Abstract
Introduction:
Neuroendocrine tumors are becoming increasingly prevalent with many patients presenting with or developing metastatic disease to the liver.
Methods:
In this landmark series paper, we highlight the critical studies that have defined the surgical management of neuroendocrine tumor liver metastases, as well as several randomized control trials which have investigated strategies for systemic control of metastatic disease.
Results:
Liver-directed surgical approaches and locally ablative procedures are recommended for patients with limited, resectable and in some cases, nonresectable tumor burden. Angiographic liver-directed techniques, such as transarterial embolization, chemoembolization, and radioembolization, offer another approach for management in patients with liver-predominant disease. Peptide receptor radionuclide therapy is a promising therapy for patients with hepatic and/or extrahepatic metastases. Various systemic medical therapies are also available as adjunct or definitive therapy for patients with metastatic disease.
Conclusion:
This article will review the current data regarding management of neuroendocrine liver metastases and highlight areas for future study.
Introduction
Neuroendocrine tumors (NETs) encompass a heterogenous group of tumors which exhibit variable clinical behavior. These tumors recapitulate various neuroendocrine cell types and are frequently categorized based on their location of origin, the foregut (bronchial, gastric, duodenal, pancreatic), midgut (small bowel, appendiceal, proximal colon), or hindgut (distal colon and rectum). A recent analysis of the Surveillance, Epidemiology, and End Results (SEER) database reported that the highest incidences of NETs are in the lung (1.49 per 100,000 persons), followed by small bowel (1.05 per 100,000), rectum (1.04 per 100,000) and pancreas (0.48 per 100,000).1 The prevalence of NET liver metastases (NETLMs) ranges between 27–60%, with approximately 12–74% of patients presenting with liver metastases (LM) at diagnosis.2–6 Although as a whole NETs are considered a more indolent cancer subtype, the presence of liver metastases is a negative predictor of survival for NET patients.7,8 The clinical manifestations of NETs are varied, ranging from asymptomatic to debilitating, and are dependent upon the secretory activity of the primary tumor and extent of hepatic tumor load. As such, management of NETLMs is a critical component of treatment in NET patients.
Neuroendocrine neoplasms are classified histologically based on tumor grade (grades 1–3) and differentiation. Most fall into one of 4 broad histologic categories: low grade, well differentiated NETs (G1); intermediate grade, well-differentiated NETs (G2); high grade, well differentiated NETs (G3); and high grade, poorly differentiated neuroendocrine carcinomas (NEC). Grade is generally defined by mitotic count and Ki-67 index and if there is discordance, the higher grade is used to assign classification [Table 1]. Most NETs are typically G1 or G2 while G3 NETs are rare and present with aggressive behavior.9 For purposes of this review, surgical management recommendations will focus primarily on G1 and G2 NETs as the mainstay of therapy, and systemic therapy is more commonly used for G3 NETs and NECs.
Table 1.
Classification for Neuroendocrine Neoplasms
| Well Differentiated NET | Ki-67 index (%) | Mitotic index |
|---|---|---|
| Grade 1 | <3 | <2/10 HPF |
| Grade 2 | 3–20 | 2–20/10 HPF |
| Grade 3 | >20 | >20/10 HPF |
| Poorly Differentiated NEC | ||
| Grade 3 (Neuroendocrine Carcinoma) | >20 | >20/10 HPF |
There are a variety of treatment modalities for NETLMs, the indications for which frequently depend on tumor characteristics such as anatomic origin, patterns of metastasis (number, location, size, tumor burden), and tumor grade. Typically, there are three broad intervention strategies for NETLM management: surgical resection and ablative techniques, non-surgical liver-directed therapies, and systemic therapies (chemotherapy, somatostatin analogue therapy, peptide receptor radionuclide therapy). This review focuses on discussing current treatment paradigms for NETLMs.
Surgical Management
Surgical Cytoreduction
Despite high recurrence rates after resection, surgery remains among the most favorable approaches for select patients with NETLMs. Surgical treatment of NETLMs comprises resection and cytoreductive surgery for symptom management and improvement of survival. The concept of surgical resection of NETLMs dates to 1977, when Foster and Berman reported on results of 44 cases of resection for control of symptoms. They noted that in the majority of patients who had at least 95% debulking and non-rapid rates of tumor growth, good symptom control was achieved.10,11 The concept of a debulking threshold was revisited by McEntee and colleagues years later, still in the era prior to the availability of somatostatin analogue (SSA) therapy. In the McEntee study, 37 patients underwent resection for symptom relief. Though the authors did not aim to achieve a specific debulking threshold, they noted that symptom control was generally achieved only if at least 90% of the grossly visible tumors were resected. No additional assessment of factors that were predictive of survival were noted.12 In a subsequent series from the Mayo Clinic, Que et al published their findings on 74 patients with NETLM who underwent resection for management of endocrinopathies. In this study, a debulking threshold of 90% was set based on McEntee’s series and 38 patients had concomitant resections of their primary tumors. They reported a four-year survival rate of 73% and a postoperative symptomatic response rate of 90%, with a mean duration of response of 19.3 months.13
In a 2003 study from the same institution, Sarmiento et al. further evaluated the impact of surgical resection utilizing a liver debulking threshold of 90%. This was now at a time where SSA therapy was available to patients and the purpose of the study was to evaluate survival. A total of 170 patients with NETLMs from functional and nonfunctional gastroenteropancreatic NETs (GEP-NETs) were included. Surgical resection was associated with a 61% 5-year survival rate, which was a significant improvement over historical controls. No survival difference was noted between patients who had functional or nonfunctional tumors, and the site of primary also had no impact. For those patients with hormonal symptoms, resection was associated with complete relief of or improvement in symptoms in 96%. This particular study was critical in that it was at this point that resection of NETLM with a 90% debulking threshold was accepted as a means of also improving patient survival in contrast to just for symptom management.14
The possibility that lower levels of cytoreduction might also be beneficial to patients with NETLM was suggested by Chambers et al in 2008, who evaluated outcomes in 66 patients with gastrointestinal (GI) NETs (excluding PNETs) with nodal and/or liver metastases. All patients in the study were symptomatic (36% had obstructive/ischemic symptoms from their primary tumor and 85% had carcinoid syndrome) and their approach was to resect primary tumors, mesenteric nodal disease when possible, and potentially cytoreduce NETLMs in select cases. Improvement in obstructive symptoms was achieved in all 24 patients and of carcinoid syndrome symptoms in 75% of patients. In these patients, 30 (45%) underwent hepatic cytoreduction, 22 with resection of their primary tumor as well, while 21 patients with miliary or widespread metastases did not have cytoreduction. Overall survival (OS) at five years was 74%. They stated that not being able to achieve 90% cytoreduction was not a contraindication to debulking surgery, and that attempting to achieve >70% NETLM debulking was still helpful for palliation of carcinoid symptoms.15
Graff-Baker and colleagues evaluated different levels of NETLM debulking in 52 patients with small bowel neuroendocrine primary tumors (SBNET) in whom more than 70% cytoreduction of NETLMs could be achieved.16 Patients with margin positive resection and extrahepatic disease were included. The study found that 21% of patients had 70–89% cytoreduction with 27% having disease progression, 42% had 90–99% cytoreduction with 27% having progression, while 37% had 100% cytoreduction and 32% progressed at a median follow-up of 37.4 months. The 5-year OS rate for the whole group was 88%, and only age <50 was identified as a negative prognostic factor for progression-free survival (PFS). Given no difference in liver PFS or disease-specific survival (DFS) in the 3 groups of patients having >70% cytoreduction, the argument was made to lower the debulking threshold for NETLM to >70%.16 Another report from the same group in Oregon evaluated 44 debulking procedures performed on 42 patients with pancreatic (34) or duodenal NETs (7, plus 1 unknown).17 In this study, 36% had the primary tumor removed first, 33% had liver debulking first followed by primary resection, 11% had resection of primary tumor and NETLM simultaneously, and 21% did not have resection of their primary tumor. Most patients (55%) reached 100% debulking, with 27% having 90–99%, and 18% with 70–89% cytoreduction. At a median follow up of 33 months, the liver specific PFS in this cohort of patients was 11 months, which was significantly lower than that described in the previous SBNET study. They also found no difference in the rates of progression between the 100%, 90–99%, or ≥70% debulking groups. In evaluating for predictors of progression, on multivariate regression analysis, only having a liver metastasis measuring ≥5 cm was found to be significant (p=0.003).
Thresholds of cytoreduction were further evaluated by Maxwell et al., who reported on 108 patients presenting with NETLMs who had liver debulking operations out of 142 evaluated in their clinic. In this study, 84% of patients had concurrent resection of their primary tumor and the mean percentage of cytoreduction determined by comparing preoperative and postoperative CTs was 80%.18 A total of 80 patients had small bowel primaries and 28 had PNETs, with a median of 10 liver lesions. The median PFS of all patients having cytoreduction was 2.2 years with a median OS of 10.5 years. Patients in this study had a range of liver debulking surgery from less than 50% to greater than 90%, and the results demonstrated that patients who had 70% or greater cytoreduction had improved OS compared to those who had less than 70% (median OS not reached vs. 6.5 years, p=0.009). Adoption of this lower debulking threshold of >70%, along with the use of parenchymal-sparing surgical techniques (wedge resections, enucleations, and ablations), allowed for more than 75% of patients to undergo hepatic cytoreduction.
In a multi-institutional study, which included 339 patients with NETLM treated with surgical intervention, most patients underwent surgical resection (77.6%), while 19.5% had a combination of resection and ablation. More than half (52.5%) of the patients included had non-anatomic liver resections. Most patients had R0 resections (54%) while 20% had R1 and 19% had R2 resections. The survival benefit was greatest in patients with functional tumors and those who had R0/R1 resection (Figure 1).19 The median OS was 125.1 months with a 5-year OS of 74%. Patients undergoing resection for palliative intent had worse OS than those treated with curative intent (77.5 vs. 156.9 months). The presence of synchronous disease at the primary site, extrahepatic disease at the time of liver resection, and nonfunctional hormonal status were independent risk factors that led to worse OS. This study did not provide information regarding the degree of liver involvement or volume of disease removed, and therefore did not contribute to determining debulking thresholds.19 Another large study by Boudreaux et al. evaluated outcomes in 189 patients with stage IV well-differentiated SBNETs who were managed at a single institution. A total of 229 operations were performed, and 89% had carcinoid symptoms. This subset of SBNET patients had 5-and 10-year survival rates of 87% and 77% after operative intervention.20
Figure 1:
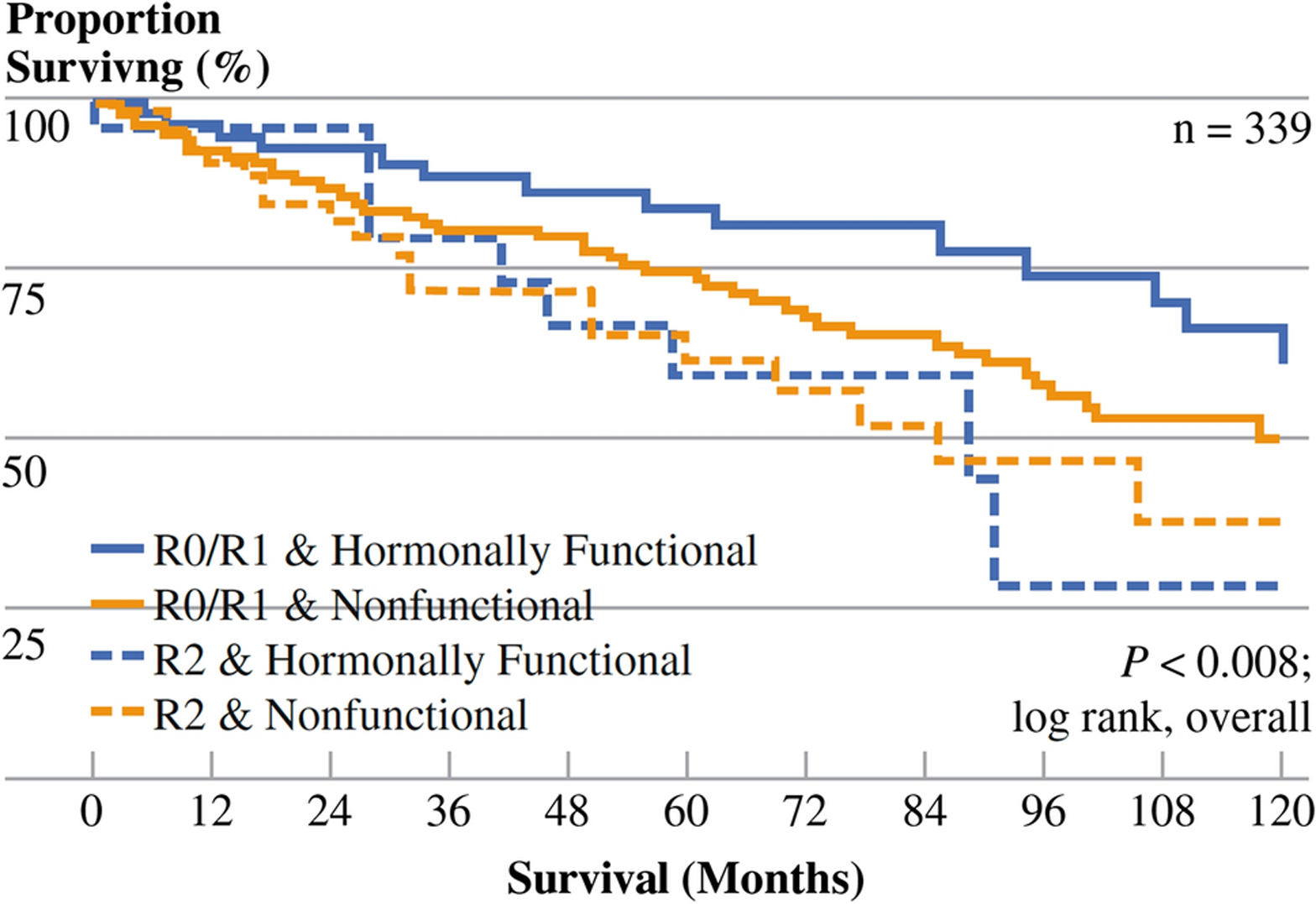
Kaplan-Meier survival stratified by margin status (R0/R1 vs. R2) after first liver directed operation and hormonal function of the NET. Patients with hormonally functioning tumors who had R0/R1 resection had greater survival than other groups (P = 0.008; reprinted from Mayo et al.19 with permission).
A recent single institutional study updating the experience of Maxwell et al evaluated outcomes from 188 total hepatic cytoreductive procedures. Most patients had SBNET primaries (128 patients) followed by pancreatic primaries (41 patients), with 74% of patients having concurrent resection of their primary tumors. Patients were divided into 3 groups based on the number of liver lesions treated (1–5, 6–10, and >10 NETLMs). The groups were noted to be well matched with the exception of shorter median follow up in patients with >10 NETLMs and administration of preoperative SSA, which was higher in the >10 NETLMs group. The mean percent tumor debulking achieved was 79% and did not differ between groups, and the mean OS did not differ between groups based upon the number of lesions treated. Overall complication rates were 52% with 15% being grade 3 or 4 complications (most commonly bleeding and intraabdominal infection) with no deaths. Upon examining OS relative to debulking thresholds, >70% debulking was associated with better OS compared to <70% (median 134.3 mos. vs. 37.6 mos., p<0.01). They found no significant difference in OS between 70–90% and >90% cytoreduction, while PFS was improved in the >90% group relative to the 70–90% and <70% groups (Figure 2). Another finding of the study was that the mean number of lesions and percent liver replacement decreased with increasing levels of cytoreduction, suggesting that it is easier to achieve better levels of cytoreduction when there is less liver involvement. This study further validated the 70% debulking threshold and demonstrated that this target can be achieved even in patients with >10 NETLMs.21
Figure 2:
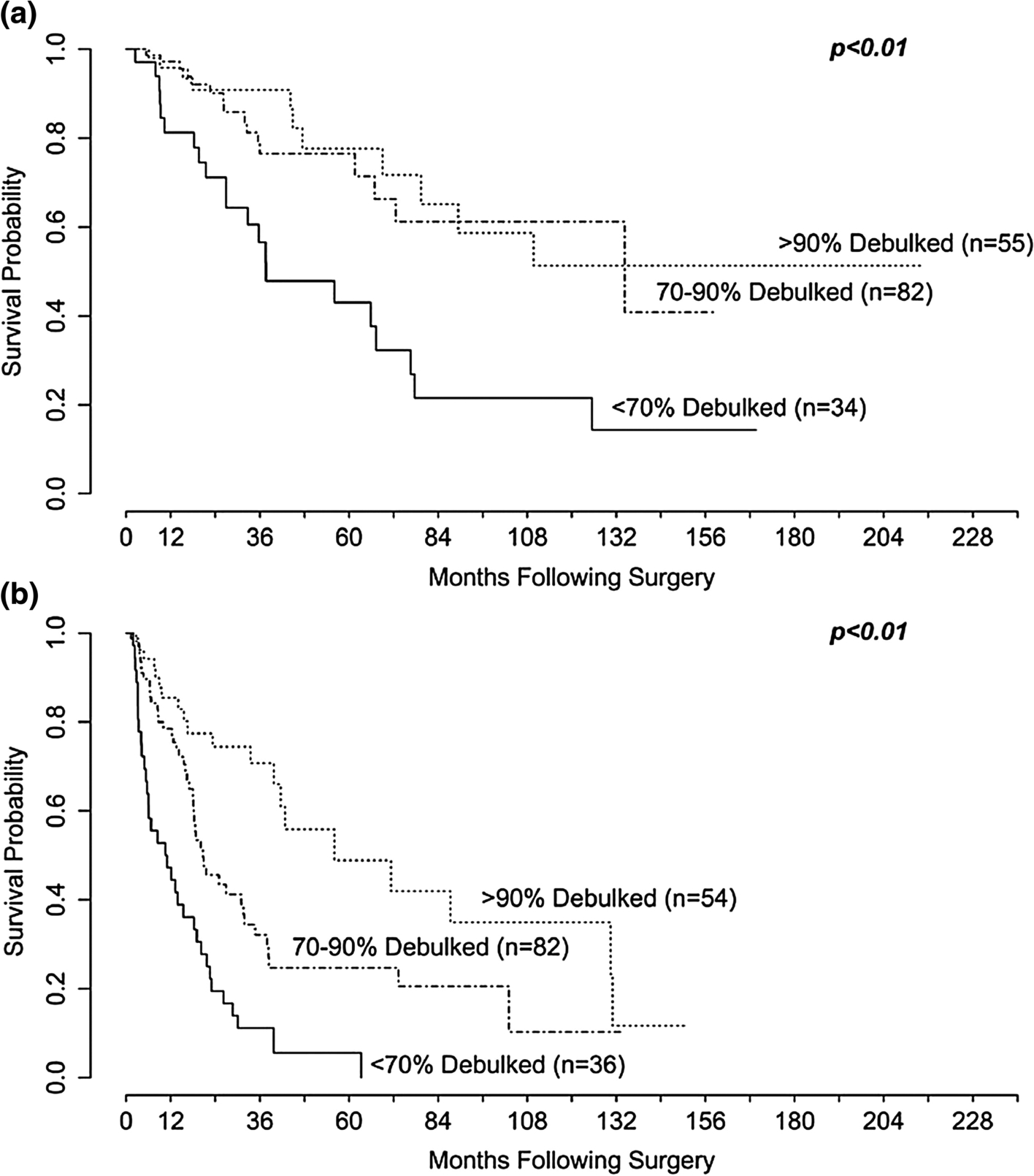
Kaplan-Meier curves for (A) Overall survival and (B) Progression free survival stratified by the amount of tumor debulked. There was a difference in (A) OS comparing <70% to 70%–90% (P < .01) but not 70%–90% to >90% (P = .6). There was a significant difference in (B) PFS comparing <70% to 70%–90% (P < .01) and 70%–90% to >90% (P < .01; reprinted from Scott et al.21 with permission).
The benefit of surgical resection in nonfunctional NETLMs remains controversial, with varying reports of disease control and survival benefit relative to historical controls. The potential for significant selection bias must be considered given the retrospective nature of the data, where patients with lower tumor burdens, fewer co-morbidities, and more favorable tumor biology may be more likely to undergo cytoreduction. Ideally, a trial randomizing patients to cytoreduction of NETLMs or SSAs, stratified by disease-site, tumor burden, and degree of cytoreduction achieved, could potentially give definitive answers to the survival benefits of cytoreduction. However, this is unlikely to be achievable for several reasons, including that patients need to agree to be randomized, hundreds would be required, and follow-up would need to be long. Numerous retrospective studies have demonstrated that good symptom control can be achieved with surgical debulking,13–15,17,20,22 as well significant reductions in hormone levels.18,21 Also, when >70% debulking is achieved despite less than complete resection (R1/R2), comparable survival outcomes are observed as for R0 resection with >70% cytoreduction.16,18,21
NANETs and ENETs guidelines suggest that treatment should be individualized based on patient age and co-morbidities, distribution of lesions and volume of liver involvement, the presence of symptoms, and rate of progression.6,23,24 Factors that generally preclude surgical cytoreduction include liver replacement >50–70%, many (>50) small bilobar metastases, carcinoid heart disease, multiple patient co-morbidities, and the presence of high-grade tumors (poorly differentiated, or well differentiated with Ki-67 well in excess of 20%).
It is important to keep in mind that even if effective debulking can be performed, recurrence rates of NETLMs are 80–95% within 5 years and 99% by 10 years, demonstrating that even though long-term survival can be achieved, few patients with NETLM are cured.14,19 The high rate of intrahepatic recurrence is due in part to the underestimation of the extent of liver disease by preoperative imaging. Elias et al. carefully evaluated pathologic specimens after NETLM resection with thin serial sections and found that fewer than 50% of NETLMs were detected by imaging preoperatively. Many of the lesions identified were smaller than 2 mm in size and the accuracy of detection by somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging were calculated to be only 24%, 38% and 49% respectively.25
Liver Transplantation
In patients who are ineligible for hepatic debulking, liver transplantation may offer the potential for curative resection and improves survival in highly selected patients.26,27 Eligibility for transplantation is variable, but the Milan criteria and ENETS guidelines require tumors to be low grade (G1/G2; Ki-67% <10 per ENETS), <50% tumor involvement of the liver, the primary tumor has been removed, no extrahepatic disease, stable disease for at least 6 months, and age <55. The potential benefits of this procedure must be weighed against the national shortage of grafts.6,27–29 Fan et al performed a literature review of 705 patients who underwent hepatic transplantation for NETLMs and reported a 5-year OS of 53% and 5-year DFS of 31%.30 A Swedish study evaluating 33 patients less than 65 years of age with low grade (G1/G2) small bowel NETLMs and resected locoregional disease who met Milan criteria found 5-year OS of 97% (±6%) when treated with liver resection/RFA and 89% (±21%) when there was no liver resection/RFA, which was better than the 76% ±21% 5-year OS of comparable patients from the literature who underwent liver transplant for NETLM. They concluded that most patients with NETLMs may have excellent long-term survival with locoregional therapies and may derive limited benefit from liver transplantation.31 More recently, Mazzaferro et al. reported outcomes in patients who underwent transplant for NETLMs who had a 1) primary tumor drained by the portal system which had been previously removed with either no extrahepatic disease, or prior curative resection of all extrahepatic disease, 2) low grade (G1/G2) histology, 3) NETLM < 50% of total liver volume, 4) stable disease or response to treatment for at least 6 months, and 5) age <60. Overall, clinical characteristics between transplant and non-transplant patients were similar with the exception of patients in the non-transplant group being older (median age 40.5 vs. 55.5, p<0.01), having more advanced T stage (p=0.048), and having fewer locoregional treatments (21.7% vs. 40.5%, p=0.003). The reported 5-and 10-year OS rates were 97.2% and 88.8% for transplant patients vs. 50.9% and 22.4% for non-transplant patients, suggesting significant long-term OS advantage in patients undergoing transplant who met these restrictive criteria.28 These studies are difficult to reconcile since they come to different conclusions and do not directly compare transplantation vs cytoreduction. At the current time, liver transplantation remains an option for patients who meet the ENETS and Milan criteria, but it is not frequently performed.
Liver Directed therapy
Ablative Techniques
Ablative methods comprise microwave ablation (MWA), radiofrequency ablation (RFA), cryotherapy, and irreversible electroporation (IRE). There are no reliable data that compare results of these methods for cytoreduction, but each has its own risks and benefits.32 These techniques can be performed percutaneously by interventional radiologists, usually requiring general anesthesia, and are limited to treatment of one or a few lesions. These same methods can be employed during open or laparoscopic surgery as well,18,21,33–35 and multiple lesions may be treated at once.21 Cryotherapy was the first of these ablative techniques advocated for liver metastases, and was later replaced with RFA. The advantage of the latter is that larger lesions could be treated, but disadvantages are that portions of tumors at the edges of larger blood vessels may not achieve high enough temperatures to kill tumor cells, and that it takes a longer time to ablate lesions. Many in the field have changed to using MWA, which is faster and therefore amenable to treating more lesions and will effectively ablate tumors even on blood vessels; this however can result in biliary strictures if applied too close to portal structures. Tumors up to 5 cm can be treated, but recurrence rates are generally higher with these larger lesions. The advantage of IRE is that it does not kill tumors by heat, but rather by creating small holes in cell membranes using electric pulses, which results in cell death. This characteristic allows for ablation to be performed along important structures with less risk of permanent injury, but its utility is limited to smaller lesions and by the cost of the machine.36
Intra-arterial liver directed therapies
Given that NETLMs are vascular and are preferentially supplied by hepatic arteries rather than portal venous blood, occlusion of the arterial supply to these tumors can lead to ischemia and necrosis. Selective arterial occlusion can be performed alone (bland embolization) or in combination with chemotherapeutic agents (chemoembolization), with chemotherapy bound to beads which slowly release the drug (drug eluting beads; DEB), or with beads bound to 90Yttrium (transarterial radioembolization; TARE). These methods are all options for symptomatic patients and for locoregional control of unresectable and disseminated NETLM. They can also be used alone or in combination with systemic medical therapies.37–39 Patients with bilobar disease are commonly treated with staged procedures to each lobe. Contraindications to intra-arterial liver directed therapies include main portal vein thrombosis, renal and/or hepatic insufficiency, bilirubin greater than 2mg/dL, and extreme caution should be used if there is >75% of liver involvement or a bilioenteric anastomosis is present.40
Transarterial embolization (TAE) involves administration of an embolic substance (with polyvinyl alcohol particles which may range from 50–500 μm with or without ethiodized oil) into the hepatic artery that is feeding the tumor, with the goal of inducing tumor ischemia and subsequent tumor cell death. Transarterial chemoembolization (TACE) combines intra-arterial embolization with a locally delivered dose of a chemotherapeutic agent (i.e. 5-fluorouracil, doxorubicin, cisplatin) into the feeding artery. Importantly, TACE has the potential for intratumoral drug concentrations being up to 20 times greater than those achieved by systemic administration. In addition to inducing ischemia, the reduction of blood flow results in a longer washout of the chemotherapy, prolonging its local effects. In a retrospective series with 123 patients with NETLMs who were treated with TACE, the 5 and 10-year OS rates were 36% and 20%.41 At present, there is no consensus regarding embolization agents, chemotherapeutic agents, or technique in TACE. Additional studies have shown essentially equivalent results for TACE vs TAE for management of NETLM, suggesting that ischemia secondary to restricted inflow is likely more critical than the effect of locally administered chemotherapy.42,43 In contrast, a phase II study of 13 patients receiving DEB TACE (using doxorubicin) resulted in 7 patients developing bilomas, 4 of whom required percutaneous drainage.44 Due to the high rate of complications, DEB TACE is no longer a recommended form of embolization.
Transarterial radioembolization (TARE) involves the delivery of radioactive microspheres for internal radiation treatment. Currently there are 2 types of radioactive microspheres available, SIR-spheres® which are 20–40 μm diameter resin polymer microspheres and Thera Sphere® which are a 20–30 μm ceramic/glass microspheres. Both use 90Yttrium as the radiation emitting isotope. These microspheres are smaller than those used for bland or chemoembolization, and therefore lodge in smaller vessels and can deliver more selective radiation of tumors, with hypothetically a more limited dose being delivered to the normal liver parenchyma. 90Yttrium is a β-emitter with a half-life of 64.2 hours, average tissue penetration of 2.5 mm, delivering relatively high doses of radiation in the range of 50 to 150 Gy.38 The efficacy of TARE is comparable to TACE, can be staged or only require one treatment, while TACE is typically repeated for bilobar disease.45,46
One analysis evaluated a matched subset of patients with high volume liver burden (>25% liver involvement) with and without carcinoid symptoms and compared surgical resection versus intra-arterial therapy. Propensity score methods were used to account for clinicopathologic differences between patients treated with surgery versus intra-arterial therapy. The study found that symptomatic patients derived a greater benefit from surgical resection than intra-arterial therapy when compared to asymptomatic patients.33 This suggests that for low volume disease or for symptom control, surgical resection should be considered, while in patients with high-volume disease and lack of symptoms, other treatment options including intra-arterial therapies should be considered.
Both the European and North American Neuroendocrine Tumor Society guidelines support the use of TAE, TACE, and TARE in appropriately selected patients as treatment options for patients with NETLM.6,23,24 Further important information will come from the randomized embolization trial for NETLM (RETNET), which is ongoing in patients with progressive or symptomatic unresectable NETLM.47 This trial set out to compare hepatic PFS in patients randomized to either TAE, lipiodol TACE, or DEB TACE, but early complications in the DEB TACE group have led to that arm being closed.
Medical Management
There have been numerous randomized controlled studies showing improvement of PFS in patients with metastatic NETs, suggesting that systemic therapy has a role in management of patients with NETLMs (Table 2). For patients with carcinoid syndrome, SSAs are the mainstay of treatment to control symptoms of hormone excess. Octreotide was the first synthetic SSA developed in the 1980s and was a short-acting formulation given either by continuous infusion or as a subcutaneous injection two to three times per day. In 1998, a long-acting formulation, octreotide long-acting repeatable (LAR), was approved by the FDA, which is typically administered at doses beginning at 20 mg as every 4 weeks by intramuscular injection and can be increased up to 60 mg per month. Although SSAs have long been used for symptom control of hormone excess in patients with NETs, only recently have they been considered as antiproliferative agents for patients with metastatic disease.
Table 2.
Major clinical trials for management of advanced NETs
| Study | Primary Endpoint | Study Population | Study Arms | Primary Endpoint Results | HR / P value |
|---|---|---|---|---|---|
| PROMID | PFS | Metastatic midgut NETs | Octreotide LAR 30 mg every 4 weeks vs. placebo | 14.3 vs 6 months | HR, 0.34; P = 0.000072 |
| CLARINET | PFS | Metastatic enteropancreatic NETs | Lanreotide 120 mg every 4 weeks vs. placebo | Median PFS not reached vs. 18 months | HR, 0.47; P < .001 |
| RADIANT-4 | PFS | Advanced nonfunctioning NETs of the lung or GI tract | Everolimus 10 mg daily vs placebo | 11 vs 3.9 months | HR, 0.48; P < .00001 |
| Sunitinib Malate | PFS | Advanced pancreatic NETs | Sunitinib 37.5 mg daily vs placebo | 11.4 vs 5.5 months | HR, 0.42; P < .001 |
| ECOG 2211 | PFS | Advanced pancreatic NETs | Temozolomide vs. Temozolomide/Capecitabin e | 14.4 vs. 22.7 months | HR, 0.58; P = .023 |
| NETTER-1 | PFS | Metastatic midgut NETs | 177Lu-Dotatate q8 weeks plus best supportive care with ocreotide LAR vs. ocreotide LAR 60 mg every 4 weeks | Median PFS not reached vs 8.4 months | HR, 0.21; P < .0001 |
PROMID
The first clinical trial to demonstrate prolonged time to tumor progression (TTP) of SSAs was the Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors (PROMID) study.48 In this phase III trial, 85 patients with locally inoperable or metastatic well-differentiated midgut NETs were randomly assigned to receive 30 mg octreotide LAR once per month via intramuscular injection or placebo. Octreotide significantly improved time to progression when compared with placebo (14.3 vs 6 months; hazard ratio [HR], 0.34; 95% CI, 0.20 to 0.59; P < .001). Functional and non-functional tumors responded equally well. Of note, the greatest favorable effect was seen in patients with resected primary tumors and low hepatic tumor burden. There was no difference noted in median OS, which will not become clearer with long-term follow-up because the majority of patients in the placebo arm were allowed to cross over to the treatment arm. As a result of this study, octreotide was widely adopted for controlling tumor growth in patients with metastatic midgut NETs.
CLARINET
The Controlled Study of Lanreotide Antiproliferative Response in Neuroendocrine Tumors (CLARINET) was a phase III evaluation of lanreotide in patients with advanced, nonfunctioning, metastatic or inoperable somatostatin receptor positive (SSTR-positive) well-or moderately-differentiated GEP-NETs. Lanreotide differs from octreotide in 3 of its 8 amino acids, and it is a water-based injectable with a nanotubule structure which can be administered as a deep subcutaneous injection (into fat or muscle); octreotide LAR is a complex polymer and must be administered specifically into the muscle for appropriate absorption. Patients were randomized to 120 mg lanreotide via a deep subcutaneous injection every 28 days or placebo. In 205 enrolled patients, lanreotide improved PFS when compared with placebo (18.0 months vs median not reached; HR, 0.47; 95% CI, 0.30 to 0.73; P < .001). There was no difference in median OS, and like in PROMID, obscured by allowing for crossover from the placebo arm to the treatment group.49 In contrast to PROMID, this study included patients with grade 1 or 2 tumors (Ki-67 < 10%), larger liver tumor volume, but patients had stable disease for 3–6 months at baseline. Lanreotide was granted FDA approval in December 2014 for advanced GEP-NETs. Although PROMID evaluated octreotide LAR in midgut NETs and CLARINET studied lanreotide in pancreatic, midgut, hindgut, and unknown primary NETs, the PFS benefit in those trials was thought to be a SSA class effect.
RADIANT-4
The Everolimus plus best supportive care vs placebo plus best supportive care in the treatment of patients with advanced Neuroendocrine Tumors Trial (RADIANT-4) was a phase III trial evaluating the efficacy of Everolimus, an mTOR inhibitor, for the treatment of advanced, non-functional neuroendocrine tumors of the lung or GI tract. Patients with advanced, progressive, nonfunctional, well-differentiated lung or GI NETs were included and randomized in a 2:1 ratio to either oral everolimus (10 mg per day) or placebo. A total of 302 patients were enrolled, with 205 receiving everolimus and 97 placebo. Median PFS in the everolimus group was 11 months (95% CI 9.2–13.3) and 3.9 months in the placebo group (95% CI 3.6–7.4). Everolimus was found to be associated with a 52% reduction in the estimated risk of progression or death with a hazard ratio of 0.48 (95% CI 0.35–0.67, p<0.00001). Additionally, OS analysis indicated that administration of everolimus might be associated with a decreased risk of death (HR 0.64 [95% CI 0.40–1.05, p=0.037).50 This was the first targeted therapy to show good anti-tumor activity with acceptable toxicity in a range of NET sites.
Sunitinib
Sunitinib, an oral VEGF tyrosine kinase inhibitor, was evaluated in a phase III study of 171 patients with advanced, progressive, well-differentiated pancreatic NETs. Patients were randomly assigned to receive 37.5 mg of oral sunitinib daily or placebo. The study was halted early after the data and safety monitoring committee noted more adverse events and deaths in the placebo arm and improved PFS in the sunitinib arm, which was 11.5 months vs 5.5 months in the placebo arm (HR 0.42; 95% CI 0.26 – 0.66, p<0.001). This trial led to FDA approval of sunitinib for advanced pancreatic NETs in 2011.51
ECOG 2211
ECOG 2211 was a two arm, randomized phase II trial which compared efficacy and response of oral temozolomide to the combination of oral capecitabine and temozolomide in 144 patients with metastatic or unresectable low to intermediate grade pancreatic NETs with progression within the prior 12 months. Median PFS was found to be an impressive 22.7 months in the capecitabine/temozolomide group as compared to 14.4 months in the temozolomide only group. At median follow up of 29 months, OS was not reached in the capecitabine/temozolomide and was 38 months for the temozolomide only group,52 with similar partial/complete response rates of 33% and 28%, respectively, using the response evaluation criteria in solid tumors (RECIST 1.1) (P. Kunz, personal communication 3/14/20). Capecitabine/temozolomide therefore appears to be a promising therapy for the management of metastatic pancreatic NETs with a favorable impact on survival and is well-tolerated. Anecdotally, this combination appears to have the greatest potential for shrinking both hepatic metastases and their primary pancreatic NETs, making it a promising combinationfor neoadjuvant treatment.53
Radiolabeled SSAs and NETTER-1
Radiolabeled SSA therapy, also known as PRRT, was initially described for NETs in the 1990s and is another form of targeted systemic therapy which allows for the delivery of radionuclides directly to tumor cells.54,55 In 2008, Kwekkeboom et a.l reported a large retrospective analysis of 504 patients with NETs (458 GEP-NETs) treated with 177Lu-Dotatate between 2000 and 2006.55 Complete and partial tumor responses occurred in 2% and 28% of the patients, respectively, and minor tumor response occurred in 16% of the patients (SWOG criteria, with partial response being >50% size reduction in cross-product measurement of index lesion on imaging, and minor response a decrease >25% and <50%). Median time to progression was 40 months, and median OS was 46 months. Serious delayed toxicities occurred in a minority of patients and included renal insufficiency (two patients), liver toxicity (three patients), and myelodysplastic syndrome (four patients). These studies from Europe showed the efficacy and safety of radiolabeled SSAs in a large number of patients, but these were phase II trials that required further validation.
The NETTER-1 trial was a randomized controlled trial evaluating the efficacy and safety of 177Lu-Dotatate in patients with advanced, progressive SSTR-positive jejunoileal and proximal colonic (midgut) NETs. This study randomized 229 patients to receive either 177Lu-Dotatate at a dose of 7.4 gigabecquerel (GBq) every 8 weeks via four intravenous infusions plus 30 mg ocreotide LAR intramuscularly (IM) every four weeks or to 60 mg ocreotide LAR IM every four weeks. The primary endpoint in this study was PFS and secondary endpoints were objective response rate (ORR), OS, safety and side effect profiles. At the interim analysis, the estimated rate of PFS at month 20 was 65.2% in the PRRT group vs 10.8% in the control group (95% CI 50–76.8 vs 95% CI 3.5–23.0). Response rates were 18% in the PRRT group vs 3% in the control group.56 At a median follow up of 24 months, myelodysplastic syndrome was reported in 2.7% of patients in the PRRT arm and the most significant adverse effects were lymphopenia and increased GGT. 177Lu-Dotatate was ultimately approved for the treatment of GEP-NETs by the FDA in January of 2018.
Although there are multiple treatment options for neuroendocrine patients, the optimal sequence of these treatments is unknown and requires further investigation. Treatment is also determined by patient preference, the clinical course of disease, and local availability of treatment modalities. It is also important to note that many of the trials described allowed patients to crossover into the treatment arm, which may obfuscate true differences in OS between therapies. With continued research to identify molecular-based markers and additional clinical trials comparing promising new therapeutics, the treatment of neuroendocrine tumors will likely continue to become more precise and individualized.
Conclusion
Hepatic metastases develop in a large number of NET patients and significantly worsen their prognosis. Current treatment algorithms include a variety of surgical and non-surgical modalities. The treatment of NETLMs lacks standardization and this is in part because of the dearth of prospective randomized studies comparing treatment modalities in homogenous cohorts of patients, especially regarding the impact of surgical interventions. In patients with primary tumors in place, surgical exploration with primary resection and an attempt to cytoreduce to ≥70–90% is a reasonable approach, depending on the tumor burden, symptoms, patient co-morbidities, and tumor grade. Surgical treatment can include major resections depending upon the distribution of disease, but parenchymal sparing procedures such as wedge resections, enucleations, and ablations are gaining in favor and yield similar results. Liver-directed therapies also play an important role for palliation of symptoms and to slow progression, especially when tumors are not amenable to surgical cytoreduction. Systemic therapy options have shown modest improvements in PFS but not OS. The relatively new modality of PRRT has shown significant promise for survival benefits in GEP-NETs, as has the combination of capecitabine/temozolomide in pancreatic NETs. Patients with NETs, especially those with extensive metastatic disease with NETLMs, should be managed in centers of expertise to enable a multidisciplinary approach to treatment.
Synopsis:
Neuroendocrine tumors frequently metastasize to the liver. Although they generally progress slowly, hepatic metastases are the leading cause of death in NET patients and frquently cause symptoms. This review highlights the multimodality management of neuroendocrine liver metastases.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Alexandra Gangi, Division of Surgical Oncology, Department of Surgery, Cedars Sinai Medical Center, 8700 Beverly Blvd; AC 1049, Los Angeles, CA 90048.
James R. Howe, Surgical Oncology and Endocrine Surgery, University of Iowa Carver College of Medicine, 4644 JCP, 200 Hawkins Drive, University of Hospitals and Clinics, Iowa City, IA, 52242.
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–445. doi: 10.1016/s1072-7515(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 5.Niederle B, Pape U-F, Costa F, et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology. 2016;103(2):125–138. doi: 10.1159/000443170 [DOI] [PubMed] [Google Scholar]
- 6.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95(2):157–176. doi: 10.1159/000335597 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16(3):885–894. doi: 10.1677/ERC-09-0042 [DOI] [PubMed] [Google Scholar]
- 8.Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(17):2372–2377. doi: 10.1200/JCO.2010.33.0688 [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Hong S-M, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11–16. doi: 10.1016/j.anndiagpath.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1–342. [PubMed] [Google Scholar]
- 11.Foster J, Lundy J. Liver Metastases. In: Current Problems in Surgery. 18 ; 1981:157–202. [DOI] [PubMed] [Google Scholar]
- 12.McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108(6):1091–1096. [PubMed] [Google Scholar]
- 13.Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169(1):36–42; discussion 42–43. doi: 10.1016/s0002-9610(99)80107-x [DOI] [PubMed] [Google Scholar]
- 14.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29–37. doi: 10.1016/S1072-7515(03)00230-8 [DOI] [PubMed] [Google Scholar]
- 15.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144(4):645–651; discussion 651–653. doi: 10.1016/j.surg.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 16.Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery. 2014;156(6):1369–1376; discussion 1376–1377. doi: 10.1016/j.surg.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery. 2018;163(1):218–225. doi: 10.1016/j.surg.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159(1):320–333. doi: 10.1016/j.surg.2015.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17(12):3129–3136. doi: 10.1245/s10434-010-1154-5 [DOI] [PubMed] [Google Scholar]
- 20.Boudreaux JP, Wang Y-Z, Diebold AE, et al. A single institution’s experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J Am Coll Surg. 2014;218(4):837–844. doi: 10.1016/j.jamcollsurg.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 21.Scott AT, Breheny PJ, Keck KJ, et al. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery. 2019;165(1):166–175. doi: 10.1016/j.surg.2018.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena A, Chua TC, Perera M, Chu F, Morris DL. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol. 2012;21(3):e131–141. doi: 10.1016/j.suronc.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 23.Howe JR, Cardona K, Fraker DL, et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–731. doi: 10.1097/MPA.0000000000000846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(1):1–33. doi: 10.1097/MPA.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elias D, Lefevre JH, Duvillard P, et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think.. Ann Surg. 2010;251(2):307–310. doi: 10.1097/SLA.0b013e3181bdf8cf [DOI] [PubMed] [Google Scholar]
- 26.Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162(3):525–536. doi: 10.1016/j.surg.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47(4):460–466. doi: 10.1016/j.jhep.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferro V, Sposito C, Coppa J, et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant. 2016;16(10):2892–2902. doi: 10.1111/ajt.13831 [DOI] [PubMed] [Google Scholar]
- 29.Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg Chic Ill 1960. 2011;146(8):953–958. doi: 10.1001/archsurg.2011.186 [DOI] [PubMed] [Google Scholar]
- 30.Fan ST, Le Treut YP, Mazzaferro V, et al. Liver transplantation for neuroendocrine tumour liver metastases. HPB. 2015;17(1):23–28. doi: 10.1111/hpb.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norlén O, Daskalakis K, Öberg K, Åkerström G, Stålberg P, Hellman P. Indication for Liver Transplantation in Young Patients with Small Intestinal NETs Is Rare? World J Surg. 2014;38(3):742–747. doi: 10.1007/s00268-013-2331-z [DOI] [PubMed] [Google Scholar]
- 32.Gamblin TC, Christians K, Pappas SG. Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg Oncol Clin N Am. 2011;20(2):273–279, vii-viii. doi: 10.1016/j.soc.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 33.Mayo SC, de Jong MC, Bloomston M, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18(13):3657–3665. doi: 10.1245/s10434-011-1832-y [DOI] [PubMed] [Google Scholar]
- 34.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142(1):10–19. doi: 10.1016/j.surg.2007.01.036 [DOI] [PubMed] [Google Scholar]
- 35.Berber E, Siperstein A. Local Recurrence After Laparoscopic Radiofrequency Ablation of Liver Tumors: An Analysis of 1032 Tumors. Ann Surg Oncol. 2008;15(10):2757–2764. doi: 10.1245/s10434-008-0043-7 [DOI] [PubMed] [Google Scholar]
- 36.Cannon R, Ellis S, Hayes D, Narayanan G, Martin RCG. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107(5):544–549. doi: 10.1002/jso.23280 [DOI] [PubMed] [Google Scholar]
- 37.Osborne DA, Zervos EE, Strosberg J, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13(4):572–581. doi: 10.1245/ASO.2006.03.071 [DOI] [PubMed] [Google Scholar]
- 38.Kennedy AS. Hepatic-directed Therapies in Patients with Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30(1):193–207. doi: 10.1016/j.hoc.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(8):1590–1602. doi: 10.1002/cncr.21389 [DOI] [PubMed] [Google Scholar]
- 40.de Baere T, Deschamps F, Tselikas L, et al. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. Eur J Endocrinol. 2015;172(4):R151–166. doi: 10.1530/EJE-14-0630 [DOI] [PubMed] [Google Scholar]
- 41.Dong XD, Carr BI. Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: a long-term follow-up in 123 patients. Med Oncol Northwood Lond Engl. 2011;28 Suppl 1:S286–290. doi: 10.1007/s12032-010-9750-6 [DOI] [PubMed] [Google Scholar]
- 42.Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control J Moffitt Cancer Cent. 2006;13(1):72–78. doi: 10.1177/107327480601300110 [DOI] [PubMed] [Google Scholar]
- 43.Ho AS, Picus J, Darcy MD, et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188(5):1201–1207. doi: 10.2214/AJR.06.0933 [DOI] [PubMed] [Google Scholar]
- 44.Bhagat N, Reyes DK, Lin M, et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol. 2013;36(2):449–459. doi: 10.1007/s00270-012-0424-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao CQ, Yan TD, Bester L, Liauw W, Morris DL. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg. 2010;97(4):537–543. doi: 10.1002/bjs.6931 [DOI] [PubMed] [Google Scholar]
- 46.Gebhard TA, Suhocki P, Engstrom BI, et al. Metastatic neuroendocrine tumors to the liver: treatment with bland embolization versus radioembolization. J Vasc Interv Radiol. 2013;24(4):S13. doi: 10.1016/j.jvir.2013.01.034 [DOI] [Google Scholar]
- 47.Chen JX, Wileyto EP, Soulen MC. Randomized Embolization Trial for NeuroEndocrine Tumor Metastases to the Liver (RETNET): study protocol for a randomized controlled trial. Trials. 2018;19(1):390. doi: 10.1186/s13063-018-2782-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinke A, Müller H-H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510 [DOI] [PubMed] [Google Scholar]
- 49.Caplin ME, Pavel M, wikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 50.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, nonfunctional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet Lond Engl. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 52.Kunz PL, Catalano PJ, Nimeiri H, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;36(15_suppl):4004–4004. doi: 10.1200/JCO.2018.36.15_suppl.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(9):759–767. doi: 10.1530/ERC-16-0147 [DOI] [PubMed] [Google Scholar]
- 54.Krenning EP, Kooij PP, Bakker WH, et al. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci. 1994;733:496–506. doi: 10.1111/j.1749-6632.1994.tb17300.x [DOI] [PubMed] [Google Scholar]
- 55.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(13):2124–2130. doi: 10.1200/JCO.2007.15.2553 [DOI] [PubMed] [Google Scholar]
- 56.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]


