Abstract
Chronic low back pain (cLBP) is a prevalent disorder. A growing body of evidence linking the pathology of the reward network to chronic pain suggests that pain sensitization may contribute to cLBP chronification via disruptions of mesocortical and mesolimbic circuits in the reward system. Resting-state (RS) functional magnetic resonance imaging (fMRI) data was acquired from 90 patients with cLBP and 74 matched pain-free controls (HCs) at baseline and after a manipulation for back pain intensification. The ventral tegmental area (VTA) was chosen as a seed region to perform RS functional connectivity (FC) analysis. Baseline rsFC of both the mesocortical (between the VTA and bilateral rostral anterior cingulate cortex (rACC) / and medial prefrontal cortex (mPFC)) and mesolimbic (between the VTA and bilateral hippocampus/parahippocampus) pathways was reduced in patients with cLBP (vs. HCs). In addition, patients exhibiting higher back pain intensity (compared to the relatively lower back pain intensity condition) also showed increases in both mesocortical and mesolimbic connectivity, implicating these pathways in pain downregulation in cLBP. Mediation analysis further isolated the mesolimbic (VTA-hippocampus/ parahippocampus) dysconnectivity as a neural mechanism mediating the association between mechanical pain sensitivity (indexed by P40 pressure) and cLBP severity. In sum, the current study demonstrates deficient mesocorticolimbic connectivity in cLBP, with the mesolimbic dysconnectivity potentially mediating the contribution of pain sensitization to pain chronification. These reward network dysfunctions and purportedly, dopaminergic dysregulations, may help us to identify key brain targets of neuromodulation in the treatment of cLBP.
Keywords: low back pain, central sensitization, pain sensitivity, quantitative sensory testing, mesocorticolimbic network, reward network, functional connectivity, ventral tegmental area
1. Introduction
Chronic low back pain (cLBP) poses a major health burden, with 50% to 85% of all people complaining of back pain at some time in their life (Andersson, 1999). The socioeconomic impact of cLBP is comparable to depression, heart disease, diabetes, and cancer (Dagenais et al., 2008; Maniadakis and Gray, 2000). In spite of such high prevalence and social burden, the pathophysiology of cLBP remains obscure, and treatment of cLBP is far from satisfactory.
Although still under investigation, literature suggests that central sensitization, which is defined as “increased responsiveness of nociceptive neurons in the central nervous system to their normal or sub-threshold afferent input”, may be a key mechanism of chronic pain (Gold and Gebhart, 2010; Latremoliere and Woolf, 2009; Woolf, 2011). For instance, using quantitative sensory testing (QST) assessments, investigators have found widespread hyperalgesia at the back, and peripheral sites of chronic back pain patients have been reported using pressure (Clauw et al., 1999; Giesecke et al., 2004; Meints et al., 2019), electrical (Flor et al., 2004), and heat stimuli (Kleinbohl et al., 1999). Such intense and long-lasting nociceptive barrage can give rise to persistent central sensitization (Price, 1991), facilitating the onset of “chronic pain” (Coderre et al., 1993). However, it remains unclear what neural mechanisms underlie central sensitization in cLBP.
In parallel, the reward system and its anomalies have been increasingly linked to the pathophysiology of chronic pain (Baliki et al., 2010; Baliki et al., 2012; Borsook et al., 2016; Navratilova and Porreca, 2014; Porreca and Navratilova, 2017). Relief from pain can be viewed as a form of reward, whereas exacerbation of pain can be viewed as greater distance from reward (Benedetti et al., 2013). It has been postulated that the systems of pain and reward are deeply intertwined to the extent that pain and reward can be considered opposing processes (Becker et al., 2012) with shared neurobiological mechanisms (Leknes and Tracey, 2008). In support of this reward hypothesis of central pain sensitization, studies have found extensive similarities between the substrates of painful sensations and those of pleasant sensations in the reward network (Martikainen et al., 2015; Schweinhardt et al., 2009) and that analgesia can activate the same mesocorticolimbic reward network that is activated by typical rewards, such as food, money, and drugs (Kalivas et al., 1999; Kender et al., 2008).
Since the dopaminergic system plays a critical role in reward (Haber and Knutson, 2010), key regions of this system have been the focus of research into reward functioning in pain (Baliki et al., 2010; Lammel et al., 2011; Vachon-Presseau et al., 2016). The VTA is the primary origin of the dopamine system, innervating widespread limbic and cortical targets via dopaminergic projections known as the mesolimbic and mesocortical pathways (Morales and Margolis, 2017; Subramaniam and Roeper, 2017). Manipulation of these dopaminergic pathways has been shown to reliably modulate the affective component of pain and motivated behavioral responses to pain relief (Navratilova et al., 2012). In addition, in chronic pain including cLBP, aberrant activity and functional connectivity involving cortical and limbic targets of the VTA (e.g., anterior cingulate cortex, medial prefrontal cortex, and hippocampus) have been repeatedly identified (Egorova et al., 2015; Gondo et al., 2012; Li et al., 2017; Mutso et al., 2014; Ploghaus et al., 2001; Tu et al., 2019; Zhang et al., 2019). Therefore, it is likely that these mesolimbic and mesocortical pathways are intimately associated with the pathophysiology of cLBP and central sensitization.
This study thus aims to test the hypothesis that 1) alterations in mesocortical and mesolimbic functional connectivity are involved in the pathophysiology and neural underpinnings of cLBP, and 2) mesocortical and mesolimbic functional connectivity may mediate the association between central sensitization (as indicated by increased pain sensitivity) and LBP severity. Specifically, we first compared VTA functional connectivity between healthy controls (HCs) and a relatively large cohort of cLBP patients to isolate VTA circuitry pathology related to chronic pain. Then, by applying pain-exacerbating maneuvers to induce pain intensification, we established the involvement of these pathways in cLBP pathology by demonstrating alterations (or lack thereof) in VTA resting-state functional connectivity. Finally, we applied a mediation model to directly test the hypothesis that aberrant mesocorticolimbic connectivity mediates increased mechanical pain sensitivity in cLBP patients. In a previous behavioral study (Meints et al., 2019), we applied quantitative sensory testing methods to investigate pain sensitization in patients with cLBP (N = 167) and pain-free controls (N = 33) (most cLBP patients and pain-free controls in this study were included in the published study). We found that cLBP patients had increased sensitivity to mechanical (deep-tissue) pressure stimuli and more lingering pain afterwards compared to pain-free controls. In addition, we found that P40 score (the pressure at which moderate pain, rated 40/100, is induced) is associated with pain bothersomeness in cLBP patients. We thus decided to perform a mediation analysis to test the hypothesis that aberrant mesocorticolimbic connectivity mediates the contribution of P40 score to cLBP severity.
2. Materials and Methods
2.1. Participants
This study was approved by the Institutional Review Board at Massachusetts General Hospital, and all subjects signed informed consent forms. Nonspecific cLBP patients (Balague et al., 2012) and age- and gender-matched pain-free HCs were recruited in this study.
Inclusion criteria: 1) 20–50 years old, 2) Presence of nonspecific cLBP for a duration of at least 6 months with the condition established by a clinical evaluation, including the use of X-ray/MRI reports, when available, 3) Pain intensity averaging at least 4 on a 0–10 visual analog scale (VAS). Exclusion criteria: 1) Specific causes of back pain (e.g., cancer-related back pain, post-surgical and traumatic back pain, neuropathic back pain, rheumatoid arthritis, widespread pain such as fibromyalgia), 2) Complicated back problems (e.g., prior back surgery, medicolegal issues), 3) Major systemic and/or psychiatric diseases or history of head injury or coma, 4) Presence of any contraindications to MRI scanning, 5) History of substance abuse or dependence.
The dataset has been used to explore the alteration in amplitude of low-frequency fluctuation (Zhang et al., 2019) and functional connectivity of the occipital cortex in patients with chronic low back pain (Shen et al., 2019). This study aims to explore alterations in mesocorticolimbic connectivity using seed-based functional connectivity methods in patients with cLBP, which has not been previously published.
2.2. Clinical assessment
Pain Bothersomeness Scale (Cherkin et al., 2009; Deyo et al., 1998; Yuan et al., 2008):
This is a self-reported measure of cLBP pain severity that is commonly used to assess clinical chronic pain. Participants rated how bothersome their LBP was in the previous week with a VAS scale (0–10) from “not at all bothersome” (0) to “extremely bothersome” (10).
Current low back pain rating:
Participants also rated the intensity of their current pain intensity using a 1–100 VAS (from “no pain” to “worst pain imaginable”). This rating was used to measure acute changes in low back pain intensity following our pain-exacerbating maneuver described below. Specifically, ratings were acquired right before and after each resting state fMRI scan and were averaged to index current pain intensity for each resting scan.
Pain sensitization:
We applied a quantitative sensory testing (QST) method to assess pain sensitization. We focused on P40 pressure, a reliable (inverted) index of deep-tissue hyperalgesia (Meints et al., 2019). P40 pressure refers to the pressure at which moderate pain, rated 40/100, is induced. Therefore, lower P40 pressure indicates higher pain sensitization. This index was obtained with cuff pressure algometry (CPA), which determines an individual’s responses to deep pressure pain using a Hokanson rapid cuff inflator (Wey et al., 2014). Cuff stimulation was applied to the left gastrocnemius muscle and a limits procedure was used to determine the P40 pressure. This pressure was maintained for a duration of 2 minutes, during which participants provided verbal ratings of pain and unpleasantness every 30 seconds as well as fifteen seconds after cuff deflation (Meints et al., 2019).
2.3. Experimental procedures
Patients with cLBP underwent two MRI scan sessions. The first MRI session included a dimensional structural T1-weighted MRI and a resting state fMRI (RS-fMRI) scan. After this session, subjects exited the scanner and performed maneuvers to increase their LBP by about 30%. The maneuvers included lumbar flexion, extension, or rotation, which were tailored to each subject based on what they reported would exacerbate LBP. Maneuvers were not performed if subjects were reluctant to increase their pain or if their pain was already very strong (greater than 70 on a 0–100 VAS)(Kong et al., 2013; Lee et al., 2018). After the maneuvers, which took around 15 minutes, subjects re-entered the scanner for the second (identical) RS-fMRI scan.
2.4. MRI data acquisition
The fMRI brain-imaging data was acquired with a 3T Siemens whole-body scanner using a 32-channel radio-frequency head coil at the Martinos Center for Biomedical Imaging. T2*-weighted functional images encompassing the whole brain were acquired with the gradient-echo EPI sequence (echo time: 30 ms, repetition time: 3,000 ms, flip angle: 90°, slice thickness: 2.6 mm, 44 slices, voxel size: 2.62×2.62×3.12 mm3, field of view: 220×220 mm2, matrix: 84×84 mm2, slice orientation: axial, order of slice accession: interleaved). During the 6-minute resting state fMRI scan, subjects were asked to keep their eyes open and blink normally. High-resolution brain structural images were also acquired with a T1-weighted three-dimensional multi-echo magnetization-prepared rapid gradient-echo (MPRAGE) sequence (repetition time: 2,530 ms, echo time: 1.69 ms, slice thickness 1 mm, flip angle: 7°, 176 sagittal slices covering the whole brain, voxel size: 1×1×1 mm3, field of view: 256×256 mm2, matrix: 256×256 mm2, inversion time: 1100 ms).
2.5. fMRI data processing and analysis
The fMRI data was preprocessed and analyzed using CONN toolbox version 18a (http://www.nitrc.org/projects/conn) in MATLAB (Math Works, Inc., Natick, MA, USA). We used the default preprocessing pipeline for volume-based analysis (direct normalization to MNI-space). The specific steps were as follows: slice timing correction; head motion correction; skull-stripping using BET; co-registration of the anatomical image to the mean functional image; segmentation of the anatomical gray matter, white matter, and CSF; normalization to MNI152 standard template; and smoothing with a 6-mm Gaussian kernel. Band-pass filtering was performed with a frequency band of 0.008–0.09 Hz.
To eliminate head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART (http://www.nitrc.org/projects/artifactdetect). For each subject, we treated images (time points) as outliers if composite movement from a preceding image exceeded 0.5 mm, or if the global mean intensity was greater than 3 standard deviations from the mean image intensity for the entire resting scan. Outliers were included as regressors in the first-level general linear model along with motion parameters. In addition, to investigate the effect of head motion during the resting state scans, mean framewise displacement (FD)(Power et al., 2012) was calculated for each participant.
No significant difference in head movement was found between the cLBP patients and HCs (p = 0.58; cLBP: 0.039 ± 0.025; HC: 0.037 ± 0.025; two sample t-test). We also did not observe a significant difference between low pain and high pain conditions (p = 0.08; LP: 0.038 ± 0.025; HP: 0.044 ± 0.028; paired t-test).
2.6. Functional connectivity (FC) analysis
The VTA was our a priori seed for the seed-based connectivity analysis. We derived the bilateral VTA ROI from a probabilistic atlas of the dopaminergic system (Murty et al., 2014). This seed has also been used in a previous FC study on chronic pain (Liu et al., 2019). First-level correlation maps were produced by extracting the residual BOLD timeseries from the VTA seed and correlating that with the timeseries of all other voxels in the brain. The obtained (Pearson’s) correlation coefficients (i.e., FC values) were further normalized into Z scores using Fisher transformation.
A whole-brain paired t-test was conducted to compare VTA FC at “low pain” and “high pain” conditions as indicated by low back pain intensity ratings during the fMRI scan and before and after the pain-exacerbating maneuvers. In addition, whole-brain Analyses of Covariance (ANCOVAs) of Group were conducted to compare VTA FC between the LBP and HC groups, with age and gender entered as covariates. Thresholds of voxel-wise p < 0.005 (uncorrected) and cluster-level p < 0.05 false discovery rate (FDR) correction were applied for whole brain analysis. Furthermore, conjunction analysis was used to identify any common VTA FC alterations in cLBP and different conditions.
2.7. Regions of Interest (ROIs)
Guided by our previous findings concerning cLBP (Li et al., 2017; Yu et al., 2014; Zhang et al., 2019), we focused on a set of pain-related ROIs, including cortical structures of the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) and subcortical limbic structures of the amygdala, hippocampus, and nucleus accumbens. The ROIs were defined using the Anatomical Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). To correct for multiple comparisons, Monte Carlo simulations using 3dFWHMx and 3dClustSim ([https://afni.nimh.nih.gov] released in July 2017) were applied to the ROIs.
2.8. Mediation analysis
In this study, we detected a significant association between P40 measurements, a pain sensitivity measurement, and LBP severity (pain bothersomeness) in cLBP patients (see Results section for details). We then performed mediation analysis to examine VTA FC as a potential mediator of this relationship, provided that a direct association between them was confirmed.
We used a simple mediation model from PROCESS Macro in SPSS for the mediation analysis (model 4) (Hayes, 2013). It is based on 10,000 bootstrap samples for a bias-corrected bootstrap confidence interval (CI). The indirect effect is considered significant when the 95% CI does not include zero (with a null hypothesis that there is no indirect effect). Three-step regression models were constructed as follows:
In these models, X is the independent variable (P40 score), Y is the dependent variable (bothersomeness), and M is the mediator (VTA FC with each brain region showing group differences between cLBP patients and HCs). Each variable was entered separately. U1 and U2 are gender and age, respectively. The direct effect is the effect of X on Y, independent of the effect of M on Y (path c’). The direct effect between X and Y is not a necessary prerequisite for mediation (Hayes, 2009). The indirect effect, or the effect of X on Y via M, is estimated as the product of the effect of X on M and the effect of M on Y, controlling for X (ab with 95% bootstrap CI). The total effect of X on Y is the sum of the direct and indirect effects (path c) (Figure 2).
Figure 2.
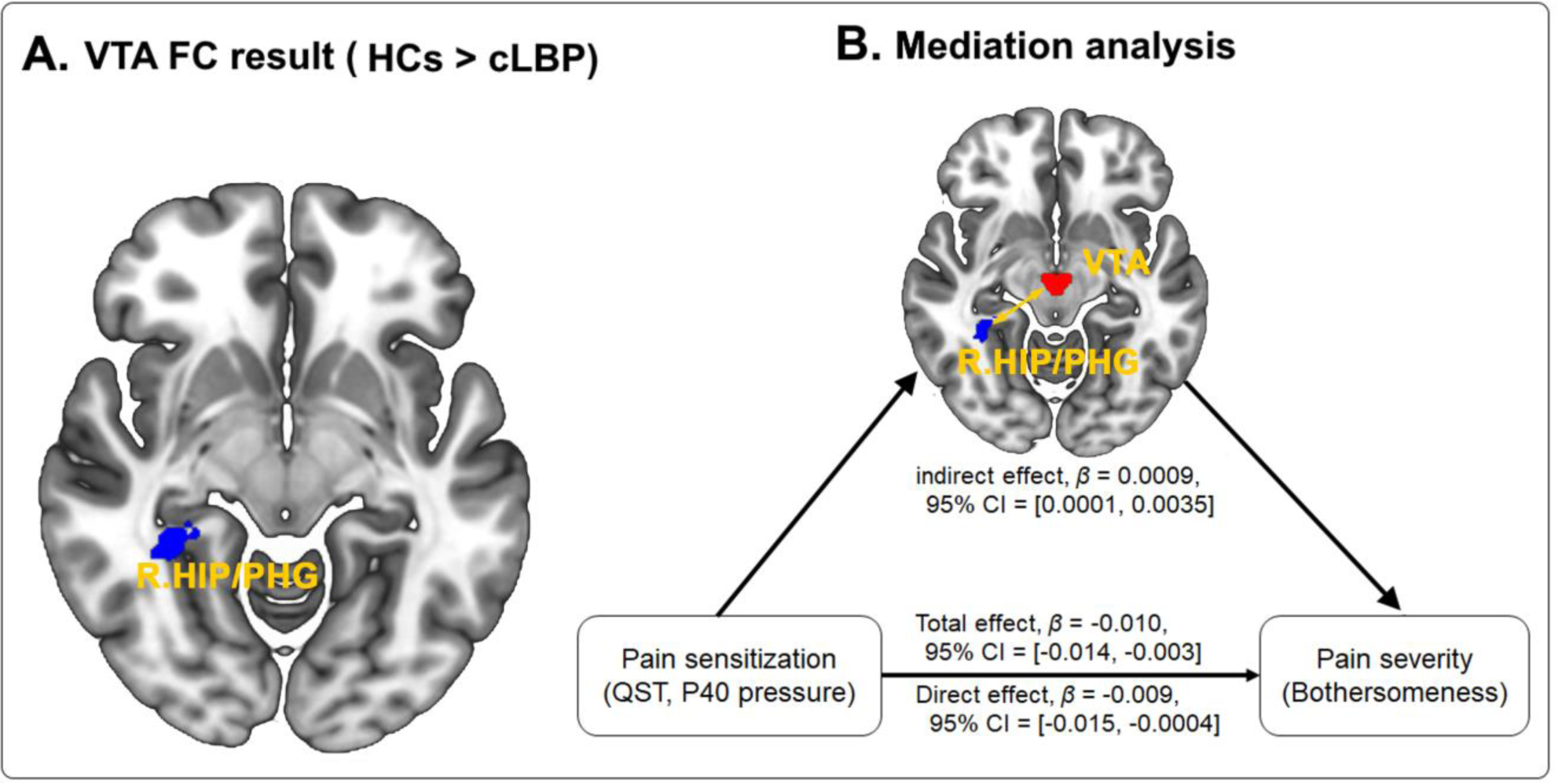
A. Group comparison between cLBP patients and HCs. Compared to healthy controls, cLBP patients had significantly lower FC in the right HIP/PHG. B. Mediation effects of VTA-HIP/PHG functional connectivity on the association between pain sensitization (P40 pressure) and pain severity (bothersomness) in cLBP patients. Abbreviations: cLBP, chronic low back pain; FC, functional connectivity; HC, healthy control; HIP, hippocampus; PHG, parahippocampus; QST, quantitative sensory testing; VTA, ventral tegmental area.
3. Results
3.1. Demographic data
Ninety nonspecific cLBP patients and 74 age/gender-matched HCs were recruited for this study. The demographic data and pain-related parameters for cLBP and HC subjects are presented in Table 1. Patients’ bothersomeness scores in the past week and duration of cLBP were 5.06 ± 1.88 (mean ± SD) and 6.94 ± 6.20 years, respectively.
Table 1.
Demographic and clinical traits for all participants (mean ± SD)
| Characteristic | cLBP (n=90) | HCs (n=74) | T or x2 | p value |
|---|---|---|---|---|
| Age | 34.46±8.97 | 32.44±8.38 | 1.47 | 0.14 |
| Gender (n, male/female) | 38/52 | 31/43 | 0.97 | 0.55†
|
| BDI | 6.12±6.00 | - | - | - |
| QST P40 pressure (mmHg)a | 171.09±68.09 | 201.94±79.81 | −2.02 | 0.04 |
| Pain bothersomenessb | 5.06±1.88 | - | - | - |
| Pain intensity (low pain condition)c | 31.33±20.02 | - | - | - |
| Pain intensity (high pain condition)c | 51.83±22.21 | - | - | - |
, the p value was obtained by a chi-square test; other p values were obtained by a two-sample t-test.
, the number of cLBP subjects was 80, and the number of HCs subjects was 33.
, pain bothersomeness: low back pain bothersomeness during the last week.
, pain intensity of 84 cLBP subjects whose pain intensity increased in the post-maneuver scan; the pain intensity of the patients in the high pain condition was significantly higher than that of patients in the low pain condition (p<0.001).
Abbreviations: BDI, Beck Depression Inventory; QST, Quantitative Sensory Testing; cLBP, chronic low back pain; HC, healthy control.
Of all patients and controls, the P40 assessments were available on 80 cLBP patients and 33 pain-free controls. A comparison of P40 scores between the two groups showed that the P40 pressure score was significantly lower in the cLBP group than in the HC group (P = 0.04).
3.2. Pain rating changes following pain-exacerbating maneuvers
Of the 90 patients who completed the study, the average pain rating at baseline (before maneuver) was 31.65 ± 20.07 (mean ± SD). 76 patients showed increased LBP following the pain-exacerbating maneuver (55.23 ± 19.56, mean ± SD). For these patients, the first RS scan was defined as the “low pain” (LP) condition and the second RS scan the “high pain” (HP) condition. Eight patients reported a reduction in low back pain during the second versus the first RS-fMRI scan due to 1) a starting pain rating over 70 (on the 0–100 VAS) that precluded the maneuver, 2) their reluctance to perform the maneuver, or 3) a reduction of pain during the second scan after the maneuver. Since the aim of this study was to explore VTA changes when back pain increased (as compared to a low pain intensity condition), we chose to include these eight patients by assigning their second scan as the LP and first scan as the HP condition to be consistent with their actual pain levels. Excluding these patients did not change the results of the analyses. Finally, six patients who reported the same pain level during the first and second RS-fMRI scans were excluded from comparisons between the HP and LP conditions. Overall, self-reported pain intensity for the LP condition (31.33 ± 20.02, mean ± SD) was significantly lower than that of the HP condition (51.83 ± 22.21, mean ± SD,) (p < 0.001).
3.3. VTA-based FC amplification due to pain intensity increase
We first established the involvement of VTA pathways in cLBP pathophysiology by examining perturbations in these pathways in response to increased pain intensity. Comparing the LP and HP conditions in patients with cLBP, we observed increased VTA FC at the bilateral mPFC, bilateral rACC, and left hippocampus in the HP condition (Table 2 and Figure 1A). These results suggest that in cLBP, the mesocortical and mesolimbic pathways remain somewhat responsive to phasic increases in back pain.
Table 2.
Results derived from whole brain and ROI VTA FC analysis
| Contrast | Brain regions | Cluster size (voxels) | MNI coordinates (x, y, z) | Peak Z- value |
|---|---|---|---|---|
| HCs > cLBP | Bilateral rACC | 189 | −4,34,−2 | 4.25 |
| Bilateral mPFC | 125 | 4,62,0 | 3.75 | |
| Left HIP* | 41 | −26,−38,−2 | 3.78 | |
| Right HIP/PHG | 132 | 34,−42,−4 | 4.73 | |
| HCs < cLBP | No region above threshold | |||
| HP > LP | Bilateral mPFC* | 80 | −2,62,2 | 3.42 |
| Bilateral rACC* | 119 | 2,40,−4 | 3.44 | |
| Left HIP* | 11 | −34,−26,−14 | 3.15 | |
| HP < LP | No region above threshold | |||
, results were significant at cluster P < 0.05 after 3dFWHMx and 3dClustSim correction. Other results were significant at cluster PFDR< 0.05 corrected at the whole brain level.
Abbreviations: cLBP, chronic low back pain; FC, functional connectivity; HC, healthy control; HP, high pain; LP, low pain; mPFC, medial prefrontal cortex; HIP, hippocampus; PHG, parahippocampus; rACC, rostral anterior cingulate cortex; VTA, ventral tegmental area.
Figure 1.
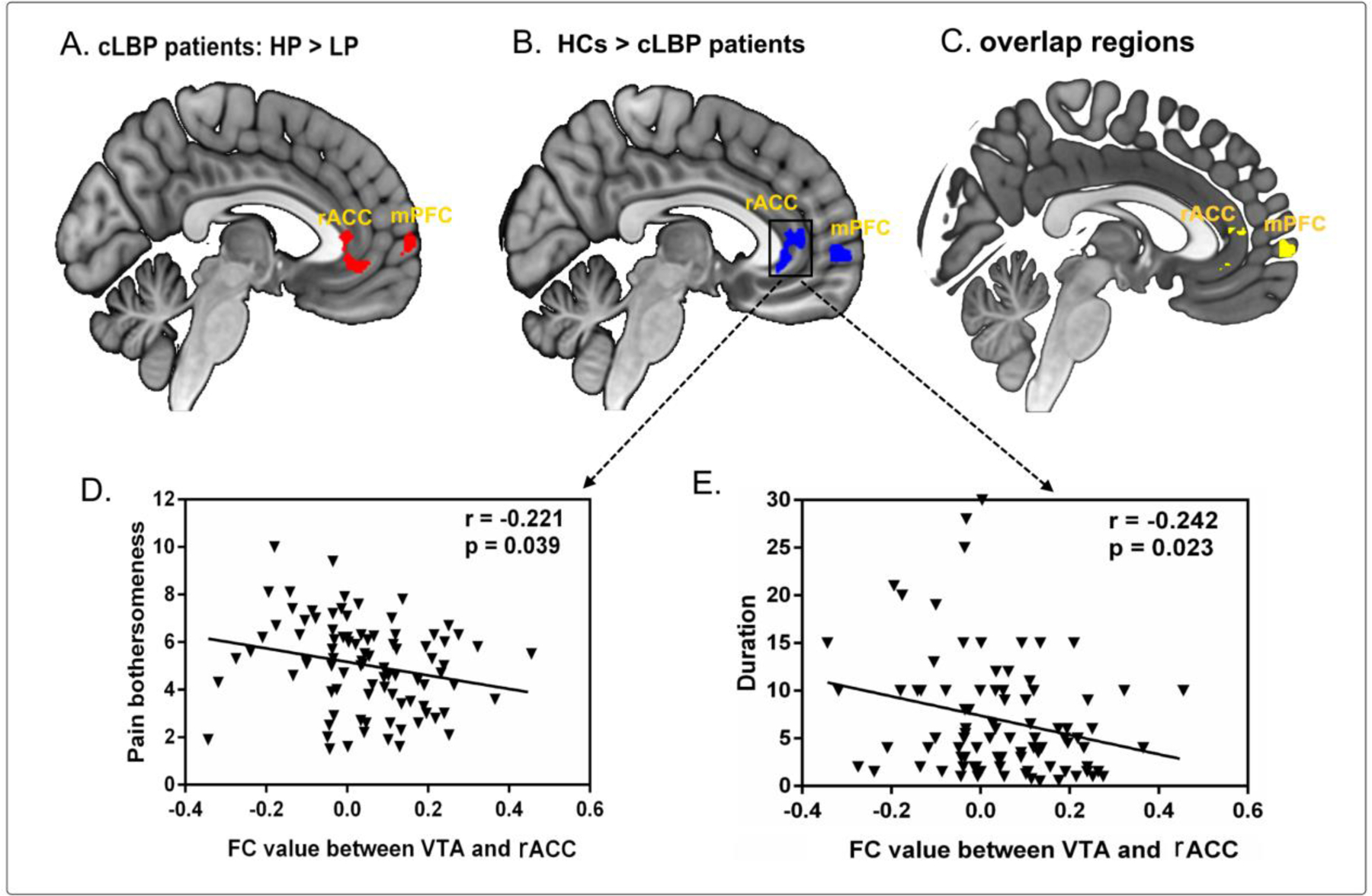
A. Group comparison between cLBP patients with high and low pain. cLBP patients in the HP condition had increased FC in the bilateral mPFC and rACC compared to cLBP patients in the LP condition. B. Group comparison between cLBP patients and HCs. Compared to healthy controls, cLBP patients had significantly lower FC in the bilateral rACC and mPFC. C. Overlap between the two analyses at the bilateral rACC and mPFC. D. Reduced FC value between the VTA and rACC was negatively associated with increased bothersomeness score (left column) and duration (right column) in cLBP patients, controlled for age and gender. Abbreviations: cLBP, chronic low back pain; FC, functional connectivity; HC, healthy control; HP, high pain; LP, low pain; mPFC, medial prefrontal cortex; rACC, rostral anterior cingulate cortex; VTA, ventral tegmental area.
3.4. VTA-based FC alterations in patients with cLBP
We then examined the difference in VTA pathways between cLBP patients and HCs to test the hypothesis of mesocortocolimbic circuitry pathology in cLBP. A comparison between the two groups (at the baseline RS scan) showed that VTA-based FC was significantly lower in cLBP patients at the bilateral mPFC, bilateral rACC, and bilateral HIP/parahippocampus (PHG) (Figure 1B, Table 2). By contrast, there was no significant VTA FC increase in patients with cLBP. Notably, these clusters in the mPFC and rACC highly overlapped with those identified in the cLBP HP-LP comparison (Figure 1C), emphasizing a paradoxical and potentially maladaptive tonic dysconnectivity of the VTA mesocortical and mesolimbic pathways that could underlie the pathophysiology of low back pain.
Findings from our previous studies suggest that the rACC plays a prominent role in the pathophysiology of chronic pain. Resting state rACC oscillations are positively correlated with pain severity in patients with cLBP (Zhang et al., 2019), and decreased rACC volume is associated with protracted duration of chronic pain (Jensen et al., 2013). We therefore explored the association between VTA-rACC FC and cLBP duration and severity. We found that VTA-rACC FC strength was negatively correlated with cLBP pain severity (pain bothersomeness; r = −0.22, p = 0.039 FDR corrected) in the previous week (Figure 1D) but not back pain intensity during the MRI scan. Furthermore, this VTA-rACC FC was also negatively correlated with cLBP duration (r = −0.24, p = 0.039 FDR corrected) (Figure 1E), highlighting its involvement in cLBP chronification.
3.5. Reduced VTA-HIP/PHG FC mediated the association between pain sensitization and cLBP severity
Correlation analysis between pain sensitization and cLBP severity confirmed their association as reported previously by our group (Meints et al., 2019) using a larger sample size (167 cLBP patients and 33 controls). Specifically, we found that LBP severity (bothersomeness in the past week) was negatively associated with pain sensitization (P40 pressure; r = −0.329, p = 0.003).
We then tested the hypothesized mediation effect of VTA-based FC on the association between pain sensitization and cLBP severity. Mediation analysis revealed a significant indirect effect of VTA-HIP/PHG FC (β = 0.0009, 95% CI = 0.0001, 0.0035) on the relationship between sensitization and pain severity in patients with cLBP (Figure 2), indicating that VTA-HIP/PHG connectivity mediated the effects of sensitization on pain bothersomeness. No mediation effects were found for VTA FC with other ROIs (rACC and mPFC).
4. Discussion
In a large sample of patients with cLBP and matched healthy controls, we observed that patients with cLBP had reduced intrinsic functional connectivity between the VTA and its cortical and limbic targets, including the bilateral mPFC, rACC, and medial temporal lobe (HIP/PHG). We further confirmed a strong association between pain sensitization (P40 pressure) and clinical pain severity (pain bothersomeness in the past week) among patients, and importantly, demonstrated that this association was mediated by reduced VTA-right-hippocampus connectivity. Critically, mesocortical and mesolimbic pathways were strengthened when low back pain intensity was higher (compared to the relatively lower back pain intensity condition). These findings thus implicate mesocorticolimbic dysconnectivity in the pathophysiology of cLBP and highlight deficient VTA-HIP/PHG connectivity as a neural underpinning for the contribution of pain sensitization in patients with chronic low back pain.
The mesocorticolimbic pathways are known to mediate reward processing. Here, we found that increased back pain intensity induced a phasic increase in mesocortical connectivity, lending further credence to the notion that the pain and reward systems share these dopaminergic VTA pathways. The rACC, a structure repeatedly identified in cLBP (Baliki et al., 2012; Jensen et al., 2013; Yu et al., 2014; Zhang et al., 2019), is densely populated with opioid receptors (Vogt et al., 2001) and is known to play a key role in the descending pain modulatory system (DPMS) (Fields, 2004; Kong et al., 2009; Kong et al., 2018; Kong et al., 2019; Li et al., 2016), which initiates the release of endogenous opioids and inhibits nociceptive signaling from the periphery (Cheriyan and Sheets, 2018; Fields, 2004).
Although verification in human trials is still needed, investigators on a rodent study (Chen et al., 2018) found that selective activation of ACC-spinal cord projecting neurons caused behavioral pain sensitization, while inhibition induced analgesic effects. As such, the VTA-rACC pathway can efficiently execute phasic analgesic modulation and motivate pursuit of pain relief (Elston and Bilkey, 2017; Vogt et al., 2001). Therefore, we speculate that when back pain intensity increases (compared to the relatively lower intensity back pain condition), the increased VTA connectivity with the rACC /mPFC may upregulate activity in these prefrontal cortices to blunt pain responses via descending analgesic signal transmission.
Interestingly, at baseline (i.e., a tonic state), patients with cLBP exhibited reduced connectivity in VTA-rACC and VTA-mPFC pathways compared to HCs. These prefrontal cortical regions were identified in both analyses, attesting to their functional relevance to low back pain processing. It is also worth noting that similar pattern changes have also been detected in primary somatosensory cortex (S1) functional connectivity in cLBP patients (i.e., S1 functional connectivity decreased when cLBP patients experienced low intensity LBP as compared with healthy controls, and S1 functional connectivity increased when cLBP patients experienced high intensity LBP as compared with the low intensity condition)(Kong et al., 2013).
This paradoxical deficit in baseline connectivity in cLBP suggests that cortical pain modulatory processes can break down due to overcompensation in response to persistent pain throughout the course of the development of chronic pain. In keeping with this notion, cLBP patients with phasic low back pain increase exhibited greater reduction in baseline VTA-rACC connectivity, and longer duration of cLBP was coupled with greater reduction in connectivity. This idea of maladaptive pain modulation via the rACC and mPFC in cLBP aligns with a previous report that the rACC and mPFC are recruited into the pain network as low back pain becomes chronic (Hashmi et al., 2013). The VTA-rACC pathway has also been found to be deficient in other chronic conditions (such as anhedonia; (Wacker et al., 2009). Future study is needed to provide a better interpretation of the finding.
Patients with cLBP also showed decreased functional connectivity of the mesolimbic (VTA-HIP/PHG) pathway. The hippocampus is a key region in learning and memory, and it has been previously identified in fMRI studies on pain and anxiety. Studies have also shown that it plays an important role in anxiety of pain (Ploghaus et al., 2001) and the nocebo effect (Kong et al., 2008). Chronic pain has been conceptualized as a type of long-term learning (Apkarian et al., 2009). A previous study found that processing reorganization within the hippocampus and between the hippocampus and cortex may contribute to the transition from subacute to chronic pain and may also underlie learning and emotional abnormalities associated with chronic pain (Mutso et al., 2014).
Mesolimbic circuits involving the hippocampus have been associated with pain relief and pain-induced analgesia (Gear et al., 1999; Gondo et al., 2012; Navratilova et al., 2012), and the reduced VTA-hippocampus connectivity in cLBP corroborates the notion that altered hippocampal functional connectivity contributes to the development of chronic back pain (Egorova et al., 2015; Gondo et al., 2012; Mutso et al., 2014; Ploghaus et al., 2001). Importantly, we found that this VTA-HIP/PHG connectivity reduction mediated the association between P40 and cLBP symptom severity, suggesting that individuals with weak VTA-HIP/PHG FC showed strong contribution of pain sensitization to cLBP severity.
This mediation effect of VTA-HIP/PHG dysconnectivity may support the stress model of chronic pain, which implicates stress-related alterations of hippocampal functioning in pain sensitization (Gondo et al., 2012; Kong et al., 2008; Ploghaus et al., 2001) and chronification (Vachon-Presseau et al., 2013). According to this model, anxiety and stress can affect hippocampus connectivity, including VTA-hippocampus connectivity (Marusak et al., 2017), thereby heightening pain sensitization by priming aversive responses to pain stimulation and impairing inhibitory modulation of midbrain activity to suppress fear responses. As a result, pain outcomes worsen, increasing clinical symptoms and precipitating the onset of chronic pain (Gondo et al., 2012; Kong et al., 2008; Ploghaus et al., 2001).
While both mesocortical (involving the rACC and mPFC) and mesolimbic (involving the hippocampus) pathways are disrupted in cLBP, current results suggest that dysfunctions in the mesolimbic pathway could be even more severe and consequential (i.e., connectivity enhancement following pain intensification). Second, the mediation effect between pain sensitization and cLBP severity was observed for VTA-HIP/PHG (but not mesocortical) dysconnectivity, suggesting a critical role of this mesolimbic pathology in pain sensitization in cLBP. These two consequences of VTA-HIP/PHG dysconnectivity, whether additive (parallel) or supra-additive (interactive), accentuate mesolimbic dysconnectivity in the chronification of cLBP. However, it is worth noting that this difference between mesocortical and mesolimbic pathways is likely quantitative as opposed to qualitative. In fact, a post hoc analysis indicated that reductions in VTA-HIP/PHG and VTA-rACC connectivity were somewhat correlated (r = .15, p = .046, one-tailed), suggesting a considerable degree of shared pathology in these circuits.
There are several limitations to this study. First, it is a cross-sectional (vs. longitudinal) design and thus cannot reveal causal relationships in the etiology and maintenance of cLBP. Second, the connectivity analysis concerns inter-regional functional coupling as opposed to directional, causal effects between regions. Thirdly, we did not collect data such as depression scores in healthy subjects, which could confound our correlation analysis. Nonetheless, as the average BDI score in our study is quite low (with an average score of 6.12 ± 6.0), less than mild depression (score > 14) as defined by the BDI manual, we believe that this confound is rather minimal. Finally, we cannot completely exclude the potential influence of medication in patients with chronic low back pain. Nevertheless, to avoid the potential influence of opiates, only low-dose opioid use was allowed in this study (less than 10 mg/per day), and only a small portion of participants were taking this medication (see Supplementary Table 1 for details of medication use). We thus do not believe medication is a major confounding factor in this study.
In conclusion, the present study demonstrated VTA-based mesocorticolimbic pathway deficits in patients with cLBP, though both mesocortical and mesolimbic pathways were still somewhat functional as indicated by increased FC (in pain-induced analgesia). This disruption in the mesolimbic (VTA-HIP/PHG) circuit facilitates pain sensitization, thereby perpetuating pain symptoms and promoting the development of chronic back pain. Overall, our findings highlight the importance of the mesocorticolimbic network in the neuropathology of cLBP. Critically, the isolation of mesolimbic dysconnectivity in the central sensitization and chronification of cLBP may help to identify key targets of neuromodulation methods (such as transcranial magnetic stimulation) that could significantly improve the treatment of this highly prevalent and debilitating disorder.
Supplementary Material
Highlight.
Mesocortical and mesolimbic connectivity decreased in cLBP patients compared to controls. Mesocortical and mesolimbic connectivity increased when back pain intensity increased. Mesolimbic connectivity mediated the association between pain sensitivity and cLBP severity.
Acknowledgements
This work is supported by P01 AT006663 to Bruce Rosen and Randy Gollub. Jian Kong is supported by R01 AT008563, R33 AT009310, R33AT009341, R34DA046635, and R21AT008707 from NIH.
Footnotes
Ethics statement
This study was approved by the Institutional Review Board at Massachusetts General Hospital, and all subjects signed informed consent forms. J.K. has a disclosure to report (holding equity in a startup company, MNT, and pending patents to develop new neuromodulation tools) but declares no conflict of interest. All other authors declare no conflicts of interest.
Data and code availability statement
The data and code will be available per request.
References
- Andersson GB, 1999. Epidemiological features of chronic low-back pain. Lancet 354, 581–585. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY, 2009. Towards a theory of chronic pain. Progress in neurobiology 87, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué F, Mannion AF, Pellisé F, Cedraschi C, 2012. Non-specific low back pain. The Lancet 379, 482–491. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV, 2010. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV, 2012. Corticostriatal functional connectivity predicts transition to chronic back pain. Nature neuroscience 15, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P, 2012. Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience Letters 520, 182–187. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C, 2013. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain 154, 361–367. [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman A, Becerra L, Elman I, 2016. Reward deficiency and anti-reward in pain chronification. Neuroscience & Biobehavioral Reviews 68, 282–297. [DOI] [PubMed] [Google Scholar]
- Chen T, Taniguchi W, Chen QY, Tozakisaitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaitosugimura Y, Wang J, 2018. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nature Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, Sheets PL, 2018. Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. Journal of Neuroscience, 2731–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkin DC, Sherman KJ, Avins AL, Erro JH, Ichikawa L, Barlow WE, Delaney K, Hawkes R, Hamilton L, Pressman A, 2009. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Archives of internal medicine 169, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, Kobrine AI, Wiesel SW, 1999. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine 24, 2035. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R, 1993. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 52, 259–285. [DOI] [PubMed] [Google Scholar]
- Dagenais S, Caro J, Haldeman S, 2008. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine Journal Official Journal of the North American Spine Society 8, 8–20. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Battie M, Beurskens A, Bombardier C, Croft P, Koes B, Malmivaara A, Roland M, Von Korff M, Waddell G, 1998. Outcome measures for low back pain research: a proposal for standardized use. Spine 23, 2003–2013. [DOI] [PubMed] [Google Scholar]
- Egorova N, Gollub RL, Kong J, 2015. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin 9, 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston TW, Bilkey DK, 2017. Anterior Cingulate Cortex Modulation of the Ventral Tegmental Area in an Effort Task. Cell Rep 19, 2220–2230. [DOI] [PubMed] [Google Scholar]
- Fields H, 2004. State-dependent opioid control of pain. Nature Reviews Neuroscience 5, 565–575. [DOI] [PubMed] [Google Scholar]
- Flor H, Diers M, Birbaumer N, 2004. Peripheral and electrocortical responses to painful and nonpainful stimulation in chronic pain patients, tension headache patients and healthy controls. Neuroscience letters 361, 147–150. [DOI] [PubMed] [Google Scholar]
- Gear RW, Aley K, Levine JD, 1999. Pain-induced analgesia mediated by mesolimbic reward circuits. Journal of Neuroscience 19, 7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ, 2004. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 50, 613–623. [DOI] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF, 2010. Nociceptor sensitization in pain pathogenesis. Nature medicine 16, 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo M, Moriguchi Y, Kodama N, Sato N, Sudo N, Kubo C, Komaki G, 2012. Daily physical complaints and hippocampal function: an fMRI study of pain modulation by anxiety. Neuroimage 63, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV, 2013. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136, 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2009. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs 76, 408–420. [Google Scholar]
- Hayes AF, 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. The Guilford Press, New York. [Google Scholar]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, 2013. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis & Rheumatism 65, 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A, 1999. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann. N. Y. Acad. Sci 877, 64–70. [DOI] [PubMed] [Google Scholar]
- Kender RG, Harte SE, Munn EM, Borszcz GS, 2008. Affective analgesia following muscarinic activation of the ventral tegmental area in rats. The Journal of Pain 9, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinböhl D, Hölzl R, Möltner A, Rommel C, Weber C, Osswald PM, 1999. Psychophysical measures of sensitization to tonic heat discriminate chronic pain patients. Pain 81, 35–43. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, LaViolette P, Vangel M, Rosen B, Kaptchuk TJ, 2008. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. Journal of Neuroscience 28, 13354–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub R, 2009. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 45, 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Spaeth RB, Wey H-Y, Cheetham A, Cook AH, Jensen K, Tan Y, Liu H, Wang D, Loggia ML, 2013. S1 is associated with chronic low back pain: a functional and structural MRI study. Molecular pain 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wang Z, Leiser J, Minicucci D, Edwards R, Kirsch I, Wasan AD, Lang C, Gerber J, Yu S, 2018. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: A functional neuroimaging study. NeuroImage: Clinical 18, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wolcott E, Wang Z, Jorgenson K, Harvey WF, Tao J, Rones R, Wang C, 2019. Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind-body intervention. Brain Imaging Behav 13, 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC, 2011. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ, 2009. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The Journal of Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, Chan S-T, Gerber J, Schmithorst VJ, Edwards RR, 2018. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Tracey I, 2008. A common neurobiology for pain and pleasure. Nat Rev Neurosci 9, 314–320. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu M, Lan L, Zeng F, Makris N, Liang Y, Guo T, Wu F, Gao Y, Dong M, 2016. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Scientific reports 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zeng F, Yin T, Lan L, Makris N, Jorgenson K, Guo T, Wu F, Gao Y, Dong M, 2017. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. NeuroImage: Clinical 15, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen L, Chen X, Hu K, Tu Y, Lin M, Huang J, Liu W, Wu J, Qiu Z, Zhu J, Li M, Park J, Wilson G, Lang C, Xie G, Tao J, Kong J, 2019. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: a randomised multimodal magnetic resonance imaging study. Br J Anaesth. [DOI] [PubMed] [Google Scholar]
- Maniadakis N, Gray A, 2000. The economic burden of back pain in the UK. Pain 84, 95–103. [DOI] [PubMed] [Google Scholar]
- Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, Stohler CS, Zubieta J-K, 2015. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. Journal of Neuroscience 35, 9957–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Hatfield JR, Thomason ME, Rabinak CA, 2017. Reduced Ventral Tegmental Area-Hippocampal Connectivity in Children and Adolescents Exposed to Early Threat. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan S-T, Wasan AD, Kaptchuk TJ, McDonnell C, Carriere J, 2019. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 160, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Margolis EB, 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nature Reviews Neuroscience 18, 73. [DOI] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter MK, Huettel SA, Adcock RA, 2014. Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 100, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV, 2014. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 111, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Porreca F, 2014. Reward and motivation in pain and pain relief. Nature neuroscience 17, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F, 2012. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proceedings of the National Academy of Sciences 109, 20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I, 2001. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21, 9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Navratilova E, 2017. Reward, motivation and emotion of pain and its relief. Pain 158, S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, 1991. Characterizing central mechanisms of pathological pain states by sensory testing and neurophysiological analysis. Pain and Central Nervous System Disease: The Central Pain Syndromes 8, 103–115. [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC, 2009. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. Journal of Neuroscience 29, 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tu Y, Gollub RL, Ortiz A, Napadow V, Yu S, Wilson G, Park J, Lang C, Jung M, 2019. Visual network alterations in brain functional connectivity in chronic low back pain: A resting state functional connectivity and machine learning study. NeuroImage: Clinical 22, 101775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Roeper J, 2017. Subtypes of midbrain dopamine neurons Handbook of Behavioral Neuroscience. Elsevier, pp. 317–334. [Google Scholar]
- Tu Y, Jung M, Gollub RL, Napadow V, Gerber J, Ortiz A, Lang C, Mawla I, Shen W, Chan S-T, Wasan AD, Edwards RR, Kaptchuk TJ, Rosen B, Kong J, 2019. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain 160, 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P, 2013. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 136, 815–827. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, 2016. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 139, 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RG, Vogt BA, 2001. Colocalization of μ-opioid receptors and activated G-proteins in rat cingulate cortex. Journal of Pharmacology and Experimental Therapeutics 299, 840–848. [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA, 2009. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey H-Y, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, Chonde DB, Rosen BR, Gollub RL, Kong J, 2014. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage 102, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, 2011. Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J, 2014. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. NeuroImage: Clinical 6, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Purepong N, Kerr DP, Park J, Bradbury I, McDonough S, 2008. Effectiveness of acupuncture for low back pain: a systematic review. Spine 33, E887–E900. [DOI] [PubMed] [Google Scholar]
- Zhang B, Jung M, Tu Y, Gollub R, Lang C, Ortiz A, Park J, Wilson G, Gerber J, Mawla I, 2019. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. British Journal of Anaesthesia 123, e303–e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


