Abstract
Underutilization of effective screening is one driver of disparities in cervical cancer incidence and mortality. Consideration of patient preferences could help to improve screening rates in populations facing substantial barriers to preventive care. We conducted a systematic review of the literature on cervical cancer screening preferences among medically underserved patients in the United States. We searched six electronic databases (PubMed, Web of Science, EMBASE, Scopus, CINAHL, and PsycINFO) for articles published through February 2019 [Prospero ID: CRD42019125431]. Among the forty-three articles included, 23 reported screening modality preferences, 11 reported preferences related to provider demographics and attributes, 6 reported screening scheduling and results delivery preferences, and 9 reported preferences related to health education and communication. This review demonstrates the wide variety of medically underserved patient preferences related to cervical cancer screening. It also draws attention to two key preference trends that emerged despite heterogeneity in study design, populations, and preference assessment. Consistent preferences for HPV self-testing over traditional Pap testing highlight a key potential mechanism for increasing cervical cancer screening uptake among medically underserved populations. Additionally, preferences for gender- and language-concordant providers underscore the need for continued efforts toward expanding diversity among medical professionals.
Keywords: Early Detection of Cancer, Patient Preference, Systematic Review, Uterine Cervical Neoplasms
Introduction
Cervical cancer incidence in the United States (US) has declined by over 70% since the introduction of routine preventive screening in the 1940’s, including Pap testing and, more recently, human papillomavirus (HPV) testing (1–4). Recommended screening protocols, when administered appropriately, are effective in decreasing cervical cancer incidence and mortality through early detection and treatment of pre-cancerous lesions (5).
Despite the success of preventive measures, cervical cancer remains a leading cause of cancer-related death in medically underserved individuals (6). Cervical cancer incidence and mortality are marked by disparities in the US (7, 8). Notably, the hysterectomy-adjusted overall mortality rate from cervical cancer among African-Americans, 10.1 per 100,000 individuals with a cervix, is almost double the rate for White individuals (9). Heightened mortality rates have also been documented among Hispanics, American Indians/Alaskan Natives (AI/AN), individuals without a usual source of care, and residents of geographically remote locations (7, 8, 10).
As over 50% of new cervical cancer cases are estimated to be due to insufficient screening (11), disparities in cervical cancer outcomes stem, in part, from disparate screening uptake across groups. Lower screening rates have been documented among Hispanic (79.4%), AI/AN (79.0%), and Asian (75.3%) women compared to Black (85.6%) and White (85.0%) women (12). Additionally, a disproportionate number of recent immigrants to the US, uninsured individuals, and individuals without a usual health care source remain unscreened (12, 13). Evidence shows that patient perceptions of low cancer risk and high screening barriers (e.g., cost, access, embarrassment) are associated with low screening uptake (14, 15). Lack of consideration of patient preferences surrounding screening, particularly in medically underserved populations, is another important factor contributing to disparate screening rates (16).
With the introduction of HPV testing, in addition to Pap testing, as a recommended primary screening mechanism, patient preferences surrounding screening modality must be considered. Various HPV testing modalities have proven to be effective, including clinician-administered HPV testing and HPV self-testing, in which the patient collects their own vaginal sample, either by direct mail or in-person delivery. The specificity and sensitivity of HPV self-testing is comparable to clinician-administered HPV testing for the detection of HPV infection and high-grade cervical lesions (17, 18). Additionally, studies have documented high patient acceptability of HPV self-collection, particularly among under-screened individuals, suggesting this may be a potentially effective and preferred method to increase screening uptake (19–21). Despite these advances, however, the US Preventive Services Task Force (USPSTF) only recommends provider-administered HPV tests, rather than self-tests, leading to limitations in HPV self-test dissemination and uptake (5, 19). In addition to screening modality, preferences for other aspects of cervical cancer screening should be assessed, including preferences about certain provider attributes, scheduling, and reminders – in the case of in-clinic screening – as well as about communication of results and delivery of screening education interventions.
To date, cervical cancer screening preferences among medically underserved individuals in the US have not been systematically reviewed. Huynh and colleagues (2010) conducted a systematic review of articles published through October 2008 about individual perceptions of HPV self-testing (22). Additionally, two studies have systematically reviewed self-sampling acceptability (23, 24). While these reviews found self-testing to be generally well received by patients, none have specifically reviewed preferences across the full range of approved screening delivery options or focused on medically underserved populations. Other systematic reviews have assessed barriers to and facilitators of cervical cancer screening among specific populations, such as immigrants, Latinas, and other racial/ethnic minorities (7, 15, 25, 26). Building on these reviews, this systematic review is unique in its assessment of medically underserved patients’ preferences for cervical cancer screening attributes and delivery, including test modality (particularly important in the era of HPV self-testing), provider demographics and attributes, scheduling and results delivery, and health education and communication efforts. This work can be used to inform future interventions, policies, and research targeting screening uptake.
Materials and Methods
We conducted a systematic review of patient preferences related to cervical cancer screening among medically underserved individuals, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27, 28). The protocol for this review is registered with the International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42019125431) (29).
We developed the inclusion and exclusion criteria for this review using an adapted version of the PICOTS (Population, Intervention, Comparison, Outcome, Timing, and Setting) framework commonly used for clinical research questions (30, 31). The Intervention criterion was not considered since we did not limit this review to studies with an experimental component. Instead, we added a Study Design criterion to capture observational and experimental studies that assessed patient preferences using quantitative, qualitative, or mixed method research designs (Supplementary Table S1).
We developed an initial list of “medically underserved” populations by consulting the Department of Health and Human Services’ National Stakeholder Strategy for Achieving Health Equity. The National Stakeholder Strategy defines “medically underserved” as groups or individuals subject to social factors known to increase the risk of adverse health outcomes, including low socioeconomic status, low educational attainment, racism, and inadequate access to quality health care (32). We then expanded this definition to include any additional groups that face economic, cultural, or linguistic barriers to accessing health care, given that these populations often have disparately low rates of cervical cancer screening (7, 8, 10, 12). Because cervical cancer screening guidelines differ internationally (33), we only included studies conducted in the US in this review to ensure consistency in the recommended screening guidelines that may shape patients’ preferences and screening behaviors. As for outcomes, studies had to include a patient-reported preference related to cervical cancer screening to be considered for inclusion; however, preference was not required to be the primary outcome assessed. The specific inclusion and exclusion criteria are outlined in Supplementary Table S1.
We searched the following databases for articles published through February 2019: Medline (PubMed), Science Citation Index (Web of Science), EMBASE, Scopus, CINAHL, and PsycINFO (Figure 1). The following basic search string was used to identify relevant articles: (HPV OR Pap OR papillomavirus OR human papillomavirus OR Papanicolaou OR cervical OR cervix OR endocervix OR endocervical) AND (Test* OR screen* OR self-test OR self-tests OR self-testing OR self-tested) AND (Preference OR perception OR perceptions OR (discrete AND choice*) OR attitude OR attitudes) AND (Neoplasms OR neoplasm OR neoplasia OR neoplasias OR neoplastic OR dysplastic OR dysplasia OR dysplasias OR cancer OR cancers OR cancerous OR malignant OR malignancy OR malignancies) AND NOT (vaccine) (Title only) AND NOT (vaccination) (Title only). Detailed search terms specific to each database can be found in the supplementary materials (Supplementary Table S2).
Figure 1. PRISMA flow diagram:
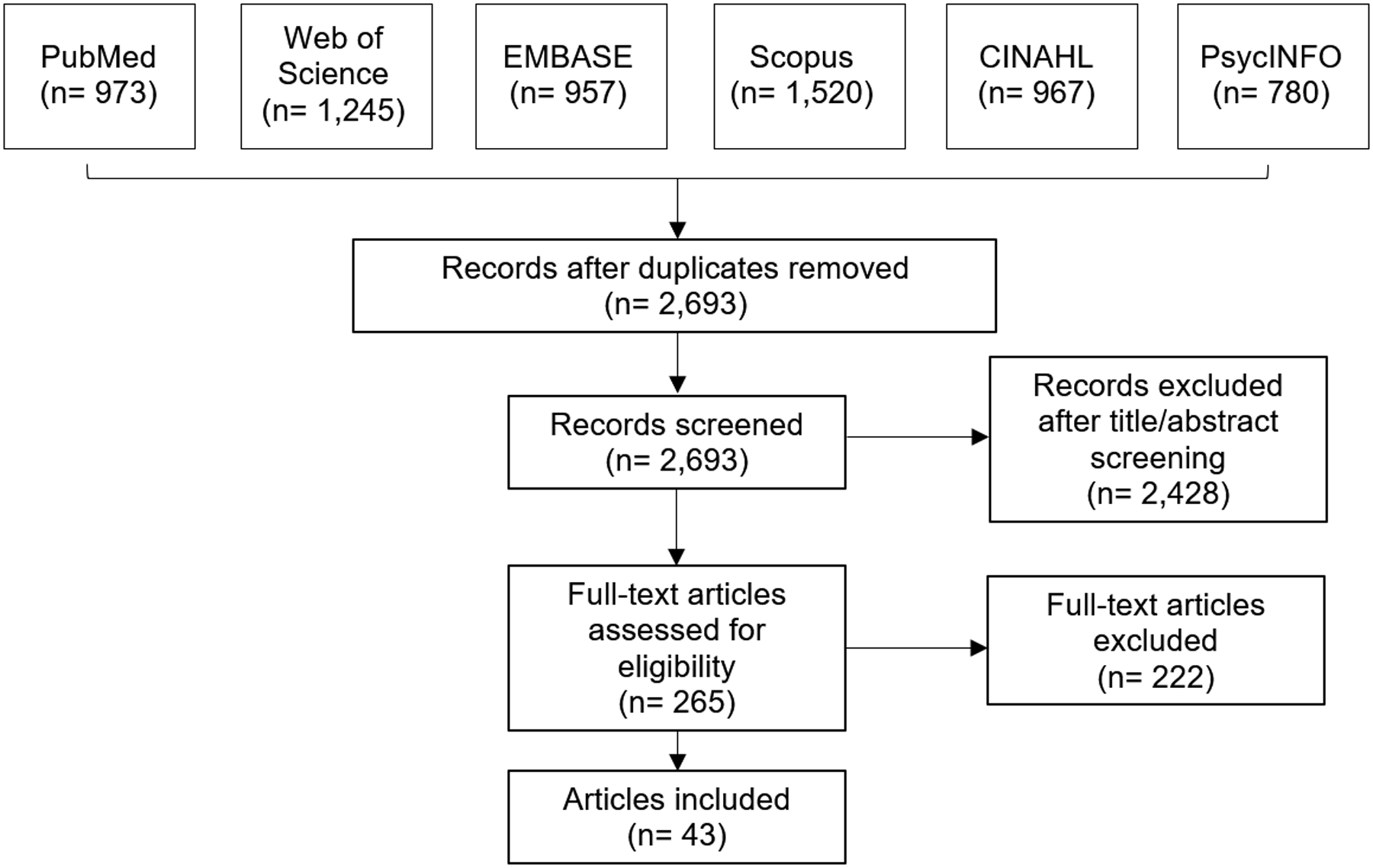
Figure 1 represents the PRISMA flow diagram, which depicts the flow of studies reviewed throughout the systematic review.
In total, 6,442 articles were identified across the six databases and imported into F1000 Workspace (Faculty of 1000 Ltd, 2019), a reference management database (Figure 1). After the removal of duplicates, 2,693 unique articles remained and were transferred to Covidence (Veritas Health Innovation Ltd, 2019) for screening. Two reviewers (CBB and MCO) independently screened all titles and abstracts, initially reviewing and comparing 20 articles to ensure screening consistency. For the review of all remaining articles, the reviewers resolved any discrepancies through discussion with one another, and with a third reviewer (LPS) when consensus could not be reached.
During the title/abstract screening, we focused on excluding articles that were not conducted in the US or not related to cervical cancer screening, such as studies related to other health conditions or cervical cancer studies related to HPV vaccination or treatment. Additionally, we excluded studies that were not conducted among patients and studies without an outcome derived from patient experience, such as studies reporting screening uptake only. During the title/abstract screening process, 2,428 articles were removed, leaving 265 articles for full-text review.
During full-text review, the two reviewers (CBB and MCO) assessed whether each record met the inclusion criteria, with discrepancies resolved by the third reviewer (LPS) as necessary. Reviewers specifically focused on excluding articles that did not include a medically underserved population or preference outcome. Assessing these criteria in full-text review allowed us to capture the broad range of cervical cancer screening preferences across diverse populations who meet our definition of medically underserved but were not included in our initial list of target populations. Of the 265 articles reviewed, 222 were excluded, 193 of which did not include a preference outcome. An additional 25 articles were excluded due to not including a medically underserved population, and the remaining 4 were excluded due to being conducted outside of the US. The remaining 43 articles identified for inclusion were abstracted using structured fields.
As a final step, we conducted a risk of bias assessment using a template designed specifically for preference studies by Purnell and colleagues (2014) (34). Each of the 43 included studies were evaluated for low versus high bias in the following six categories: (1) well-defined study question, (2) well-described inclusion criteria, (3) comprehensive description of alternatives, (4) appropriate measure of preferences, (5) appropriate analysis, and (6) pre-specified analysis.
Results
We included a total of 43 studies assessing cervical cancer screening preferences among medically underserved patients in the US. Twenty-three of these studies used a quantitative design, 17 qualitatively assessed preferences, and 3 employed mixed methods. Of the quantitative studies, 22 administered a cross-sectional survey, and 1 elicited patient preferences as a part of a randomized controlled trial (RCT). The majority of qualitative studies (n=11) conducted focus groups, and the other 6 studies conducted one-on-one interviews. Among the mixed method studies, 2 used a combination of interviews and surveys, while 1 paired focus groups and surveys.
Medically underserved populations are categorized broadly as racial and ethnic minorities in 30 studies, low-income populations in 7 studies, LGBTQ populations in 4 studies, rural residents in 3 studies, homeless women in 1 study, and women residing in domestic violence shelters in 1 study. Three of these studies reported preferences for intersectional populations, such as individuals who self-identified as being Black or African American, as well as lesbian, gay, or bisexual.
Tables 1–4 organize results by the type of preference outcome assessed. Table 1 includes 23 studies assessing preferences regarding screening modality (e.g., Pap test, HPV test, co-testing, HPV self-test). Table 2 includes 11 studies reporting preferences for provider demographics and attributes (e.g., gender, ethnicity, type of training). Table 3 presents 6 studies about screening scheduling and results delivery (e.g., reminders, timing) preferences, and Table 4 identifies 9 studies that report preferences regarding health education and communication (e.g. information source, educational material attributes). Studies reporting preferences falling into more than one category are included in each of the relevant tables. Variables associated with preference were only consistently reported across screening modality preference studies; therefore, associations are only reported in Table 1. Associations with other preference outcomes are reported in the text.
Table 1.
Characteristics of studies assessing screening modality preference (n=23)
| Study | Medically Underserved Population | Study Design | Sample Size | Findings | Factors Associated with Screening Modality Preference |
|---|---|---|---|---|---|
| Anhang 2005 | Low-income populationsa | Cross-sectional Survey | 172 | 32% preferred HPV self-test to Pap test “Which would increase screening likelihood…” Pap test (47%), HPV self-test (21%), HPV self-test + pelvic exam (19%), none (12%) |
Those recruited at a STD clinic (versus a cancer screening clinic), non-Hispanics (versus Hispanics), and those with at least some college education (versus less than college education) were more likely to prefer the HPV self-test. |
| Barbee 2010 | Racial/ethnic minorities | Cross-sectional Survey | 189 (Haitian) | 87% preferred HPV self-test to Pap test | -- |
| Cina 2017 | Racial/ethnic minorities | Cross-sectional Survey | 93 (AI/AN) | 63% preferred HPV self-test to Pap test | -- |
| Crosby 2015b | Rural residents | Cross-sectional Survey | 400 (Appalachian Kentucky) | 89% preferred HPV self-test to Pap test | -- |
| Crosby 2017 | Racial/ethnic minorities | Cross-sectional Survey | 88 (non-Hispanic Black Mississippians) | 78% preferred HPV self-test to Pap test | No associations with preference for HPV self-test. |
| Hatcher 2011 | Rural residents | Cross-sectional Survey | 345 (Appalachian Kentucky) | 66% preferred HPV self-test to Pap test | Rarely or never screened individuals (versus recently screened individuals) were more likely to prefer the HPV self-test. |
| Winer 2016 | Racial/ethnic minorities | Cross-sectional Survey | 306 (Hopi Tribe members) | 62% preferred HPV self-test to Pap test | Those without a Pap test within the past 3 years and individuals not employed full-time were more likely to prefer the HPV self-test. |
| Ilangovan 2016 | Racial/ethnic minorities | Cross-sectional Survey | 121 (Haitian: 40 Hispanic: 81) | 77% preferred HPV self-test, 20% had no preference, 2% preferred Pap test, 1% did not respond | Hispanics (versus Haitians) were more likely to prefer the HPV self-test. |
| Jones 2012 | Racial/ethnic minorities | Cross-sectional Survey | 197 (Hispanic: 166 Black: 25 Other: 6) | 79% preferred HPV self-test, 14% reported no preference, 8% preferred Pap testc | Black individuals (versus Hispanic individuals) were more likely to prefer the HPV self-test. |
| Kilfoyle 2018 | Low-income populationsd | Cross-sectional Survey | 227 | 51% preferred HPV self-test, 27% had no preference,19% preferred Pap testc | Black women and other women of color (versus White women) were more likely to prefer the HPV self-test. |
| Levinson 2016 | Women staying in domestic violence shelters | Cross-sectional Survey | 142 | 10% preferred HPV self-test, 39% had no preference, 42% preferred Pap testc | Women not up-to-date on screening were more likely to prefer the HPV self-test. |
| Litton 2013 | Racial/ethnic minorities | Cross-sectional Survey | 516 (AA) | 43% preferred HPV self-test, 25% preferred Pap test, 15% preferred HPV test at doctor’s office, 17% did not know | Women who had recently received a colposcopy or Pap test were more likely to prefer a Pap test. |
| Molokwu 2018 | Racial/ethnic minorities | Randomized controlled trial | 195 (Hispanic) | 32% preferred HPV self-test, 45% had no preference, 23% preferred Pap test | -- |
| Penaranda 2015 | Racial/ethnic minorities | Cross-sectional Survey | 110 (Hispanic) | 30% preferred HPV self-test, 43% had no preference, 26% preferred Pap testc | -- |
| Seay 2017 | LGBTQ populations | Cross-sectional Survey | 91 (transgender men) | 57% preferred HPV self-test, 21% preferred Pap test, 14% had no preference, 7% would refuse both testsc | Individuals who reported avoiding preventive health care due to cost and/or discrimination were more likely to prefer the HPV self-test. Individuals with health insurance and a history of screening were less likely to prefer the HPV self-test. |
| Galbraith 2014 | Low-income populationsd | Cross-sectional Survey | 199 | When asked which protects women’s health better… 75% had no preference, 19% preferred Pap test, 6% preferred HPV self-test |
-- |
| McDowell 2017 | LGBTQ populations | Mixed Methods (Interviews + Surveys) | 63 (transmasculine individualsf) | 79% preferred HPV self-test to Pap test (across survey and interviews) |
Self-test benefits: less invasive, more comfortable, less likely to provoke gender discordance, greater agency Pap test benefits: more thorough The degree of comfort participants felt with their providers influenced their willingness to have Pap test. (n=31, interviews only) |
| Pieters 2013 | Homeless women | Qualitative (Interviews) | 17 | 65% preferred HPV self-test, 29% preferred Pap test, 6% reported no preference |
Self-test benefits: easy, comfortable, unintrusive Cytology still seen as necessary. |
| Katz 2017 | Low-income populationsf | Qualitative (Focus Groups) | 15 | Majority preferred HPV self-tests |
Self-test benefits: convenience, cost, no doctor’s office visit Self-test concerns: pain |
| Penaranda 2014 | Racial/ethnic minorities | Qualitative (Focus Groups) | 21 (Hispanicg) | No clear preference consensus |
Participants value: choice, ease, accuracy, cost Self-test benefits: ease, convenience, practicality, less embarrassment, not needing child care Self-test concerns: performing test incorrectly |
| Reisner 2018 | LGBTQ populations | Qualitative (Interviews) | 131 (transmasculine individualsf) | >90% preferred HPV self-test to Pap test |
Self-test benefits: ease, privacy, self-empowerment Self-test concerns: performing test incorrectly, distrust of accuracy, gender dysphoria triggered by interacting with genitals |
| Scarinci 2013 | Racial/ethnic minorities | Qualitative (Focus Groups) | 96 (AA) | Most women would be willing to perform HPV self-test |
Self-test benefits: convenient, private Self-test concerns: performing test incorrectly, cost |
| Anhang 2004 | Low-income populationsh & Racial/ethnic minorities | Qualitative (Focus Groups) | 48 (Hispanic, AA, AI/AN, White) | Most women preferred physician- administered HPV test or preferred physician examination in addition to self-test | -- |
Defined as women attending inner-city publicly funded health clinics in New York City
Preference measured as which type of test participants would be more likely to complete on a regular basis
Due to rounding and/or missing data, percentages do not add up to 100%
Defined as individuals who had children in federal school lunch program, had Medicaid or Medicare Part B, were uninsured, or had income <200% of the federal poverty level
Defined as those assigned female at birth who identify with a gender other than female
Income <$20,000 (87% of individuals had incomes less than $10,000)
Residing on the US-Mexico border, majority Hispanic
Majority had income <$15,000, not otherwise defined
Abbreviations: African American (AA); American Indian/Alaskan Native (AI/AN)
Table 4.
Characteristics of studies assessing preferences related to health education and communication (n=9)
| Study | Medically Underserved Population | Study design | Sample size | Delivery Preference Assessed | Findings |
|---|---|---|---|---|---|
| Yu 2001 | Racial/ethnic minorities | Cross-sectional Survey | 332 (Chinese) | Class location | Preferred community service center (54.7%), local school (21.3%), church (20.9%), home (6.2%); senior centers, hospitals, clubs, and work places (all <3%). |
| Yemane 2016a | LGBTQ populations | Cross-sectional Survey | 28 (WSW) | Information source | Preferred media (46%), pamphlet (21%), personal conversation with healthcare provider (18%), workshop at a community center (7%). |
| Haworth 2014 | Racial/ethnic minorities | Mixed Methods (Survey + Focus Groups) | 69 (Bhutanese refugeesb) | Information source | Preferred community health workers who spoke native language over other information sources. |
| Sharpe 2013 | Racial/ethnic minorities | Qualitative (Interviews) | 32 (Cherokee) | Information source | Preferred receiving information from a health care provider; reading HPV educational information in private; printed materials available to take home that include informational websites. |
| Lee 2015 | Racial/ethnic minorities | Cross-sectional Survey | 62 (Vietnamese American: 30 Korean American: 32) | Information source (within the context of ethnic beauty salons) | Cosmetologists preferred videos (31.3%) and 1-on-1 talks (25%) for receiving health information, and 1-on-1 talks (47.1%) and pamphlets (29.4%) for information delivery. Customers preferred videos (20.5%) and 1-on-1 talks (23.1%) for receiving health information. Customers were interested in talking about cervical cancer (75%) and willing to learn about it from cosmetologists (89%). |
| Kenya 2015 | Racial/ethnic minorities | Qualitative (Focus Groups) | 21 (Haitianc) | Screening information source Printed material attributes | Preferred community health workers to HIV case managers for information delivery. Preferred communication of cervical cancer and HPV information through Haitian radio. Preferred flip-charts to written materials. |
| Christopher 2009 | Racial/ethnic minorities | Qualitative (Focus Groups) | 68 (Apsáalooke – Crow Nation) | Printed material attributes | Preferred English brochure with native words interspersed; pictures (not drawings); pamphlets with white space. |
| Reed 2002 | Racial/ethnic minorities | Qualitative (Focus Groups) | 26 (Somalian: 10, Central American: 6, Vietnamese: 10) | Printed material attributes | Preferred visual presentations of health care information (including pictures, diagrams, and stories) and group educational sessions. |
| Hunter 2012 | Racial/ethnic minorities | Qualitative (Interviews) | 45 (Mexicand) | Printed material attributes | Preferred written descriptions focused on prevention and finding early changes instead of cervical cancer (98%); preferred more realistic illustrations of pelvic exam. |
Letter to the editor
Surveys: 42, Focus groups: 27
Born in Haiti
Born in Mexico, lived in the US for five years or less, completed nine grades or less of formal education, and speak predominantly Spanish
Abbreviations: African American (AA); Women who have sex with women (WSW); American Indian/Alaskan Native (AI/AN)
Table 2.
Characteristics of studies assessing preferences for provider demographics and attributes (n=11)
| Study | Medically Underserved Population | Study design | Sample Size | Provider Preference Assessed | Findings |
|---|---|---|---|---|---|
| Carey Jackson 2000 | Racial/ethnic minorities | Mixed Methods (Survey + Interviews) | 455 (Cambodian-American) | Gender | 77% preferred a female provider, 73% preferred a female interpreter. (n=413, survey only) |
| Haworth 2014a | Racial/ethnic minorities | Mixed Methods (Survey + Focus Groups) | 69 (Bhutanese refugeesb) | Gender | Women almost unanimously preferred female doctors. (n = 27, focus groups only) |
| Lanier 1999 | Racial/ethnic minorities | Cross-sectional Survey | 481 (AI/ANc) | Gender | 48% preferred a female provider, 2% preferred a male provider, 50% had no preference. 18% would refuse a male provider. |
| Ma 2012 | Racial/ethnic minorities | Cross-sectional Survey | 1416 (Vietnamese) | Gender | 60% preferred female provider, 3.5% preferred male provider, 36.5% had no preference. |
| Reed 2002a | Racial/ethnic minorities | Qualitative (Focus Groups) | 26 (Somalia: 10, Central America: 6, Vietnam: 10) | Gender | Somali women preferred female providers and interpreters. |
| Nguyen 2002 | Racial/ethnic minorities | Cross-sectional Survey | 1566 (Vietnamese, Vietnamese-American, or Vietnamese-Chinesed) | Gender Language |
57.2% preferred a female doctor for a Pap test. 52.9% preferred a female standby if male doctor performs a Pap test. 64.4% preferred a Vietnamese-speaking doctor for a Pap test. |
| Kim 2017 | Racial/ethnic minorities | Qualitative (Interviews) | 32 (KIW) | Gender Communication style Language |
Majority preferred a female doctor. Some KIW wanted more dialogue with their doctors about Pap test results, rather than a letter or phone message. Majority preferred a Korean-speaking doctor. |
| Torres 2013 | Racial/ethnic minorities | Qualitative (Interviews) | 45 (Hispanic) | Gender Language Examiner type |
Most expressed comfort in speaking to another woman about women’s health issues but preferred a doctor, who was generally perceived to be male, over a nurse, who was perceived as female, for screening. Majority preferred healthcare professional who speaks Spanish. Preferred receiving information from a doctor versus a nurse. |
| McAlearney 2012 | Rural residents | Qualitative (Focus Groups) | 36 (Appalachian Ohio) | Gender Communication style |
Majority preferred female providers. Preferred patient-centered communication. |
| Alexander 1981e | Racial/ethnic minorities | Cross-sectional Survey | 509 (Mexican-American) | Gender, Examiner type | 44% preferred female physician, 4% male physician, 10% nurse or nurse practitioner, 8% female person, 34% no preference. |
| Low-income | Cross-sectional Survey | 1956 | Gender, Examiner type | 33% preferred female physician, 6% male physician, 12% nurse or nurse practitioner, 9% female person, 40% no preference. | |
| Agénor 2015 | Racial/ethnic minorities & LGBTQ populations | Qualitative (Focus Groups) | 18 (Black or AA and LBQ) | Gender, Race/Ethnicity Examiner type Communication style |
Majority preferred providers who identified as a woman, person of color, and/or LGBTQ. Many preferred to receive care from a physician’s assistant, registered nurse, or nurse practitioner. Preferred providers who had experience and felt comfortable serving LBQ patients and were willing to explain the Pap test process with a “calm demeanor and gentle touch.” |
Included in more than one results table
Surveys: 42, Focus groups: 27
Members of a federally recognized AI/AN group or tribe
Ethnic Chinese who were born or had lived in Vietnam
Stratified results by racial/ethnic minority and income
Abbreviations: Korean immigrant women (KIW); African-American (AA); Lesbian, bisexual, or queer (LBQ); AI/AN (American Indian/Alaskan Native)
Table 3.
Characteristics of studies assessing preferences related to screening scheduling and results delivery (n=6)
| Study | Medically Underserved Population | Study design | Sample size | Delivery Preference Assessed | Findings |
|---|---|---|---|---|---|
| Brandzel 2016 | Racial/ethnic minorities | Qualitative (Focus Groups) | 39 (Black: 24 Hispanic: 15) | Screening reminder delivery | No consensus on screening reminder timing. Black women preferred reminders from community-based advocates (versus HCPs). Hispanic women preferred smart phone reminders. |
| Greaney 2014 | Racial/ethnic minorities | Qualitative (Focus Groups) | 40 (Hispanic) | Screening reminder delivery | No consensus on screening reminder source (IVR, personal call, letters). Preferred IVR messages recorded by member of clinic staff, native speaker, and community representative. Preferred brief messages emphasizing screening importance. |
| McAlearney 2012 | Rural residents | Qualitative (Focus Groups) | 36 (Appalachian Ohio) | Screening reminder delivery | Preferred timely reminders in the form of a letter or postcard in the mail. |
| Hatcher 2011 | Rural residents | Cross-sectional Survey | 345 (Appalachian Kentucky) | Appointment time | 63.8% did not prefer weekend appointments. |
| Hunter 2012 | Racial/ethnic minorities | Qualitative (Interviews) | 45 (Mexicana) | Results delivery | Preferred detailed explanations of Pap test results accompanied by images of cell changes. |
| Katz 2017 | Low-income populationsb | Qualitative (Focus Groups) | 15 | Results delivery | Mixed preferences on how to receive HPV self-test results (phone, mail, in person). Preferred to avoid receiving results in an office visit. |
Born in Mexico, lived in the US for five years or less, completed nine grades or less of formal education, and speak predominantly Spanish
Those assigned female at birth who identify with a gender other than female
Abbreviations: Healthcare Professional (HCP); Interactive Voice Response (IVR)
Screening Modality (n=23)
A total of 23 studies reported preference outcomes related to cervical cancer screening modality, specifically assessing patient preference between HPV self-testing and traditional cytology (Pap test) (Table 1). Various study designs were used to elicit this preference, including 15 cross-sectional survey studies (35–49), 6 qualitative studies (4 focus group studies (50–53), 2 interview studies (54, 55)), 1 RCT (56), and 1 mixed methods study (57).
All non-focus group studies reported the percentage of participants who preferred the HPV self-test to the Pap test (35–49, 54–57). Percentages ranged from 10% (45) to over 90% (54). Only 2 studies reported a greater percentage of participants preferring the Pap test compared to the HPV self-test (42.3% vs. 9.9% (45); 67.6% vs. 32.4% (35)). While about half of these studies posed the preference question as a dichotomous choice between the two modalities (36–41, 57), the remaining 9 studies provided additional options, such as “no preference” (35, 42–45, 47–49, 55, 56), “would refuse either” (48), and “do not know” (46). Preference for HPV self-testing over Pap testing ranged from 6% (55) to 45% (56) in these studies. Of note, Galbraith and colleagues (2014) assessed preference differently than the studies discussed above in that participants were asked which test they believed provides the highest level of protection. Only 6% believed the HPV self-test offered greater protection than the Pap test; however, 75% of participants believed the tests protected them equally well, and the remaining 19% favored the Pap test (49).
On the whole, low-income populations (35, 44) and women staying in domestic violence shelters (45) preferred the HPV self-test over the Pap test less than other groups. The majority of rural residents studied (89% (39), 66% (40)) preferred the HPV self-test to the Pap test. The percentage of individuals who preferred the HPV self-test among Hispanic populations ranged from 30% among individuals residing along the US-Mexico border (47) to 89% (42). Similarly ranging estimates were seen across studies assessing the preferences of non-Hispanic Black individuals and African-Americans, among other racial/ethnic minorities. Both studies assessing AI/AN populations reported just over 60% of individuals preferring self-testing over the Pap test (37, 41).
The remaining studies (n=4) used focus groups to probe on reasons individuals may prefer one test over the other. In focus groups of low-income, minority individuals, Anhang and colleagues (2004) found that most participants preferred that the physician administer the HPV test, rather than performing a self-test, due to fear of performing the test incorrectly (53). Alternatively, Scarinci and colleagues (2013) and Katz and colleagues (2017) found that the majority of low-income and minority participants preferred HPV self-testing over Pap testing due to convenience and privacy (50, 52). The focus groups conducted by Penaranda and colleagues (2014) with 21 individuals residing along the US-Mexico border did not reach a clear preference consensus, reflecting both the negative and positive self-testing attributes posed by the other three focus group studies (51). Two studies probed further regarding participant preferences for HPV self-testing device instructions. Trans-masculine individuals preferred both video and written instructions tailored to trans-masculine individuals (54). Similarly, African-American individuals echoed the preference for take-home video instructions; however, participants indicated a preference for in-person instructions as well (52).
Of the quantitative studies included, 11 used bivariate and multivariate regression analyses to identify variables associated with a preference for HPV self-testing over Pap testing. Variables identified as significantly associated with a preference for HPV self-testing included: more education (35), older age (49), less frequent screening history (40, 45, 48), and self-reported avoidance of preventive care due to cost or discrimination (48). Additionally, lack of health insurance was associated with preferring HPV self-testing among transgender men (48), whereas having health insurance was associated with preferring HPV self-testing among low-income women (49). Race and ethnicity were significantly associated with HPV self-testing preference in 4 studies, with the following groups having a higher likelihood of preferring self-testing to Pap testing: Hispanics versus Haitians (42), Blacks versus Hispanics (43), Blacks versus Whites (44), and non-Hispanics versus Hispanics (35).
Across the 23 screening modality preference studies, study designs varied regarding whether participants performed the HPV self-test, received a Pap test, or completed both tests prior to stating their preference. Participants underwent both HPV self-testing and a provider-administered Pap test at the time of the survey in only 6 studies (35, 43, 44, 46, 54, 55). In 8 studies, participants received descriptions of the HPV self-test and Pap test but did not undergo either screening procedure (40, 45, 48, 50–53, 57). In the remaining 9 studies, participants self-administered an HPV self-test but did not have a Pap test (36–39, 41, 42, 47, 49, 56). However, in synthesizing study results by test(s) performed, it does not appear that significant differences in preferences existed based on whether participants performed either or both screening modalities prior to being assessed.
Provider Demographics and Attributes (n=11)
Eleven studies reported patient preferences for provider demographics and attributes (Table 2). All 11 studies assessed preferences related to provider gender. Additional outcomes assessed included preferences for the type of examiner (n=3) (58–60), language spoken by the provider (n=3) (58, 61, 62), provider’s communication style (n=3) (60, 61, 63), and provider’s race or ethnicity (n=1) (60).
Gender
All but one study found a preference for female providers over male providers. Of the 5 studies that elicited patients’ preference for a female rather than male provider quantitatively, percentages ranged from 41% (59) to 77% (64). Three of these studies included the option of “no preference”, with between 34% (59) and 50% (65) of respondents selecting this option. Lanier and colleagues (1999) also found that 18% of the AI/AN respondents would refuse a male provider (65), while Nguyen and colleagues (2002) reported that 53% of Vietnamese, Vietnamese-American, or Vietnamese-Chinese participants would prefer to have a female standby if a Pap test was performed by a male provider (62).
Qualitative research provided some insight into the reasons for the female provider preference in select groups. Participants in rural Appalachian Ohio reported trusting female physicians more, and expressed discomfort with male physicians (63). Among Bhutanese refugees, preference for a female provider was the result of negative experiences with male doctors in refugee camps (66). Somali women identified a preference for both female providers and interpreters, in part, due to their sensitivity to female issues (67). In the single study that reported a preference for male over female providers, Hispanic participants often described doctors as male and nurses as female, with most preferring to receive information from a doctor than a nurse. However, Hispanic participants still expressed comfort in speaking to another woman about women’s health issues (58).
Type of Examiner
A clear consensus for preferred examiner type (e.g. physician, nurse, etc.) when being screened for cervical cancer was not found. Alexander and McCullough (1981) provided Mexican-American and low-income women with a choice between the following examiner categories: female physician, male physician, nurse or nurse practitioner, female person, no preference. Forty-eight percent of Mexican-Americans and 39% of low-income study participants preferred a physician (with the majority preferring a female over a male), whereas just 10% of Mexican-Americans and 12% of low-income participants preferred a nurse or nurse practitioner. Of note, 34% of Mexican-Americans and 40% of low-income participants reported no preference (59). Torres and colleagues (2013) also found that Hispanic interviewees typically preferred receiving information from a doctor instead of a nurse (58). In contrast, Agénor and colleagues (2015) reported that Black lesbian, bisexual, or queer (LBQ) focus group participants preferred to receive care from a physician’s assistant, registered nurse, or nurse practitioner, who they felt were more likely to have increased time and attention available (60).
Language
Three studies assessed whether ethnic minority women preferred to have a provider who speaks their own non-English language. In a cross-sectional survey study of Vietnamese, Vietnamese-American, and Vietnamese-Chinese individuals, nearly two-thirds (64%) preferred having a provider who spoke Vietnamese when completing a Pap test (62). Torres and colleagues (2013) similarly found that the majority of Hispanics interviewed preferred a Spanish-speaking healthcare professional (58). Although Kim and colleagues (2017) found that the majority of Korean immigrant women interviewed preferred a female provider who spoke Korean, their provider language preference changed if seeing a male doctor, preferring a male provider who did not speak Korean out of concern for privacy (61).
Communication Style
Among the three studies reporting communication style preferences, McAlearney and colleagues (2012) conducted focus groups and found that patient-centered communication is preferred among individuals residing in rural Appalachian Ohio (63). Kim and colleagues (2017) found that, instead of receiving the results of their Pap test by letter or phone, most Korean immigrant women preferred to receive their results through in-person conversations with their doctor (61). Black LBQ individuals, in particular, articulated the importance of having an examiner with a calm demeanor who described the process of the Pap test before and during the exam (60).
Race/Ethnicity
Only one study examined preferences related to the provider’s race or ethnicity. The majority of Black LBQ participants preferred providers who shared similar characteristics and backgrounds, stating preferences for clinicians who are persons of color, female, and/or from the LGBTQ community. These participants also noted their preference for providers who have had experience with and feel comfortable serving LBQ patients, even if they do not identify as LBQ (60).
Associations with Provider Preferences
Variables associated with preferences for provider demographics and attributes were reported in three studies. Ma and colleagues (2012) found that Vietnamese survey participants who preferred male providers over female providers, as well as those who had no gender preference, were more likely to report a prior Pap test (67). Nguyen and colleagues (2002) reported that older study participants, defined as 65 years of age and above, were more likely to prefer a doctor of the same ethnicity (Vietnamese) but less likely to prefer a female provider (62). Alexander and McCullough (1981) found that Mexican-American and low-income participants were more likely to prefer a female physician over a male physician, nurse, or nurse practitioner compared to other participants attending the free Pap test clinics (59).
Screening Scheduling and Results Delivery (n=6)
A total of 6 studies reported preference outcomes related to cervical cancer screening scheduling and results delivery (Table 3). Outcomes assessed related to logistical aspects of the screening appointment, including reminder delivery (n=3) (63, 68, 69), appointment time (n=1) (40), and results delivery (n=2) (50, 70)).
Reminder Delivery
In their assessment of screening reminder preferences, Brandzel and colleagues (2016) found that African-American participants most often preferred that reminders, whether mailed or sent electronically, came from community-based advocates over health care professionals. Latina participants did not express a preference regarding who the reminder came from but preferred smart phone reminders over mailed letters (68). In contrast, McAlearney and colleagues (2012) reported that rural residents preferred to receive provider encouragement in the form of mailed, versus electronic, reminders (63). Though Greaney and colleagues (2014) did not find a preference consensus on reminder medium among study participants, when asked about interactive voice response (IVR) messages, Latina women preferred brief messages left by a member of their community to more detailed messages recorded by an outside health care professional, and believed that screening details should be limited for confidentiality reasons (69).
Appointment Time and Results Delivery
Only one study assessed patient preferences regarding screening appointment time. Hatcher and colleagues (2011) reported that 36% of rural residents surveyed preferred weekend appointments over weekday appointments, with recently screened individuals more likely to report this preference than individuals who had not been screened in the past five years (40). Lastly, two studies reported preferences related to the delivery of screening results. Both found that participants preferred comprehensive descriptions of results, with Mexican women preferring that images of cell changes accompany their results compared to having no images with their results (70), and low-income women preferring to receive results outside of the doctor’s office as opposed to in the clinic due to anxiety associated with office waiting rooms (50).
Health Education and Communication (n=9)
A total of 9 studies reported preferences related to health education and communication efforts aimed to increase screening uptake in underserved communities (Table 4). Whereas all prior preference results related to the screening encounter, the studies presented in Table 4 considered patient preferences when developing interventions to increase screening uptake. Outcomes assessed related to three broad categories: information source (n=5) (66, 71–74), health education class location (n=1) (75), and printed material attributes (n=4) (70, 74, 76, 77).
Information Source and Education Class Location
Among the 5 studies that assessed preferences related to the source of health information prior to screening, two studies indicated informational preferences in favor of printed materials. Sharpe and colleagues (2013) documented that members of the Cherokee tribe preferred to receive printed educational materials over in-person information since printed materials can be taken home and read in private (72). Similarly, Yemane and colleagues (2016) found that women who have sex with women (WSW) preferred to receive information through the media and pamphlets over conversations with health care providers or community workshops (71).
Three studies noted patients’ preferences to receive information about screening within their community as compared to health care settings. Haworth and colleagues (2014) demonstrated that Bhutanese refugees preferred to learn about screening from community health workers who spoke their native language over other information sources (66), and Kenya and colleagues (2015) found that Haitian focus group participants preferred community health workers to HIV case managers for the delivery of information about screening benefits and guidelines (74). Lee and colleagues (2015) specifically studied preferences and acceptability related to screening information delivery in Vietnamese-American and Korean-American beauty salons; preferences for videos and one-on-one conversations over websites, workshops, and pamphlets were shared by both cosmetologists and customers (73). Relatedly, Yu and colleagues (2001) assessed participant preferences for the location of a health education class, finding that Chinese participants most often preferred community service centers, followed by schools and churches (75).
Printed Material Attributes
Four studies provided participants with sample printed educational materials and probed on preferred attributes. Participants in all four studies emphasized the importance of visual components, including realistic pictures and diagrams, particularly in the case of limited literacy (70, 74, 76, 77). More specifically, Hunter and Kelly (2012) found that Mexican immigrant women preferred screening descriptions and pictures focused on prevention rather than cancer (70), and Christopher and colleagues (2009) noted that, though Apsáalooke women preferred realistic pictures to unrealistic illustrations, they urged against depicting medical procedures in realistic detail (76).
Quality Assessment
The supplementary materials present the results of the quality assessment, identifying either low or high bias for each of the six evaluated categories: (1) study question well-defined, (2) inclusion criteria well-defined, (3) comprehensive description of alternatives, (4) appropriate measure of preferences, (5) appropriate analysis, and (6) pre-specified analysis (Supplementary Table S3). Of the 43 studies, 14 (33%) were identified as having high bias in one category, 7 (16%) as having high bias in two categories, and 3 (7%) as having high bias in three categories.
Among the 24 studies with some type of bias identified, 14 were reported as having high bias specifically related to the appropriateness of the analysis, most commonly due to having an unknown or low (less than 60%) participation rate among quantitative studies. Twelve studies had high bias with respect to the description of alternatives provided to participants. Generally, these studies assessed participants’ preference for screening modality after only administering one of the modality options as part of the study (i.e. self-testing was completed prior to evaluating preferences, but Pap testing was not completed as part of the study). Six studies had potential for bias due to providing minimal description of the primary inclusion criteria, and 5 studies were identified as biased with respect to the appropriateness of the preference measure employed (e.g. acceptability vs. preference). None of the studies were identified as having high bias related to the study question or pre-specified analysis categories.
Of note, many of the studies included did not assess patient preferences as their primary objective but rather to supplement other results. Thus, the results of the quality assessment should not be assumed to apply to the study as a whole, but rather to the assessment of preferences specifically.
Discussion
This systematic review highlights several themes in cervical cancer screening-related preferences among medically underserved populations, including documented preferences for HPV self-testing over Pap testing and providers who share patient socio-demographic characteristics, such as gender and language spoken. This review also demonstrates the variation in patient preferences related to screening scheduling, results delivery, and the communication of health information across the medically underserved populations studied. These findings can be utilized in the development of programs and policies designed to increase screening uptake and reduce disparities in cervical cancer incidence and mortality.
A preference for HPV self-testing over Pap testing emerged as a primary theme, with more participants preferring the HPV self-test in over 90% of screening modality studies. This overwhelming preference for self-testing over Pap testing is consistent with findings in prior systematic reviews of self-testing acceptability, which all found that participants in the majority of included studies preferred self-testing (22–24). The consistency of these results, viewed in concert with other reviews, has important implications for the incorporation of HPV self-testing into primary screening practice. In 2018, the USPSTF approved provider-administered HPV testing as a primary screening strategy, but self-testing was not included (5, 78). The majority of studies that we reviewed assessed patient preferences before HPV testing had been approved as a primary screening mechanism. As such, the novelty of self-testing may have contributed to patient concerns about test accuracy and inability to properly perform the test, which were expressed consistently across study populations. Similar concerns were also identified by prior reviews of self-testing acceptability (22–24). Particularly, Morgan and colleagues (2019) found that participants in 42% (8/19) of studies preferred provider-administered sampling, primarily due to concerns about correctly conducting the test independently (24). These concerns highlight the importance of communication, particularly related to device instructions, as the HPV self-test is increasingly studied, disseminated, and incorporated into national screening guidelines.
A second theme that emerged, of particular relevance to medically underserved populations, was that individuals generally preferred providers who share their demographic characteristics and life experience. In the absence of available providers with gender, racial/ethnic, sexual orientation, and language concordance, individuals valued providers with experience and who demonstrated comfort in serving patients like them. Participants in multiple studies commented on the difficulty of finding providers with whom they felt comfortable (60, 61), underlining the need for increased diversity among the full range of healthcare. professionals, including physicians, nurses, medical assistants, and community health workers. These findings are in line with a prior review of perceived psychosocial barriers to cervical cancer screening, which identified unsatisfactory experiences with physicians, particularly male physicians, as common barriers (15).
Additionally, this review documents the heterogeneity of patient preferences relating to screening communication, which may be attributed to varying degrees of English language ability, comfort with providers, and familiarity with technology among different populations and individuals. This variation highlights the importance of assessing patient preference prior to designing screening intervention programs or adapting evidence-based interventions to new populations (79). It is also important to recognize the individual nature of preferences and the difficulty of generalizing the preferences of one group of patients to the larger population of interest.
It is possible that the introduction of the HPV vaccine in 2006 may have affected cervical cancer screening preferences through changes in risk perception, and thus attitudes toward screening. However, given the age range of participants in the studies reviewed (21–65 years) and the fairly recent introduction of the HPV vaccine, most participants would not have received the HPV vaccine within the recommended age interval of 10–12 years. Additionally, we did not find differences in preferences between participants of studies conducted pre-2006 and those of studies conducted post-2006. As vaccination rates increase over time, though, it may be interesting to assess the influence of vaccination on attitudes toward screening, particularly among underserved groups.
There are several limitations of this review, including the inability to fully characterize the populations studied. The population categorizations reported in Tables 1–4 reflect the population groups identified in each study’s inclusion criteria; however, in many cases, a large majority of patients could be classified as medically underserved along other axes as well, such as being uninsured, having low educational attainment, or having immigrated to the US. Additionally, due to the heterogeneity of patient preferences among individuals, few results could be generalized to medically underserved populations as a whole. This review thus serves, not to draw conclusions about the preferences held by underserved groups but rather to document the variety of preferences reported in the literature to date. It also must be mentioned that studies of cervical cancer screening-related preferences are not currently available for several notable medically underserved groups, such as veterans, individuals with disabilities, and the uninsured. This dearth in the literature represents areas for future research.
This review also demonstrates a need for standardized preference elicitation studies to better capture trends in patient preference and assess potential differences between underserved populations. Of note, no studies utilized formal preference elicitation methods, such as discrete-choice experiments or best-worst scaling (80). Additionally, almost half of cross-sectional survey studies across all preference outcomes did not offer participants a “no preference” option or the ability to not prefer any of the options presented, which is a legitimate preference that is important to consider. A formalized preference elicitation approach recognizing all potential preference options could help various stakeholders - including providers, policy-makers, and researchers - to understand why screening rates remain so much lower among underserved populations, informing future interventions and policies. Interventions that fail to account for the wide range of patient preferences regarding screening will fall short of effectively improving cervical cancer screening uptake, and thus health outcomes, for medically underserved individuals.
This systematic review provides a compilation and synthesis of medically underserved patient preferences relating to cervical cancer screening documented in the current literature. Findings suggest that there is significant heterogeneity of patient preferences across populations and individuals, pointing to the importance of assessing preferences among individuals designed to benefit from a given intervention. That being said, synthesis of the 43 studies included in this review revealed two overarching themes: preference for HPV self-tests over Pap tests and preference for providers who reflect patient gender, language, and life experience. These preferences must be recognized and leveraged by relevant stakeholders in the development of programs and policies to increase cervical cancer screening uptake among individuals most at risk. Failing to account for the specific preferences of medically underserved individuals will allow the disparities in cervical cancer incidence and mortality to continue widening.
Supplementary Material
Acknowledgements
We are grateful to the following individuals for their guidance and feedback on our systematic review protocol: Melissa B. Gilkey, PhD, Rebecca McCall, MSLS, Kate Miele, MD, MA, Victoria Petermann, BSN, RN, Mary White, MS, MSHI.
Funding
This study was supported, in part, by Cooperative Agreement Number U48‐DP005017 from the Centers for Disease Control and Prevention (CDC) Prevention Research Centers (PRC) Program and the National Cancer Institute (NCI), as part of the Cancer Prevention and Control Research Network (CPCRN). LPS and MCO are supported by the Cancer Care Quality Training Program, University of North Carolina at Chapel Hill (UNC-CH), Grant No. T32-CA-116339. CBB was supported by the National Institutes of Health (NIH) Big Data 2 Knowledge (BD2K) Biomedical Graduate Training Program, UNC-CH, Grant No 5-T32-LM-12420-4. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the funders.
Footnotes
Conflicts of Interest
SBW receives unrelated grant funding to her institution from Pfizer. All other authors declare no conflicts of interest.
References
- 1.Yang DX, Soulos PR, Davis B, Gross CP, Yu JB. Impact of widespread cervical cancer screening: number of cancers prevented and changes in race-specific incidence. Am J Clin Oncol. 2018;41(3):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97(12 Suppl):3133–275. [DOI] [PubMed] [Google Scholar]
- 3.Safaeian M, Solomon D. Cervical Cancer Prevention - Cervical Screening: Science in Evolution. Obstet Gynecol Clin North Am. 2007;34(4):739–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin LM, Parker SL, Wingo PA, Heath CW Jr. Cervical cancer incidence and screening: status report on women in the United States. Cancer Pract. 1996;4(3):130–4. [PubMed] [Google Scholar]
- 5.USPSTF. Final Recommendation Statement: Cervical Cancer: Screening. 2018.
- 6.NIH. NIH Fact Sheets - Cervical Cancer.
- 7.Chan DNS, So WKW. A Systematic Review of the Factors Influencing Ethnic Minority Women’s Cervical Cancer Screening Behavior. Cancer Nursing. 2017;40(6):E1–E30. [DOI] [PubMed] [Google Scholar]
- 8.Ackerson K, Gretebeck K. Factors influencing cancer screening practices of underserved women. Journal of the American Academy of Nurse Practitioners. 2007;19(11):591–601. [DOI] [PubMed] [Google Scholar]
- 9.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123(6):1044–50. [DOI] [PubMed] [Google Scholar]
- 10.Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007–2012. MMWR Morbidity and mortality weekly report. 2014;63(44):1004–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–83. [DOI] [PubMed] [Google Scholar]
- 12.Goding Sauer A, Siegel RL, Jemal A, Fedewa SA. Current Prevalence of Major Cancer Risk Factors and Screening Test Use in the United States: Disparities by Education and Race/Ethnicity. Cancer Epidemiol Biomarkers Prev. 2019;28(4):629–42. [DOI] [PubMed] [Google Scholar]
- 13.Watson M, Benard V, King J, Crawford A, Saraiya M. National assessment of HPV and Pap tests: Changes in cervical cancer screening, National Health Interview Survey. Preventive medicine. 2017;100:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCaul KD, Tulloch HE. Cancer screening decisions. Journal of the National Cancer Institute Monographs. 1999(25):52–8. [DOI] [PubMed] [Google Scholar]
- 15.Bukowska-Durawa A, Luszczynska A. Cervical cancer screening and psychosocial barriers perceived by patients. A systematic review. Contemporary oncology (Poznan, Poland). 2014;18(3):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiviniemi MT, Hay JL, James AS, Lipkus IM, Meissner HI, Stefanek M, et al. Decision making about cancer screening: an assessment of the state of the science and a suggested research agenda from the ASPO Behavioral Oncology and Cancer Communication Special Interest Group. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(11):3133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart DE, Gagliardi A, Johnston M, Howlett R, Barata P, Lewis N, et al. Self-collected samples for testing of oncogenic human papillomavirus: a systematic review. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2007;29(10):817–28. [DOI] [PubMed] [Google Scholar]
- 18.Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105(2):530–5. [DOI] [PubMed] [Google Scholar]
- 19.Smith JS, Des Marais AC, Deal AM, Richman AR, Perez-Heydrich C, Yen-Lieberman B, et al. Mailed Human Papillomavirus Self-Collection With Papanicolaou Test Referral for Infrequently Screened Women in the United States. Sexually transmitted diseases. 2018;45(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones HE, Brudney K, Sawo DJ, Lantigua R, Westhoff CL. The Acceptability of a Self-Lavaging Device Compared to Pelvic Examination for Cervical Cancer Screening Among Low-Income Women. Journal of Women’s Health. 2012;21(12):1275–81. [DOI] [PubMed] [Google Scholar]
- 21.Tisci S, Shen YH, Fife D, Huang J, Goycoolea J, Ma CP, et al. Patient acceptance of self-sampling for human papillomavirus in rural china. Journal of lower genital tract disease. 2003;7(2):107–16. [DOI] [PubMed] [Google Scholar]
- 22.Huynh J, Howard M, Lytwyn A. Self-Collection for Vaginal Human Papillomavirus Testing. Journal of Lower Genital Tract Disease. 2010;14(4):356–62. [DOI] [PubMed] [Google Scholar]
- 23.Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sexually transmitted infections. 2017;93(1):56–61. [DOI] [PubMed] [Google Scholar]
- 24.Morgan K, Azzani M, Khaing SL, Wong YL, Su TT. Acceptability of Women Self-Sampling versus Clinician-Collected Samples for HPV DNA Testing: A Systematic Review. J Low Genit Tract Dis. 2019;23(3):193–9. [DOI] [PubMed] [Google Scholar]
- 25.Corcoran J, Crowley M. Latinas’ attitudes about cervical cancer prevention: a meta-synthesis. Journal of cultural diversity. 2014;21(1):15–21. [PubMed] [Google Scholar]
- 26.Johnson CE, Mues KE, Mayne SL, Kiblawi AN. Cervical Cancer Screening Among Immigrants and Ethnic Minorities. Journal of Lower Genital Tract Disease. 2008;12(3):232–41. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute for Health Research. PROSPERO: International prospective register of systematic reviews. University of York Centre for Reviews and Dissemination; 2019. [Google Scholar]
- 30.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics and Decision Making. 2007;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA. Using the PICOTS Framework to Strengthen Evidence Gathered in Clinical Trials - Guidance from the AHRQ’s Evidence-based Practice Centers Program | FDA. 2019.
- 32.USDHHS. National Stakeholder Strategy for Improving Health Equity: Office of Minority Health; 2019. [Available from: https://www.minorityhealth.hhs.gov/npa/files/Plans/NSS/NSS_05_Section1.pdf.
- 33.Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purnell TS, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37(7):2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. Journal of women’s health (2002). 2005;14(8):721–8. [DOI] [PubMed] [Google Scholar]
- 36.Barbee L, Kobetz E, Menard J, Cook N, Blanco J, Barton B, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer causes & control : CCC. 2010;21(3):421–31. [DOI] [PubMed] [Google Scholar]
- 37.Cina KR, Omidpanah AA, Petereit DG. Assessing HPV and cervical knowledge, preference and HPV status among urban american indian women. S D Med. 2017;70(10):439–43. [PubMed] [Google Scholar]
- 38.Crosby RA, Hagensee ME, Fisher R, Stradtman LR, Collins T. Self-collected vaginal swabs for HPV screening: An exploratory study of rural Black Mississippi women. Prev Med Rep. 2017;7:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosby RA, Hagensee ME, Vanderpool R, Nelson N, Parrish A, Collins T, et al. Community-Based Screening for Cervical Cancer: A Feasibility Study of Rural Appalachian Women. Sexually transmitted diseases. 2015;42(11):607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. Journal of Health Care for the Poor and Underserved. 2011;22(1):176–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winer RL, Gonzales AA, Noonan CJ, Cherne SL, Buchwald DS, Collaborative to Improve Native Cancer O. Assessing Acceptability of Self-Sampling Kits, Prevalence, and Risk Factors for Human Papillomavirus Infection in American Indian Women. J Community Health. 2016;41(5):1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilangovan K, Kobetz E, Koru-Sengul T, Marcus EN, Rodriguez B, Alonzo Y, et al. Acceptability and Feasibility of Human Papilloma Virus Self-Sampling for Cervical Cancer Screening. Journal of women’s health (2002). 2016;25(9):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones HE, Brudney K, Sawo DJ, Lantigua R, Westhoff CL. The acceptability of a self-lavaging device compared to pelvic examination for cervical cancer screening among low-income women. Journal of women’s health (2002). 2012;21(12):1275–81. [DOI] [PubMed] [Google Scholar]
- 44.Kilfoyle KA, Des Marais AC, Ngo MA, Romocki L, Richman AR, Barclay L, et al. Preference for Human Papillomavirus Self-Collection and Papanicolaou: Survey of Underscreened Women in North Carolina. J Low Genit Tract Dis. 2018;22(4):302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levinson KL, Jernigan AM, Flocke SA, Tergas AI, Gunderson CC, Huh WK, et al. Intimate partner violence and barriers to cervical cancer screening: A gynecologic oncology fellow research network study. J Low Genit Tract Dis. 2016;20(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litton AG, Castle PE, Partridge EE, Scarinci IC. Cervical cancer screening preferences among African American women in the Mississippi Delta. J Health Care Poor Underserved. 2013;24(1):46–55. [DOI] [PubMed] [Google Scholar]
- 47.Penaranda E, Molokwu J, Flores S, Byrd T, Brown L, Shokar N. Women’s Attitudes Toward Cervicovaginal Self-Sampling for High-Risk HPV Infection on the US-Mexico Border. J Low Genit Tract Dis. 2015;19(4):323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seay J, Ranck A, Weiss R, Salgado C, Fein L, Kobetz E. Understanding Transgender Men’s Experiences with and Preferences for Cervical Cancer Screening: A Rapid Assessment Survey. LGBT Health. 2017;4(4):304–9. [DOI] [PubMed] [Google Scholar]
- 49.Galbraith KV, Gilkey MB, Smith JS, Richman AR, Barclay L, Brewer NT. Perceptions of mailed HPV self-testing among women at higher risk for cervical cancer. J Community Health. 2014;39(5):849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz ML, Zimmermann BJ, Moore D, Paskett ED, Reiter PL. Perspectives from health-care providers and women about completing human papillomavirus (HPV) self-testing at home. Women Health. 2017;57(10):1161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penaranda E, Molokwu J, Hernandez I, Salaiz R, Nguyen N, Byrd T, et al. Attitudes toward self-sampling for cervical cancer screening among primary care attendees living on the US-Mexico border. South Med J. 2014;107(7):426–32. [DOI] [PubMed] [Google Scholar]
- 52.Scarinci IC, Litton AG, Garcés-Palacio IC, Partridge EE, Castle PE. Acceptability and usability of self-collected sampling for HPV testing among African-American women living in the Mississippi Delta. Womens Health Issues. 2013;23(2):e123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anhang R, Wright TC, Smock L, Goldie SJ. Women’s desired information about human papillomavirus. Cancer. 2004;100(2):315–20. [DOI] [PubMed] [Google Scholar]
- 54.Reisner SL, Deutsch MB, Peitzmeier SM, White Hughto JM, Cavanaugh TP, Pardee DJ, et al. Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PloS one. 2018;13(3):e0190172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pieters HC, Wiley DJ. Decision-making about cervical cancer screening methods by homeless women. J Natl Black Nurses Assoc. 2013;24(1):9–15. [PubMed] [Google Scholar]
- 56.Molokwu JC, Penaranda E, Dwivedi A, Mallawaarachchi I, Shokar N. Effect of Educational Intervention on Self-Sampling Acceptability and Follow-Up Paps in Border Dwelling Hispanic Females. J Low Genit Tract Dis. 2018;22(4):295–301. [DOI] [PubMed] [Google Scholar]
- 57.McDowell M, Pardee DJ, Peitzmeier S, Reisner SL, Agénor M, Alizaga N, et al. Cervical Cancer Screening Preferences Among Trans-Masculine Individuals: Patient-Collected Human Papillomavirus Vaginal Swabs Versus Provider-Administered Pap Tests. LGBT Health. 2017;4(4):252–9. [DOI] [PubMed] [Google Scholar]
- 58.Torres E, Erwin DO, Treviño M, Jandorf L. Understanding factors influencing Latina women’s screening behavior: a qualitative approach. Health Educ Res. 2013;28(5):772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexander K, McCullough J. Women’s preferences for gynecological examiners: sex versus role. Women Health. 1981;6(3–4):123–34. [DOI] [PubMed] [Google Scholar]
- 60.Agénor M, Bailey Z, Krieger N, Austin SB, Gottlieb BR. Exploring the Cervical Cancer Screening Experiences of Black Lesbian, Bisexual, and Queer Women: The Role of Patient-Provider Communication. Women Health. 2015;55(6):717–36. [DOI] [PubMed] [Google Scholar]
- 61.Kim K, Kim S, Gallo JJ, Nolan MT, Han H-R. Decision making about Pap test use among Korean immigrant women: A qualitative study. Health Expect. 2017;20(4):685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen TT, McPhee SJ, Nguyen T, Lam T, Mock J. Predictors of cervical Pap smear screening awareness, intention, and receipt among Vietnamese-American women. Am J Prev Med. 2002;23(3):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAlearney AS, Oliveri JM, Post DM, Song PH, Jacobs E, Waibel J, et al. Trust and distrust among Appalachian women regarding cervical cancer screening: a qualitative study. Patient Educ Couns. 2012;86(1):120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carey Jackson J, Taylor VM, Chitnarong K, Mahloch J, Fischer M, Sam R, et al. Development of a cervical cancer control intervention program for Cambodian American women. J Community Health. 2000;25(5):359–75. [DOI] [PubMed] [Google Scholar]
- 65.Lanier AP, Kelly JJ, Holck P. Pap prevalence and cervical cancer prevention among Alaska Native women. Health Care Women Int. 1999;20(5):471–86. [DOI] [PubMed] [Google Scholar]
- 66.Haworth RJ, Margalit R, Ross C, Nepal T, Soliman AS. Knowledge, attitudes, and practices for cervical cancer screening among the Bhutanese refugee community in Omaha, Nebraska. J Community Health. 2014;39(5):872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma GX, Fang CY, Feng Z, Tan Y, Gao W, Ge S, et al. Correlates of cervical cancer screening among Vietnamese American women. Infect Dis Obstet Gynecol. 2012;2012:617234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brandzel S, Chang E, Tuzzio L, Campbell C, Coronado N, Bowles EJA, et al. Latina and black/african american women’s perspectives on cancer screening and cancer screening reminders. J Racial Ethn Health Disparities. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greaney ML, De Jesus M, Sprunck-Harrild KM, Tellez T, Bastani R, Battaglia TA, et al. Designing audience-centered interactive voice response messages to promote cancer screenings among low-income Latinas. Prev Chronic Dis. 2014;11(3):E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunter J, Kelly PJ. Imagined anatomy and other lessons from learner verification interviews with Mexican immigrant women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E1–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yemane RE, Brueggmann D, Brown N, Church T, Jaque JM. Perceptions and patterns of cervical cancer screening among predominately Hispanic women who have sex with women: a cross-sectional study. Eur J Obstet Gynecol Reprod Biol. 2016;198:163–4. [DOI] [PubMed] [Google Scholar]
- 72.Sharpe PA, Brandt HM, McCree DH, Owl-Myers E, Taylor B, Mullins G. Development of culturally tailored educational brochures on HPV and pap tests for American Indian women. J Transcult Nurs. 2013;24(3):282–90. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Carvallo M, Lee E. Feasibility of utilizing ethnic beauty salons for cervical cancer screening education. West J Nurs Res. 2015;37(11):1489–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kenya S, Carrasquillo O, Fatil M, Jones J, Jean C, Huff I, et al. Human Papilloma Virus and Cervical Cancer Education Needs among HIV-Positive Haitian Women in Miami. Womens Health Issues. 2015;25(3):262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu ESH, Kim KK, Chen EH, Brintnall RA. Breast and Cervical Cancer Screening among Chinese American Women. Cancer Pract. 2001;9(2):81–91. [DOI] [PubMed] [Google Scholar]
- 76.Christopher S, Smith A, McCormick AKH, Christopher S, Smith A, McCormick AKHG. Participatory development of a cervical health brochure for Apsáalooke women. Journal of Cancer Education. 2009;20(3):173–6. [DOI] [PubMed] [Google Scholar]
- 77.Reed SD, Assefi NP, Gooding TD, Teklemariam M. Knowledge and attitudes regarding routine health screening and prevention in Somali, Vietnamese, and Latina women. Clin J Womens Health. 2002;2(3):105–11. [Google Scholar]
- 78.Smith JS, Des Marais AC, Deal AM, Richman AR, Perez-Heydrich C, Yen-Lieberman B, et al. Mailed Human Papillomavirus Self-Collection With Papanicolaou Test Referral for Infrequently Screened Women in the United States. Sexually transmitted diseases. 2018;45(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wheeler SB, Basch E. Translating Cancer Surveillance Data Into Effective Public Health Interventions. Jama. 2017;317(4):365–7. [DOI] [PubMed] [Google Scholar]
- 80.Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


