Abstract
Withdrawal from Δ9-tetrahyrocannibidol (THC) is associated with a host of dysphoric symptoms that increase probability of relapse. To date, many animal models of THC withdrawal rely on withdrawal-induced somatic withdrawal signs leaving withdrawal-suppressed behavior relatively unexplored. As compared with withdrawal-induced behaviors, ongoing behavior that is suppressed by withdrawal is a useful behavioral endpoint because it 1) more effectively models the subjective aspects of withdrawal and 2) identifies pharmacotherapies that restore behavior to baseline levels, rather than eliminate behavior induced by withdrawal. The current study assessed effects of spontaneous and rimonabant-precipitated THC withdrawal in mice responding on a progressive-ratio (PR) schedule of sucrose water reinforcement. Once behavior stabilized, male and female mice were administered THC (10 mg/kg, s.c.) or vehicle for five or six days. THC was either discontinued and behavior monitored for three days during abstinence, or the CB1 antagonist rimonabant (2 mg/kg, i.p.) was used to precipitate withdrawal. Whereas spontaneous THC withdrawal had no effect on PR performance, THC-treated mice were differentially sensitive to rimonabant administration via large decreases in break point, overall response rate, and run rate relative to vehicle-treated mice. Importantly, pretreatment with the CB1 positive allosteric modulator ZCZ011 (10 mg/kg, i.p.) did not prevent precipitated-withdrawal-induced behavioral impairment. These extend findings of earlier studies suggesting operant baselines are useful tools to study subjective effects of cannabinoid withdrawal. Additionally, operant baselines allow withdrawal pharmacotherapies to be tested in a restoration-of-function context, which may be more sensitive, selective, and clinically relevant.
Keywords: cannabinoid withdrawal, progressive ratio, drug addiction, CB1 positive allosteric modulator, cannabis use disorder, THC
1. Introduction
Cannabis is the most widely used federally illicit substance in the United States (SAMHSA, 2018). Cessation of cannabis use in heavy users is associated with a withdrawal syndrome(American Psychiatric Association, 2013; Budney, Hughes, Moore, & Vandrey, 2004; Montoya & Weiss, 2019) characterized by sleep disturbances, decreased appetite, irritability, increased anxiety, and depressed mood (Haney et al., 1999). While these symptoms are not life-threatening, they can contribute to relapse following cessation of use (Haney et al., 2013).
The array of symptoms associated with cannabis withdrawal, spanning somatic, cognitive, and mood-related disturbances necessitates a corresponding array of preclinical models to probe these facets of withdrawal. Preclinical models of THC withdrawal typically focus on somatic withdrawal elicited by CB1 antagonist challenge (Aceto et al., 1995; Cook et al., 1998; Lichtman et al., 1998; Tsou et al., 1995). At least two reasons for targeting somatic withdrawal signs are that 1) these models serve as historical precedents allowing for comparison across studies and 2) outcomes of this model are robust and replicable (e.g., Schlosburg et al., 2009). Despite the reliability of these measures, somatic signs do not translate readily to cannabinoid withdrawal in humans, namely emotional and motivational components of the syndrome (APA, 2013; Herrmann et al., 2015). We previously reported an array of behavioral tests that target emotionality-related changes during spontaneous and antagonist-precipitated withdrawal (Trexler et al., 2018). In these studies, either spontaneous or antagonist-precipitated THC withdrawal routinely increased struggling in the tail suspension test or decreased marble burying (antagonist-precipitated only). Coupled with consistent increases in plasma corticosterone (Trexler et al., 2018), it can be inferred that THC withdrawal is a stressor (Rodríguez De Fonseca et al., 1997), which manifests as disruption of behavior that mice normally display under baseline conditions.
One advantage of moving toward novel behavioral models of THC withdrawal is that withdrawal-depressed behavior can begin to be assessed more closely. Interestingly, behavior that is elicited by withdrawal (i.e., somatic signs) is blocked more consistently by pharmacological challenge than behavior that is disrupted or suppressed by withdrawal (e.g., marble burying). For example, pretreatment with THC, the monoacylglycerol lipase inhibitor JZL184, or the CB1 positive allosteric modulator (PAM) ZCZ011 blocks withdrawal-elicited increases in somatic signs in mice (Schlosburg et al., 2009; Trexler, Eckard, & Kinsey, 2019; Trexler et al., 2018) without a corresponding effect in withdrawal-suppressed marble burying (Trexler et al., 2018). This suggests that disruptions in behavior that mice normally engage in (i.e., digging) may be more resistant to pharmacological intervention. This issue has also been highlighted in preclinical pain research (Negus, 2019). Similar to our observations of withdrawal-stimulated and withdrawal-suppressed behavior, experimental analgesics tend to show robust clinical efficacy in assays of pain-stimulated behavior but lack efficacy in pain-suppressed behavior (Negus, 2019). Thus, there may be utility in assessing withdrawal-suppressed behavior due to this process being more reflective of affective changes during cannabinoid withdrawal.
One overarching paradigm of withdrawal-suppressed behavior that is largely missing from the characterization of cannabinoid withdrawal is tests of operant behavior. To date, only two studies have shown disruption in ongoing operant behavior as a consequence of THC withdrawal in rodents (Beardsley et al., 1986; Beardsley & Martin, 2000). There are a few accounts of drug discrimination procedures being used to assess the subjective experience of THC withdrawal in monkeys (McMahon & France, 2003; Stewart & McMahon, 2010); however, these procedures are not intended to model disruption of ongoing behavior as emphasized here. For example, Beardsley et al. (1986) showed that response rates of rhesus monkeys working on a fixed-ratio schedule of reinforcement decreased dramatically following spontaneous withdrawal from a 10-15 day intravenous THC regimen and persisted for several days. More recently, Beardsley et al. (2000) showed that rimonabant-precipitated THC withdrawal decreased response rates of rats working on a variable-interval schedule. While these studies are not directly comparable, they do suggest operant behavior is sensitive to both spontaneous and antagonist-precipitated THC withdrawal via suppressed responding.
A common operant procedure for assessing motivational disruptions during drug withdrawal is the progressive-ratio (PR) schedule. In this task, the work requirement for a reinforcer (e.g., sucrose water) increases after each reinforcer delivery (Hodos & Kalman, 1963). Both the final ratio completed (i.e., breaking point) and rate of responding during the task provide indices of a subject’s motivation to earn reinforcers. Thus, in the context of drug withdrawal, disruptions in PR performance are generally considered to be reflective of anhedonia or reduced reinforcer efficacy (Barr & Phillips, 1999). Progressive-ratio schedules have been used to characterize decreased motivation for sucrose water during withdrawal from nicotine (Kirshenbaum et al., 2015), amphetamine (Barr & Phillips, 1999; Orsini et al., 2001), methamphetamine (Hoefer et al., 2006), and morphine (Zhang et al., 2007). Importantly, drug withdrawal manifests as decreased break point for several days following cessation of repeated drug administration indicating the sensitivity of PR schedules to detect extended motivational disruption. Furthermore, in the case of withdrawal from psychostimulants, administration of partial dopamine agonists restores break point to baseline levels suggesting the utility of PR schedules to assess pharmacological interventions of withdrawal-suppressed operant behavior (Hoefer et al., 2006; Orsini et al., 2001).
There were three primary aims of the current set of studies. The first two aims were to characterize possible motivational disruption during both spontaneous and antagonist-precipitated THC withdrawal, respectively, using a PR schedule. The third aim was to challenge either spontaneous or antagonist-precipitated THC withdrawal with a novel CB1 positive allosteric modulator (ZCZ011) to assess its therapeutic potential in withdrawal-suppressed operant behavior (Trexler et al., 2019).
2. Methods
2.1. Subjects
Eighteen experimentally naïve female (n = 9) and male (n = 9) C57/BL6J mice (Jackson Labs, Bar Harbor, ME) approximately 2 months old were used for each experiment (See Exp. 2 for an exception). Mice were housed 4-5 per cage with free access to water in a temperature-controlled, AAALAC-accredited vivarium on a 12:12 h light:dark cycle with lights on at 0600 hours. Food was restricted to approximately 2g of chow per mouse per day. Body weights were recorded daily. Females and males weighed between 16-20 g and 21-25 g, respectively. The Animal Care and Use Committee at West Virginia University approved all experimental protocols.
2.2. Drugs
The mixed CB receptor agonist Δ9-tetrahydrocannabinol (THC) and CB1 selective inverse agonist rimonabant (SR141716A) were generously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). ZCZ011 was purchased from Axon Medchem (Reston, VA). All drugs were dissolved in a vehicle solution of 5% ethanol, 5% Cremophor (Sigma-Aldrich, St. Louis, MO), and 90% physiological saline (0.9% sodium chloride) (Kinsey and Cole, 2013). All solutions were warmed to room temperature before injection at a volume of 10 μl/g body mass.
2.3. Apparatus
Nine MED-Associates® operant-conditioning chambers for mice were used for data collection (17.8 cm L x 15.2 cm W x 18.4 cm H). The work panel of the chambers consisted of two nose-poke holes spaced 9 cm apart, each illuminated by a small, yellow LED. Head entries into the active nose-poke aperture were detected by breaks in an infrared photobeam. Sucrose water (15% wt/v; 20 μl/delivery) was accessed from a dipper cup equidistant between both nose-poke holes. The floor of each chamber consisted of a stainless-steel grid of 19 horizontal bars. A houselight centered at the top of the back wall opposite the work panel illuminated the interior of the chamber during sessions. Each chamber was enclosed in a sound-attenuating box with a wall-mounted fan that provided ventilation and white noise during sessions. All experimental events were controlled by a MED-Associates® interface and desktop computer in an adjacent room using MED-PC notation.
2.4. Behavioral Procedures
2.4.1. Pre-training.
Nose poking was established using an autoshaping procedure (Eckard & Kyonka, 2018). Sucrose water was made available according to a conjoint fixed-time (FT) 90 s, fixed-ratio (FR) 1 schedule of reinforcement. Each trial began with the illumination of the houselight and active nose-poke aperture. During reinforcer presentations, the nose-poke light was extinguished and the houselight flashed on and off in 0.5-s increments for a total of five seconds of reinforcer presentation. Autoshaping sessions terminated after three hours. Once responding was reliable, an FR 1 schedule was in place the following session. Once 60 reinforcers had been earned for three consecutive sessions, an FR 3 was in place followed by an FR 5 with similar reinforcement requirements. After 60 reinforcers had been earned for three consecutive sessions on the FR 5 schedule, the PR schedule was introduced.
2.4.2. Progressive ratio schedule.
Each PR session began with a response requirement of one (FR 1). Thereafter, the response requirement increased by two responses after each reinforcer delivery (i.e., 1, 3, 5, 7, 9, etc.) (Hodos & Kalman, 1963). This step size was chosen to avoid potential floor effects in responding following THC administration and/or withdrawal. The break point criterion was set at 10 min. That is, if a 10-min pause in responding occurred, then the session was terminated. Session duration was capped at 2 hours. Thus, sessions ended either after a 10-min pause in responding or 2 hours elapsed, whichever occurred first. Sessions took place at approximately the same time each day between 0900 and 1300 seven days per week. A minimum of 21 baseline PR sessions were conducted prior to any drug administration.
2.5. Pharmacological Procedures
2.5.1. Spontaneous THC withdrawal.
Mice were separated into two groups (n = 9) matched for sex, break point, and response rate following stable responding (THC: F = 4; M = 5; Vehicle: F = 5, M = 4) (see Data Analysis). Groups received twice-daily injections of either THC (10 mg/kg; s.c., b.i.d.; n = 9) or vehicle (n = 9) for six days (Trexler et al., 2018). Morning injections occurred 1 h prior to sessions, and evening injections occurred approximately 12 h later. The final THC injection occurred 12 h prior to the following session to allow for withdrawal to be assessed at 12, 36, and 60 h post-THC. The 36-h time point was of primary interest, as somatic signs of spontaneous THC withdrawal peak at 36 h post-THC (Trexler et al., 2018).
2.5.2. Acute rimonabant challenge and precipitated THC withdrawal.
In addition to blocking the effects of THC at the CB1 receptor, rimonabant per se also blocks endocannabinoid signaling at CB1 reducing PR performance (Rasmussen & Huskinson, 2008) and inducing pruritus at high doses (Schlosburg, Boger, et al., 2009). Thus, prior to any THC administration, rimonabant (0, 1, 2, or 3 mg/kg, i.p.) was administered to a subset of mice (n = 9) in descending doses to determine possible dose-dependent effects of rimonabant on PR performance. Rimonabant was administered 30 min prior to sessions with at least a 3-day washout between doses. The highest dose that did not significantly disrupt responding was used for the subsequent withdrawal experiments. Repeated THC (10 mg/kg, s.c. b.i.d; n = 7) or vehicle (n = 9) administration began following a 9-day washout after the last rimonabant dose. Groups were matched for sex and PR performance with the added constraint of matching for rimonabant experience (THC: M = 3, F = 4; Vehicle: M = 5, F = 4). THC administration was identical to that described above. Seven days after THC administration began, rimonabant (2 mg/kg, i.p.) was administered to all mice 30 min after the final THC or vehicle injection and testing began 30 min after rimonabant.
2.5.3. Acute ZCZ011 challenge of precipitated THC withdrawal.
Prior to any THC administration, the CB1 selective positive allosteric modulator ZCZ011 was administered to a subset of mice (n = 9) in descending doses to identify a subthreshold dose to attenuate rimonabant-precipitated withdrawal. ZCZ011 (0, 5, 10, or 20 mg/kg, i.p.) administration was identical to that of rimonabant except for a 75 min pretreatment (Ignatowska-Jankowska et al., 2015). As with the above experiment, repeated THC (10 mg/kg, s.c. b.i.d) or vehicle administration began following a 9-day washout after the last ZCZ011 dose. Mice were separated into three groups (n = 6; 3/sex/group). Two groups received THC for 6 days while the other group received vehicle. THC administration was identical to that described above, however, due to the rapid tolerance to THC observed in our initial experiments, withdrawal was precipitated on Day 6. On Day 6, ZCZ011 (10 mg/kg, i.p.) was administered to one THC-treated group 75 min prior to testing with the other groups receiving vehicle. THC and rimonabant administration were otherwise identical to that described above.
2.6. Dependent Measures and Data Analysis
The three primary dependent measures were break point, overall response rate, and run rate. Break point was defined as the ratio in which the 10-min pause in responding occurred. Overall response rate was calculated by recording the total elapsed time to complete each ratio requirement and dividing the ratio value by the recorded duration. Thus, response rate was calculated for each ratio within each session. These single-ratio response rates were then averaged across the session to yield an average response rate for each session expressed as responses per second. Run rate was calculated similarly but omitted the latency to first response from each ratio duration. The first ratio completed (FR 1) and the last, unfinished ratio were excluded from response rate calculations. Response rates were included as an additional measure because they have been described as a more sensitive indicator of PR schedule performance relative to break point alone (Bradshaw & Killeen, 2012; Rasmussen & Huskinson, 2008).
Because repeated THC or vehicle administration began at the same time for all mice in each experiment, stability criteria were applied at the group level using the natural baseline dichotomy of sex (n = 9 unless otherwise specified). Stability criteria were as follows: mean break point and response rates were calculated for the previous nine sessions. Then, means within each 3-session block were compared to the 9-session grand mean. Stability was defined as no more than 15% deviation in each 3-session block compared to the 9-session grand mean for both sexes. Upon reaching stability, mice were assigned to receive repeated THC (10 mg/kg, s.c.) or vehicle injections. Groups were matched with respect to sex, break point, and response rate using matched random assignment. Specifically, mice were ranked highest to lowest on break point, and mice from each descending pair (Exp. 1 and 2) or trio (Exp. 3) were then randomly assigned to vehicle or THC groups. No sex differences were detected throughout any experiment.
All data are expressed as a proportion of baseline (last three sessions) mean ± SEM (see Supplement for raw baseline values). Rimonabant and ZCZ011 dose-effect data were analyzed using one-way repeated-measures analysis of variance (RMANOVA) with drug dose as the within-subjects factor. Significant main effects of dose were followed by Dunnett’s post hoc tests to compare vehicle and drug administration to baseline. THC withdrawal data were analyzed using two-way RMANOVA with Group as the between-subjects factor and Session as the within-subjects factor. For rimonabant-precipitated withdrawal experiments, analyses of repeated THC administration and withdrawal included the last session of baseline up to and including the day withdrawal was precipitated (Session 7-14 for Exp. 2 and 3). In the spontaneous THC withdrawal experiment, analyses included the last day of baseline up to and including the final time point of abstinence (Session 7-16 for Exp. 1). All significant interactions were followed by Tukey’s HSD post hoc tests. Mauchly’s test was used to evaluate violations of sphericity. Greenhouse-Geisser adjustments were applied for sphericity violations. Comparisons were considered significant if p < .05.
3. Results
3.1. Experiment 1: Spontaneous THC withdrawal.
Figure 1 shows PR dependent measures during baseline, repeated THC (10 mg/kg, s.c.) administration, and three days of THC abstinence. Overall, behavior was stable during baseline with no systematic differences between groups. Analysis of break point and response rates yielded a significant main effect of Session [break point: F(9,144) = 4.27, p < 0.05, η2 = 0.21; overall response rate: F(9,144) = 7.20, p < 0.05, η2 = 0.31; run rate: F(9,144) = 10.13, p < 0.05, η2 = 0.39] as well as a significant Session x Group interaction [break point: F(9,144) = 5.37, p < 0.05, η2 = 0.25; response rate: F(9,144) = 10.06, p < .05, η2 = 0.39; run rate: F(5,80) = 9.63, p < 0.05, η2 = 0.37]. Post hoc analyses revealed that acute THC (10 mg/kg, s.c.) administration decreased break point, response rate, and run rate relative to vehicle and the previous baseline session [all p’s < .05]. Vehicle administration had no effect on responding [all p’s > 0.95]. Tolerance to THC’s suppressive effects was evident by the second [p > 0.95] or third [p > 0.84] session of repeated administration as indicated by break point and response rates, respectively. Discontinuation of THC or vehicle administration did not affect any dependent measure [all p’s >0.23].
Figure 1.
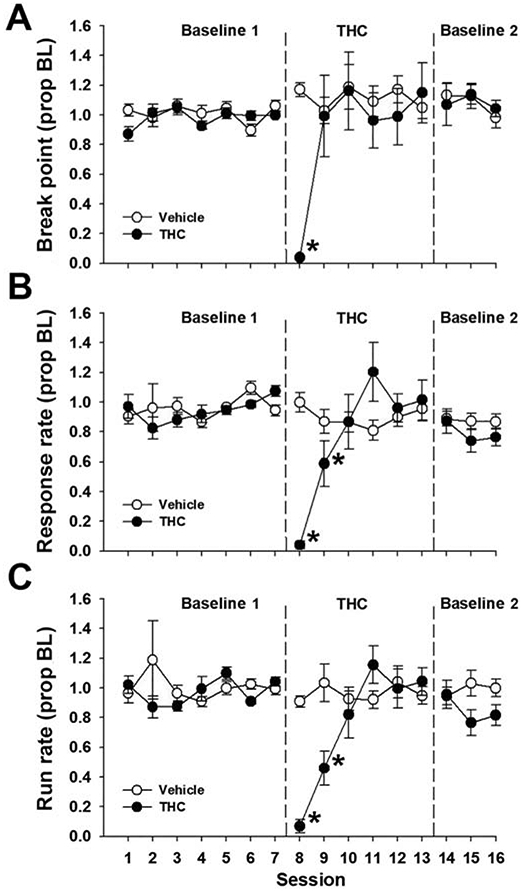
Spontaneous THC withdrawal does not affect PR performance. Male and female mice were trained on a PR 2 schedule of reinforcement and administered THC (10 mg/kg, s.c.) or vehicle for six days. The final THC injection occurred such that 12, 36, and 60 h abstinence time points aligned with regular operant testing sessions. Spontaneous THC withdrawal did not affect break point (A), overall response rate (B), or run rate (C). Data represent proportion of baseline mean ±SEM (n = 9; THC 4F/5M; Vehicle 5F/4M). Baseline 1 = pre-THC baseline; Baseline 2 = post-THC abstinence baseline * p < 0.05 vs. Session 7.
3.2. Experiment 2: Acute rimonabant and precipitated THC withdrawal.
Due to the lack of spontaneous THC withdrawal effects on PR performance, we next investigated effects of rimonabant-precipitated THC withdrawal (Aceto et al., 1995). Figure 2 shows results from the rimonabant dose response. Acute rimonabant administration decreased break point [F(4,32) = 7.17, p < 0.05, η2 = 0.47] but not response rate [F(4, 32) = 0.70, p = 0.60, η2 = 0.08]. Post hoc analyses of break point revealed that the 3 mg/kg dose decreased break point relative to baseline. Thus, 2 mg/kg rimonabant was used in the subsequent experiments to precipitate withdrawal.
Figure 2.
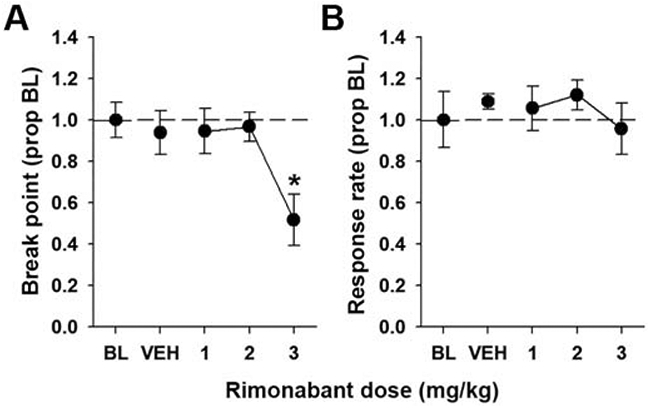
Rimonabant decreases break point. Male and female mice were trained on a PR 2 schedule of reinforcement and acutely administered rimonabant (0-3 mg/kg, i.p.) in descending doses with at least three days between each administration. Rimonabant (3 mg/kg) decreased break point (A) but not response rate (B). Data represent proportion of baseline mean ±SEM (n = 9). * p < 0.05 vs. BL.
Prior to THC (10 mg/kg) administration, baseline responding was stable with no difference between matched groups [Fig. 3]. Analysis of break point and response rate throughout repeated THC administration and withdrawal showed a significant main effect of Session [break point: F(7, 98) = 7.33, p < .05, η2 = 0.34; response rate: F(7, 98) = 7.72, p < .05, η2 = 0.36; run rate: F(7, 98) = 8.92, p < .05, η2 = 0.39 ] as well as a Session x Group interaction [break point: F(7, 98) = 3.25, p < .05, η2 = 0.19; response rate: F(7, 98) = 9.35, p < .05, η2 = 0.40; run rate: F(7, 98) = 736, p < .05, η2 = 0.34]. Post hoc analyses revealed that acute THC administration decreased break point, response rate, and run rate relative to the previous baseline session [both p’s < .05]. Vehicle administration had no effect on responding [all p’s > 0.66]. Tolerance to THC was again observed by the second day of THC administration for all dependent measures. After six days of THC, acute rimonabant administration (2 mg/kg, i.p.) selectively decreased break point and response rate measures in THC-treated mice relative to the final THC session as well as the final pre-THC baseline session [all p’s < .05]. Rimonabant had no effect in vehicle-treated mice [all p’s > 0.80].
Figure 3.
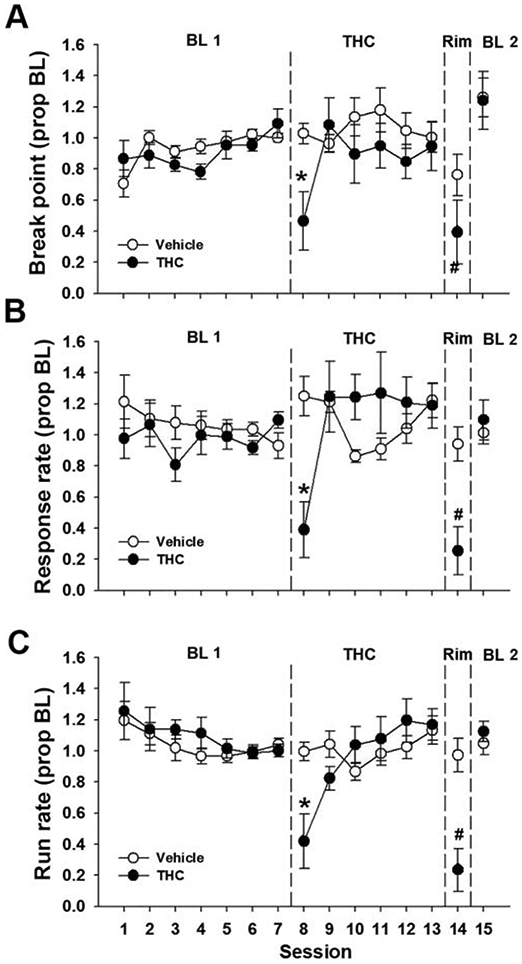
Rimonabant-precipitated THC withdrawal disrupts PR performance. Male and female mice were trained on a PR 2 schedule of reinforcement and administered THC (10 mg/kg, s.c.) or vehicle for six days. On the seventh day, all mice were administered rimonabant (2 mg/kg, i.p.). Rimonabant administration selectively decreased break point (A), response rate (B), and run rate (C) in THC-treated mice. Data represent proportion of baseline mean ±SEM (n = 7-9; THC 4F/3M; Vehicle 4F/5M). BL 1 = pre-THC baseline. BL 2 = post-withdrawal baseline. * p < 0.05 vs. Session 7; # p < 0.05 vs. Session 13.
3.3. Experiment 3: Acute ZCZ011 challenge of precipitated THC withdrawal.
After we found that a low step-size PR schedule was sensitive to precipitated THC withdrawal, we were next interested in restoring PR performance by using the CB1 PAM ZCZ011 (Trexler et al., 2019). Figure 4 shows results of the ZCZ011 dose response. There was a main effect of ZCZ011 dose on both break point [F(4,32) = 11.27, p < .05, η2 = 0.58] and response rate [F(4,32) = 6.18, p < .05, η2 = 0.44]. Post hoc analyses revealed that 20 mg/kg ZCZ011 selectively decreased break point and response rate relative to baseline [p < .05]. Thus, 10 mg/kg ZCZ011 was used in the subsequent THC withdrawal experiment.
Figure 4.
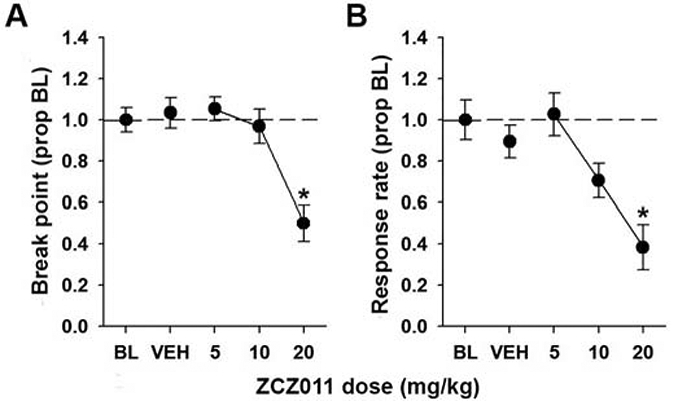
ZCZ011 decreases break point and response rate. Male and female mice were trained on a PR 2 schedule of reinforcement and acutely administered ZCZ011 (0-20 mg/kg, i.p.) in descending doses with at least three days between each administration. ZCZ011 (20 mg/kg) decreased break point (A) and response rate (B). Data represent proportion of baseline mean ±SEM (n = 9). * p < 0.05 vs. BL.
Similar to the experiments detailed above, there were no baseline differences between matched groups before repeated THC administration [Fig. 5]. Sphericity violations were detected across sessions for break point [χ2(20) = 59.90, p < 0.05] and run rate [χ2(20) = 47.21, p < 0.05]. Across repeated THC administration sessions and withdrawal, there was a significant main effect of Session [break point: F(6, 90) = 14.84, p < .05, η2 = 0.50; response rate: F(6, 90) = 31.51, p < .05, η2 = 0.68; run rate: F(6, 90) = 19.47, p < .05, η2 = 0.56] and a Session x Group interaction [break point: F(12, 90) = 4.22, p < .05, η2 = 0.36; response rate: F(12, 90) = 7.6, p < .05, η2 = 0.50; run rate: F(6, 90) = 4.49, p < .05, η2 = 0.37]. Post hoc analyses revealed that, like the previous experiments, acute THC decreased break point and response rate measures relative to baseline and vehicle [all p’s < .05]. Tolerance to THC was again observed by the third session of THC administration for both THC groups and behavior was stable thereafter. Because of this relatively robust tolerance, withdrawal was precipitated six days after the first THC administration. Six days after THC administration began, acute rimonabant administration reduced response rates for both THC-treated groups, and break point for the THC-only group, relative to the previous THC session [all p’s < .05]. For THC-treated mice pretreated with ZCZ011 (10 mg/kg), break point during withdrawal was not significantly different from the previous THC session [p = .08] but was different from the last session of baseline [p < .05]. Thus, ZCZ011 (10 mg/kg) was ineffective at restoring PR performance to baseline levels.
Figure 5.
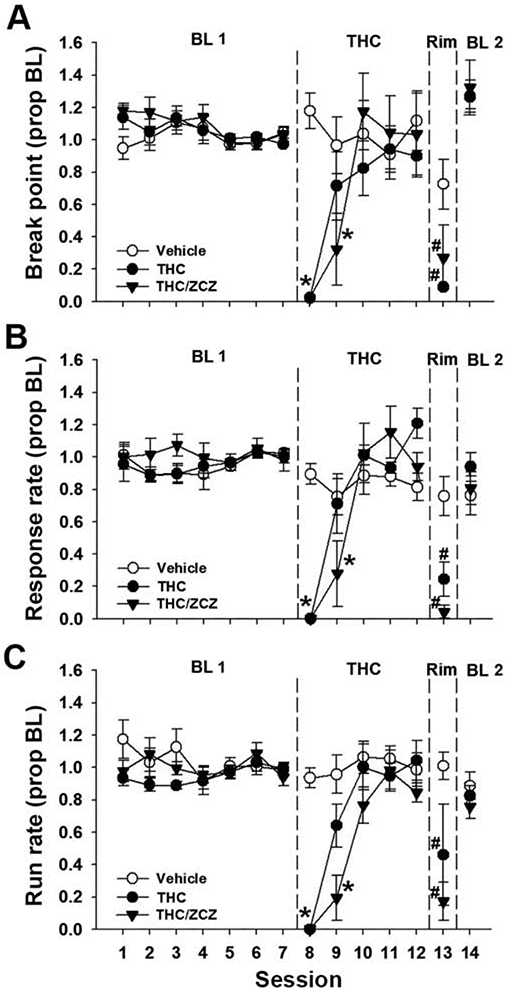
ZCZ011 does not attenuate rimonabant-precipitated THC withdrawal on a PR schedule. Male and female mice were trained on a PR 2 schedule of reinforcement and administered THC (10 mg/kg, s.c.) or vehicle for five days. On the sixth day, mice were pretreated with ZCZ011 (10 mg/kg, i.p.) or vehicle, and all mice were administered rimonabant (2 mg/kg, i.p.). Rimonabant administration selectively decreased break point (A), response rate (B), and run rate (C) in THC-treated mice. ZCZ011 pretreatment did not affect performance in THC-treated mice. Data represent proportion of baseline mean ±SEM (n = 6; 3/sex/group). BL 1 = pre-THC baseline. BL 2 = post-withdrawal baseline. * p < 0.05 vs. Session 7; # p < 0.05 vs. Session 12.
4. Discussion
The present series of experiments demonstrate that a low-step size PR schedule is sensitive to rimonabant-precipitated THC (10 mg/kg) withdrawal after five or six days of twice daily THC injections, as evidenced by decreases in break point and/or response rates. Interestingly, spontaneous THC withdrawal had no effect on PR performance, whereas rimonabant-precipitated withdrawal produced a robust but transient effect, with behavior returning to baseline levels the day following rimonabant administration. Furthermore, suppression of break point and response rates by rimonabant-precipitated THC withdrawal was not reversed by the CB1 PAM ZCZ011, which was effective at blocking somatic signs of rimonabant-precipitated THC withdrawal in mice (Trexler et al., 2019).
The lack of disruption in PR performance following spontaneous THC withdrawal in the present study coincides with a general finding that spontaneous THC withdrawal is relatively difficult to detect in animal models. Operant behavioral methods have been proposed as a more sensitive and objective alternative to traditional observational techniques for assessing drug withdrawal (Beardsley et al., 1986; Beardsley & Martin, 2000; Stewart & McMahon, 2010; Thompson & Schuster, 1964). Yet, despite this perceived advantage, the overall findings of spontaneous THC withdrawal in an operant context are mixed, with some studies reporting presence of a withdrawal effect (Beardsley et al., 1986; Branch et al., 1980; Stewart & McMahon, 2010) while others report no signs of withdrawal (Harris et al., 1974; McMillan et al., 1970). This inconsistency highlights the subtly of behavioral signs of spontaneous THC withdrawal, which may be due to the relatively long duration of action and slow elimination of THC (Grotenhermen, 2003). Beardsley et al. (1986) did not detect response rate decreases until the second day of THC abstinence. Likewise, we have previously reported that somatic signs of spontaneous THC withdrawal in mice peak at 36 h post-abstinence (Trexler et al., 2018). Although, it should be noted that somatic signs of spontaneous THC withdrawal range 20%-50% that of precipitated THC withdrawal in our studies (Trexler et al., 2018).
Similar to Beardsley et al. (2000), THC-treated mice were differentially sensitive to low-dose rimonabant administration, indicating a PR schedule is sensitive to rimonabant-precipitated THC withdrawal. Not only was break point suppressed, but also overall response rate and run rate were suppressed. Furthermore, these outcomes were replicated in Experiment 3, in which ZCZ011 (10 mg/kg) did not reverse these THC-induced reductions in break point and response rates. Break point suppression is a common outcome of drug withdrawal on a PR schedule, and is interpreted as decreased motivation (Barr & Phillips, 1999; Hoefer et al., 2006; Kirshenbaum et al., 2015). Response rate is not assessed often on PR schedules; however, relative to break point, it is a continuous rather than categorical measure potentially allowing for greater measurement sensitivity (Bradshaw & Killeen, 2012; Rasmussen & Huskinson, 2008). Response rate measures also provide some information about overall task engagement. While break point only indicates the point at which responding ceased, overall response rate and run rate give some indication of how responding was paced before the break point was reached. Because overall response rate includes the post-reinforcement pause, decreases in overall rate can be interpreted as mice taking longer to initiate responding on a given ratio. Branch et al. (1980) observed a similar phenomenon in squirrel monkeys working on a response alternation task during spontaneous THC withdrawal. While overall accuracy was unaffected by THC abstinence, trial initiation rate decreased by nearly 50% for several days possibly indicating decreased motivation to engage in the task (Branch et al., 1980). Furthermore, reductions in run rate suggest that, even after responding on a given ratio began, it was relatively sporadic.
In contrast to our previous data using a different behavioral paradigm (Trexler et al., 2019), ZCZ011 (10 mg/kg) did not reverse the behavioral suppression observed during rimonabant-precipitated THC withdrawal in the current study. The 10 mg/kg ZCZ011 dose was chosen due to its lack of behavioral disruption when administered alone. Thus, the response rate suppression observed in THC-treated mice given ZCZ011 does not reflect sedative action of ZCZ011. The primary interest in screening ZCZ011 for its preclinical efficacy stems from its relatively recent synthesis and characterization (Ignatowska-Jankowska et al., 2015) as well as a continued interest in developing novel, cannabinoid-based pharmacotherapies for various CB1-mediated disorders, including withdrawal (Nguyen et al., 2017). To date, only two studies have evaluated ZCZ011 for its preclinical efficacy in mice (Ignatowska-Jankowska et al., 2015; Trexler et al., 2019). Importantly, each endpoint in these studies evaluated pain- or withdrawal-stimulated behaviors. Therefore, the preclinical efficacy of ZCZ011 in a restoration-of-function context is still largely unknown. If the current study is any indication, then ZCZ011 may lack efficacy in restoring motivation-related outcomes in cannabinoid withdrawal. Furthermore, withdrawal-elicited increases in somatic signs of THC withdrawal may lack pharmacological specificity relative to other, more sensitive assays (Stewart & McMahon, 2010). For example, Stewart and McMahon (2010) showed that the GABAA PAM diazepam or the indirect dopamine agonist cocaine both reduced rimonabant-precipitated increases in head twitches in THC-treated rhesus monkeys without a therapeutic effect on rimonabant-lever responding in a drug discrimination procedure. Future studies should evaluate ZCZ011 and other CB1 PAMs in restoration-of-function contexts, including cannabinoid withdrawal, to address these limitations.
The present study expands on previous work, for example that of Beardsley et al. (2000) albeit several methodological considerations and limitations that warrant mention. First, to limit any difficulty in interpreting rimonabant-induced decreases in responding with the suppressive effects of THC alone, a single dose of THC (10 mg/kg) was administered repeatedly in the present study, rather than escalating the THC dose to achieve greater withdrawal. This allowed for THC habituation to develop during repeated THC administration to delineate a clear withdrawal effect. Perhaps spontaneous withdrawal could have been detected using a higher THC dose (e.g., 50 mg/kg) rather than escalating the dose; however somatic signs of spontaneous withdrawal from high-dose THC (50 mg/kg) do not differ from low-dose THC (10 mg/kg) suggesting a possible threshold effect of spontaneous withdrawal (Trexler et al., 2018). PR sessions also continued following rimonabant administration to determine the possible duration, in days, of the withdrawal effect. Similar to Beardsley et al. (2000), we observed behavior return to baseline and, in some cases (e.g., Exp. 3 break point), exceed baseline levels the day following rimonabant administration. Second, an important limitation is the lack of a positive control drug that attenuates withdrawal in the current paradigm. Following a similar THC regimen to that used here, high-dose THC (50 mg/kg) partially restored withdrawal-induced tail suspension struggling to vehicle levels (Trexler et al., 2018). Notably, this effect did not occur following administration of the indirect endocannabinoid agonist JZL184 or PF-3845. Due to robust THC habituation we observe, perhaps high-potency synthetic cannabinoids, like HU-210 or CP 55,940, may be useful in demonstrating cannabinoid-dependent attenuation of withdrawal in an operant context. Third, it is unclear if the current withdrawal regimen was sufficient to produce somatic withdrawal signs, which would presumably impact the ability of the mice to engage in nose-poking behavior independent of motivational effects, per se. Beardsley et al. (2000) informally noted the absence of overt withdrawal signs at any stage of testing, both with vehicle administration at the beginning of the withdrawal session and with subsequent injections of rimonabant throughout the session. Similarly, Wise et al. (2011) treated mice with THC (10 mg/kg) for 4 days and then precipitated withdrawal with 1, 3 or 10 mg/kg rimonabant. They observed spatial navigation deficits beginning at 1 mg/kg rimonabant without significant increases in paw tremors or head shakes. Because we did not record somatic withdrawal signs in the current study, it is plausible that they may have played a role in the observed behavioral disruption. Regarding human use patterns, a fourth limitation is the use of experimenter-administered drug, specifically experimenter-injected drug in the current study. However, self-administration of cannabinoids, including THC, has been difficult to establish in rodents (e.g., Barrus et al., 2018). There have been a few reports of learning impairment and withdrawal signs from exposure to cannabis smoke in mice (Niyuhire et al., 2007; Wilson et al., 2006), but not in an operant context representing an interesting goal of future studies.
As many of the DSM-V criteria for Cannabis Use Disorder (CUD) involve some aspect of uncontrolled self-administration of cannabis (APA, 2013), the two criteria most applicable to animal studies are tolerance and withdrawal, the latter of which was targeted in the current study using an operant paradigm. However, it is noteworthy that alleviating withdrawal symptoms alone is often not sufficient to promote abstinence in treatment-seeking individuals (Werneck et al., 2018). Psychosocial interventions, in addition to managing withdrawal symptoms, are ultimately the most effective strategy in treating cannabis dependence (Gates et al., 2016). In this regard, predictive animal models can aid in identifying pharmacotherapies that may serve as an adjuvant to effective psychosocial intervention.
Experimental animal models of cannabinoid withdrawal are often critiqued for using antagonist-precipitated withdrawal rather than arising spontaneously via abstinence. Indeed, people experience cannabinoid withdrawal as a result of reducing or terminating their use rather than via ingesting a cannabinoid antagonist. However, as this study and many others show, spontaneous THC withdrawal is often difficult to detect in animal behavior, thereby limiting the mechanistic utility of spontaneous withdrawal studies. Apart from behavioral indices, one role of spontaneous cannabinoid withdrawal studies may lie in the neurological and neuroendocrine withdrawal response. For example, spontaneous withdrawal from the synthetic agonist CP 55,940 produces robust increases in cannabinoid receptor sensitization and plasma corticosterone in mice (Oliva et al., 2003). While this neuroendocrine effect of cannabinoid withdrawal is difficult to detect in humans (Cohen, 1974; Goodwin et al., 2012), these stress effects may be of sufficient magnitude for probing pharmacological intervention in a preclinical context. In the case of precipitated withdrawal, assessments of behavioral symptoms in an operant or learning context appear to be more sensitive than standard observational techniques, such as somatic signs. A rimonabant dose of 10 mg/kg is typically administered in precipitated THC withdrawal to observe somatic signs after a relatively high- (50 mg/kg) or lower-dose (10 mg/kg) THC withdrawal (Schlosburg et al., 2009; Wise et al., 2011). While our laboratory has used a lower rimonabant dose to precipitate withdrawal (3 mg/kg; Trexler et al., 2018), rimonabant doses in operant or learning contexts induce impairment at as low as 1 mg/kg (i.p.) (Beardsley et al., 2000; Wise et al., 2011) in rodents and 0.32 mg/kg (i.v.) in monkeys. To the extent that behavioral indices of cannabinoid withdrawal have utility, more sensitive paradigms allowing for lower antagonist doses, such as the regimen used in the present study, may be more predictive of effective pharmacotherapies for human studies.
Despite these limitations and overarching issues, these data show that rimonabant-precipitated THC withdrawal impairs behavior on a PR schedule of sucrose reinforcement in mice. Although often overlooked in favor of more naturalistic or observational assays, operant behavioral techniques are sensitive tools for the study of drug withdrawal states in animals (Barr & Phillips, 1999; Emmett-Oglesby et al., 1990). These methods also allow for assessing disruption of ongoing behavior that may be useful models of motivational/emotional perturbation as a consequence of THC withdrawal in humans. Finally, withdrawal-induced disruption of operant behavior serves as a target for putative withdrawal pharmacotherapies with regard to restoration of function.
Supplementary Material
Highlights.
A progressive-ratio (PR) schedule is sensitive to antagonist-precipitated, but not spontaneous, THC withdrawal.
The CB1 positive allosteric modulator ZCZ011 does not attenuate withdrawal-induced suppression of PR performance.
Withdrawal-suppressed behavior may be more selective than withdrawal-induced behavior in identifying pharmacotherapies for cannabinoid dependence.
Acknowledgements:
We would like to thank Ethan Mick, Asia Taylor, Elijah Cheeks, Kristyn Campbell, and the WVU OLAR staff for their technical and husbandry assistance. This work was supported by NIH grants DA039335, AR066806, GM081741, and GM103434.
Funding: DA039335, AR066806, GM081741, and GM103434
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References.
- Aceto MD, Scates SM, Lowe JA, & Martin BR (1995). Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. European Journal of Pharmacology, 252(1–3), 1–2. 10.1016/0014-2999(95)00447-S [DOI] [PubMed] [Google Scholar]
- Barr AM, & Phillips AG (1999). Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology, 141(1), 99–106. 10.1007/s002130050812 [DOI] [PubMed] [Google Scholar]
- Barrus DG, Lefever TW, & Wiley JL (2018). Evaluation of reinforcing and aversive effects of voluntary Δ 9 -tetrahydrocannabinol ingestion in rats. Neuropharmacology, 137, 133–140. 10.1016/j.neuropharm.2018.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Baslter RL, & Harris LS (1986). Dependence on Tetrahydrocannabinol in Rhesus Monkeys. The Journal of Pharmacology and Experimental Therapeutics, 239. [PubMed] [Google Scholar]
- Beardsley PM, & Martin BR (2000). Effects of the cannabinoid CB 1 receptor antagonist, SR141716A , after D 9 -tetrahydrocannabinol withdrawal. European Journal of Pharmacology, 387, 47–53. [DOI] [PubMed] [Google Scholar]
- Bradshaw CM, & Killeen PR (2012). A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology, 222(4), 549–564. 10.1007/s00213-012-2771-4 [DOI] [PubMed] [Google Scholar]
- Branch MN, Dearing ME, & Lee DM (1980). Acute and chronic effects of Δ9-Tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology, 71(3), 247–256. 10.1007/BF00433059 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, & Vandrey R (2004). Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry, 161, 1967–1977. 10.1176/appi.ajp.161.11.1967 [DOI] [PubMed] [Google Scholar]
- Cohen S (1974). The 94-day cannabis study. Annals of the New York Academy of Science, 282, 211–220. [DOI] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, & Martin BR (1998). CB1 receptor antagonist precipitates withdrawal in mice. The Journal of Pharmacology and Experimental Therapeutics, 285, 1150–1156. [PubMed] [Google Scholar]
- Eckard ML, & Kyonka EGE (2018). Differential reinforcement of low rates differentially decreased timing. Behavioural Processes, 151(March), 111–118. 10.1016/j.beproc.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Mathis DA, Moon RTY, & Lai H (1990). Animal models of drug withdrawal symptoms. Psychopharmacology, 101(3), 292–309. 10.1007/BF02244046 [DOI] [PubMed] [Google Scholar]
- Gates PJ, Sabioni P, Copeland J, Le Foll B, & Gowing L (2016). Psychosocial interventions for cannabis use disorder ( Review ). Cochrane Database of Systematic Reviews, 5, 1–101. 10.1002/14651858.CD005336.pub4.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RS, Baumann MH, Gorelick DA, Schwilke E, Schwope DM, Darwin WD, Kelly DL, Schroeder JR, Ortemann-Renon C, Bonnet D, & Huestis MA (2012). CB1 cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. American Journal of Drug and Alcohol Abuse, 38(1), 114–119. 10.3109/00952990.2011.600398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics, 42(4), 327–360. 10.1093/jat/19.6.459 [DOI] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper Z, Glass A, Vosburg SK, Comer SD, & Foltin RW (2013). Predictors of marijuana relpase in the human laboratory: Robust impact of tobacco cigarette smoking status. Biological Psychiatry, 32, 736–740. 10.1371/journal.pone.0178059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, & Fischman MW (1999). Abstinence symptoms following smoked marijuana in humans. Psychopharmacology, 141(4), 395–404. 10.1007/s002130050849 [DOI] [PubMed] [Google Scholar]
- Harris RT, Waters W, & McLendon D (1974). Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia, 37(1), 23–29. 10.1007/BF00426679 [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Weerts EM, & Vandrey R (2015). Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Experimental and Clinical Psychopharmacology, 23, 415–421. 10.1037/pha0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer ME, Voskanian SJ, Koob GF, & Pulvirenti L (2006). Effects of terguride, ropinirole, and acetyl-l-camitine on methamphetamine withdrawal in the rat. Pharmacology Biochemistry and Behavior, 83(3), 403–409. 10.1016/j.pbb.2006.02.023 [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj IM, Poklis J, Wiley JL, Zanda M, Zanato C, Greig IR, Lichtman AH, & Ross RA (2015). A cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 40(13), doi: 10.1038/npp.2015.148. 10.1038/npp.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum A, Green J, Fay M, Parks A, Phillips J, Stone J, & Roy T (2015). Reinforcer devaluation as a consequence of acute nicotine exposure and withdrawal. Psychopharmacology, 232(9), 1583–1594. 10.1007/s00213-014-3792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Wiley JL, Lavecchia KL, Neviaser ST, Arthur DB, Wilson DM, & Martin BR (1998). Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. European Journal of Pharmacology, 357, 139–148. 10.1016/S0014-2999(98)00558-5 [DOI] [PubMed] [Google Scholar]
- McMahon LR, & France CP (2003). Discriminative Stimulus Effects of the Cannabinoid Antagonist, SR 141716A, in Δ9-Tetrahydrocannabinol-Treated Rhesus Monkeys. Experimental and Clinical Psychopharmacology, 11(4), 286–293. 10.1037/1064-1297.11.4.286 [DOI] [PubMed] [Google Scholar]
- McMillan DE, Harris LE, Frankenheim JM, & Kennedy JS (1970). Tetrahydrocannabinol in Pigeons : Tolerance to the Behavioral Effects. Science, 169, 501–503. [DOI] [PubMed] [Google Scholar]
- Montoya ID, & Weiss SRB (Eds.). (2019). Cannabis Use Disorders. Springer. [Google Scholar]
- Negus SS (2019). Core outcome measures in preclinical assessment of candidate analgesics. Pharmacological Reviews, 71(2), 225–266. 10.1124/pr.118.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Li J-X, Thomas BF, Wiley JL, Kenakin TP, & Zhang Y (2017). Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor. Medicinal Research Reviews, 37(3), 441–474. 10.1002/med.21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, & Lichtman AH (2007). Exposure to marijuana smoke impairs memory retrieval in mice. Journal of Pharmacology and Experimental Therapeutics, 322(3), 1067–1075. 10.1124/jpet.107.119594 [DOI] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, & Manzanares J (2003). Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. Journal of Neurochemistry, 85(1), 94–104. 10.1046/j.1471-4159.2003.01627.x [DOI] [PubMed] [Google Scholar]
- Orsini C, Koob GF, & Pulvirenti L (2001). Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology, 25(5), 789–792. 10.1016/S0893-133X(01)00270-6 [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, & Huskinson SL (2008). Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behavioural Pharmacology, 7, 735–742. 10.1097/FBP.0b013e3283123cc2 [DOI] [PubMed] [Google Scholar]
- Rodríguez De Fonseca F, Carrera MRA, Navarro M, Koob GF, & Weiss F (1997). Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science, 276(5321), 2050–2054. 10.1126/science.276.5321.2050 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Boger DL, Cravatt BF, & Lichtman AH (2009). Endocannabinoid Modulation of Scratching Response in an Acute Allergenic Model: A New Prospective Neural Therapeutic Target for Pruritus. Journal of Pharmacology and Experimental Therapeutics, 329(1), 314–323. 10.1124/jpet.108.150136.logical [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BLA, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, & Lichtman AH (2009). Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. The AAPS Journal, 11, 342–352. 10.1208/s12248-009-9110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, & McMahon LR (2010). Rimonabant-induced Δ9-tetrahydrocannabinol withdrawal in rhesus monkeys: Discriminative stimulus effects and other withdrawal signs. Journal of Pharmacology and Experimental Therapeutics, 334(1), 347–356. 10.1124/jpet.110.168435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, & Schuster CR (1964). Morphine self-administration, food-reinforced, and avoidance behaviors in rhesus monkeys. Psychopharmacologia, 5, 87–94. [DOI] [PubMed] [Google Scholar]
- Trexler KR, Eckard ML, & Kinsey SG (2019). CB1 positive allosteric modulation attenuates Δ9-THC withdrawal and NSAID-induced gastric inflammation. Pharmacology Biochemistry and Behavior, 177, 27–33. 10.1016/j.pbb.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler Kristen R., Nass SR, Crowe MS, Gross JD, Jones MS, McKitrick AW, Siderovski DP, & Kinsey SG (2018). Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug and Alcohol Dependence, 191(November 2017), 14–24. 10.1016/j.drugalcdep.2018.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Patrick SL, & Walker JM (1995). Physical withdrawal in rats tolerant to Δ 9 - tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. European Journal of Pharmacology, 280, 14–16. 10.1016/0014-2999(95)00360-W [DOI] [PubMed] [Google Scholar]
- Werneck MA, Trevizan G, Arthur K, & De. Andrade G (2018). A Systematic Review of the Efficacy of Cannabinoid Agonist Replacement Therapy for Cannabis Withdrawal Symptoms. CNS Drugs, 32(12), 1113–1129. 10.1007/s40263-018-0577-6 [DOI] [PubMed] [Google Scholar]
- Wilson DM, Varvel SA, Harloe JP, Martin BR, & Lichtman AH (2006). SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacology Biochemistry and Behavior, 85(1), 105–113. 10.1016/j.pbb.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Wise LE, Varvel SA, Selley DE, Wiebelhaus JM, Long KA, Middleton LS, Sim-Selley LJ, & Lichtman AH (2011). δ 9-Tetrahydrocannabinol-dependent mice undergoing withdrawal display impaired spatial memory. Psychopharmacology, 217(4), 485–494. 10.1007/s00213-011-2305-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhou X, Wang X, Xiang X, Chen H, & Hao W (2007). Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addiction Biology, 12(2), 152–157. 10.1111/j.1369-1600.2007.00068.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


