Abstract
BACKGROUND:
Major prevention trials for Alzheimer’s disease (AD) are now focusing on multidomain lifestyle interventions. However, the exact combination of behavioral factors related to AD pathology remains unclear. In 2 cohorts of cognitively unimpaired individuals at risk of AD, we examined which combinations of personality traits, neuropsychiatric symptoms, and cognitive lifestyle (years of education or lifetime cognitive activity) related to the pathological hallmarks of AD, amyloid-β. and tau deposits.
METHODS:
A total of 115 older adults with a parental or multiple-sibling family history of sporadic AD (PREVENT-AD [PRe-symptomatic EValuation of Experimental or Novel Treatments for AD] cohort) underwent amyloid and tau positron emission tomography and answered several questionnaires related to behavioral attributes. Separately, we studied 117 mutation carriers from the DIAN (Dominant Inherited Alzheimer Network) study group cohort with amyloid positron emission tomography and behavioral data. Using partial least squares analysis, we identified latent variables relating amyloid or tau pathology with combinations of personality traits, neuropsychiatric symptoms, and cognitive lifestyle.
RESULTS:
In PREVENT-AD, lower neuroticism, neuropsychiatric burden, and higher education were associated with less amyloid deposition (p = .014). Lower neuroticism and neuropsychiatric features, along with higher measures of openness and extraversion, were related to less tau deposition (p = .006). In DIAN, lower neuropsychiatric burden and higher education were also associated with less amyloid (p = .005). The combination of these factors accounted for up to 14% of AD pathology.
CONCLUSIONS:
In the preclinical phase of both sporadic and autosomal dominant AD, multiple behavioral features were associated with AD pathology. These results may suggest potential pathways by which multidomain interventions might help delay AD onset or progression.
Keywords: Alzheimer’s, PET, Prevention, Reserve, Resistance, Risk factors
Given the limited successes of pharmacological treatments for Alzheimer’s disease (AD), attention has shifted toward risk or protective factors that might prevent or postpone disease onset (1, 2). Estimates suggest that a multidomain lifestyle intervention that achieved a 10% reduction in risk factors could prevent more than a million cases worldwide (3). The mechanisms that link protective factors and AD risk are not well understood, but current notions of resilience and resistance may be helpful (4). While “resilience” refers to the preservation of cognitive abilities in the presence of AD pathology, “resistance” refers to avoidance of the pathology in the first place (5,6). These concepts, which are not mutually exclusive, have been tested in the sporadic form of the disease, given the causative genetic mutation in autosomal dominant AD (ADAD). Here, we describe investigations in both disease forms of the relationships between several personality and behavioral features associated with AD risk and presence of AD pathology. Such relationships, tested in asymptomatic individuals, might suggest sources of resistance pathway, thereby hinting at modifiable pathways to postpone manifestation of brain pathology.
In sporadic AD, as much as a third of AD risk appears to be related to modifiable factors such as level of education, depression, and cognitive or physical activity (1, 7). Education and midlife cognitive activity have been associated with lower levels of pathology in the preclinical phase of the disease and with increased resilience to pathology in later stages (8,9). Neuropsychiatric symptoms like depression and apathy are known to increase over the course of the disease (10–12). While some such features are likely a consequence of the disease, midlife neuropsychiatric symptoms have been associated with increased AD risk in later life (13). Personality traits like neuroticism and conscientiousness have also been associated with cognitive decline and risk of sporadic AD (14, 15). Admittedly, personality traits may change as a consequence of the disease process, but a recent study showed that personality traits in adolescence-a time when AD pathology is unlikely-are associated with incident dementia 54 years later (16). Furthermore, personality traits usually remain stable in the early stages of the disease (17).
Fewer studies have explored the associations between behavioral/personality features and AD risk in ADAD. Higher resilience has been noted in individuals having higher levels of education, using the estimated years to symptom onset as a proxy for disease severity (18). Less physical activity and lower levels of education have also been associated with increased AD pathology and cognitive decline in preclinical ADAD (19–21). While personality has been studied less in ADAD, neuropsychiatric symptoms such as depression and anxiety have been found to remain stable in asymptomatic individuals but to increase in individuals with cognitive impairment (22). When compared with noncarriers, asymptomatic ADAD mutation carriers have been found to exhibit fewer depressive symptoms (22).
During the presymptomatic phase of either disease, individuals remain cognitively normal despite their accumulation of AD pathological hallmarks, amyloid-β (Aβ) and tau proteins (23,24). This silent phase, which can span more than 2 decades, represents an ideal window of opportunity for preventive strategies (25). Given the complex etiology of AD, targeting multidomain factors is rapidly becoming the norm in prevention trials (26). We therefore used multivariate analyses to investigate combinations of personality traits, neuropsychiatric symptoms, and cognitive lifestyle in relation to Aβ and tau deposition in cognitively normal older adults at increased risk of sporadic or autosomal dominant forms of AD (mutation carriers; for the latter, Aβ only). We expected to find similar associations in both disease forms, but perhaps weaker associations in ADAD, given the latter’s overwhelming genetic diathesis. We reasoned that discovery of such associations in the asymptomatic phase of the disease could suggest that preventive behavioral interventions may be useful in at-risk persons that are still free from pathology.
METHODS AND MATERIALS
Participants
We studied 232 cognitively unimpaired participants, including 115 individuals at risk of sporadic AD from the PRe-symptomatic EValuation of Experimental or Novel Treatments for AD (PREVENT-AD) study and 117 asymptomatic individuals with ADAD from the Dominant Inherited Alzheimer Network (DIAN) study group. PREVENT-AD enrolls older adults having intact cognition but a parent or 2 siblings diagnosed with AD-like dementia, who are therefore at increased risk of sporadic AD (27). Participants were above 60 years of age, or between 55 and 59 if their age was fewer than 15 years from their parent’s age of symptom onset. Participants were free of major neurological and psychiatric diseases at enrollment. Inclusion criteria included intact cognition based on the Montreal Cognitive Assessment (score above 25) (28) and a 45-minute standardized neuropsychological evaluation using the Repeatable Battery for the Assessment of Neuropsychological Status (29). The cognitive status of individuals with questionable neuropsychological status was reviewed in consensus meetings of neuropsychologists (including SV) and/or psychiatrists (including JCSB). Only participants with Aβ positron emission tomography (PET), tau PET, and data on behavioral factors were included, resulting in 115 participants (out of 324 active PREVENT-AD participants as of May 2019). The DIAN study group enrolls individuals over 18 years old with a family history of ADAD. We selected mutation carriers who were cognitively normal as evidenced by Clinical Dementia Rating (30) score of 0 and who had Aβ PET and behavioral data available. Those studied comprised 117 participants (85 PSEN1 mutation carriers, 17 PSEN2 mutation carriers, and 15 APP mutation carriers) out of 146 mutation carriers archived in the DIAN data freeze of May 2016.
Behavioral Factors
All participants filled out questionnaires to assess various behavioral factors plausibly related to AD risk (Supplemental Table S1). For ease of interpretation, we grouped these factors into 3 categories: “Big Five” personality traits (neuroticism, openness, extraversion, agreeableness, conscientiousness), neuropsychiatric symptoms (depression, anxiety, stress, apathy), and features of cognitive lifestyle (years of education, lifetime cognitive activity). In PREVENT-AD, all questionnaires were answered at home 6 months to a year before PET (mostly electronically, but 10% responded by paper version). Follow-up questionnaires were sent to participants every year or so, resulting in 3 time points for neuropsychiatric symptoms (2016, 2017, 2018) and 2 for personality (2016 and 2018). Intraclass correlation coefficients and their 95% confidence intervals, based on absolute agreement in 2-way mixed-effects models, were computed using SPSS, version 20 (IBM Corp., Armonk, NY) (31). Supplemental Figures S1 and S2 display correlations between these scores at the different time points.
In DIAN, all questionnaires were answered at the baseline visit, which also included Aβ PET. The DIAN personality questionnaire (International Personality Item Pool Representation of the Revised NEO Personality Inventory [IPIP-NEO-120]) (32) was more detailed than the Big Five Inventory used in PREVEN-AD and yielded scores on 30 personality facets along with the Big Five personality domains. The 30 facets were used only in complementary analyses.
Image Acquisition
PREVENT-AD participants underwent PET using [18F]NAV4694 to assess Aβ burden and flortaucipir ([18F]AV1451) to assess tau deposition. DIAN participants underwent Aβ PET only using Pittsburgh compound B ([11C]PIB). A T1-weighted structural image was also acquired using a similar magnetization prepared rapid acquisition gradient-echo sequence in both studies (greater detail available in the Supplement).
Image Processing
Both PREVEN-AD and DIAN scans were processed locally using the same pipeline (see https://github.com/villeneuvelab/vlpp for more details and the Supplement for parameters used). Aβ and tau PET images were registered to the T1-weighted scan of each participant, which had been segmented with the Desikan-Killiany atlas using FreeSurfer version 5.3 (33). Images were then masked to remove the scalp and cerebrospinal fluid, to reduce contamination by non-gray and non-white matter voxels. In PREVENT-AD, PET images were smoothed with a Gaussian kernel of 6 mm. Standardized uptake value ratios (SUVRs) were obtained using the whole cerebellum as reference region for Aβ PET (34) and the inferior cerebellar gray matter for tau PET (35). DIAN PET images were smoothed with a Gaussian kernel of 8 mm to diminish multisite effect (36), and Aβ PET SUVRs were obtained using the whole cerebellum as reference region. Mean SUVR from the left and right hemispheres in each Desikan-Killiany region was used for further analyses. The frontal pole region was excluded owing to weaker registration to the structural scan. Only a subset of sensitive regions was included for each modality in the analyses. For Aβ. bilateral SUVRs in the lateral and medial prefrontal, parietal, lateral temporal, and cingulate cortical regions were included in multivariate analyses because these are key regions of Aβ deposit in the preclinical and clinical phases of AD (37,38). The weighted average across all these regions is referred to here as global Aβ index SUVR (38,39) and used in univariate analyses. For tau PET, bilateral SUVRs in the regions of Braak stages I (entorhinal cortex), III, and IV were included in the multivariate analysis since those stages capture regions up to early tau accumulation (40,41) (Supplemental Table S2). Average SUVR in separate Braak stages was also computed and used in univariate analyses. Braak stage II (hippocampus) was excluded, however, owing to signal contamination from the choroid plexus (42), and regions of Braak stages V and VI were also excluded, given that they represent later stages of AD progression (43,44).
Statistical Analyses
Univariate Analyses.
We first estimated univariate parametric correlations between each individual behavioral feature and pathology. We used global Aβ index SUVR in both PREVENT-AD and DIAN. Tau SUVR in Braak stages I, III, and IV was used in PREVENT-AD. Also, to evaluate the extent to which behavioral features were related to one another, we calculated the parametric correlation between all factors. We considered p values < .05 significant. Associations surviving false discovery rate (FDA) of 5% are also reported to account for multiple comparisons.
Multivariate Analyses.
The main statistical approach was partial least squares (PLS) analysis (45,46), implemented using PLS Software v6.15.1 (https://www.rotman-baycrest.on.ca/index.php?section=84) on MATLAB (The MathWorks, Inc., Natick, MA) v2016a. This approach allowed investigation of relationships between combinations of behavioral factors and AD pathology across the brain. PREVENT-AD permitted 2 PLS analyses, relating these behavioral features with Aβ and tau independently. Two PLS analyses were also performed in DIAN: the primary analysis relating similar behavioral features with Aβ and a complementary one further detailing personality after including the 30 personality facets available exclusively in DIAN.
Figure 1 explains these analyses (greater detail is available in the Supplement). Briefly, PLS finds linear combinations of 2 sets of variables (organized in 2 matrices) that correlate maximally with each other. The first matrix enters the behavioral factors in columns with entries corresponding to the score on the various questionnaires; the rows correspond to individual participants. The behavioral data were z scored columnwise since all questionnaires were on different scales. The second matrix contains either regional Aβ or tau SUVR in columns and rows corresponding to participants. The output from the PLS analyses are sets of latent variables relating behavioral features and AD pathology. The number of latent variables is equal to the smallest dimension of the matrices, here the number of behavioral factors. Permutation tests were used to identify which latent variables were significant, with p value < .05 being considered significant. The latent variables are a triplet of 1) a singular value, 2) a vector of weights attributed to each behavioral factor, and 3) a vector of weights attributed to the various cortical regions. In the significant latent variable(s), bootstrap resampling was used to identify the most stable features and brain regions contributing to the behavioral factors-pathology relationship. Lastly, the vectors of weights from each behavioral factor and each brain region were multiplied by the original data of each participant. These 2 values correspond to each participant’s weighted score of behavioral factors and weighted score of pathology. Correlating these 2 scores across participants provided an estimate of the strength of the multivariate relationship between the behavioral and pathology features.
Figure 1.
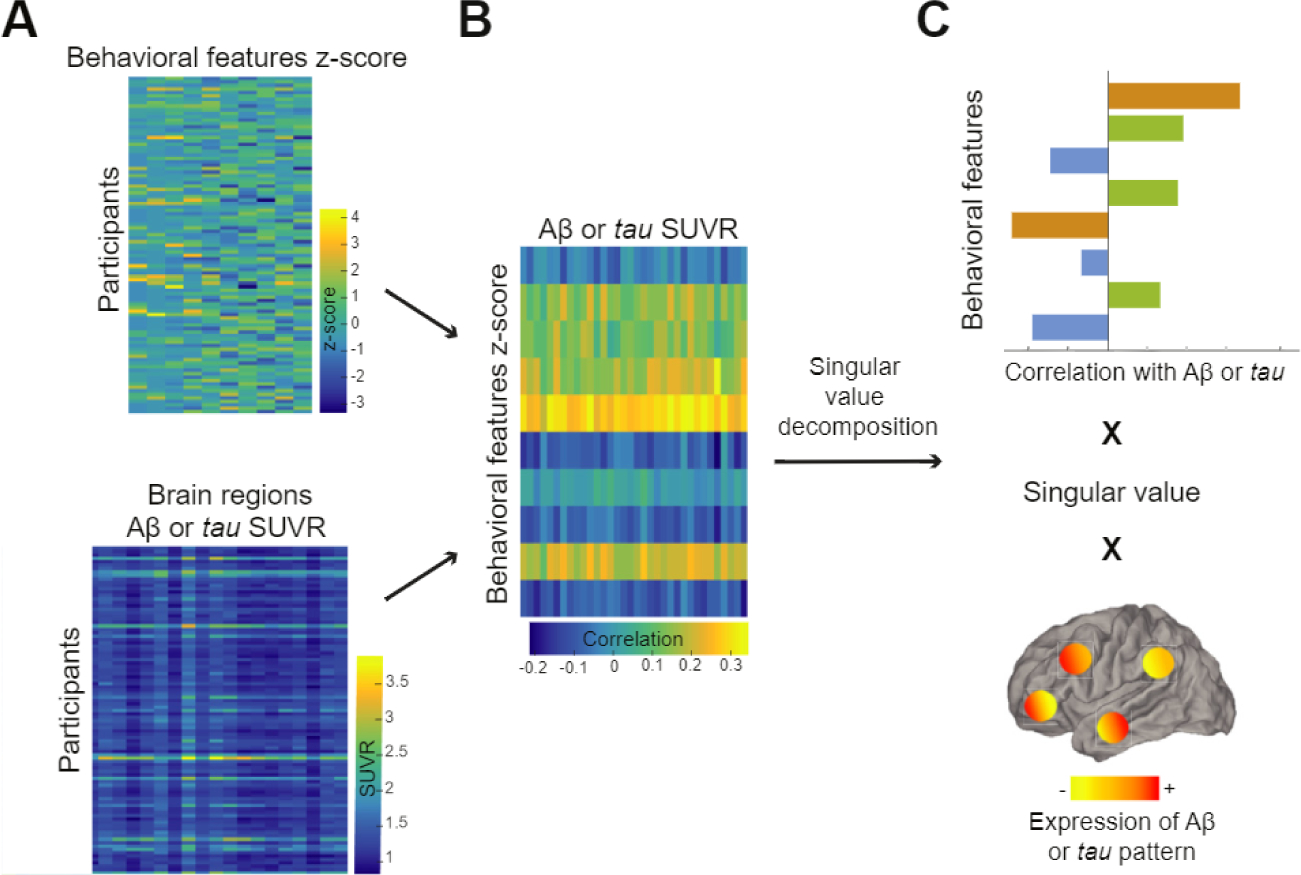
Partial least squares analysis finds maximally correlated linear combinations of two input matrices, one with behavioral features (top matrix in A) and the other with Alzheimer’s disease pathology across defined cortical regions (bottom matrix in A). These two matrices are then correlated together, and this latter matrix (B) is decomposed into multiple latent variables using singular value decomposition. (C) An example of a latent variable. Briefly, each latent variable consists of a singular value (related to the covariance between the 2 input matrices) and 2 vectors of weights representing how much each behavioral feature and each brain region contribute the overall multivariate relationship. Aβ. amyloid-β; SUVR, standardized uptake value ratio.
Complementary Analyses.
One complementary question is whether behavioral factors influence AD pathology, pathology influences behavioral factors, or these relationships are bidirectional. This question is particularly relevant for neuropsychiatric symptoms, inasmuch as education level and lifetime cognitive activity typically precede AD pathology and personality traits generally remain stable over time, even in individuals with AD-related cognitive impairment (15). Longitudinal PET scans will be needed to address this question more fully. Nonetheless, we sought to take advantage of 3-year follow-up for neuropsychiatric symptoms and evaluated whether Aβ (global Aβ index SUVR) and tau (entorhinal tau SUVR) were associated with change in neuropsychiatric symptom scores. To do this, we used linear mixed-effects models having random slope and intercept, in which a time by-Aβ or time-by-tau SUVR interaction predicted longitudinal neuropsychiatric symptom scores. These mixed-effects analyses used the R package lme4 version 1.1–15 (Vienna, Austria).
RESULTS
Sample characteristics are detailed in Table 1. Information on cognitive data in both cohorts is available in Supplemental Tables S3 and S4.
Table 1.
Participants’ Demographics and Behavioral Features
| Demographics and Behavioral Features | PREVENT-AD (n = 115) | DIAN (n = 117) |
|---|---|---|
| Age, Years | 67.6 ± 5.0 (58.6 to 83.3) | 34.6 ± 9.4 (18.0 to 61.0) |
| Estimated Years to Onseta | −5.7 ± 7.8 (−20.8 to 16.8) | −12.9 ± 8.0 (−31.5 to 11.8) |
| Gender, F:M, n (%F) | 86:29 (75%) | 64:53 (55%) |
| APOE ε4 Carriers, n (%) | 44 (38%) | 36 (31%) |
| Global Aβ SUVRb | 1.1 ± 0.3 (0.9 to 2.3) | 0.9 ± 0.2 (0.8 to 1.6) |
| Tau Braak I SUVR | 1.1 ± 0.1 (0.7 to 1.7) | - |
| Tau Braak III SUVR | 1.2 ± 0.1 (0.8 to 1.7) | - |
| Tau Braak IV SUVR | 1.1 ± 0.1 (0.9 to 1.6) | - |
| MMSE | 28.8 ± 1.3 (24 to 30) | 29.1 ± 1.2 (24 to 30) |
| Cognitive Lifestyle | ||
| Education, years | 15.0 ± 3.2 (7.0 to 22.0) | 15.2 ± 3.0 (10.0 to 24.0) |
| Lifetime cognitive activity | 2.6 ± 0.7 (1.2 to 4.4) | - |
| Neuropsychiatric Symptoms | ||
| Depression | 1.3 ± 1.9 (0 to 10.0) | 1.5 ± 1.8 (0 to 9.0) |
| Anxiety | 2.1 ± 3.6 (0 to 18.0) | - |
| Stress | 4.7 ± 5.2 (0 to 24.0) | - |
| Apathy | 27.8 ± 6.2 (18.0 to 46.0) | - |
| NPI-Q | - | 0.7 ± 1.8 (0 to 11.0) |
| Personalityc | ||
| Openness | 38.9 ± 6.5 (21.0 to 50.0) | 79.5 ± 11.8(49.0 to 107.0) |
| Neuroticism | 17.6 ± 6.1 (8.0 to 35.0) | 60.1 ± 13.8 (31.0 to 94.0) |
| Conscientiousness | 37.4 ± 5.4 (19.0 to 45.0) | 96.0 ± 12.3 (67.0 to 120.0) |
| Agreeableness | 39.2 ± 4.0 (26.0 to 45.0) | 95.8 ± 10.2 (62.0 to 115.0) |
| Extraversion | 26.7 ± 5.6 (14.0 to 40.0) | 85.6 ± 12.1 (47.0 to 109.0) |
Data presented as mean ± SD (range) unless noted otherwise.
Aβ, amyloid-β; DIAN, Dominant Inherited Alzheimer Network; F, female; M, male; MMSE, Mini-Mental State Evaluation; NPI-Q, Neuropsychiatric Inventory Questionnaire; PREVENT-AD, PRe-symptomatic EValuation of Experimental or Novel Treatments for AD; SUVR, standardized uptake value ratio.
For PREVEN-AD participants, data are sporadic estimated years to onset (data available for 111 participants). For DIAN participants, data are estimated years to onset (age of participant - age of the parent at symptom onset).
[18F]NAV4694 is used in PREVENT-AD and [11C]PIB is used in DIAN.
Personality traits are assessed with the Big Five Inventory in PREVENT-AD and the International Personality Item Pool Representation of the Revised NEO Personality Inventory in DIAN, and the 2 questionnaires have different scales.
Univariate Relationship Between Behavioral Features and Aβ and Tau
In PREVENT-AD, only neuroticism was related the global Aβ index SUVR, with higher scores on neuroticism related with higher Aβ deposition (R = .21, p = .02, but does not survive FOR correction; Supplemental Table S5). Tau SUVR in Braak I (entorhinal cortex) was related with different behavioral features (personality traits, apathy, and lifetime cognitive activity [R = .21–.34, p < .001–.02]), while only lifetime cognitive activity was related to tau SUVR in Braak III or IV (Supplemental Table S5). All associations with tau SUVR in Braak I survived FOR correction.
In DIAN, fewer behavioral features were available for analyses (8 rather than 11), and some of the questionnaires differed from those in PREVENT-AD (Supplemental Table S1). Higher level of education correlated with lower global Aβ index (R = −.19, p = .04, but does not survive FDR correction; Supplemental Table S5). Of note, mutation type had virtually no effect on behavioral features (the only difference being that PSEN2 mutation carriers had lower extraversion scores than PSEN1 carriers in post hoc testing, p = .04). There was also no difference on any behavioral features between asymptomatic mutation carriers and 127 noncarriers.
Intercorrelation Among Behavioral Features
Intercorrelations among behavioral features revealed associations between about half of the features in PREVENT-AD (Figure 2A). The neuropsychiatric symptoms were themselves intercorrelated, and neuropsychiatric symptoms were (unsurprisingly) associated mainly with higher neuroticism and lower extraversion. Furthermore, education, cognitive activity, and openness were positively correlated with one another. In DIAN, more years of education was also associated with increased openness, and intercorrelations were found between different personality traits (Figure 2B). These numerous intercorrelations suggest that a wide variety of behavioral features relate to one another, thus justifying our decision to investigate them in combination.
Figure 2.
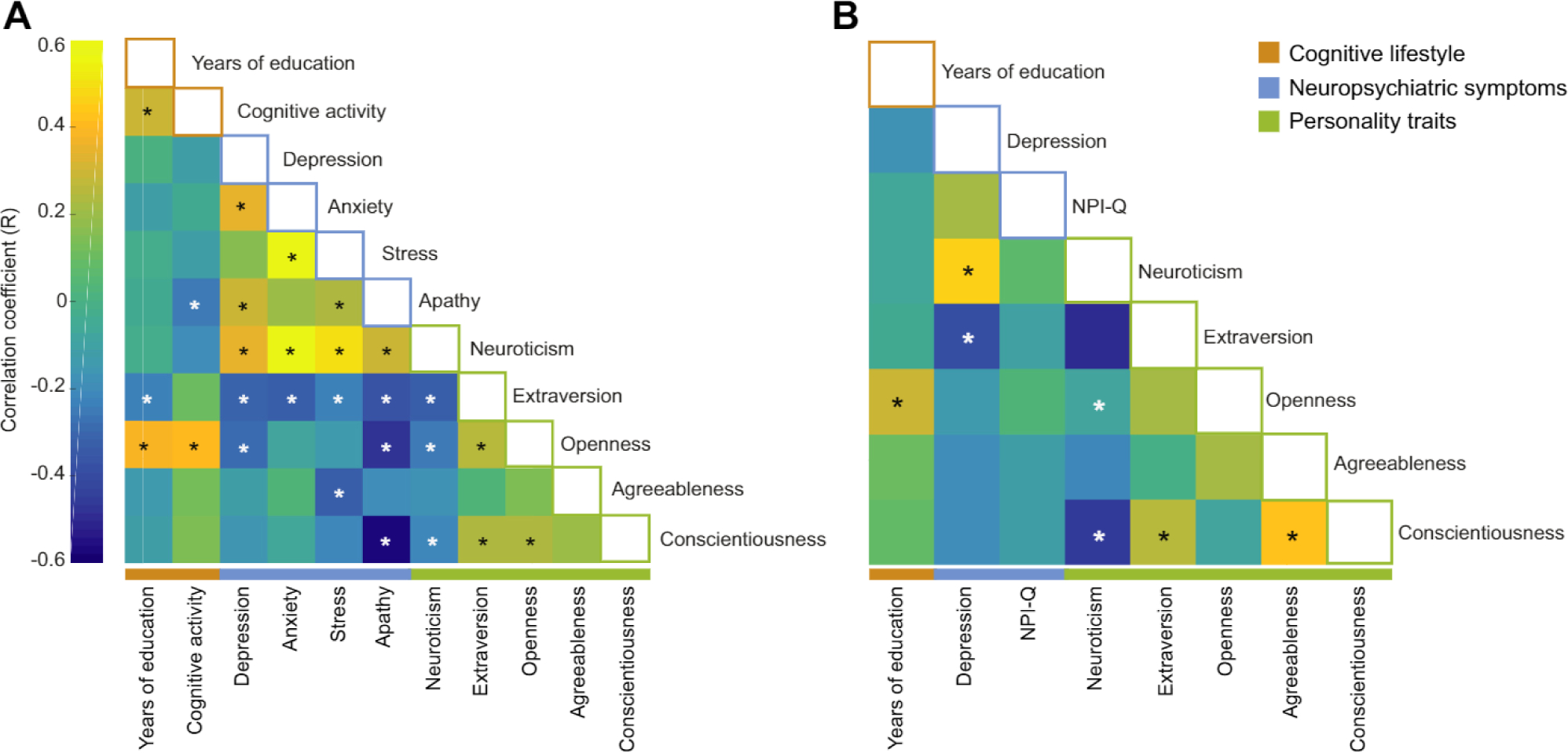
Correlations between behavioral features in both cohorts. Intercorrelation (Pearson correlation) between behavioral factors in PRe-symptomatic EValuation of Experimental or Novel Treatments for AD (PREVENT-AD) (A) and Dominant Inherited Alzheimer Network (DIAN) (B) study groups. White stars correspond to negative correlations and black stars to positive correlations that remained significant after false discovery rate correction. NPI-Q, Neuropsychiatric Inventory Questionnaire.
Relationship of AD Pathology With Multidomain Behavioral Features
In PREVENT-AD there was one significant latent variable relating behavioral features with Aβ (p = .014, 95% of the PLS variance being explained by this variable). Figure 3A displays the different weights of the behavioral features and brain regions that form this latent variable. A combination of lower neuroticism, anxiety, and apathy along with higher education and openness were the features that were most strongly associated with lower Aβ burden. All regions of the global Aβ index contributed to the relationship. The correlation between the weighted scores of the behavioral features and of regional Aβ pathology across participants was R = .23, p = .013, accounting for 5.3% of the Aβ variance.
Figure 3.
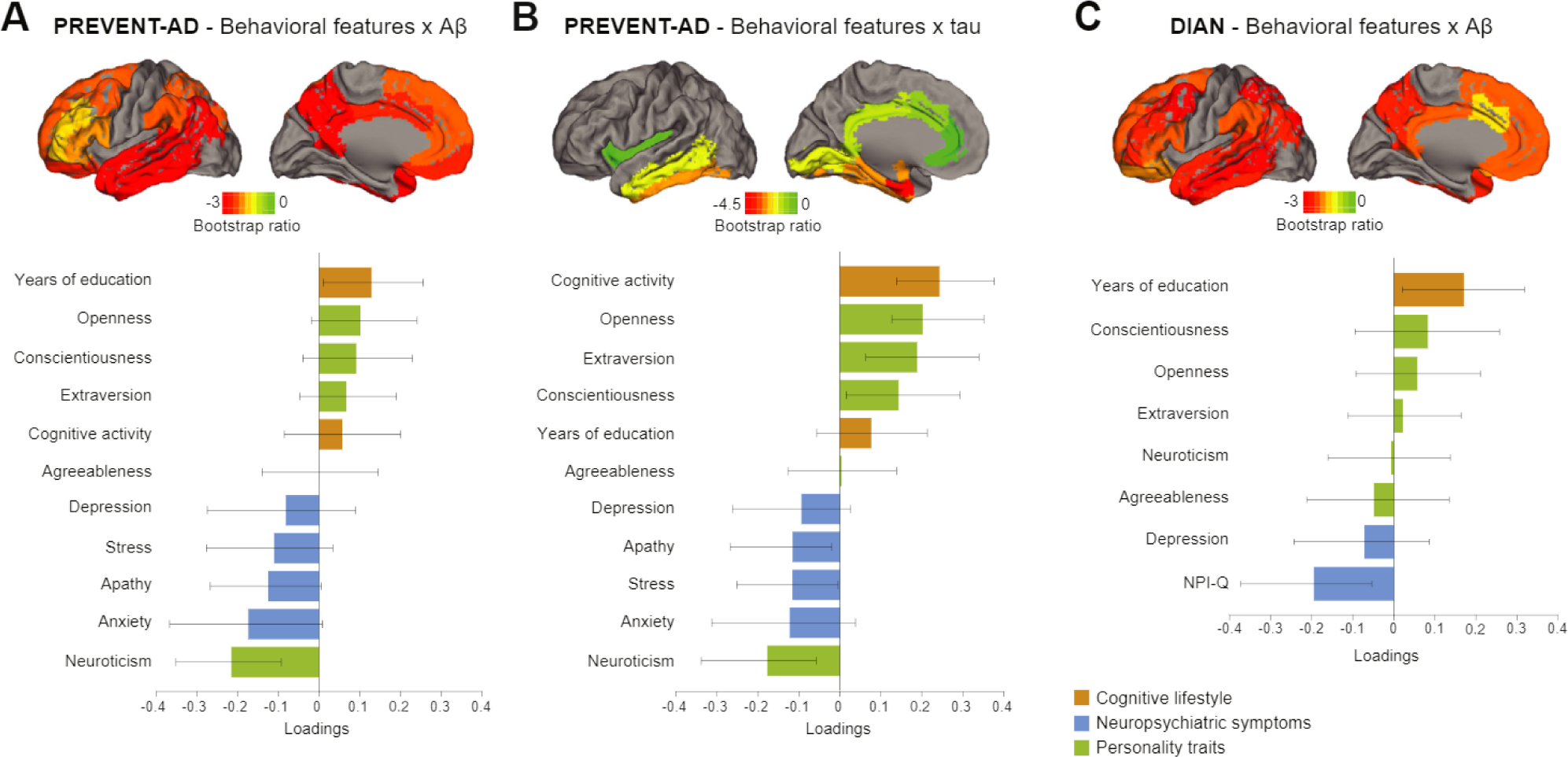
Latent variables from partial least squares analysis relating behavioral features and Alzheimer’s disease (AD) pathology in both cohorts. Results from the different partial least squares analyses representing which combinations of behavioral features relate to amyloid-β (Aβ) pathology in PRe-symptomatic EValuation of Experimental or Novel Treatments for AD (PREVENT-AD) study group (A), tau pathology in PREVENT-AD study group (B), and Aβ pathology in Dominant Inherited Alzheimer Network (DIAN) study group (C). Bar graphs represent the weight of each behavioral feature to the multivariate relationship. Confidence intervals are derived from bootstrap resampling. All brain regions included in the partial least squares analyses are projected on the brains. Bootstrap ratios correspond to the importance of each region to the behavioral feature-pathology relationship. NPI-Q, Neuropsychiatric Inventory Questionnaire.
The multivariate analysis with tau also revealed one latent variable relating behavioral features with regional tau SUVR (p = .006, 82% of the PLS variance was explained by this variable). Figure 3B displays the different weights of the behavioral features and brain regions forming this latent variable. Almost all behavioral variables contributed to this relationship, with a combination of higher scores on openness and extraversion, higher cognitive activity, lower neuropsychiatric symptoms, and neuroticism being related to less tau burden. The top region related to behavioral features was the entorhinal cortex (Braak I), followed by others in the medial and lateral temporal lobe. Regions outside the temporal lobe did not contribute, which is in keeping with the known deposition pattern of tau in the asymptomatic phase of AD (40,47). The correlation between the weighted scores of the behavioral features and regional tau pathology across participants was R = .29, p = .002, accounting for 8.4% of variance explained.
In DIAN, one latent variable related behavioral features with Aβ (p = .005, 91% of the PLS variance explained; Figure 3C). More years of education and a lower score on the Neuropsychiatric Inventory Questionnaire were the factors that related most strongly to lower Aβ burden. All regions included in the global Aβ index contributed to this relationship. The correlation between weighted scores of the behavioral features and regional Aβ pathology across participants was R = .26, p = .005, accounting for 6.7% of variance explained. To obtain a more fine-grained picture of these associations in DIAN, the PLS was repeated, substituting the Big Five personality traits with the 30 personality facets. Again, one latent variable (p = .004, 88% of PLS variance explained) related behavioral features and Aβ. Higher intellect (a facet of the openness trait), along with more years of education and a low score on Neuropsychiatric Inventory Questionnaire, were related to lower Aβ burden (Figure 4). The correlation between the weighted scores of the behavioral features and pathology was R = .37, p < .001, accounting for 14% of the variance.
Figure 4.
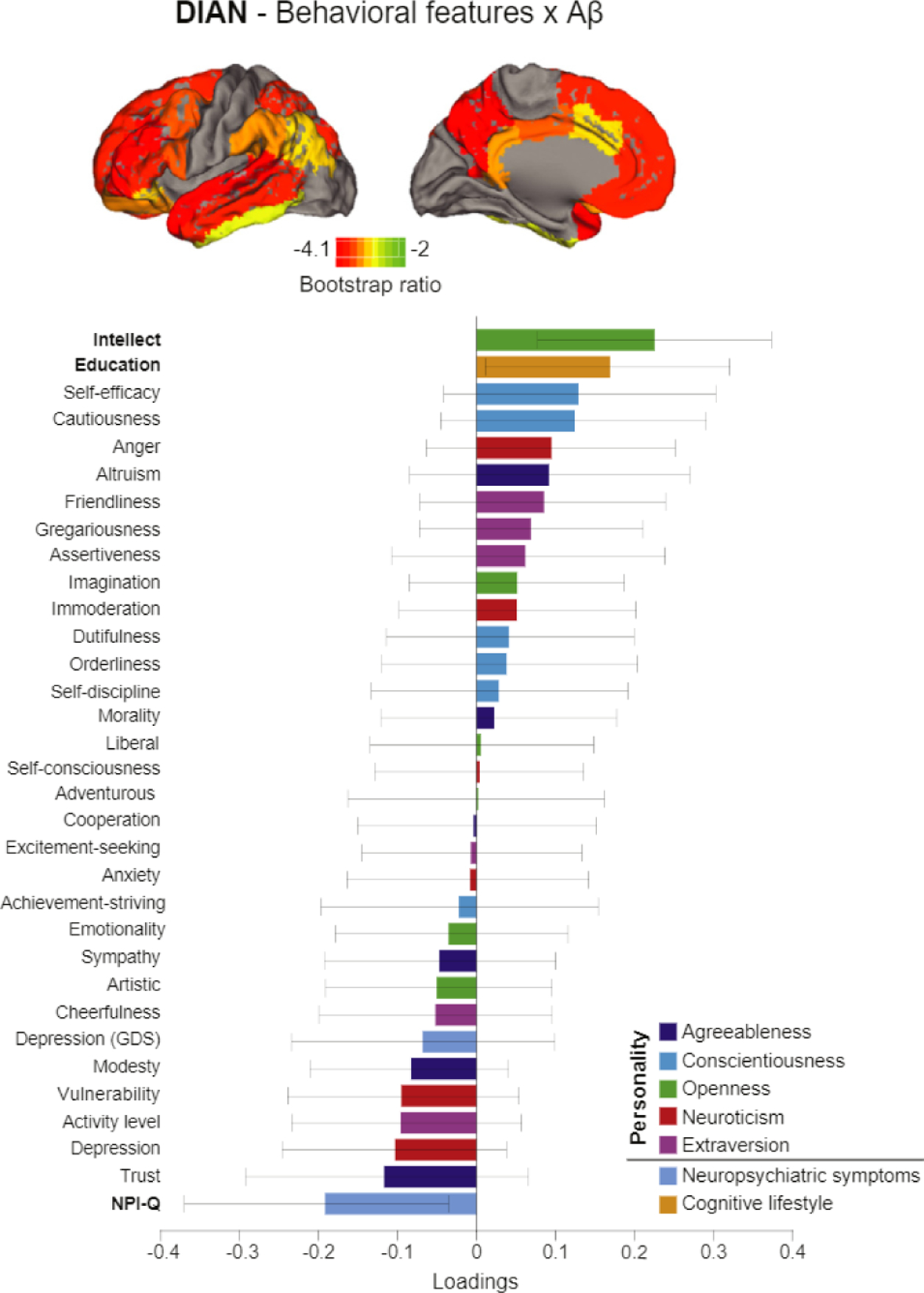
Latent variable from partial least squares analysis relating personality facets and behavioral features with amyloid-β (Aβ) in Dominant Inherited Alzheimer Network (DIAN) study group. Result from the partial least squares analysis relating behavioral features including the 30 personality facets and AP pathology across brain regions in DIAN. Bar graphs represent the weight of each behavioral feature to the multivariate relationship. Confidence intervals are derived from bootstrap resampling. All brain regions included in the analysis are projected on the brain. Bootstrap ratios correspond to the importance of each region to the behavioral feature-pathology relationship. GDS, Geriatric Depression Scale; NPI-Q, Neuropsychiatric Inventory Questionnaire.
Stability Over Time of Behavioral Features in PREVENT-AD
All analyses presented thus far included the behavioral feature assessments nearest in time to the PET scans. We also evaluated the stability of such self-reported questionnaire responses, taking advantage of the longitudinal assessment of 3 years for neuropsychiatric symptoms and 2 years for personality (Supplemental Figures S1 and S2). Education and lifetime cognitive activity were only assessed once as they are typically fixed. Overall, there was moderate stability of neuropsychiatric symptoms over 3 years (intraclass correlation coefficient between 0.55 and 0.73; Supplemental Figure S1) and, predictably, better stability of personality traits over 2 years (intraclass correlation coefficient between 0.76 and 0.81; Supplemental Figure S2). Using the 3-year data available on neuropsychiatric symptoms, we also found no apparent influence of the level of Aβ or tau on change of neuropsychiatric symptoms over time (Supplemental Table S6; only the relationship of tau and stress had a p value of .03, but this did not survive correction for multiple comparisons).
DISCUSSION
It has been estimated that up to 35% of AD risk is modifiable by health and behavioral factors such as physical health, psychological health, education, and cognitive activity (1, 7). Beyond these factors, facets of personality, such as neuroticism (15), and other behaviors, such as sleep dysregulation (48), have also been associated with a risk of AD, suggesting that even more than 35% of AD risk may be modifiable. Working in the asymptomatic stage of the sporadic and autosomal dominant forms of AD, we tested whether combinations of multidomain behavioral features were related to AD pathology and to what extent. In cognitively unimpaired late-middle-aged individuals at increased risk of sporadic AD, several combinations of factors encompassing personality traits, neuropsychiatric symptoms, and cognitive lifestyle were related to Aβ and tau deposition in the brain. In asymptomatic ADAD mutation carriers, education and psychiatric symptoms were related to Aβ. Across analyses, the variance explained from behavioral feature-pathology relationships ranged from 5% to 14%. Although this might appear modest, reduction of AD risk factors by such percentages could have a major impact on future disease prevalence, preventing millions of cases (3).
In sporadic AD, personality traits had been described previously as being related to the incidence of dementia (14, 15). Little was known, however, about associations with Aβ and tau pathology in the earliest phases of the disease (49,50). In PREVENT-AD, a higher score on neuroticism was among the key factors related to the presence of both pathologies. Our results are in accord with the aforementioned studies in which neuroticism, characterized by negative emotions (51), is the dominating trait associated with increased risk of AD. Neuropsychiatric symptoms-which are correlated with neuroticism-were also associated with Aβ and tau burden. Other personality traits such as openness and extraversion also related to tau pathology in both univariate and multivariate analyses.
Our results add to an abundant literature reporting increased prevalence of neuropsychiatric symptoms with disease progression (52–55) and suggest that neuropsychiatric features may be related to pathology even in cognitively normal individuals (56–58). Given that our findings are only correlational and that pathology accumulates over many years, reverse causality is also possible (i.e., that pathology has already affected the magnitude of neuropsychiatric symptoms even in cognitively unimpaired individuals). Neuropsychiatric symptoms are frequent in individuals with dementia (52,55), and at that late disease stage they are most certainly a consequence of the disease. Longer follow-up and longitudinal PET scans will be needed to clarify which behavioral features cause, and which are a consequence of, AD pathology. By contrast, given that personality traits are abiding characteristics of an individual, we postulate that they are probably true risk factors of the disease. Clarifying such relationships might help target the right factors at the optimal time for prevention.
In DIAN mutation carriers, the main factors related to Aβ deposition were fewer years of education, lower scores on the intellect personality facet, and higher neuropsychiatric symptom burden. Here, personality traits did not appear to be driving factors related to the pathology. The importance of personality traits in sporadic AD might be due to a lifelong effect of personality, which influences lifestyle choices and how one copes with situations throughout life, eventually affecting pathology accumulation in old age. DIAN mutation carriers, being much younger, may not exhibit such an effect of personality traits on Aβ burden. This idea remains in line with recent studies suggesting the influence of lifestyle factors such as physical activity and education on (later) AD progression in the presence of a fully penetrant genetic mutation (19).
Perhaps importantly, the current work assesses multiple behavioral features in the same analytic design. As shown in Figure 2, many behavioral features are, in fact, highly correlated. The net sum of these factors, rather than one factor alone, may therefore be associated with an altered risk of developing AD pathology. We included protective factors that might contribute to higher cognitive reserve or brain maintenance (4,59) but also risk factors that might contribute to cognitive debt. The concept of cognitive debt refers to the constellation of behaviors (mainly stress and neuropsychiatric symptoms) that increase individual risk to AD (60). As postulated by this hypothesis, lower neuroticism and neuropsychiatric symptoms might be a way to reduce vulnerability to Alzheimer’s dementia. Along with high cognitive reserve, modulating these risk factors might be important targets to resist pathology accumulation.
Other important limitations of this study include relatively modest sample sizes in the 2 samples. It will be important to test whether such findings generalize to populations without the added risk conferred by a family history of AD. Most participants were also highly educated, and it will be of interest to know which associations would still be found in individuals with less education. For example, certain associations between high school personality traits and dementia in late life have been reported as being stronger in individuals with higher socioeconomic status (16). Also, associations with openness (or intellect) and education could reflect an underlying relationship with different intelligence measurements (61, 62), which, unfortunately, were not available in either cohort. Furthermore, in PREVENT-AD, behavioral and PET data were not collected at the same time. We did, however, show that the self-reported behavioral features had good stability over 2 to 3 years.
Given the failures of many clinical trials, new avenues are needed to prevent or slow AD progression. Multidomain lifestyle interventions have shown some promises in delaying cognitive decline. We suggest here that such interventions might also postpone accumulation of AD pathology in both sporadic and autosomal dominant forms of AD. In the former, behavioral interventions might focus on aspects of personality and/or emotional regulation, as those features were strongly related to both Aβ and tau deposition. Beyond this, acting on personality traits could have a positive impact on lifestyle changes (63). While more work is needed to understand the mechanisms by which behavioral features may influence AD risk, our results may suggest that personality, neuropsychiatric symptoms, and lifestyle features should be considered when assessing multidomain interventions to postpone the accumulation of AD pathology and its related clinical expression.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | 115 mutation carriers of autosomal dominant Alzheimer’s disease (86 female) | PRe-symptomatic EValuation of Experimental or Novel Treatments for AD (PREVENT-AD) http://www.douglas.qc.ca/page/prevent-alzheimer-the-centre | ||
| Biological Sample | 117 mutation carriers of autosomal dominant Alzheimer’s disease (64 female) | Dominantly Inherited Alzheimer Network (DIAN) http://www.dian-info.org/default.htm | SCR_000812 | Data access granted through project DIAN-D1624 The role of heredity in pre-clinical AD biomarkers: comparison of sporadic AD and autosomal dominant AD |
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | Partial least squares software | https://www.rotman-baycrest.on.ca/index.php?section=84 | ||
| Software; Algorithm | MATLAB R2016a | Mathworks; https://www.mathworks.com/ | RRID:SCR_001622 | |
| Transfected Construct | ||||
| Other-Radiotracer | [11C]-Pittsbrugh compound B | DIAN study only (produced at various sites) | Radiotracer for amyloid positron emission tomography | |
| Other-Radiotracer | [18F]-NAV4694 | StoP-AD study, produced at the McConnell Brain Imaging Centre at the Montreal Neurological Institute | Radiotracer for amyloid positron emission tomography | |
| Other-Radiotracer | [18F]-AV1451 (flortaucipir) | StoP-AD study, produced at the McConnell Brain Imaging Centre at the Montreal Neurological Institute | Radiotracer for tau positron emission tomography | |
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a Canada Research Chair, a Canadian Institutes of Health Research Foundation Grant, a Canada Fund for Innovation Grant, and an Alzheimer’s Association Grant (to SV) and a joint Alzheimer Society of Canada and Fonds de recherche Santé Québec fellowship (to APB). PREVENT-AD was launched in 2011 as a $13.5 million, 7-year public-private partnership using funds provided by McGill University, the Fonds de Recherche du Québec-santé, an unrestricted research grant from Pfizer Canada, the Levesque Foundation, the Douglas Hospital Research Centre and Foundation, the Government of Canada, and the Canada Fund for Innovation. Private sector contributions are facilitated by the Development Office of the McGill University Faculty of Medicine and by the Douglas Hospital Research Centre Foundation (http://www.douglas.qc.ca/). Data collection and sharing for this project was supported by DIAN (UF1AG032438) funded by the National Institute on Aging, the Gemnan Center for Neurodegenerative Diseases, Raul Carrea Institute for Neurological Research, partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute.
This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study. We acknowledge the staff of the PREVENT-AD as well as the Brain Imaging Centre of the Douglas Mental Health University Institute and the PET and cyclotron units of the Montreal Neurological Institute. We also acknowledge the participants of the PREVENT-AD cohort for dedicating their time and energy to helping us collect these data.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Department of Psychiatry (APB, JP, JCSB, SV) and Department of Anesthesia (ÉV-P), Faculty of Medicine; Faculty of Dentistry (ÉV-P); McConnell Brain Imaging Center (DLC, SV), Montreal Neurological Institute, McGill University; Douglas Mental Health University Institute (APB, JP, JCSB, SV); and Alan Edwards Centre for Research on Pain (ÉV-P), McGill University, Montreal, Quebec, Canada; Knight Alzheimer’s Disease Research Center (JM, RB, TB) and Department of Neurology (JM, RB) and Department of Radiology (TB), Washington University School of Medicine, St. Louis, Missouri.
The DIAN consortium members are Ricardo Allegri, Fatima Amtashar, Randy Bateman, Tammie Benzinger, Sarah Berman, Courtney Bodge, Susan Brandon, William (Bill) Brooks, Jill Buck, Virginia Buckles, Sochenda Chea, Jasmeer Chhatwal, Patricio Chrem, Helena Chui, Jake Cinco, Jack Clifford, Carlos Cruchaga, Mirelle D’Mello, Tamara Donahue, Jane Douglas, Noelia Edigo, Nilufer Erekin-Taner, Anne Fagan, Marty Farlow, Angela Farrar, Howard Feldman, Gigi Flynn, Nick Fox, Erin Franklin, Hisako Fujii, Cortaiga Gant, Samantha Gardener, Bernardino Ghetti, Alison Goate, Jill Goldman, Brian Gordon, Neill Graff-Radford, Julia Gray, Jenny Gurney, Jason Hassenstab, Mie Hirohara, David Holtzman, Russ Hornbeck, Siri Houeland DiBari, Takeshi lkeuchi, Snezana lkonomovic, Gina Jerome, Mathias Jucker, Celeste Karch, Kensaku Kasuga, Takeshi Kawarabayashi, William (Bill) Klunk, Robert Koeppe, Elke Kuder-Buletta, Christoph Laske, Jae-Hong Lee, Johannes Levin, Daniel Marcus, Ralph Martins, Neal Scott Mason, Colin Masters, Denise Maue-Dreyfus, Eric McDade, Lucy Montoya, Hiroshi Mori, John Morris, Akem Nagamatsu, Katie Neimeyer, James Noble, Joanne Norton, Richard Perrin, Marc Raichle, John Ringman, Jee Hoon Roh, Stephen Salloway, Peter Schofield, Hiroyuki Shimada, Tomoyo Shiroto, Mikio Shoji, Wendy Sigurdson, Hamid Sohrabi, Paige Sparks, Kazushi Suzuki, Laura Swisher, Kevin Taddei, Jen Wang, Peter Wang, Mike Weiner, Mary Wolfsberger, Chengjie Xiong, Xiong Xu.
The PREVENT-AD members are Angela Tam, Anne Labonté, Alexa Pichet Binette, Anne-Marie Faubert, Axel Mathieu, Cécile Madjar, Charles Edouard Carrier, Christian Dansereau, Christina Kazazian, Claude Lepage, Cynthia Picard, David Maillet, Diane Michaud, Doris Couture, Doris Dea, Claudio Cuello, Alan Barkun, Alan Evans, Blandine Courcot, Christine Tardif, Clément Debacker, Clifford R. Jack, David Fontaine, David S. Knopman, Gerhard Maultaup, Jamie Near, Jeannie-Marie Leoutsakos, Jean-Robert Maltais, Jason Brandt, Jens Pruessner, John C. Morris, John C.S. Breitner, Judes Poirier, Laksanun Cheewakriengkrai, Lisa-Marie Münter, Louis Collins, Mallar Chakravarty, Mark A. Sager, Marina Dauar-Tedeschi, Mark Eisenberg, Natasha Rajah, Paul Aisen, Joanne Toussaint, Pedro Rosa-Neto, Pierre Bellec, Penelope Kostopoulos, Pierre Etienne, Pierre N. Tariot, Pierre Orban, Reisa A. Sperling, Rick Hoge, Ronald G. Thomas, Serge Gauthier, Suzanne Craft, Sylvia Villeneuve, Thomas J. Montine, Vasavan Nair, Véronique Bohbot, Vinod Venugopalan, Vladimir Fonov, Vasser Ituria-Medina, Zaven S. Khachaturian, Eduard Teigner, Elena Anthal, Elsa Yu, Fabiola Ferdinand, Galina Pogossova, Ginette Mayrand, Guerda Duclair, Guylaine Gagné, Holly Newbold-Fox, IIIana Leppert, Isabelle Vallée, Jacob W. Vogel, Jennifer Tremblay-Mercier, Joanne Frenette, Josée Frappier, Justin Kat, Justin Miron, Karen Wan, Laura Mahar, Leopoldina Carmno, Louise Théroux, Mahsa Dadar, Marianne Dufour, Marie-Elyse Lafaille-Magnan, Melissa Appleby, Mélissa Savard, Miranda Tuwaig, Mirela Petkova, Pierre Rioux, Pierre-François Meyer, Rana EI-Khoury, Renee Gordon, Renuka Giles, Samir Das, Seqian Wang, Shirin Tabrizi, Sulantha Mathotaarachchi, Sylvie Dubuc, Tanya Lee, Thomas Beaudry, Valérie Gervais, Véronique Pagé, Julie Gonneaud, Gülebru Ayranci, Tharick A. Pascoal, René Desautels, Fatiha Benbouhoud, Eunice Farah Saint-Fort, Sander C.J. Verfaillie, Sarah Farzin, Alyssa Salaciak, Stephanie Tullo, Etienne Vachon-Presseau, Leslie-Ann Daoust, Theresa Köbe, Nathan Spreng, Melissa McSweeney, Nathalie Nilsson, Morteza Pishnamazi, Christophe Bedetti. The listing of the PREVENT-AD Research Group members with their current status is available at https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=(2019-08-12].
REFERENCES
- 1.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. (2017): Dementia prevention, intervention, and care. Lancet 390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Mangialasche F, Ngandu T (2017): Can lifestyle changes prevent cognitive impairment? Lancet Neurol 16:338–339. [DOI] [PubMed] [Google Scholar]
- 3.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014): Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neural 13:788–794. [DOI] [PubMed] [Google Scholar]
- 4.Arenaza-Urquijo EM, Vemuri P (2018): Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 90:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stem Y (2009): Cognitive reserve. Neuropsychologia 47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arenaza-Urquijo EM, Wirth M, Chételat G (2015): Cognitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Front Aging Neurosci 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes DE, Yaffe K (2011): The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ (2014): Gene-environment interactions: Lifetime cognitive activity, APOE genotype, and β-amyloid burden. J Neurosci 34:8612–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenaza-Urquijo EM, Bejanin A, Gonneaud J, Wirth M, La Joie R, Mutlu J, et al. (2017): Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: Neuroimaging evidence for protection and compensation. Neurobiol Aging 59:72–79. [DOI] [PubMed] [Google Scholar]
- 10.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, et al. (2008): Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: Population-based study. Arch Gen Psychiatry 65:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutin RA, Stephan Y, Terracciano A (2018): Psychological distress, self-beliefs, and risk of cognitive impairment and dementia. J Alzheimers Dis 65:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters ME, Rosenberg PB, Steinberg M, Norton MC, Welsh-Bohmer KA, Hayden KM, et al. (2013): Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: The Cache County Study. Am J Geriatr Psychiatry 21:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimson A, Schlosser M, Huntley JD, Marchant NL (2018): Support for midlife anxiety diagnosis as an independent risk factor for dementia: A systematic review. BMJ Open 8:e019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson L, Guo X, Duberstein PR, Hällström T, Waem M, Östling S, et al. (2014): Midlife personality and risk of Alzheimer disease and distress: A 38-year follow-up. Neurology 83:1538–1544. [DOI] [PubMed] [Google Scholar]
- 15.Terracciano A, An Y, Sutin AR, Thambisetty M, Resnick SM (2017): Personality change in the preclinical phase of Alzheimer disease. JAMA Psychiatry 74:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman BP, Huang A, Peters K, Horner E, Manly J, Bennett DA, et al. (2019): Association between high school personality phenotype and dementia 54 years later in results from a national US sample (published online ahead of print October 16]. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terracciano A, Sutin AR (2019): Personality and Alzheimer’s disease: An integrative review. Personal Disord 10:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzmeier N, Düzel E, Jessen F, Buerger K, Levin J, Duering M, et al. (2018): Left frontal hub connectivity delays cognitive impairment in autosomal-dominant and sporadic Alzheimer’s disease. Brain 141:1186–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown BM, Sohrabi HR, Taddei K, Gardener SL, Rainey-Smith SR, Peiffer JJ, et al. (2017): Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement 13:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Munoz C, Giraldo M, et al. (2016): Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: A retrospective cohort study. JAMA Neurol 73:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonneaud J, Bedetti C, Pichet Binette A, Benzinger T, Morris JC, Bateman RJ, et al. (In press): Education is associated with Aβ burden in preclinical familial and sporadic Alzheimer’s disease. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringman JM, Liang LJ, Zhou Y, Vangala S, Teng E, Kremen S, et al. (2015): Early behavioural changes in familial Alzheimer’s disease in the dominantly inherited Alzheimer network. Brain 138:1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. (2012): Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. (2013): Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. (2011): Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kivipelto M, Mangialasche F, Ngandu T (2018): Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14:653–666. [DOI] [PubMed] [Google Scholar]
- 27.Breitner JCS, Poirier J, Etienne PE, Leoutsakos JM, Group P-ADR (2016): Rationale and Structure for a New Center for Studies on Prevention of Alzheimer’s Disease (StoP-AD). J Prev Alzheimers Dis 3:236–242. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005): The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- 29.Randolph C, Tierney MC, Mohr E, Chase TN (1998): The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol 20:310–319. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC (1993): The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 31.Koo TK, Li MY (2016): A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JA (2014): Measuring thirty facets of the five factor model with a 120-item public domain inventory: Development of the IPIP-NE0–120. J Res Pers 51:78–89. [Google Scholar]
- 33.Desikan RS, Ségonne F, Fischl B (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 34.Jagust WJ, Landau SM, Koeppe RA, Reiman EM, Chen K, Mathis CA, et al. (2015): The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement 11:757–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker SL, Maass A, Jagust WJ (2017): Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief 15:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi A, Koeppe RA, Fessler JA (2009): Reducing between scanner differences in multi-center PET studies. Neuroimage 46:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmqvist S, Schöll M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, et al. (2017): Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 8:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. (2015): Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain 138:2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, et al. (2012): Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 59:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maass A, Landau S, Baker SL, Horng A, Lockhart SN, Joie RL, et al. (2017): Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage 157:448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. (2016): Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neural 79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marquie M, Verwer EE, Meltzer AC, Kim SJW, Aguero C, Gonzalez J, et al. (2017): Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol Commun 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. (2016): PET imaging of tau deposition in the aging human brain. Neuron 89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF, et al. (2018): Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcintosh AR, Bookstein FL, Haxby JV, Grady CL (1996): Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3:143–157. [DOI] [PubMed] [Google Scholar]
- 46.McIntosh AR, Misic B (2013): Multivariate statistical analyses for neuroimaging data. Ann Rev Psychol 64:499–525. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Thai DR, Ghebremedhin E, Del Tredici K (2011): Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neural 70:960–969. [DOI] [PubMed] [Google Scholar]
- 48.Mander BA, Winer JR, Jagust WJ, Walker MP (2016): Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci 39:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes AD, Aizenstein HJ, et al. (2015): Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 23:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz SA, Gordon BA, Mishra S, Su Y, Morris JC, Ances BM, et al. (2019): Association between personality and tau-PET binding in cognitively normal older adults (published online ahead of print September 5]. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR (2014): The origins of neuroticism. Perspect Psychol Sci 9:481–496. [DOI] [PubMed] [Google Scholar]
- 52.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S(2002): Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 288:1475–1483. [DOI] [PubMed] [Google Scholar]
- 53.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJH, Pankratz VS, et al. (2014): Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am J Psychiatry 171:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krell-Roesch J, Vassilaki M, Mielke MM, Kremers WK, Lowe VJ, Vemuri P, et al. (2019): Cortical beta-amyloid burden, neuropsychiatric symptoms, and cognitive status: The Mayo Clinic Study of Aging. Transl Psychiatry 9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leoutsakos JM, Forrester SN, Lyketsos CG, Smith GS (2015): Latent classes of neuropsychiatric symptoms in NACC controls and conversion to mild cognitive impairment or dementia. J Alzheimers Dis 48:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.d’Oieire Uquillas F, Jacobs HIL, Biddle KD, Properzi M, Hanseeuw B, Schultz AP, et al. (2018): Regional tau pathology and loneliness in cognitively normal older adults. Transl Psychiatry 8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. (2018): Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry 175:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, et al. (2016): Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry 73:1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stem Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, et al. (2018): Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance [published online ahead of print September 14]. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchant NL, Howard RJ (2015): Cognitive debt and Alzheimer’s disease. J Alzheimers Dis 44:755–770. [DOI] [PubMed] [Google Scholar]
- 61.DeYoung CG, Quilty LC, Peterson JB, Gray JR (2014): Openness to experience, intellect, and cognitive ability. J Pers Assess 96:46–52. [DOI] [PubMed] [Google Scholar]
- 62.Nishita Y, Tange C, Tomida M, Otsuka R, Ando F, Shimokata H (2019): Positive effects of openness on cognitive aging in middle-aged and older adults: A 13-year longitudinal study. Int J Environ Res Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutin AR, Stephan Y, Luchetti M, Artese A, Oshio A, Terracciano A (2016): The five-factor model of personality and physical inactivity: A meta-analysis of 16 samples. J Res Pers 63:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


