Abstract
In this review, we highlight Professor John Rothwell’s contribution towards understanding basal ganglia function and dysfunction, as well as the effects of subthalamic nucleus deep brain stimulation (STN DBS). The first section summarizes the rate and oscillatory models of basal ganglia dysfunction with a focus on the oscillation model. The second section summarizes the motor, gait, and cognitive mechanisms of action of STN DBS. In the final section, we summarize the effects of STN DBS on motor and cognitive tasks. The studies reviewed in this section support the conclusion that high frequency STN DBS improves the motor symptoms of Parkinson’s disease. With respect to cognition, STN DBS can be detrimental to performance especially when the task is cognitively demanding. Consolidating findings from many studies, we find that while motor network oscillatory activity is primarily correlated to the beta band, cognitive network oscillatory activity is not confined to one band but is subserved by activity in multiple frequency bands. Because of these findings, we propose a modified motor and associative/cognitive oscillatory model that can explain the consistent positive motor benefits and the negative and null cognitive effects of STN DBS. This is clinically relevant because STN DBS should enhance oscillatory activity that is related to both motor and cognitive networks in order to improve both motor and cognitive performance.
Introduction
Professor John Rothwell’s contribution towards understanding basal ganglia function and dysfunction, as well as the effects of subthalamic nucleus deep brain stimulation (STN DBS) in Parkinson’s disease (PD), is broad reaching and significant (Ashby and Rothwell 2000; Berardelli et al. 2001; Mahlknecht et al. 2017; Rothwell and Edwards 2013). His early work provided partial support for the rate model of basal ganglia dysfunction and contributed to the refinement of the rate model (Jahanshahi et al. 2000; Thompson et al. 1988). He also conducted key experiments whose findings did not support the rate model and were hinting at perhaps an alternate model (Brown et al. 1999; Thompson et al. 1988), now more commonly referred to as the pattern model or oscillatory model of basal ganglia dysfunction (Bergman et al. 1994; Hutchison et al. 2004; Nambu et al. 2015). In addition, his work has significantly contributed to our understanding of the therapeutic effects of deep brain stimulation and some of the mechanisms that underlie the therapeutic effect of deep brain stimulation (Ashby and Rothwell 2000; Jahanshahi et al. 2000; MacKinnon et al. 2005; Mahlknecht et al. 2017; Nowak et al. 2005; Prodoehl et al. 2007). Much of this review will focus on the subthalamic nucleus (STN) and the effects of STN DBS. First, two models of basal ganglia function and dysfunction will be briefly summarized: the rate and oscillation model. In this section, we will focus on the oscillation model of basal ganglia dysfunction. Second, we will summarize what is known about the mechanisms of action of STN DBS, highlight the importance of the STN, and propose ideas to address gaps in our understanding especially in the context of the effect of STN DBS on oscillations in the cognitive network. Finally, we will summarize the beneficial and detrimental effects of STN DBS on motor and cognitive tasks.
Models of basal ganglia dysfunction
Models of basal ganglia dysfunction have evolved over the past several decades. Initial models were based on our growing understanding of neuroanatomy, neurochemistry, and neurophysiology of the basal ganglia. This guided the development of much of the early models including the parallel circuit model, the direct and indirect pathway model, and the center surround models of basal ganglia function/dysfunction (Albin et al. 1989; Alexander and Crutcher 1990; Alexander et al. 1986; DeLong 1990; Mink and Thach 1993). The nature of the neuronal output of the basal ganglia, namely its rate and pattern, has been one focus of later models of basal ganglia dysfunction (Bergman et al. 1994; Gatev et al. 2006; Hutchison et al. 2004; Nelson and Kreitzer 2014; Salvade et al. 2016; Wichmann 2019). The most recent models have focused on much more than the rate and pattern of the output nuclei (e.g. external globus pallidus interactions with the striatum and the STN in rodents (Abdi et al. 2015; Glajch et al. 2016; Mastro et al. 2017), and the significance of striatal microcircuitry and synaptic plasticity (Plotkin and Goldberg 2019)). It should be noted that neuroanatomy dependent models are closely linked with the output dependent models of basal ganglia dysfunction. For instance, both the classic anatomy dependent model of the direct and indirect pathways and the output dependent rate model would predict an increased firing rate in the basal ganglia output nuclei in persons with PD (Hutchison et al. 1994). Table 1 lists the popular models of basal ganglia dysfunction. Even though significant progress has been made in understanding basal ganglia dysfunction in the past several decades, none of the models listed in Table 1 fully explain basal ganglia dysfunction and not all predictions from these models are supported by empirical findings. This review will focus on the rate and pattern or oscillation models, with a particular focus on the oscillation model. Because the focus of this review and a central point of discussion in current models of basal ganglia dysfunction are abnormal oscillations, we will refer to the pattern model as the oscillation model henceforth in this review. For recent detailed reviews and updates on the anatomical models of basal ganglia dysfunction, the readers are directed elsewhere (Calabresi et al. 2014; McGregor and Nelson 2019; Nelson and Kreitzer 2014; Wichmann 2019).
Table 1.
Models of Basal Ganglia Dysfunction
| 1. Parallel circuit model (Alexander et al. 1986) |
| 2. Classic direct and indirect pathway model (Albin et al. 1989; Alexander and Crutcher 1990; DeLong 1990) |
| 3. Rate model (Albin et al. 1989; DeLong 1990; Nelson and Kreitzer 2014) |
| 4. Center surround or action selection model (Mink 1996; Mink and Thach 1993) |
| 5. Oscillation/pattern model (Bergman et al. 1994; Gatev et al. 2006; Hutchison et al. 2004; Salvade et al. 2016; Wichmann 2019) |
Rate model
The rate model is closely tied with the idea of the direct and indirect pathways of the basal ganglia (Albin et al. 1989; DeLong 1990; Nelson and Kreitzer 2014). Simply put, given that the output of the basal ganglia is inhibitory, activating the direct pathway is rate decreasing resulting in movement initiation, while activating the indirect pathway is rate increasing resulting in movement inhibition (Albin et al. 1989; DeLong 1990). Basal ganglia output affects cortical activity via the ventrolateral thalamus (Albin et al. 1989; Alexander and Crutcher 1990). The excitatory output of the thalamus, which affects cortical output and movement initiation, is disinhibited by the decrease in the mean firing rate of the internal globus pallidus or inhibited by the increase in the mean firing rate of the internal globus pallidus (Albin et al. 1989). In PD, degeneration of the substantia nigra pars compacta leads to the over activity of the indirect pathway and under activity of the direct pathway (Albin et al. 1989). This leads to an increased firing rate that inhibits thalamocortical neurons. Consequently, impairments in initiating movement and slowness of movement ensue. The main driving force for the increased discharge of the internal globus pallidus and the substantia nigra pars reticulata is posited to be an hyperactive STN (Limousin et al. 1997).
An additional factor which could contribute to the increased basal ganglia discharge rate is neuro-plastic changes that accompany dopaminergic degeneration (Wichmann 2019). The neuro-plastic changes include 1) a partial loss of the hyper direct cortico-subthalamic projections in parkinsonian monkeys (Mathai et al. 2015), as well as reduced cortico-subthalamic transmission in parkinsonian mice (Chu et al. 2017), and 2) an increase in the number of synaptic connections between the external globus pallidus and STN per axon terminal which results in the strengthening of the external globus pallidus – STN pathway in parkinsonian rats and mice (Fan et al. 2012). While the neuro-plastic changes are triggered by dopaminergic loss, the processes that regulate the loss of cortico-subthalamic transmission or improve the strength of the external globus pallidus-STN transmission and affect basal ganglia output are unknown. One candidate mechanism is the activation of STN N-Methyl-d-aspartate receptors, which is likely to affect the neuro-plastic changes at the synapses between the external globus pallidus and the STN (Chu et al. 2017; Chu et al. 2015; Fan et al. 2012). Neuro-plastic changes following dopaminergic degeneration is an evolving area of research and is likely to significantly contribute to updating models of basal ganglia dysfunction.
One of the limitations of the rate model is that ablative surgery and neurostimulation have similar therapeutic effects (Hutchison et al. 2004). Furthermore, ablative surgery and neurostimulation have the same effects in hypokinetic (Parkinson’s disease) and hyperkinetic (Huntington’s disease) movement disorders (Nelson and Kreitzer 2014). Another limitation is the presence of hypo- and hyperkinetic symptoms (i.e., bradykinesia and chorea respectively) in a hyperkinetic disorder such as Huntington’s disease (Thompson et al. 1988). Similarly, bradykinesia, a hypokinetic symptom, and dystonia, a hyperkinetic symptom, can be found together in PD (Nelson and Kreitzer 2014). Finally, although abnormality in firing rates are often found in the output nuclei of the basal ganglia, these changes can be considered small in magnitude (Nelson and Kreitzer 2014). The firing rate in the internal globus pallidus neurons increases by 10–22% in the Parkinsonian state (Hutchison et al. 2004). The changes observed in the basal ganglia output firing rate, while significant, are argued to be insufficient to explain the motor dysfunction observed in PD (McGregor and Nelson 2019; Nelson and Kreitzer 2014; Wichmann 2019).
Oscillation model
The oscillation model of basal ganglia dysfunction puts suggests that abnormalities in firing rates are insufficient at explaining the motor dysfunction observed in PD. Instead, the oscillation model postulates that abnormalities in neuronal oscillations may be linked with the motor dysfunction observed in PD (Brown 2003; Eusebio and Brown 2007; Hutchison et al. 2004). In animal models of PD and in persons with PD, abnormalities in oscillations are observed both within and between neuronal populations (Bevan et al. 2002; McGregor and Nelson 2019). The source of these pathological oscillations in PD is unknown. Hypothesized sources include the striatum, the subthalamic nucleus, and the cortex (Bevan et al. 2002).
The oscillation model is related to the idea of PD affecting multiple neuronal networks (Wichmann 2019). It is thought that information transfer across different areas in the motor network occurs through oscillations. Neuronal populations oscillating at the same frequency are thought to share similar information content (Akam and Kullmann 2010). As a result, areas that share similar information content tend to be coherent in either phase or amplitude or both (Fries 2005; Siegel et al. 2012). Beta band oscillations and synchronizations within the motor network are well documented in PD (Hammond et al. 2007). Beta band oscillations are increased in PD and reduced with dopaminergic medication indicating the dependence of beta band synchronization on the integrity of dopaminergic inputs to the striatum (Brown et al. 2001; Hammond et al. 2007). In PD, beta band synchronization in the motor network is considered anti-kinetic and linked with movement suppression (Brown 2003). On the other hand, gamma synchronization is considered pro-kinetic and is linked with movement initiation (Brown 2003).
The oscillation model can serve as a mechanism for action selection (Brittain et al. 2014). Beta activity underlies action selection by promoting the current state over a novel action (Brittain and Brown 2014). Within the basal ganglia-thalamocortical motor network, beta band activity is hypothesized to maintain a tonic state that corresponds to maintaining posture at rest prior to initiating movement (Jenkinson and Brown 2011). This tonic state, which could be considered a ‘beta threshold’ created by synchronization in the beta band, has to be overcome by phasic activity when movement needs to be initiated. A similar view was postulated by Courtemanche and colleagues (2003) and their findings suggest that beta band activity in the basal ganglia is non-pathological. Synchronization in the beta band across the basal ganglia-thalamocortical network is hypothesized to act as a spatiotemporal filter in healthy awake behaving monkeys. In order for the cortical input to the striatum to propagate to downstream structures of the pallidum and thalamus, the cortical input should exceed this beta synchronization at focused striatal areas that relate to the movement that needs to be initiated. Focused/modular cortical input can cause movement related neurons in the striatum to phasically burst and desynchronize from the population level beta band enhancement (Courtemanche et al. 2003; Hutchison et al. 2004). This results in information being propagated to downstream basal ganglia and cortical structures. In this way, action that is needed is selected by beta band desynchronization while unwanted actions remain suppressed by beta band synchronization. In PD, degenerative loss of dopaminergic neurons results in the alteration of the sensorimotor mapping of the surviving neurons (McGregor and Nelson 2019). This adversely affects the focused/modular input-output organization of the basal ganglia network. While segregation remains, receptive fields are less specific and more diffuse leading to a reduction in functional segregation in the basal ganglia networks (McGregor and Nelson 2019). Consequently, more neurons may be entrained in the beta band and overcoming the widespread beta band enhancement to initiate movement becomes more difficult (Hutchison et al. 2004).
Most of the research examining STN beta band synchronization, its relationship to motor symptoms of PD, and its alteration by dopaminergic medication has been conducted at rest and is quite well understood as it applies to the resting state (Alonso-Frech et al. 2006; Kuhn et al. 2006; Kühn et al. 2005; Kuhn et al. 2009; Marceglia et al. 2006; Priori et al. 2004; Weinberger et al. 2006). This research concludes that, in persons with PD, beta band 1) synchronization is exaggerated at rest, 2) synchronization at rest is associated with motor impairment while off medication, 3) synchronization at rest is suppressed by dopaminergic medication, and 4) suppression at rest following dopaminergic medication is associated with motor improvement (Brittain et al. 2014; Brown and Williams 2005; Hammond et al. 2007).
On the other hand, beta band activity during movement is not as well understood. This is important because bradykinesia, the major deficit in PD, occurs during movement (Hammond et al. 2007). Research examining STN beta band activity prior to and during movement in persons with PD has found that beta band activity is suppressed prior to movement, this suppression continues during movement, and is reversed, i.e., beta band enhancement, later in movement (Kühn et al. 2004). In addition, when movement requires to be unexpectedly inhibited, beta band enhancement is observed earlier relative to trials that did not require movement to be inhibited (Kühn et al. 2004). This finding supports the idea that beta band suppression facilitates movement while beta band enhancement inhibits movement. Kühn et al. (2004) also showed that persons with PD were able to modulate beta band activity, i.e. beta band was suppressed prior to movement onset and was enhanced when movement was instructed to be inhibited. So the question remains, if persons with PD can modulate beta band activity, why is bradykinesia observed in PD? One theory is that, while persons with PD are able to modulate beta band activity, the amount of modulation is insufficient and therefore bradykinesia is observed. In support of this idea, one study has shown that relative to healthy controls, persons with PD exhibit reduced beta desynchronization prior to movement onset and during movement in the contralateral sensorimotor cortices (Heinrichs-Graham et al. 2014). However, two studies have shown the opposite effect, i.e., increased beta desynchronization during movement in persons with PD relative to healthy controls (Chung et al. 2018; Stegemöller et al. 2016). Chung et al. (2018) state that increased beta desynchronization during movement can imply that the cortex is not functioning normally and that increased beta-band desynchronization can in fact result in bradykinesia. The fact that increasing beta band activity was positively correlated to movement velocity, accompanied with the fact that administration of levodopa decreased beta-band desynchronization was further evidence that increased desynchronization is associated with bradykinesia (Chung et al. 2018). To summarize these findings, even though people with PD modulate beta oscillations when they move, the amount of suppression is possibly not optimal, so their movements remain impaired. One way in which STN DBS could improve bradykinesia is by optimally modulating the beta activity during movement. Further research is required to determine the extent to which beta band modulation during movement is impaired in persons with PD. Additionally, more research is required to determine the relationship between beta activity and bradykinesia with respect to the effects of dopaminergic medication and STN DBS.
In addition to synchrony in the beta band, more recently the duration of beta bursting has acquired considerable attention in PD. This is because short duration beta bursts are considered to be non-pathological (Feingold et al. 2015). In healthy monkeys, short duration beta bursts are observed following movement and are thought to realign circuit activity so as to modify or maintain the strengths of the connections involved in task performance according to contextual demands (Feingold et al. 2015). In persons with PD, prolongation of beta bursts are predicted to impair such flexibility in the neural control of behavior (Feingold et al. 2015) and this could manifest in the form of motor symptoms. In line with this prediction, in PD, beta activity is not constantly increased, but varies as a function of both duration and amplitude (Tinkhauser et al. 2017). Furthermore, empirical evidence indicates that short duration beta bursts in the STN are associated with motor improvement while longer duration bursts were related to motor impairment (Tinkhauser et al. 2017). Another reason for beta band duration receiving considerable attention is because of its importance in adaptive STN DBS. Adaptive STN DBS is a novel method of stimulation where STN DBS is turned on or off based on a predetermined beta band amplitude threshold. STN DBS is turned on when beta band activity exceeds this threshold and turned off when beta band activity falls below this threshold (Little et al. 2013). Preliminary studies show that adaptive STN DBS is better than traditional STN DBS (Little et al. 2013) and the mechanism of action differs between the two forms of STN DBS. Adaptive STN DBS reduces beta burst duration while traditional STN DBS attenuates amplitude (Tinkhauser et al. 2017). Crucially, duration of beta bursting is correlated with motor symptoms of bradykinesia, rigidity, and tremor (Tinkhauser et al. 2017). Thus beta burst duration appears to be of greater importance than beta burst amplitude in the pathophysiology of PD (Starr and Ostrem 2013). These ideas, in the context of basal ganglia dysfunction, are emerging and are likely to significantly improve our understanding of models of basal ganglia dysfunction in the years to come.
One potential limitation of the oscillatory model is that bradykinesia and akinesia appear prior to the onset of oscillatory abnormalities at the level of individual neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treated monkeys using a progressive dopamine depleting process (Leblois et al. 2007). This suggests that oscillatory abnormalities at the level of individual neurons might not cause the early symptoms of bradykinesia or akinesia, however this does not rule out the possibility that oscillatory abnormalities might still drive later symptoms such as rigidity, postural disturbance, and freezing of gait (Leblois et al. 2007). In addition, oscillatory abnormalities at the level of the individual neuron may appear to be quite different from population level oscillatory abnormalities (Leblois et al. 2007). Further evidence that questions the causal effect of abnormal oscillations on akinesia/bradykinesia comes from an experiment conducted in a rodent model of PD. Mallet et al. (2008) studied the acute and chronic behavioral response and occurrence of beta band oscillations in the STN and frontal cortex in 6-hydroxydopamine lesioned rodents. They found that the acute response to systemic dopamine receptor antagonists included akinesia/bradykinesia but did not include abnormal beta oscillations (Mallet et al. 2008). Abnormal beta oscillations appeared only as a chronic response to dopaminergic disruption (Mallet et al. 2008). This raises the possibility that the process by which beta oscillations are increased is a slow, long-term adaptive or compensatory process. It is likely that dopaminergic degeneration results in some yet to be discovered causal process that drives both bradykinesia and beta oscillations.
Inconsistencies between model predictions and empirical observations
Professor John Rothwell has often pointed out the inconsistencies between predictions from basal ganglia models of dysfunction and observed experimental data. Models of basal ganglia dysfunction predict decreased cortical excitability in hypokinetic disorders such Professor John Rothwell has often pointed out the inconsistencies between predictions from basal ganglia models of dysfunction and observed experimental data. Models of basal as PD. However, in persons with PD, some transcranial magnetic stimulation measures of corticospinal and intra cortical excitability are greater than in healthy controls, which is inconsistent with this prediction (Bologna et al. 2018). Another inconsistency is the improvement of dystonia following internal globus pallidus DBS (Vidailhet et al. 2007). Dystonia, which is considered as a hyperkinetic movement disorder associated with reduced basal ganglia output, is improved by internal globus pallidus DBS (Vidailhet et al. 2007). The rate model predicts that reduction of basal ganglia output should make dystonia worse, but this is not the case (Jahanshahi and Rothwell 2017). Finally, yet another inconsistency is the improvement of drug-induced dyskinesias following a pallidotomy (Rothwell 2011). Dyskinesias, or excess movements, are linked with reduced basal ganglia output, yet a pallidotomy that reduces basal ganglia output results in improving dyskinesias instead of making them worse (Rothwell 2011). These critical insights on inconsistencies between model based predictions and actual observed data has led several groups to reassess models of basal ganglia dysfunction.
STN DBS
The subthalamic nucleus is a critical structure in the oscillatory model of basal ganglia function and dysfunction. This is because, first, it is uniquely positioned to drive basal ganglia output via the globus pallidus internus and the substantia nigra pars reticulata exerting its influence on cortical and brain stem nuclei (Wichmann and DeLong 1999). Second, it is a zone of cortical input via the hyperdirect pathway receiving projections from the primary motor cortex, supplementary motor area, premotor cortices and prefrontal cortices (Nambu et al. 2002). Third, by means of its reciprocal connections to the globus pallidus externus, the STN forms a localized feedback system that can support rhythmic firing even in the absence of cortical and striatal input (Plenz and Kital 1999). Consequently, the STN may function as the intrinsic pacemaker of the basal ganglia (Bevan et al. 2002; Plenz and Kital 1999) and could drive oscillatory activity in the basal ganglia-thalamocortical networks. Fourth, given that the STN is anatomically and functionally segregated into motor, associative, and limbic areas (Hamani 2004), it has the capability to modulate motor, oculomotor, cognitive, and limbic function. Finally, the STN has been shown to have the ability to integrate activity from the motor and cognitive networks (Delaville et al. 2015).
The STN has emerged as the most targeted location for effective deep brain stimulation in advanced PD. Following the initial United States Food and Drug Administration (FDA) approval of STN DBS in 2002 for advanced PD, STN DBS was approved by the FDA for PD with early motor complications in 2016. This approval followed the findings of a 2-year clinical trial that reported significant improvements in the quality of life of persons with PD with early motor complications (Schuepbach et al. 2013). Because of this, the use of STN DBS in persons with PD is likely to increase, yet our understanding of the mechanisms of action of STN DBS is incomplete. Understanding the mechanism of action of STN DBS is critical to maintain motor benefits, reduce detrimental effects, and extend DBS to other conditions. The next sections will discuss what we know about the motor and cognitive mechanisms of action of STN DBS and the effect of STN DBS on motor and cognitive behavior.
Motor, gait, and cognitive mechanisms
Given that the STN is divided into motor, associative, and limbic sections (Hamani 2004), it follows that STN DBS is likely to affect motor, cognitive, and limbic function. The limbic mechanisms are beyond the scope of this paper (for a review see, Marceglia et al. 2011; Temel et al. 2005). The primary mechanism by which STN DBS is thought to bring about the observed clinical effect is by modulating oscillatory activity in the basal ganglia-thalamocortical motor network (Kuhn et al. 2008). This is most likely due to STN DBS causing axonal excitation (Holsheimer et al. 2000). In addition, activation of dendrites and the cell body of the neuron may also contribute to the modulation of oscillatory activity (Montgomery 2017). We will first list the mechanisms that are thought to underlie altered motor function. Second, we will list the mechanisms that underlie altered gait function. And third, we will list the mechanisms that underlie altered cognitive function.
Motor mechanisms
High frequency STN DBS has been shown to modify oscillatory activity in the basal ganglia-thalamocortical motor network. More specifically high frequency STN DBS facilitates beta power desynchronization and gamma power synchronization in the basal ganglia-thalamocortical motor network and this is associated with the observed clinical benefit (Cao et al. 2017; Eusebio et al. 2012; Müller and Robinson 2018). The excessive beta power synchronization in the premotor and motor cortices is significantly reduced by bilateral STN DBS relative to off stimulation in participants with PD (Gulberti et al. 2015; Kang and Lowery 2014; Whitmer et al. 2012). The strongest beta power desynchronization occurred over the area of cortex identified as the origin of the hyperdirect pathway fibers projecting to the STN (Whitmer et al. 2012). In addition to modifying activity in beta and gamma bands, STN DBS has been shown to modify activity in the mu or alpha band. During movement tasks, STN DBS reduced power in the mu or alpha band over frontal areas and this was correlated with a decrease in Unified Parkinson’s Disease Rating Scale motor score and faster movement initiation (Devos et al. 2004; Spay et al. 2018).
STN DBS may also affect more complex oscillation patterns than solely changing power in a given frequency band. STN stimulation reduced the magnitude of motor cortex beta-high gamma phase-amplitude coupling at rest, which was excessive in participants with PD who were off stimulation compared to participants with dystonia and epilepsy (de Hemptinne et al. 2015). Increased phase-amplitude coupling is thought to impair information flow in the cortex resulting in motor dysfunction (de Hemptinne et al. 2013). The reduction of beta-high gamma phase-amplitude coupling at rest with STN DBS is highly correlated with the beta waveform sharpness ratio in motor cortex (Cole et al. 2017). The sharpness ratio evaluates the asymmetry of the waveform shape by comparing the sharpness between the beta peaks and troughs. In PD with no stimulation, motor cortex beta oscillations have a high sharpness ratio, meaning more asymmetry, which is reduced with STN DBS making the waveform more symmetric or sinusoidal. A decrease in the sharpness ratio is thought to represent a decrease in the synchronous synaptic input to motor cortex, which is hypothesized to enhance neural communication (Cole et al. 2017; Sherman et al. 2016). Additionally, the beta sharpness ratio was correlated with the clinical rigidity score, with a decrease in sharpness ratio relating to an improvement in rigidity score (Cole et al. 2017). Before and during an upper limb movement task, beta-high gamma phase-amplitude coupling was reduced with no stimulation compared to at rest, however, phase-amplitude coupling was still excessive compared to participants with dystonia and epilepsy (de Hemptinne et al. 2013). During STN stimulation, beta-high gamma phase-amplitude coupling reduced even further during movement, suggesting a facilitation of movement with STN DBS compared to off stimulation through a reduction in phase-amplitude coupling (de Hemptinne et al. 2015). High frequency STN DBS modulates oscillations within the motor network in several ways during rest and movement. These modulations can be linked to improvements in motor symptoms and performance.
The motor response to STN DBS depends on the frequency of stimulation (McConnell et al. 2012). High frequency stimulation (~>130 Hz) provides the greatest benefit with respect to tremor, akinesia, bradykinesia, and rigidity (Benabid et al. 2009). It is thought that stimulating at high frequencies suppresses pathological oscillations in the beta band and partially entrains neuronal discharge at stimulation frequencies in downstream and upstream targets (Brown et al. 2004; Hashimoto et al. 2003; Kang and Lowery 2014; Swann et al. 2016). Brown and colleagues propose that high frequency STN DBS, i.e. >70 Hz, is pro-kinetic while stimulation frequencies <30 Hz are anti-kinetic (Brown 2003; Hutchison et al. 2004). Therefore, stimulating in pro-kinetic frequencies will tend to facilitate movement while stimulating in anti-kinetic frequencies will tend to impair movement. Several authors have shown that stimulating in frequencies of >130 Hz abolishes abnormal oscillations in the beta band in the basal ganglia-thalamocortical motor network (Kuhn et al. 2008; Whitmer et al. 2012). On the other hand, stimulating at low frequencies has no beneficial effect and at some frequencies can exacerbate motor symptoms of PD. STN DBS at beta band frequencies (20 Hz) has been shown to slow movement when compared to stimulating at higher frequencies (50 Hz) (Chen et al. 2007), as well as when compared to no stimulation or lower than 20 Hz (Eusebio et al. 2008) stimulation in participants with PD. This frequency dependent mechanism of modulating oscillatory activity is thought to mediate the clinical motor benefit that accompanies STN DBS.
In summary, the oscillatory mechanisms that underlie the motor benefit include, modifying power, modifying phase-amplitude coupling, and are dependent on the frequency of STN DBS.
Gait mechanisms
Parkinsonian gait is a hypokinetic gait disorder characterized by reduced amplitude of movements, reduced velocity, and increased stride-to-stride variability (Mirelman et al. 2019; Pötter-Nerger and Volkmann 2013). With the progression of the disease gait symptoms worsen with greater incidences of freezing of gait and falls (Mirelman et al. 2019). If beta band oscillatory activity is involved in the pathophysiology of gait symptoms observed in PD, then it follows that 1) beta band activity will be enhanced at rest and is likely to be sub-optimally suppressed during gait relative to rest, 2) beta band synchrony between neural regions will be increased at rest and possibly insufficiently reduced during gait relative to rest, 3) persons with PD who are classified as freezers are likely to manifest with greater beta band activity than those who are non-freezers, and 4) persons with PD who are classified as freezers are likely to manifest with greater beta band activity during freezing episodes of gait compared to non-freezing episodes of gait. As predicted by basal ganglia models of dysfunction, one study has shown attenuation of STN beta band activity during gait compared to rest in persons with PD (Hell et al. 2018). In addition, they also found that correlation of beta band activity between the left and right STN was reduced during gait relative to rest suggesting reduced beta band synchrony between neural regions during gait (Hell et al. 2018). In another study conducted while at rest, in persons with PD who are classified as freezers, and whose freezing responds to dopaminergic medication, STN beta band power in the high beta frequency band was greater than that of non-freezers (Toledo et al. 2014). In one more study conducted while walking on a treadmill, persons with PD who are freezers presented with greater STN beta band power in the low beta frequency compared to non-freezers (Singh et al. 2013). In contrast to the findings reported in the above studies, a more recent study has found that during non-freezing gait in freezers relative to non-freezers, beta band activity was reduced (Syrkin-Nikolau et al. 2017). Three out the four studies reviewed here extend the anti-kinetic effect of oscillations in the beta frequency to gait symptoms in PD and support predictions from the oscillatory model of basal ganglia dysfunction.
The effect of STN DBS, for the most part, is similar to the effect of levodopa on hypokinetic gait deficits. Most of the levodopa responsive gait symptoms are STN DBS responsive and those that are levodopa resistant are STN DBS resistant as well. Similar to levodopa, STN DBS improves spatial characteristics and has no effect on temporal characteristics of gait in persons with PD (Cossu and Pau 2017; Pötter-Nerger and Volkmann 2013). Improvement in spatial characteristics are likely to be driven by improved amplitude scaling, possibly related to beta band attenuation, as a result of STN DBS. This improvement in amplitude scaling, i.e., increasing the amplitude of movements, is observed both during gait initiation (Follett et al. 2010), as well as during gait (Cossu and Pau 2017; Pötter-Nerger and Volkmann 2013). The improvement in amplitude scaling is observed in the range of motion of arm swing, trunk rotation, trunk lateral flexion, pelvic rotation, and hip, knee, and ankle range of motion (Cossu and Pau 2017; Pötter-Nerger and Volkmann 2013). It is likely that the increase in amplitude scaling drives the increase in gait velocity, as there is a documented relationship between lower-limb range of motion and gait velocity (Oberg et al. 1994).
STN DBS treatment of levodopa resistant gait symptoms is complex. One review (Pötter-Nerger and Volkmann 2013) and one meta-analysis (Cossu and Pau 2017) conclude that temporal characteristics of gait that are typically levodopa resistant are also resistant to STN DBS. These symptoms include stance time, swing time, and cadence (Cossu and Pau 2017; Pötter-Nerger and Volkmann 2013). There is evidence that points to the lack of an effect of STN DBS on gait parameters such as cadence (Faist 2001), stride-to-stride variability, and freezing of gait (Hausdorff et al. 2009). But, there is also evidence that points to the beneficial effect of STN DBS on these gait parameters (Fasano et al. 2011). Poor control of cadence and stride-to-stride variability are seen in persons who manifest freezing of gait (Hausdorff et al. 2003). One hypothesis is that because PD is an asymmetric disease, the lateralized nature of the disease causes deficits in inter-limb coordination that leads to poor control of cadence and stride-to-stride variability during gait (Fasano et al. 2011). Therefore, in theory, if STN DBS parameters can be programmed to reduce asymmetry, then inter-limb coordination could improve, resulting in improved control of cadence and stride-to-stride variability, which in turn would result in improving freezing of gait. This hypothesis was tested by Fasano and colleagues (2011), and they found that reducing stimulation intensity on the side contralateral to the limb with the larger stride length resulted in reduced stride-to-stride variability, improved inter-limb coordination, and reduced duration and frequency of freezing of gait episodes. In addition to STN DBS intensity, location of the active contact and stimulation frequency affect gait symptoms. Careful targeting of the STN DBS lead especially the dorsal aspect of the STN are correlated with the best gait outcomes (Johnsen et al. 2010), but a later study contradicts this finding (Hilliard et al. 2011). With respect to stimulation frequency, some studies show that low frequency stimulation at 60Hz improves gait symptoms, specifically it reduced frequency of freezing episodes while maintaining motor benefit relative to 130Hz stimulation frequency (Moreau et al. 2008; Xie et al. 2017; Xie et al. 2015). However, other studies show no difference between low frequency stimulation and high frequency stimulation with respect to improving gait symptoms (Phibbs et al. 2014; Sidiropoulos et al. 2013; Vallabhajosula et al. 2015).
While there are similarities in the effects of levodopa and STN DBS, there are differences as well. In persons with STN DBS, the effect of levodopa appears to be greater than the effect of STN DBS (Ferrarin et al. 2005; Ferrarin et al. 2004; Krystkowiak et al. 2003). Some studies show that the combined effect of levodopa and STN DBS results in the greatest improvement of gait symptoms (Ferrarin et al. 2005; Ferrarin et al. 2004; Krystkowiak et al. 2003), while one study has shown that STN DBS provides no additive benefits (McNeely and Earhart 2013). This suggests while there are overlapping mechanisms of action of levodopa and STN DBS, there are also non-overlapping mechanisms of action unique to each levodopa and STN DBS.
In summary, the oscillatory mechanisms that underlie the gait benefit are largely driven by beta band attenuation, which in turn drives amplitude scaling of 1) arm swing of upper limbs, 2) trunk rotation, and 3) hip, knee, and ankle range of motion. Location of the active contact and frequency of stimulation may be additional factors that can influence gait symptoms.
Cognitive mechanisms
Few studies have examined the effect of STN DBS on oscillatory activity in the basal ganglia-thalamocortical cognitive network. Cavanagh et al. (2011) found that performance on high-conflict trials of a decision-making task was impaired with STN DBS due to faster response times, which reflect impulsivity. In PD with no stimulation and healthy controls, cue-related medial prefrontal cortex theta band power predicted slower response times but with STN stimulation this relationship was inverted (Cavanagh et al. 2011). With STN DBS, high medial prefrontal cortex theta band power corresponded with faster response times, suggesting that this could be a mechanism for impulsivity that occurs with STN DBS (Cavanagh et al. 2011). In addition, Hatz and colleagues (2018) found that for individuals whose verbal fluency performance worsened with STN DBS, there was an increase in delta power over the left temporal lobe.
While enhanced delta and theta power with STN DBS has been related to worsened cognitive performance, Kelley and colleagues (2018) found that high frequency STN DBS had no effect on interval timing performance compared to off stimulation. However, when they stimulated STN at 4 Hz, interval timing performance was improved while midfrontal delta power was enhanced. This suggests that the STN and cortex communicate in the delta and theta frequency ranges to affect aspects of cognitive control. High frequency stimulation may disrupt this communication leading to worsened cognitive performance while stimulation at delta frequency may improve cognitive performance.
Interestingly, Swann and colleagues reported that high frequency STN DBS improved performance on the stop signal task, a measure of inhibitory control, by improving stopping speed (Swann et al. 2011). This improvement corresponded with enhanced beta power around the time of stopping in the right frontal cortex with STN DBS compared to no stimulation (Swann et al. 2011). The stop signal task relies on the cognitive control of motor response inhibition and is generally interpreted as a cognitive task (Logan et al. 1984; Verbruggen and Logan 2009). If this task was viewed as a motor task, then the enhancement of power in the beta band could be easily predicted. However, as a cognitive task, this result seems contradictory to the previously described effects of STN DBS on cognitive performance. However, one could postulate that for cognitive tasks that rely heavily on motor responses, the benefits of STN DBS on movement may result in improvement of aspects of cognitive performance, such as reaction time.
STN DBS has task-dependent effects on cortical oscillatory activity, resulting in power changes in delta, theta, and beta bands. DBS-induced cortical power changes during cognitive tasks were mostly related to detrimental or no effects on performance. Further research on the effects of STN DBS on cortical activity related to cognitive tasks, motor tasks, and tasks that require a motor and cognitive component is needed to corroborate and expand on current findings.
An oscillation model for movement and cognition
A hallmark of basal ganglia neuronal organization is that different nuclei of the basal ganglia are made up of repeating neuronal architectures, are reciprocally connected, and innervate functionally related regions in the basal ganglia output nuclei (Bevan et al. 2002). The STN, given its unique position within the basal ganglia as an input and output zone, anatomically segregated in to motor, cognitive, and limbic portions, can serve to integrate information from different networks, such as the motor and cognitive networks.
Studies suggest that the STN has the ability to integrate activity from motor and cognitive networks (Brunenberg et al. 2012; Delaville et al. 2015; Kolomiets et al. 2001). One line of evidence for the STN integrating input from motor and cognitive networks comes from a study conducted in anesthetized healthy rats (Kolomiets et al. 2001). Kolomiets and colleagues (2001) found that, in addition to the convergence of inputs from the motor and prefrontal cortices in the STN, the same neurons in the STN responded to inputs from both the motor and prefrontal cortices (Kolomiets et al. 2001). This allows for interactions between input from the motor and cognitive networks (Kolomiets et al. 2001). Another line of evidence comes from structural and resting state functional connectivity using MRI in healthy humans (Brunenberg et al. 2012). Brunenberg and colleagues (2012) showed that there was a mediolateral gradient with respect to structural and functional connectivity in the STN. Medial parts of the STN were more connected to non-motor regions of the cortex while lateral parts were more connected to motor regions of the cortex. However, they did not find a complete segregation of motor and non-motor regions within the STN. Therefore, they state that motor and non-motor circuits are partially integrated within the STN (Brunenberg et al. 2012). Yet another line of evidence comes from recording local field potentials from rats lesioned with 6-hydroxydopamine performing a task that involves motor and cognitive processes (Delaville et al. 2015). They showed that during the task coherence between the STN and motor cortex was in the high beta band while coherence between the STN and medial frontal cortex (cognitive) was in the low gamma band. They also found no coherence between the motor cortex and the medial prefrontal cortex. Taken together these findings suggested that the motor and medial prefrontal areas were participating in functionally distinct networks, each with a link to the STN and support an integrative role for the STN between cognitive and motor networks.
The integrative role played by the STN in movement and cognition is important because stimulating the STN via high frequency deep brain stimulation is more than likely to influence both motor and cognitive networks. Whether this is beneficial or detrimental to cognitive function depends on the oscillatory frequency that serves a particular cognitive function. In figure 1A, we provide an oscillatory model that is a modification of Brown and colleagues’ pro-kinetic and anti-kinetic oscillation model (Brown 2003; Hutchison et al. 2004). The model illustrates that oscillatory activity <10 Hz, 10–30 Hz, and >70Hz subserve normal function. Oscillatory activity is context specific and optimized depending on the motor and cognitive requirements of the task. In figure 1B, we provide an oscillatory model that is a modification of Brown and colleagues’ pro-kinetic and anti-kinetic oscillation model (Brown 2003; Hutchison et al. 2004), and extend the idea to the associative/cognitive domain. We have added pro-associative (thick blue dashed arrow) and anti-associative neural oscillations to their model (thin red dashed arrow). In figure 1C, we provide a motor and associative oscillatory model that shows the hypothesized effects of high frequency STN DBS. Similar to the idea of a pro-kinetic (thick blue arrow) and anti-kinetic (thin red arrow) effect of high frequency of STN DBS in the movement domain, it is likely that there are pro-associative (thin dashed blue arrow) and anti-associative (thick red dashed arrow) effects of high frequency STN DBS in the cognitive domain. When the frequency of STN DBS aligns with pro-kinetic and pro-cognitive neural oscillations, either motor or cognitive or both behaviors are enhanced. On the other hand, when the frequency of STN DBS only aligns with pro-kinetic oscillations and not with pro-cognitive oscillations, motor behaviors are enhanced and cognitive behaviors are likely to be impaired. This modified motor and associative/cognitive oscillatory model explains the consistent positive motor benefits found with STN DBS that are outlined in the next section. It may also explain the negative and null cognitive effects that we outlined in the previous section in terms of brain mechanisms and expand upon in the next section in terms of behavior.
Fig 1.
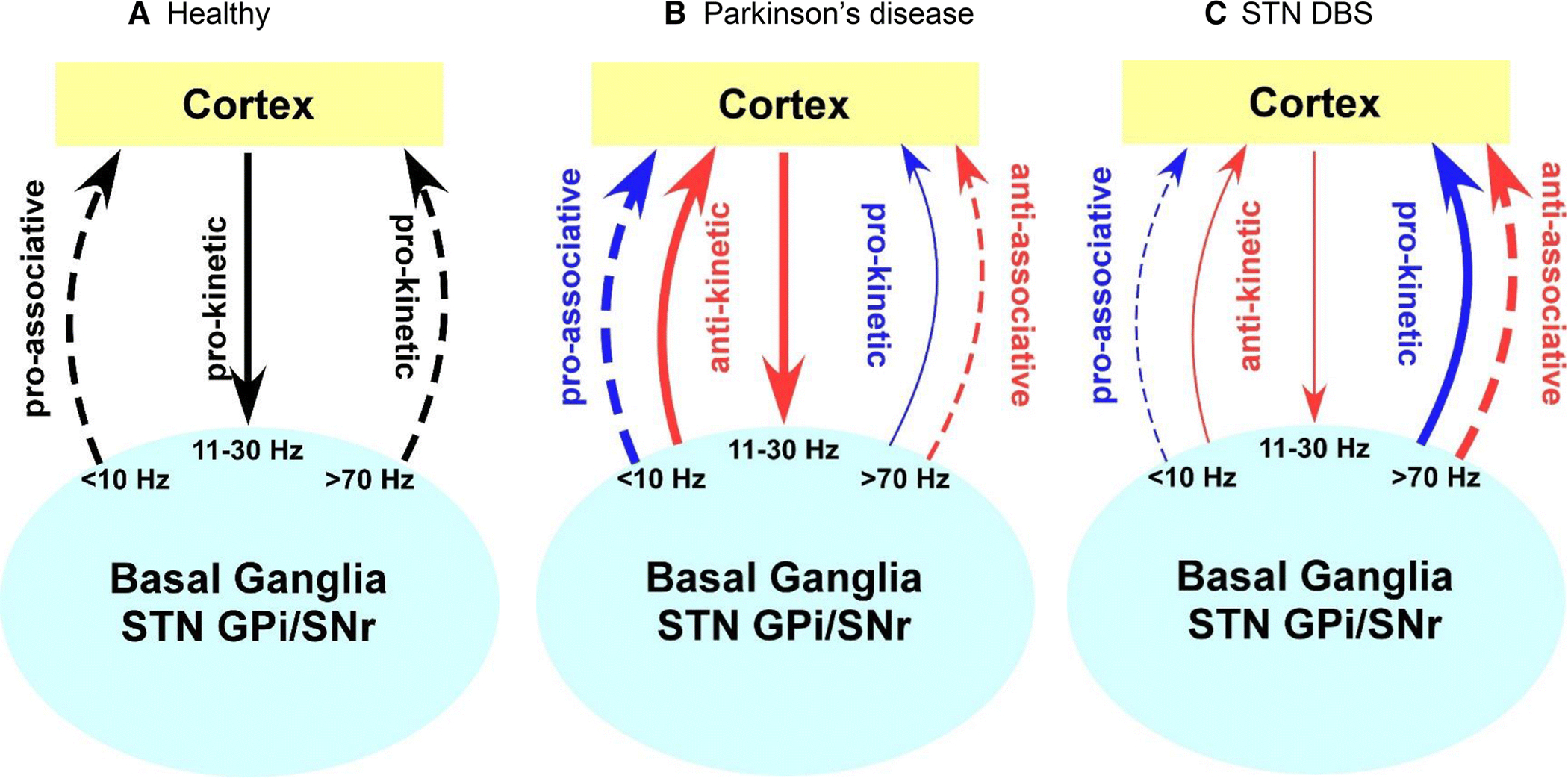
A. Healthy motor and associative oscillatory model. Oscillatory activity subserves normal motor and cognitive function. B. The Parkinson’s disease motor and associative oscillatory model. Dopaminergic degeneration results in low frequency oscillations being enhanced and high frequency oscillations being suppressed. C. STN DBS motor and associative oscillatory model. High frequency STN DBS may result in opposite effects in terms of enhancing or impairing motor and cognitive behavior in the motor and associative networks respectively. STN, subthalamic nucleus; GPi, internal globus pallidus; SNr, substantia nigra pars reticulata. Adapted from Hutchison et al. (2004).
Motor and cognitive tasks
In this section, we review the effects of STN DBS on both motor and cognitive tasks. We also address the extent to which tasks have both motor and cognitive components since many of the experimental tasks employed have both motor and cognitive components. Examples of such tasks include dual tasks in which a motor task is performed at the same time as concurrent memory tasks (Alberts et al. 2008) and the stop signal task (Logan et al. 1984; Verbruggen and Logan 2009).
It is important to note that one source of variability in both motor and cognitive results could be due to variability in STN DBS lead placement and active contacts used. This variability has been linked to declines in cognition (Welter et al. 2014; York et al. 2009). In fact, reoperation to correct DBS lead placement has been shown to improve outcomes (Ellis et al. 2008). In the future, the development of new neuroimaging techniques to accurately target the STN (Verhagen Metman et al. 2016) and directional DBS to shape the electric field (Dembek et al. 2017) may result in an improvement in the motor and cognitive effects of STN DBS during initial programming.
Motor tasks
The motor response to STN DBS is quite remarkable in persons with advanced PD and is therapeutically beneficial. STN DBS is effective at improving movement initiation (Brown et al. 1999), movement speed (Brown et al. 1999; Nowak et al. 2005; Sturman et al. 2010; Vaillancourt et al. 2004), force generation (Brown et al. 1999; Nowak et al. 2005; Prodoehl et al. 2007; Sturman et al. 2010), reducing rigidity (Shapiro et al. 2007), reducing tremor (Sturman et al. 2004; Sturman et al. 2007), and significantly reducing the score on the motor section of Unified Parkinson’s Disease Rating Scale (Brown et al. 1999; Limousin et al. 1998; Nowak et al. 2005; Prodoehl et al. 2007).
Participants with PD display more difficulties with complex motor tasks compared to simple motor tasks (Benecke et al. 1987b). They present with added performance deficits when executing more complex simultaneous or sequential motor tasks (Benecke et al. 1986; Benecke et al. 1987a). Simple and complex motor tasks have some aspects of control that are common. One way in which to consider simple and complex tasks is in terms of whether their control is driven by modulating intensive or coordinative parameters (Hening et al. 2009). Intensive aspects of control are common to both simple and complex motor tasks (Hening et al. 2009). Intensive aspects of control comprise a single major dimension of intensity. These include movement speed, movement amplitude, peak force, and scaling (Hening et al. 2009; Schettino et al. 2006; Snider et al. 2014). STN DBS effectively treats deficits in intensive aspects of control and significantly improves movement speed and peak torque (Brown et al. 1999; Nowak et al. 2005; Sturman et al. 2010). Complex movements are characterized by coordinative aspects of control that include processing and integration of different neural inputs as well as different motor components (Hening et al. 2009; Schettino et al. 2006; Snider et al. 2014). STN DBS is not as effective at treating deficits in coordinative aspects of control and can be detrimental to performance (Alberts et al. 2008; David et al. 2018).
The reason why STN DBS could impair simultaneous or sequential motor tasks is possibly because of the greater cognitive requirements that underlie coordinative aspects of control while performing these tasks. The next section will discuss the effect of STN DBS on cognition and motor tasks with greater cognitive demands.
Cognitive tasks
Research studies examining the cognitive effects of STN DBS have been on the rise because of the significant incidence of cognitive symptoms in advanced PD (Emre et al. 2007; Hely et al. 2008), the medically refractory nature of cognitive symptoms in advanced PD (Hely et al. 2005), and the impact cognitive symptoms have on the quality of lives of persons with PD and their caregivers (Goldman et al. 2018; Jones et al. 2017; Schrag et al. 2000). Not only are the cognitive deficits observed in persons with PD heterogeneous, they also have both, varying neural representations and varying neuropathologies (Biundo et al. 2016).
A general pattern of studies examining the effect of STN DBS on cognition is that the efficacy of STN DBS reduces or becomes detrimental cognitive task increases. Alberts when the complexity or difficulty of the and colleagues (2008) reported that simple dual-task performance was unchanged with STN DBS. However, on a complex dual-task requiring simultaneous performance of a force maintenance task and the n-back task (2-back), a test of working memory, cognitive performance was worsened with bilateral STN DBS compared to unilateral and no stimulation. Similarly, compared to no stimulation, STN DBS impaired working memory and response inhibition performance on a more difficult version of the tasks, the spatial delayed response task and Go-NoGo task, respectively, but had no effect on performance of the simpler versions (Hershey et al. 2004). Impaired response inhibition on the most difficult version of a task with STN DBS has been reported again on the Go-NoGo task and during paced Random Number Generation (Georgiev et al. 2016; Williams et al. 2015). In addition, STN DBS impaired performance on decision-making tasks during high-conflict trials but not on low-conflict trials (Cavanagh et al. 2011; Frank et al. 2007). Together these studies suggest that to reveal the cognitive deficits that occur with STN DBS, it is necessary to use a cognitively demanding task. This could be the Go-NoGo task with a high level of prepotency, the fast-paced Random Number Generation task, the n-back or spatial delayed response task with a high memory load, or a high-conflict decision-making task.
During a cognitively demanding oculomotor task with high levels of prepotency affecting inhibition, we have shown that STN DBS is detrimental to performance (Goelz et al. 2017). We found that during the antisaccade task, relative to no stimulation and age and sex matched healthy controls, STN DBS significantly increased prosaccade errors, the prepotent response that was required to be inhibited (Goelz et al. 2017). The finding that STN DBS increases prosaccade errors relative to no stimulation is quite robust and has been replicated by Bakhtiari and colleagues (2019). In a follow-up analysis we found that STN DBS significantly impaired measures of preparatory set and this was related to the observed failure in inhibition, i.e., increase in prosaccade errors during the antisaccade task (Goelz et al. 2019). It is known that the STN receives input from the supplementary eye field, frontal eye field (Nambu et al. 2002), and the dorsolateral prefrontal cortex (Benarroch 2008; Morris et al. 2017), which are primary cortical areas associated with preparatory set of the antisaccade task (DeSouza et al. 2003; Everling and Munoz 2000; Schlag-Rey et al. 1997; Sweeney et al. 1996). It has also been shown that STN DBS alters activity in the very same frontal (Hilker et al. 2004; Mayer et al. 2016) and prefrontal areas such as the DLPFC (Campbell et al. 2008; Kalbe et al. 2009; Limousin et al. 1997). We could hypothesize that STN DBS could disrupt frontal activity underlying preparatory set and drive the inhibitory impairment seen during the antisaccade task.
Similarly, during a sequential reaching task with a high spatial memory load, we showed that STN DBS worsened cognitive performance (David et al. 2018). Participants were asked to visually encode 3 sequential targets, after a brief delay they were asked to look and point as accurately as possible to the remembered targets in the order that they were presented. STN DBS, relative to no stimulation, did not affect eye error but significantly increased pointing error. This showed that STN DBS probably adversely affected neural processes related to the transfer of information from eye-centered coordinates to limb centered coordinates (David et al. 2018). This transfer of information from eye to limb centered coordinates requires normal cognitive executive function (Inzelberg et al. 2008), and is thought to take place in the posterior parietal cortex (Batista et al. 1999; Buneo et al. 2002). Previous studies have shown that STN DBS alters activity in the posterior parietal cortex (Hilker et al. 2004; Trost et al. 2006; Vafaee et al. 2004). It is probable that STN DBS disrupts visuomotor transformations of this area and this could be related to the increased spatial error observed during pointing. The studies conducted in our lab consistently show that STN DBS disrupts cognitive aspects of motor tasks that are cognitively demanding.
Recent reviews on the effects of STN DBS on cognition concluded that the worsening of cognitive function was rare (Mehanna et al. 2017) and that STN DBS effects on cognition were heterogeneous (Cernera et al. 2019). These are both valid conclusions, especially as both reviews focused on the effects of STN DBS on many different standardized neuropsychological tests to examine cognitive functions. Studies comparing STN stimulation to no stimulation have reported no effect of stimulation on a variety of neuropsychological tests measuring executive function, memory, verbal fluency, and visuospatial processing (Fraraccio et al. 2008; Hälbig et al. 2004; Morrison et al. 2004; Tremblay et al. 2015; Witt et al. 2004). Studies that examined the pre vs post-surgery effects of STN DBS, comparing cognitive performance to PD controls, showed no effect of STN DBS on most neuropsychological tasks tested, however, each study also showed at least one worsened measure of cognition. The cognitive functions that have been reported to worsen are verbal fluency, executive function, and memory (Alegret et al. 2004; Castelli et al. 2010; De Gaspari et al. 2006; Demeter et al. 2017; Marshall et al. 2012; Merola et al. 2014; Moretti et al. 2003; Morrison et al. 2004; Sáez-Zea et al. 2012; Smeding et al. 2011; Smeding et al. 2006; Williams et al. 2011; Witt et al. 2008; York et al. 2008; Zangaglia et al. 2009). However, it should be noted that many of these studies were pre vs. post-surgery comparisons, where the post-surgery time point lacked an off vs. on stimulation comparison. Therefore, STN stimulation effects could not be separated from the lesion effects, making it difficult to conclude that these changes are due solely from STN stimulation.
Three studies comparing STN stimulation to no stimulation report an improvement on some cognitive tasks that measure executive function (Castner et al. 2007; Jahanshahi et al. 2000; Page and Jahanshahi 2007). However, some tasks on which performance was improved with STN DBS, such as the Stroop Test and the Wisconsin Card Sorting Test (Jahanshahi et al. 2000; Page and Jahanshahi 2007), have been shown not to be affected by STN DBS in other studies (Fraraccio et al. 2008; Hälbig et al. 2004). Additionally, controlled longitudinal studies discussed in the previous paragraph have not shown any significant improvement on cognitive tasks over time with STN DBS.
STN DBS has no effect on the majority of standardized neuropsychological tests and when an effect is reported, the change is not consistent across studies. This is in contrast to the repeated detrimental effects found in cognitively demanding tasks and motor tasks that rely heavily on cognitive control. This discrepancy may occur due to the limited insight most neuropsychological tests can have on clinically relevant behavior. Problems associated with using neuropsychological tests include that the tests were not designed to measure clinically relevant deficits, precise task design affects the results, the tests involve multiple complex cognitive constructs that could each affect the results, and that the tests are typically not measuring behavioral function (Burgess et al. 2006). Future studies would benefit from using tasks directly relevant to behavior instead of neuropsychological tests to evaluate the cognitive effects of STN DBS.
Conclusion
The studies reviewed here support the conclusion that with respect to motor symptoms of Parkinson’s disease, high frequency STN DBS attenuates activity in the beta band and improves the motor symptoms of PD. With respect to cognitive symptoms, evidence is accumulating to support the statement that STN DBS can be detrimental to performance especially when the task is cognitively demanding. We have provided a modified oscillatory model of basal ganglia dysfunction and suggest that similar to pro-kinetic and anti-kinetic frequencies that underlie motor function there are pro-cognitive and anti-cognitive frequencies that underlie cognitive function. While motor network oscillatory activity is primarily correlated to the beta band, cognitive network oscillatory activity is not confined to one band. Cognitive function is subserved by activity in multiple frequency bands. In order to observe both motor and cognitive benefits, STN DBS should enhance oscillatory activity that is related to both motor and cognitive function.
With the advent of STN DBS for the treatment of PD, we have gained a significant amount of knowledge about models of basal ganglia dysfunction, STN DBS mechanisms of action, and the motor and cognitive effects of STN DBS. Professor John Rothwell has contributed significantly to each of these domains during his illustrious and productive career. He has been an archetype of collaborative effort that spans continents in the pursuit of empirical evidence to test the many predictions from the very early models of basal ganglia dysfunction. As we move in to the era of adaptive STN DBS, models of basal ganglia function need to be updated, STN DBS mechanisms of actions need to be better understood, and the predictions of updated models of basal ganglia dysfunction need to be tested in the associative and limbic domains as well as the motor domains. The most effective way moving forward is to follow in the footsteps of Professor John Rothwell’s collaborative approach to moving the science forward and seek out collaborative efforts in the form of animal models, computational modeling, and human studies to address the many unanswered questions that remain.
Sources of funding:
This paper was supported by National Institutes of Health (R01NS09295001 and T32 NS047987). The sponsors were not involved in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Fabian J. David, Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, USA
Miranda J. Munoz, Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, USA
Daniel M. Corcos, Department of Physical Therapy and Human Movement Sciences, Northwestern University; Department of Neurological Sciences, Rush University Medical Center, Chicago, USA
References
- Abdi A et al. (2015) Prototypic and Arkypallidal Neurons in the Dopamine-Intact External Globus Pallidus Journal of Neuroscience 35:6667–6688 doi: 10.1523/jneurosci.4662-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam T, Kullmann DM (2010) Oscillations and Filtering Networks Support Flexible Routing of Information Neuron 67:308–320 doi: 10.1016/j.neuron.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL (2008) Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients Brain : a journal of neurology 131:3348–3360 doi: 10.1093/brain/awn238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB (1989) The Functional-Anatomy of Basal Ganglia Disorders Trends in neurosciences 12:366–375 [DOI] [PubMed] [Google Scholar]
- Alegret M, Valldeoriola F, Martí M, Pilleri M, Junqué C, Rumià J, Tolosa E (2004) Comparative cognitive effects of bilateral subthalamic stimulation and subcutaneous continuous infusion of apomorphine in Parkinson’s disease Mov Disord 19:1463–1469 doi: 10.1002/mds.20237 [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing Trends Neurosci 13:266–271 doi: 10.1016/0166-2236(90)90107-l [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986) Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex Annual Review of Neuroscience 9:357–381 [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F et al. (2006) Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease Brain 129:1748–1757 doi: 10.1093/brain/awl103 [DOI] [PubMed] [Google Scholar]
- Ashby P, Rothwell JC (2000) Neurophysiologic aspects of deep brain stimulation Neurology 55:S17–20 [PubMed] [Google Scholar]
- Bakhtiari S, Altinkaya A, Pack CC, Sadikot AF (2019) The Role of the Subthalamic Nucleus in Inhibitory Control of Oculomotor Behavior in Parkinson’s Disease. Cold Spring Harbor Laboratory. doi: 10.1101/606897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA (1999) Reach plans in eye-centered coordinates Science (New York, NY: ) 285:257–260 [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease Lancet neurology 8:67–81 doi: 10.1016/S1474-4422(08)70291-6 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2008) Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation Neurology 70:1991–1995 doi: 10.1212/01.wnl.0000313022.39329.65 [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1986) Performance of simultaneous movements in patients with Parkinson’s disease Brain 109 ( Pt 4):739–757 doi: 10.1093/brain/109.4.739 [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1987a) Disturbance of sequential movements in patients with Parkinson’s disease Brain 110 ( Pt 2):361–379 doi: 10.1093/brain/110.2.361 [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1987b) Simple and complex movements off and on treatment in patients with Parkinson’s disease J Neurol Neurosurg Psychiatry 50:296–303 doi: 10.1136/jnnp.50.3.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallet M (2001) Pathophysiology of bradykinesia in Parkinson’s disease Brain 124:2131–2146 [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism J Neurophysiol 72:507–520 doi: 10.1152/jn.1994.72.2.507 [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ (2002) Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network Trends Neurosci 25:525–531 doi: 10.1016/s0166-2236(02)02235-x [DOI] [PubMed] [Google Scholar]
- Biundo R, Weis L, Antonini A (2016) Cognitive decline in Parkinson’s disease: the complex picture NPJ Parkinsons Dis 2:16018 doi: 10.1038/npjparkd.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M et al. (2018) Neurophysiological correlates of bradykinesia in Parkinson’s disease Brain 141:2432–2444 doi: 10.1093/brain/awy155 [DOI] [PubMed] [Google Scholar]
- Brittain J-S, Brown P (2014) Oscillations and the basal ganglia: Motor control and beyond NeuroImage 85:637–647 doi: 10.1016/j.neuroimage.2013.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Sharott A, Brown P (2014) The highs and lows of beta activity in cortico-basal ganglia loops Eur J Neurosci 39:1951–1959 doi: 10.1111/ejn.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease Mov Disord 18:357–363 doi: 10.1002/mds.10358 [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V (2004) Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease Experimental Neurology 188:480–490 doi: 10.1016/j.expneurol.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V (2001) Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease J Neurosci 21:1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Williams D (2005) Basal ganglia local field potential activity: character and functional significance in the human Clin Neurophysiol 116:2510–2519 doi: 10.1016/j.clinph.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Brown RG et al. (1999) Impact of deep brain stimulation on upper limb akinesia in Parkinson’s disease Ann Neurol 45:473–488 doi: [DOI] [PubMed] [Google Scholar]
- Brunenberg EJL et al. (2012) Structural and Resting State Functional Connectivity of the Subthalamic Nucleus: Identification of Motor STN Parts and the Hyperdirect Pathway PLoS ONE 7:e39061 doi: 10.1371/journal.pone.0039061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA (2002) Direct visuomotor transformations for reaching Nature 416:632–636 doi: 10.1038/416632a [DOI] [PubMed] [Google Scholar]
- Burgess PW et al. (2006) The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology J Int Neuropsychol Soc 12:194–209 doi: 10.1017/S1355617706060310 [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal Nat Neurosci 17:1022–1030 doi: 10.1038/nn.3743 [DOI] [PubMed] [Google Scholar]
- Campbell MC et al. (2008) Neural correlates of STN DBS-induced cognitive variability in Parkinson disease Neuropsychologia 46:3162–3169 doi: 10.1016/j.neuropsychologia.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C-Y, Zeng K, Li D-Y, Zhan S-K, Li X-L, Sun B-M (2017) Modulations on cortical oscillations by subthalamic deep brain stimulation in patients with Parkinson disease: A MEG study Neuroscience Letters 636:95–100 doi: 10.1016/j.neulet.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Castelli L, Rizzi L, Zibetti M, Angrisano S, Lanotte M, Lopiano L (2010) Neuropsychological changes 1-year after subthalamic DBS in PD patients: A prospective controlled study Parkinsonism & Related Disorders 16:115–118 doi: 10.1016/j.parkreldis.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Castner JE, Copland DA, Silburn PA, Coyne TJ, Sinclair F, Chenery HJ (2007) Lexical-semantic inhibitory mechanisms in Parkinson’s disease as a function of subthalamic stimulation Neuropsychologia 45:3167–3177 doi: 10.1016/j.neuropsychologia.2007.06.019 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ (2011) Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold Nature Neuroscience 14:1462–1467 doi: 10.1038/nn.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernera S, Okun MS, Gunduz A (2019) A Review of Cognitive Outcomes Across Movement Disorder Patients Undergoing Deep Brain Stimulation Front Neurol 10 doi: 10.3389/fneur.2019.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC et al. (2007) Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease Experimental Neurology 205:214–221 doi: 10.1016/j.expneurol.2007.01.027 [DOI] [PubMed] [Google Scholar]
- Chu H-Y, McIver EL, Kovaleski RF, Atherton JF, Bevan MD (2017) Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons Neuron 95:1306–1318.e1305 doi: 10.1016/j.neuron.2017.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Atherton JF, Wokosin D, Surmeier DJ, Bevan MD (2015) Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex Neuron 85:364–376 doi: 10.1016/j.neuron.2014.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JW et al. (2018) Beta-band oscillations in the supplementary motor cortex are modulated by levodopa and associated with functional activity in the basal ganglia Neuroimage Clin 19:559–571 doi: 10.1016/j.nicl.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, van der Meij R, Peterson EJ, de Hemptinne C, Starr PA, Voytek B (2017) Nonsinusoidal Beta Oscillations Reflect Cortical Pathophysiology in Parkinson’s Disease J Neurosci 37:4830–4840 doi: 10.1523/JNEUROSCI.2208-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Pau M (2017) Subthalamic nucleus stimulation and gait in Parkinson’s Disease: a not always fruitful relationship Gait & Posture 52:205–210 doi: 10.1016/j.gaitpost.2016.11.039 [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM (2003) Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys J Neurosci 23:11741–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FJ, Goelz LC, Tangonan RZ, Metman LV, Corcos DM (2018) Bilateral deep brain stimulation of the subthalamic nucleus increases pointing error during memory-guided sequential reaching Exp Brain Res 236:1053–1065 doi: 10.1007/s00221-018-5197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gaspari D et al. (2006) Clinical and neuropsychological follow up at 12 months in patients with complicated Parkinson’s disease treated with subcutaneous apomorphine infusion or deep brain stimulation of the subthalamic nucleus Journal of Neurology, Neurosurgery, and Psychiatry 77:450–453 doi: 10.1136/jnnp.2005.078659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C et al. (2013) Exaggerated phase–amplitude coupling in the primary motor cortex in Parkinson disease PNAS 110:4780–4785 doi: 10.1073/pnas.1214546110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA (2015) Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease Nature Neuroscience 18:779–786 doi: 10.1038/nn.3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaville C, McCoy AJ, Gerber CM, Cruz AV, Walters JR (2015) Subthalamic nucleus activity in the awake hemiparkinsonian rat: relationships with motor and cognitive networks J Neurosci 35:6918–6930 doi: 10.1523/JNEUROSCI.0587-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR (1990) Primate Models of Movement-Disorders of Basal Ganglia Origin Trends in neurosciences 13:281–285 [DOI] [PubMed] [Google Scholar]
- Dembek TA et al. (2017) Directional DBS increases side-effect thresholds-A prospective, double-blind trial Mov Disord 32:1380–1388 doi: 10.1002/mds.27093 [DOI] [PubMed] [Google Scholar]
- Demeter G, Valálik I, Pajkossy P, Szőllősi Á, Lukács Á, Kemény F, Racsmány M (2017) The effect of deep brain stimulation of the subthalamic nucleus on executive functions: impaired verbal fluency and intact updating, planning and conflict resolution in Parkinson’s disease Neuroscience Letters 647:72–77 doi: 10.1016/j.neulet.2017.03.026 [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S (2003) Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI J Neurophysiol 89:1016–1023 doi: 10.1152/jn.00562.2002 [DOI] [PubMed] [Google Scholar]
- Devos D et al. (2004) Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson’s disease Brain: A Journal of Neurology 127:408–419 doi: 10.1093/brain/awh053 [DOI] [PubMed] [Google Scholar]
- Ellis T-M et al. (2008) Reoperation for suboptimal outcomes after deep brain stimulation surgery Neurosurgery 63:754–760; discussion 760–761 doi: 10.1227/01.NEU.0000325492.58799.35 [DOI] [PubMed] [Google Scholar]
- Emre M et al. (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease Mov Disord 22:1689–1707; quiz 1837 doi: 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- Eusebio A, Brown P (2007) Oscillatory activity in the basal ganglia Parkinsonism & Related Disorders 13:S434–S436 doi: 10.1016/s1353-8020(08)70044-0 [DOI] [PubMed] [Google Scholar]
- Eusebio A, Cagnan H, Brown P (2012) Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Frontiers in Integrative Neuroscience 6 doi: 10.3389/fnint.2012.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A et al. (2008) Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease Exp Neurol 209:125–130 doi: 10.1016/j.expneurol.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field J Neurosci 20:387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M (2001) Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson’s disease Brain 124:1590–1600 doi: 10.1093/brain/124.8.1590 [DOI] [PubMed] [Google Scholar]
- Fan KY, Baufreton J, Surmeier DJ, Chan CS, Bevan MD (2012) Proliferation of external globus pallidus-subthalamic nucleus synapses following degeneration of midbrain dopamine neurons J Neurosci 32:13718–13728 doi: 10.1523/JNEUROSCI.5750-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A et al. (2011) Modulation of gait coordination by subthalamic stimulation improves freezing of gait Movement Disorders 26:844–851 doi: 10.1002/mds.23583 [DOI] [PubMed] [Google Scholar]
- Feingold J, Gibson DJ, Depasquale B, Graybiel AM (2015) Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks PNAS 112:13687–13692 doi: 10.1073/pnas.1517629112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, Lopiano L (2005) Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson’s disease Exp Brain Res 160:517–527 doi: 10.1007/s00221-004-2036-5 [DOI] [PubMed] [Google Scholar]
- Ferrarin M, Rizzone M, Lopiano L, Recalcati M, Pedotti A (2004) Effects of subthalamic nucleus stimulation and L-dopa in trunk kinematics of patients with Parkinson’s disease Gait Posture 19:164–171 doi: 10.1016/S0966-6362(03)00058-4 [DOI] [PubMed] [Google Scholar]
- Follett KA et al. (2010) Pallidal versus Subthalamic Deep-Brain Stimulation for Parkinson’s Disease New England Journal of Medicine 362:2077–2091 [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism Science (New York, NY) 318:1309–1312 doi: 10.1126/science.1146157 [DOI] [PubMed] [Google Scholar]
- Fraraccio M, Ptito A, Sadikot A, Panisset M, Dagher A (2008) Absence of cognitive deficits following deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease Arch Clin Neuropsychol 23:399–408 doi: 10.1016/j.acn.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence Trends in Cognitive Sciences 9:474–480 doi: 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T (2006) Oscillations in the basal ganglia under normal conditions and in movement disorders Mov Disord 21:1566–1577 doi: 10.1002/mds.21033 [DOI] [PubMed] [Google Scholar]
- Georgiev D, Dirnberger G, Wilkinson L, Limousin P, Jahanshahi M (2016) In Parkinson’s disease on a probabilistic Go/NoGo task deep brain stimulation of the subthalamic nucleus only interferes with withholding of the most prepotent responses Experimental Brain Research 234:1133–1143 doi: 10.1007/s00221-015-4531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch KE et al. (2016) Npas1(+) Pallidal Neurons Target Striatal Projection Neurons Journal of Neuroscience 36:5472–5488 doi: 10.1523/jneurosci.1720-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz LC, Cottongim M, Metman LV, Corcos DM, David FJ (2019) Bilateral subthalamic nucleus deep brain stimulation increases fixational saccades during movement preparation: evidence for impaired preparatory set Exp Brain Res 237:2841–2851 doi: 10.1007/s00221-019-05636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz LC, David FJ, Sweeney JA, Vaillancourt DE, Poizner H, Metman LV, Corcos DM (2017) The effects of unilateral versus bilateral subthalamic nucleus deep brain stimulation on prosaccades and antisaccades in Parkinson’s disease Exp Brain Res 235:615–626 doi: 10.1007/s00221-016-4830-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG et al. (2018) Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health NPJ Parkinsons Dis 4:19 doi: 10.1038/s41531-018-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulberti A et al. (2015) Predictive timing functions of cortical beta oscillations are impaired in Parkinson’s disease and influenced by L-DOPA and deep brain stimulation of the subthalamic nucleus Neuroimage Clin 9:436–449 doi: 10.1016/j.nicl.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hälbig TD et al. (2004) Subthalamic stimulation differentially modulates declarative and nondeclarative memory Neuroreport 15:539–543 doi: 10.1097/00001756-200403010-00031 [DOI] [PubMed] [Google Scholar]
- Hamani C (2004) The subthalamic nucleus in the context of movement disorders Brain 127:4–20 doi: 10.1093/brain/awh029 [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P (2007) Pathological synchronization in Parkinson’s disease: networks, models and treatments Trends in neurosciences 30:357–364 doi:S0166-2236(07)00123-3 [pii] [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL (2003) Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons The Journal of Neuroscience 23:1916–1923 doi: 10.1523/jneurosci.23-05-01916.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatz F, Meyer A, Roesch A, Taub E, Gschwandtner U, Fuhr P (2018) Quantitative EEG and Verbal Fluency in DBS Patients: Comparison of Stimulator-On and -Off Conditions Front Neurol 9:1152 doi: 10.3389/fneur.2018.01152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D (2009) Deep brain stimulation effects on gait variability in Parkinson’s disease Movement Disorders 24:1688–1692 doi: 10.1002/mds.22554 [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N (2003) Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait Exp Brain Res 149:187–194 doi: 10.1007/s00221-002-1354-8 [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E et al. (2014) Neuromagnetic Evidence of Abnormal Movement-Related Beta Desynchronization in Parkinson’s Disease Cerebral Cortex 24:2669–2678 doi: 10.1093/cercor/bht121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell F, Plate A, Mehrkens JH, Botzel K (2018) Subthalamic oscillatory activity and connectivity during gait in Parkinson’s disease Neuroimage Clin 19:396–405 doi: 10.1016/j.nicl.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years Mov Disord 20:190–199 doi: 10.1002/mds.20324 [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WGJ, Adena MA, Halliday GA, Morris JGL (2008) The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years Movement Disorders 23:837–844 doi: 10.1002/mds.21956ER [DOI] [PubMed] [Google Scholar]
- Hening W, Harrington DL, Poizner H (2009) Basal ganglia: motor functions of In: Binder MD, Hirokawa N, Windhorst U (eds) Encyclopedia of Neuroscience. Springer, Berlin, Heidelberg, [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS (2004) Stimulation of STN impairs aspects of cognitive control in PD Neurology 62:1110–1114 [DOI] [PubMed] [Google Scholar]
- Hilker R et al. (2004) Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease J Cereb Blood Flow Metab 24:7–16 doi: 10.1097/01.WCB.0000092831.44769.09 [DOI] [PubMed] [Google Scholar]
- Hilliard JD, Frysinger RC, Elias WJ (2011) Effective Subthalamic Nucleus Deep Brain Stimulation Sites May Differ for Tremor, Bradykinesia and Gait Disturbances in Parkinson’s Disease Stereotactic and Functional Neurosurgery 89:357–364 doi: 10.1159/000331269 [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Demeulemeester H, Nuttin B, De Sutter P (2000) Identification of the target neuronal elements in electrical deep brain stimulation European Journal of Neuroscience 12:4573–4577 doi: 10.1111/j.1460-9568.2000.01306.x [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P (2004) Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings J Neurosci 24:9240–9243 doi: 10.1523/JNEUROSCI.3366-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Lozano AM, Davis KD, Saint-Cyr JA, Lang AE, Dostrovsky JO (1994) Differential neuronal activity in segments of globus pallidus in Parkinson’s disease patients Neuroreport 5:1533–1537 doi: 10.1097/00001756-199407000-00031 [DOI] [PubMed] [Google Scholar]
- Inzelberg R, Schechtman E, Hocherman S (2008) Visuo-motor coordination deficits and motor impairments in Parkinson’s disease PLoS One 3:e3663 doi: 10.1371/journal.pone.0003663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M et al. (2000) The impact of deep brain stimulation on executive function in Parkinson’s disease Brain 123 (Pt 6):1142–1154 doi: 10.1093/brain/123.6.1142 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Rothwell JC (2017) Inhibitory dysfunction contributes to some of the motor and non-motor symptoms of movement disorders and psychiatric disorders Philos Trans R Soc Lond B Biol Sci 372 doi: 10.1098/rstb.2016.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Brown P (2011) New insights into the relationship between dopamine, beta oscillations and motor function Trends in Neurosciences 34:611–618 doi: 10.1016/j.tins.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Johnsen EL, Sunde N, Mogensen PH, Østergaard K (2010) MRI verified STN stimulation site - gait improvement and clinical outcome Eur J Neurol 17:746–753 doi: 10.1111/j.1468-1331.2010.02962.x [DOI] [PubMed] [Google Scholar]
- Jones AJ et al. (2017) Caregiver burden is increased in Parkinson’s disease with mild cognitive impairment (PD-MCI) Transl Neurodegener 6:17 doi: 10.1186/s40035-017-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E et al. (2009) Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease Neurology 72:42–49 doi: 10.1212/01.wnl.0000338536.31388.f0 [DOI] [PubMed] [Google Scholar]
- Kang G, Lowery MM (2014) Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson’s disease: a simulation study Front Comput Neurosci 8:32 doi: 10.3389/fncom.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R et al. (2018) A human prefrontal-subthalamic circuit for cognitive control Brain 141:205–216 doi: 10.1093/brain/awx300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Menetrey A, Glowinski J, Thierry AM (2001) Segregation and convergence of information flow through the cortico-subthalamic pathways J Neurosci 21:5764–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystkowiak P et al. (2003) Effects of Subthalamic Nucleus Stimulation and Levodopa Treatment on Gait Abnormalities in Parkinson Disease Archives of Neurology 60:80 doi: 10.1001/archneur.60.1.80 [DOI] [PubMed] [Google Scholar]
- Kuhn AA et al. (2008) High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance The Journal of neuroscience : the official journal of the Society for Neuroscience 28:6165–6173 doi: 10.1523/JNEUROSCI.0282-08.2008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P (2006) Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease Eur J Neurosci 23:1956–1960 doi: 10.1111/j.1460-9568.2006.04717.x [DOI] [PubMed] [Google Scholar]
- Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G-H, Brown P (2005) The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease 194:212–220 doi: 10.1016/j.expneurol.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Kuhn AA et al. (2009) Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity Experimental neurology 215:380–387 doi: 10.1016/j.expneurol.2008.11.008 [doi] [DOI] [PubMed] [Google Scholar]
- Kühn AA et al. (2004) Event‐related beta desynchronization in human subthalamic nucleus correlates with motor performance Brain 127:735–746 doi: 10.1093/brain/awh106 [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T (2007) Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism Eur J Neurosci 26:1701–1713 doi: 10.1111/j.1460-9568.2007.05777.x [DOI] [PubMed] [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R (1997) Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease Annals of Neurology 42:283–291 doi: 10.1002/ana.410420303 [doi] [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease N Engl J Med 339:1105–1111 doi: 10.1056/NEJM199810153391603 [DOI] [PubMed] [Google Scholar]
- Little S et al. (2013) Adaptive deep brain stimulation in advanced Parkinson disease Annals of Neurology 74:449–457 doi: 10.1002/ana.23951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA (1984) On the ability to inhibit simple and choice reaction time responses: a model and a method J Exp Psychol Hum Percept Perform 10:276–291 doi: 10.1037//0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Webb RM, Silberstein P, Tisch S, Asselman P, Limousin P, Rothwell JC (2005) Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson’s disease Eur J Neurosci 21:1394–1402 doi: 10.1111/j.1460-9568.2005.03952.x [DOI] [PubMed] [Google Scholar]
- Mahlknecht P et al. (2017) Pyramidal tract activation due to subthalamic deep brain stimulation in Parkinson’s disease Mov Disord 32:1174–1182 doi: 10.1002/mds.27042 [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ (2008) Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex J Neurosci 28:4795–4806 doi: 10.1523/JNEUROSCI.0123-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S, Foffani G, Bianchi AM, Baselli G, Tamma F, Egidi M, Priori A (2006) Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease J Physiol 571:579–591 doi: 10.1113/jphysiol.2005.100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S, Fumagalli M, Priori A (2011) What neurophysiological recordings tell us about cognitive and behavioral functions of the human subthalamic nucleus Expert Rev Neurother 11:139–149 doi: 10.1586/ern.10.184 [DOI] [PubMed] [Google Scholar]
- Marshall DF, Strutt AM, Williams AE, Simpson RK, Jankovic J, York MK (2012) Alternating verbal fluency performance following bilateral subthalamic nucleus deep brain stimulation for Parkinson’s disease Eur J Neurol 19:1525–1531 doi: 10.1111/j.1468-1331.2012.03759.x [DOI] [PubMed] [Google Scholar]
- Mastro KJ, Zitelli KT, Willard AM, Leblanc KH, Kravitz AV, Gittis AH (2017) Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice Nature Neuroscience 20:815–+ doi: 10.1038/nn.4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Ma Y, Pare JF, Villalba RM, Wichmann T, Smith Y (2015) Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys 138:946–962 doi: 10.1093/brain/awv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Neimat J, Folley BS, Bourne SK, Konrad PE, Charles D, Park S (2016) Deep brain stimulation of the subthalamic nucleus alters frontal activity during spatial working memory maintenance of patients with Parkinson’s disease Neurocase 22:369–378 doi: 10.1080/13554794.2016.1197951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM (2012) Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns J Neurosci 32:15657–15668 doi: 10.1523/JNEUROSCI.2824-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor MM, Nelson AB (2019) Circuit Mechanisms of Parkinson’s Disease Neuron 101:1042–1056 doi: 10.1016/j.neuron.2019.03.004 [DOI] [PubMed] [Google Scholar]
- McNeely ME, Earhart GM (2013) Medication and subthalamic nucleus deep brain stimulation similarly improve balance and complex gait in Parkinson disease Parkinsonism & Related Disorders 19:86–91 doi: 10.1016/j.parkreldis.2012.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna R, Bajwa JA, Fernandez H, Wagle Shukla AA (2017) Cognitive Impact of Deep Brain Stimulation on Parkinson’s Disease Patients Parkinson’s Disease [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola A et al. (2014) Medical therapy and subthalamic deep brain stimulation in advanced Parkinson’s disease: a different long-term outcome? Journal of Neurology, Neurosurgery, and Psychiatry 85:552–559 doi: 10.1136/jnnp-2013-305271 [DOI] [PubMed] [Google Scholar]
- Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs Prog Neurobiol 50:381–425 doi: 10.1016/s0301-0082(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT (1993) Basal ganglia intrinsic circuits and their role in behavior Curr Opin Neurobiol 3:950–957 doi: 10.1016/0959-4388(93)90167-w [DOI] [PubMed] [Google Scholar]
- Mirelman A et al. (2019) Gait impairments in Parkinson’s disease The Lancet Neurology 18:697–708 doi: 10.1016/s1474-4422(19)30044-4 [DOI] [PubMed] [Google Scholar]
- Montgomery EB (2017) Deep Brain Stimulation Programming: Mechanisms, Principles, and Practice. 2nd edn Oxford University Press, New York [Google Scholar]
- Moreau C et al. (2008) STN-DBS frequency effects on freezing of gait in advanced Parkinson disease Neurology 71:80–84 doi: 10.1212/01.wnl.0000303972.16279.46 [DOI] [PubMed] [Google Scholar]
- Moretti R et al. (2003) Neuropsychological changes after subthalamic nucleus stimulation: a 12 month follow-up in nine patients with Parkinson’s disease Parkinsonism & Related Disorders 10:73–79 doi: 10.1016/s1353-8020(03)00073-7 [DOI] [PubMed] [Google Scholar]
- Morris LS, Baek K, Voon V (2017) Distinct cortico-striatal connections with subthalamic nucleus underlie facets of compulsivity Cortex 88:143–150 doi: 10.1016/j.cortex.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CE et al. (2004) Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease Arch Clin Neuropsychol 19:165–181 doi: 10.1016/S0887-6177(03)00004-0 [DOI] [PubMed] [Google Scholar]
- Müller EJ, Robinson PA (2018) Suppression of Parkinsonian Beta Oscillations by Deep Brain Stimulation: Determination of Effective Protocols Front Comput Neurosci 12 doi: 10.3389/fncom.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tachibana Y, Chiken S (2015) Cause of parkinsonian symptoms: Firing rate, firing pattern or dynamic activity changes? 5:1–6 doi: 10.1016/j.baga.2014.11.001 [DOI] [Google Scholar]
- Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo pallidal ‘hyperdirect’ pathway Neurosci Res 43:111–117 doi: 10.1016/s0168-0102(02)00027-5 [DOI] [PubMed] [Google Scholar]
- Nelson AB, Kreitzer AC (2014) Reassessing models of basal ganglia function and dysfunction Annu Rev Neurosci 37:117–135 doi: 10.1146/annurev-neuro-071013-013916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Topka H, Tisch S, Hariz M, Limousin P, Rothwell JC (2005) The beneficial effects of subthalamic nucleus stimulation on manipulative finger force control in Parkinson’s disease Exp Neurol 193:427–436 doi: 10.1016/j.expneurol.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Oberg T, Karsznia A, Oberg K (1994) Joint angle parameters in gait: reference data for normal subjects, 10–79 years of age J Rehabil Res Dev 31:199–213 [PubMed] [Google Scholar]
- Page D, Jahanshahi M (2007) Deep brain stimulation of the subthalamic nucleus improves set shifting but does not affect dual task performance in Parkinson’s disease IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society 15:198–206 doi: 10.1109/TNSRE.2007.897074 [DOI] [PubMed] [Google Scholar]
- Phibbs FT, Arbogast PG, Davis TL (2014) 60-Hz Frequency Effect on Gait in Parkinson’s Disease With Subthalamic Nucleus Deep Brain Stimulation 17:717–720 doi: 10.1111/ner.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kital ST (1999) A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus Nature 400:677–682 doi: 10.1038/23281 [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Goldberg JA (2019) Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders The Neuroscientist 25:359–379 doi: 10.1177/1073858418807887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pötter-Nerger M, Volkmann J (2013) Deep brain stimulation for gait and postural symptoms in Parkinson’s disease Movement Disorders 28:1609–1615 doi: 10.1002/mds.25677 [DOI] [PubMed] [Google Scholar]
- Priori A et al. (2004) Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease Experimental Neurology 189:369–379 doi: 10.1016/j.expneurol.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Rothwell JC, Metman LV, Bakay RAE, Vaillancourt DE (2007) Effects of STN DBS on memory guided force control in Parkinson’s disease (June 2007) Ieee Transactions on Neural Systems and Rehabilitation Engineering 15:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC (2011) The motor functions of the basal ganglia J Integr Neurosci 10:303–315 doi: 10.1142/S0219635211002798 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Edwards MJ (2013) Parkinson’s disease. In. Elsevier, pp 535–542. doi: 10.1016/b978-0-444-53497-2.00042-5 [DOI] [PubMed] [Google Scholar]
- Sáez-Zea C, Escamilla-Sevilla F, Katati MJ, Mínguez-Castellanos A (2012) Cognitive effects of subthalamic nucleus stimulation in Parkinson’s disease: a controlled study European Neurology 68:361–366 doi: 10.1159/000341380 [DOI] [PubMed] [Google Scholar]
- Salvade A et al. (2016) Distinct roles of cortical and pallidal beta and gamma frequencies in hemiparkinsonian and dyskinetic rats Exp Neurol 275 Pt 1:199–208 doi: 10.1016/j.expneurol.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H (2006) Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state Exp Brain Res 168:186–202 doi: 10.1007/s00221-005-0080-4 [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J (1997) Antisaccade performance predicted by neuronal activity in the supplementary eye field Nature 390:398–401 doi: 10.1038/37114 [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N (2000) What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 69:308–312 doi: 10.1136/jnnp.69.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepbach WM et al. (2013) Neurostimulation for Parkinson’s disease with early motor complications N Engl J Med 368:610–622 doi: 10.1056/NEJMoa1205158 [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Vaillancourt DE, Sturman MM, Metman LV, Bakay RA, Corcos DM (2007) Effects of STN DBS on rigidity in Parkinson’s disease IEEE Trans Neural Syst Rehabil Eng 15:173–181 doi: 10.1109/TNSRE.2007.896997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MA et al. (2016) Neural mechanisms of transient neocortical beta rhythms: Converging evidence from humans, computational modeling, monkeys, and mice Proceedings of the National Academy of Sciences of the United States of America 113:E4885–4894 doi: 10.1073/pnas.1604135113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulos C, Walsh R, Meaney C, Poon YY, Fallis M, Moro E (2013) Low-frequency subthalamic nucleus deep brain stimulation for axial symptoms in advanced Parkinson’s disease 260:2306–2311 doi: 10.1007/s00415-013-6983-2 [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012) Spectral fingerprints of large-scale neuronal interactions Nature Reviews Neuroscience 13:121–134 doi: 10.1038/nrn3137 [DOI] [PubMed] [Google Scholar]
- Singh A, Plate A, Kammermeier S, Mehrkens JH, Ilmberger J, Bötzel K (2013) Freezing of gait-related oscillatory activity in the human subthalamic nucleus 3:25–32 doi: 10.1016/j.baga.2012.10.002 [DOI] [Google Scholar]
- Smeding HMM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B (2011) Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s Disease Journal of Neurology, Neurosurgery, and Psychiatry 82:754–760 doi: 10.1136/jnnp.2007.140012 [DOI] [PubMed] [Google Scholar]
- Smeding HMM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, van Laar T, Schmand B (2006) Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study Neurology 66:1830–1836 doi: 10.1212/01.wnl.0000234881.77830.66 [DOI] [PubMed] [Google Scholar]
- Snider J, Lee D, Harrington DL, Poizner H (2014) Scaling and coordination deficits during dynamic object manipulation in Parkinson’s disease J Neurophysiol 112:300–315 doi: 10.1152/jn.00041.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spay C et al. (2018) Clonidine modulates the activity of the subthalamic-supplementary motor loop: evidence from a pharmacological study combining deep brain stimulation and electroencephalography recordings in Parkinsonian patients J Neurochem 146:333–347 doi: 10.1111/jnc.14447 [DOI] [PubMed] [Google Scholar]
- Starr PA, Ostrem JL (2013) Commentary on “Adaptive deep brain stimulation in advanced Parkinson disease” Annals of Neurology 74:447–448 doi: 10.1002/ana.23966 [DOI] [PubMed] [Google Scholar]
- Stegemöller EL, Allen DP, Simuni T, Mackinnon CD (2016) Motor cortical oscillations are abnormally suppressed during repetitive movement in patients with Parkinson’s disease Clinical Neurophysiology 127:664–674 doi: 10.1016/j.clinph.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman MM, Vaillancourt DE, Metman LV, Bakay RA, Corcos DM (2004) Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease Brain 127:2131–2143 doi: 10.1093/brain/awh237 [DOI] [PubMed] [Google Scholar]
- Sturman MM, Vaillancourt DE, Metman LV, Bakay RA, Corcos DM (2010) Effects of five years of chronic STN stimulation on muscle strength and movement speed Exp Brain Res 205:435–443 doi: 10.1007/s00221-010-2370-8 [DOI] [PubMed] [Google Scholar]
- Sturman MM, Vaillancourt DE, Metman LV, Sierens DK, Bakay RA, Corcos DM (2007) Deep brain stimulation and medication for parkinsonian tremor during secondary tasks Mov Disord 22:1157–1163 doi: 10.1002/mds.21518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N et al. (2011) Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease The Journal of neuroscience : the official journal of the Society for Neuroscience 31:5721–5729 doi: 10.1523/JNEUROSCI.6135-10.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC et al. (2016) Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease The Journal of Neuroscience 36:6445–6458 doi: 10.1523/jneurosci.1128-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR (1996) Positron emission tomography study of voluntary saccadic eye movements and spatial working memory Journal of neurophysiology 75:454–468 [DOI] [PubMed] [Google Scholar]
- Syrkin-Nikolau J et al. (2017) Subthalamic neural entropy is a feature of freezing of gait in freely moving people with Parkinson’s disease Neurobiology of Disease 108:288–297 doi: 10.1016/j.nbd.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Steinbusch HWM, Visser-Vandewalle V (2005) The functional role of the subthalamic nucleus in cognitive and limbic circuits 76:393–413 doi: 10.1016/j.pneurobio.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Thompson PD, Berardelli A, Rothwell JC, Day BL, Dick JP, Benecke R, Marsden CD (1988) The coexistence of bradykinesia and chorea in Huntington’s disease and its implications for theories of basal ganglia control of movement Brain 111 (Pt 2):223–244 doi: 10.1093/brain/111.2.223 [DOI] [PubMed] [Google Scholar]
- Tinkhauser G, Pogosyan A, Little S, Beudel M, Herz DM, Tan H, Brown P (2017) The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease Brain 140:1053–1067 doi: 10.1093/brain/awx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB et al. (2014) High beta activity in the subthalamic nucleus and freezing of gait in Parkinson’s disease Neurobiology of Disease 64:60–65 doi: 10.1016/j.nbd.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Tremblay C, Macoir J, Langlois M, Cantin L, Prud’homme M, Monetta L (2015) The effects of subthalamic deep brain stimulation on metaphor comprehension and language abilities in Parkinson’s disease Brain Lang 141:103–109 doi: 10.1016/j.bandl.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Trost M et al. (2006) Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease Neuroimage 31:301–307 doi: 10.1016/j.neuroimage.2005.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, K OS, Sunde N, Gjedde A, Dupont E, Cumming P (2004) Focal changes of oxygen consumption in cerebral cortex of patients with Parkinson’s disease during subthalamic stimulation Neuroimage 22:966–974 doi: 10.1016/j.neuroimage.2004.02.019 [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM (2004) Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease Brain : a journal of neurology 127:491–504 doi: 10.1093/brain/awh057 [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S, Haq IU, Hwynn N, Oyama G, Okun M, Tillman MD, Hass CJ (2015) Low-frequency Versus High-frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study Brain Stimulation 8:64–75 doi: 10.1016/j.brs.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2009) Models of Response Inhibition in the Stop-Signal and Stop-Change Paradigms Neuroscience and biobehavioral reviews 33:647–661 doi: 10.1016/j.neubiorev.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen Metman L, Pal G, Slavin K (2016) Surgical Treatment of Parkinson’s Disease Current Treatment Options in Neurology 18:49 doi: 10.1007/s11940-016-0432-3 [DOI] [PubMed] [Google Scholar]
- Vidailhet M et al. (2007) Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study Lancet Neurol 6:223–229 doi: 10.1016/S1474-4422(07)70035-2 [DOI] [PubMed] [Google Scholar]
- Weinberger M et al. (2006) Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease J Neurophysiol 96:3248–3256 doi: 10.1152/jn.00697.2006 [DOI] [PubMed] [Google Scholar]
- Welter M-L et al. (2014) Optimal target localization for subthalamic stimulation in patients with Parkinson disease Neurology 82:1352–1361 doi: 10.1212/WNL.0000000000000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H (2012) High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease Front Hum Neurosci 6:155 doi: 10.3389/fnhum.2012.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T (2019) Changing views of the pathophysiology of Parkinsonism Mov Disord 34:1130–1143 doi: 10.1002/mds.27741 [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR (1999) Oscillations in the basal ganglia Nature 400:621–622 doi: 10.1038/23148 [DOI] [PubMed] [Google Scholar]
- Williams AE, Arzola GM, Strutt AM, Simpson R, Jankovic J, York MK (2011) Cognitive outcome and reliable change indices two years following bilateral subthalamic nucleus deep brain stimulation Parkinsonism & Related Disorders 17:321–327 doi: 10.1016/j.parkreldis.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IA, Wilkinson L, Limousin P, Jahanshahi M (2015) Load-Dependent Interference of Deep Brain Stimulation of the Subthalamic Nucleus with Switching from Automatic to Controlled Processing During Random Number Generation in Parkinson’s Disease Journal of Parkinson’s Disease 5:321–331 doi: 10.3233/JPD-140355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K et al. (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study Lancet Neurol 7:605–614 doi: 10.1016/S1474-4422(08)70114-5 [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P (2004) Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease Archives of Neurology 61:697–700 doi: 10.1001/archneur.61.5.697 [DOI] [PubMed] [Google Scholar]
- Xie T, Padmanaban M, Bloom L, Maccracken E, Bertacchi B, Dachman A, Warnke P (2017) Effect of low versus high frequency stimulation on freezing of gait and other axial symptoms in Parkinson patients with bilateral STN DBS: a mini-review Translational Neurodegeneration 6 doi: 10.1186/s40035-017-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T et al. (2015) Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD Neurology 84:415–420 doi: 10.1212/WNL.0000000000001184 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, Jankovic J (2008) Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease Journal of Neurology, Neurosurgery, and Psychiatry 79:789–795 doi: 10.1136/jnnp.2007.118786 [DOI] [PubMed] [Google Scholar]
- York MK, Wilde EA, Simpson R, Jankovic J (2009) Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location Journal of the Neurological Sciences 287:159–171 doi: 10.1016/j.jns.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangaglia R et al. (2009) Deep brain stimulation and cognitive functions in Parkinson’s disease: A three-year controlled study Mov Disord 24:1621–1628 doi: 10.1002/mds.22603 [DOI] [PubMed] [Google Scholar]


