Abstract
Objectives:
Individuals from different socioeconomic status (SES) backgrounds may respond variably to stressful events, and such differences are likely to contribute to health disparities. The current study leveraged data collected before and after a petrochemical explosion and aimed to investigate how individuals from different SES backgrounds responded to this unexpected stressor in terms of perceived social support, perceived stress, and systemic inflammation.
Methods:
Data were drawn from 124 participants (Mage = 55.9 ± 16.1 years, 69.4% female, 29.0% White) living close to a petrochemical complex where the explosion occurred in 2005. SES was assessed at baseline, and perceived stress and inflammatory markers (i.e., C-reactive protein [CRP], interleukin-6 [IL-6]) were assessed at both pre- and post-explosion. Perceived social support was assessed at post-explosion.
Results:
Lower SES was associated with less perceived social support. Lower SES was also associated with a larger increase in perceived stress and higher levels of IL-6, but not CRP. Perceived social support did not moderate or mediate the effects of SES on changes in perceived stress, IL-6, or CRP. The associations between SES and inflammatory markers were also not explained by changes in perceived stress.
Conclusion:
Findings from this study support the idea that individuals from different SES backgrounds respond differently to stressors both at the psychosocial (perceived social support and perceived stress) and biological (inflammation) levels. Our findings also suggest that these two processes appear to act independently from each other.
Keywords: Socioeconomic status, inflammation, perceived stress, perceived social support
1. Introduction
Socioeconomic status (SES) disparities in health are widespread and persistent over time (Link & Phelan, 1995). Low SES has been robustly associated with poor health and premature mortality (Elo, 2009; Stringhini et al., 2017). SES is commonly measured by education, income, occupation, or a combination of these dimensions that are usually overlapping but not interchangeable (Adler et al., 1994). For example, education and income, the two most common indicators assessed in studies of SES inequalities in the United States (Elo, 2009), may capture somewhat different aspects of SES; education reflects the requisite knowledge and skills for acquiring economic and psychosocial resources, whereas income more directly indicates the accessibility to economic resources (Winkleby, Jatulis, Frank, & Fortmann, 1992). Despite these potential differences, indicators of SES are intercorrelated and have all been linked to health and mortality, although education appears to be the most consistent predictor of health (for a review, see Elo, 2009). To alleviate health disparities across SES groups, it is critical to understand the complex biopsychosocial pathways through which SES impacts health.
Exposure to stressors has been proposed as one of such factors (Cundiff, Boylan, & Muscatell, 2020). Individuals from lower SES often report experiencing more stressors than higher SES individuals (Businelle et al., 2014). Some studies, however, have also shown that lower SES is associated with less but more severe stressors on a daily basis (Grzywacz, Almeida, Neupert, & Ettner, 2004). As succinctly depicted in the Stage Model of Stress and Disease (Cohen, Gianaros, & Manuck, 2016), exposure to stressors elicits stress appraisal (e.g., perceived stress) and negative emotional responses, which subsequently affects biological processes (e.g., inflammation) relevant to physical health.
In addition to exposure to stressors, SES also contributes to how individuals construe stressful events. For example, people from low SES backgrounds display increased psychological vulnerability following major life stressors (McLeod & Kessler, 1990) and experience aberrant elevated inflammatory responses to acute laboratory stressors (e.g., a larger increase in interleukin-6 [IL-6], for a review, see Marsland, Walsh, Lockwood, & John-Henderson, 2017). However, reports have also shown no associations between SES and inflammatory responses to stress (Steptoe, Owen, Kunz-Ebrecht, & Mohamed-Ali, 2002). As indicated by the Reserve Capacity Model (RCM, Gallo & Matthews, 2003), these SES differences in stress response can be attributed to SES discrepancies in reserve capacity (i.e., a general resource bank). The RCM suggests that individuals with low SES are not only confronted with more stressors but also have fewer resources at their disposal to deal with such stressors (e.g., perceived social support). A depleted pool of resources may exacerbate biopsychosocial reactivity to stress, and ultimately, lead to poor health. Notably, the RCM also acknowledges that SES can affect health directly and indirectly through one’s reserve capacity of resources. Recent studies have corroborated these theoretical predictions. For example, using data from the Midlife in the United States, Elliot and Chapman (2016) reported that a cluster of psychosocial resources moderated the association between SES and inflammation, though such moderation effects were evident only among men (Elliot & Chapman, 2016). In another study among Hispanic/Latino adults, McCurley et al. (2017) found that psychosocial resources mediated the association between SES and metabolic syndrome. However, Gallo et al. (2019) only found direct associations between SES and psychosocial resources and between SES and allostatic load, but not significant buffering or indirect effects in a sample of Hispanic/Latino youths.
The current study aimed at examining how SES affects individuals’ biopsychosocial responses (i.e., perceived social support, perceived stress, inflammation) to a human-caused disaster. Prior studies on this topic largely focused on laboratory-induced stress (e.g., Marsland et al., 2017), and few studies have examined the effects of SES on these stress responses in naturalistic settings. We hypothesized that individuals with lower SES reported lower perceived social support and experienced a more prolonged elevation in perceived stress and systemic inflammation (i.e., IL-6, C-reactive protein [CRP]) in response to the explosion. Based on RCM, we also predicted that perceived social support mediated and moderated the effects of SES on changes in perceived stress and systemic inflammation. Lastly, we hypothesized that perceived stress mediated the association between SES and changes in systemic inflammation.
2. Method
2.1. Participants and Procedure
Data for the current analysis were drawn from the Texas City Stress and Health study, a study on the social determinants of health among Hispanics, Whites, and African Americans (for details, see Cutchin, Martin, Owen, & Goodwin, 2008; Peek, Cutchin, Freeman, Stowe, & Goodwin, 2009). On March 23, 2005, a petrochemical explosion occurred in the study site, which prompted the research team to administer an additional, post-explosion survey. The explosion killed 15 oil workers and injured over 170 others. At the time of the explosion, 550 participants had completed their face-to-face interviews, of which were invited to complete a telephone post-explosion survey. A total of 315 (57%) agreed and completed the post-explosion survey between May and August 2005. Of the 315 participants, 124 participants (69.4% female, 25–86 years of age) provided blood samples at both baseline and post-explosion assessments, constituting the final sample for the current study. All participants were living in the community in which the explosion occurred. The time between the two assessments ranged from one month to 13 months (M = 5.9 ± 2.7 months). The study protocol was approved by the Institutional Review Board at the University of Texas Medical Branch, and informed consent was obtained from all participants.
2.2. Measure
2.2.1. Socioeconomic status (SES)
SES was assessed by education and annual household income at baseline. Participants were asked to report years of schooling completed and annual household income on an 8-point scale ranging from 1 = less than 10,000 to 8 = 75,000 or more. A composite index of SES was calculated by averaging z scored education and annual household income, given the moderate association between education and income (r = 0.44, p < .001) and that combining indicators of SES is a common practice when studies have an interest in determining the general ingredient of SES in health (e.g., Elliot & Chapman, 2016).
2.2.2. Pre- and post-explosion perceived stress
The 10-item Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983) was used to assess perceived stress. Participants were asked to rate how often they felt or thought in a certain way (e.g., felt nervous and stressed) during the past month on a 5-point scale, ranging from 0 = never to 4 = very often. Four positively stated items were reversely coded, and a composite score was calculated by summing responses of the 10 items. The Cronbach alpha for this scale was 0.77 at baseline.
2.2.3. Post-explosion perceived social support
Perceived social support was assessed by a four-item scale developed for this study. This scale was administered only after the explosion. Participants were asked to report whether, since the explosion, there had always been someone to (1) ask and rely on for advice, (2) tell about the mistakes that could put you into trouble, (3) go for comfort if they were treated badly and were upset about it, and (4) go for comfort if they engaged in conflicts on a binary response (0 = no, 1 = yes). Responses were summed to calculate an overall score. The Cronbach alpha for this scale was 0.83.
2.2.4. Pre- and post-explosion systemic inflammation.
CRP and IL-6 were assayed using enzyme-linked immunosorbent assays. Specifically, CRP was determined using a commercial assay kit (Diagnostic Systems Laboratories, Webster, TX), with an assay sensitivity of 0.1 mg/L and an average coefficient of variation (CV) of less than 5%. IL-6 was determined using the OptEIA assay kit (BD Pharmingen, San Diego, CA), with an assay sensitivity of 2.2 pg/ml and an average CV of less than 10%. Raw values for CRP and IL-6 were log-transformed to correct for positive skew.
2.2.5. Demographic variables.
At baseline, participants self-reported age, sex (0 = male, 1 = female), race/ethnicity (dummy coded: White vs. Hispanic; White vs. African American), and marital status (0 = other, 1 = married). Body mass index (BMI) was calculated using participants’ height and weight assessed by trained research staff. One participant’s BMI was assessed using self-reported height and weight.
2.2.6. Exposure variables
Two variables reflecting exposure to the disaster were included as covariates (Cutchin et al., 2008). Distance in miles from the explosion site was operationalized as the shortest distance from the participant’s home address to the isomerization unit at the refinery that exploded. Also, three items were used to assess if participants had heard the explosion, felt the explosion, or saw the smoke from the explosion (0 = no, 1 = yes). An index for heard/felt/saw the explosion was created by summing responses on these three items.
2.3. Statistical Analyses
Regression analyses were employed to test the main effects of SES on each post-explosion outcome, as well as to test the moderation effects of perceived social support on the associations between SES and perceived stress and inflammation. Path analyses were employed to test the mediation effect of perceived social support on these associations, as well as to test the mediation effect of perceived stress on the association between SES and inflammation. The mediation model would be considered to fit the data well if the comparative fit index (CFI) ≥ 0.95 and root mean square error of approximation (RMSEA) ≤ 0.06 (Hu & Bentler, 1999). Indirect effects were tested using the bootstrapping method (MacKinnon, Lockwood, & Williams, 2004). This method allows deriving 95% bias-corrected bootstrap confidence intervals (CI), which are indicative of a significant indirect effect if they do not contain 0 (i.e., 1,000 resamples). All models were analyzed using the maximum likelihood estimator with robust standard errors in Mplus 7.0 (Muthén & Muthén, 2012). Missing data were about 1% and were handled with multiple imputation with 10 imputed datasets, and results from each imputed dataset were combined using Rubin’s formula (Rubin, 2004).
3. Results
Results showed that participants at post-explosion reported higher levels of perceived stress than at baseline (t = − 2.15, p = .034, Cohen’s d = 0.23). IL-6 or CRP levels did not significantly change from pre-explosion to post-explosion (t = 0.18, p = .86; t = 0.80, p = .42; respectively). Table 1 displayed the detailed descriptive results and bivariate correlations between variables.
Table 1.
Means, standard deviations, and bivariate correlations between study variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Education | - | ||||||||||||||||
| 2. Income | 0.44*** | - | |||||||||||||||
| 3. T1 PS | −0.01 | −0.19* | - | ||||||||||||||
| 4. T2 PS | −0.21* | −0.29** | 0.37*** | - | |||||||||||||
| 5. T2 Perceived SS | 0.17 | 0.18 | 0.06 | −0.02 | - | ||||||||||||
| 6. T1 CRP | −0.05 | 0.07 | 0.05 | 0.12 | 0.04 | - | |||||||||||
| 7. T2 CRP | −0.08 | 0.05 | 0.10 | 0.13 | 0.03 | 0.69*** | - | ||||||||||
| 8. T1 IL-6 | −0.03 | −0.22* | 0.16 | 0.03 | −0.06 | 0.21* | 0.16 | - | |||||||||
| 9. T2 IL-6 | −0.20* | −0.33*** | 0.08 | 0.21* | −0.04 | 0.16 | 0.10 | 0.51*** | - | ||||||||
| 10. Female | 0.02 | −0.07 | 0.05 | −0.02 | 0.04 | 0.08 | 0.11 | −0.04 | 0.03 | - | |||||||
| 11. African American | 0.04 | −0.20* | 0.16 | 0.13 | 0.09 | 0.17 | 0.16 | 0.08 | −0.09 | 0.02 | - | ||||||
| 12. Hispanic | −0.36*** | −0.09 | −0.10 | 0.00 | −0.05 | 0.05 | 0.06 | 0.00 | 0.07 | 0.12 | −0.43*** | - | |||||
| 13. Married | −0.03 | 0.28** | −0.07 | −0.04 | 0.18 | 0.06 | −0.01 | −0.06 | 0.01 | −0.21* | −0.12 | 0.13 | - | ||||
| 14. Age | −0.07 | −0.03 | −0.22* | −0.22* | −0.02 | 0.03 | −0.01 | 0.15 | 0.06 | −0.19* | 0.14 | −0.33*** | −0.21* | - | |||
| 15. BMI | 0.08 | 0.01 | 0.03 | 0.10 | 0.03 | 0.29** | 0.28** | 0.02 | 0.14 | 0.22* | 0.16 | −0.08 | −0.05 | −0.03 | - | ||
| 16. Distance from explosion | 0.20* | 0.07 | 0.18 | −0.11 | 0.16 | −0.19* | −0.17 | −0.10 | −0.04 | −0.02 | 0.10 | −0.22* | −0.16 | 0.14 | −0.07 | - | |
| 17. Heard/felt/saw explosion | −0.19* | −0.19* | 0.15 | −0.03 | 0.07 | 0.28** | 0.26** | 0.12 | 0.19* | 0.03 | 0.05 | 0.11 | 0.08 | −0.00 | 0.16 | −0.17 | - |
| M | 10.96 | 3.61 | 12.63 | 14.12 | 2.69 | 11.97a | 12.06a | 3.04b | 2.36b | 86c | 14c | 73c | 65c | 55.91 | 30.89 | 2.06e | 2.43 |
| SD | 3.33 | 2.21 | 6.44 | 8.75 | 1.52 | 12.71 | 15.34 | 6.34 | 4.94 | 69.4d | 11.3d | 58.9d | 52.4d | 16.10 | 7.12 | 0.72 | 1.01 |
Note. T1 = baseline visit; T2 = follow-up visit; SES = socioeconomic status; PS = perceived stress; SS = social support; CRP = C-reactive protein; IL = interleukin; AA = African American; BMI = body mass index.
Unit mg/dL
Unit pg/mL
Displayed as N
Displayed as percentage
Unit mile.
p < .05;
p < .01;
p < .001.
Controlling for sex, race/ethnicity, age, marital status, BMI, distance from the explosion, heard/felt/saw the explosion, and baseline outcomes, regression analyses indicated that SES was associated with post-explosion perceived social support (β = .21, p = .033) and changes in perceived stress (β = −.27, p = .001) and IL-6 (β = −.29, p < .001), but not CRP (β = .03, p = .73)1. To further visualize changes in the outcomes from pre- to post-explosion as a function of SES, Figure 1 depicted mean changes in perceived stress, IL-6, and CRP across the two assessments across individuals with low (below 1 SD), middle (mean), and high (above 1 SD) SES. Perceived social support did not moderate the effects of SES on changes in perceived stress (β = −.02, p = .77), IL-6 (β = −.07, p = .36), or CRP (β = −.09, p = .17). Of the two hypothesized mediators, only post-explosion perceived stress correlated with both SES and post-explosion IL-6. Thus, path analyses were only performed to test the mediation effect of post-explosion perceived stress on the association between SES and post-explosion IL-6. The mediation model fitted the data well, χ2(2) = 1.75, CFI = 1.00, RMSEA = 0.01. SES was associated with perceived stress (β = −.27, p = .001); however, perceived stress was not associated with IL-6 (β = .15, p = .058). The indirect effect from SES to IL-6 through perceived stress was not statistically significant (indirect effect = −0.04, 95% CI [−0.09, 0.01]). Notably, the association between SES and post-explosion IL-6 remained significant in the path analysis (β = −.24, p = .002).
Figure 1.
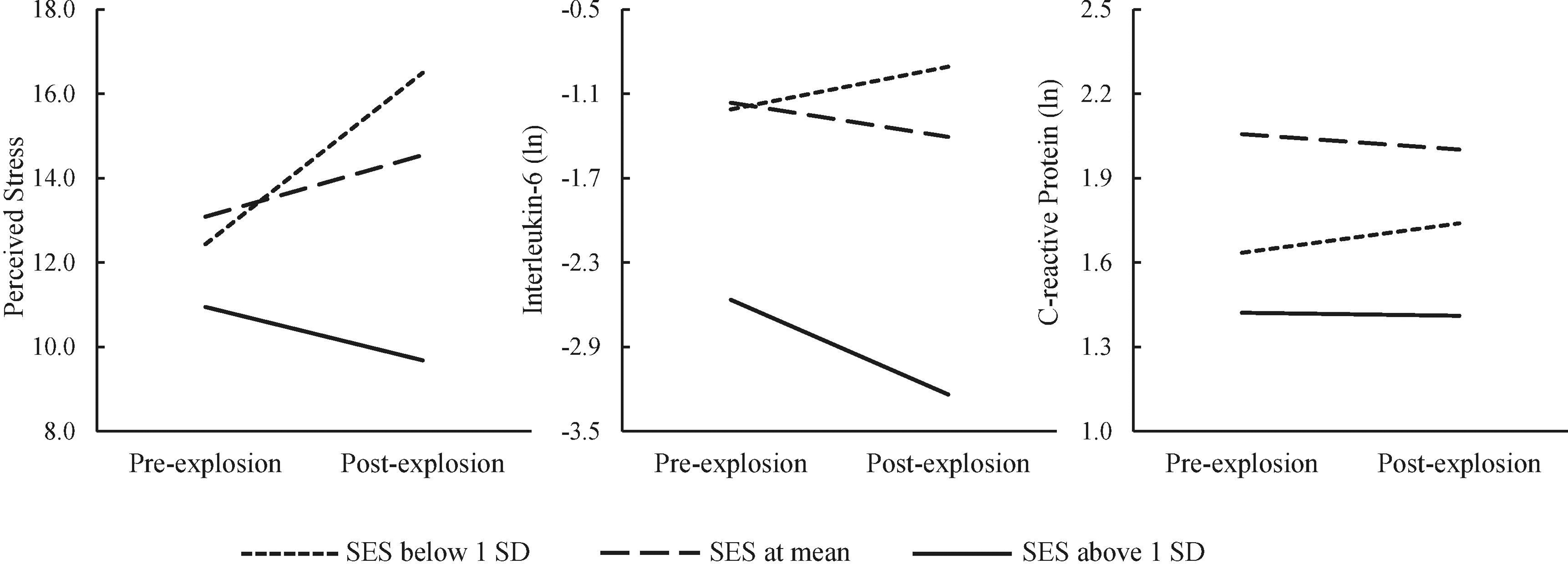
Mean changes from pre- to post-explosion in perceived stress, interleukin-6, and C-reactive protein across individuals from different socioeconomic status (SES) backgrounds (split at mean and below and above one standard deviation [SD]).
4. Discussion
This study showed that individuals with low SES reported low levels of perceived social support and increased levels of perceived stress following a human-caused disaster. Moreover, although IL-6 and CRP levels did not significantly change from before to after the explosion, SES negatively correlated with changes in IL-6, but not CRP, suggesting that changes in IL-6 were more prolonged among individuals with lower SES. Also, perceived social support did not moderate or mediate the effects of SES on changes in perceived stress or inflammation. Lastly, the association between SES and inflammation was not mediated by perceived stress.
Our results align with the prediction of the Reserve Capacity Model (Gallo & Matthews, 2003), according to which SES influences the appraisal of the threat value of potentially stressful situations (i.e., perceived stress) and accessibility of coping resources (i.e., perceived social support). The RCM model postulates direct, indirect, and moderation effects of SES on biological risk through an individual’s reserve capacity of resources. Consistent with a recent study documenting the direct effect of SES on allostatic load (Gallo et al., 2019), our results corroborate the direct association between SES and inflammation but do not confirm the potential indirect or buffering effects of perceived social support on this association. The results also suggest that SES-variability in appraisal appears not to impact changes in IL-6 associated with SES. Our study, however, does not simply replicate previous findings but extends them in two significant ways. First, our study is among the first to test the predictions of the RCM using a prospective design in which changes in stress appraisal and inflammatory markers through time were assessed. Second, such changes were measured in relation to a naturalistic stressor (vs. a laboratory stressor; e.g., Marsland et al., 2017), allowing us to infer how SES predicts psychological and inflammatory responses to stress in a naturalistic setting. Overall, these findings suggest that increases in perceived stress and systemic inflammation following a stressful event act as potential mechanisms contributing to SES disparities in health and mortality (Elo, 2009; Stringhini et al., 2017).
We found no effects of SES on CRP. One potential explanation for this null finding has to do with the smaller variation in pre- and post-explosion CRP levels compared to IL-6, potentially suggesting that CRP levels are less easily disturbed by environmental stressors than IL-6. Previous studies suggest that CRP levels are fairly stable over time when compared to IL-6 (Ferrari et al., 2013). Another possible explanation is that IL-6 is more sensitive to stress exposure than CRP, though IL-6 activates downstream production of CRP. Accordingly, evidence from a previous meta-analysis showed more consistent elevated productions of IL-6 than CRP following laboratory-induced stress (Marsland et al., 2017).
There are some limitations of this study. First, this study did not include a control group (i.e., individuals without explosion exposure), which limits our ability to demonstrate that the changes in perceived stress and inflammatory markers were caused by the explosion. Second, the absence of data on pre-explosion perceived social support limits the interpretation of the result regarding the SES-perceived social support association. Third, the limited statistical power due to a small sample size may reduce the opportunities to detect some true effects in this study. Despite these limitations, this study provides novel evidence to indicate that low SES contributes to low levels of perceived social support and prolonged elevated perceived stress and production of IL-6 following a naturalistic stressor. These biopsychosocial factors may represent critical pathways contributing to persistent SES disparities in health reported in the literature.
Highlights.
Low SES was associated with low levels of perceived social support following a human-caused disaster
Low SES was associated with larger changes in perceived stress and interleukin-6 from pre-disaster to post-disaster
The association between SES and changes in interleukin-6 was not mediated by perceived stress or perceived social support
Funding:
Data collection for the present study was supported by the National Cancer Institute (P50 CA105631). The preparation of the current data analysis was partly supported by a Faculty Competition for Postdoctoral Fellowship from Wayne State University to Dr. Samuele Zilioli and the NIGMS/NIH grant R25 GM 058905-20 to Jacqueline Rodriguez-Stanley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We separately tested the effects of education and income on post-explosion outcomes, and the results were similar. Specifically, both education and income were associated with changes in perceived stress (β = −.25, p = .001; β = −.24, p = .015, respectively) and IL-6 (β = −.22, p = .007; β = −.30, p < .001, respectively), but not CRP (β = −.04, p = .59; β = .08, p = .34, respectively). Neither education nor income was associated with post-explosion perceived social support (β = .17, p = .11; β = .14, p = .14, respectively).
We also examined the association between SES and perceived social support and perceived stress among 315 participants who completed the post-survey data, regardless of the availability of blood samples. The results were very similar to the findings reported in the main text. SES was significantly associated with changes in perceived stress (β = −0.18, p = .002) and post-explosion perceived social support (β = .23, p < .001).
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, & Syme SL (1994). Socioeconomic status and health: The challenge of the gradient. American Psychologist, 49(1), 15–24. [DOI] [PubMed] [Google Scholar]
- Businelle M, Mills B, Chartier K, Kendzor D, Reingle J, & Shuval K (2014). Do stressful events account for the link between socioeconomic status and mental health? Journal of Public Health, 36(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gianaros PJ, & Manuck SB (2016). A stage model of stress and disease. Perspectives on Psychological Science, 11(4), 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Cundiff JM, Boylan JM, & Muscatell KA (2020). The pathway from social status to physical health: Taking a closer look at stress as a mediator. Current Directions in Psychological Science, 29(2), 147–153. [Google Scholar]
- Cutchin MP, Martin KR, Owen SV, & Goodwin JS (2008). Concern about petrochemical health risk before and after a refinery explosion. Risk Analysis: An International Journal, 28(3), 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, & Chapman BP (2016). Socioeconomic status, psychological resources, and inflammatory markers: Results from the MIDUS study. Health Psychology, 35(11), 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo IT (2009). Social class differentials in health and mortality: Patterns and explanations in comparative perspective. Annual Review of Sociology, 35, 553–572. [Google Scholar]
- Ferrari R, Tanni SE, Caram LM, Corrêa C, Corrêa CR, & Godoy I (2013). Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respiratory Research, 14(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, & Matthews KA (2003). Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin, 129(1), 10–51. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Roesch SC, Bravin JI, Savin KL, Perreira KM, Carnethon MR, … Isasi CR (2019). Socioeconomic adversity, social resources, and allostatic load among Hispanic/Latino youth: The study of Latino Youth. Psychosomatic Medicine, 81(3), 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz JG, Almeida DM, Neupert SD, & Ettner SL (2004). Socioeconomic status and health: A micro-level analysis o exposure and vulnerability to daily stressors. Journal of Health and Social Behavior, 45(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Hu L. t., & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. [Google Scholar]
- Link BG, & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 80–94. [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley JL, Penedo F, Roesch SC, Isasi CR, Carnethon M, Sotres-Alvarez D, … Camacho A (2017). Psychosocial factors in the relationship between socioeconomic status and cardiometabolic risk: the HCHS/SOL Sociocultural Ancillary Study. Annals of Behavioral Medicine, 51(4), 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, & Kessler RC (1990). Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior, 31(2), 162–172. [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus: Statistical analysis with latent variables, user’s guide: Muthén & Muthén. [Google Scholar]
- Peek MK, Cutchin MP, Freeman D, Stowe RP, & Goodwin JS (2009). Environmental hazards and stress: Evidence from the Texas City Stress and Health Study. Journal of Epidemiology & Community Health, 63(10), 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (2004). Multiple imputation for nonresponse in surveys. New York: Wiley. [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, & Mohamed-Ali V (2002). Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain, Behavior, and Immunity, 16(6), 774–784. [DOI] [PubMed] [Google Scholar]
- Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, … Bochud M (2017). Socioeconomic status and the 25× 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1· 7 million men and women. The Lancet, 389(10075), 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, & Fortmann SP (1992). Socioeconomic status and health: How education, income, and occupation contribute to risk factors for cardiovascular disease. American Journal of Public Health, 82(6), 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]


