and N3 at 12 or 15 Hz can increase the number of sleep spindles (Antony & Paller, 2017). And applying oscillating electrical potentials to the scalp at 0.75 Hz has been used to augment slow oscillations in N3, resulting in improved memory performance the following day (Marshall, Helgadóttir, Mölle, & Born, 2006).
Finally, experimental protocols have combined pre-sleep and within-sleep stimulation to induce ‘targeted reactivation’ of specific content. In Targeted Memory Reactivation (TMR), a sensory stimulus is associated with a learning task prior to sleep, and is then re-presented during sleep to trigger associated memory reactivation (Lewis & Bendor, 2019; Rudoy, Voss, Westerberg, & Paller, 2009). For instance, presenting subjects with an olfactory cue during NREM sleep that was previously presented during an object location task enhances memory consolidation (Rasch, Büchel, Gais, & Born, 2007; Rihm, Diekelmann, Born, & Rasch, 2014). Auditory cues, including speech, can target reactivation of declarative memories and have frequently been used to enhance language learning (Oudiette & Paller, 2013). Re-presenting an auditory or odor cue during sleep can even enhance fear extinction in both mice and humans (Hauner, Howard, Zelano, & Gottfried, 2013; Oudiette, Antony, & Paller, 2014).
Targeted reactivation techniques can also be applied to dreaming. For instance, Targeted Dream Reactivation (TDR) pairs a stimulus with pre-sleep priming or dream incubation, and re-presents the stimulus during sleep to trigger relevant dream content. An early example combined pre-sleep thirst with presentation of liquid-related words in REM sleep: Dreams about liquids increased, and those who dreamt of quenching their thirst drank less once awake than those who dreamt of being thirsty (Bokert, 1968). De Koninck and Koulack (1975) presented a stressful film prior to sleep, then re-presented the film music during sleep: this led to increased film elements in dreams, and increased emotionality the following day. Induction of lucid dreams has also been facilitated through audio and visual stimulation during sleep (e.g. via DreamLight mask, LaBerge & Levitan, 1995). Within a Targeted Lucidity Reactivation (TLR) protocol, audio and visual stimuli are associated with a lucid mind-state prior to sleep, and then re-presented during REM sleep; this technique induced lucid dreams in 50% of participants in a morning nap (Carr et al., 2020). In addition, novel protocols are emerging all the time; for instance, Targeted Dream Incubation creatively combines components of targeted reactivation with intentional incubation at the hypnagogic border between sleep and wake (Horowitz, Cunningham, Maes, & Stickgold, 2020). Applying targeted protocols to dream content has potential for enhancing sleep functions, e.g., incubating creativity or ameliorating mood via pleasant dreams.
In sum, techniques from rhythmic entrainment to targeted reactivation, pre-sleep priming, dream incubation and dream direction each offer a sense that stimulation of specific sleep and dream content is both experimentally feasible and potentially functional (see Table 1). We posit that 1) an understanding of dreaming in circuit with the body and 2) an application of contemporary technology from the field of HCI can make the systematic induction of themes into simulated dream worlds a reality. HCI researchers have spent decades exploring how to generate simulations through a variety of sensory stimulation, which have potential to be adapted to dream engineering purposes.
Table 1. Overview of Stimulation Techniques.
Pre-sleep priming presents a stimulus before sleep, e.g., a video or music to influence dream content in subsequent sleep. Dream incubation involves pre-sleep rehearsal of content, such as visualization of a rescripted nightmare, repeating an intention to become lucid (lucid dream incubation), or focusing on a personal problem to incubate a creative solution. Dream direction applies a sensory stimulus during sleep and relies on implicit associations to sensation to direct dreams: a pleasant scent to ameliorate dream emotion, muscle stimulation to augment dream movement, speech to direct dream narrative. Of note, pre-sleep priming, dream incubation, and dream direction may affect dream content in relatively metaphorical or idiosyncratic, but nonetheless functional ways. Rhythmic entrainment acts on physiological rhythms during sleep, from fast rhythms like neural oscillations to slower rhythms like respiration or circadian changes in temperature; while entrainment does not necessarily influence dream content, it may improve sleep functions. Finally, in targeted reactivation, a stimulus is paired with specific content during wake, and when the stimulus is re-presented during sleep its associated content is reactivated. Targeted reactivation can enhance consolidation of specific memory traces (targeted memory reactivation), trigger imagery that was rehearsed prior to sleep (targeted dream reactivation), or induce lucidity (targeted lucidity reactivation).
| Wake |
Sleep |
|||
|---|---|---|---|---|
| Stimulus | Content | Stimulus | Content | |
| Pre-Sleep Priming | ✔ | X | X | ✔ |
| Dream Incubation | X | ✔ | X | ✔ |
| Dream Direction | X | X | ✔ | ✔ |
| Rhythmic Entrainment | X | X | ✔ | X |
| Targeted Reactivation | ✔ | ✔ | ✔ | ✔ |
2. Simulating worlds through sensory stimulation
The field of HCI has a storied history of building devices to create and alter experiences of simulated worlds. Virtual Reality (VR), specifically, is a medium that allows the user to experience a simulated but immersive reality, typically created through Head-Mounted Displays or projected environments. A successful VR experience relies on maintaining the illusion of being in the simulated environment, which is accomplished by continuous injection of sensory information via interactive devices, especially wearables. Each of these devices is designed for a particular sensory manipulation (e.g., one particular wearable might simulate the smell of an experience); therefore, we posit that these interactive devices are also reliable candidates to engineer the sensory experience of the dreamer’s simulated world.
The perspective of HCI researchers on creation and maintenance of illusory worlds can likewise enrich neuroscientific accounts of dream generation. Existing theoretical dream science portrays dreaming as a form of simulated reality (Hobson et al., 2014; Nielsen, 2010; Valli & Revonsuo, 2009); Hobson et al. (2014) called dreams “an innate virtual reality”. The perspective of dream theorists has been primarily to deconstruct dream features and memory sources and identify similarities with waking reality. HCI researchers instead approach the building of illusory worlds from the ground up, using technology to construct plausible and immersive realities. The mechanisms that enable this illusion generation could thus shed light on the organic mechanisms that make the dream world immersive and plausible.
Many VR researchers have studied the underlying brain mechanisms that enable illusions in VR. Gonzalez-Franco and Lanier (2017) proposed a model explaining the cognitive and perceptual mechanisms of simulation generation: (1) Bottom-up multisensory processing (Blanke, 2012; Calvert, Spence, & Stein, 2004); (2) Top-down prediction manipulations (Haggard, Clark, & Kalogeras, 2002); and, (3) Sensorimotor self-awareness frameworks (Gallagher, 2000). They also propose three main types of illusions which account for simulation maintenance, based on previous VR work (Spanlang et al., 2014): (1) Plausibility illusion – participants feel that the events happening in the virtual world are real; (2) Embodiment illusion – participants feel that they inhabit a virtual person; and, (3) Place illusion – participants feel they inhabit a virtual location. These mechanisms of generating and maintaining illusory worlds in VR echo the accounts of dream generation described above, lending theoretical support for the translation of technologies from HCI to dream engineering.
2.1. The sensory interface in dreaming and VR
In both dreaming and VR simulations there is limited access to the “real world”. In the field of HCI, achieving more realistic simulations (as well as more immersive virtual environments) required researchers to tackle this limitation. The typical approach is to develop stimulation technologies that allow users to perceive more sensory cues while interacting in a simulated world; these cues add up to immersion, a sense of belief in the VR experience, which is caused by a predictability of the effects of the virtual world in the user’s senses. While the early decades of VR research focused on attaining visual realism, today’s research focuses instead on the other senses, with a particular emphasis on somatosensation (touch, forces), vestibular sense, thermosensation and olfaction. These are particularly interesting for dream research because of the aforementioned close analogy between virtual and dream worlds.
Nevertheless there are obvious constraints to simulating dream worlds. First, unlike in VR, sensory processing in sleep is limited by both gating and arousal mechanisms. Gating mechanisms act to selectively filter sensory information during sleep and vary by sleep stages and type of stimulation (Andrillon & Kouider, 2019). For instance, cortical processing is selectively amplified for relevant as opposed to irrelevant speech (Legendre, Andrillon, Koroma, & Kouider, 2019) and speech related to current concerns, but not unrelated speech, has been shown to influence dream content (Hoelscher, Klinger, & Barta, 1981). At the same time, sleep is a fragile state, and an abundance of sensory stimulation will lead to microarousals or full awakenings. A second limitation that distinguishes dreaming from virtual simulations is the level of control the experimenter has over the simulation. In VR, an entire multisensory world can be scripted into a predefined narrative. In dreaming, the multiplicity of idiosyncratic autobiographical memories, recent experiences, socio-cultural and evolutionary pressures cause the dream to emerge in a relatively unpredictable manner.
Thus, continued basic experimental research is necessary to further unveil which stimuli cross the threshold of sleep, whether these stimuli enter into dream content, and how the impact of stimuli on dream content varies within and between individuals. The same auditory or olfactory stimuli could transform into various dream images based on the user’s memories, cultural background, emotional state, etc. These limitations at a physiological and phenomenological level guide our approach to dream engineering: we aim to affect simple attributes of dreaming via sensory stimulation, e.g. to increase movement or ameliorate emotion without attempting to control the manifest content of such dreamed movement or emotion. We here discuss simple sensory HCI technologies that, we believe, can be used for dream engineering.
2.2. Stimulating the sense of touch and force (Somatosensation)
HCI researchers have built a series of interactive devices that stimulate the haptic sense in order to convey the physicality of the virtual world, which could be applied to incorporate tactile stimulation in dreams (Brooks, 1999). There has been progress on two fronts: (1) simulating the cutaneous qualities of interacting with lightweight objects, such as contact with surfaces or textures (Culbertson, López Delgado, & Kuchenbecker, 2014), and (2) simulating the proprioceptive side of interaction with objects that arises from their weight, i.e., the forces that arise when we push or lift a heavy object (Gu, Zhang, Sun, Bian, Zhou, & Kristensson, 2016).
Vibration is a common modality in VR haptics because vibration actuators are cheap and relatively small, fitting into wearables such as gloves (Burdea, 2000) or vests (Lindeman, Yanagida, Noma, & Hosaka, 2006). Generally, vibration emulates touching an object (Kron & Schmidt, 2003) or conveys the texture of objects (Culbertson et al., 2014), with high frequency vibration conveying smooth texture, and low frequency conveying rough textures. Vibration is not typically used to simulate the sense of pressure, although devices can create a pseudo sense of directional pressure using an asymmetric vibration pattern (Rekimoto, 2013). Other tactile devices create small forces sufficient to displace the user’s skin laterally (Chen, Anderson, Walker, & Besier, 2016), e.g. Pneumatic Gloves contain air pockets that inflate when the user’s fingertips touch a virtual object (Benali-Khoudja, Hafez, Alexandre, & Kheddar, 2004).
While technologies for simulating contact with objects has been maturing over the past decades of HCI research, simulating large forces that arise from interactions with objects is still a focus of much VR research. The main approach employs mechanical actuation, such as pulling on tethers attached to the user (SPIDAR; Murayama et al., 2004), e.g. to simulate hitting a virtual baseball (Jeong, Hashimoto, & Makoto, 2004). While mechanically actuated devices offer high precision, they are still large and cumbersome devices. Therefore, HCI researchers explored actuating the user’s muscles with electrical impulses as a means of creating force feedback. This technique, called electrical muscle stimulation (EMS), originated in the field of rehabilitation medicine where it is applied to regain lost motor function (Strojnik, Kralj, & Ursic, 1979). Tamaki, Miyaki, and Rekimoto (2011) used EMS in an interface that guided users in learning a new instrument and Pfeiffer, Dünte, Schneegass, Alt, and Rohs (2015) used an EMS-based device to steer users while walking. Unarguably, EMS’ largest application area in HCI is as a wearable to create force-feedback in virtual simulations: to add forces to mobile devices (Lopes & Baudisch, 2013); to render the sensation of a ball hitting a racket in an augmented reality tennis game (Farbiz, Yu, Manders, & Ahmad, 2007); to simulate touching virtual walls and objects (Lopes, You, Cheng, Marwecki, & Baudisch, 2017; Lopes, You, Ion, & Baudisch, 2018); and to simulate the sensation of hitting or being hit in VR sports, such as boxing (Lopes, Ion, & Baudisch, 2015). The latter wearable device uses a combination of tactile stimuli (a solenoid tapping the skin) and force feedback (electrical muscle stimulation) to create more realistic illusions of feeling a virtual object, i.e., not only touching the object’s surface but also feeling the object’s inertia.
2.2.1. Somatosensory stimulation in dream engineering
While the effect of somatosensation on dream content has been described earlier, the use of small wearables presents an avenue for conducting novel studies potentially in home users. For instance, Pneumatic Gloves provide a wearable similar to the pressure cuff used in (Nielsen, 1993).
Importantly, the application of EMS presents a relatively new avenue of dream direction (though see Koulack, 1969). EMS application could functionally simulate twitches that naturally occur during REM sleep. Recent work suggests that sensorimotor feedback from twitches during REM sleep is critical for development and maintenance of motor coordination (Blumberg, Marques, & Iida, 2013). An open question is whether targeted application of low-level EMS could potentially enhance motor learning during sleep, in parallel to the rehabilitative effects of EMS on motor function in wake (Strojnik et al., 1979). Further, EMS could trigger motor imagery during sleep, which likewise has been associated with enhanced motor learning (e.g., in lucid dreams, Schädlich et al., 2017). In one recent study, Debarnot, Perrault, Sterpenich, Legendre, Huber, Guillot, and Schwartz (2019) found that waking motor imagery practice during arm immobilization led to increased subsequent REM sleep and improved adaptation the next day. The use of EMS to augment motor dream imagery thus offers an intriguing application of dream engineering for physical rehabilitation.
As an initial pilot test of the concept, we applied EMS to one pilot subject (approved by MIT’s Committee on the Use of Humans as Experimental Subjects) using an FDA approved electrical muscle stimulator device with a maximum voltage and current of 70 V and 0.72 mA respectively. EMS was applied during a nap on the upper and lower calf muscle after at least 5 min of REM sleep, defined as minutes in which eye movements exceeded 10 eye movements per minute (Takahashi & Atsumi, 1997). The subject was awakened for a report after the first stimulation, then the procedure was repeated for a second trial. The EMS application did not waken the subject, and dream reports obtained after awakening did contain reference to the limb stimulation. The first report contained reference to running: “Was like a beach…just looking at them, the rocks…I can see my feet…I had a small image of running in a field. And then feeling the grass hit on my feet.” In the second report, the stimulation seemed to transform into an audiovisual experience in the dream: “I didn’t get any dreams until I started feeling the device. Yeah it was cool, at some point you can anticipate the increase of the, you know, da-da-da-da-da-da [‘the shock’] and then once it started to get stronger you kind of will be waiting for like boom this is the peak and then at the peak you get an image.” Although preliminary, we suggest EMS as a technology for REM sleep dream direction is worthy of future investigation.
2.3. Stimulating the sense of temperature (thermoception)
To improve the realism of VR experiences, researchers in the field of HCI have been developing and adding more haptic sensations. More recently, attention has been given to simulating the temperature of a virtual experience (Peiris, Peng, Chen, Chan, & Minamizawa, 2017). This is typically achieved by means of heating or cooling the air around the user’s skin, e.g., using air conditioning units (Xu, Kuroda, Yoshimoto, & Oshiro, 2018) or heat lamps (Hülsmann, Fröhlich, Mattar, & Wachsmuth, 2014) or by directly heating/cooling the skin using thermoelectric elements, also known as Peltier elements (Peiris et al., 2017). The latter are especially relevant since they are smaller and fit into wearables, i.e., they attach directly to the user and not to the user’s environment. Peltier-based thermal devices create a temperature difference across two plates in the presence of an electric current. These thermal devices have been used for realism and for transmitting information in general purpose interfaces, for instance adding thermal navigation cues on a steering wheel (Vito, 2019). The ThermalBracelet (Peiris, Feng, Chan, & Minamizawa, 2019a, 2019b) attached Peltiers to the user’s wrist to explore eyes-free feedback, and Peiris et al. (2017) added Peltiers to a VR headset, creating temperature sensations directly on to the user’s face. Season Traveller (Ranasinghe & Tung, 2018) utilized heating elements on the back of the user’s neck combined with an olfactory display to generate realistic scented environments; lastly, Thermotaxis (Narumi, Tomohiro, Seong, & Hirose, 2009) used Peltier elements attached to the user’s ears to render temperature changes.
Alternative technologies that stimulate thermoception include: hydraulics (i.e., pushing hot/cold liquids through tubes that are in contact with the user’s skin; Han, Anderson, Irani, & Grossman, 2018), gel packs (Jain et al., 2016), resistive heating (Wettach, Behrens, Danielsson, & Ness, 2007) and, more recently, a thermal illusion based on projecting scents, such as mint or capsaicin, that are perceived as cooling or warming by the user’s trigeminal nerve when inhaled (Brooks, Nagels, & Lopes, 2020).
2.3.1. Temperature in dream engineering
Sleep is closely tied to the circadian rhythm of core body temperature (Lack & Lushington, 1996). To date, researchers have explored passive body heating through the skin to decrease sleep onset latency (Raymann, Swaab, & Van Someren, 2007) and warming of the periocular skin to improve subjective sleep quality (Sakamoto et al., 2017) and even increase delta power during sleep (Igaki et al., 2018). The authors of the latter study developed a heat- and steam-generating sheet to warm the skin, either around the eyes, neck or abdomen, which proved effective for improving sleep quality.
While warmth helps individuals to fall asleep, core body temperature drops during deep sleep, with optimal sleep temperature reported to be somewhere between 60 and 68 degrees Fahrenheit (16–20 degrees Celsius) (Harding, Franks, & Wisden, 2019). Thus, cooling the body has also been explored as a means of enhancing deep sleep. For instance, the Kryo Chilipad is a temperature-controlled cooling mattress topper that can cool to 60 degrees Fahrenheit using a water-based thermoregulator (Inc, 2019).
HCI concepts, such as the development of small actuators that can heat or cool in a matter of milliseconds (Peiris et al., 2017; Peiris et al., 2019a, 2019b), or even stimuli such as scents that are associated with sensations of heating or cooling (such as capsaicin or peppermint), provide simple technologies for dream engineering. Ideally, temperature devices with application to sleep would be able to heat or cool depending on need and would be safe and easy to use throughout the night. One possibility is integrating wearable HCI devices with sleep tracking software. In fact, warming/cooling scents could be presented via Essence, an olfactory device described later.
2.4. Stimulating the sense of balance (vestibular)
Vestibular proprioception consists of both the sense of movement and the position of the body, creating a sense of bodily balance called the vestibular sense. A good amount of progress has been made towards simulating the body’s vestibular sense in VR, especially walking. Our approach (Sra, Jain, & Maes, 2019) to simulating movement in virtual environments is to provide proprioceptive feedback related to virtual motion by directly stimulating the user’s vestibular system (see Fig. 1; based on Fitzpatrick, Burke, & Gandevia, 1994). The Galvanic Vestibular Stimulation system sits on a user’s neck with electrodes attached behind the ear, on the mastoid bone of the user. A small amount of electrical signal is delivered in a controlled manner, giving balance signals across the vestibular system, which corresponds to virtual movements. These balance signals significantly increase the sense of realism in the VR experience, and the system enables induction of a full body sensation of motion in 4 relative directions (forward, backward, left and right).
Fig. 1.
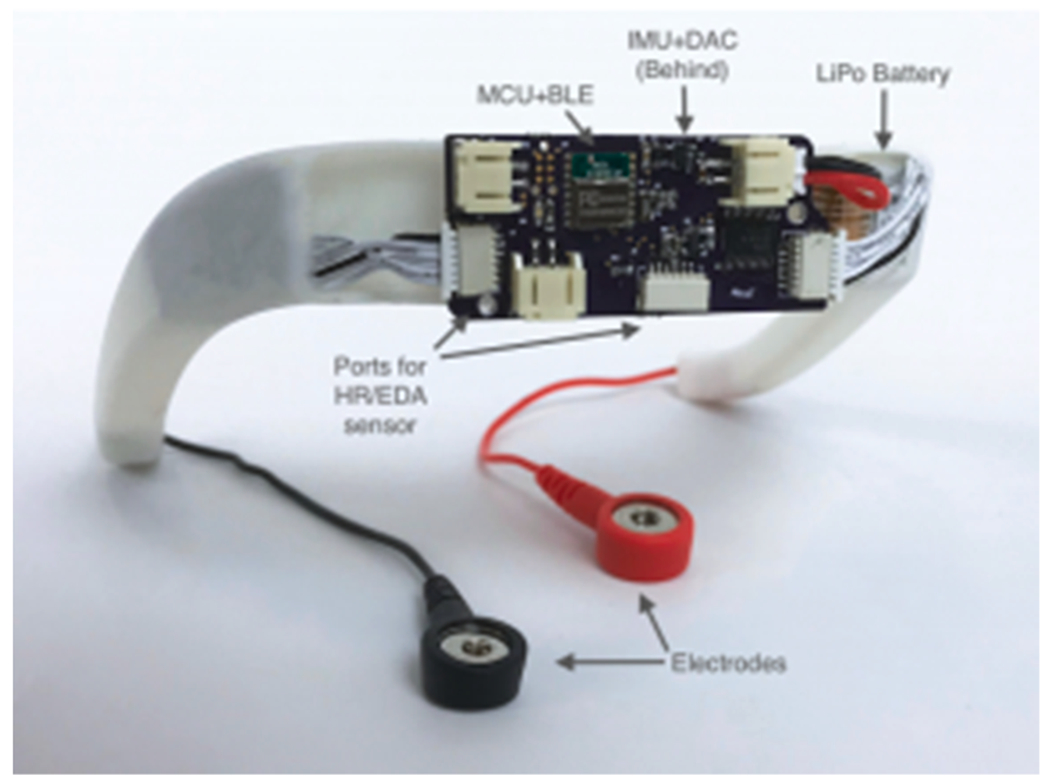
Electrodes attached behind the ear are used to transmit a small electrical current to the vestibular system, providing proprioceptive information to the user.
2.4.1. Vestibular sense in dream engineering
Electrical stimulation of the vestibular sense opens up opportunities for rocking subjects to sleep with wearable electronics and influencing sense of balance in simulated dream bodies. Sleep neuroscience research has shown that sensations of swinging can modulate physiological parameters of sleep, easing sleep onset and inducing a sustained boosting of slow oscillations and spindle activity (Bayer et al., 2011). It is proposed that the swinging motion exerts a synchronizing action in the brain that reinforces endogenous sleep rhythms—yet this study required physically rocking a whole bed. Our Galvanic Vestibular Stimulation device could potentially deliver rocking sensations without the construction of a mobile bed, enabling targeted direction and timing of vestibular sensations in a small wearable device. Other devices have shown simple movement signals can significantly influence sleep. The Breathing Bear (Ingersoll & Thoman, 1994) uses rhythmic movement stimulation from an actuated toy to entrain infant breath, easing sleep onset. Haptic interfaces such as NightShift have been used to alleviate sleep apnea by delivering vibration to make users change from unhealthy body positions during sleep (Scarlata, Bartoli, Santangelo, Giannunzio, & Incalzi, 2015). Thus, bodily movements influence both sleep physiology and quality, and are a viable target for sleep manipulation technologies.
2.5. Stimulating the olfactory sense
The history of olfactory technological research can be dated to the late 50’s when scents were released during the viewing of films to associate certain smells with scenes of a movie (Smell-O-Vision and Sensorama; Heilig, 1961). A decade ago, the HCI research community started looking into the challenges and possibilities for smell-based technology. Recent HCI research and product developer efforts have focused on enabling scent to become part of digital communications. Most systems use off the shelf aromas in their prototypes, focusing research efforts on the device itself rather than facing the chemical engineering challenge of capturing odors. Although limited work has been published in the field of olfactory displays for VR, some researchers (Ischer et al., 2014), showed that the addition of smell in VR significantly enhances the sense of immersion. Li and Bailenson (2018) demonstrated that olfactory and haptic cues in VR have satiation effects. Pleasant ambient odors have also been used to relieve stress and improve relaxation when coupled with VR (Carskadon & Herz, 2004), or as a biofeedback tool for mindfulness and meditation (Amores, Dotan, & Maes, 2019, Amores, Wang, Dotan, & Maes, 2019). Keller, Kouzes, Kangas, and Hashem (1995) created an electronic nose that can identify certain odors and transmit olfactory information for telepresence VR systems. Tijou, Richard, and Richard (2006) explored the use of olfactory cues for educational purposes using large-scale fan-based devices while students learned the structures of organic molecules. More recently, Radvansky and Dombeck (2018) developed the first olfactory VR system for mice, to study odor-guided virtual navigation behavior. Such research will open new translational opportunities to study olfactory learning and sleep in humans.
2.5.1. Scent in dream engineering
In comparison to sight or audio, odors presented during sleep are less likely to cause arousal. Olfactory information passes directly from the olfactory epithelium in the nose to the olfactory bulb in the forebrain and then on to the olfactory cortex, whereas other sensory modalities go through the thalamus which is linked to arousal. The awareness of odors during sleep is relatively low, although the use of very putrid, fish-like odors like Pyridine, as well as arousing scents like peppermint can wake people up given increased intensity (Stuck et al., 2007). These two types of smells activate the trigeminal nerve, and therefore, even if the person is entirely anosmic (incapable of smelling), they can differentiate these smells based on physical sensation. In contrast, using non-trigeminal olfactory stimuli, an odor with increased intensity does not cause nocturnal arousals (Stuck et al., 2007). Although odors might not lead to awakenings, they are still processed by the brain (Badia, Wesensten, Lammers, Culpepper, & Harsh, 1990). Some studies suggest that smells can modulate the circadian rhythm (Granados-Fuentes, Tseng, & Herzog, 2006) or reduce sleep onset latency and improve sleep quality (Field et al., 2008). A study by Arzi et al. (2010) showed how odors influenced respiration during sleep: decreased inhalation and increased exhalation following an odor release. Another interesting study found that triggering scents during sleep increased delta frequencies and sleep spindles proportional to smell duration (Perl et al., 2016).
Other researchers have assessed the impact of smell on dreams. Schredl et al. (2009) compared the results of using the smell of roses, rotten eggs, or placebo on dreams reported from REM sleep. Dream emotions were more positive in the case of the rose scent, and negative with that of rotten eggs. The same authors conducted a more recent study showing that if an odor is associated to images during wakefulness, and re-presented while in REM sleep, subjects report having dreams of those images (Schredl, Hoffmann, Sommer, & Stuck, 2014). Rasch et al. (2007) and Klinzing et al. (2018) used odor cues to enhance learning of visuospatial locations via targeted memory reactivation during N2/N3, which enhanced memory retrieval of 2D object-locations after sleep. In these studies, researchers used a full PSG setup and a nasal mask or nasal cannula connected via long Teflon tubes to a computer-controlled olfactometer. The scent-release device is placed in an adjacent room because the air pumps used to release scent are noisy. Thus, current olfactometers are expensive and not wearable; therefore, HCI researchers (Amores, 2016) created a miniaturized, silent, wearable olfactometer, the Essence device, which we present in detail in the following as we believe it is a particularly useful device for dream engineering.
2.5.2. The Essence device
Essence is a wearable that can release bursts of scent based on physiological information of the user. It can be worn as a necklace or a clip and can release odors based on heart rate and brain activity (Fig. 2, Amores & Maes, 2017). The device is wireless and connects to physiological sensors as well as the Muse EEG headband; it monitors heart and breathing rate from sensors integrated into the device (Amores, Hernandez, Dementyev, Wang, & Maes, 2018). The device can release multiple fragrances that can be remotely controlled by an Android app, and can release scent based on individual parameters, for example ‘if HR greater than 80, release pleasant scent’. The system can connect to the Muse EEG headband and can incorporate sleep-staging algorithms described in this paper (Koushik, Amores, & Maes, 2019). The device has been successfully tested with more than 100 participants for use during the day or in combination with VR for relaxation (Amores, Richer, Zhao, Maes, & Eskofier, 2018).
Fig. 2.
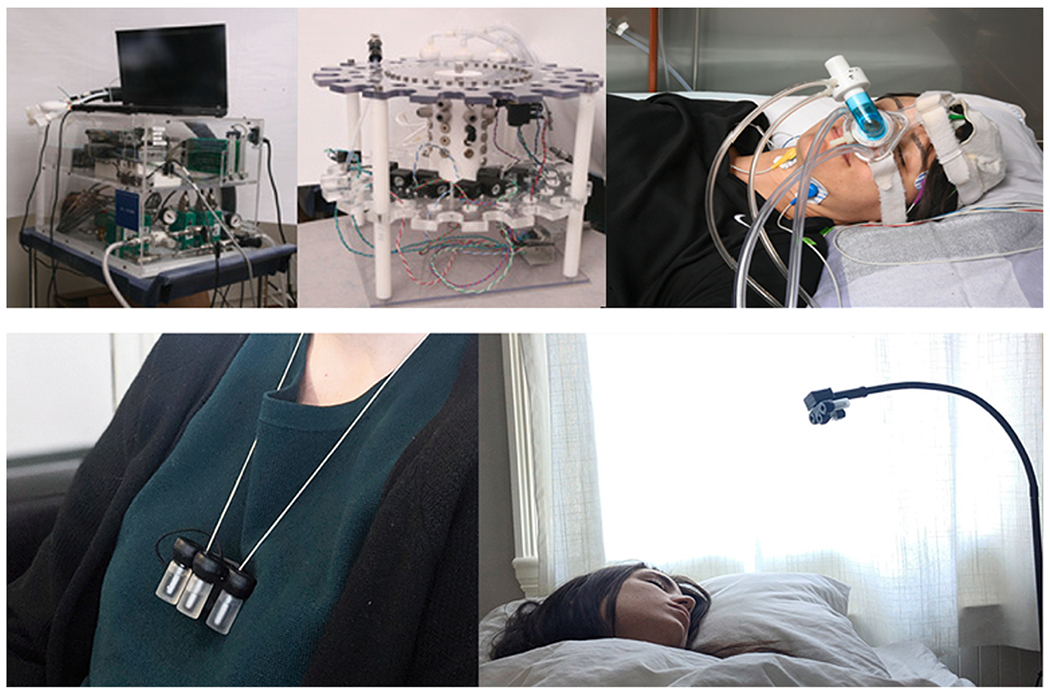
(top) Traditional PSG and olfactometers used for sleep studies. (bottom) The Essence prototype can be worn during the day and clipped to a flexible holder at night. The scent parameters and position can be adjusted to the user’s or experimenter’s preferences.
Essence was proposed as a “sleep user interface” with olfactory cues to open a new interaction opportunity in HCI (Amores, Dotan, & Maes, 2019, Amores, Wang, Dotan, & Maes, 2019). The device could be used to release pleasant scent based on physiological indicators of nightmares; or used as a device for targeted reactivation protocols. A pilot experiment was conducted with a participant who suffered PTSD, with the intention of positively mitigating traumatic nightmares using olfactive and sound cues during slow-wave sleep (Amores, 2016). For this pilot case study, unpleasant trauma-related sounds were paired with pleasant odors or presented without odor; following sleep all sounds were rated as less arousing and more pleasant than prior to sleep, though this difference was larger for sounds that had been paired with pleasant odors. While preliminary, our ultimate goal is to open new opportunities for sleep and HCI researchers to conduct at-home sleep-olfactory studies.
2.6. Stimulating the auditory sense
Audio has always been used in tandem with visual stimulation in VR simulations. Spatialized audio has been used to increase sense of immersion in simulated virtual space (Naef, Staadt, & Gross, 2002), while real-time audio has been used to enable sense of presence for virtual collaborations (Monahan, McArdle, & Bertolotto, 2008). Technologies used for audio have ranged from headsets to bone-conduction speakers (Walker & Lindsay, 2005). A series of technologies have also been built to probe and alter the dreaming mind using audio. Simple apps, like SleepBot, provide interfaces for dream logging in the morning after a night of sleep via text or audio recording (Ong & Gillespie, 2016). One device that builds on these simple audio interfaces is Dormio (Horowitz, Grover, Reynolds-Cuéllar, Breazeal, & Maes, 2018), which we present in the following as a useful device for dream engineering.
2.6.1. The Dormio system
The Dormio system is a combined sleep tracker and dream incubator, focusing on incubation of sleep onset dreams using auditory semantic cues. The Dormio device is wrist-worn (see Fig. 3) with an associated app used to communicate with users and record dream reports via laptop or cellphone. The system tracks sleep onset and then initiates serial awakenings, inserting a dream incubation theme during each inertia-laden awakening, creating a serial dream incubation paradigm.
Fig. 3.
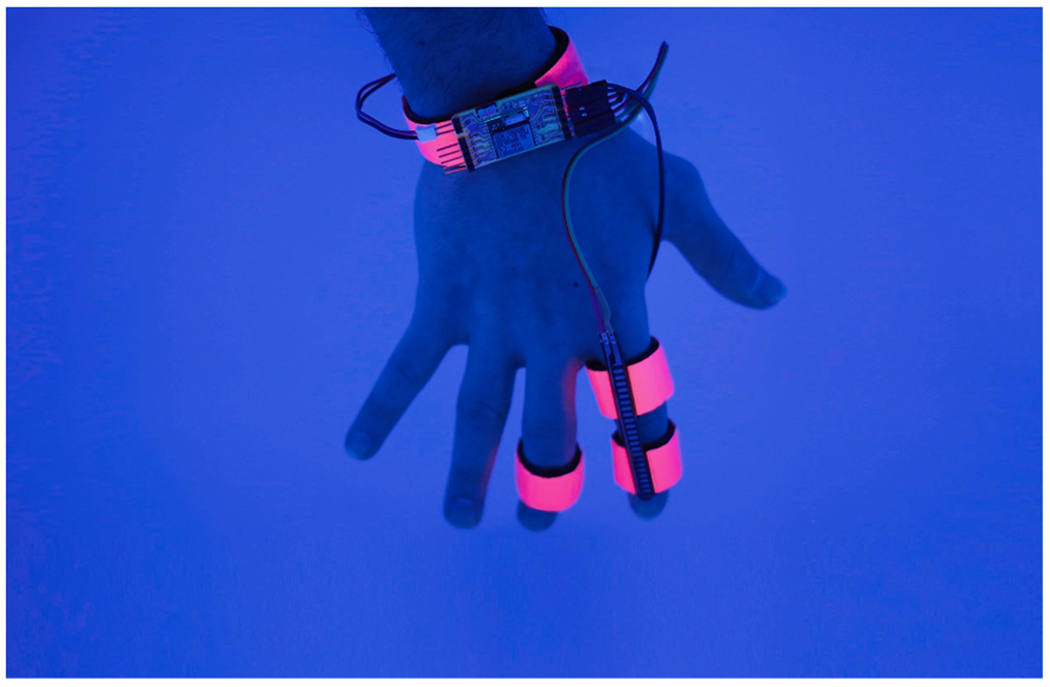
The Dormio, design led by Tomás Vega, and handworn system, collaboration with Oscar Rosello, dorsal side. Photo credit Oscar Rosello.
To achieve this, Dormio uses three physiological indicators of sleep onset in conjunction. First, users are asked to gently close their hand when they lie down to sleep, allowing a flex sensor to monitor progressive loss of muscle tone via hand opening. This is a passive behavioral measure of sleep onset (Kelly, Strecker, & Bianchi, 2012; Prerau et al., 2014), as loss of muscle tone is temporally tied to onset of hypnagogic imagery. Recent papers have also demonstrated that drops in heartrate and shifts in electrodermal activity (EDA) coincide with loss of muscle tone to confirm descent into hypnagogia (Herlan, Ottenbacher, Schneider, Riemann, & Feige, 2019; Ogilvie, 2001). The user’s heart rate is monitored on the middle finger, muscle tone is tracked using a sensor wrapped around the index finger, and EDA is measured between two electrodes placed on the bottom of the wrist. The Dormio glove is designed to be free of wires, breathable, lightweight and comfortable for sleep.
The Dormio app (ios, web) provides an interface for users to control their hypnagogic experiences. There are features for recording the incubation prompts, and for inputting the desired number of hypnagogic rounds. On each awakening, the user verbally reports their dream, which is recorded by the app, and a silence detection feature stops the recording once the report is finished. To gather and store sensor data, the Dormio Web App makes use of OpenSleep, a framework built for biosignal tracking and analysis. Results in Horowitz et al. (2018) and Horowitz (2019) show that Dormio increases direct inclusion of target words in reported dream content, and suggests that inclusion of target words in dreams is linked to improved performance on a range of creativity tasks related to these targets.
2.7. Physiological tracking for HCI
A key step in creating effective virtual world illusions is tracking behavior and physiology of the user, so that stimulation timing and type is appropriate to increase immersion (predictable, believable, etc.) by being coherent with virtual simulations. In addition to a VR visual Head-Mounted Display (HMD), sensors can be added to enable real-time monitoring of a user’s physiological and cognitive state, for instance, heart rate and EDA can be physiological indicators of emotional arousal (Sequeira, Hot, Silvert, & Delplanque, 2009), EEG can provide data regarding cognition (Klimesch, 1999), and facial EMG can be used to detect emotional facial expressions (Van Boxtel, 2010). HCI research has developed hardware and software solutions to provide accurate, real-time information regarding a user’s response to content in a virtual environment in devices that are affordable, wearable, and easy-to-use. For instance, even though the main method for automatic emotion recognition is audio-visual analysis (De Silva, 2004; Wang & Guan, 2008), HCI has looked to EMG to decode emotional expressions (Branco, Firth, Encarnação, & Bonato, 2005; Mahlke & Minge, 2006), removing the need for a front-facing camera, and allowing expression detection regardless of illumination or noise (essential for translation to sleep applications).
2.7.1. Sleep tracking
Although PSG is the gold standard for sleep staging, it is both expensive and impractical for users at home (Ravichandran, Sien, Patel, Kientz, & Pina, 2017). In recent years, there has been an increase in development of low-cost sensors aimed at tracking sleep outside of the laboratory without the need of a PSG setup (de Zambotti et al., 2016). Research shows nine out of ten Americans report using a technological device in the hour before sleep, facilitating the introduction of technological interventions for the bedroom (Gradisar et al., 2013). Many sleep trackers are based on mobile apps, although wearables, smart pillows and smart sheets exist as well (Heise, Rosales, Sheahen, Su, & Skubic, 2013; Lawson et al., 2013; Schreiner & Staresina, 2019). For example, Tal, Shinar, Shaki, Codish, and Goldbart (2017) presented a novel contact-free, under-the-mattress piezoelectric sensor that senses heart, breath and body movement patterns and shows 90.5% sleep stage detection accuracy. Sensors like the Oura ring offer non-intrusive, wireless sleep tracking with adequate staging sensitivity compared to PSG (de Zambotti, Rosas, Colrain, & Baker, 2019). These trackers show promise for users getting information about their sleep, but without the real time sleep staging which is necessary for interventions or interaction in sleep.
The Nightcap home sleep monitoring system enabled a host of important home-based studies in the 1990s (Ajilore, Stickgold, Rittenhouse, & Hobson, 1995; Cantero, Atienza, Stickgold, & Hobson, 2002; Mamelak & Hobson, 1989; Stickgold & Hobson, 1994; Stickgold, Pace-Schott, & Hobson, 1994). The Nightcap used eyelid, eyeball and head movement to track sleep onset, REM sleep and deep NREM sleep in real time and trigger automatic collection of dream reports by sleep stage, with only a 5–10% decrease in accuracy in sleep state identification compared to PSG (Ajilore et al., 1995). However, it relied on custom and uncomfortable sensors stuck to the eyelid and remained wired with a bulky amplification circuit, neither of which is ideal for sleeping settings. We recently modernized the Nightcap into a device called Masca (Vega, 2019), which uses piezoresistive fabric sensors to detect eye movement; these sensors can be placed in an eye mask instead of affixed to the eyelid. Custom-built miniature circuit boards allow signal amplification and measurement of head movement, and Bluetooth transmits data, eliminating the wires and bulky amplification unit. This is just one example of how previous sleep tracking technologies can be modernized and miniaturized using common tools in HCI. While effective for sleep tracking, the use of additional sensors could enable recording of physiological information related to dream content, such as emotional expression.
2.7.2. Fascia
To further improve on the recording of multiple physiological signals, we developed Fascia, a smart sleep mask that simultaneously collects EEG, EOG, EMG, heart rate, head movement and skin temperature. This device is an update of a similar device built to collect physiological data from within a VR facemask, the PhysioHMD (Bernal, Yang, Jain, & Maes, 2018). The Fascia device is a flexible circuit in the shape of a sleep mask (Fig. 4), which aims to gather all relevant PSG data without disturbing sleep quality. The device integrates the sensors close to the skin, while two printed circuit boards house the components for signal processing and storage farther away from the skin. The prototype is designed to maximize the quantity and quality of sensor signals, as well as user comfort, to produce accurate data and reduce the first night effect (Agnew, Webb, & Williams, 1966). The prototype can also detect emotional expression via facial EMG and we previously demonstrated a system for recording the emotion of a VR user to an avatar, by mapping the user’s facial expression during a VR experience to a 3d rigged model avatar. This presents a possibility for recording emotional expressions and displaying them on a dream avatar, since prior research has demonstrated that frowning and smiling muscle tension during sleep corresponds with dreamed emotional content (Perlis, 1991; Rivera-García et al., 2019)
Fig. 4.

(top) The Fascia device, a smart sleep mask that collects EEG, EOG, EMG, heart rate, head movement and skin temperature. (bottom) The user’s real-time expression and emotion are mapped into the user’s VR avatar.
2.7.3. Sleep scoring
There has been a significant increase in research devoted to machine learning algorithms for automatic sleep staging using wearable devices and even radio signals (Zhao, Yue, Katabi, Jaakkola, & Bianchi, 2017), or different types of EEG, such as stickers or in-ear EEG (Nakamura, Goverdovsky, Morrell, & Mandic, 2017; Stochholm, Mikkelsen, & Kidmose, 2016). However, most of these provide offline and not real-time sleep staging that is necessary for dream engineering techniques. Therefore, we developed a Deep Convolutional Neural Network that automatically computes sleep stages in real-time from single channel EEG. It has an overall accuracy of 83.5% for 5-stage classification of sleep stages ( + wake) on 4 test nights using power spectral analysis using the open Sleep-EDF dataset (Koushik et al., 2019). The algorithm processes the EEG data streamed via Bluetooth to a smartphone app. It scores in real-time sleep stages (30-second epochs) and does not require an offline analysis or server-client architecture as used in commercially available EEG headbands. The current algorithm has been tested with a customized EEG based on the commercially available Muse EEG. The app and algorithm are open-sourced and can be adapted to take in single-channel recordings from any wearable EEGs such as Fascia. We aim to use this algorithm for real-time interventions and integrated with other devices for sensory stimulation described throughout this paper.
2.8. Summary
We reviewed a wealth of devices from HCI designed and built to generate and maintain simulated virtual worlds, and provided examples of specific HCI devices that have been adapted to the sleep science field to experimentally modulate the sleeping body and track concurrent changes in dream content. In Table 2 we list existing dream engineering techniques and devices alongside ideas for future developments at the sensory interface of sleep, and describe possible functions of these technologies for improving sleep quality, enhancing memory, or generating specific dream content. Importantly, these devices could provide low-cost options to manipulate dreaming in the wild.
Table 2.
Dream Engineering Techniques, Technologies and Functional Targets.
| Sense | Technique | Technology | Function |
|---|---|---|---|
| Sound | Targeted Reactivation1 (TMR, TDR, TLR), Dream Direction (DD), Rhythmic Entrainment1 (RE) | Bone conduction, Dormio, Bluetooth speaker | Improving sleep depth and sleep efficiency (RE), alleviating nightmares (TDR, TLR), augmenting creativity (TDR), ameliorating dream valence (TLR, DD), enhancing memory (TMR) |
| Visual | Targeted Reactivation (TLR), Pre-Sleep Priming (PSP), Rhythmic Entrainment (RE) | Light stimulation masks (e.g. DreamLight), Head-Mounted Displays, video monitors | Augmenting creativity (TLR), ameliorating dream valence (TLR, PSP), alleviating nightmares (TLR, PSP), improving sleep depth and efficiency (RE) |
| Temperature | Dream Direction (DD), Rhythmic Entrainment (RE) | ChilliPad, thermal sheet, heated eyemasks, hand warmers | Ameliorating dream valence (DD), alleviating nightmares (DD), reducing sleep onset latency (RE), improving sleep depth and efficiency (RE) |
| Haptic/Proprioceptive | Dream Direction (DD), Rhythmic Entrainment (RE) | Pressure cuff, Electrical Muscle Stimulation, Galvanic Vestibular Stimulation, rocking | Ameliorating dream valence (DD), reducing sleep onset latency (RE), motor learning (DD) |
| Smell | Targeted Reactivation (TMR, TDR), Rhythmic Entrainment (RE), Dream Direction (DD) | Essence, olfactometer, scent diffuser | Enhancing memory (TMR), improving sleep depth and efficiency (TMR, RE), alleviating nightmares (TDR, DD), reducing sleep onset latency (RE), ameliorating dream valence (DD) |
Targeted Reactivation includes Targeted Memory Reactivation (TMR), Targeted Dream Reactivation (TDR) and Targeted Lucidity Reactivation (TLR). Rhythmic Entrainment (RE) can act on neural oscillations, respiratory, or circadian rhythms among others. Pre-Sleep Priming (PSP) presents a stimulus prior to sleep intending to influence subsequent dreams, and Dream Direction (DD) applies a stimulus only during sleep to influence dreams.
3. Future visions, ethical considerations and conclusions
3.1. Nighttime neuroprosthetics and BCI control
It was recently shown that controlling a Brain-Computer Interface (BCI) device is possible from within a lucid dream. Mallett (2019) showed that using a consumer BCI, a lucid dreamer was able to, while asleep, control a moving block on a computer screen as instructed. While preliminary, it is worth considering the application of BCIs to developments in dream engineering. For instance, the use of BCIs are increasingly common for neuroprosthetics, e.g. a BCI can detect motor commands (‘move arm’) from the sensorimotor cortex and translate this signal to a neuroprosthetic limb (Müller-Putz, Scherer, Pfurtscheller, & Rupp, 2005). BCI neuroprosthetics have even been used to restore walking in individuals with spinal cord injury (King, Wang, McCrimmon, Chou, Do, & Nenadic, 2014). However a major area of current research is to develop BCIs that are able to integrate more seamlessly with the body schema. Currently the use of BCI neuroprosthetics requires long periods of motor imagery practice, as individuals must re-learn how to send a motor command to control an external limb. VR has been used as a more immersive way to train individuals to control a virtual avatar using a BCI, prior to learning to control a real neuroprosthetic (De Mauro et al., 2010). Given the benefits of motor imagery and VR training for BCIs, a future possibility may be to use lucid dreaming to practice controlling a BCI while also exploring the use of the dreaming body in the dreamworld. Blumberg and Dooley (2017) have likewise suggested that adaptation to neuroprosthetics may benefit from nighttime stimulation similar to twitching, in order to fully integrate the sensory feedback of a neuroprosthetic limb into the body schema, essentially completing the dream engineering circuitry from bottom-up to top-down.
While considering such exciting prospects for the future for dream engineering, we also must consider the potential risks of developing dream engineering to such an extent. For instance, dreams could be directly recorded to a BCI avatar (which highlights the importance of consent), or technology could be directly controlled from within a dream (which highlights the role of agency of a dreamer acting in the real world). These are important ethical considerations that parallel others in the field of neuroengineering, which we discuss further below.
3.2. Ethical considerations
The ethics of dream engineering and sleep manipulation is a critical public discussion to address as experimental approaches and commercial products are increasingly developed. Perhaps of unique importance to the future of dream engineering is a consideration of the vulnerable state of a sleeping person, and the intimate relationship between a person and their dreams. Experimental approaches to influencing dreams in clinical settings, at home or in a sleep laboratory must consider potential threats to avoid the misuse of these technologies, prevent harmful sleep interventions and protect the safety of an individual.
Can dream engineering be harmful? Concepts of mind-control and inception, introducing ideas into an individual’s memory without their consent or even conscious awareness, harken back to public concerns around subliminal persuasion. At present, most dream engineering applications require priming in wakefulness, i.e. within an individual’s awareness, in order to influence dreams or reactivate memories during sleep (see Table 1). However, evidence is now emerging that sleep-learning, i.e. forming new memories while asleep, is possible. For instance, Arzi et al. (2014) paired the scent of cigarettes with that of rotten fish to participants who were smokers; presenting these scent-pairs during sleep alone (but not during wake) led to a reduction in cigarette smoking, meaning the negative association was learned during sleep and influenced subsequent behavior. While this is a positive outcome, there are real concerns that manipulating dreams or sleep-learning can have negative outcomes. These range from simple possibilities of inducing nightmares via unpleasant sensory stimulation, to more complex possibilities of selectively enhancing or weakening implicit associations – creating political bias or sexual attraction, among others.
At a more basic level, even dream engineering that is beneficial for an individual may be at the expense of other natural dreaming processes. For instance, if dream engineering is used on a nightly basis to trigger pleasant rose-scented dreams, does it interfere with the natural process of emotion regulation, the ‘overnight therapy’ of normal dreaming? If targeting learning a new language, could other declarative memories weaken? Thus, a critical avenue of research is evaluating the extent to which repeated or prolonged application of stimulation techniques can interfere with natural sleep function. Similar cautions have recently been raised in the field of lucid dreaming. Despite the numerous benefits afforded by lucid dreams, researchers argue that repeatedly practicing techniques of lucid dream induction, such as questioning reality during the day (termed reality checks) or disrupting the normal sleep pattern could have detrimental effects on psychology (Soffer-Dudek, 2020).
Finally, the state of immobility and absence of voluntary control make a sleeping person particularly vulnerable to external influences. Indeed, dream engineering approaches are designed to be nearly imperceptible in order to not waken an individual, and in experimental studies participants are unaware of whether they have been exposed to stimulation during sleep or not. Thus, individuals by design are unaware of and have no control over the application of dream engineering as it is occurring. It is necessary to continue to evaluate the potential misuses of dream engineering technology, including purposeful misuses for personal or political gain, but also unintentional misuses that interfere with natural sleep emotion or memory processing functions. In parallel with these ethical considerations, we suggest that a minimalist approach to dream engineering using simple sense stimulation techniques, technologies, and targets carries promise for numerous positive outcomes, and we encourage future research that keeps ethical responsibility at the forefront of dream engineering development.
3.3. Conclusions
In this paper, we offer a vision for the application of a wide range of sensory stimulation technologies to the area of sleep and dream engineering. We begin by emphasizing the causal role of the body in dream generation, outlining bodily sensations that serve as a bottom-up source of dreaming and identifying isomorphisms between physiology of the sleeping body and phenomenology of the dream. We further outline top-down processes which shape dream content: that memories are used as material for creation of dream content, with current emotion as a driving force behind associative connections, and generative sense making as an updating process to incorporate current sensations. Accordingly, we justify our approach of dream engineering via bodily stimulation, moving beyond neurocognitive brain in a vat models of dreaming. The dream is understood as in circuit with the body. We then identify past protocols for influencing dream content, many of which have taken advantage of dreaming in circuitry with the body, i.e. linking thirst to dreams of water. Considering other areas that might afford tools for engineering sensory content in simulated worlds, we turn to Virtual Reality. We elucidate parallels between dreaming as a simulation of waking experiences and VR as a simulated world and we describe the development of new VR technologies, such as haptic and olfactory stimulation devices designed to simulate waking sensations and engineer plausible world simulations. We propose that an understanding of these Human Computer Interaction technologies, in the context of sleep and dream research, will enable developments in dream engineering. We outline a collection of relevant VR technologies from the HCI field, categorizing them by sense, including those which potentiate auditory, olfactory, temperature and haptic stimulation. We hope these technologies, which have been engineered for high mobility and low cost, can be transferred directly to the field of dream engineering. We also describe relevant tools created to interface with sleep and dreams, including sleep tracking devices that offer clear links for integration with VR technologies. We close by discussing possible future directions in sleep engineering, dream direction and nighttime neuroprosthetics, and the ethics of a world in which targeted dream engineering and sleep manipulation are feasible.
Acknowledgement
This work was supported by the National Institute of General Medical Sciences grant: K12 GM106997 (MC).
References
- Agnew HW Jr., Webb WB, & Williams RL (1966). The first night effect: An Eeg study of sleep. Psychophysiology, 2(3), 263–266. [DOI] [PubMed] [Google Scholar]
- Ajilore O, Stickgold R, Rittenhouse CD, & Hobson JA (1995). Nightcap: Laboratory and home-based evaluation of a portable sleep monitor. Psychophysiology, 32(1), 92–98. [DOI] [PubMed] [Google Scholar]
- Amores J (2016). Essence: Olfactory interfaces for unconscious influence of mood and cognitive performance [MSc Thesis]. Massachusetts Institute of Technology. [Google Scholar]
- Amores J, Dotan M, & Maes P (2019). An Exploration of Form Factors for Sleep-Olfactory Interfaces. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 1456–1460). [DOI] [PubMed] [Google Scholar]
- Amores J, Hernandez J, Dementyev A, Wang X, & Maes P (2018). BioEssence: A Wearable Olfactory Display that Monitors Cardio-respiratory Information to Support Mental Wellbeing. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 5131–5134). [DOI] [PubMed] [Google Scholar]
- Amores J, & Maes P (2017). Essence: Olfactory Interfaces for Unconscious Influence of Mood and Cognitive Performance 28–34. 10.1145/3025453.3026004. [DOI]
- Amores J, Richer R, Zhao N, Maes P, & Eskofier BM (2018). Promoting relaxation using virtual reality, olfactory interfaces and wearable EEG. In 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN) (pp. 98–101). [Google Scholar]
- Amores J, Wang J, Dotan M, & Maes P (2019). Lotuscent: Targeted memory reactivation for wellbeing using scent and VR biofeedback. In IEEE EMBS Symposium and Workshop on Brain, Mind, and Body: Cognitive Neuroengineering for Health and Wellness. [Google Scholar]
- Andrillon T, & Kouider S (2019). The vigilant sleeper: Neural mechanisms of sensory (de) coupling during sleep. Current Opinion Physiology. [Google Scholar]
- Antony JW, & Paller KA (2017). Using oscillating sounds to induce sleep spindles. Sleep, 40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, & Sobel N (2014). Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. Journal of Neuroscience, 34(46), 15382–15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Sela L, Green A, Givaty G, Dagan Y, & Sobel N (2010). The influence of odorants on respiratory patterns in sleep. Chemical Senses, 35(1), 31–40. [DOI] [PubMed] [Google Scholar]
- Aserinsky E, & Kleitman N (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science, 118(3062), 273–274. [DOI] [PubMed] [Google Scholar]
- Badia P, Wesensten N, Lammers W, Culpepper J, & Harsh J (1990). Responsiveness to olfactory stimuli presented in sleep. Physiology & Behavior, 48(1), 87–90. [DOI] [PubMed] [Google Scholar]
- Barrett D (1993). The” committee of sleep”: A study of dream incubation for problem solving. Dreaming, 3(2), 115. [Google Scholar]
- Bayer L, Constantinescu I, Perrig S, Vienne J, Vidal P-P, Mühlethaler M, & Schwartz S (2011). Rocking synchronizes brain waves during a short nap. Current Biology, 21(12), R461–R462. 10.1016/j.cub.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Benali-Khoudja M, Hafez M, Alexandre J-M, & Kheddar A (2004). Tactile interfaces: A state-of-the-art survey. International Symposium on Robotics 10. [Google Scholar]
- Bernal G, Yang T, Jain A, & Maes P (2018). PhysioHMD: A conformable, modular toolkit for collecting physiological data from head-mounted displays. Proceedings of the 2018 ACM International Symposium, on Wearable Computers (pp. 160–167). [Google Scholar]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, & Vaughn BV (2012). The AASM manual for the scoring of sleep and associated events: Rules, Terminology and Technical Specifications, Version 2.2. American Academy of Sleep Medicine. [Google Scholar]
- Blanke O (2012). Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews Neuroscience, 13(8), 556. [DOI] [PubMed] [Google Scholar]
- Blumberg Mark S., & Dooley James C. (2017). Phantom limbs, neuroprosthetics, and the developmental origins of embodiment. Trends in neurosciences, 40(10), 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS (2010). Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology, 1, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Marques HG, & Iida F (2013). Twitching in Sensorimotor Development from Sleeping Rats to Robots. Current Biology, 23, R532–R537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, & Plumeau AM (2016). A new view of “dream enactment” in REM sleep behavior disorder. Sleep Medicine Reviews, 30, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokert E (1968). The Effects of Thirst and a Related Verbal Stimulus on Dream Reports. Dissertation Abstracts International, 28(11-B), 4753. [Google Scholar]
- Branco P, Firth P, Encarnação LM, & Bonato P (2005). Faces of emotion in human-computer interaction. CHI’05 Extended Abstracts on Human Factors. Computing Systems, 1236–1239. [Google Scholar]
- Brooks FP (1999). What’s real about virtual reality? IEEE Computer Graphics and Applications, 19(6), 16–27. 10.1109/38.799723. [DOI] [Google Scholar]
- Brooks J, Nagels S, & Lopes P (2020). Trigeminal-based Temperature Illusions. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI ‘20). [Google Scholar]
- Brueckner AL (1986). Brains in a Vat. The Journal of Philosophy, 83(3), 148–167. [Google Scholar]
- Burdea GC (2000). Haptics issues in virtual environments. Proceedings Computer Graphics International, 2000, 295–302. 10.1109/CGI.2000.852345. [DOI] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, & Mednick SC (2009). REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences, 106(25), 10130–10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA, Spence C, & Stein BE (2004). The handbook of multisensory processing. [Google Scholar]
- Campbell RL, & Germain A (2016). Nightmares and posttraumatic Stress disorder (PTSD). Current Sleep Medicine Reports, 2(2), 74–80. [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, & Hobson JA (2002). Nightcap: A reliable system for determining sleep onset latency. Sleep, 25(2), 238–245. [DOI] [PubMed] [Google Scholar]
- Carr M, Konkoly K, Mallett R, Edwards C, Appel K, & Blagrove M (2020). Combining pre-sleep cognitive training and REM-sleep stimulation in a laboratory morning nap for lucid dream induction. Psychology of Consciousness: Theory, Research, and Practice. [Google Scholar]
- Carr M, & Nielsen T (2015). Morning REM sleep naps facilitate broad access to emotional semantic networks. Sleep, 38(3), 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M, & Nielsen T (2017). A novel Differential Susceptibility framework for the study of nightmares: Evidence for trait sensory processing sensitivity. Clinical Psychology Review, 58, 86–96. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, & Herz RS (2004). Minimal olfactory perception during sleep: Why odor alarms will not work for humans. Sleep, 27(3), 402–405. [PubMed] [Google Scholar]
- Cavallero C, Cicogna P, Natale V, Occhionero M, & Zito A (1992). Slow wave sleep dreaming. Sleep, 15(6), 562–566. [DOI] [PubMed] [Google Scholar]
- Chen DKY, Anderson IA, Walker CG, & Besier TF (2016). Lower Extremity Lateral Skin Stretch Perception for Haptic Feedback. IEEE Transactions on Haptics, 9(1), 62–68. 10.1109/TOH.2016.2516012. [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Newby-Clark IR, & Rueffer SD (1999). Relations among hypnagogic and hypnopompic experiences associated with sleep paralysis. Journal of Sleep Research, 8(4), 313–317. [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Rueffer SD, & Newby-Clark IR (1999). Hypnagogic and hypnopompic hallucinations during sleep paralysis: Neurological and cultural construction of the night-mare. Consciousness and Cognition, 8(3), 319–337. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Becker J, García L, Ibarra R, Morales M, & Souza M (1986). Dream content after using visual inverting prisms. Perceptual and Motor Skills, 63(2), 415–423. [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Garfinkel SN (2018). The influence of physiological signals on cognition. Current Opinion in Behavioral Sciences, 19, 13–18. [Google Scholar]
- Culbertson H, López Delgado JJ, & Kuchenbecker KJ (2014). One hundred data-driven haptic texture models and open-source methods for rendering on 3D objects. IEEE Haptics Symposium (HAPTICS), 2014, 319–325. 10.1109/HAPTICS.2014.6775475. [DOI] [Google Scholar]
- De Koninck J-M, & Koulack D (1975). Dream content and adaptation to a stressful situation. Journal of Abnormal Psychology, 84(3), 250. [DOI] [PubMed] [Google Scholar]
- De Mauro A, Carrasco E, Oyarzun D, Ardanza A, Paloc C, Gil A, & Florez J (2010). Virtual reality system in conjunction with neurorobotics and neuroprosthetics for rehabilitation in cerebrovascular accidents and spinal cord injuries. Proceedings of the 10th IEEE International Conference on Information Technology and Applications in Biomedicine (pp. 1–3). [Google Scholar]
- De Silva LC (2004). Audiovisual emotion recognition. In 2004 IEEE International Conference on Systems, Man and Cybernetics (IEEE Cat. No. 04CH37583) (Vol. 1, pp. 649–654). [Google Scholar]
- de Zambotti M, Godino JG, Baker FC, Cheung J, Patrick K, & Colrain IM (2016). The boom in wearable technology: Cause for alarm or just what is needed to better understand sleep? Sleep, 39(9), 1761–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Rosas L, Colrain IM, & Baker FC (2019). The sleep of the ring: Comparison of the OURA sleep tracker against polysomnography. Behavioral Sleep Medicine, 17(2), 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarnot U, Perrault A, Sterpenich V, Legendre G, Huber C, Guillot A, & Schwartz S (2019). Motor imagery practice during arm-immobilization benefits sensorimotor cortical functions and plasticity-related sleep features. BioRxiv, 828889. [Google Scholar]
- Dement W, & Kleitman N (1957a). Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalography and Clinical Neurophysiology, 9(4), 673–690. [DOI] [PubMed] [Google Scholar]
- Dement W, & Kleitman N (1957b). The relation of eye movements during sleep to dream activity: An objective method for the study of dreaming. Journal of Experimental Psychology, 53(5), 339. [DOI] [PubMed] [Google Scholar]
- Dement W, & Wolpert EA (1958). The relation of eye movements, body motility, and external stimuli to dream content. Journal of Experimental Psychology, 55(6), 543. [DOI] [PubMed] [Google Scholar]
- Di Campli San Vito P, Shakeri G, Brewster S, Polliek F, Brown E, Skrypehuk L, & Mouzakitis A (2019). Haptic Navigation Cues on the Steering Wheel. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems - CHI ’9 (pp. 1–11). 10.1145/3290605.3300440. [DOI] [Google Scholar]
- Domhoff GW (2017). The emergence of dreaming Mind-wandering, embodied simulation, and the default network, Oxford University Press. [Google Scholar]
- Dresler Martin, Kock Stefan P., Wehrle Renate, Spoormaker Victor I., Holsboer Florian, Steiger Axel, … Czisch Michael (2011). Dreamed movement elicits activation in the sensorimotor cortex. Current Biology, 21 (21), 1833–1837. [DOI] [PubMed] [Google Scholar]
- Farbiz F, Yu ZH, Manders C, & Ahmad W (2007). An Electrical Muscle Stimulation Haptic Feedback for Mixed Reality Tennis Game. In ACM SIGGRAPH 2007 Posters. 10.1145/1280720.1280873. [DOI] [Google Scholar]
- Field T, Field T, Cullen C, Largie S, Diego M, Schanberg S, & Kuhn C (2008). Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Human Development, 84(6), 399–401. [DOI] [PubMed] [Google Scholar]
- Fisher C, Byrne J, Edwards A, & Kahn E (1970). A psychophysiological study of nightmares. Journal of the American Psychoanalytic Association, 18(4), 747–782. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, & Gandevia SC (1994). Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. The Journal of Physiology, 478(2), 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes D (1985). Dreaming: A cognitive-psychological analysis. A Cognitive-Psychological Analysis. New York: Hillsdale. [Google Scholar]
- Freud S (1965). The interpretation of dreams (Strachey J, Trans.). New York: Avon.(Original Work Published 1900; ). [Google Scholar]
- Gallagher S (2000). Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences, 4(1), 14–21. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Franco M, & Lanier J (2017). Model of illusions and virtual reality. Frontiers in Psychology, 8, 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DR, Witkin HA, Koulack D, & Cohen H (1975). The effects of stress films on dream affect and on respiration and eye-movement activity during rapid-eye-movement sleep. Psychophysiology, 12(3), 313–320. [DOI] [PubMed] [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, & Czeisler CA (2013). The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America poll. Journal of Clinical Sleep Medicine, 9(12), 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, & Herzog ED (2006). A circadian clock in the olfactory bulb controls olfactory responsivity. Journal of Neuroscience, 26(47), 12219–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Zhang Y, Sun W, Bian Y, Zhou D, & Kristensson PO (2016). Dexmo: An Inexpensive and Lightweight Mechanical Exoskeleton for Motion Capture and Force Feedback in VR. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems (pp. 1991–1995). 10.1145/2858036.2858487. [DOI] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, & Walker MP (2010). A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cerebral Cortex, 21(1), 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P, Clark S, & Kalogeras J (2002). Voluntary action and conscious awareness. Nature Neuroscience, 5(4), 382. [DOI] [PubMed] [Google Scholar]
- Han T, Anderson F, Irani P, & Grossman T (2018). Hydro Ring: Supporting Mixed Reality Haptics Using Liquid Flow. In The 31st Annual ACM Symposium on User Interface Software and Technology - UIST ‘18 (pp. 913–925). 10.1145/3242587.3242667. [DOI] [Google Scholar]
- Harding EC, Franks NP, & Wisden W (2019). The Temperature Dependence of Sleep. Frontiers in Neuroscience, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E (1996). Outline for a theory on the nature and functions of dreaming. Dreaming 6(2), 147. [Google Scholar]
- Hartmann E, & Kunzendorf R (2013). Thymophor in dreams, poetry, art and memory: Emotion translated into imagery as a basic element of human creativity. Imagination, Cognition and Personality, 33(1), 165–191. [Google Scholar]
- Hauner KK, Howard JD, Zelano C, & Gottfried JA (2013). Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nature Neuroscience, 16(11), 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne KM (1978). Lucid dreams: An elecro-physiological and psychological study [PhD Thesis], Liverpool University. [Google Scholar]
- Heilig M (1961). ‘Sensorama: A Totally Mechanical VR Device (One Person Theater). Invention. [Google Scholar]
- Heise D, Rosales L, Sheahen M, Su B-Y, & Skubic M (2013). Non-invasive measurement of heartbeat with a hydraulic bed sensor progress, challenges, and opportunities. In 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) (pp. 397–402). [Google Scholar]
- Hennevin E, Huetz C, & Edeline J-M (2007). Neural representations during sleep: From sensory processing to memory traces. Neurobiology of Learning and Memory, 87(3), 416–440. [DOI] [PubMed] [Google Scholar]
- Herlan A, Ottenbacher J, Schneider J, Riemann D, & Feige B (2019). Electrodermal activity patterns in sleep stages and their utility for sleep versus wake classification. Journal of Sleep Research, 28(2), Article e12694. [DOI] [PubMed] [Google Scholar]
- Herman JH, Erman M, Boys R, Peiser L, Taylor ME, & Roffwarg HP (1984). Evidence for a directional correspondence between eye movements and dream imagery in REM sleep. Sleep, 7(1), 52–63. [DOI] [PubMed] [Google Scholar]
- Hobson JA (2009). REM sleep and dreaming: Towards a theory of protoconsciousness. Nature Reviews Neuroscience, 10(11), 803. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Hong CC-H, & Friston KJ (2014). Virtual reality and consciousness inference in dreaming. Frontiers in Psychology, 5, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, & McCarley R (1997). The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. American Journal of Psychiatry, 134, 1335–1348. [DOI] [PubMed] [Google Scholar]
- Hoelscher TJ, Klinger E, & Barta SG (1981). Incorporation of concern-and nonconcern-related verbal stimuli into dream content. Journal of Abnormal Psychology, 90(1), 88. [Google Scholar]
- Horowitz A (2019). Incubating Dreams [PhD Thesis]. Massachusetts Institute of Technology. [Google Scholar]
- Horowitz A, Grover I, Reynolds-Cuéllar P, Breazeal C, & Maes P (2018). Dormio: Interfacing with Dreams. Extended Abstracts of. the 2018 CHI Conference on Human Factors in Computing Systems (pp. alt10). [Google Scholar]
- Hufford D (1982). The terror that comes in the night: An experience-centered study of supernatural assault traditions (Vol. 7). University of Pennsylvania Press. [Google Scholar]
- Horowitz Adam, Cunningham Tony, Maes Pattie, & Stickgold Robert (2020). Dormio: A targeted dream incubation device. Consciousness and Cognition, 83, 102938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann F, Fröhlich J, Mattar N, & Wachsmuth I (2014). Wind and warmth in virtual reality: Implementation and evaluation. Proceedings of the 2014 Virtual Reality International Conference (pp. 1–8). . 10.1145/2617841.2620712. [DOI] [Google Scholar]
- Igaki M, Suzuki M, Sakamoto I, Ichiba T, Kuriyama K, & Uchiyama M (2018). Effects of bedtime periocular and posterior cervical cutaneous warming on sleep status in adult male subjects: A preliminary study. Sleep and Biological Rhythms, 16(1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll EW, & Thoman EB (1994). The breathing bear: Effects on respiration in premature infants. Physiology & Behavior, 56(5), 855–859. [DOI] [PubMed] [Google Scholar]
- Ischer M, Baron N, Mermoud C, Cayeux I, Porcherot C, Sander D, & Delplanque S (2014). How incorporation of scents could enhance immersive virtual experiences. Frontiers in Psychology, 5, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, Feldman M, & Bender MB (1972). Are the eye movements of dreaming sleep related to the visual images of the dreams? Psychophysiology, 9(4), 393–401. [DOI] [PubMed] [Google Scholar]
- Jain D, Sra M, Guo J, Marques R, Wu R, Chiu J, & Schmandt C (2016). Immersive Terrestrial Scuba Diving Using Virtual Reality. Proceedings of the 2016 CHI Conference Extended Abstracts on Human Factors in Computing Systems (pp. 1563–1569). . 10.1145/2851581.2892503. [DOI] [Google Scholar]
- Jalal B, Simons-Rudolph J, Jalal B, & Hinton DE (2014). Explanations of sleep paralysis among Egyptian college students and the general population in Egypt and Denmark. Transcultural Psychiatry, 51(2), 158–175. [DOI] [PubMed] [Google Scholar]
- Jeong S, Hashimoto N, & Makoto S (2004). A Novel Interaction System with Force Feedback between Real—And Virtual Human: An Entertainment System: “Virtual Catch Ball” Proceedings of the 2004 ACM SIGCHI International Conference on Advances in Computer Entertainment Technology (pp. 61–66).. 10.1145/1067343.1067350. [DOI] [Google Scholar]
- Keller PE, Kouzes RT, Kangas LJ, & Hashem S (1995). Transmission of Olfactory Information. Interactive Technology and the New Paradigm for Healthcare, 18, 168. [Google Scholar]
- Kelly JM, Strecker RE, & Bianehi MT (2012). Recent developments in home sleep-monitoring devices. ISRN Neurology, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Wang PT, McCrimmon CM, Chou CC, Do AH, & Nenadic Z (2014). Brain-computer interface driven functional electrical stimulation system for overground walking in spinal cord injury participant. In 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (pp. 1238–1242). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (1999). EEC alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2–3), 169–195. [DOI] [PubMed] [Google Scholar]
- Klinzing JG, Kugler S, Soekadar SR, Rasch B, Born J, & Diekelmann S (2018). Odor cueing during slow-wave sleep benefits memory independently of low cholinergic tone. Psychopharmacology, 235(1), 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulack D (1969). Effects of somatosensory stimulation on dream content. Archives of General Psychiatry, 20(6), 718–725. [DOI] [PubMed] [Google Scholar]
- Koushik A, Amores J, & Maes P (2019). Real-time Smartphone-based Sleep Staging using 1-Channel EEG. In 2019 IEEE 16th International Conference on Wearable and Implantable Body Sensor Networks (BSN) (pp. 1–4). [Google Scholar]
- Krakow B, & Zadra A (2010). Imagery rehearsal therapy: Principles and practice. Sleep Medicine Clinics, 5(2), 289–298. [Google Scholar]
- Kramer M (1991). The nightmare: A failure in dream function. Dreaming, 1(4), 277. [Google Scholar]
- Kron A, & Schmidt G (2003). Multi-fingered tactile feedback from virtual and remote environments. In 11th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, 2003. HAPTICS 2003. Proceedings (pp. 16–23). 10.1109/HAPTIC.2003.1191219. [DOI] [Google Scholar]
- Kryo Inc. (2019). Chili, http://chilitechnology.com.
- LaBerge S (1980). Lucid Dreaming: A Study of Consciousness During Sleep [PhD Thesis]. Ph. D. [Google Scholar]
- LaBerge S, & Levitan L (1995). Validity established of DreamLight cues for eliciting lucid dreaming. Dreaming 5(3), 159–168. [Google Scholar]
- LaBerge S, Nagel LE, Dement WC, & Zarcone VP Jr (1981). Lucid dreaming verified by volitional communication during REM sleep. Perceptual and Motor Skills, 52(3), 727–732. [DOI] [PubMed] [Google Scholar]
- Lack L, & Lushington K (1996). The rhythms of human sleep propensity and core body temperature. Journal of Sleep Research, 5(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Lawson S, Jamison-Powell S, Garbett A, Linehan C, Kucharczyk E, Verbaan S, … Morgan K (2013). Validating a mobile phone application for the everyday, unobtrusive, objective measurement of sleep. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (pp. 2497–2506). . [Google Scholar]
- Legendre G, Andrillon T, Koroma M, & Kouider S (2019). Sleepers track informative speech in a multitalker environment. Nature Human Behaviour, 3(3), 274–283. [DOI] [PubMed] [Google Scholar]
- Levin R, & Nielsen T (2009). Nightmares, bad dreams, and emotion dysregulation: A review and new neurocognitive model of dreaming. Current Directions in Psychological Science, 18(2), 84–88. [Google Scholar]
- Lewis PA, & Bendor D (2019). How Targeted Memory Reactivation Promotes the Selective Strengthening of Memories in Sleep. Current Biology, 29( 18), R906–R912. [DOI] [PubMed] [Google Scholar]
- Li BJ, & Bailenson JN (2018). Exploring the influence of haptic and olfactory cues of a virtual donut on satiation and eating behavior. Presence, 26(03), 337–354. [Google Scholar]
- Lindeman RW, Yanagida Y, Noma H, & Hosaka K (2006). Wearable vibrotactile systems for virtual contact and information display. Virtual Reality, 9(2), 203–213. 10.1007/s10055-005-0010-6. [DOI] [Google Scholar]
- Lopes P, & Baudisch P (2013). Muscle-propelled Force Feedback: Bringing Force Feedback to Mobile Devices. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (pp. 2577–2580). . 10.1145/2470654.2481355. [DOI] [Google Scholar]
- Lopes P, Ion A, & Baudisch P (2015). Impacto: Simulating Physical Impact by Combining Tactile Stimulation with Electrical Muscle Stimulation. Proceedings of the 28th Annual ACM Symposium on User Interface Software & Technology (pp. 11–19). . 10.1145/2807442.2807443. [DOI] [Google Scholar]
- Lopes P, You S, Cheng L-P, Marwecki S, & Baudisch P (2017). Providing Haptics to Walls & Heavy Objects in Virtual Reality by Means of Electrical Muscle Stimulation. Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems (pp. 1471–1482). . 10.1145/3025453.3025600. [DOI] [Google Scholar]
- Lopes P, You S, Ion A, & Baudisch P (2018). Adding Force Feedback to Mixed Reality Experiences and Games Using Electrical Muscle Stimulation. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems (Vol. 446, pp. 1–446:13). 10.1145/3173574.3174020. [DOI] [Google Scholar]
- Ma S, Wu T, & Pi G (2014). Sleep paralysis in Chinese adolescents: A representative survey. Sleep and Biological Rhythms, 12(1), 46–52. [Google Scholar]
- Mahlke S, & Minge M (2006). Emotions and EMG measures of facial muscles in interactive contexts. Cognition and Emotion, 6, 169–200. [Google Scholar]
- Mallett R (2019). A pilot investigation into brain-computer interface use during a lucid dream. [Google Scholar]
- Mamelak A, & Hobson JA (1989). Nightcap: A home-based sleep monitoring system. Sleep, 12(2), 157–166. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, & Born J (2006). Boosting slow oscillations during sleep potentiates memory. Nature, 444(7119), 610. [DOI] [PubMed] [Google Scholar]
- McKellar P (1995). Creative imagination: Hypnagogia and surrealism. Journal of Mental Imagery.. [Google Scholar]
- McNally RJ, & Clancy SA (2005). Sleep paralysis, sexual abuse, and space alien abduction. Transcultural Psychiatry, 42(1), 113–122. [DOI] [PubMed] [Google Scholar]
- Monahan T, McArdle G, & Bertolotto M (2008). Virtual reality for collaborative e-learning. Computers & Education, 50(4), 1339–1353. [Google Scholar]
- Moskowitz E, & Berger RJ (1969). Rapid eye movements and dream imagery: Are they related? Nature, 224(5219), 613. [DOI] [PubMed] [Google Scholar]
- Müller-Putz GR, Scherer R, Pfurtscheller G, & Rupp R (2005). EEG-based neuroprosthesis control: A step towards clinical practice. Neuroscience Letters, 382(1–2), 169–174. [DOI] [PubMed] [Google Scholar]
- Murayama J, Bougrila L, Luo Y, Akahane K, Hasegawa S, Hirsbrunner B, & Sato M (2004). SPIDAR G&G: A Two-Handed Haptic Interface for Bimanual VR Interaction. Eurohaptics, 9. [Google Scholar]
- Naef M, Staadt O, & Gross M (2002). Spatialized audio rendering for immersive virtual environments. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, 65–72. [Google Scholar]
- Nakamura T, Goverdovsky V, Morrell MJ, & Mandic DP (2017). Automatic sleep monitoring using ear-EEG. IEEE Journal of Translational Engineering in Health and Medicine, 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi T, Tomohiro A, Seong YA, & Hirose M (2009). Characterizing the Space by Thermal Feedback through a Wearable Device. In Shumaker R (Vol. Ed.), Virtual and Mixed Reality: Vol 5622, (pp. 355–364). Berlin Heidelberg: Springer, 10.1007/978-3-642-02771-0_40. [DOI] [Google Scholar]
- Nielsen T (1993). Changes in the kinesthetic content of dreams following somatosensory stimulation of leg muscles during REM sleep. Dreaming, 3(2), 99. [Google Scholar]
- Nielsen T (2010). Dream analysis and classification: The reality simulation perspective. Principles and Practice of Sleep Medicine, 595–603. [Google Scholar]
- Nielsen T (2012). Dream incubation: Ancient techniques of dream influence. Www.Dreamscience.Ca.
- Nielsen T (2017). Microdream neurophenomenology. Neuroscience of Consciousness, 2017(1), nix001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T, & Carr M (2017). Nightmares and nightmare function. Principles and practice of sleep medicine (pp. 546–554). Elsevier. [Google Scholar]
- Nielsen T, Carr M, Blanchette-Carrière C, Marquis L-P, Dumel G, Solomonova E, … Paquette T (2017). NREM sleep spindles are associated with dream recall. Sleep Spindles & Cortical Up States, 1(1), 27–41. [Google Scholar]
- Nielsen T, & Powell RA (1992). The day-residue and dream-lag effects: A literature review and limited replication of two temporal effects in dream formation. Dreaming, 2(2), 67. [Google Scholar]
- Nielsen T, & Powell RA (2015). Dreams of the Rarebit Fiend: Food and diet as instigators of bizarre and disturbing dreams. Frontiers in Psychology, 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T, & Zadra A (2005). Nightmares and other common dream disturbances. Principles and Practice of Sleep Medicine, 4, 926–935. [Google Scholar]
- Noreika V, Windt JM, Kern M, Valli K, Salonen T, Parkkola R, & Lenggenhager B (2020). Modulating dream experience: Noninvasive brain stimulation over the sensorimotor cortex reduces dream movement. Scientific Reports, 10(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie RD (2001). The process of falling asleep. Sleep Medicine Reviews, 5(3), 247–270. [DOI] [PubMed] [Google Scholar]
- Olunu E, Kimo R, Onigbinde EO, Akpanobong M-AU, Enang IE, Osanakpo M, … Fakoya A0J (2018). Sleep paralysis, a medical condition with a diverse cultural interpretation. International Journal of Applied and Basic Medical Research, 8(3), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Nell CW (1965). A cross-cultural study of hunger and thirst motivation manifested in dreams. Human Development, 181–193. [Google Scholar]
- Ong AA, & Gillespie MB (2016). Overview of smartphone applications for sleep analysis. World Journal of Otorhinolaryngology-Head and Neck Surgery, 2(1), 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Antony JW, & Paller KA (2014). Fear not: Manipulating sleep might help you forget. Trends in Cognitive Sciences, 18(1), 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, De Cock VC, Lavault S, Leu S, Vidailhet M, & Arnulf I (2009). Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology, 72(6), 551–557. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Dodet P, Ledard N, Artru E, Rachidi I, Similowski T, & Arnulf I (2018). REM sleep respiratory behaviours match mental content in narcoleptic lucid dreamers. Scientific Reports, 8(1), 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, & Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences, 17(3), 142–149. [DOI] [PubMed] [Google Scholar]
- Peiris RL, Feng Y-L, Chan L, & Minamizawa K (2019). ThermalBraeelet: Exploring Thermal Haptic Feedback Around the Wrist. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems - CHI ’19 (pp. 1–11). 10.1145/3290605.3300400. [DOI] [Google Scholar]
- Peiris RL, Peng W, Chen Z, Chan L, & Minamizawa K (2017). ThermoVR: Exploring Integrated Thermal Haptic Feedback with Head Mounted Displays. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems - CHI ’17 (pp. 5452–5456). 10.1145/3025453.3025824. [DOI] [Google Scholar]
- Peiris, Roshan Lalitha, Feng Y-L, Chan L, & Minamizawa K (2019). ThermalBraeelet: Exploring Thermal Haptic Feedback Around the Wrist. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems - CHI ’9 (pp. 1–11). 10.1145/3290605.3300400. [DOI] [Google Scholar]
- Perl O, Arzi A, Sela L, Secundo L, Holtzman Y, Samnon P, … Hairston IS (2016). Odors enhance slow-wave activity in non-rapid eye movement sleep. Journal of Neurophysiology, 115(5), 2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML (1991). Sustained facial muscle activity during. REM sleep. [DOI] [PubMed] [Google Scholar]
- Perogamvros L, Park H-D, Bayer L, Perrault AA, Blanke O, & Schwartz S (2019). Increased heartbeat-evoked potential during REM sleep in nightmare disorder. Neuroimage: Clinical, 22, Article 101701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Dünte T, Schneegass S, Alt F, & Rohs M (2015). Cruise Control for Pedestrians: Controlling Walking Direction Using Electrical Muscle Stimulation. Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems (pp. 2505–2514). . 10.1145/2702123.2702190. [DOI] [Google Scholar]
- Prerau MJ, Hartnack KE, Obregon-Henao G, Sampson A, Merlino M, Gannon K, … Purdon PL (2014). Tracking the sleep onset process: An empirical model of behavioral and physiological dynamics. PLoS Computational Biology, 10(10), Article e1003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radvansky BA, & Dombeck DA (2018). An olfactory virtual reality system for mice. Nature Communications, 9(1), 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe N, Eason Wai Tung C, Yen CC, Do EY-L, Jain P, Thi Ngoc Tram N, …, Shamaiah K (2018). Season Traveller: Multisensory Narration for Enhancing the Virtual Reality Experience. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems (pp. 1–13). 10.1145/3173574.3174151. [DOI] [Google Scholar]
- Rasch B, Büchel C, Gais S, & Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315(5817), 1426–1429. [DOI] [PubMed] [Google Scholar]
- Ravichandran R, Sien S-W, Patel SN, Kientz JA, & Pina LR (2017). Making sense of sleep sensors: How sleep sensing technologies support and undermine sleep health. Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems (pp. 6864–6875). . [Google Scholar]
- Raymann RJ, Swaab DF, & Van Someren EJ (2007). Skin temperature and sleep-onset latency: Changes with age and insomnia. Physiology & Behavior, 90(2–3), 257–266. [DOI] [PubMed] [Google Scholar]
- Rekimoto J (2013). Traxion: A tactile interaction device with virtual force sensation. In Proceedings of the 26th Annual ACM Symposium on User Interface Software and Technology - UIST ℉13 (pp. 427–432). 10.1145/2501988.2502044. [DOI] [Google Scholar]
- Revonsuo A (2000). The reinterpretation of dreams: An evolutionary hypothesis of the function of dreaming. Behavioral and Brain Sciences, 23(6), 877–901. [DOI] [PubMed] [Google Scholar]
- Rihm JS, Diekelmann S, Born J, & Rasch B (2014). Reactivating memories during sleep by odors: Odor specificity and associated changes in sleep oscillations. Journal of Cognitive Neuroscience, 26(8), 1806–1818. [DOI] [PubMed] [Google Scholar]
- Rivera-García AP, López Ruiz IE, Ramírez-Salado I, González-Olvera JJ, Ayala-Guerrero F, & Jiménez-Anguiano A (2019). Emotional facial expressions during REM sleep dreams. Journal of Sleep Research, 28(1), Article e12716. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, & Kreber LS (2000). Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience, 114(2), 328. [DOI] [PubMed] [Google Scholar]
- Rozen N, & Soffer-Dudek N (2018). Dreams of Teeth Falling Out: An Empirical Investigation of Physiological and Psychological Correlates. Frontiers in Psychology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch S, Markes O, Duss SB, Oppliger D, Reber TP, Koenig T, … Henke K (2012). Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia, 50(10), 2389–2396. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, & Paller KA (2009). Strengthening individual memories by reactivating them during sleep. Science, 326(5956), 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Ichiro, Igaki Michihito, Ichiba Tomohisa, Suzuki Masahiro, Kuriyama Kenichi, & Uchiyama Makoto (2017). Effects of Bedtime Periocular Warming on Sleep Status in Adult Female Subjects: A Pilot Study. Evidence-Based Complementary and Alternative Medicine, 2017, 1–5. 10.1155/2017/6419439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saredi R, Baylor GW, Meier B, & Strauch I (1997). Current concerns and REM-dreams: A laboratory study of dream incubation. Dreaming, 7(3), 195. [Google Scholar]
- Scarlata S, Bartoli IR, Santangelo S, Giannunzio G, & Incalzi RA (2015). Short term effects of neck-based treatment and monitoring device for positional obstructive sleep apnea, Eur Respiratory Soc. [Google Scholar]
- Schädlich M, Erlacher D, & Schredl M (2017). Improvement of darts performance following lucid dream practice depends on the number of distractions while rehearsing within the dream–a sleep laboratory pilot study. Journal of Sports Sciences, 35(23), 2365–2372. [DOI] [PubMed] [Google Scholar]
- Schredl M, Atanasova D, Hörmann K, Maurer JT, Hummel T, & Stuck BA (2009). Information processing during sleep: The effect of olfactory stimuli on dream content and dream emotions. Journal of Sleep Research, 18(3), 285–290. [DOI] [PubMed] [Google Scholar]
- Schredl M, & Erlacher D (2020). Fever dreams: An online study. Frontiers in Psychology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl M, Hoffmann L, Sommer JU, & Stuck BA (2014). Olfactory stimulation during sleep can reactivate odor-associated images. Chemosensory Perception, 7(3–4), 140–146. [Google Scholar]
- Schreiner T, & Staresina BP (2019). Sleep: Rock and Swing versus Toss and Turn. Current Biology, 29(3), R86–R88. [DOI] [PubMed] [Google Scholar]
- Sequeira H, Hot P, Silvert L, & Delplanque S (2009). Electrical autonomic correlates of emotion. International Journal of Psychophysiology, 71(1), 50–56. [DOI] [PubMed] [Google Scholar]
- Siclari F, Baird B, Perogamvros L, Bernardi G, LaRocque JJ, Riedner B, … Tononi G (2017). The neural correlates of dreaming. Nature Neuroscience, 20(6), 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypińska D, & Slodka M (2014). Who are the people in my dreams? Self-awareness and character identification in dreams. [Google Scholar]
- Smith C (2001). Sleep states and memory processes in humans: Procedural versus declarative memory systems. Sleep Medicine Reviews, 5(6), 491–506. [DOI] [PubMed] [Google Scholar]
- Soffer-Dudek N (2020). Are Lucid Dreams Good for Us? Are We Asking the Right Question? A Call for Caution in Lucid Dream Research. Frontiers in Neuroscience, 13, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonova E (2018). 31 Sleep Paralysis: Phenomenology, Neurophysiology, and Treatment. The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming, 435. [Google Scholar]
- Solomonova E, Frantova E, & Nielsen T (2011). Felt presence: The uncanny encounters with the numinous Other. AI & SOCIETY, 26(2), 171–178. [Google Scholar]
- Solomonova E, Stenstrom P, Paquette T, & Nielsen T (2015). Different temporal patterns of memory Incorporations into dreams for laboratory and virtual reality experiences: Relation to dreamed locus of control. International Journal of Dream. Research, 10–26. [Google Scholar]
- Spanlang B, Normand J-M, Borland D, Kilteni K, Giannopoulos E, Pomés A, … Muncunill XN (2014). How to build an embodiment lab: Achieving body representation illusions in virtual reality. Frontiers in Robotics and AI, 1, 9. [Google Scholar]
- Speth C, & Speth J (2018). A New Measure of Hallucinatory States and a Discussion of REM Sleep Dreaming as a Virtual Laboratory for the Rehearsal of Embodied Cognition. Cognitive Science, 42(1), 311–333. [DOI] [PubMed] [Google Scholar]
- Sra M, Jain A, & Maes P (2019). Adding Proprioceptive Feedback to Virtual Reality Experiences Using Galvanic Vestibular Stimulation. Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems (pp. 1–14). . [Google Scholar]
- Stenstrom P, Fox K, Solomonova E, & Nielsen T (2012). Mentation during sleep onset theta bursts in a trained participant: A role for NREM stage 1 sleep in memory processing? International Journal of Dream Research. [Google Scholar]
- Stickgold R (2001). Watching the sleeping brain watch us-sensory processing during sleep. TRENDS in Neurosciences, 24(6), 307–308. [DOI] [PubMed] [Google Scholar]
- Stickgold R, & Hobson JA (1994). Home monitoring of sleep onset and sleep-onset mentation using the nightcap√. [Google Scholar]
- Stickgold R, Malia A, Maguire D, Roddenberry D, & O’Connor M (2000). Replaying the game: Hypnagogic images in normals and amnesics. Science, 290(5490), 350–353. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Pace-Schott E, & Hobson JA (1994). A new paradigm for dream research: Mentation reports following spontaneous arousal from REM and NREM sleep recorded in a home setting. Consciousness and Cognition, 3(1), 16–29. [Google Scholar]
- Stickgold R, Scott L, Rittenhouse C, & Hobson JA (1999). Sleep-induced changes in associative memory. Journal of Cognitive Neuroscience, 11(2), 182–193. [DOI] [PubMed] [Google Scholar]
- Stochholm A, Mikkelsen K, & Kidmose P (2016). Automatic sleep stage classification using ear-EEG. In 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 4751–4754). [DOI] [PubMed] [Google Scholar]
- Strojnik P, Kralj A, & Ursic I (1979). Programmed Six-Channel Electrical Stimulator for Complex Stimulation of Leg Muscles During Walking. IEEE Transactions on Biomedical Engineering, BME-26(2), 112–116. https://doi.org/l0.1109/TBME.1979.326520. [DOI] [PubMed] [Google Scholar]
- Stuck BA, Stieber K, Frey S, Freiburg C, Hörmann K, Maurer JT, & Hummel T (2007). Arousal responses to olfactory or trigeminal stimulation during sleep. Sleep, 30(4), 506–510. [DOI] [PubMed] [Google Scholar]
- Takahashi K, & Atsumi Y (1997). Precise measurement of individual rapid eye movements in REM sleep of humans. Sleep, 20(9), 743–752. [DOI] [PubMed] [Google Scholar]
- Tal A, Shinar Z, Shaki D, Codish S, & Goldbart A (2017). Validation of contact-free sleep monitoring device with comparison to polysomnography. Journal of Clinical Sleep Medicine, 13(03), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki E, Miyaki T, & Rekimoto J (2011). PossessedHand: Techniques for Controlling Human Hands Using Electrical Muscles Stimuli. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (pp. 543–552). . 10.1145/1978942.1979018. [DOI] [Google Scholar]
- Tart CT (1987). The world simulation process in waking and dreaming: A systems analysis of structure. Journal of Mental Imagery.. [Google Scholar]
- Tijou A, Richard E, & Richard P (2006). Using olfactive virtual environments for learning organic molecules. International Conference on Technologies for E-Learning and Digital Entertainment, 1223–1233. [Google Scholar]
- Tononi G, & Cirelli C (2003). Sleep and synaptic homeostasis: A hypothesis. Brain Research Bulletin, 62(2), 143–150. [DOI] [PubMed] [Google Scholar]
- Tononi G, & Cirelli C (2006). Sleep function and synaptic homeostasis. Sleep Medicine Reviews, 10(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, & Fishbein W (2006). A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory, 86(2), 241–247. [DOI] [PubMed] [Google Scholar]
- Valli K, Frauscher B, Peltomaa T, Gschliesser V, Revonsuo A, & Högl B (2015). Dreaming furiously? A sleep laboratory study on the dream content of people with Parkinson’s disease and with or without rapid eye movement sleep behavior disorder. Sleep Medicine, 16(3), 419–427. [DOI] [PubMed] [Google Scholar]
- Valli K, & Revonsuo A (2009). The threat simulation theory in light of recent empirical evidence: A review. The American Journal of Psychology, 17–38. [PubMed] [Google Scholar]
- Van Boxtel A (2010). Facial EMC as a tool for inferring affective states. Proceedings of Measuring Behavior, 104–108. [Google Scholar]
- Van Der Helm E, Yao J, Dutt S, Rao V, Saletin JM, & Walker MP (2011). REM sleep depotentiates amygdala activity to previous emotional experiences. Current Biology, 21(23), 2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kolk BA (1994). The body keeps the score: Memory and the evolving psychobiology of posttraumatic stress. Harvard Review of Psychiatry, 1(5), 253–265. [DOI] [PubMed] [Google Scholar]
- Vega T (2019). Masca, REM Tracking Eye Movement prototype. Talk at Dream Engineering Workshop, MIT Media Laboratory. [Google Scholar]
- Walker BN, & Lindsay J (2005). Navigation performance in a virtual environment with bonephones. [Google Scholar]
- Walker MP, Liston C, Hobson JA, & Stickgold R (2002). Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research, 14(3), 317–324. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Perry K, Djonlagic I, Reaven LB, & Stickgold R (2010). Cognitive replay of visuomotor learning at sleep onset: Temporal dynamics and relationship to task performance. Sleep, 33(1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker M, Payne JD, Benavides JA, & Stickgold R (2010). Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Current Biology, 20(9), 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Guan L (2008). Recognizing human emotional state from audiovisual signals. IEEE Transactions on Multimedia, 10(5), 936–946. [Google Scholar]
- Wettach R, Behrens C, Danielsson A, & Ness T (2007). A thermal information display for mobile applications. In Proceedings of the 9th International Conference on Human Computer Interaction with Mobile Devices and Services - MobileHCI Ό7 (pp. 182–185). 10.1145/1377999.1378004. [DOI] [Google Scholar]
- Windt JM (2018). Predictive brains, dreaming selves, sleeping bodies: How the analysis of dream movement can inform a theory of self-and world-simulation in dreams. Synthese, 195(6), 2577–2625. [Google Scholar]
- Xu J, Kuroda Y, Yoshimoto S, & Oshiro O (2018). Cold air generation system using a vortex tube to present a non-contact cold sensation. Proceedings of System Integration Division of Society of Instruments and Control Engineers, 546–548. [Google Scholar]
- Yu CK-C (2010). Recurrence of typical dreams and the instinctual and delusional predispositions of dreams. Dreaming 20(4), 254. [Google Scholar]
- Zhao M, Yue S, Katabi D, Jaakkola TS, & Bianchi MT (2017). Learning sleep stages from radio signals: A conditional adversarial architecture. In Proceedings of the 34th International Conference on Machine Learning-Volume 70 (pp. 4100–4109). [Google Scholar]


