Abstract
Background.
The vast majority of individuals diagnosed with diabetes are low/middle income and may have access to only three of the 11 oral hypoglycemic medications (OHMs) due to cost: metformin intermediate release (IR) or extended release (ER), sulfonylureas (glimepiride, glipizide, glyburide), and pioglitazone. Sulfonylureas and pioglitazone have had significant controversy related to potential adverse events, but it remains unclear whether these negative outcomes are class-, drug-, or dose-related.
Objective.
We conducted a narrative review of low-cost OHMs.
Methods.
We evaluated the maximum recommended (MAX) compared to the most effective (EFF) daily dose, time-to-peak change in HbA1c levels, and adverse events of low-cost oral hypoglycemic medications.
Results.
We found that the MAX was often greater than the EFF: metformin IR/ER (MAX: 2,550/2,000 mg, EFF: 1,500–2,000/1,500–2,000 mg), glipizide IR/ER (MAX: 40/20 mg, EFF: 20/5 mg), glyburide (MAX: 20 mg, EFF: 2.5–5.0 mg), pioglitazone (MAX: 45 mg, EFF: 45 mg). Time-to-peak change in HbA1c levels occurred at weeks 12–20 (sulfonylureas), 25–39 (metformin), and 25 (pioglitazone). Glimepiride was not associated with weight gain, hypoglycemia, or negative cardiovascular events relative to other sulfonylureas. Cardiovascular event rates did not increase with lower glyburide doses (p<0.05). Glimepiride and pioglitazone have been successfully used in renal impairment.
Conclusions.
Metformin, glimepiride, and pioglitazone are safe and efficacious OHMs. Prescribing at the EFF rather than the MAX may avoid negative dose-related outcomes. OHMs should be evaluated as individual drugs, not generalized as a class, due to different dosing and adverse-event profiles; Glimepiride is the preferred sulfonylurea since it is not associated with the adverse events as others in its class.
Keywords: diabetes, medication, low-income or underserved, hypoglycemic medication, metformin, sulfonylurea, thiazolidinediones
INTRODUCTION
Diabetes is a major source of morbidity and mortality worldwide and has a strong association with poverty.1 Of the 425 million people living with diabetes, the majority (80%) reside in low- or middle-income countries.2 In high-income countries most (67%) individuals diagnosed with diabetes are from low- or middle-income socioeconomic communities.3 Of the 11 oral hypoglycemic medications (OHMs), three are available at low-cost (<$10/month) in the US (metformin, sulfonylureas, thiazolidinediones (pioglitazone]).1 The other eight classes (alpha-glucosidase inhibitors, amylin analogs, bile acid sequestrants, quick-release dopamine-2 agonists, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide (GLP)-1 receptor agonists, meglitinides, sodium-glucose Cotransporter (SGLT)-2 inhibitors) average $570/month ($6,840/year).1 These data reveal that the vast majority of individuals diagnosed with diabetes may have access to only three low-cost options for OHM therapy. Sulfonylureas and pioglitazone have had substantial controversy regarding adverse events4–6, but it is not clear whether these negative outcomes are class-, drug-, or dose-related.
Diabetes is associated with tremendous individual and system burdens, largely due to increasing costs of insulins and most OHM options.1,7 Physician prescribing practices lack of transparent insurance rebate and discount pricing, and limited to no payer price negotiations have led to surging hypoglycemic medications costs in the US ($10 to $22 billion, 2002 to 2012).7 In recent years there has been a paradigm shift of more international organizations recommending multiple OHMs regimens, treating even markedly elevated hemoglobin (Hb)A1c levels (>9%).1,8–10 However beyond metformin as a first-line therapy, there has been significant controversy in deciding the most appropriate subsequent OHM.1,8–10 Concerns for low-cost OHMs including the sulfonylureas and thiazolidinediones have related to potential adverse events (i.e., beta-cell loss, hypoglycemia, negative cardiovascular effects).5,10,11 Strong evidence also suggests that pharmaceutical companies have influenced the discussion regarding therapeutics.12 This may have contributed to less attention to older, low-cost OHMs and resulted in few residual options for many individuals and major public health burdens.5,8
In spite of substantial diversity of OHMs within a class5,13,14, there is a paucity of data comparing these drugs individually. Additionally, low-cost OHM studies have had a diversity of methodology including dosing, adverse events, and duration of therapy. Further, though the maximum recommended daily dose (MAX) is established for OHMs1,15,16, the most effective daily dose (EFF) is not, making it difficult to decipher whether adverse events would decrease or be eliminated if appropriate doses were used. These factors hinder the ability to conduct a robust systematic review or meta-analysis comparing individual OHMs within classes.
METHODS
In this study, we conducted a narrative review of low-cost OHMs by searching PubMed (until June 2019) for randomized controlled trials, systematic reviews, metanalyses, and observational studies that evaluated the MAX, EFF, time-to-peak change in HbA1c level, and adverse events associated with metformin (intermediate release (IR), extended release (ER)), sulfonylureas (glyburide, glipizide, glimepiride), and thiazolidinediones (pioglitazone). We structured the review using the PICOS model: participants (individuals with type 2 diabetes), intervention (OHM), comparison (OHMs-individually and by class), outcomes (MAX, EFF, HbA1c, adverse events), and study design (randomized, observational, systematic review, meta-analysis).17
RESULTS
Diabetes medication therapy is costly, placing many individuals at risk for suboptimal or no treatment. SGLT2 inhibitors and GLP-1 receptor agonists are among the most expensive ($399/month and $711/month, respectively).1 Most insulins are also not available at low-cost (mean: $265/month).1 The Prospective Urban Rural Epidemiology study (N=156,625; 110,803 households in 22 countries) revealed that insulin was available in 93.8% of pharmacies in high-income countries, differing from rates in middle-income (40.2%) and low-income (10.3%) countries.18 Additionally, 63% of households in low-income countries could not afford insulin.18
NPH, Regular, and 70/30 insulin are available at a lower cost ($25/vial) from limited US pharmaceutical companies, but this may still be very expensive. For example, an individual prescribed 50 units of insulin twice daily would spend $75/month ($900/year) even at this reduced price. Also, unlike OHMs where cost is typically a reflection of the number of pills rather than an increase in dose, insulin prices escalate with higher levels. For instance, 30 tablets of metformin 500 mg/day are the same cost as 30 tablets of metformin 1000mg/day, whereas insulin NPH 50 units/day is twice as much as 25 units/day. Furthermore, additional costs of insulin supplies for self-monitoring and syringes make annual expenditures more than $2,000.19
Insulin concerns
In addition to cost-barriers associated with insulin, patient and system burdens are substantial.20 Hypoglycemia has been associated with lower income and less education.21 OHMs are reportedly as efficacious as insulin in treating severe uncontrolled diabetes (HbA1c >11%, insulin: ˗5.1%, OHMs: ˗4.6%, p=0.846) and with less system burden.22 US national statistics have attributed nearly 100,000 annual emergency room visits to insulin-related issues.23 Needle phobia and negative patient perceptions have resulted in increased non-adherence.24
Biguanides (metformin)
In concurrence with lifestyle modifications, metformin is clearly the recommended first-line therapy in type 2 diabetes.1,8,9 Metformin reduces hepatic gluconeogenesis, increases skeletal muscle glucose uptake, suppresses lipogenic enzymes, and induces fatty acid oxidation, all of which positively effect both glycemic and lipid control.25 There are two formulations of metformin: immediate release (IR) and extended release (ER or XL). Metformin ER is now offered at prices comparable to IR, and both have similar effects on glycemic control.1,26,27 However, the median time to reach peak plasma concentration is longer for ER (3 hours vs. 7 hours, respectively),28 which may reduce the frequency of gastrointestinal side effects and thus improve adherence.29,30 One study found that patients prescribed metformin ER had better adherence than those taking IR (80% vs. 72%, p=0.0026), and those switched from IR to ER exhibited improved adherence (62% to 81%, p<0.0001) and glycemic control (HbA1c: 9.1% to 8.4%).29
Maximum recommended (MAX) and most effective (EFF) dosing.
Metformin IR/ER is initiated at 500 mg or 850 mg daily, increased in 500 mg/day increments weekly, and has a maximum dose of 2,550 mg/day (ER: 2,000 mg/day).1,31 A clinical trial (N=451) that evaluated metformin 500–2,500 mg in 500-mg increments found a linear dose response for HbA1c improvement (500 mg: ˗0.6% to 2,000 mg: ˗2.0%) and that each 500-mg increment increase was significant until 2,000 mg/day (p<0.05).32 Although the MAX is 2,550 mg/day, studies have shown that the EFF is between 1,500–2,000 mg/day.32,33 Metformin has been shown to improve glycemic control linearly at least until 25–39 weeks,27,33 but further studies are needed beyond this timeframe to verify time-to-peak HbA1c effects.
Adverse events.
Adverse-event profiles are similar for metformin IR and ER, most commonly involving gastrointestinal effects (abdominal pain, decreased appetite, diarrhea, heartburn, flatulence, nausea, and vomiting).26,27 However, the ER formulation is associated with fewer gastrointestinal effects due to slower absorption and therefore delayed maximum plasma concentration.30 These effects may play a role in weight loss associated with metformin (7% loss of total body weight).34
Other adverse events have been linked to B12 deficiency and negative metabolic effects. Although not entirely clear, B12 deficiency in metformin users may be related to dietary and enterohepatic absorption as well as proton pump inhibitor use.35–37 It is now recommended to test B12 levels in patients prescribed metformin.1,9,35 Metformin use is contraindicated for patients with increased risk of lactic acidosis (i.e., renal impairment), but if prescribed appropriately dose, this risk approaches zero.31,38 Prior guidelines recommended against metformin use if creatinine levels were elevated (men: 1.5 mg/dL; women: 1.4 mg/dL), but it is now contraindicated based on glomerular filtration rate (<30 mL/min/1.73 m2).38 Although there have been concerns related to its use in patients with liver disease and congestive heart failure, metformin has been shown to reduce all-cause mortality in patients with moderate liver and heart disease.39
Sulfonylureas
Sulfonylureas are the oldest class of OHMs, emerging in 1942. Although they constitute a single class, they differ in terms of receptors and binding sites (SUR1, SUR2A; A, B; respectively) on pancreatic beta-cells, route of elimination, duration of action, and rate of absorption.40 Sulfonylureas stimulate insulin secretion by pancreatic beta-cells and decrease hepatic insulin clearance;40 thus, their efficacy depends on functioning beta-cells.40 Although a shorter duration of diabetes may be suggestive of sulfonylurea efficacy, individual variations in glycemic control over time would make this difficult to determine. Although glipizide and glimepiride metabolites are renally excreted, they exhibit less hypoglycemic activity than glyburide and are therefore more frequently used in patients with renal impairment.40
The seven sulfonylureas can be categorized into two generations (first-generation: chlorpropamide, tolbutamide; second-generation: glipizide, gliclazide, gliquidone, glimepiride, glyburide/glibenclamide).40 For the purposes of this review, the most common clinically used second-generation drugs are reviewed.
Glyburide (glibenclamide).
Glyburide is initiated at a dose of 2.5–5 mg and has a MAX of 20 mg/day.1,20 Although the MAX is 20 mg/day,1 studies have failed to demonstrate efficacy at this dose. One study found no improvement in fasting glucose levels beyond 2.5 mg/day to 5 mg/day and reported no effect on insulin stimulation beyond 5 mg/day (2 mg: 51.4%; 5 mg: 58.3%; 10 mg: 44.4%; 20 mg: 33.5%).41,42 Another study found that increasing glyburide from 10 to 20 mg/day did not significantly affect HbA1c (p=0.08), but lower doses were not evaluated.43 There are limited data on glyburide’s effect on HbA1c prior to 12 weeks. However, a systematic review showed that HbA1c changed ˗1.1% to ˗1.2% between 12–20 weeks but did not improve thereafter.15,33,44 Since studies titrated sulfonylureas to achieve glycemic goals, data are limited to compare various doses over time.
Glipizide.
Glipizide is available in IR and ER (extended/sustained/controlled release). These formulations do not differ in terms of HbA1c reduction, but the controlled release formulation has shown lower C-peptide (p<0.05) and fasting insulin (p<0.01) levels compared to the IR formulation.45 Glipizide IR/ER are initiated at 5 mg/day.1,38 Glipizide IR’s MAX is 40 mg/day but had an EFF of 20 mg/day in a 10–40 mg/day evaluation.43 Glipizide ER’s MAX is 20 mg/day, but a 16-week investigation of 5–60 mg/day doses determined the EFF to be 5 mg/day.46 Data suggest that glipizide reduces HbA1c (˗0.9% to ˗1.8%), and time-to-peak HbA1c change occurs by 16 weeks,46,47 although further studies are needed beyond this timeframe to make definitive conclusions.
Glimepiride.
Glimepiride is initiated at 1–2 mg/day and has a MAX of 8 mg.1,11,48 A 14-week study (N=304) revealed that glimepiride exhibits dose-related responses at 2–8 mg/day, although there are small HbA1c differences between 4 and 8 mg/day (˗1.8% vs. ˗1.9%, respectively).49 No significant differences in glycemic control have been demonstrated beyond 8 mg daily.48 The time-to-peak HbA1c reduction (˗1.0% to ˗1.9%) is 12–14 weeks.44
Adverse events.
Concerns regarding adverse events associated with sulfonylureas were recently highlighted.5,50,51 However, sulfonylureas are generally well tolerated, and growing evidence has shown that adverse events vary by drug and should not be generalized to the class.5,11,40 Several studies evaluating only glyburide or a first-generation medication have attributed adverse events to the entire class of sulfonylureas.15,50–52
Beta-cell loss.
Potential lack of beta-cell preservation or expedition of their failure are concerns related to this class though investigations often do not delineate differences between individual sulfonlyureas.40 In vitro, glyburide has been associated with substantially greater damage to islet cells compared to glimepiride and glipizide.11 Sulfonylureas are routinely prescribed at doses higher than their EFF43, and this practice may expedite beta cell loss.
Cardiovascular.
In the setting of diabetes, some sulfonylureas have been found to inhibit KATP channels in cardiac monocytes, resulting in ischemic preconditioning inhibition.53 Glyburide has more affinity for cardiac monocytes than glipizide and glimepiride.5 This provides an explanation for the negative cardiovascular outcomes seen with glyburide.53 It is important to note, however, that negative cardiovascular outcomes have not been demonstrated with glyburide doses ≤10 mg/day, which is above its highest EFF (2.5–5 mg/day).4,41,42 Among 14,213 new users of glyburide or gliclazide, major adverse cardiovascular events were evident in patients taking high (>10 mg/day) but not low (≤10 mg/day) doses.4 This may have provided a rationale for a meta-analysis of randomized controlled trials that did not find an increase in all-cause mortality, myocardial infarction, or cardiovascular mortality associated with glyburide, glimepiride, or glipizide.54
Weight gain.
A study of glipizide ER at doses ranging from 5 to 60 mg/day did not observe patient weight gain, regardless of dose.46 Glimepiride demonstrated a dose-related weight gain at 1-, 4-, and 8-mg doses (0.0 kg, 0.5 kg, and 0.9 kg, respectively) compared to placebo (˗1.82 kg) in one study.49 Another study found glimepiride to have neutral effects on weight or to induce weight loss.55 The latter findings were attributed to extra-pancreatic, glucose-lowering effects, including increased peripheral glucose uptake and decreased endogenous glucose production.55 Another review reported weight gain; however, sulfonylureas were generalized as a class, making it difficult to delineate individual drug effects.40 Furthermore, the average weight gain associated with sulfonylureas (2 kg) was lower than that associated with insulin (4 kg).40
Hypoglycemia.
Hypoglycemic effects are markedly greater with glyburide compared to glipizide and glimepiride, particularly in certain populations (those with renal impairment and the elderly).5 Patients taking glyburide have been shown to exhibit delayed plasma glucose recovery compared to controls (p=0.0001), and during recovery from hypoglycemia, glyburide inappropriately stimulated insulin secretion, whereas glimepiride did not.56 In a 14-week study (N=304), patients treated with glimepiride (range 1–8 mg/day vs. placebo) did not report hypoglycemia.49
Thiazolidinediones (pioglitazone)
Thiazolidinediones, introduced in 1996, have an attractive mechanism of action with many putative metabolic benefits, but their initial popularity plummeted with concerns over adverse events. Pioglitazone decreases peripheral and liver insulin resistance, resulting in less hepatic glucose output and peripheral insulin resistance.16 Its benefits of improved lipid profiles and less hypoglycemia compared to sulfonylureas increase its value to those with hyperlipidemia and at risk for low blood glucose.57
MAX and EFF.
Pioglitazone is initiated at 15–30 mg, and its MAX is 45 mg/day.1,16,58 Pioglitazone exhibits a dose response, with an EFF of 45 mg/day (HbA1c: ˗0.7% to ˗1.9%), with a greater response in treatment-naïve patients.16,33 The glycemic effects of pioglitazone improve linearly until at least 26 weeks,16,58 but longitudinal studies are needed for definitive determination of timeframes. This review focuses on pioglitazone, as rosiglitazone is not a low-cost medication.
Adverse events
Bladder cancer.
Concerns about bladder cancer resulted in the removal of or radical reduction in pioglitazone use in several countries and prompted litigation in the US.6 However, many of these concerns were found to be unwarranted, and prescribing of pioglitazone resurged.59 A possible association with bladder cancer was noted in male but not female rats or in either sex for mice, dogs, and monkeys.59 In one randomized, controlled trial, there appeared to be increased incidence of bladder cancer in the pioglitazone arm the first year of the study, but after 7.8 years, there were more cases in the placebo group (21 vs. 14 cases, respectively).60 In the most-recent large epidemiologic studies, no associations with bladder cancer have been found.61,62 However, the early years resulted in international concern, and despite a lack of evidence, a large pharmaceutical company established a $2.4 billion pool to settle lawsuits.6 Yet the warning for bladder cancer remains active, requiring healthcare professionals to disclose potential risks to avoid liability.16
Vascular.
In a meta-analysis of 19 trials (N=16,390), myocardial infarction and stroke prevalence was less in the pioglitazone arm than placebo arm (4.4% vs. 5.7%, RR 0.82, 95% CI 0.72–0.94, p=0.005).63 Serious heart failure was associated with pioglitazone use but without associated mortality.63 In addition, pioglitazone has also been shown to reduce negative cardiovascular outcomes among patients with insulin resistance and prediabetes.64 Furthermore, a recent meta-analysis showed that pioglitazone reduces the risk of recurrent stroke (HR 0.68, 95% CI 0.5–0.92).65
Weight gain.
The effect of weight gain includes components of fluid retention in addition to increased adiposity. Although there is an increase in adipose tissue, it is selectively peripheral and not visceral and risks including stroke, cardiovascular disease, and metabolic syndrome are associated with the latter.66–68 Weight changes have been found to follow a dose response (placebo: ˗1.4 kg; 15 mg: 0.9 kg; 30 mg: 1.0 kg; 45 mg: 2.6 kg).16
Fractures.
Two meta-analyses, which were primarily rosiglitazone trials, suggested a doubled risk of fractures in women, but this was not observed in men.13,14 A more-recent meta-analysis analyzing only pioglitazone trials found no increase in fractures in patients with type 2 diabetes.69 A 5-year, non-diabetes clinical trial for secondary stroke prevention found no increased risk of fractures in males or females taking pioglitazone versus placebo (13.6% vs. 8.8%, respectively).70 Avoiding pioglitazone in high-risk patients, including those with a history of tobacco abuse or low BMI, could promote its potential benefits.
The Table and Figure summarize data regarding low-cost OHMs.
Table.
Overview of low-cost oral hypoglycemic medications (OHMs)
| OHM | Initial dose (mg/day) | MAXa(mg/day) | EFFb(mg/day) | Time-to-peak HbA1c effect(week) | Change in HbA1c (%)(range)c | Adverse events |
|---|---|---|---|---|---|---|
| Glimepiride | 1–2 | 8 | 4–8 | 12–14 | −1.0 to −1.9 |
|
|
Glipizide IRe ERf |
|
|||||
| Glyburide/Glibenclamide | 2.5–5 | 20 | 2.5–5 | 12–20 | −1.1 to −1.2 | |
| Metformin |
|
|||||
| Pioglitazone | 15–30 | 45 | 45 | 26 | −0.7 to −1.9 |
|
MAX: Maximum recommended daily dose.
EFF: Most effective daily dose.
Range of EFF for all studies.
Severe hypoglycemia <50 mg/dL23.
IR: Intermediate release.
ER: Extended release.
CVD: Cardiovascular disease.
CI: Contraindicated.
RI: Renal insufficiency.
Figure.
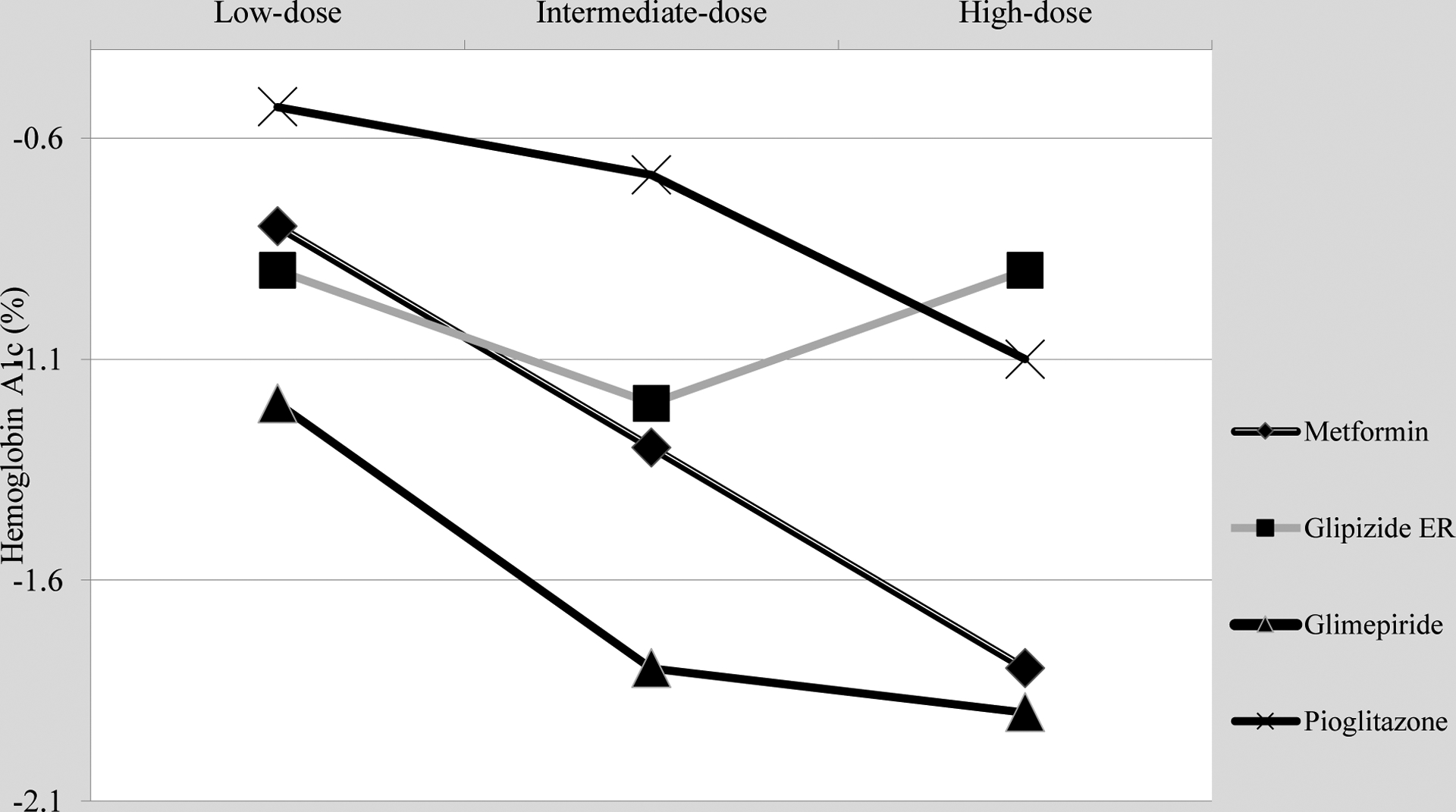
Dose-response of low-cost oral hypoglycemic agents.
DISCUSSION
Low- and middle-income individuals are significantly and disproportionately affected by diabetes.2,3,8 This is complicated by the scare options of low-cost therapy available and the controversy of potential adverse events amongst these medications. It is critical to provide sustainable, safe medications to these individuals to reduce sequelae.9,20 The findings of this study are encouraging; Metformin, glimepiride, and pioglitazone are safe, efficacious, and low-cost options. Further, prescribing the EFF rather than the MAX, such as for glyburide, may reduce or eliminate negative dose-related outcomes including cardiovascular events.1,41,42
Since there is limited data comparing low-cost OHMs individually, we compare our findings to other class-based rather than individual drug-based studies. Metformin as a first-line OHM is consistent with current recommendations1,8,9, but glimepiride and pioglitazone remain controversial second-line therapies due to perceived adverse events.5,10,50 Sulfonylureas exhibited drug- rather than class-specific adverse events, and the main concern of bladder cancer associated with pioglitazone was not supported by significant evidence.5,6,55,59–62 Furthermore, adverse events were often dose-related, and patients are frequently prescribed doses greater than those which were effective.4,41–43 For example, the MAX for glyburide was 20 mg/day, but the EFF was 2.5–5 mg/day, and significant negative cardiovascular outcomes have not been reported at doses ≤10 mg/day.1,41,42 The statins provide a similar example of wide variation between individual drugs within a class; They have demonstrated dose-related adverse events, and their MAX may be significantly higher than their EFF.1,71,72 Further research is warranted to evaluate the adverse-event profiles of sulfonylureas at the EFF rather than MAX.
The reported HbA1c effects of OHMs (~1–2%) (Table) may be a function of baseline glycemic control, duration of treatment, and time since diagnosis.58 For example, studies are typically <30 weeks and include patients with average baseline HbA1c near 8%.15,16,33 A 52-week study with participant HbA1c levels >11% revealed that OHMs decreased HbA1c levels to 4.6% compared to insulin.22
The time to achieve peak HbA1c reduction should also be considered. For instance, peak HbA1c reductions with sulfonylureas were evident by 12–20 weeks, whereas those with metformin and pioglitazone lasted beyond 25 weeks.16,27,33,44,46,47 These findings suggest that current recommendations assuming full therapeutic effects by 12 weeks are premature.1,10 A meta-analysis of OHM randomized controlled trials found that <30% of studies lasted beyond 24 weeks.33 Longitudinal studies are needed to fully determine the time-to-peak HbA1c reduction.
Some investigations have shown value of OHMs beyond and, at times, independent of glycemic control.73 A meta-analysis of eight RCTs evaluating six OHM combinations with metformin found that almost all (n=5/6) improved cholesterol values and two (exenatide/metformin, vildagliptin/metformin) increased insulin sensitivity.74 Investigators have stated the need for a paradigm shift in diabetes care from the predominantly glucocentric view of management to holistic care including sustainable access, individualized glycemic targets, minimizing complications and treatment burdens, and improving quality of life.73
Conclusions
This study highlighted several key findings for safe, efficacious, and affordable OHMs: metformin, glimepiride, and pioglitazone. Sulfonylureas and thiazolidinediones should be evaluated as individual drugs and not generalized as a class because their dosing and adverse-event profiles differ. Glimepiride is the preferred sulfonylurea because its adverse event profile differs from others in its class. Awareness of the EFF compared to the MAX is critical to avoid negative dose-related outcomes while optimizing therapy. Further studies are needed to determine OHM protocols for patients in low- and middle-income settings.
ACKNOWLEDGEMENTS
Funding: This work was supported by the National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases. Federal Award Identification Number-DK110341 (Vaughan, PI).
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes 2019. Diabetes Care. 2019;42(Supplement 1):S1–S193.30559224 [Google Scholar]
- 2.International Diabetes Federation International diabetes atlas-8th edition. Brussels, BE: International Diabetes Federation; 2017:1–29. [Google Scholar]
- 3.Beckles GL, Chou CF. Disparities in the Prevalence of Diagnosed Diabetes - United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep. November 18 2016;65(45):1265–1269. [DOI] [PubMed] [Google Scholar]
- 4.Abdelmoneim AS, Eurich DT, Senthilselvan A, Qiu W, Simpson SH. Dose-response relationship between sulfonylureas and major adverse cardiovascular events in elderly patients with type 2 diabetes. Pharmacoepidemiol Drug Saf. October 2016;25(10):1186–1195. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson MJ. Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? Yes, they continue to serve us well! Diabetes Care. January 2015;38(1):166–169. [DOI] [PubMed] [Google Scholar]
- 6.Davidson MB. Pioglitazone (Actos) and bladder cancer: Legal system triumphs over the evidence. J Diabetes Complications. August 2016;30(6):981–985. [DOI] [PubMed] [Google Scholar]
- 7.McEwen LN, Casagrande SS, Kuo S, Herman WH. Why Are Diabetes Medications So Expensive and What Can Be Done to Control Their Cost? Curr Diab Rep. September 2017;17(9):71. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. ed. Geneva: CH: World Health Organization; 2018:. [PubMed] [Google Scholar]
- 9.Qaseem A, Barry MJ, Humphrey LL, Forciea MA, Physicians CGCotACo. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med. February 21 2017;166(4):279–290. [DOI] [PubMed] [Google Scholar]
- 10.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2019 Executive Summary. Endocr Pract. January 2019;25(1):69–100. [DOI] [PubMed] [Google Scholar]
- 11.Del Guerra S, Marselli L, Lupi R, et al. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications. Jan-Feb 2005;19(1):60–64. [DOI] [PubMed] [Google Scholar]
- 12.Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. September 27 2017;7(9):e016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. January 6 2009;180(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. November 2014;68:115–123. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration, Company B-MS. Glucovance: Highlights of prescibing information. Princeton, NJ: US Food and Drug Administration; 2018. [Google Scholar]
- 16.US Food and Drug Administration, Takeda Pharmaceuticals. Highlights of prescribing information: Actos. Deerfield, IL: US Food and Drug Administration, Takeda Pharmaceuticals; 2011. [Google Scholar]
- 17.Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Eden, Levit L, Berg A, Morton S Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC)2011. [PubMed] [Google Scholar]
- 18.Chow CK, Ramasundarahettige C, Hu W, et al. Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol. October 2018;6(10):798–808. [DOI] [PubMed] [Google Scholar]
- 19.Yeaw J, Lee WC, Aagren M, Christensen T. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm. Jan-Feb 2012;18(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Essential medicines and health products surveying insulin availability and pricing: vital to treating diabetes. 2019; https://www.who.int/medicines/areas/access/webstory_diabetes/en/. Accessed December 24, 2019.
- 21.Berkowitz SA, Karter AJ, Lyles CR, et al. Low socioeconomic status is associated with increased risk for hypoglycemia in diabetes patients: the Diabetes Study of Northern California (DISTANCE). J Health Care Poor Underserved. May 2014;25(2):478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan EM, Moreno JP, Hyman D, Chen TA, Foreyt JP. Efficacy of oral versus insulin therapy for newly diagnosed diabetes in low-income settings. Arch Gen Intern Med. 2017;1(2):17–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. May 2014;174(5):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ. Nov-Dec 2009;35(6):1014–1022. [DOI] [PubMed] [Google Scholar]
- 25.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. September 2017;60(9):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal N, Singla A, Mathieu C, et al. Metformin extended-release versus immediate-release: An international, randomized, double-blind, head-to-head trial in pharmacotherapy-naive patients with type 2 diabetes. Diabetes Obes Metab. February 2018;20(2):463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz S, Fonseca V, Berner B, Cramer M, Chiang YK, Lewin A. Efficacy, tolerability, and safety of a novel once-daily extended-release metformin in patients with type 2 diabetes. Diabetes Care. April 2006;29(4):759–764. [DOI] [PubMed] [Google Scholar]
- 28.Timmins P, Donahue S, Meeker J, Marathe P. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet. 2005;44(7):721–729. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly LA, Morris AD, Pearson ER. Adherence in patients transferred from immediate release metformin to a sustained release formulation: a population-based study. Diabetes Obes Metab. April 2009;11(4):338–342. [DOI] [PubMed] [Google Scholar]
- 30.Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med. January 2011;123(1):15–23. [DOI] [PubMed] [Google Scholar]
- 31.Samson SL, Garber AJ. Metformin and Other Biguanides: Pharmacology and Therapeutic Usage, 4th edition of the International Textbook of Diabetes Mellitus. Hoboken, NJ: Wiley-Blackwell; 2015. [Google Scholar]
- 32.Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med. December 1997;103(6):491–497. [DOI] [PubMed] [Google Scholar]
- 33.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. August 2010;33(8):1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. February 7 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. April 2016;101(4):1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. March 1 2003;67(5):979–986. [PubMed] [Google Scholar]
- 37.Long AN, Atwell CL, Yoo W, Solomon SS. Vitamin B(12) deficiency associated with concomitant metformin and proton pump inhibitor use. Diabetes Care. December 2012;35(12):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. June 2011;34(6):1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann Intern Med. February 7 2017;166(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. August 12 2015;11(4):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambiritch V, Maharaj B, Naidoo P. Glibenclamide in patients with poorly controlled type 2 diabetes: a 12-week, prospective, single-center, open-label, dose-escalation study. Clin Pharmacol. 2014;6:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rambiritch V, Naidoo P, Pillai G. Glibenclamide population pharmacokinetic/pharmacodynamic modeling in South African type 2 diabetic subjects. Clin Pharmacol. 2016;8:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurren KM, Bartley EP, O’Neill JL, Ronis DL. Effect of sulfonylurea dose escalation on hemoglobin A1c in Veterans Affairs patients with type 2 diabetes. Acta Diabetol. April 2013;50(2):261–265. [DOI] [PubMed] [Google Scholar]
- 44.Dills DG, Schneider J. Clinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. Glimepiride/Glyburide Research Group. Horm Metab Res. September 1996;28(9):426–429. [DOI] [PubMed] [Google Scholar]
- 45.Berelowitz M, Fischette C, Cefalu W, Schade DS, Sutfin T, Kourides IA. Comparative efficacy of a once-daily controlled-release formulation of glipizide and immediate-release glipizide in patients with NIDDM. Diabetes Care. December 1994;17(12):1460–1464. [DOI] [PubMed] [Google Scholar]
- 46.Simonson DC, Kourides IA, Feinglos M, Shamoon H, Fischette CT. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. The Glipizide Gastrointestinal Therapeutic System Study Group. Diabetes Care. April 1997;20(4):597–606. [DOI] [PubMed] [Google Scholar]
- 47.Archer M, Oderda G, Richards K, Turpin S. Sulfonylurea agents & combination products: Drug class review. Salt Lake City, Utah: University of Utah;2013. [Google Scholar]
- 48.Rosenstock J, Samols E, Muchmore DB, Schneider J. Glimepiride, a new once-daily sulfonylurea. A double-blind placebo-controlled study of NIDDM patients. Glimepiride Study Group. Diabetes Care. November 1996;19(11):1194–1199. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg RB, Holvey SM, Schneider J. A dose-response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agents. The Glimepiride Protocol #201 Study Group. Diabetes Care. August 1996;19(8):849–856. [DOI] [PubMed] [Google Scholar]
- 50.Douros A, Dell’Aniello S, Yu OHY, Filion KB, Azoulay L, Suissa S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ July 18 2018;362:k2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riddle MC. Modern Sulfonylureas: Dangerous or Wrongly Accused? Diabetes Care. May 2017;40(5):629–631. [DOI] [PubMed] [Google Scholar]
- 52.Cheng V, Kashyap SR. Weight considerations in pharmacotherapy for type 2 diabetes. J Obes. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee TM, Chou TF. Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab. February 2003;88(2):531–537. [DOI] [PubMed] [Google Scholar]
- 54.Varvaki Rados D, Catani Pinto L, Reck Remonti L, Bauermann Leitao C, Gross JL. The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials. PLoS Med. April 2016;13(4):e1001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations (GIFTS). [corrected]. Vasc Health Risk Manag. 2012;8:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szoke E, Gosmanov NR, Sinkin JC, et al. Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism. January 2006;55(1):78–83. [DOI] [PubMed] [Google Scholar]
- 57.Kim JM, Kim SS, Kim JH, et al. Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study. Diabetes Metab J. July 11 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. November 2000;23(11):1605–1611. [DOI] [PubMed] [Google Scholar]
- 59.Ryder RE. Pioglitazone has a dubious bladder cancer risk but an undoubted cardiovascular benefit. Diabet Med. March 2015;32(3):305–313. [DOI] [PubMed] [Google Scholar]
- 60.Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. March 2016;18(3):266–273. [DOI] [PubMed] [Google Scholar]
- 61.Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. July 21 2015;314(3):265–277. [DOI] [PubMed] [Google Scholar]
- 62.Korhonen P, Heintjes EM, Williams R, et al. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. BMJ. August 16 2016;354:i3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. September 12 2007;298(10):1180–1188. [DOI] [PubMed] [Google Scholar]
- 64.Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. January 5 2017;7(1):e013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for Secondary Stroke Prevention: A Systematic Review and Meta-Analysis. Stroke. February 2017;48(2):388–393. [DOI] [PubMed] [Google Scholar]
- 66.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. January 2005;54(1):24–32. [DOI] [PubMed] [Google Scholar]
- 67.Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. June 2007;6(2):51–59. [DOI] [PubMed] [Google Scholar]
- 68.Finelli C, Sommella L, Gioia S, La Sala N, Tarantino G. Should visceral fat be reduced to increase longevity? Ageing Res Rev. September 2013;12(4):996–1004. [DOI] [PubMed] [Google Scholar]
- 69.Pavlova V, Filipova E, Uzunova K, Kalinov K, Vekov T. Pioglitazone Therapy and Fractures: Systematic Review and Meta- Analysis. Endocr Metab Immune Disord Drug Targets. 2018;18(5):502–507. [DOI] [PubMed] [Google Scholar]
- 70.Viscoli CM, Inzucchi SE, Young LH, et al. Pioglitazone and Risk for Bone Fracture: Safety Data From a Randomized Clinical Trial. J Clin Endocrinol Metab. March 1 2017;102(3):914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu M, Tomlinson B. Current Perspectives on rosuvastatin. Integr Blood Press Control. 2013;6:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tayal U, Carroll R. Should anyone still be taking simvastatin 80 mg? BMJ Case Rep August 8 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Zuniga-Hernandez JA, McCoy RG. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ. November 5 2019;367:l5887. [DOI] [PubMed] [Google Scholar]
- 74.Peng Y, Chen SH, Liu XN, Sun QY. Efficacy of different antidiabetic drugs based on metformin in the treatment of type 2 diabetes mellitus: A network meta-analysis involving eight eligible randomized-controlled trials. J Cell Physiol. March 2019;234(3):2795–2806. [DOI] [PubMed] [Google Scholar]


