Abstract
Disrupted maternal interaction in early infancy is associated with maladaptive child outcomes. Thus, identifying early risk factors for disrupted interaction is an important challenge. Research suggests that maternal depressive symptoms and maternal cortisol dysregulation are associated with disrupted maternal interaction, but both factors have rarely been considered together as independent or interactive predictors of disrupted interaction. In a sample of 51 women, hair cortisol concentrations (HCC) and depressive symptoms were assessed during pregnancy, and depressive symptoms were assessed again at 4-month postpartum. Maternal disrupted interaction was assessed during the Still-Face Paradigm at 4 months. Results indicated that HCC and depressive symptoms interacted to predict both maternal withdrawing and inappropriate/intrusive interaction. Withdrawing interaction was associated with high levels of HCC in pregnancy in the context of high depressive symptoms at 4 months; inappropriate/intrusive interaction was associated with high levels of HCC in the context of low depressive symptoms. Thus, high HCC potentiated both forms of disrupted interaction. Results raised questions about the meaning of very low reported depressive symptoms, and underscored the importance of chronic stress physiology and maternal depressed mood as risk factors for distinct forms of maternal disrupted interaction, both of which are deleterious for infant development.
Keywords: disrupted maternal interaction, hair cortisol, maternal depressive symptoms, perinatal period, Still-Face Paradigm
1 |. INTRODUCTION
The quality of early mother–infant interactions has long-term implications for child social, emotional, and psychological development (Groh, Fearon, van IJzendoorn, Bakermans-Kranenburg, & Roisman, 2016; Lyons-Ruth et al., 2013; Saint-Georges et al., 2013). Given these developmental effects, explicating the factors that shape maternal disrupted interactions is critical. Extensive research indicates that postpartum depression is associated with disrupted maternal–infant interactions (for review see Field, 2010; Lovejoy, Graczyk, O’Hare, & Neuman, 2000). In addition, theory and research suggest that physiological stress in the period from pregnancy to the first-year postpartum, indexed by activation of the hypothalamic–pituitary–adrenal (HPA) axis and its end product cortisol, is linked to disruptions in the mother–infant relationship (Barrett & Fleming, 2010; Finegood, Blair, Granger, Hibel, & Mills-Koonce, 2016; Tarullo, John, & Meyer, 2017). However, research has yet to investigate the joint roles of maternal depressive symptoms and HPA axis activity in predicting disrupted maternal interaction. Moreover, relevant research to date has re-lied heavily on salivary cortisol measures, which provide short-term characterization of HPA axis activity. Measures of cortisol concentration in hair offer potential insights into how longer term HPA axis activity may impact maternal behavior. The goal of the current study was to examine how longer term HPA axis activity during pregnancy, measured through hair cortisol, and maternal depressive symptoms during pregnancy and in early infancy jointly influence forms of disrupted maternal interaction that have been associated with poor child outcomes (Lyons-Ruth et al., 2013; Madigan et al., 2006).
1.1 |. Maternal depression and maternal interactions
Approximately 10%–15% of mothers experience postpartum depression (Bell et al., 2016; Gaynes et al., 2005; O’Hara & McCabe, 2013). Importantly, not only maternal diagnoses established by clinical interview, but also dimensional depression measures indexed by depressive symptom scores, are associated with maternal and infant risk. Maternal risks include lower self-efficacy in the mothering role (e.g., Leahy-Warren & McCarthy, 2011) and less effective mother–infant interactions (e.g., Letourneau, Watson, Duffett-Leger, Hegadoren, & Tryphonopoulos, 2011). Infant risks include insecure attachment (meta-analyses: Atkinson et al., 2000; Martins & Gaffan, 2000), disrupted stress regulation (Feldman et al., 2009; Khoury et al., 2016), psychopathology (e.g., Allen, Oshri, Rogosch, Toth, & Cicchetti, 2019; Bureau, Easterbrooks, & Lyons-Ruth, 2009; Silk, Shaw, Forbes, Lane, & Kovacs, 2006), and enlarged amygdala volume and altered amygdala functional connectivity in offspring (e.g., Buss et al., 2011; Qiu et al., 2015; Wen et al., 2017).
Depressed mothers may show a number of forms of disrupted interaction with their infants, such as intrusive, abrupt, or controlling actions and gestures, displays of negative affect (or less positive affect), the absence of timely or appropriate responses to infant cues, and/or failure to facilitate infant arousal regulation or to engage the infant in interaction (for reviews see Bernard, Nissim, Vaccaro, Harris, & Lindhiem, 2018; Field, 2010; Lovejoy, Graczyk, Ohare, & Neuman, 2000). Elevated maternal depressive symptoms have been associated with reduced responsiveness and sensitivity (Bernard et al., 2018; Stanley, Murray, & Stein, 2004), less frequent and affectionate physical touch (Ferber, Feldman, & Makhoul, 2008; Field et al., 2007), more difficulty regulating emotions (e.g., Riva Crugnola et al., 2016), less responsive speech (e.g., Murray, Kempton, Woolgar, & Hooper, 1993; Shannon & Leider, 2011), fewer verbal references to their infants’ behavior (e.g., Herrera, Reissland, & Shepherd, 2004; Kaplan, Bachorowski, & Zarlengo-Strouse, 1999), less smiling (e.g., Field et al., 2007), greater physical distance during interaction (Vaever, Krogh, Smith-Nielsen, Harder, & Køppe, 2013), and more negative and intrusive behavior (Lyons-Ruth, Connell, Grunebaum, & Botein, 1990; Tronick & Reck, 2009; for reviews see Bernard et al., 2018; Field, 2010; Lovejoy et al., 2000).
Many studies on maternal depression and maternal–infant interaction have focused on the effects of postpartum depression. However, given that depressive symptoms during pregnancy predict depressive symptoms in the postpartum period (Heron, O’Connor, Evans, Golding, & Glover, 2004), measures of maternal depressive symptoms in pregnancy may show similar associations to maternal behavior as depressive symptoms in the postpartum period. For example, one study found that depressive symptoms during pregnancy were associated negatively with postnatal maternal sensitivity (Edwards & Hans, 2015). Depressive symptoms during pregnancy may be an important target for early prevention efforts given their predictive association with later problematic maternal behavior. However, research has yet to compare the potential prediction from depressive symptoms during pregnancy versus depressive symptoms postpartum to a range of maternal disrupted interactions. Thus, we assessed whether maternal depressive symptoms both during pregnancy and during the postpartum period were associated with aspects of maternal disrupted interaction in early infancy.
1.2 |. Maternal HPA axis activity and maternal interactions
As stated by Barrett and Fleming (2010), “mothering is not unitary, but is instead complex, and comprised of many behavioral propensities that are mediated, moderated, and regulated by multiple physiological and brain systems” (pp. 369). Specifically, Barrett and Fleming highlight how mothering behavior in the postpartum period is likely to result from an interplay between maternal mood (e.g., depression) and maternal physiology, including maternal HPA axis activity. Thus, it is important to consider maternal depression in the context of maternal stress physiology, although relatively few studies have done so.
A number of studies have demonstrated an association between maternal salivary cortisol activity and parenting. For example, some studies have found that mothers with higher baseline salivary cortisol levels (Mills-Koonce et al., 2009) and higher cortisol in response to emotionally challenging tasks (Finegood et al., 2016; Thompson & Trevathan, 2008) display lower levels of sensitivity (Finegood et al., 2016; Thompson & Trevathan, 2008) and higher levels of maternal intrusiveness with their infants (Mills-Koonce et al., 2009). Other studies have found that lower maternal salivary cortisol output (lower AUC with respect to ground) is associated both with negative maternal behaviors (defined as intrusive behavior, neutral vocalizations, or noninfant-focused engagement; Wolf et al., 2018) and high levels of overall maternal disrupted interaction (Crockett, Holmes, Granger, & Lyons-Ruth, 2013; Schechter et al., 2004). These associations with both high and low salivary cortisol values are consistent with the larger literature on HPA axis function, which shows that both high and low cortisol levels are related to adversity and to maladaptive outcomes (Guilliams & Edwards, 2010; Khoury, Bosquet Enlow, Plamondon, & Lyons-Ruth, 2019; Miller, Chen, & Zhou, 2007; Stadler et al., 2017; Staufenbiel, Penninx, Spijker, Elzinga, & van Rossum, 2013).
All of the aforementioned studies on the HPA axis and maternal interaction utilized salivary cortisol, which is an acute index of HPA axis activity and is influenced by a number of factors, including time of day, food and caffeine consumption, and current contextual stressors, such as coming to an unfamiliar laboratory (Dickerson & Kemeny, 2004; Gibson et al., 1999; Spiga, Walker, Terry, & Lightman, 2014). Thus, there are methodological challenges in relying on assessments of salivary cortisol at a single point in time as a measure of more enduring HPA axis activity. In contrast, cortisol sampled from hair provides a reliable measure of HPA axis functioning across several months (Russell, Koren, Rieder, & Van Uum, 2012; Stalder & Kirschbaum, 2012), with high degrees of intraindividual stability (Stalder & Kirschbaum, 2012). For example, a recent meta-analysis indicated that individuals who had experienced some form of chronic stress exhibited 22%–43% increased HCC compared to those who were not chronically stressed (Stalder et al., 2017). Nonetheless, researchers must consider sample-related characteristics (e.g., body mass index, gender) and methodological features (e.g., proximity to scalp, length of hair sample; Stalder & Kirschbaum, 2012) when using hair cortisol as a chronic index of HPA axis activity. Despite these limitations, using hair cortisol, as a more stable index of chronic cortisol levels in comparison to salivary cortisol, may enhance our understanding of how HPA axis activity impacts caregiving.
Given the recent development of hair cortisol sampling methods, the role of more enduring HPA axis activation on maternal behavior is not yet well studied. Two studies have assessed hair cortisol concentrations (HCC) in relation to maternal interactions. Tarullo and colleagues (2017) found that higher maternal HCC was associated with greater maternal intrusiveness and with less positive engagement synchrony during a free play interaction at infant age 5–7 months. Nyström-Hansen and colleagues (2019) found that, in a sample of mothers with severe mental illness and controls, HCC in pregnancy was highly correlated with HCC at infant age 4 months and both assessments of HCC were related to greater overall disrupted maternal interaction at 4 months. It is not known whether these findings would replicate in samples with milder symptoms of psychological distress.
When examining the role of HPA axis functioning in pregnancy on maternal and child outcomes, it is important to consider normative, expected changes in cortisol output that occur over the course of pregnancy. Generally, cortisol levels increase during pregnancy (physiological mechanisms reviewed by de Weerth & Buitelaar, 2005). Several researchers have shown that hair cortisol levels reliably increase throughout pregnancy (Bosquet Enlow et al., 2019; Bowers et al., 2018; Braig et al., 2015; D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011; Scharlau et al., 2018). Taking into account these normative physiological changes, researchers have assessed the effects of pregnancy cortisol on various outcomes. Atypical cortisol levels during pregnancy are associated with a pleth-ora of negative perinatal outcomes, including, but not limited to, miscarriage (Nepomnaschy et al., 2006), premature birth, decreased birth weight (Field & Diego, 2008), and elevated child cortisol levels (Gutteling, Weerth, & Buitelaar, 2005). Thus, although cortisol output is expected to increase over the course of pregnancy, individual differences in HCC during pregnancy persist and may provide information regarding longer term maternal stress that influences parenting behavior in the postpartum period.
1.3 |. Maternal HPA axis activity and depression
Depression has been associated consistently with dysregulated HPA axis functioning, including atypical diurnal salivary cortisol patterns and both high and low salivary cortisol reactivity in response to stressors (e.g., Burke, Davis, Otte, & Mohr, 2005; Dettenborn et al., 2011; Gillespie & Nemeroff, 2005; Staufenbiel et al., 2013; Wei et al., 2015). Depressive symptoms occurring during pregnancy or in the first-year postpartum (Seth, Lewis, & Galbally, 2016) are strongly associated with atypical cortisol functioning (see Seth et al., 2016 for a review). For example, maternal depressive symptoms in pregnancy have been associated with greater cortisol reactivity to stressor tasks (Evans, Myers, & Monk, 2008; Nierop et al., 2006). Due to the lack of studies that consider both maternal depressive symptoms and HPA axis functioning in relation to maternal behavior, it is unknown how these risk factors may jointly influence maternal interaction.
1.4 |. The current study: Maternal depressive symptoms, hair cortisol concentrations, and disrupted maternal interaction
The extant literature indicates that (a) maternal depressive symptoms are associated with maladaptive maternal interaction, (b) dysregulated cortisol activity is associated with disrupted maternal interaction, and (c) maternal depression is linked with atypical cortisol levels. Despite the consistent association between maternal depression and disrupted maternal interaction, modest effect sizes indicate that maternal depression is not invariably associated with deviations in maternal behavior. As proposed by Barrett and Fleming (2010), the impact of maternal depression on maternal behavior is not uniform and is likely modulated by maternal physiology.
To date, studies have not specifically examined whether maternal depressive symptoms and hair cortisol levels are independent contributors to disrupted maternal behavior or whether maternal HPA axis activity may interact with depression to predict maternal disrupted interaction. However, related work has found that maternal sensitivity moderates the impact of prenatal anxiety, but not depression, on infant cortisol responses to a laboratory stressor, the Still-Face Paradigm (SFP) (Grant, McMahon, Reilly, & Austin, 2010). In a second study, prenatal anxiety and maternal sensitivity were found to contribute in an additive fashion to infant cortisol reactivity to the SFP, and these results were independent of maternal prenatal and postnatal depressive symptoms (Grant et al., 2009). In addition, the extant literature has documented evidence for both independent and interactive effects of depression and maternal cortisol levels in predicting outcomes other than maternal interaction. For example, maternal cortisol levels have been found to interact with maternal depressive symptoms to predict infant cortisol levels at age 16–17 months (Khoury et al., 2016). A major objective of the current study was to assess whether maternal depressive symptoms and more chronic HPA axis activity have independent or interactive effects on disrupted maternal interactions.
1.5 |. Aims and hypotheses
The primary objectives of this study were as follows: (a) to assess the association between maternal depressive symptoms, both in pregnancy and at 4 months infant age, and maternal disrupted interaction with her infant at 4 months, (b) to assess the association between chronic maternal HPA axis activity, assessed via HCC in pregnancy, and maternal disrupted interaction at 4 months, and (c) to assess whether maternal depressive symptoms and maternal HCC act as independent or interactive contributors to disrupted maternal interaction. Our main hypotheses were that (a) higher maternal depressive symptoms, both in pregnancy and at 4-month postpartum, are related to more disrupted maternal interaction; (b) higher maternal HCC in pregnancy is related to more disrupted maternal interaction, and (c) higher maternal HCC and higher maternal depressive symptoms have an interactive effect on disrupted maternal interaction.
2 |. METHODS
2.1 |. Participants
Fifty-one women were assessed during the third trimester of pregnancy as part of the Harvard Mother-Infant Neurobiological Development (MIND) study. Six participants dropped out after the pregnancy study session prior to completing the 4-month assessment, leaving 45 women who completed both the pregnancy and 4-month study sessions. However, missing data were estimated so that all 51 participants were included in the current analyses. Participants were recruited through prenatal classes, community flyers, and local birth records. Exclusion criteria were: (a) English not a primary language spoken at home, (b) maternal age over 44 years at time of infant birth, (c) infant born before 36-weeks gestation or weighing less than 2500 g at birth, and (d) infant has congenital developmental disorder or birth defects. Mothers ranged in age from 20 to 39 years (M = 32.53, SD = 4.24). Infants (29.4% male) were approximately 4 months of age (M = 4.47 months, SD = 0.73 months) at the time of assessment. Table 1 displays the sample sociodemographic characteristics.
TABLE 1.
Sample characteristics (N = 51)
| Sample characteristics (%) | |
|---|---|
| Maternal ethnicity/race | |
| Hispanic White | 5.9 |
| Hispanic Non-White | 9.8 |
| Non-Hispanic White | 66.7 |
| Non-Hispanic Black | 3.9 |
| Non-Hispanic Asian | 11.8 |
| Non-Hispanic Bi/Multi-racial | 2.0 |
| Maternal education | |
| High school | 13.7 |
| Associate degree | 11.8 |
| Bachelor’s degree | 12.7 |
| Master’s degree | 39.2 |
| Doctoral degree | 21.6 |
| Annual family income | |
| $0–$15,000 | 7.8 |
| $16,000–$25,000 | 7.8 |
| $26,000–$50,000 | 9.8 |
| $51,000–$75,000 | 17.6 |
| $76,000–$100,000 | 13.7 |
| $101,000–$150,000 | 27.5 |
| $151,000+ | 15.6 |
| Relationship status/living situation | |
| Married/living with partner | 88.2 |
| Single, living with family | 3.9 |
| Single, living alone | 7.8 |
2.2 |. Procedure
Assessments were conducted at two time points: during the third trimester of pregnancy at a laboratory visit and at approximately 4-month postpartum during a home visit. During the pregnancy assessment, women completed measures of sociodemographic characteristics and current depressive symptoms and maternal hair samples were collected for extracting cortisol. At the 4-month visit, mothers and infants participated in a video-recorded Still-Face Paradigm (SFP; Tronick, Als, Adamson, Wise, & Brazelton, 1978), and mothers completed a measure of current depressive symptoms.
2.3 |. Measures
2.3.1 |. Still-Face Paradigm (SFP)
Mothers and infants participated in the SFP (Tronick et al., 1978), during which the mother interacted with her infant in face-to-face interaction for 3 minutes (play period), then displayed a neutral face and did not interact with her infant for 2 minutes (still-face period), then engaged in a final period of typical interaction for 5 minutes (reunion period). A meta-analysis has validated that the SFP serves as a mild stressor for the infant, reducing infant positive affect and increasing infant negative affect (Mesman, van IJzendoorn, & Bakermans-Kranenburg, 2009).
2.3.2 |. Maternal depressive symptoms
Maternal depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden, & Sagovsky, 1987) during pregnancy (EPDS-P) and at infant age 4 months (EPDS-4M). The EPDS consists of 10 items that assess a range of depressive symptoms, with possible total scores ranging from 0 to 30. The recommended cutoff score of ≥13 has been identified as indicating a clinically significant risk for postpartum depression (Cox, Chapman, Murray, & Jones, 1996; Matthey, Henshaw, Elliott, & Barnett, 2006). The EPDS is a widely used measure for screening for depression and has been validated for use in pregnant (Murray & Cox, 1990), postnatal (Cox et al., 1987), and nonpostnatal women (Cox et al., 1996).
2.3.3 |. Hair cortisol
Hair cortisol was assessed from participants’ scalp hair, collected during the third trimester of pregnancy (M = 34.90 weeks, SD = 3.38 weeks). A small bundle of hair, approximately 2–3 mm in diameter, was cut using scissors from the posterior vertex region of women’s scalps, a region of the head that has the most uniform hair growth (Pragst & Balikova, 2006). Hair samples were stored at room temperature, out of direct sunlight, in aluminum foil inside manila envelopes (D’Anna-Hernandez et al., 2011). Hair samples were analyzed in the Kirschbaum laboratory at the Department of Psychology, Technical University of Dresden. Hair washing and cortisol extraction, using liquid chromatography–tan-dem mass spectrometry (Gao et al., 2013), was conducted using an established protocol (Stalder et al., 2013). Beginning at the scalp, the first 4-cm segment of hair was used for analyses, which corresponds to hair grown over an approximately 4-month period, assuming a growth rate of 1 cm/month (Stalder & Kirschbaum, 2012; Wennig, 2000). Given that hair was sampled in the third trimester, hair cortisol levels would index cortisol levels from the second to the third trimesters of pregnancy. Hair cortisol concentrations (HCC) were standardized based on sample weight and are reported in pg/mg. Average inter- and intra-assay variabilities were 7.98% and 6.36%, respectively.
2.3.4 |. Maternal disrupted interaction
Maternal disrupted interaction with her infant during the reunion period of the SFP was scored by two trained coders using the Atypical Maternal Behavior Instrument for Assessment and Classification (AMBIANCE) coding system (Lyons-Ruth, Bronfman, & Parsons, 1999). The reunion phase was chosen for coding maternal behavior because it represents the mother’s ability to repair interaction with her infant after the mild stress of the still-face portion. The AMBIANCE rates five aspects of disrupted interaction on 7-point scales (1–7), with higher scores indicating greater disrupted communication: (a) Affective communication errors, defined as incongruent affective signals to the infant (e.g., using a sweet tone of voice with a derogatory or demanding message) or inappropriate or inadequate responses to the infant’s cues (e.g., not responding to a crying infant; rating 1 = consistently responds appropriately to infant signals of distress or proximity-seeking behavior; rating 7 = rarely responds appropriately to infant’s distress and nondistress signals); (b) Role-confusion, defined as the mother soliciting the infant’s attention or affection to herself in ways that override or ignore the infant’s signals (rating 1 = consistently maintains a clear adult role with infant during distress and nondistress interactions; rating 7 = demonstrates persistent need for the infant to focus on the caregiver or the infant to provide comfort to the caregiver); (c) Frightened/disoriented behavior, defined as fearful, hesitant, deferential or disoriented behavior toward the infant (e.g., significant hesitation or tension at moments of heightened attachment needs of infant, frightened voice or expressions; rating 1 = shows no signs of fear, tension, apprehension, or disorientation in interactions; rating 7 = clear and pervasive pattern of frightened, fearful, hesitant behavior and/or disoriented behavior); (d) Negative–intrusive behavior, defined as harsh or critical verbal communication and/or intrusive physical behavior (e.g., pushing or restraining infant, making negative comments about infant; rating 1 = does not display physically or verbally harsh or intrusive behaviors; rating 7 = displays persistent intrusive, negative, and/or hostile behaviors toward the infant); (e) Withdrawing behavior, defined by creating physical or emotional distance from the infant (e.g., silent interactions, no greeting, failure to verbally scaffold interactions, delayed responses to infant cues, awkward holding, and persistent avoidance of interaction with the infant; rating 1 = appears to be verbally and physically comfortable with the infant during nondistressed or distressed interactions; rating 7 = presence of several physical and/or verbal components of withdrawal).
The AMBIANCE has good validity, stability, and reliability as assessed by a meta-analysis (Madigan et al., 2006). Interrater reliability between two expert coders was strong on all scales based on 20% of the videos: affective communication errors ICC = 0.88, role confusion ICC = 0.88, negative–intrusive behavior ICC = 0.94, disorientation ICC = 0.94, and withdrawal ICC = 0.91.
2.3.5 |. Sociodemographics
Mothers self-reported their weight and height prior to pregnancy, age, race, ethnicity, educational attainment, household income level, and relationship status/current living situation. Weight and height were used to calculate prepregnancy body mass index (BMI), a potential correlate of hair cortisol (Stalder et al., 2017).
2.4 |. Statistical analyses
Descriptive, correlational, and principal component analyses were conducted using IBM SPSS Statistics 25. To reduce the number of tests, principal component analysis (PCA) was conducted to determine whether the five aspects of maternal disrupted interaction clustered together and whether factor scores, rather than individual subscale scores, could be used to represent the five types of disrupted maternal interaction. Multiple linear regression analyses were then conducted to assess whether maternal depression and hair cortisol had independent effects on, and/or interacted, to predict disrupted maternal interaction. Each model predicting disrupted maternal interaction was built by including independent variables (maternal depressive symptoms, hair cortisol), interaction effects of relevant independent variables (maternal depressive symptoms × maternal hair cortisol), and covariates that were associated with maternal disrupted interaction. Separate analyses were conducted to examine the similarity of effects of pregnancy versus postpartum assessment of depressive symptoms. Regression analyses were conducted in Mplus Version 8 using full information maximum likelihood (FIML) and bootstrapping to account for missing data, nonnormality, and small sample size (Fox, 2015).1 Bias-corrected confidence intervals (CIs) were used; CIs that do not contain zero are significant at p < .05.
2.4.1 |. Missing data
Analyses were conducted on 51 mothers. The percent of missing values was 13.7% for the AMBIANCE behavior coding (11.8% [k = 6] due to drop-out and 1.9% [k = 1] due to a malfunction in video equipment), 0% for the EPDS scores during pregnancy, 13.7% for the EPDS scores at 4 months (11.8% [k = 6] due to drop-out and 1.9% [k = 1] due to an incomplete questionnaire), and 0% for HCC. Based on Little’s Missing Completely at Random (MCAR) test, χ2(3) = 0.15, p = .99, these data were deemed to be missing completely at random and thus are appropriate for the use of FIML. Because FIML does not delete cases with missing data, this statistical procedure avoids biased parameter estimates likely to occur if pairwise or listwise deletion were used for missing data (Graham, 2003; Wolthke,2000). Therefore, use of statistical methods such as FIML are recommended to account for missing data when data are missing completely at random (McCartney, Burchinal, & Bub, 2006).
3 |. RESULTS
3.1 |. Descriptive statistics
Descriptive statistics are presented in Table 2. Hair cortisol values (pg/mg) were skewed and thus log-transformed to reduce skewness. EPDS scores during pregnancy ranged from 0 to 29, with 9.1% over the clinical cutoff of ≥13; EPDS scores at 4 months ranged from 0 to 28, with 9.8% over the clinical cutoff of ≥13. Ratings for the five aspects of maternal disrupted interaction were normally distributed. Several of these scales were moderately to highly correlated, with affective communication errors, role confusion, negative–intrusive behavior, and disorientation all strongly positively correlated, rs = 0.50–0.68 (ps < .001). In contrast, withdrawal was only associated (negatively) with role confusion, r = −.34, p < .05.
TABLE 2.
Descriptive statistics for main study variables
| Mean ± SD, range | |
|---|---|
| Hair cortisol | |
| Raw values | 12.03 ± 24.72, 1.18–155.19 |
| Log10-transformed values | 0.80 ± 0.41, 0.07–2.19 |
| Depression (EPDS) | |
| EPDS pregnancy | 6.41 ± 4.96, 0–29 |
| EPDS postnatal | 5.48 ± 5.62, 0–28 |
| Disrupted maternal interaction | |
| Role confusion | 2.70 ± 1.47, 1–6 |
| Affective communication errors | 4.02 ± 1.92, 1–7 |
| Negative-intrusive behavior | 3.98 ± 1.55, 1–7 |
| Fearful-disoriented behavior | 3.27 ± 1.76, 1–7 |
| Withdrawal | 2.27 ± 1.65, 1–7 |
Abbreviation: EPDS, Edinburgh Postnatal Depression Scale.
3.2 |. Principal components analysis (PCA) for aspects of disrupted maternal interaction
PCA, using oblique rotation (oblimin), was conducted on the five AMBIANCE scales. Component loadings >0.40 were interpreted (Field, 2009). Component retention was based on eigenvalue >1 (Guadagnoli & Velicer, 1988) and scree plot criteria (Cattell, 1966). The PCA resulted in a two-factor solution. The first factor, interpreted as the Inappropriate/Intrusive factor, had high loadings on affective communication errors, disorientation, negative/intrusive behavior, and role confusion, and explained 53.18% of the variance (Table 3). The second factor, interpreted as the Withdrawing factor, had a high loading on maternal withdrawal and a negative loading on maternal role confusion, and explained an additional 24.86% of the variance (Table 3). Together, this two-factor solution accounted for 78.04% of the matrix variance. The high percentage of variance explained indicated that all five aspects of disrupted maternal interaction are captured by this two-dimensional factor solution. In addition, the Withdrawing Factor and the Inappropriate/Intrusive Factor were essentially uncorrelated (r = −.051, p = .741). Based on these PCA results, subsequent analyses used the two maternal interaction factor scores as the main outcomes.
TABLE 3.
Factor loadings resulting from principal component analysis (PCA) results for the five AMBIANCE Disrupted Maternal Interaction Scales (N = 46)
| Factor 1 Inappropriate/intrusive interaction | Factor 2 Withdrawing interaction | |
|---|---|---|
| Affective communication | 0.854 | 0.051 |
| Disorientation | 0.833 | 0.355 |
| Negative/intrusive behavior | 0.831 | −0.093 |
| Role confusion | 0.731 | −0.410 |
| Withdrawal | 0.081 | 0.973 |
Note: Bold signifies interpretable factor loadings, based on a loading >.40 (Field, 2009)
3.3 |. Exploration of covariates
Maternal age, hispanic/nonhispanic ethnicity, white/nonwhite race, level of education, relationship status, and prepregnancy BMI, family income, and infant sex were assessed as potential covariates.2
Ethnicity (r = −.40, p < .01) and lower income (r = −.33, p < .05) were associated with higher ratings on the Inappropriate/Intrusive interaction factor. Maternal nonwhite race (r = .44, p < .01), lower education (r = −.31, p < .05) and lower income (r = −.38, p < .05)
3.4 |. Effects of maternal hair cortisol and maternal depressive symptoms on maternal withdrawing and inappropriate/intrusive interaction
Correlational analyses were used to test the bivariate associations among maternal HCC in pregnancy, depressive symptoms in pregnancy (EPDS-P), depressive symptoms at 4-month postpartum (EPDS-4M), and Inappropriate/Intrusive and Withdrawing maternal interaction (Table 4). The bivariate relations indicated a positive association between HCC levels in pregnancy and Withdrawing interaction at 4 months. There were no significant bivariate relations between HCC and Inappropriate/Intrusive interaction nor between HCC and depressive symptoms in pregnancy or at 4 months (Table 4). Depressive symptoms in pregnancy were highly positively correlated with depressive symptoms at 4 months (r = .78, p < .01).
TABLE 4.
Correlation coefficients among maternal disrupted interaction factor scores, hair cortisol concentrations, and maternal pregnancy and postpartum depressive symptoms
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. Inapp/Intrus interaction | — | ||||
| 2. Withdrawing interaction | −0.05 | — | |||
| 3. HCC | 0.06 | 0.37* | — | ||
| 4. EPDS-P | −0.001 | 0.28 | 0.18 | — | |
| 5. EPDS-4M | −0.09 | 0.25 | 0.11 | 0.78** | — |
Note: N = 51 for pregnancy data and N = 46 for 4-month data.
Abbreviations: EPDS-4M, Edinburgh Postnatal Depression Scale at the 4-month visit; EPDS-P, Edinburgh Postnatal Depression Scale during pregnancy; Inapp/Intrus interaction, inappropriate/intrusive interaction factor score; Withdrawing interaction, withdrawing interaction factor score.
p < .05,
p ≤ .01.
3.4.1 |. Maternal depressive symptoms during pregnancy
Separate regression models, with bootstrap CIs, were conducted to predict maternal Withdrawing interaction and Inappropriate/Intrusive interaction. Models included maternal depressive symptoms in pregnancy (EPDS-P), HCC in pregnancy, the interaction of EPDS-P and HCC, and household income and race/ethnicity as predictors. The individual predictors and the interaction term between hair cortisol and EPDS-P were not significant in either the model predicting maternal Withdrawing behavior or the model predicting Inappropriate/Intrusive behavior (Table 5).
TABLE 5.
Multiple regression results predicting disrupted maternal interaction factor scores (N = 51)
| Factor 1 Inappropriate/Itntrusive Interaction | Factor 2 Withdrawing Interaction | ||||||
|---|---|---|---|---|---|---|---|
| β | Std. error | Bootstrap CI [95%] | β | Std. error | Bootstrap CI [95%] | ||
| EPDS in pregnancy | |||||||
| Income | −0.303** | 0.138 | −0.508, −0.063 | Income | −0.299** | 0.144 | −0.515, −0.058 |
| Ethnicity | −0.340* | 0.146 | −0.538, −0.041 | Race | 0.192 | 0.153 | −0.045, 0.444 |
| HCC | 0.116 | 0.357 | −0.439, 0.686 | HCC | −0.269 | 0.487 | −0.982, 0.544 |
| EPDS-P | 0.106 | 0.426 | −0.530, 0.842 | EPDS-P | −0.473 | 0.591 | −1.419, 0.444 |
| EPDS-P × HCC | −0.233 | 0.628 | −1.340, 0.676 | EPDS-P × HCC | 0.939 | 0.718 | −0.208, 2.057 |
| EPDS at 4 months | |||||||
| Income | −0.387** | 0.142 | −0.614, −0.139 | Income | −0.197 | 0.143 | −0.424, 0.033 |
| Ethnicity | −0.351** | 0.126 | −0.534, −0.127 | Race | 0.256* | 0.138 | 0.040, 0.479 |
| HCC | 0.495 | 0.250 | 0.117, 0.936 | HCC | −0.172 | 0.245 | −0.578, 0.234 |
| EPDS-4M | 0.735 | 0.400 | 0.115, 1.373 | EPDS-4M | −0.491* | 0.410 | −1.529, −0.017 |
| EPDS-4M × HCC | −1.077** | 0.463 | −1.857, −0.362 | EPDS-4M × HCC | 0.921** | 0.415 | 0.412, 1.803 |
Note: Results include bootstrap CIs.
Abbreviations: EPDS-4M, Edinburgh Postnatal Depression Scale at the 4-month visit; EPDS-P, Edinburgh Postnatal Depression Scale at the pregnancy visit; HCC, hair cortisol concentrations.
p < .01.
p < .05.
3.4.2 |. Postpartum maternal depressive symptoms
Similar regression models were conducted using maternal depressive symptoms assessed at 4 months to predict maternal Withdrawing interaction and Inappropriate/Intrusive interaction.
Withdrawing interaction
In the analysis predicting maternal Withdrawing interaction, the model included maternal postpartum depressive symptoms (EPDS-4M), HCC, the interaction of EPDS-4M and HCC, and relevant control variables (Table 5). The overall model was significant, F (5, 43) = 6.18, p < .001, adjusted R2 = .455. The main effects will not be interpreted given the significant interaction between HCC and EPDS-4M (Table 5).
To illustrate the interaction effect, a figure was created using the unstandardized regression coefficients (Preacher, Curran, & Bauer, 2006). While continuous EPDS and HCC values were used for regression results, following recommended procedures, meaningful conditional values of the EPDS were selected for probing and graphing the interaction (Preacher, Curran, & Bauer, 2004, 2006). In addition to the mean value of 5.48, scores of 13 and 0 on the EPDS were chosen for graphing, both because they were meaningful in relation to the EPDS and because they represented scores close to 1 SD above and below the mean. A score of 13 represents the clinical cut score for the EPDS and is 1.34 SD above the mean. The score of 0 was chosen because 1 SD below the mean would be a negative value and a negative value is not possible on the EPDS. A score of 0 is 0.98 SD below the mean and the lowest possible score on the EPDS. As shown in Figure 1, mothers who scored at or above 13 on the EPDS and who also had higher HCC displayed relatively higher levels of maternal Withdrawing interaction. Simple slope analyses revealed that the simple slope of the high depressive symptoms group (t = 3.092, p < .01) was significantly different from zero. The simple slope of the low (t = −0.60, p = .55) and moderate (t = 0.549, p = .10) depressive symptoms groups were not significantly different from zero. In addition, the slopes of the high depressive symptoms group (b = 1.805, SE = 0.584) and of the low depressive symptoms group (b = −0.375, SE = 0.625) were significantly different from each other, t = 2.55, p < .05. Thus, based on these simple slope analyses, mothers with both high HCC and high depressive symptoms exhibited significant elevations in withdrawing interactions with their infants. Mothers with EPDS scores at or below the mean did not show significantly elevated withdrawal, regardless of HCC values.
FIGURE 1.
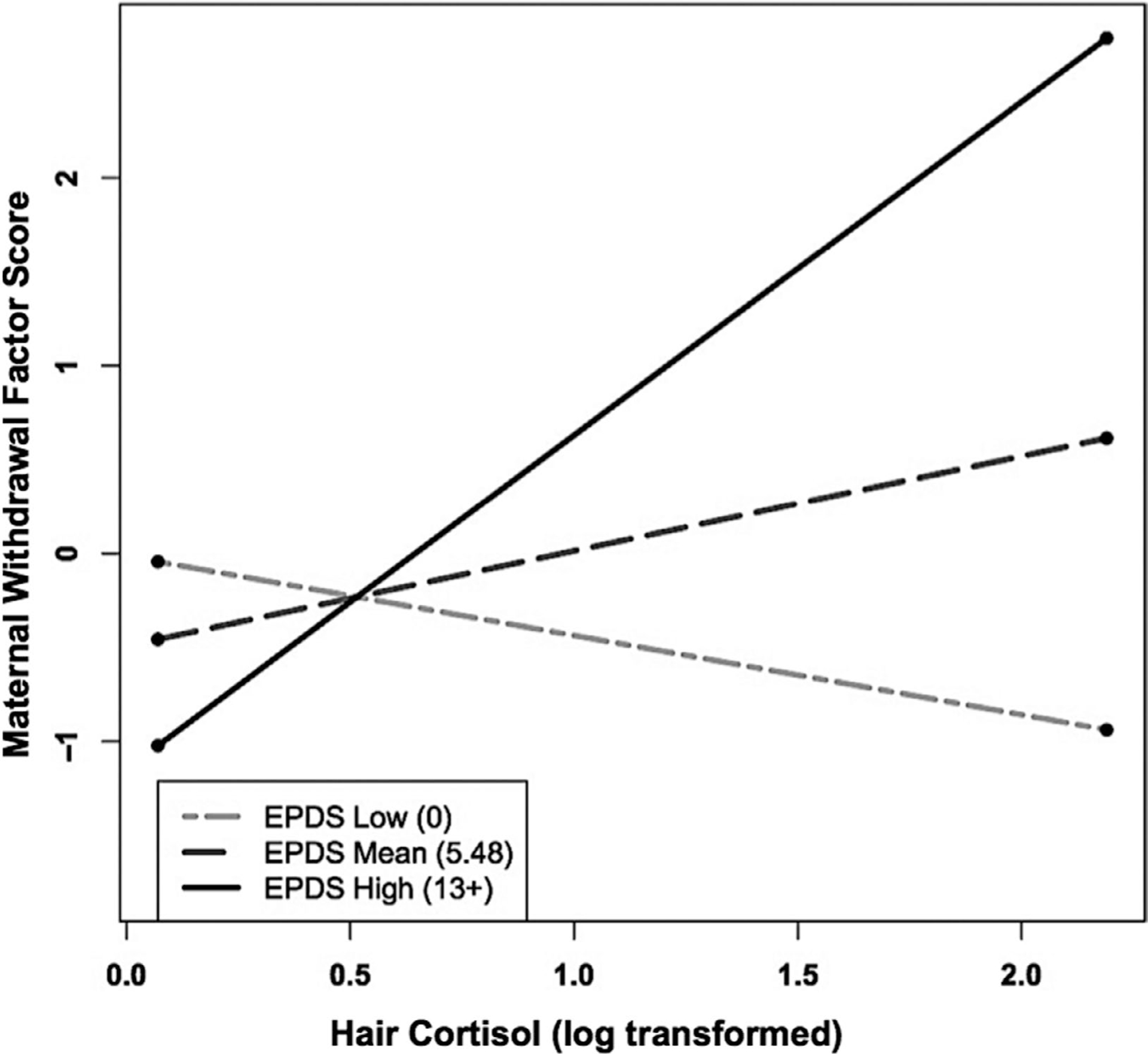
Maternal depressive symptoms at infant age 4 months moderates the relation between maternal hair cortisol concentration (HCC) in pregnancy and maternal withdrawing behavior during the Still-Face Paradigm at infant age 4 months. As shown, mothers who endorsed greater postpartum depressive symptoms (EPDS high) and had higher HCC displayed relatively greater Withdrawing interaction behaviors, whereas mothers who endorsed lower postpartum depressive symptoms (EPDS low) and had higher HCC displayed relatively low levels of Withdrawing interaction behaviors. Only the simple slope of the high depressive symptoms group was significantly different from zero. The slopes of the high depressive symptoms group and the low depressive symptoms group were significantly different from each other
Inappropriate/intrusive interaction
The model predicting maternal Inappropriate/Intrusive interaction was also significant, F (5, 43) = 3.42, p < .05, adjusted R2 = .316. The interaction between maternal depressive symptoms and HCC predicting the maternal Inappropriate/Intrusive factor score was, in some ways, the inverse of the interaction predicting the maternal Withdrawing factor score (Table 5). As shown in Figure 2, as HCC levels increased, mothers with the lowest levels of depressive symptoms (scores of zero) displayed the highest scores on Inappropriate/Intrusive interaction. Simple slope analyses revealed that the simple slope of the low depressive symptoms group (t = 2.105, p < .05) and the high depressive symptoms group (t = −2.083, p < .05) were both significantly different from zero. The simple slope of the moderate depressive symptoms group was not significantly different from zero (t = 0.360, p = .72). In addition, the slopes of the low depressive symptoms group (b = 1.220, SE = 0.579) and the high depressive symptoms group (b = −1.372, SE = 0.658) differed significantly from each other, t = 2.96, p < .05. Thus, these simple slope analyses indicate that with higher levels of HCC, mothers with very low EPDS scores became more intrusive, but mothers with high EPDS scores became less intrusive. Mothers with EPDS scores at the mean (5.48) did not vary significantly in intrusiveness as a function of HCC levels, as seen in Figure 2.
FIGURE 2.
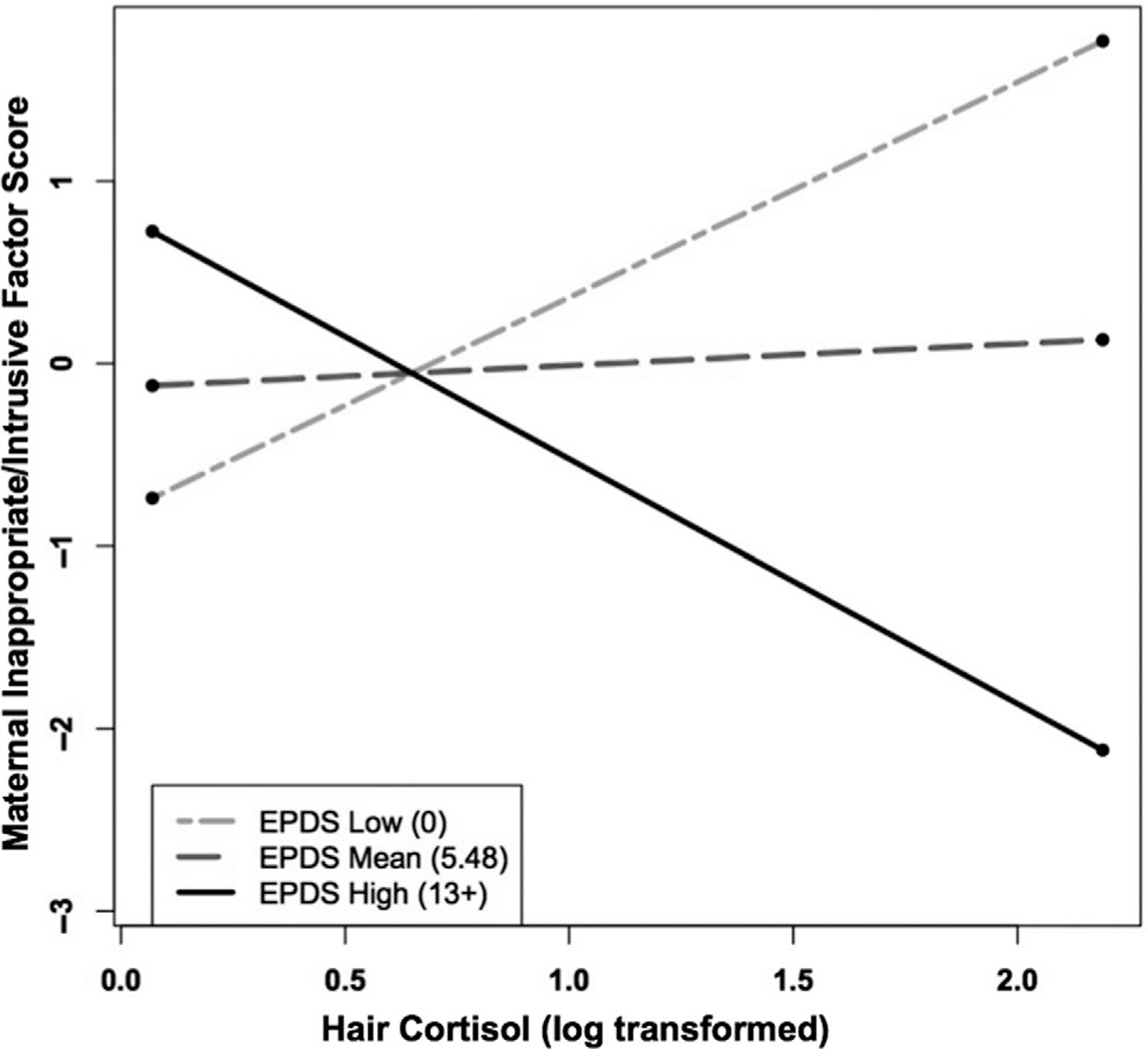
Maternal depressive symptoms at infant age 4 months moderates the relation between maternal hair cortisol concentration (HCC) in pregnancy and maternal inappropriate/intrusive interaction during the Still-Face Paradigm at infant age 4 months. As shown, mothers who endorsed greater postpartum depressive symptoms (EPDS high) and who had higher HCC displayed relatively low levels of Inappropriate/Intrusive interaction behaviors, whereas mothers who endorsed lower postpartum depressive symptoms (EPDS low) and had higher HCC displayed relatively high levels of Inappropriate/Intrusive interaction behaviors. Only the simple slope of the low depressive symptoms group was significantly different from zero. The slopes of the high depressive symptoms group and the low depressive symptoms
Similar effects were obtained after controlling for prenatal depressive symptoms. The interaction term between postnatal depressive symptoms (EPDS-4M) and HCC remained a significant predictor of both maternal Withdrawing interaction (b = 0.926, SE = 0.440, 95% CI = 0.334, 1.833) and of maternal Inappropriate/Intrusive interaction (b = −1.081, SE = 0.459, 95% CI = −1.880, −0.368).3
Taken together, these results indicate that, in the context of higher HCC levels in pregnancy, mothers are likely to exhibit higher levels of disrupted interaction with their infants at 4 months. However, the form of the disrupted behavior was quite different at different levels of depressive symptoms. Higher depressive symptoms in the context of higher HCC were associated with Withdrawing interaction, while very low depressive symptoms in the context of higher HCC were associated with Inappropriate/Intrusive interaction.
4 |. DISCUSSION
The aim of the current study was to increase our understanding of how hair cortisol concentrations (HCC) in pregnancy and maternal pre- and postpartum depressive symptoms jointly influence a mother’s interactions with her infant. Results build on a small but growing literature using hair cortisol as an indicator of more chronic maternal HPA axis activity and suggest that this measure has promise for increasing our understanding of factors influencing the quality of early maternal interaction.
The first finding of the study was that there was a strong positive correlation between the level of maternal depressive symptoms during the third trimester of pregnancy and the level of depressive symptoms at 4-month postpartum (Table 3). This finding adds to the literature on the continuity of depressive symptoms over the first year and for several years postpartum (e.g., Alpern & Lyons-Ruth, 1993; Edwards & Hans, 2015; Lyons-Ruth, Zoll, Connell, & Grunebaum, 1986) and indicates that mothers likely to experience postpartum depressive symptoms may already be showing elevated symptoms during pregnancy. Similarly, other work provides evidence of strong continuity in more general symptomatic functioning from the third trimester of pregnancy to 4-month postpartum (Nyström-Hansen et al., 2019). This continuity in symptomatic functioning from pre- to postpartum periods supports the identification of at-risk women in pregnancy, as well as the implementation of preventive interventions prior to the birth of the child to minimize disruption in the mother’s early interactions with her infant.
The second major finding, based on the PCA, was that there were two separable components of disrupted maternal interaction with the infant, namely withdrawing interaction and inappropriate/intrusive interaction. Indexing both components of disrupted interaction proved to be quite important because, in the context of elevated hair cortisol, postnatal depressive symptoms had very different relations to maternal withdrawing interaction versus maternal inappropriate/intrusive interaction. This aspect of the results reveals the importance of coding for both components of maternal behavior separately, rather than coding maternal behavior on a single scale, such as a sensitivity scale or an overall disruption scale.
The third and most striking finding was that, in the context of higher HCC, higher postnatal depressive symptoms were associated with increased maternal withdrawing interaction, while lower depressive symptoms were associated with increased maternal inappropriate/intrusive interaction. Equally important, in the absence of elevated HCC, maternal depressive symptoms were not associated with elevated levels of either form of disrupted interaction (see Figures 1 and 2). Thus, elevated HCC emerges as an important risk factor that may potentiate the effects of other associated risks on maternal behavior.
In the context of higher HCC, high depressive symptoms predicted increased levels of mother’s withdrawing behavior, thus impeding her ability to engage effectively with her infant. Notably, the mother’s withdrawing behavior was coded during the reunion phase of the SFP, a time when active maternal behavior is often needed to assist the infant in regulating negative emotions resulting from the stress of the still-face phase (Mesman, van IJzendoorn, & Bakermans-Kranenburg, 2009). Thus, higher levels of maternal depressive symptoms in combination with more chronic physiological stress may substantially interfere with a mother’s ability to assist in the regulation of her infant’s state.
Conversely, low levels of maternal depressive symptoms in the context of elevated prenatal HCC values were associated with increased inappropriate/intrusive interaction. Again, when prenatal HCC levels were low, levels of inappropriate/intrusive interaction were low, regardless of the level of depressive symptoms. Thus, high hair cortisol was a contributor to both forms of disrupted interaction, but the particular form of disruption depended on the level of the mother’s depressive symptoms.
Although these results among mothers with low depressive symptom scores may appear counterintuitive, at least two previous studies have noted similar relations. Early work by Lyons-Ruth and colleagues (1986) found that low-income mothers scoring zero on the Center for Epidemiologic Studies Scale of depressive symptoms (5% of the sample) were rated particularly high on hostility and interfering manipulation in interaction with their 12-month-old infants and were more likely to have infants who were insecurely attached, similar to mothers above the cut point for possible depression. Similarly, Field and colleagues (1991), observing face-to-face interactions of low-income mothers and their 5-month-old infants, found that mothers with a score of zero on the Beck Depression Inventory (15% of the sample) spent more time in the ‘anger/poke’ state with their infants and less time in the play state than other nondepressed mothers. Extremely low or zero scores on self-report depressive symptom scales are relatively uncommon because each symptom is rated on a severity scale (e.g., 0–3). Most mothers endorse at least some depressive symptom items at low levels (e.g., in response to the item “I have been anxious or worried,” a score of 1 is “hardly ever” whereas a score of 0 is “not at all”). Thus, scores of zero on depressive symptom scales may represent the lack of insight or avoidance of accepting or acknowledging negative feelings. Given these findings, additional work in larger samples is warranted to assess the predictors and outcomes associated with extremely low scores on maternal self-report symptom inventories. Future work that combines self-report measures and clinical interviews is needed to further explore whether some mothers with very low self-report depression scores lack insight or avoid reporting negative or vulnerable feelings despite clinically significant impairment. Additional work is also needed regarding how such avoidance in the mother might impact her behavior with her infant.
This finding of differing effects of low and high depressive symptoms in the context of high HCC identifies one factor, among many others, that may contribute to why prior literature has been so varied in relation to stress and maternal behavior. For example, Wolf et al. (2018) found that mothers with high diurnal cortisol output (i.e., flatter diurnal cortisol decline, thought to index chronic stress) displayed less negative behavior during the reunion episode of the SFP. In a similar vein, Field et al. (2007) found that mothers with higher levels of depressive symptoms were less interactive with their infants during the reunion period of the SFP. In contrast, Tarullo and colleagues (2017) found that mothers with higher hair cortisol at 6-month postpartum were more intrusive in interactions with their infants, and Lyons-Ruth et al. (1990) found that mothers with higher depressive symptom scores were more intrusive with their infants at 18 months. While these studies also differ in other aspects of their methods which might explain the above inconsistencies (e.g., measure of parenting, type of interaction-based stressor), the current results suggest that chronic cortisol levels and depressive symptoms should also be considered in concert when predicting withdrawing or inappropriate/intrusive interaction in order to capture such potential interactions.
Additional research supports the role of the HPA axis in modulating the effects of maternal depression on adverse mother–child outcomes. For example, in a community sample of mother–infant dyads, higher maternal salivary cortisol levels interacted with higher maternal depressive symptoms to result in elevated infant cortisol levels (Khoury et al., 2016). In addition, higher child evening salivary cortisol levels increased the impact of maternal and paternal depression on child externalizing behavior, as well as the impact of paternal depression on child internalizing behavior (Laurent et al., 2012). These studies, taken together with the current findings, demonstrate that greater activation of the HPA axis can exacerbate the impact of parental depressive symptoms on parent and child physiological and behavioral difficulties.
Understanding joint effects of maternal HPA axis activity and depressive symptoms on maternal interaction is important, given the widespread impact of early maternal interaction on offspring neurobiological and psychopathological outcomes. The later consequences for the child from both types of disruption are far-reaching, with maternal hostile-intrusiveness, but not withdrawal, consistently associated with childhood aggression, conduct disorder, and overall externalizing problems (Campbell, 1991; Lyons-Ruth, Alpern, & Repacholi, 1993; Lyons-Ruth, Easterbrooks, & Cibelli, 1997; Trentacosta & Shaw, 2008; for reviews see Loeber & Dishion, 1983; Patterson & Bank, 1989). In contrast, early maternal withdrawal, but not early intrusiveness, is associated with increased adult hippocampal volume (Khoury, Pechtel, Andersen, Teicher, & Lyons-Ruth, 2019), increased borderline personality disorder features, increased incidence of suicidality/self-injury (Lyons-Ruth et al., 2013), and increased substance abuse (Pechtel, Woodman, & Lyons-Ruth, 2012).
It should be underscored that, in the present study, hair cortisol was only assessed in pregnancy. Thus, the present results must be understood in relation to the specific circumstances that impact cortisol during pregnancy. Hormonal and physiological changes contribute to relatively elevated cortisol levels during pregnancy. Thus, absolute HCC levels in pregnancy likely must be interpreted differently in relation to level of chronic stress compared to HCC levels in the postpartum period. Nonetheless, the relative values of prenatal cortisol among pregnant women are still likely to be meaningful as an index of chronic stress (e.g., D’Anna-Hernandez et al., 2011; Scharlau et al., 2018).
The current results cannot speak to the potential continuity in HCC from pregnancy to the postpartum period. However, Nyström-Hansen et al. (2019) found robust continuity in HCC levels from the third trimester of pregnancy to infant age 4 months, and hair cortisol levels at both time points were associated with maternal disrupted interaction in the SFP. Thus, the effects of elevated HCC during pregnancy obtained here may possibly be linked to the continuation of elevated HCC into the postpartum period. Additional studies with repeated assessments of HCC are needed to explore the continuity in maternal HCC from pre- to postnatal periods.
The findings of this study must be considered within the context of the study’s limitations. First, the current sample size is relatively small. Thus, findings should be interpreted with caution and the results should be replicated in larger samples to assess generalizability. Second, mother–infant interaction was only observed at 4-month infant age. Child age and assessment setting are likely to influence the form of maternal disrupted interaction observed. In the early part of the first year, the infant has a limited repertoire to engage the mother, so maternal withdrawal may be a more prominent feature of at-risk interactions. By toddler-hood, the child is much more capable of initiating overtures to the mother, and thus mothers who had previously withdrawn from interaction may be more likely to exhibit inappropriate or intrusive responses when pressed for interaction by the child. Third, we did not assess the potential impact of infant behavior on maternal behavior. If the mother experienced higher levels of stress and depression during pregnancy, those risks could influence infant arousal and soothability through multiple routes, with further effects on maternal behavior. Thus, future work is needed to assess how infant behavior might further mediate or moderate the relations among maternal HCC, depressive symptoms, and disrupted behavior. Fourth, the current study did not assess clinical levels of depression with a psychiatric interview, and a psychiatric diagnosis of depression might be associated with different outcomes than the depressive symptoms assessed here. Nonetheless, these finding are informative given the well-replicated negative developmental outcomes associated with maternal depressive symptoms (e.g., Goldney, Phillips, Fisher, & Wilson, 2004; Horwath, Johnson, Klerman, & Weissman, 1994).
In summary, these results are consistent with a psychobiological theory of mothering (Barrett & Fleming, 2010). Barrett and Fleming (2010) proposed that the quality and nature of maternal behavior is influenced by individual differences in both maternal physiological stress and postpartum depression. Here, maternal chronic physiological stress, indexed by high maternal hair cortisol levels, interacted with maternal mood to influence the mother’s ability to interact with her infant in an engaged and nonintrusive fashion. In the absence of high chronic cortisol levels, high maternal depressive symptoms were not associated with disrupted maternal behavior. Thus, lower cortisol levels may protect mothers from the potentially negative effects of high depressive symptoms during the postpartum period. Given the long-term effects of maternal disrupted interaction on the child, these results point to the potential benefits of developing interventions for reducing high levels of stress and depression during pregnancy, in order to alter long-term trajectories toward maladaptation.
ACKNOWLEDGMENTS
This research was supported by National Institute of Child Health and Human Development R01HD079484 to KLR and MBE.
Footnotes
Results are presented with FIML. Regression analyses were also conducted without FIML, using only the sample with complete data (N = 46). Results without FIML are consistent with FIML results.
Race and ethnicity are shown in Table 1 using finer discrim-inations. However, that six-category variable was converted into two binary variables for entry into the correlation/regression analyses.
Results controlling for prenatal depression must be considered in light of the very high correlation between prenatal and postnatal depressive symptoms (r = .78). This high correlation leaves a very small portion of variance in postnatal scores unexplained after prenatal scores are controlled. Thus, these latter results should be considered with caution.
DATA AVAIL ABILIT Y STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Allen TA, Oshri A, Rogosch FA, Toth SL, & Cicchetti D (2019). Offspring personality mediates the association between maternal depression and childhood psychopathology. Journal of Abnormal Child Psychology, 47(2), 345–357. 10.1007/s10802-018-0453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern L, & Lyons-Ruth K (1993). Preschool children at social risk: Chronicity and timing of maternal depressive symptoms and child behavior problems at school and at home. Development and Psychopathology, 5, 369–385. 10.1017/S0954579400004478 [DOI] [Google Scholar]
- Atkinson L, Paglia A, Coolbear J, Niccols A, Parker KC, & Guger S (2000). Attachment security: A meta-analysis of maternal mental health correlates. Clinical Psychology Review, 20(8), 1019–1040. 10.1016/S0272-7358(99)00023-9 [DOI] [PubMed] [Google Scholar]
- Barrett J, & Fleming AS (2010). Annual Research Review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52(4), 368–397. 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- Bell AF, Carter CS, Davis JM, Golding J, Adejumo O, Pyra M, … Rubin LH (2016). Childbirth and symptoms of postpartum depression and anxiety: A prospective birth cohort study. Archives of Women’s Mental Health, 19(2), 219–227. 10.1007/s00737-015-0555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Nissim G, Vaccaro S, Harris JL, & Lindhiem O (2018). Association between maternal depression and maternal sensitivity from birth to 12 months: A meta-analysis. Attachment & Human Development, 20(6), 578–599. 10.1080/14616734.2018.1430839 [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, Sideridis G, Chiu YHM, Nentin F, Howell EA, Le Grand BA, & Wright RJ (2019). Associations among maternal socioeconomic status in childhood and pregnancy and hair cortisol in pregnancy. Psychoneuroendocrinology, 99, 216–224. 10.1016/j.psyneuen.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Ding L, Gregory S, Yolton K, Ji H, Meyer J, … Folger A (2018). Maternal distress and hair cortisol in pregnancy among women with elevated adverse childhood experiences. Psychoneuroendocrinology, 95, 145–148. 10.1016/j.psyneuen.2018.05.024 [DOI] [PubMed] [Google Scholar]
- Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, … Rothenbacher D (2015). Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology, 52, 289–296. 10.1016/j.psyneuen.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Bureau JF, Easterbrooks MA, & Lyons-Ruth K (2009). Maternal depressive symptoms in infancy: Unique contribution to children’s depressive symptoms in childhood and adolescence? Development and Psychopathology, 21(2), 519–537. 10.1017/S0954579409000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Pruessner JC, Muftluer TL, Head K, Hasso A, & Sandman CA (2011). Increased risk for affective disorders programmed in utero? High prenatal maternal cortisol concentrations and volumes of the amygdala and hippocampus in the offspring at 6–9 years of age. Journal of Developmental Origins of Health and Disease, 2, S37. [Google Scholar]
- Campbell SB (1991). Longitudinal studies of active and aggressive pre-schoolers: Individual differences in early behavior and in outcome In Cicchetti D, & Toth SL (Eds.), Internationalizing and externalizing expression of dysfunction: Rochester Symposium on Developmental Psychopathology (pp. 57–89). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cattell RB (1966). The scree test for the number of factors. Multivariate Behavioral Research, 1(2), 245–276. 10.1207/s15327906mbr0102_10 [DOI] [PubMed] [Google Scholar]
- Cox JL, Chapman G, Murray D, & Jones P (1996). Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. Journal of Affective Disorders, 39(3), 185–189. 10.1016/0165-0327(96)00008-0 [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150(6), 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Crockett EE, Holmes BM, Granger DA, & Lyons-Ruth K (2013). Maternal disrupted communication during face-to-face interaction at 4 months: Relation to maternal and infant cortisol among at-risk families. Infancy, 18(6), 1111–1134. 10.1111/infa.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, & Laudenslager ML (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology and Behavior, 104(2), 348–353. 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, … Otte C (2011). Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress, 15(3), 348–353. 10.3109/10253890.2011.619239 [DOI] [PubMed] [Google Scholar]
- De Weerth C, & Buitelaar JK (2005). Physiological stress reactivity in human pregnancy—a review. Neuroscience & Biobehavioral Reviews, 29(2), 295–312. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Edwards RC, & Hans SL (2015). Prenatal depressive symptoms and toddler behavior problems: The role of maternal sensitivity and child sex. Child Psychiatry & Human Development, 47(5), 696–707. 10.1007/s10578-015-0603-6 [DOI] [PubMed] [Google Scholar]
- Evans LM, Myers MM, & Monk C (2008). Pregnant women’s cortisol is elevated with anxiety and depression – but only when comorbid. Archives of Womens Mental Health, 11(3), 239–248. 10.1007/s00737-008-0019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, & Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 919–927. 10.1097/chi.0b013e3181b21651 [DOI] [PubMed] [Google Scholar]
- Ferber SG, Feldman R, & Makhoul IR (2008). The development of maternal touch across the first year of life. Early Human Development, 84(6), 363–370. 10.1016/j.earlhumdev.2007.09.019 [DOI] [PubMed] [Google Scholar]
- Field A (2009). Discovering statistics using SPSS: Introducing statistical method (3rd ed.). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Field T (2010). Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behavior and Development, 33(1), 1–6. 10.1016/j.infbeh.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, & Diego M (2008). Cortisol: The culprit prenatal stress variable. International Journal of Neuroscience, 118(8), 1181–1205. 10.1080/00207450701820944 [DOI] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Feijo L, Vera Y, Gil K, & Sanders C (2007). Still-face and separation effects on depressed mother-infant interactions. Infant Mental Health Journal, 28(3), 314–323. 10.1002/imhj.20138 [DOI] [PubMed] [Google Scholar]
- Field T, Morrow C, Healy B, Foster T, Adlestein D, & Goldstein S (1991). Mothers with zero Beck depression scores act more “depressed” with their infants. Development and Psychopathology, 3(3), 253–262. 10.1017/S0954579400005290 [DOI] [Google Scholar]
- Finegood ED, Blair C, Granger DA, Hibel LC, & Mills-Koonce R (2016). Psychobiological influences on maternal sensitivity in the context of adversity. Developmental Psychology, 52(7), 1073–1087. 10.1037/dev0000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J (2015). Applied regression analysis and generalized linear models. Los Angeles: Sage Publications. [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, & Kirschbaum C (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. Journal of Chromatography B, 928, 1–8. 10.1016/j.jchromb.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … Miller WC (2005). Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment No. 119 (Prepared by the RTI-University of North Carolina Evidence-based Practice Center, under Contract No. 290–02–0016.) AHRQ Publication No. 05-E006–2 Rockville, MD: Agency for Healthcare Research and Quality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, & Wardle J (1999). Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosomatic Medicine, 61(2), 214–224. 10.1097/00006842-199903000-00014 [DOI] [PubMed] [Google Scholar]
- Gillespie CF, & Nemeroff CB (2005). Hypercortisolemia and depression. Psychosomatic Medicine, 67, 10.1097/01.psy.0000163456.22154.d2 [DOI] [PubMed] [Google Scholar]
- Goldney RD, Phillips PJ, Fisher LJ, & Wilson DH (2004). Diabetes, depression, and quality of life: A population study. Diabetes Care, 27(5), 1066–1070. 10.2337/diacare.27.5.1066 [DOI] [PubMed] [Google Scholar]
- Graham JW (2003). Adding missing-data-relevant variables to FIML-based structural equation models. Structural Equation Modeling, 10(1), 80–100. 10.1207/S15328007SEM1001_4 [DOI] [Google Scholar]
- Grant KA, McMahon C, Austin MP, Reilly N, Leader L, & Ali S (2009). Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Developmental Psychobiology, 51(8), 625–637. 10.1002/dev.20397 [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Reilly N, & Austin MP (2010). Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behavior and Development, 33(4), 453–462. 10.1016/j.infbeh.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Groh AM, Fearon RM, Ijzendoorn MH, Bakermans-Kranenburg MJ, & Roisman GI (2016). Attachment in the early life course: Meta-analytic evidence for its role in socioemotional development. Child Development Perspectives, 11(1), 70–76. 10.1111/cdep.12213 [DOI] [Google Scholar]
- Guadagnoli E, & Velicer WF (1988). Relation of sample size to the stability of component patterns. Psychological Bulletin, 103(2), 265–275. 10.1037/0033-2909.103.2.265 [DOI] [PubMed] [Google Scholar]
- Guilliams TG, & Edwards L (2010). Chronic stress and the HPA axis: Clinical assessment and therapeutic considerations. The Standard, 9, 1–12. [Google Scholar]
- Gutteling BM, de Weerth C, & Buitelaar JK (2005). Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology, 30(6), 541–549. 10.1016/j.psyneuen.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, & Glover V (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80(1), 65–73. 10.1016/j.jad.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Herrera E, Reissland N, & Shepherd J (2004). Maternal touch and maternal child-directed speech: Effects of depressed mood in the postnatal period. Journal of Affective Disorders, 81(1), 29–39. 10.1016/j.jad.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, & Weissman MM (1994). What are the public health implications of subclinical depressive symptoms? Psychiatric Quarterly, 65(4), 323–337. 10.1007/BF02354307 [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Bachorowski JA, & Zarlengo-Strouse P (1999). Child-directed speech produced by mothers with symptoms of depression fails to promote associative learning in 4-month-old infants. Child Development, 70(3), 560–570. 10.1111/1467-8624.00041 [DOI] [PubMed] [Google Scholar]
- Khoury JE, Bosquet Enlow M, Plamondon A, & Lyons-Ruth K (2019). The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology, 103, 104–117. 10.1016/j.psyneuen.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury JE, Pechtel P, Andersen CM, Teicher MH, & Lyons-Ruth K (2019). Relations among maternal withdrawal in infancy, borderline features, suicidality/self-injury, and adult hippocampal volume: A 30-year longitudinal study. Behavioural Brain Research, 374, 112139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury JE, Gonzalez A, Levitan R, Masellis M, Basile V, & Atkinson L (2016). Maternal self-reported depressive symptoms and maternal cortisol levels interact to predict infant cortisol levels. Infant Mental Health Journal, 37(2), 125–139. 10.1002/imhj.21554 [DOI] [PubMed] [Google Scholar]
- Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, … Reiss D (2012). Effects of parental depressive symptoms on child adjustment moderated by hypothalamic pituitary adrenal activity: Within- and between-family risk. Child Development, 84(2), 528–542. 10.1111/j.1467-8624.2012.01859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy-Warren P, & McCarthy G (2011). Maternal parental self-efficacy in the postpartum period. Midwifery, 27(6), 802–810. 10.1016/j.midw.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Letourneau N, Stewart M, Dennis C, Hegadoren K, Duffett-Leger L, & Watson B (2011). Effect of home-based peer support on maternal-infant interactions among women with postpartum depression: A randomized, controlled trial. International Journal of Mental Health Nursing, 20(5), 345–357. 10.1111/j.1447-0349.2010.00736.x [DOI] [PubMed] [Google Scholar]
- Loeber R, & Dishion T (1983). Early predictors of male delin-quency: A review. Psychological Bulletin, 93, 68–99. 10.1037/0033-2909.94.1.68 [DOI] [PubMed] [Google Scholar]
- Lovejoy M, Graczyk PA, Ohare E, & Neuman G (2000). Maternal depression and parenting behavior. Clinical Psychology Review, 20(5), 561–592. 10.1016/s0272-7358(98)00100-7 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Alpern L, & Repacholi B (1993). Disorganized infant attachment classification and maternal psychosocial problems as predictors of hostile-aggressive behavior in the pre-school classroom. Child Development, 64, 572–585. 10.2307/1131270 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Bronfman E, & Parsons E (1999). Chapter IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monographs of the Society for Research in Child Development, 64, 67–96. 10.1111/1540-5834.00034 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Bureau J-F, Easterbrooks MA, Obsuth I, Hennighausen K, & Vulliez-Coady L (2013). Parsing the construct of maternal insensitivity: Distinct longitudinal pathways associated with early maternal withdrawal. Attachment & Human Development, 15, 562–582. 10.1080/14616734.2013.841051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Connell DB, Grunebaum HU, & Botein S (1990). Infants at social risk: Maternal depression and family support services as mediators of infant development and security of attachment. Child Development, 61(1), 85–98. 10.2307/1131049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Easterbrooks MA, & Cibelli CD (1997). Infant attachment strategies, infant mental lag, and maternal depressive symptoms: Predictors of internalizing and externalizing problems at age 7. Developmental Psychology, 33(4), 681–692. 10.1037/0012-1649.33.4.681 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Zoll D, Connell D, & Grunebaum HU (1986). The depressed mother and her one-year-old infant: Environment, interaction, attachment, and infant development. New Directions for Child and Adolescent Development, 1986(34), 61–82. 10.1002/cd.23219863407 [DOI] [PubMed] [Google Scholar]
- Madigan S, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Moran G, Pederson DR, & Benoit D (2006). Unresolved states of mind, anomalous parental behavior, and disorganized attachment: A review and meta-analysis of a transmission gap. Attachment and Human Development, 8(2), 89–111. 10.1080/14616730600774458 [DOI] [PubMed] [Google Scholar]
- Martins C, & Gaffan EA (2000). Effects of early maternal depression on patterns of infant-mother attachment: A meta-analytic investigation. Journal of Child Psychology and Psychiatry, 41(6), 737–746. 10.1111/1469-7610.00661 [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliott S, & Barnett B (2006). Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale – implications for clinical and research practice. Archives of Women’s Mental Health, 9(6), 309–315. 10.1007/s00737-006-0152-x [DOI] [PubMed] [Google Scholar]
- McCartney K, Burchinal MR, & Bub KL (2006). Missing data: What to do with or without them. Best Practices in Quantitative Methods for Developmentalists, 71(3), 42–64. [DOI] [PubMed] [Google Scholar]
- Mesman J, van IJzendoorn MH, & Bakermans-Kranenburg MJ (2009). The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review, 29(2), 120–162. 10.1016/j.dr.2009.02.001 [DOI] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocorti-cal axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper C, Gariepy J, Barnett M, Moore GA, Calkins S, & Cox MJ (2009). Psychophysiological correlates of parenting behavior in mothers of young children. Developmental Psychobiology, 51(8), 650–661. 10.1002/dev.20400 [DOI] [PubMed] [Google Scholar]
- Murray D, & Cox JL (1990). Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). Journal of Reproductive and Infant Psychology, 8(2), 99–107. 10.1080/02646839008403615 [DOI] [Google Scholar]
- Murray L, Kempton C, Woolgar M, & Hooper R (1993). Depressed Mothers speech to their infants and its relation to infant gender and cognitive development. Journal of Child Psychology and Psychiatry, 34(7), 1083–1101. 10.1111/j.1469-7610.1993.tb01775.x [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, & England BG (2006). Cortisol levels and very early pregnancy loss in humans. Proceedings of the National Academy of Sciences, 103(10), 3938–3942. 10.1073/pnas.0511183103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, & Ehlert U (2006). Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. The Journal of Clinical Endocrinology and Metabolism, 91, 1329–1335. 10.1210/jc.2005-1816 [DOI] [PubMed] [Google Scholar]
- Nyström-Hansen M, Andersen MS, Khoury JE, Davidsen K, Gumley A, Lyons-Ruth K, … Harder S (2019). Hair cortisol in the perinatal period mediates associations between maternal adversity and disrupted maternal interaction in early infancy. Developmental Psychobiology, 61(4), 543–556. 10.1002/dev.21833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW, & McCabe JE (2013). Postpartum depression: Current status and future directions. Annual Review of Clinical Psychology, 9, 379–407. 10.1146/annurev-clinpsy-050212-185612 [DOI] [PubMed] [Google Scholar]
- Patterson GR, & Bank L (1989). Some amplifying mechanisms for pathologic processes in families In Gunnar MR, & Thelen E (Eds.), Systems and development (pp. 167–209). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Pechtel P, Woodman A, & Lyons-Ruth K (2012). Early maternal withdrawal and non-verbal childhood IQ as precursors for substance abuse diagnosis in young adulthood: Results of a 20-year prospective study. International Journal of Cognitive Therapy, 5, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragst F, & Balikova MA (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370(1–2), 17–49. 10.1016/j.cca.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2004). Simple intercepts, simple slopes, and regions of significance in MLR 2-way interactions. [Computer software]. [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, … Meaney MJ (2015). Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry, 5(2), e508 10.1038/tp.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva Crugnola C, Ierardi E, Ferro V, Gallucci M, Parodi C, & Astengo M (2016). Mother-infant emotion regulation at three months: The role of maternal anxiety, depression and parenting stress. Psychopathology, 49(4), 285–294. 10.1159/000446811 [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, & Van Uum S (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Saint-Georges C, Chetouani M, Cassel R, Apicella F, Mahdhaoui A, Muratori F, … Cohen D (2013). Motherese in interaction: At the cross-road of emotion and cognition? (A systematic review). PLoS ONE, 8(10), 10.1371/journal.pone.0078103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharlau F, Pietzner D, Vogel M, Gaudl A, Ceglarek U, Thiery J, … Kiess W (2018). Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress, 21(1), 43–50. 10.1080/10253890.2017.1392507 [DOI] [PubMed] [Google Scholar]
- Schechter DS, Zeanah CH, Myers MM, Brunelli SA, Liebowitz MR, Marshall RD, … Hofer MA (2004). Psychobiological dysregulation in violence-exposed mothers: Salivary cortisol of mothers with very young children pre- and post-separation stress. Bulletin of the Menninger Clinic, 68, 319–336. 10.1521/bumc.68.4.319.56642 [DOI] [PubMed] [Google Scholar]
- Seth S, Lewis AJ, & Galbally M (2016). Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: A systematic literature review. BMC Pregnancy and Childbirth, 16(1). 10.1186/s12884-016-0915-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K, & Leider CN (2011). Acoustic parameters of infant-directed singing in mothers with depressive symptoms. Infant Behavior and Development, 34(2), 248–256. 10.1016/j.infbeh.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Forbes EE, Lane TL, & Kovacs M (2006). Maternal depression and child internalizing: The moderating role of child emotion regulation. Journal of Clinical Child & Adolescent Psychology, 35(1), 116–126. 10.1207/s15374424jccp3501_10 [DOI] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, & Lightman SL (2014). HPA Axis-Rhythms. Comprehensive Physiology, 1273–1298. 10.1002/cphy.c140003 [DOI] [PubMed] [Google Scholar]
- Stalder T, & Kirschbaum C (2012). Analysis of cortisol in hair – State of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, … Fischer JE (2013). Cortisol in hair and the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 98(6), 2573–2580. 10.1210/jc.2013-1056 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, … Miller R (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. 10.1016/j.psyneuen.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Stanley C, Murray L, & Stein A (2004). The effect of postnatal depression on mother–infant interaction, infant response to the still-face perturbation, and performance on an instrumental learning task. Development and Psychopathology, 16(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, & van Rossum EF (2013). Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology, 38(8), 1220–1235. 10.1016/j.psyneuen.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Tarullo AR, & St. John AM, & Meyer JS (2017). Chronic stress in the mother-infant dyad: Maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant Behavior & Development, 47, 92–102. 10.1016/j.infbeh.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LA, & Trevathan WR (2008). Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behavior & Development, 31(1), 92–106. 10.1016/j.infbeh.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta C, & Shaw D (2008). Maternal predictors of rejecting parenting and early adolescent antisocial behavior. Journal of Abnormal Child Psychology, 36(2), 247–259. 10.1007/s10802-007-9174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, & Brazelton TB (1978). The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child & Adolescent Psychiatry, 17, 1–13. 10.1016/S0002-7138(09)62273-1 [DOI] [PubMed] [Google Scholar]
- Tronick E, & Reck C (2009). Infants of depressed mothers. Harvard Review of Psychiatry, 17(2), 147–156. 10.1080/10673220902899714 [DOI] [PubMed] [Google Scholar]
- Vaever MS, Krogh MT, Smith-Nielsen J, Harder S, & Køppe S (2013). Measuring spatial proximity in mother–infant interaction: A kinematic approach for an examination of the effects of maternal postpartum depression. Infant Behavior and Development, 36(3), 427–431. 10.1016/j.infbeh.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Wei J, Sun G, Zhao L, Yang X, Liu X, Lin D, … Ma X (2015). Analysis of hair cortisol level in first-episodic and recurrent female patients with depression compared to healthy controls. Journal of Affective Disorders, 175, 299–302. 10.1016/j.jad.2015.01.023 [DOI] [PubMed] [Google Scholar]
- Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, … Qiu A (2017). Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Translational psychiatry, 7(4), e1103–e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennig R (2000). Potential problems with the interpretation of hair analysis results. Forensic science international, 107(1–3), 5–12. [DOI] [PubMed] [Google Scholar]
- Wolf IAC, Gilles M, Peus V, Scharnholz B, Seibert J, Jennen-Steinmetz C, … Laucht M (2018). Impact of prenatal stress on mother-infant dyadic behavior during the still-face paradigm. Borderline personality disorder and emotion dysregulation, 5(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthke W (2000). Longitudinal and multi-group modeling with missing data In Little TD, Schnabel K, & Baumert J (Eds.), Modeling longitudinal and multiple group data: Practical issues, applied approaches and specific examples (pp. 269–281). Mahwah, NJ: Erlbaum. [Google Scholar]


