Abstract
Guignas (Leopardus guigna) are small felids closely associated with native forest habitats. In fragmented landscapes, they use vegetation corridors and forest remnants to move across the landscape. In these contexts, guignas may increase contact probabilities with domestic animals, being therefore relevant to assess their pathogens and parasites. The aim of this study was to characterize the helminth fauna in the gastrointestinal tract and cardiorespiratory system of guignas from central and southern Chile. Between 2015 and 2018, 33 dead free-ranging guignas were found road-killed or were collected from wildlife rescue centers. Thirty-two gastrointestinal tracts and 32 cardiorespiratory organs were analyzed through direct analysis and artificial digestion. We found 81.8% (27/33) guignas were positive for helminth endoparasites (84.4% (27/32) positive for gastrointestinal parasites, 37.5% (12/32) positive for cardiorespiratory parasites). Fourteen parasites were identified (7 at genus level and 7 at species level), with Angiostrongylus sp., Molineus sp., Oslerus sp. and Troglostrongylus sp. as first records in guignas. The most prevalent parasites were the species Toxascaris leonina, Toxocara cati and Uncinaria stenocephala. Uncinaria stenocephala showed the highest intensity of infection. Multiparasitism was observed in 76% of the animals. Significant differences in richness of endoparasites and prevalence of cardiorespiratory parasites were found between geographic zones; higher values in the southern zone are possibly due to favorable environmental characteristics for endoparasite development. There were no statistically significant differences between sexes. All the parasites found in this study have been previously reported in domestic cats. These results are valuable to understand parasite transmission at the domestic-wildlife interface; the possibility of endoparasite transmission between domestic cats and guignas should be clarified with molecular analysis.
Keywords: Leopardus guigna, endoparasite; Helminth; Necropsy
Graphical abstract
Highlights
-
•
The 81.8% of analyzed Leopardus guigna were positive for helminth parasites.
-
•
First report of Angiostrongylus sp., Molineus sp., Oslerus sp. and Troglostrongylus sp. in guignas.
-
•
Higher prevalence and diversity of cardiorespiratory parasites in guignas from southern Chile.
1. Introduction
Parasites are important for biodiversity; they belong to biological communities and have an impact on food chains (Hudson et al., 2006; Dobson et al., 2008). The diversity and abundance of parasites can reflect the diversity and abundance of the community of hosts, thus, parasites may be indicative of the health of the ecosystems (Hudson et al., 2006), shedding light on disturbances in the hosts and their environments (Marcogliese, 2005; Thompson et al., 2010).
The current climate change and human perturbation of the environment could modify the dynamics of pathogen transmission (Seguel and Gottdenker, 2017). This would be more relevant for native species in fragmented habitats, where they could increase their contact probability with domestic species and the infectious agents they may carry (Fiorello et al., 2006). The guigna (Leopardus guigna), a small wild felid, is currently affected by human perturbation, being classified as Vulnerable by the IUCN Red List (Napolitano et al., 2015). Guignas are closely associated with native forests of Chile and Argentina, but they can adapt to move across fragmented landscapes using vegetation corridors (Sanderson et al., 2002; Acosta-Jamett and Simonetti, 2004; Gálvez et al., 2013, 2018; Fleschutz et al., 2016). Thus, this felid could be an indicator species for the consequences of habitat fragmentation on forest-dwelling species, and it may be possible to study the contact between guignas and domestic carnivores through the pathogens and parasites they could share.
Previous studies on endoparasites of guignas were based on coprological analysis (Cortés, 2006; González-Acuña et al., 2010; Vallverdú, 2014; Acosta-Jamett et al., 2018), direct techniques with necropsy in a small number of animals (Wolffhügel, 1949; Álvarez, 1963; Álvarez et al., 1970; Fernández and Villalba, 1984; González-Acuña et al., 2010; Moleón et al., 2015), and mainly targeting gastrointestinal parasites. The aim of this study was to characterize the helminth fauna in the gastrointestinal tract and cardiorespiratory system of guignas from central and southern Chile. Our objective was to describe prevalence, richness and diversity of parasites in free-ranging guignas, assessed for the first time with a direct technique in a larger number of animals.
2. Material and methods
2.1. Sampling
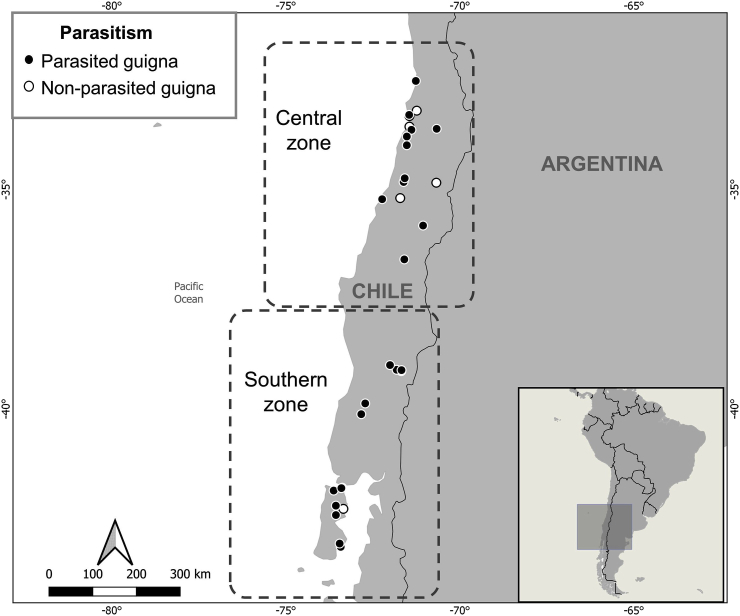
From 2015 to 2018, 33 dead free-ranging guignas were collected, found road-killed or after death or being euthanized in wildlife rescue centers. All guignas came from anthropized areas. Seventeen (51.5%) guignas were from central Chile (hereinafter central zone, Valparaíso region to Biobío region, 32°S 71.6°W - 38°S 72.4°W) and 16 (48.5%) from southern Chile (hereinafter southern zone, Araucanía region to Los Lagos region, 38°S 72.4°W - 43°S 73.6°W) (Fig. 1, Supplementary Table S1).). Eighteen females and 15 males were analyzed, 31 adults and 2 juveniles. Trachea, lungs and heart were collected. The gastrointestinal tracts were removed, knotting each portion separately (esophagus, stomach, small intestine and large intestine) with a cotton thread. The organs were frozen and stored at −20 °C until subsequent analysis.
Fig. 1.
Geographical locations and parasitism status of analyzed guigna samples in Chile. Dotted line boxes delimit the two geographic zones (Center, South) studied in Chile.
In two different road-killed guignas, the cardiorespiratory organs (in one individual) and the gastrointestinal tract (in the other) were destroyed. Therefore 32 gastrointestinal tracts and 32 hearts and lungs were analyzed. Carcass collection was approved by Animal Ethics Committee of the Institute of Ecology and Biodiversity of the Universidad de Chile (resolution of 20 November 2015) and SAG (Agriculture and Livestock Service) (capture permits 7624/2015, 2288/2016, 2185/2017, 4072/2018).
2.2. Sample analysis
Carcasses, organs and helminths were analyzed at the Parasitology Laboratory of the Department of Animal Preventive Medicine at the Faculty of Veterinary and Animal Sciences of University of Chile.
Each portion of the gastrointestinal tract was dissected for macroscopic analysis. The content was washed into a 63 μm mesh stainless steel sieve, and the liquid obtained was observed under a stereo microscope (Zeiss Stemi 1000 Stereo Microscope), according to Tagle (1970). The heart and respiratory tract (trachea and bronchial tree) were dissected and observed under a stereo microscope. Then, for a detailed inspection for undetected helminths, we performed an enzymatic digestion with hydrochloric acid and pepsin to the lungs and hearts, according to Martínez-Rondán et al. (2017). All the retrieved helminths were stored in 70% ethanol at room temperature.
Nematodes were cleared with lactophenol, while cestodes were dehydrated in a series of alcohol concentrations (30°, 40°, 50°, 60°, 70° and 99.9°) and cleared with xylol. The helminths were observed under a light microscope (Miotic BA310 Led Binocular) at 10X and 40X. Parasites were identified according to morphological keys of Soulsby (1987), Khalil et al. (1994) and Anderson et al. (2009).
2.3. Statistical analysis
The observed prevalence (infected guignas/analyzed guignas), intensity of infection (number of parasites/guigna), abundance of infection and parasite species richness were obtained according to Bush et al. (1997), and compared between geographic zones (central/south) and sex of the guignas.
We conducted a Pearson's chi-squared test (statistical significance of p ≤ 0.05) to compare the prevalence of parasite infection between sexes and geographic zones. Associations between intensity of infection and species richness, with sex or geographic zone, were analyzed with the Mann-Whitney U test. The Shannon-Wiener index was used to estimate diversity.
Statistical analyses were conducted with the software IBM SPSS Statistics 20 and PAST 3.2.
3. Results
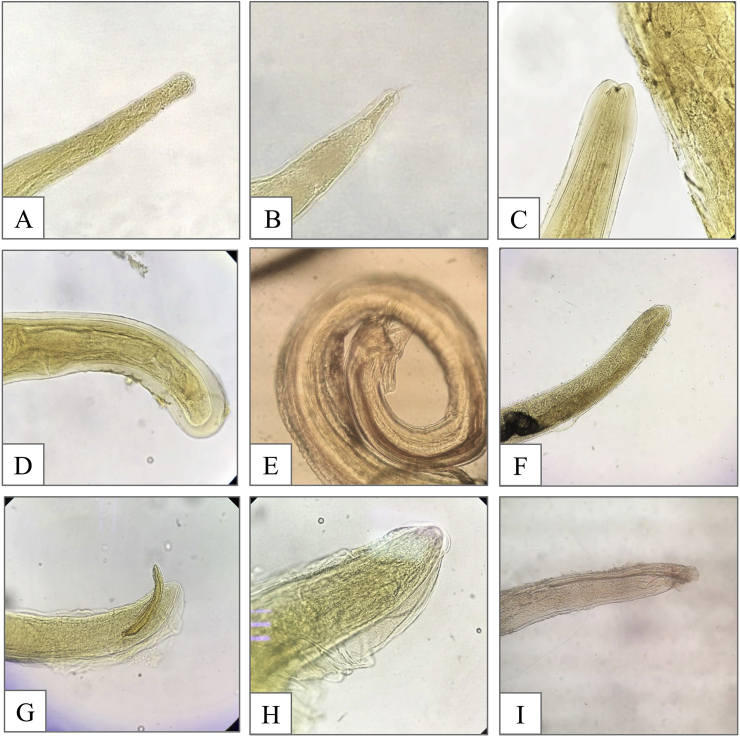
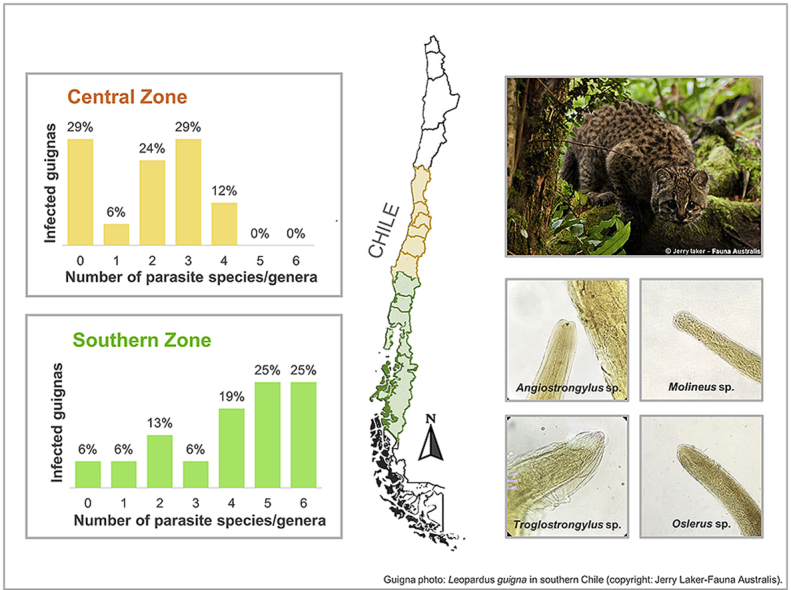
Helminth endoparasites were recorded in 27 (81.8%) of 33 analyzed guignas. Twenty-seven (84.4%) of 32 gastrointestinal tracts were parasitized, and 12 (37.5%) of 32 guignas were positive for cardiorespiratory helminths, with parasites in four hearts (12.5%) and 11 lungs (34.4%). Fourteen parasites were identified, 7 at genus level and 7 at species level, in addition to immature proglottids of Diphyllobothriidea cestodes that could not be identified (Table 1). This is the first record in guignas of Angiostrongylus sp. (Strongylida: Angiostrongylidae), Molineus sp. (Strongylida: Molineidae), Oslerus sp. (Strongylida: Filaroididae) and Troglostrongylus sp. (Strongylida: Crenosomatidae) (Fig. 2). Angiostrongylus sp. was present in the heart of four guignas from southern Chile (Pucón and Villarrica). Molineus sp. was found in the small intestine of a guigna from Pucón. Oslerus sp. was detected in the peribronchial tissue and lung parenchyma of guignas from Pucón and Paillaco (southern zone). Troglostrongylus sp. was retrieved in the lungs of four guignas from southern Chile (Pucón and Quellón) and one guigna from the central zone (Constitución).
Table 1.
Observed prevalence, mean abundance (±SE), mean infection intensity (±SE) and intensity of infection range (min-max), of helminths retrieved in the sampled guignas.
| Organsa |
Prevalence |
Mean abundance |
Mean infection intensity |
Infection intensity range |
||
|---|---|---|---|---|---|---|
| IG/AGb |
% (CI)c |
|||||
| Parasitized guignas | 27/33 | 81.8 (67.9–95.7) | ||||
| Gastrointestinal parasites | 27/32 | 84.4 (71.1–97.7) | ||||
| Toxascaris leonina | E, S, SI, LI | 18/32 | 56.3 (38.1–74.4) | 6.25 ± 3.31 | 11.11 ± 5.68 | 1–103 |
| Toxocara cati | E, SI, LI | 12/32 | 37.5 (19.8–55.2) | 1.28 ± 0.38 | 3.42 ± 0.67 | 1–8 |
| Uncinaria stenocephala | SI, LI | 12/32 | 37.5 (19.8–55.2) | 7.5 ± 3.62 | 20 ± 8.71 | 1–105 |
| Taenia sp. | E, SI, LI | 9/32 | 28.1 (11.7–44.6) | 0.31 ± 0.09 | 1.11 ± 0.11 | 1–2 |
| Hydatigera taeniaeformis | E SI | 9/32 | 28.1 (11.7–44.6) | 0.72 ± 0.31 | 2.56 ± 0.85 | 1–9 |
| Spirometra sp. | SI | 6/32 | 18.8 (4.5–33) | 0.44 ± 0.17 | 2.33 ± 0.33 | 1–3 |
| Spirometra mansonoides | E, SI, LI | 4/32 | 12.5 (0.4–24.6) | 0.78 ± 0.49 | 6.25 ± 2.84 | 2–14 |
| Diphyllobothriidea | SI, LI | 4/32 | 12.5 (0.4–24.6) | 0.31 ± 0.22 | 2.5 ± 1.5 | 1–7 |
| Molineus sp. | SI | 1/32 | 3.1 (0–9.5) | 0.03 ± 0.03 | 1 | 1 |
| Capillarinae gen. sp. | E | 1/32 | 3.1 (0–9.5) | 0.03 ± 0.03 | 1 | 1 |
| Cardiorespiratory parasites | 12/32 | 37.5 (19.8–55.2) | ||||
| Aelurostrongylus abstrusus | L | 6/32 | 18.8 (4.5–33) | 1.53 ± 1.16 | 8.17 ± 5.8 | 1–37 |
| Troglostrongylus sp. | T, L | 5/32 | 15.6 (2.3–28.9) | 1.16 ± 0.79 | 7.4 ± 4.41 | 1–24 |
| Angiostrongylus sp. | H | 4/32 | 12.5 (0.4–24.6) | 0.13 ± 0.06 | 1 | 1 |
| Eucoleus aerophilus | T, L | 3/32 | 9.4 (0–20.1) | 0.25 ± 0.19 | 2.67 ± 1.67 | 1–6 |
| Oslerus sp | L | 2/32 | 6.3 (0–15.1) | 2.78 ± 2.42 | 44.5 ± 32.5 | 12–77 |
Anatomic location of the endoparasites: E, esophagus; S, stomach; SI, small intestine; LI, large intestine; T, trachea; L, lungs; H, heart.
Infected guignas/Analyzed guignas.
Prevalence of infection expressed in percentage (Confidence interval with 95% confidence level).
Fig. 2.
Parasites recovered from analyzed guignas in Chile: A-anterior extremity of female Molineus sp. (40x); B- caudal extremity of female Molineus sp. with caudal spine (40x); C- oral extremity of female Angiostrongylus sp. (40x); D-lateral view of caudal extremity of female Angiostrongylus sp. (40x); E-caudal extremity of male Angiostrongylus sp., caudal bursa and spicules can be observed (40x); F- lateral view of the rounded caudal extremity of female Oslerus sp. (40x); G-lateral view of the caudal extremity of male Oslerus sp., with characteristic short and stout spicules (40x); H- oral extremity of female Troglostrongylus sp. (100x); I- lateral view of the caudal extremity of male Troglostrongylus sp., long spicules and caudal bursa can be observed (40x).
The most prevalent parasites were the species Toxascaris leonina (Ascaridida: Ascarididae) (56.3%), Toxocara cati (Ascaridida: Toxocaridae) (37.5%) and Uncinaria stenocephala (Strongylida: Ancylostomatidae) (37.5%). The highest intensity of infection was shown by U. stenocephala (105 helminths).
The most frequent parasite associations were T. leonina with T. cati and T. leonina with U. stenocephala in nine guignas, followed by T. leonina with Hydatigera taeniaeformis (Cyclophyllidea: Taeniidae) and T. cati with U. stenocephala, in six guignas. Nevertheless, these associations were not statistically significant. The parasites that showed positive associations and significant values were Troglostrongylus sp. with Angiostrongylus sp. (in three guignas, p = 0.0005) and U. stenocephala with Oslerus sp. (in two guignas, p = 0.049). A non-significant negative association was found between T. cati and Troglostrongylus sp. (p = 0.052), with no cases of coinfection.
Analyzing prevalence by geographic zone, we found a significant difference in the prevalence of cardiorespiratory parasites (center: 5.9% and south: 73.3%; χ2 = 15.469; p = 0.00008), with higher values in the south. No significant difference was found in parasite prevalence between sexes (Table 2, Supplementary Table S2). Mean abundance of parasites and general mean infection intensity showed significant differences between geographic zones, with higher values in guignas from the southern zone (Table 2).
Table 2.
Parasitism prevalence, mean abundance (±standard error), and mean infection intensity (±standard error) of helminths retrieved, by geographic zone and sex of the guignas.
| Geographic zone |
Sex |
|||||
|---|---|---|---|---|---|---|
| Center | South | p value | Females | Males | p value | |
| Parasitism prevalence | 12 (70.6%) | 15 (93.8%) | NSa | 14 (77.8%) | 13 (86.7%) | NS |
| GI prevalenceb | 12 (75.0%) | 15 (93.8%) | NS | 14 (77.8%) | 13 (92.9%) | NS |
| CR prevalencec | 1 (5.9%) | 11 (73.3%) | 0.00008 | 4 (23.5%) | 8 (53.3%) | NS |
| Mean abundance | 4.88 ± 5.55 | 41.81 ± 66.41 | 0.0004 | 23.89 ± 13.74 | 21.47 ± 9.78 | NS |
| Mean infection intensity | 6.92 ± 1.57 | 44.6 ± 67.77 | 0.001 | 30.71 ± 17.36 | 24.77 ± 11.05 | NS |
NS = non-significant p value (p > 0.05).
Guignas positive for gastrointestinal parasites.
Guignas positive for cardiorespiratory parasites.
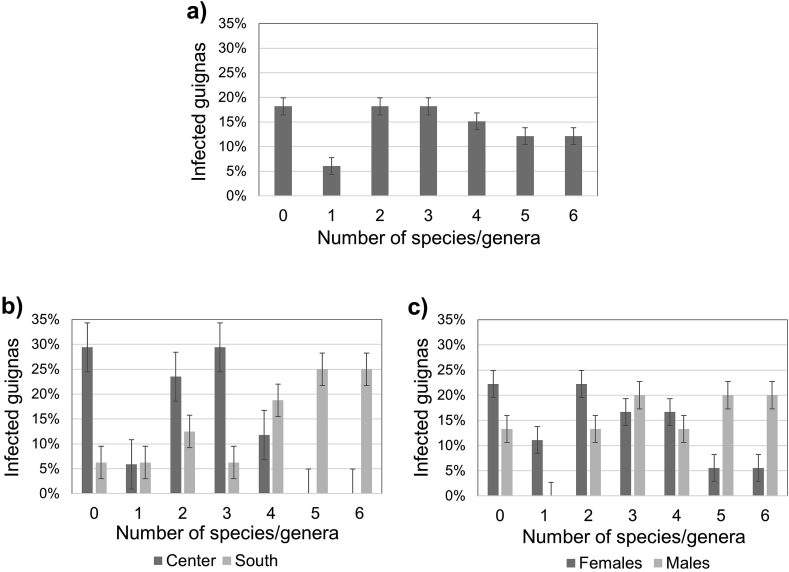
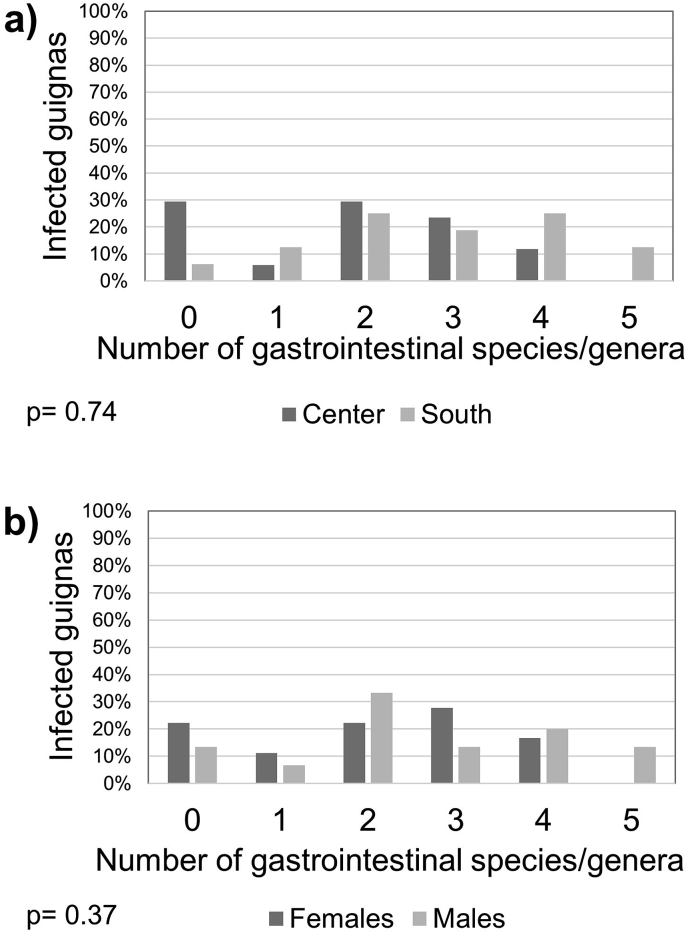
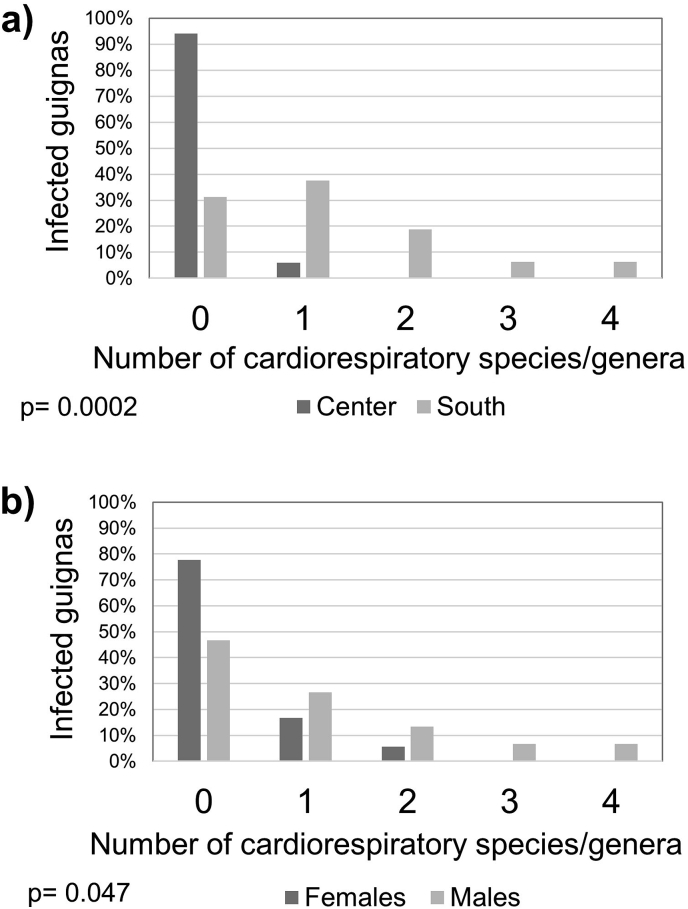
Multiparasitism was present in 76% of the guignas, with a maximum of six parasite species/genera in four cases. There was a significant difference in parasite richness between geographic zones (Mann–Whitney U = 51.5; p = 0.002), being higher in southern guignas (Fig. 3, Supplementary Table S3). Regarding parasite richness per system (gastrointestinal and cardiorespiratory organs) we found significantly higher cardiorespiratory parasite richness in guignas from the southern zone (Mann–Whitney U = 48.0; p = 0.0002) and in males (Mann–Whitney U = 88.0; p = 0.047) (Fig. 4, Fig. 5).
Fig. 3.
Parasite richness (number of species/genera) in the analyzed guignas from Chile (a) overall, (b) by geographic zone and (c) by sex. Error bars represent standard error of 5%.
Fig. 4.
Richness of gastrointestinal parasites in guignas from Chile, compared (a) by geographic zone and (b) by sex. p values show the statistical significance in the Mann-Whitney U test between (a) center-south and (b) females-males.
Fig. 5.
Richness of cardiorespiratory parasites in guignas from Chile, compared by (a) geographic zone and (b) by sex. p values show the statistical significance in the Mann-Whitney U test between (a) center-south and (b) females-males.
The Shannon-Wiener diversity index showed similar values between sexes and geographic zones. Nevertheless, the mean richness was different between them (Table 3).
Table 3.
Specific richness (number of parasite species/genera), mean richness (±SD) (mean of parasite species/genera per sample) and Shannon-Wiener index of the helminths retrieved, by geographic zone and sex of the analyzed guignas.
| Parameter | n | Specific richness | Mean richness | Shannon-Wiener (H′) |
|---|---|---|---|---|
| Geographic zone | ||||
| Center | 17 | 8 | 1.88 ± 1.45 | 1.882 |
| South | 16 | 14 | 4.0 ± 1.9 | 1.848 |
| Sex | ||||
| Females | 18 | 12 | 2.33 ± 1.81 | 1.723 |
| Males | 15 | 13 | 3.6 ± 1.99 | 1.881 |
4. Discussion
We report higher parasite richness compared to previous studies, with a maximum of 8 parasite species in Vallverdú (2014) and Acosta-Jamett et al. (2018). This could be due to the larger number of guignas sampled and the direct analysis applied to the organs. With a direct analysis to the organs, we could detect adult helminths, in contrast to previous coprological studies, where detection of parasites is affected by variations in egg and larvae excretion in the feces of the host (Houpin et al., 2016).
The Shannon-Wiener index considers species richness and abundance (Smith and Smith, 2001). In our study, the Shannon index showed similar values despite the difference in parasite richness between geographic zones. This could be due to greater variation in parasite abundance among guignas in the south, decreasing the diversity index. Guignas from the central zone have lower parasite richness, but abundance is similar among animals. The higher index in males could be due to the higher species/genera richness found.
Four parasite genera were detected for the first time in L. guigna. Molineus is a nematode genus with a direct transmission cycle; the definitive hosts (carnivores and primates) acquire the infective larvae from the environment (Bowman et al., 2002). Four species/genera have been previously reported in felids: Molineus sp. in jaguar (Panthera onca), cougar (Puma concolor) and ocelot (Leopardus pardalis) in Peru (Aranda et al., 2013); M. barbatus in bobcat (Lynx rufus) from USA (Hiestand et al., 2014); M. felineus in cougar from Argentina (Moleón et al., 2015) and M. cati in domestic cat (Felis catus) from South Africa (Durette-Desset et al., 2000). In this study the identification at species level could not be achieved because the only nematode found was an adult female.
The other three newly detected genera in guigna: Angiostrongylus sp., Oslerus sp. and Troglostrongylus sp. (Nematoda: Metastrongyloidea) are endoparasites with an indirect life cycle. The intermediate hosts are gastropods, but rodents, birds and reptiles can become paratenic hosts (Bowman et al., 2002). According to the dietary composition of guignas (Freer, 2004; Correa and Roa, 2005; Zúñiga et al., 2005; Astorga, 2013; Figueroa et al., 2018), paratenic hosts are possibly their main source of infection, as suggested in other felids (Bowman et al., 2002; Brianti et al., 2013). None of these three genera have been previously reported in the genus Leopardus. In the case of Troglostrongylus sp., our report is the first detection in a South American felid.
In our study, the four guignas positive for Angiostrongylus sp., had only one adult helminth in their hearts. These results differ from previous studies in felids (Vieira et al., 2013; Traversa et al., 2015; Diakou et al., 2016; Gherman et al., 2016), where the hosts carry several helminths; however, Varcasia et al. (2014) found only one female adult in the pulmonary artery of a domestic cat.
Metastrongyloid mixed infections were detected in five guignas from the southern zone; one of them, a male guigna from Pucón, was infected by the four metastrongyloids detected in this study (Aelurostrongylus abstrusus (Strongylida: Angiostrongylidae), Angiostrongylus sp., Oslerus sp. and Troglostrongylus sp). These co-infections have been previously reported in domestic cats (Traversa et al., 2015; Varcasia et al., 2015) and European wildcat (Felis silvestris silvestris) (Veronesi et al., 2016) in Europe. This could be due to the occurrence of shared intermediate and paratenic hosts for these parasites in the same area (Traversa et al., 2015).
The lack of previous reports of these parasites in guignas, may be due to difficulties in the diagnosis based on coprological analysis, where the larval stages of metastrongyloids are similar in size and morphology (Brianti et al., 2014b). Moreover, previous studies did not adopt the Baermann test, regarded as the gold standard technique for detection of metastrongyloid larvae in feces (Traversa and Di Cesare, 2013). The direct dissection and enzymatic digestion used in this study could reduce the false negative hosts compared to other parasitological analyses (Houpin et al., 2016; Martínez-Rondán et al., 2019). An underdiagnosis of Metastrongyloidea parasites has been proposed by other authors (Otranto et al., 2013; Traversa and Di Cesare, 2013; Penagos-Tabares et al., 2018), due to difficulties in identifying the larval stages in coprological studies, or by misdiagnosing them as A. abstrusus (Traversa and Di Cesare, 2013). Nevertheless, global warming, changes in host population dynamics and domestic animal contact with natural landscapes, among others, are also proposed as factors for the rise of reports of these parasites in various geographic areas (Traversa et al., 2010; Vieira et al., 2013; Brianti et al., 2014a; Veronesi et al., 2016; Lange et al., 2018). Awareness of these endoparasites is necessary because of their high pathogenic potential in felids and canids, especially in younger animals or in high worm burdens (Traversa et al., 2010).
Three statistically significant associations were found in this study. The presence of factors that favor or avoid coinfection with these parasites is unknown. Nevertheless, both positive associations are parasites with higher prevalence in southern zone, where intermediate hosts (for Troglostrongylus sp.-Angiostrongylus sp.) or paratenic hosts (for U. stenocephala-Oslerus sp.) could possibly be shared. Co-infection of cardiorespiratory parasites in intermediate hosts was reported by Lange et al. (2018) and Penagos-Tabares et al. (2019), finding larvae of two metastrongyloid species in the same slug. Thus, intermediate hosts with mixed infections could be eaten by paratenic hosts and then be transmitted to guignas. Parasite associations should be further explored in the future, to assess if this phenomenon is present in other carnivore species and its possible causes.
In our study, cardiorespiratory parasite prevalence, intensity of infection, abundance of infection and parasite richness showed statistically significant differences between geographic zones, with higher values in the south. High humidity and low temperature, such as those present in southern Chile (Chilean Meterological Office, http://www.meteochile.gob.cl), are favorable conditions for the viability and abundance of gastropods (intermediate hosts of metastrongyloids) (Morgan et al., 2009; Brianti et al., 2014b). This could explain the higher prevalence of cardiorespiratory parasites found in southern guignas. Previous coprological studies in domestic cats have shown geographic differences in cardiorespiratory infection: in Santiago (central zone), Alcaíno et al. (1992) found 10% prevalence of A. abstrusus, while López et al. (2006) and García (2014) did not find cardiorespiratory parasites, despite the large sample size they used (230 and 300 samples), while in Los Ríos Region (southern Chile), A. abstrusus showed prevalence of 10% (Oyarzún, 2013), 34.1% (Bonilla, 1980) and 38% (Escobar et al., 1984), whilst, Eucoleus aerophilus (Enoplida: Capillariidae) had a prevalence of 20% (Bonilla, 1980). This higher presence of cardiorespiratory helminths and the report of Molineus sp. and capillarid worms only in guignas from the southern area, could explain the higher parasite richness we reported in southern Chile.
The higher intensity and abundance of infection we observed in guignas from the south, could be due to the fact that the species with higher intensity of infection, U. stenocephala and T. leonina, showed higher intensity in the southern zone. Uncinaria stenocephala had a total intensity of 8 helminths in the central zone and 232 in the south, while T. leonina showed a total intensity of 22 in the central zone and 178 helminths in the south. Both species can develop at low temperatures. The optimal temperature for U. stenocephala larval development is 20 °C, and their eggs can remain viable after a week at 0 °C (Bowman et al., 2002). Toxascaris leonina develops at higher temperatures than U. stenocephala, but it can also resist lower temperatures (Bowman et al., 2002). These characteristics and the auspicious humidity for the eggs and larvae, could make the presence and development of these species more feasible in southern Chile.
In this study there were no statistically significant differences in parasitism between sexes, but prevalence of infection and parasite richness were slightly higher in males. Larger home ranges and higher movement patterns of male guignas, who can move across the territories of different females (Dunstone et al., 2002; Sanderson et al., 2002; Schüttler et al., 2017), may increase male exposure to endoparasites.
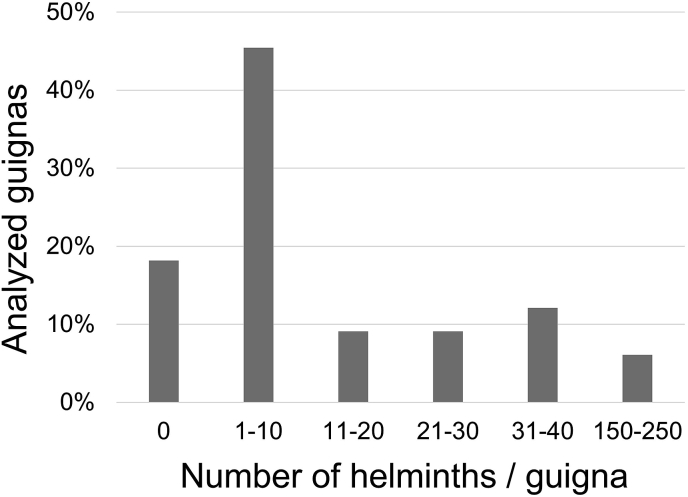
Six guignas (20% of the guignas analyzed) carried 74% of the helminths found in this study (Fig. 6). These values resemble the 20/80 rule (Anderson and May 1985; Woolhouse et al., 1997) which postulates that less than 20% of the hosts harbor 80% of the helminth population, being responsible for most parasite transmission and persistence in the environment (Anderson and May 1985; Woolhouse et al., 1997). It would be interesting in the future, to analyze this rule in studies with a larger sample size and other carnivore species who could share endoparasites with guignas.
Fig. 6.
Distribution of parasite infection intensity (total number of helminths per guigna) in the analyzed guignas from Chile.
All the endoparasites that we found in guigna in this study have been previously reported in domestic cats (Bowman et al., 2002). The parasite species/genera in common between guignas and domestic cats may be shared between these two felids, and the domestic cat could represent a reservoir species for parasite transmission (Millán and Casanova, 2007; Otranto et al., 2015). This theory must be confirmed with molecular identification techniques, as has already been done for parasites shared by domestic and wild hosts (Epe et al., 1999; Millán and Blasco-Costa, 2012; Di Cesare et al., 2014; Hodžić et al., 2016). Cross-species transmission of feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) has been reported between free-roaming domestic cats and guignas in fragmented landscapes in Chile (Mora et al., 2015). This infectious agent transmission between domestic and wild species is probably facilitated by current habitat fragmentation and changes in land use (Napolitano et al., 2015), promoting proximity and contact between domestic cats and guignas, either by direct contact or by other species which could play the role of vectors or intermediate and paratenic hosts.
5. Conclusion
A large part of the analyzed guignas were parasitized, with higher parasite richness compared to previous studies, probably due to the analysis by necropsy that we used. Sex had no influence on parasitism, while geographic zone could be an important factor for infection, possibly due to favorable environmental conditions for the life cycles of the parasites.
For a more accurate identification at the species level of the parasites found in this study, we suggest conducting further analysis using molecular techniques, and also phylogenetic assessments of shared parasites between guigna and domestic cat, exploring the possibility of domestic-wildlife transmission.
The results of this study must be considered by veterinarians and researchers, to develop new research opportunities and detect if these helminths parasitize other wild and domestic carnivores in Chile, clarifying their life cycle and epidemiology. These results will be valuable to inform conservation decisions for threatened carnivores like guignas and their habitat.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful to CONAF, especially Patricio Contreras, Patricia Barría; SAG, especially to Diego Ramírez, Rodrigo Villalobos and Luis Sepúlveda for logistic support and valuable samples. We thank Patricio Toro for technical support in laboratory work. Special thanks to Nicolás Gálvez, Jorge Valenzuela, Eduardo Silva, Brayan Zambrano, Javier Cabello, Gerardo Morales, Ricardo Pino, Daniel González, Nicole Sallaberry, Angelo Espinoza, Diego Peñaloza, Mario Alvarado, Aitor Cevidanes, Frederick Toro, Paulette Abarca, Alfredo Catalán, Gabriella Svensson, Jaime Rau, Andrea Roa, Tomás Valdés and Manuel Valdés for their valuable support in sample collection. Our work was funded by CONICYT FONDECYT Iniciación 11150934 (CN), Morris Animal Foundation (MAF) D15ZO-413 (CN), National Geographic Society C309-15 (CN), Mohamed bin Zayed Species Conservation Fund 152510351 (CN), ANID PAI 77190064 (CN), the Wild Felid Association (IS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.07.013.
Contributor Information
Francisca Acuña-Olea, Email: francisca.acuna@veterinaria.uchile.cl.
Constanza Napolitano, Email: constanza.napolitano@ulagos.cl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Acosta-Jamett G., Contreras S., Muñoz P., Briceño C., Chirgwin C., Hernández F. Description of gastrointestinal parasitism through coprologic survey in Darwin's fox, Lycalopex fulvipes (Martin 1837), and kodkod, Leopardus guigna (Molina 1782), in Chiloé Island, Chile. Gayana. 2018;82:160–165. doi: 10.4067/S0717-65382018000200160. [DOI] [Google Scholar]
- Acosta-Jamett G., Simonetti J.A. Habitat use by Oncifelis guigna and Pseudalopex culpaeus in a fragmented forest landscape in central Chile. Biodivers. Conserv. 2004;13:1135–1151. doi: 10.1023/B:BIOC.0000018297.93657.7d. [DOI] [Google Scholar]
- Alcaíno H., Gorman T., Larenas I. Fauna endoparasitaria del gato doméstico en una zona urbana marginal de la Región Metropolitana de Chile. Parasitol. al día. 1992;16:139–142. [Google Scholar]
- Álvarez V. Echinococcosis silvestre en Chile. Arch. Int. la Hidatid. 1963;21:156–159. [Google Scholar]
- Álvarez V., Rivera G., Neghme A., Schenone H. Tiquinosis en animales en Chile. Bol. Chil. Parasitol. 1970;25:83–86. [PubMed] [Google Scholar]
- Anderson R.C., Chabaud A.G., Willmott S. CAB International; London, UK: 2009. Keys to the Nematode Parasites of Vertebrates. [Google Scholar]
- Anderson R.M., May R.M. Helminth infections of humans: mathematical models, population dynamics, and control. Adv. Parasitol. 1985;24:1–101. doi: 10.1016/S0065-308X(08)60561-8. [DOI] [PubMed] [Google Scholar]
- Aranda C., Serrano-Martínez E., Tantaleán M., Quispe M., Casas G. Identification and frequency of gastrointestinal parasites in captive wild cats in Peru. Rev. investig. vet. Perú. 2013;24(3) [Google Scholar]
- Astorga D. Universidad Austral de Chile; Chile: 2013. Comparación de la dieta de dos carnívoros silvestres, güiña (Leopardus guigna) y zorro chilla (Pseudalopex griseus), en el Parque Nacional Nahuelbuta, Región de la Araucanía. [Google Scholar]
- Bonilla C. Universidad Austral de Chile; Chile: 1980. Estudio de la fauna helmintológica del gato en la ciudad de Valdivia. [Google Scholar]
- Bowman D.D., Hendrix C.M., Lindsay D.S., Barr S.C. first ed. Iowa State University Press Blackwell Science Company; Iowa, EE. UU: 2002. Feline Clinical Parasitology. [Google Scholar]
- Brianti E., Gaglio G., Napoli E., Falsone L., Giannelli A., Annoscia G., Varcasia A., Giannetto S., Mazzullo G., Otranto D. Feline lungworm Oslerus rostratus (Strongylida: filaridae) in Italy: first case report and histopathological findings. Parasitol. Res. 2014;113:3853–3857. doi: 10.1007/s00436-014-4053-z. [DOI] [PubMed] [Google Scholar]
- Brianti E., Gaglio G., Napoli E., Falsone L., Giannetto S., Latrofa M.S., Giannelli A., Dantas-Torres F., Otranto D. Evidence for direct transmission of the cat lungworm Troglostrongylus brevior (Strongylida: Crenosomatidae) Parasitology. 2013;140:821–824. doi: 10.1017/S0031182013000188. [DOI] [PubMed] [Google Scholar]
- Brianti E., Giannetto S., Dantas-Torres F., Otranto D. Lungworms of the genus Troglostrongylus (strongylida: Crenosomatidae): neglected parasites for domestic cats. Vet. Parasitol. 2014;202:104–112. doi: 10.1016/j.vetpar.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology Meets Ecology on Its Own Terms: margolis et al. Revisited. J. Parasitol. 1997;83 doi: 10.2307/3284227. 575. [DOI] [PubMed] [Google Scholar]
- Correa P., Roa A. Relaciones tróficas entre Oncifelis guigna, Lycalopex culpaeus, Lycalopex griseus y Tyto alba en un ambiente fragmentado de la zona central de Chile. Mastozool. Neotrop. 2005;12:57–60. [Google Scholar]
- Cortés M. Universidad de Concepción; Chile: 2006. Identificación de formas reproductivas de parásitos gastrointestinales, en mamíferos nativos presentes en el Buin Zoo. [Google Scholar]
- Di Cesare A., Otranto D., Latrofa M.S., Veronesi F., Perrucci S., Lalosevic D., Gherman C.M., Traversa D. Genetic variability of Eucoleus aerophilus from domestic and wild hosts. Res. Vet. Sci. 2014;96:512–515. doi: 10.1016/j.rvsc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Diakou A., Psalla D., Migli D., Di Cesare A., Youlatos D., Marcer F., Traversa D. First evidence of the European wildcat (Felis silvestris silvestris) as definitive host of Angiostrongylus chabaudi. Parasitol. Res. 2016;115:1235–1244. doi: 10.1007/s00436-015-4860-x. [DOI] [PubMed] [Google Scholar]
- Dobson A., Lafferty K.D., Kuris A.M., Hechinger R.F., Jetz W. Homage to linnaeus: how many parasites? How many hosts? Light Evol. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstone N., Durbin L., Wyllie I., Freer R., Jamett G.A., Mazzolli M., Rose S. Spatial organization, ranging behaviour and habitat use of the kodkod (Oncifelis guigna) in southern Chile. J. Zool. 2002;257:1–11. doi: 10.1017/S0952836902000602. [DOI] [Google Scholar]
- Durette-Desset M.C., Boomker J., Malan F.S. Molineus cati n. sp. (Nematoda, Trichostrongylina, Molineoidea), a parasite of feral cats, Felis catus Linnaeus, 1758 in South Africa. Onderstepoort J. Vet. Res. 2000;67:173–177. [PubMed] [Google Scholar]
- Epe C., Meuwissen M., Stoye M., Schnieder T. Transmission trials, ITS2-PCR and RAPD-PCR show identity of Toxocara canis isolates from red fox and dog. Vet. Parasitol. 1999;84:101–112. doi: 10.1016/S0304-4017(99)00080-1. [DOI] [PubMed] [Google Scholar]
- Escobar R., Illanes O., Fuentealba I., Cubillos V. Nematodiasis pulmonar en el gato doméstico. Arch. Med. Vet. 1984;16:47–49. [Google Scholar]
- Fernández J., Villalba C. Helmintos parásitos de Felis guigna Molina , 1782. Bol. Soc. Biol. Concepción, Chile. 1984;55:161–164. [Google Scholar]
- Figueroa R.A., Corales E.S., Rau J.R. Prey of the güiña (Leopardus guigna) in an Andean mixed southern beech forest, southern Chile. Stud. Neotrop. Fauna Environ. 2018;53:211–218. doi: 10.1080/01650521.2018.1477032. [DOI] [Google Scholar]
- Fiorello C.V., Robbins R.G., Maffei L., Wade S.E. Parasites of free-ranging small canids and felids in the Bolivian chaco. J. Zoo Wildl. Med. 2006;37:130–134. doi: 10.1638/05-075.1. [DOI] [PubMed] [Google Scholar]
- Fleschutz M.M., Gálvez N., Pe’er G., Davies Z.G., Henle K., Schüttler E. Response of a small felid of conservation concern to habitat fragmentation. Biodivers. Conserv. 2016;25:1447–1463. doi: 10.1007/s10531-016-1118-6. [DOI] [Google Scholar]
- Freer R.A. Dep. Biol. Sci. University of Durham; Chile: 2004. The Spatial Ecology of the Güiña (Oncifelis Guigna) in Southern. [Google Scholar]
- Gálvez N., Guillera-Arroita G., St John F.A.V., Schüttler E., Macdonald D.W., Davies Z.G. A spatially integrated framework for assessing socioecological drivers of carnivore decline. J. Appl. Ecol. 2018;55:1393–1405. doi: 10.1111/1365-2664.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez N., Hernández F., Laker J., Gilabert H., Petitpas R., Bonacic C., Gimona A., Hester A., MacDonald D.W. Forest cover outside protected areas plays an important role in the conservation of the Vulnerable guiña Leopardus guigna. Oryx. 2013;47:251–258. doi: 10.1017/S0030605312000099. [DOI] [Google Scholar]
- García M. Universidad de Chile; Chile: 2014. Helmintos y protozoos gastrointestinales de gatos (Felis catus) de la ciudad de Santiago. [Google Scholar]
- Gherman C.M., Ionică A.M., D'Amico G., Otranto D., Mihalca A.D. Angiostrongylus chabaudi (Biocca, 1957) in wildcat (Felis silvestris silvestris, S) from Romania. Parasitol. Res. 2016;115:2511–2517. doi: 10.1007/s00436-016-5032-3. [DOI] [PubMed] [Google Scholar]
- González-Acuña D., Moreno L., Ardiles K., Flores M., Duclos M., Kinsella M. Endoparasites of the kodkod, Oncifelis guigna (carnivora, felidae) in Chile. Rev. Chil. Hist. Nat. 2010;83:619–622. doi: 10.4067/S0716-078X2010000400015. [DOI] [Google Scholar]
- Hiestand S.J., Nielsen C.K., Jiménez F.A. Epizootic and zoonotic helminths of the bobcat (Lynx rufus) in Illinois and a comparison of its helminth component communities across the American Midwest. Parasite. 2014;21 doi: 10.1051/parasite/2014005. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A., Alić A., Klebić I., Kadrić M., Brianti E., Duscher G.G. Red fox (Vulpes vulpes) as a potential reservoir host of cardiorespiratory parasites in Bosnia and Herzegovina. Vet. Parasitol. 2016;223:63–70. doi: 10.1016/J.VETPAR.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Houpin E., Mccarthy G., Ferrand M., Waal T De, Neill E.J.O., Zintl A. Comparison of three methods for the detection of Angiostrongylus vasorum in the final host. Vet. Parasitol. 2016;220:54–58. doi: 10.1016/j.vetpar.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Lafferty K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Khalil L.F., Jones A., Bray R.A. CAB International; Wallingford, Oxon, UK: 1994. Keys to the Cestode Parasites of Vertebrates. [Google Scholar]
- Lange M.K., Penagos-Tabares F., Hirzmann J., Failing K., Schaper R., Van Bourgonie Y.R., Backeljau T., Hermosilla C., Taubert A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018;254:120–130. doi: 10.1016/j.vetpar.2018.03.011. [DOI] [PubMed] [Google Scholar]
- López J., Abarca K., Paredes P., Inzunza E. Parásitos intestinales en caninos y felinos con cuadros digestivos en Santiago, Chile. Consideraciones en Salud Pública. Rev. Med. Chil. 2006;134:193–200. doi: 10.4067/s0034-98872006000200009. [DOI] [PubMed] [Google Scholar]
- Marcogliese D.J. International Journal for Parasitology. 2005. Parasites of the superorganism: are they indicators of ecosystem health? pp. 705–716. [DOI] [PubMed] [Google Scholar]
- Martínez-Rondán F.J., Ruiz de Ybáñez M.R., López-Beceiro A.M., Fidalgo L.E., Berriatua E., Lahat L., Sacristán I., Oleaga A., Martínez-Carrasco C. Cardiopulmonary nematode infections in wild canids: does the key lie on host-prey-parasite evolution? Res. Vet. Sci. 2019;126:51–58. doi: 10.1016/j.rvsc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Martínez-Rondán F.J., Ruiz de Ybáñez M.R., Tizzani P., López-Beceiro A.M., Fidalgo L.E., Martínez-Carrasco C. The American mink (Neovison vison) is a competent host for native European parasites. Vet. Parasitol. 2017;247:93–99. doi: 10.1016/j.vetpar.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Millán J., Blasco-Costa I. Molecular evidence of shared hookworm Ancylostoma tubaeforme haplotypes between the critically endangered Iberian lynx and sympatric domestic cats. Vet. Parasitol. 2012;186:518–522. doi: 10.1016/j.vetpar.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Millán J., Casanova J.C. Helminth parasites of the endangered Iberian lynx (Lynx pardinus) and sympatric carnivores. J. Helminthol. 2007;81:377–380. doi: 10.1017/S0022149X07869203. [DOI] [PubMed] [Google Scholar]
- Moleón M.S., Kinsella J.M., Moreno P.G., Del Valle Ferreyra H., Pereira J., Pía M., Beldomenico P.M. New hosts and localities for helminths of carnivores in Argentina. Zootaxa. 2015;4057:106–114. doi: 10.11646/zootaxa.4057.1.6. [DOI] [PubMed] [Google Scholar]
- Mora M., Napolitano C., Ortega R., Poulin E., Pizarro-Lucero J. Feline immunodeficiency virus and feline leukemia virus infection in free-ranging guignas (Leopardus guigna) and sympatric domestic cats in human perturbed landscapes on Chiloé Island, Chile. J. Wildl. Dis. 2015;51:199–208. doi: 10.7589/2014-04-114. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Jefferies R., Krajewski M., Ward P., Shaw S.E. Canine pulmonary angiostrongylosis: the influence of climate on parasite distribution. Parasitol. Int. 2009;58:406–410. doi: 10.1016/j.parint.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Napolitano C., Gálvez N., Bennett M., Acosta-Jamett G., Sanderson J. Leopardus guigna, guiña view on. 2015. www.iucnredlist.orgTHE IUCN RED LIST OF THREATENED SPECIESTM 8235. [DOI]
- Otranto D., Brianti E., Dantas-Torres F. Troglostrongylus brevior and a nonexistent “dilemma. Trends Parasitol. 2013;29:517–518. doi: 10.1016/j.pt.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: helminths and arthropods. Vet. Parasitol. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Oyarzún J. Universidad Austral de Chile; Chile: 2013. Pesquisa de nemátodos pulmonares en perros y gatos de las ciudades de Río Bueno y La Unión, Provincia del Ranco. [Google Scholar]
- Penagos-Tabares F., Lange M.K., Chaparro-Gutiérrez J.J., Taubert A., Hermosilla C. Angiostrongylus vasorum and Aelurostrongylus abstrusus: neglected and underestimated parasites in South America. Parasites Vectors. 2018;11:1–13. doi: 10.1186/s13071-018-2765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagos-Tabares F., Lange M.K., Vélez J., Hirzmann J., Gutiérrez-Arboleda J., Taubert A., Hermosilla C., Chaparro Gutiérrez J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Neglected Trop. Dis. 2019;13:1–18. doi: 10.1371/journal.pntd.0007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson J., Sunquist M.E., Iriarte A.W. Natural history and landscape-use of guignas (Oncifelis guigna) on isla grande de Chiloé, Chile. J. Mammal. 2002;83:608–613. doi: 10.1644/1545-1542(2002)083<0608.nhaluo>20.co;2. [DOI] [Google Scholar]
- Schüttler E., Klenke R., Galuppo S., Castro R.A., Bonacic C., Laker J., Henle K. Habitat use and sensitivity to fragmentation in America's smallest wildcat. Mamm. Biol. 2017;86:1–8. doi: 10.1016/j.mambio.2016.11.013. [DOI] [Google Scholar]
- Seguel M., Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int. J. Parasitol. Parasites Wildl. 2017;6:177–194. doi: 10.1016/j.ijppaw.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.L., Smith T.M. fourth ed. Pearson Educacion; Madrid, España: 2001. Ecologia. [Google Scholar]
- Soulsby E. seventh ed. Nueva Editorial Interamericana, Mexico D.F.; Mexico: 1987. Parasitología y Enfermedades Parasitarias de los Animales Domésticos. [Google Scholar]
- Tagle I. first ed. Editorial Andrés Bello; Santiago, Chile: 1970. Enfermedades parasitarias de los animales domésticos. [Google Scholar]
- Thompson R.C.A., Lymbery A.J., Smith A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010;40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A. Feline lungworms: what a dilemma. Trends Parasitol. 2013 doi: 10.1016/j.pt.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A., Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasites Vectors. 2010;3:1–22. doi: 10.1186/1756-3305-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Lepri E., Veronesi F., Paoletti B., Simonato G., Diaferia M., Di Cesare A. Metastrongyloid infection by Aelurostrongylus abstrusus, Troglostrongylus brevior and Angiostrongylus chabaudi in a domestic cat. Int. J. Parasitol. 2015;45:685–690. doi: 10.1016/j.ijpara.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Vallverdú A. Universidad Austral de Chile; Chile: 2014. Descripción de parásitos gastrointestinales en güiña, zorro chilla, zorro culpeo y puma, mediante análisis coprológicos, en Parque Nacional Nahuelbuta, Región de la Araucanía. [Google Scholar]
- Varcasia A., Brianti E., Tamponi C., Pipia A.P., Cabras P.A., Mereu M., Dantas-Torres F., Scala A., Otranto D. Simultaneous infection by four feline lungworm species and implications for the diagnosis. Parasitol. Res. 2015;114:317–321. doi: 10.1007/s00436-014-4207-z. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Tamponi C., Brianti E., Cabras P.A., Boi R., Pipia A.P., Giannelli A., Otranto D., Scala A. Angiostrongylus chabaudi Biocca, 1957: a new parasite for domestic cats? Parasites Vectors. 2014;7 doi: 10.1186/s13071-014-0588-1. 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi F., Traversa D., Lepri E., Morganti G., Vercillo F., Grelli D., Cassini R., Marangi M., Iorio R., Ragni B., Di Cesare A. Occurrence of lungworms in European wildcats (Felis silvestris silvestris) of central Italy. J. Wildl. Dis. 2016;52:270–278. doi: 10.7589/2015-07-187. [DOI] [PubMed] [Google Scholar]
- Vieira F.M., Muniz-Pereira L.C., de Souza Lima S., Neto A.H.A.M., Guimarães E.V., Luque J.L. A new metastrongyloidean species (Nematoda) parasitizing pulmonary arteries of Puma (Herpailurus) yagouaroundi (É. Geoffroy, 1803) (Carnivora: felidae) from Brazil. J. Parasitol. 2013;99:327–331. doi: 10.1645/GE-3171.1. [DOI] [PubMed] [Google Scholar]
- Wolffhügel K. ¿Es autóctono el Diphyllobothrium en Chile? Bol. Soc. Biol. Concepc. 1949;24:85–89. [Google Scholar]
- Woolhouse M.E.J., Dye C., Etard J.-F., Smith T., Charlwood J.D., Garnett G.P., Hagan P., Hii J.L.K., Ndhlovu P.D., Quinnell R.J., Watts C.H., Chandiwana S.K., Anderson R.M. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94:338–342. doi: 10.1073/PNAS.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga A., Quintana V., Fierro A. Relaciones tróficas entre depredadores en un ambiente fragmentado del sur de Chile. Gestión Ambient. 2005;11:31–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.