Abstract
Purpose:
Adverse childhood experiences (ACEs) have been shown to be associated with increased risk of mortality. The biobehavioral mechanisms linking adverse events and survival in cancer patients remain unclear. The aims of the study were to: (1) examine the rates and types of early adverse events in patients diagnosed with cancer; (2) investigate the association of adverse events with circulating cytokines, representing immune status of the patient; and (3) test whether immune markers mediated the association between early adverse events and survival while adjusting for other factors that are associated with immunity (e.g., fatigue) and survival (e.g., depression).
Patients and Methods:
The patients were recruited from an outpatient oncology clinic. Patients were administered a battery of questionnaires including the Traumatic Events Survey and the Center for Epidemiological Studies-Depression scale. Blood was collected and serum levels of cytokines were assessed to characterize immune status. Descriptive statistics, Mann-Whitney U tests and Cox regression were performed to address study aims.
Results:
Of the 408 patients, 66% reported at least one ACE. After adjusting for demographic, disease-specific factors, and psychological/behavioral factors; having had a major upheaval between parents during childhood or adolescence was associated with poorer survival [β=−0.702, HR=0.496, p=0.034]. Lower levels of interleukin-2 (IL-2) explained, in part, the link between this early adverse event and poorer survival as when IL-2 was entered into the model, a major upheaval between one’s parents and survival was no longer significant [β=−0.612, HR=0.542, p=0.104].
Conclusion:
Having experienced an ACE was associated with lower IL-2 levels-a growth factor for anti-inflammatory T- regulatory lymphocytes-central in contemporary immunotherapy, as well as poorer survival in those diagnosed with cancer. Since lower IL-2 levels also explained, in part, the link between the ACE involving parental upheaval and survival, there is support for a psychoneuroimmunological model of disease course in this vulnerable population.
Introduction
Adverse childhood experiences (ACEs) such as child physical or sexual abuse, neglect, and poverty are linked with increased risk of psychological sequelae such as depression and post-traumatic stress disorder (PTSD).1–4 Several studies have shown that ACEs and the psychological sequelae have also been linked to hypothalamic-pituitary-adrenal (HPA) axis dysregulation, poorer health-related quality of life, cancer, cardiovascular disease, and risk of early mortality.5–12
While the mechanisms linking ACEs with disease are not well understood, in the context of cancer, health behaviors and/or immune system dysfunction has been theorized as a potential mediator liking ACEs to increased risk for mortality. A recent meta-analysis concluded that ACEs were moderately associated with smoking, heavy alcohol use and related health outcomes such as cancer, heart and respiratory disease.12 Experiencing early adverse events has also been associated with elevations in serum levels of pro-inflammatory cytokines interleukin 6 (IL-6) and Tumor Necrosis Factor (TNF)-α, and shortened telomere length, after adjusting for age, body mass index, exercise, and sleep.2–3 The psychological sequelae of adverse events, such as depression and PTSD, have also been linked to increased levels of circulating pro-inflammatory cytokines including IL-6, TNFα, and Interleukin-1. No study to our knowledge has disentangled the link between early adverse events, psychological sequelae, and immune status in cancer patients.
Low socioeconomic status (SES) as a child, which may also be considered an ACE, has been associated with decreased glucocorticoid receptor sensitivity and increased pro-inflammatory signaling in adulthood.13 Miller and colleagues found that lower childhood SES was associated with an up-regulation of genes bearing transcription factors that convey adrenergic signals to leukocytes, and a down-regulation of glucocorticoid receptor genes, which transduce anti-inflammatory actions.14 These response patterns could favor increased inflammatory signaling, which could ultimately contribute to the progression of chronic diseases, including cancer.14
Adversity, such as chronic stress and social isolation, may be associated with poorer cell-mediated and anti-viral immunity. As a result an upregulation of leukocyte SNS-associated gene expression may occur which in turn is associated with diminished surveillance of circulating cancer cells.15,16 A leukocyte profile characterized by less anti-viral cellular immune gene expression and greater inflammatory gene expression during treatment for breast cancer predicted shorter disease-free interval over an 11-year follow-up.17 Thus, adversity could affect the immune system in multiple ways, which could impact the course of cancer after diagnosis.
Investigators have explored the role of ACEs in the progression of cancer. In one study, early adverse events were linked to tumor growth and disease progression in women with metastatic breast cancer.18 but neither early traumatic events nor distress were associated with disease progression after adjusting for demographic and disease specific factors.18,19 It remains unclear whether cancer patients with a history of ACEs have a poorer course of disease and whether factors such as immune status could explain this association.
The aims of this study were to: (1) examine the rate and type of early adverse events in patients diagnosed with cancer; (2) investigate the association of adverse events with circulating cytokines, representing immune status of the patient; and (3) test whether immune markers mediated the link between early adverse events and survival while adjusting for other factors that are associated with immunity (e.g., fatigue) and survival (e.g., depression).
Methods
Design and Participants
Design
This is a secondary analysis of two prospective studies conducted at a large tertiary medical center with patients diagnosed with cancers affecting the hepatobiliary and pancreatic system. The studies included in this analysis involved the examination of psychosocial, behavioral, and biological factors in patients diagnosed with cancer (R21CA127046; R01CA176809). For the purposes of this study, the assessment of psychological and biological markers was performed at baseline only to avoid measuring the acute effects of current cancer treatment. The samples of blood used to measure circulating cytokines were collected at least 3 months after surgery and/or regional chemotherapy, reducing any acute changes in cytokines from the cancer treatment. Only patients who were not receiving active systemic chemotherapy were included in the analyses for this study.
Participants
The sample was drawn from the Division of Hepatobiliary and Pancreatic Surgery at the University of Pittsburgh Medical Center, which evaluates and treats patients with cancers including hepatocellular, cholangiocarcinoma, gallbladder, as well as pancreatic carcinoma and other primary tumors that have metastasized to the liver. Patients were enrolled in the study between April 2007 to October 2011 (K07CA118576, R21CA127046, and P30CA047904) and November 2012 to October 2013 (R01CA176809). Inclusion criteria and exclusion criteria for both studies were: (1) biopsy or radiographically-proven diagnosis of cancer affecting the hepatobiliary or pancreatic system; (2) age 21 years or older; (3) fluency in English; and (4) no evidence of thought disorder, hallucinations, or delusions.
Procedure
Both studies from which patient data were drawn were approved by the University of Pittsburgh’s Institutional Review Board. Patients were referred to the study team by their medical team. If the patient agreed to speak to a member of the study team, the individual was explained the risks and benefits of the study and written informed consent was obtained from the patient prior to completing the questionnaires.
Assessments
Demographic, Disease, and Treatment Specific Factors
Sociodemographic data included patients’ age, gender, race, ethnicity, educational level, occupation, income, and health insurance status, and was reported on a questionnaire designed specifically for these studies. Disease-specific and treatment-related information was gathered from patients’ electronic medical records including diagnosis, body mass index, presence or absence of cirrhosis, maximum tumor size, number of lesions, vascularity of lesions, and vascular invasion. Survival was measured from the time of diagnosis of cancer until death. Death was determined by records in the electronic medical record or the Social Security Death Index and the patients were followed for up to 10 years post-diagnosis.
Adverse Childhood Experiences
The Traumatic Events Survey (TES) evaluates a wide range of childhood and adult traumas.20,21 This 14-item instrument is a valid measure of exposure to potentially traumatic events during childhood and adolescence as well as adulthood. Only the six items measuring adverse childhood and adolescent experiences (prior to age 17) were used due to the focus of this study. The ACEs that were included in the childhood and adolescent portion of this survey included “Prior to the age of 17 years… (1) did you experience a death of a very close friend or family member, (2) was there a major upheaval between your parents, (3) did you have a traumatic sexual experience, (4) were you a victim of violence, (5) were you extremely ill or injured, and (6) did you experience any other major upheaval that you think may have shaped your life or personality significantly?20
Depressive Symptoms
The Center for Epidemiologic Studies-Depression (CES-D) is a 20-item self-report questionnaire designed to assess depressive symptoms.22 The patient responds on a 4 point scale by reporting weekly frequency of depressive symptoms (“rarely,” ”some days,” ”occasionally,” or ”most days”).22 A score of 16 or greater represents depressive symptoms in the clinical range.22 The CES-D has demonstrated adequate construct validity and reliability in cancer patients.23
Serum Cytokines
Serum levels of cytokines including Interleukin (IL)-1α, IL-1β, IL-2, IL-10, TNFα, and Interferon (IFN)-γ were measured. These cytokines provide a broad characterization of immune system status including pro-inflammatory (IL-1α, IL-1β, TNF-α), anti-inflammatory (IL-10), and anti-viral/anti-tumor (IL-2, IFN-γ) functions. The blood draws were performed between 8am.and noon when possible. For serum, blood was drawn into red-top vacutainer tubes without anticoagulant and processed in a local laboratory at the University of Pittsburgh upon receipt, allowing >30 minutes for clot formation. Serum aliquots were stored in temperature monitored −80 °C freezers without thawing. The samples were thawed once before testing using Luminex™ (Millipore, Billerica, MA).
Data Analysis
Data were entered, verified, and analyzed using SPSS version 23 (IBM Corp, Armonk, NY). Descriptive statistics were performed to obtain measures of central tendency, distribution, and proportions for each variable. Mann-Whitney U tests and the Spearman Rho were used to test associations between ACEs, number of ACEs and biomarkers. Predictor variables that were included in the multivariable Cox regression survival analyses were based on significant factors (p <.05) found to be associated with survival in univariate analyses. Mediation was examined using Baron and Kenny’s mediational analyses, which tests whether the association between adverse childhood events and survival after controlling for putative intervening variables (e.g., depression, immune status). See Figure below.
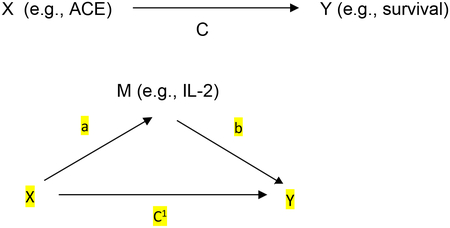
First, we used Y as the criterion variable in a regression equation and X as a predictor.24 Next, we used M as the criterion variable in the regression equation and X as a predictor. Then we used Y as the criterion variable in a regression equation and X and M as predictors (estimate and test path b); and each of these links are required to be statistically significant.24 Finally, to establish that M completely mediates the X-Y relationship, the effect of X on Y controlling for M (path c’) should no longer be significant.24 All four steps must be satisfied for a factor to be considered mediational including a total effect [c=ab+c1] and direct effect [c1=c-ab] and an indirect effect [c-c1=ab].24
Results
Sociodemographic and Disease Specific Factors
A total of 408 participants with cancer affecting the hepatobiliary-pancreatic system were included in the study. Sociodemographic characteristics can be found in Table 1. The majority of the patients were male (64.5%) and Caucasian (91.9%). The mean age was 62 (SD=11.3). Forty-four percent of patients were diagnosed with primary liver cancer or cancer of the bile ducts followed by other primary cancers with liver metastases (34.9%); neuroendocrine carcinoma (11.8%); and cancers of the gallbladder, stomach, and pancreas (8.8%). Table 1 provides details of demographic and disease specific information and Table 2 provides descriptive statistics for the serum cytokines.
Table 1:
Sociodemographic and disease specific characteristics of the Sample (n=408)
| 62 (11.3) | |
| Gender (n, %) | |
| Male | 263 (64.5) |
| Female | 145 (35.5) |
| Completed High School (n, %) | 391 (94.4) |
| Caucasian (n, %) | 373 (91.9) |
| Diagnosis (n, %) | |
| Gallbladder/stomach/pancreatic/appendix cancer | 36 (8.8) |
| Hepatocellular/cholangiocarcinoma | 181 (44.5) |
| Other primary cancer with liver metastases | 142 (34.9) |
| Neuroendocrine carcinoma | 48 (11.8) |
| Largest tumor size (Mean, SD in cm) | 3.51 (3.67) |
| Number of lesions (Mean, SD) | 3.72 (2.50) |
| Cirrhosis (n, %) | 141 (34.8) |
| Vascular Invasion (n, %) | 69 (17.6) |
Table 2:
Univariate Cox regression analyses of survival on demographic, disease related behavioral, psychological, biological factors (n=299–408)
| Chi-square | p-value | |
|---|---|---|
| Age | 10.436 | 0.001 |
| Tumor Size | 69.386 | <0.001 |
| Number of Lesions | 0.072 | 0.789 |
| Pain | 0.180 | 0.180 |
| Depression | 25.332 | <0.001 |
| Fatigue | 18.808 | <0.001 |
| Interleukin-2 | 24.372 | <0.001 |
| Interleukin-1 α | 0.862 | 0.353 |
| Interleukin-1 β | 0.449 | 0.503 |
| Tumor Necrosis Factor-α | 0.270 | 0.603 |
| Interferon-γ | 1.876 | 0.171 |
Rates of Early Adverse events and Quality of Life
Of the 408 patients, 66% reported at least one early adverse event during childhood or adolescence (Figure 1). Of the patients who reported early adverse events, 58% reported the death of a close friend or family member, 19% had a major upheaval between their parents, 8.5% reported a traumatic sexual experience, 9.3% reported being a victim of violence, and 19.9% were extremely ill or injured as a child. With regard to cumulative trauma, of the 66% who reported ACEs, 37% (n=148) reported one ACE, 19.8% (n=79) reported two ACEs, 8.3% (n=33) reported three ACEs, 4.3% (n=8) reported four ACEs, 2% (n=8) reported five ACEs, and 1 patient (<1%) reported all six ACEs.
Figure 1:
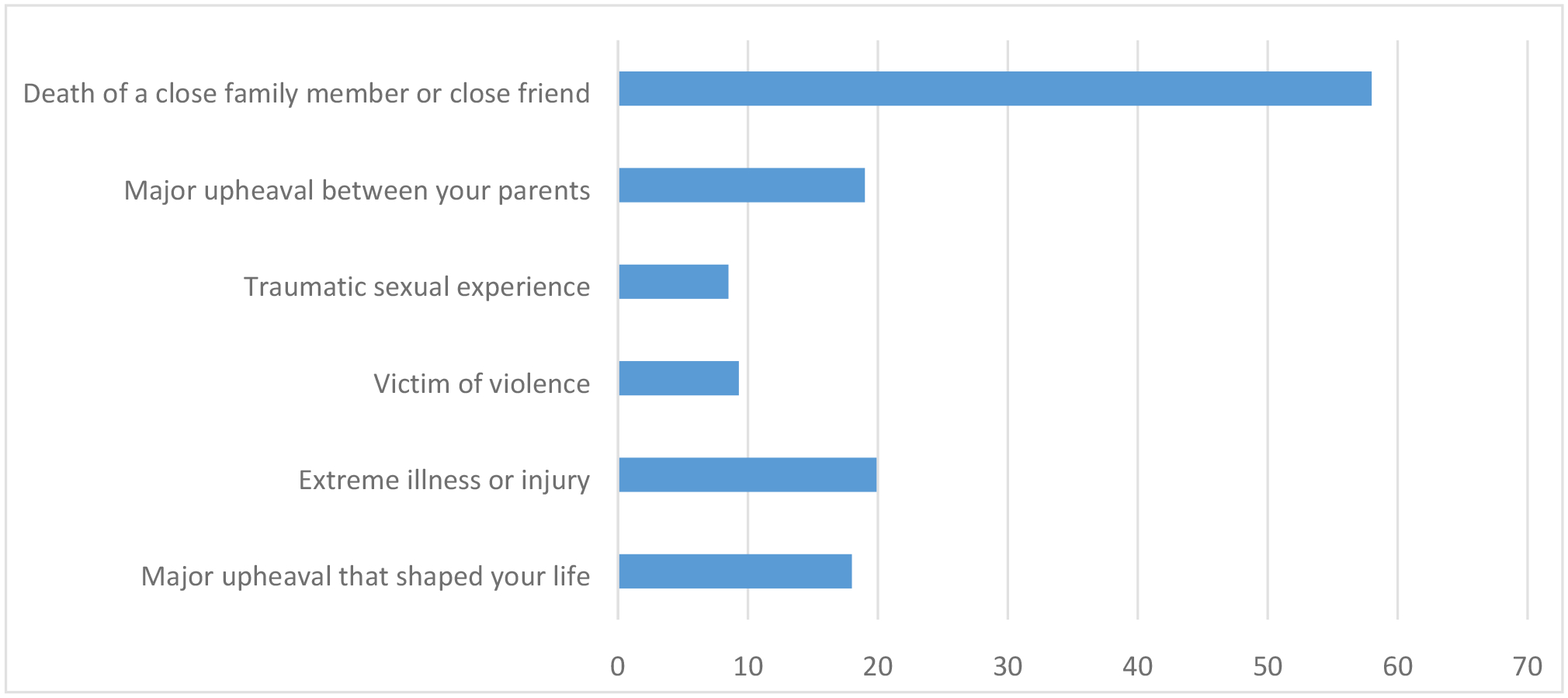
Percent of patients who reported adverse life events prior to the age of 17 years (n=408)
Adverse childhood experiences and survival
We first examined each of the established variables that have been linked to survival using univariate Kaplan Meier survival analyses and Cox regression survival analyses. We found that being older [Cox regression χ2 =10.436, p=0.001], male [Kaplan Meier χ2 =14.685, p<0.001], having a pancreatic, gallbladder, or stomach cancer diagnosis [Kaplan Meier χ2 =36.502, p<0.001], cirrhosis [Kaplan Meier χ2 =26005, p<0.001], larger tumor size [Cox regression χ2 =69.386, p<0.001], vascular invasion [Kaplan Meier χ2 =35.047, p<0.001], higher levels of depressive symptoms [Cox regression χ2 =25.332, p<0.001] and fatigue [Cox regression χ2 =18.808, p<0.001] and lower levels of IL-2 [Cox regression χ2 =24.372, p<0.001] were associated with poorer survival in univariate tests. The only early adverse event that was significantly associated with poorer survival was having had a major upheaval between one’s parents during childhood or adolescence [β=−0.702, HR=0.496, p=0.034]. Cumulative exposure to ACEs (number of ACEs) was also significantly associated with better survival [β=0.138 HR=1.013, p=0.031]. See Tables 2 and 3.
Table 3:
Univariate Kaplan Meier analyses of survival on demographic and disease specific factors (n=299–408)
| Chi-square | p-value | |
|---|---|---|
| Gender | 14.685 | <0.001 |
| Cirrhosis | 26.005 | <0.001 |
| Diagnosis | 36.502 | <0.001 |
| Vascular Invasion | 35.047 | <0.001 |
A multivariate model was tested which included the factors that were significantly linked to survival in the univariate analyses. We found that after adjusting for these demographic (gender, age) factors, disease-specific factors (diagnosis, tumor size, treatment, cirrhosis, vascular invasion), as well as depressive symptoms, that having a major upheaval between one’s parents as a child or adolescent predicted poorer survival [β=−0.702, HR=0.496, p=0.034]. Table 4. Post hoc analyses also revealed that major upheaval between your parents was significantly correlated with a traumatic sexual experience (Spearman rho=0.244, p=0.01) and being a victim of violence (Spearman rho=284, p=0.01).
Table 4:
Adjusted Cox regression survival analyses on childhood upheaval of parents (n=299)
| B | Sig. | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | −.253 | .457 | .777 | .399 | 1.511 |
| Age | −.002 | .887 | .998 | .968 | 1.029 |
| Tumor size | .067 | .103 | 1.069 | .986 | 1.159 |
| Diagnosis | .081 | ||||
| Hepatocellular/Cholangio | 1.291 | .075 | 3.635 | .876 | 15.086 |
| Other primary cancers with liver metastases | −.703 | .414 | .495 | .092 | 2.673 |
| Neuroendocrine | 1.119 | .095 | 3.061 | .822 | 11.399 |
| Treatment | .306 | ||||
| Systemic | .641 | .445 | 1.899 | .367 | 9.840 |
| Regional | 1.224 | .122 | 3.402 | .721 | 16.050 |
| Surgery | .511 | .502 | 1.667 | .375 | 7.406 |
| Cirrhosis | −.940 | .093 | .391 | .131 | 1.170 |
| Vascular Invasion | .515 | .149 | 1.674 | .832 | 3.370 |
| Depression | −.013 | .447 | .987 | .955 | 1.020 |
| Fatigue | .042 | .006 | 1.043 | 1.012 | 1.075 |
| Major upheaval of family as a child | −.702 | .034 | .496 | .259 | .947 |
We also found that after adjusting for demographic, disease-specific factors, depression, and fatigue the cumulative exposure to early adverse events was associated with improved survival [β=0.166, HR=1.180, p=0.041]. We then examined each of the 5 other ACEs individually to test their association with survival, after adjusting for demographic, disease-specific, and psychosocial predictors. We observed that experiencing a death of a close friend or family member (β=0.353, HR=1.423, p=0.099) and being extremely ill or injured prior to the age of 17 years was not significantly associated with better survival but a trend toward significance was observed (β=0.448, HR=1.564, p=0.053). Prior to the age of 17, a traumatic sexual experience (β=−0.224, HR=0.799, p=0.569), being a victim of violence (β=0.072, HR=1.075, p=0.831), and major upheaval that shaped your life or personality significantly (β=0.163, HR=1.177, p=0.554) was not significantly associated with survival. Fifty-eight percent of patients reported the death of a close friend or family member before the age of 17 years and 19.9% reported an extreme illness or injury prior to the age of 17 years which may explain the reasons cumulative ACEs was associated with better survival since this was a large percentage of the patients and these events are not often included in measures of cumulative ACE exposure.27
Links between Adverse Childhood Experiences and Biological Mediators
We next focused on analyses between biomarkers associated with cancer progression and having a major upheaval between one’s parents as related to survival. We found that this early adverse event was associated with lower levels of serum IL-2 [Mann-Whitney U test=6945, p=0.05] when compared to patients who did not report having had a major upheaval between their parents during childhood or adolescence. A major upheaval between parents was not associated with any other cytokines. Cumulative exposure of ACEs was also not correlated with any of the biomarkers. Using Spearman’s Rho cumulative exposure to ACEs was not associated with IL-2 [rho=0.039, p=0.489], IFN γ [rho=−0.003, p=0.953], IL-10 [rho=0.046 p=0.0416], IL1α [rho=−0.053, p=0.341], IL-1β [rho=−0.0.43, p=0.442], and TNFα [rho=0.017, p=0.767]. As a result of the lack of association between cumulative exposure to ACEs and the biomarkers, a mediational model was not tested for cumulative exposure to ACEs.24
Mediational Model of Adverse Childhood Experiences and Survival
Since IL-2 was associated with survival and having a major upheaval between one’s patients during childhood or adolescence, we entered IL-2 into the multivariate model. Having a major upheaval between your parents as a child or adolescent was no longer significantly associated with survival (β=−0.612, HR=0.542, p=0.104) after IL-2 was included in the model. This suggests that IL-2 may mediate the link between having a major upheaval between one’s parents as a child or adolescent and survival. See Table 5.
Table 5:
Cox regression survival analyses on major upheaval of parents and IL-2 as a mediator (n=299)
| B | Sig. | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | −.304 | .455 | .738 | .333 | 1.637 |
| Age | −.001 | .973 | .999 | .966 | 1.035 |
| Tumor size | .080 | .069 | 1.083 | .994 | 1.180 |
| Diagnosis | .239 | ||||
| Hepatocellular/Cholangio | .628 | .427 | 1.873 | .398 | 8.811 |
| Other primary cancer with metastases | −1.728 | .137 | .178 | .018 | 1.733 |
| Neuroendocrine | .288 | .702 | 1.334 | .306 | 5.817 |
| Treatment | .786 | ||||
| Systemic | .495 | .563 | 1.641 | .306 | 8.799 |
| Regional | .743 | .373 | 2.102 | .410 | 10.773 |
| Surgery | .305 | .694 | 1.357 | .297 | 6.192 |
| Cirrhosis | −.118 | .845 | .889 | .272 | 2.900 |
| Vascular Invasion | .245 | .557 | 1.278 | .564 | 2.896 |
| Depression | −.020 | .335 | .980 | .942 | 1.021 |
| Fatigue | .048 | .008 | 1.049 | 1.012 | 1.086 |
| Interleukin- 2 | −.034 | .273 | .967 | .911 | 1.027 |
| Major upheaval of family as a child | −.612 | .104 | .542 | .259 | 1.134 |
Discussion
The majority of cancer patients reported at least one ACE. This is higher than the general population which was reported to be 45% in 2018.25 The ACE that was significantly related to poorer survival in this cohort was “a major upheaval within the family.” A large epidemiological study in Sweden showed that men of divorced parents had a significantly increased risk of all-cause mortality26 Parental divorce during childhood has also been found to be the single strongest social predictor of early death.11 In the present study we observed that a major upheaval between one’s parents was linked to poorer survival after adjusting for demographic, disease specific, behavioral, and psychological factors (depression) in cancer patients. Although many studies have shown that divorce is associated with increased risk of mortality, no study to our knowledge found a potential biological mediator of childhood adversity and survival in cancer patients. D’Andrea and colleagues also found that a single exposure to an ACE was associated with health outcomes.31
With regard to cumulative exposure to ACEs, our findings were inconsistent with prior research that has suggested cumulative exposure to ACEs was associated with increased mortality.27,28 First, it should be noted that the mortality and survival are different outcomes and are measured differently.29 Secondly, the risk of those with cumulative exposure had a very small increased risk of better survival. Post hoc analyses demonstrated that the death of a close friend or family member and extreme illness or injury prior to the age of 17 years was driving this link between cumulative ACEs and better survival. Prior studies focused on ACEs include various definitions of ACEs and therefore may explain the differences in findings. Jakubowski has noted that the lack of a consistent operational definition of adversity and the types, timing, and duration of these adversities is a major limitation of research focused on ACEs and health outcomes.30
Furthermore, prior research included a limited number of covariates in their analyses while our study adjusted for demographic, disease-specific, and psychosocial covariates known to be associated with survival. The outcomes for many of the studies examining the link between ACEs and mortality or either using all-cause mortality or cardiometabolic disease related outcomes.30 While there are studies evaluating the role of ACEs in the risk of developing cancer31, there are no studies to our knowledge that have examined the link of ACEs with survival in those already diagnosed with cancer.
Finally, one explanation why we may have observed that cumulative ACE exposure was associated with better survival, albeit a small risk, may be the role of moderators that are often not included in studies focusing on the link between ACEs and mortality. For example, Edmonds and colleagues observed that the association between childhood betrayal traumas and leukocyte telomere length was larger for those higher on conscientiousness.32 Elliot and colleagues also observed that a strong sense of mastery may buffer the association between ACEs and mortality.28 This may be especially true for the two ACEs that seemed to drive the association between cumulative exposure to ACEs and better survival. When we examined the ACEs individually, those patients who reported a major upheaval in their family before the age of 17 years of age were more than twice as likely to die from cancer when compared to those who reported a major upheaval prior to the age of 17 years.
We did not find a link with most ACEs and circulating cytokines in this cohort of cancer patients. A recent meta-analysis examining childhood adversity with immune and inflammatory biomarkers concluded that childhood adversity was not consistently related to immune or inflammatory markers.32 However, we did observe a link between a major upheaval between parents during childhood or adolescence being associated with lower serum levels of IL-2. Interleukin-2 also mediated the link between a major upheaval between parents and survival. Since IL-2 is known to drive the differentiation and activation of tumor-infiltrating lymphocytes (and lymphokine-activated killer cells), then IL-2 levels may differentiate patients’ ability to mount anticancer responses (e.g., blocking tumorigenesis, metastasis) which may lead to disease progression and poorer survival.
Interleukin-2 plays a key role in cell-mediated immunity and anti-inflammatory regulatory functions, and has been used to treat some cancers and extend life.33–36 This cytokine is a master regulator of the immune system, acting as a growth factor for many immune cells, especially the anti-inflammatory regulatory T cells (Tregs) at low doses and other cell types at high doses.33 These mechanisms are reflected in recent advances in understanding the role of PD1, PD-L1, and PD-L2 antibodies to which block T regulatory cell activation in the context of immunotherapy of non-small-cell lung cancer, melanoma, or renal-cell cancers.37–40
One explanation regarding why a major upheaval between one’s parents may result in dysregulation of IL-2 is that the major upheaval in a child’s parents may be a result, or result, in chronic stress. The parental unit creates a sense of security for a child with regard to trust in dependency and increased marital conflict before, during and after a separation and/or divorce. Children may also suffer from decreased household income as a result of divorce and can be exposed to neglect, physical and sexual abuse.41 Post hoc analyses revealed that a major upheaval between parents was significantly associated with being a victim of violence and sexual abuse. Further research is needed to understand the timing of the ACEs and whether the sexual abuse or violence occurred before or after the major upheaval between the parents. Fagan and Churchill (2012) reported separation and/or divorce may result in a child being less able to manage conflict, having diminished social skills which may lead to problematic relationships with others, leaving home earlier, having more conflictual marriages and intimate relationships, diminished religious faith, lower educational achievement, greater alcohol, drug and tobacco use, and lower household income as an adult.41 All of these factors can lead to chronic stress over an individuals’ lifetime. This lifetime of chronic stress is likely to result in immune dysfunction including that of the master regulator IL-2.
As with other studies, we examined each cytokine independently, the immune system is complex and the interactions between cytokines are often not considered due to limitations in biostatistics. Dynamic Bayesian Network (DBN) analyses has begun to be used by some investigators as it addresses the complex interactions of cytokines over time.42–44 However at this time, DBN cannot utilize censored data. As advances in biostatistical modeling are made we will be able to better understand these complex interactions.
The present study has many strengths, including the large sample size and the ability to adjust for demographic, disease-specific, psychological, and behavioral factors that have been shown to influence survival in prior cancer studies.41–43 The study sample, which was comprised of a heterogeneous cohort of cancer patients may be considered a strength of the study as the findings will be more generalizable. We did not collect data regarding psychiatric treatment during childhood or adolescence, but we did collect data related to psychiatric treatment at the time of presentation. However, our rationale for including the severity of depressive symptoms, rather than psychiatric treatment, in the multivariable model as a person who is in psychiatric treatment may continue to have high levels of depressive symptoms. Depressive symptoms also have been linked both to inflammation and survival in cancer patients44–48; whereas there has been an inconsistent link between psychiatric treatment, inflammation, and survival in cancer patients.49–52
Several concerns that have been raised using Kenny and Baron’s mediational approach, however our study addressed many of these issues including testing the direct effects prior to indirect effects, having a theoretically driven model, and inclusion of covariates.53 Limitations of this approach remain with regard to the confidence intervals as they included a zero in the final model testing mediation.54 However, we did not observe confidence intervals including zero in our analyses of indirect effects.55
Future research may include the examination of the ACE in greater depth such as the age in which the individual was exposed to the ACE or ACEs, the timing of those who were exposed to multiple ACEs, and the duration of the ACE may also influence the impact the ACE or ACEs has on the immune system and long-term health. Inclusion of a leukocyte challenge study to assess IL-2 production may further support the findings of the present study. We know in prior studies of stress and stress management in breast cancer patients that adversity is associated with poorer T-cell responses to challenge and that interventions teaching patients stress management skills in the weeks after surgery is associated with improvements in lymphocyte proliferation and IL-2 production.56–58
Primary prevention of ACEs during childhood and adolescence is recommended first and foremost. Prevention of poverty; child physical, sexual and emotional abuse; neglect; and parental discord and dissolution might decrease negative psychological and health consequences later in life. Unfortunately, it is not likely that all ACEs can be prevented; therefore, early intervention designed to mitigate the consequences of these events to prevent downstream development of psychological and health morbidity and mortality is recommended. Including assessment and treatment of trauma, and immune status in survivorship care planning could be added to the comprehensive assessment and treatment that is currently completed with patients and may reduce health care utilization and costs.59
Funding:
National Cancer Institute R21CA127046; R01CA176809
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
Contributor Information
Jennifer L. Steel, University of Pittsburgh, Department of Surgery, Psychiatry, and Psychology
Michael Antoni, University of Miami, Department of Psychology.
Ritambhara Pathak, University of Pittsburgh, Department of Surgery.
Lisa H. Butterfield, University of Pittsburgh, Department of Immunology
Yoram Vodovotz, University of Pittsburgh, Department of Surgery.
Alexandra Savkova, University of Pittsburgh, Department of Surgery.
Marsh Wallis, University of Pittsburgh, Department of Surgery.
Yisi Wang, University of Pittsburgh, Department of Surgery.
Hui Jing, University of Pittsburgh, Department of Surgery.
Elizabeth Grammer, University of Pittsburgh, Department of Surgery.
Robin Burke, University of Pittsburgh, Department of Surgery.
Mya Brady, University of Pittsburgh, Department of Surgery.
David A. Geller, University of Pittsburgh, Department of Surgery
References
- 1.Copeland J Traumatic events and post traumatic stress in childhood. JAMA Psychiatry 2007;64:577–84. [DOI] [PubMed] [Google Scholar]
- 2.Steel Z. Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: a systematic review and meta-analysis. JAMA 2009;302:537–49. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen Leslie K. SM S,Kosten Thomas R.. Substance Use Disorders in Patients With Posttraumatic Stress Disorder: A Review of the Literature AJP 2001;158:1184–90 [DOI] [PubMed] [Google Scholar]
- 4.Golen-Kruetz DM. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol 2005;24:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flett RA, Kazantzis N, Long NR, MacDonald C, Millar M. Traumatic events and physical health in a New Zealand community sample. J Trauma Stress 2002;15:303–12. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33. [DOI] [PubMed] [Google Scholar]
- 7.Duszynski KR, Shaffer JW, Thomas CB. Neoplasm and traumatic events in childhood. Arch Gen Psychiatry 1981;38:327–31. [DOI] [PubMed] [Google Scholar]
- 8.Garssen B Psychological factors and cancer development: evidence after 30 years of research. Clinical Psychology Review 2004;24:315–38. [DOI] [PubMed] [Google Scholar]
- 9.Peled R Camil D, Siboni-Samocha O, Shoham-Vardi I. Breast cancer, psychological distress and life events among young women. BMC Cancer 2008; 8:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinder LS Bradley KA, Katon WJ, Ludman E, McDonnel MB & Bryson CL. Depression, posttraumatic stress disorder, and mortality. Psychosomatic Medicine 2008;70:20–6. [DOI] [PubMed] [Google Scholar]
- 11.Stein DJ, Chiu WT, Hwang I, Kessler RC, Sampson N, Alonso J, Borges G, Bromet E, Bruffaerts R, de Girolamo G, Florescu S, Gureje O, He Y, Kovess-Masfety V, Levinson D, Matschinger H, Mneimneh Z, Nakamura Y, Ormel J, Posada-Villa J, Sagar R, Scott KM, Tomov T, Viana MC, Williams DR, Nock MK. Cross-national analysis of the associations between traumatic events and suicidal behavior: findings from the WHO World Mental Health Surveys. PloS One 2010;5:e10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosomatic Medicine 2008;70:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusslock R Miller G Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biological Psychiatry 2015;4:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller GE Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences 2009;106:14716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer: 2015, 15(9): 563–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoni MH DF. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer, 2019;125:1417–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoni MH J J, Bouchard LC, Lechner SC, Jutagir DR, Gudenkauf LM, Blomberg BB, Glück S, Carver CS. Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. General Hospital Psychaitry 2016;44:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler LD, Koopman C, Classen C, Spiegel D. Traumatic stress, life events, and emotional support in women with metastatic breast cancer: cancer-related traumatic stress symptoms associated with past and current stressors. Health Psychol 1999;18:555–60. [DOI] [PubMed] [Google Scholar]
- 19.Epping-Jordan JE Compass B, Howell DC. Predictors of cancer progression in young adult men and women: avoidance, intrusive thoughts, and psychological symptoms. Health Psychol 1994;13:539–47. [DOI] [PubMed] [Google Scholar]
- 20.Elliot DM. Traumatic Events Survey. University of California Los Angeles, School of Medicine; 1992. [Google Scholar]
- 21.Elliott DM & Briere J. Sexual abuse trauma among professional women: validating the Trauma Symptom Checklist-40 (TSC-40). Child Abuse Negl 1992;16:391–8. [DOI] [PubMed] [Google Scholar]
- 22.Radoff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas 1977;1:385–401. [Google Scholar]
- 23.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). Journal of Psychosomatic Research 1999;46:437–43. [DOI] [PubMed] [Google Scholar]
- 24.Kenny DA, Kashy DA, Bolger N. Data analysis in social psychology. New York, NY: McGraw-Hill; 1998. [Google Scholar]
- 25.Sacks V & Murphey D. The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity. https://wwwchildtrendsorg/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity 2018. [Google Scholar]
- 26.Hemminki K, Chen B. Lifestyle and cancer: effect of parental divorce. Eur J Cancer Prev 2006;15:524. [DOI] [PubMed] [Google Scholar]
- 27.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH.,. Adverse childhood experiences and the risk of premature mortality. ameican journal of preventative medicine 2009;37:389–96. [DOI] [PubMed] [Google Scholar]
- 28.Elliot AJ, Turiano NA, Infurna FI, Lachman ME, Chapman BP. Lifetime trauma, perceived control, and all-cause mortality: Results from the Midlife in the United States Study. Health Psychol 2018;37:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis L W L, Estève J, Eloranta S, Coleman MP, Rachet B. Cancer incidence, survival and mortality: Explaining the concepts. International Journal of Cancer 2014;135: 1774–82. [DOI] [PubMed] [Google Scholar]
- 30.Jakubowski KP, Cundiff J, Matthews KA. Cumulative childhood adversity and adult cardiometabolic disease: A meta-analysis. Health Psychol 2018;37:701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holman DM, Ports K, Buchanan ND, Hawkins NA, Merrick MT, Metzler M, Trivers KF. The Association Between Adverse Childhood Experiences and Risk of Cancer in Adulthood: A Systematic Review of the Literature. Pediatrics, 2016;138:S81–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmonds GW, Hamon SE, Côté HC, Hill PL, Klest B. Childhood Personality, Betrayal Trauma, and Leukocyte Telomere Length in Adulthood: A Lifespan Perspective on Conscientiousness and Betrayal Traumas as Predictors of a Biomarker of Cellular Aging. european journal of personality 2016;30:426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol 2015;36:763–77. [DOI] [PubMed] [Google Scholar]
- 34.Berghella AM, Pellegrini P, Piancatelli D, Maccarone D, Del Beato T, Giubilei D, Pomidori A, Adorno D, Casciani CU. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunology, immunotherapy :Cancer Imunology and Immunotherapy. 1994;38 (3):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eklund JW & Kuzel TM. A review of recent findings involving interleukin-2-based cancer therapy. Current opinion in oncology 2004;16:542–6. [DOI] [PubMed] [Google Scholar]
- 36.Gasteiger G, Hemmers S, Firth MA, LeFloc A, Huse M, Sun JC, and Rudensky AY. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. JEM 2013;210:1167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res 2015;21:976–84. [DOI] [PubMed] [Google Scholar]
- 38.Bustamante Alvarez JG, Gonzalez-Cao M, Karachaliou N, et al. Advances in immunotherapy for treatment of lung cancer. Cancer biology & medicine 2015;12:209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sgambato A, Casaluce F, Sacco PC, et al. Anti PD-1 and PDL-1 Immunotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC): A Review on Toxicity Profile and its Management. Current drug safety 2016;11:62–8. [DOI] [PubMed] [Google Scholar]
- 40.Smit EF, van den Heuvel MM. PD-L1 in non-small-cell lung cancer: the third target for immunotherapy. Lancet 2016;387:1795–6. [DOI] [PubMed] [Google Scholar]
- 41.Collins KP, Geller DA, Antoni M, et al. Sleep duration is associated with survival in advanced cancer patients. Sleep Med 2017;32:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krane A, Terhorst L, Bovbjerg D, Scheier MF, Kucinski B, Geller DA, Marsh W, Tsung A, Steel JL. Putting the Life in Lifestyle: Lifestyle choices after a diagnosis of cancer predicts overall survival. Cancer 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology 2007;25:2397–405. [DOI] [PubMed] [Google Scholar]
- 44.Steel JL, Geller D, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology 2007;25:2397–405. [DOI] [PubMed] [Google Scholar]
- 45.Satin JR, Linden W, Phillips MJ. Deprression as a predictor of disease progression and mortality in cancer patients. Cancer 2009;115:5349–61. [DOI] [PubMed] [Google Scholar]
- 46.Pinquart PR, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychological Medicine 2010;40:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 2007;21:229–37. [DOI] [PubMed] [Google Scholar]
- 48.Morris AA, Zhao L, Ahmed Y, et al. Association between depression and inflammation-Differences by race and sex: The META-Health Study. Psychosomatic Med 2011;73:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busby J, Millis K, Zhang SD, Liberante FG, Cardwell CR. Selective serotonin reuptake inhibitor use and breast cancer survival: a population-based cohort study. Breast Cancer Res 2018;20:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011;36:2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc 2004;52:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression.[see comment]. Biological Psychiatry 2003;54:269–82. [DOI] [PubMed] [Google Scholar]
- 53.Agler R & DeBoeck P. On the interpretation and use of mediation: Multiple perspectives on mediational analysis. Frontiers in Psychology 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cumming G Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-Analysis. New York, NY: Routledge; 2012. [Google Scholar]
- 55.Shrout PE & Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods 2002;7:422–55. [PubMed] [Google Scholar]
- 56.Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor B, Carver CS, Blomberg B. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun 2009;23:580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. Journal of Psychosomatic Research 2004;56:1–8. [DOI] [PubMed] [Google Scholar]
- 58.Andersen BL, Farrar W, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE 3rd. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol 2004;22:3570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koball AM, Rasmussen C, Olson-Dorff D, Klevan J, Ramirez L, Domoff SE. The relationship between adverse childhood experiences, healthcare utilization, cost of care and medical comorbidities. Child Abuse Negl 2019;90:120–6. [DOI] [PubMed] [Google Scholar]


