Abstract
Group 2 innate lymphoid cells (ILC2s) are implicated in host defense and inflammatory disease, but these potential functional roles need more precise definition, particularly using advanced technologies to better target ILC2s and engaging experimental models that better manifest both acute infection and chronic even life-long disease. Here we use a mouse model that applies improved genetic definition of ILC2s via IL7r-conditional Rora gene targeting and takes advantage of a distinct progression from acute illness to chronic disease based on a persistent type 2 immune response to respiratory infection with a natural pathogen (Sendai virus). We first show that ILC2s are activated but are not required to handle acute illness after respiratory viral infection. In contrast, we find that this type of infection also activates ILC2s chronically for IL-13 production and consequent asthma-like disease traits that peak and last long after active viral infection is cleared. However, to manifest this type of disease, Csf1-dependent myeloid-macrophage lineage are also active at two levels: first, at a downstream level, this lineage provides lung tissue macrophages (interstitial macrophages and tissue monocytes) that represent a major site of Il13 gene expression in the diseased lung; and second, at an upstream level, this same lineage is required for Il33 gene induction that is necessary to activate ILC2s for participation in disease at all, including IL-13 production. Together, these findings provide a revised scheme for understanding and controlling the innate immune response leading to long-term post-viral lung diseases with features of asthma and related progressive conditions.
1. Introduction
The innate immune system is proving to have new cellular components that orchestrate tissue homeostasis under normal conditions and the response to environmental insults that lead to injury and disease. In particular, there has been a surge in reports on the role of innate lymphoid cells (ILCs) and the special role of group 2 ILCs (designated ILC2s) in the response to infectious and allergenic agents (1). Indeed, some of the first examples of ILC existence and behavior included the immune response to helminth infection and allergen challenge at gut and airway sites in mice (2-8) as a model for what might occur in corresponding human inflammatory diseases such as asthma (9-12). Together, the picture emerged for ILC2s as a key immune component for the development of allergic asthma (13-15). The ILC2 population was also reported to defend against injury due to respiratory viral infections such as influenza A virus (IAV) (16) that is also linked to asthma (17).
However, these initial studies of ILC2s were challenged by the difficulty in specific definition of ILC2 function, generally based on genetic deletion. This challenge has continued into the latest round of studies using Rag2−/−Il2rg−/−, Bcl11bflox/−, or Plzf−/− mice (13, 18-25) and Il7ra-Cre mice without an additional cell-specific floxed-target gene (26, 27). A recent advancement has been the combination of Il7ra-Cre crossed to Rora-flox transgenic mice to more selectively eliminate ILC2s (28, 29), taking advantage of requirements specific for ILC2s (30-32) and avoiding complications of Rorα-deficiency at other cell sites (33). Thus, the application of Il7ra-Cre–Rora-flox mice was used successfully to define a role for ILC2s to partner with CD4+ Th2 cells in the type 2 immune response against helminth infection and with Th2 and dendritic cells in the immune memory response to allergen (28, 29). However, this technical advance has not been applied to the key issues of host defense and chronic inflammatory disease. Indeed, the role of ILC2s remains undefined in long-term models in general, leaving uncertainty for the pathological role for ILC2s alone and in combination with other immune and stromal cell populations (1).
To address these issues, the current study engaged mice that were bred as Il7rawt/Cre-Rorafl/fl mice to achieve more selective and efficient deletion of ILC2s. In addition, we employed a mouse model that manifests both acute illness and subsequent progression to chronic inflammatory disease after infection with the natural mouse pathogen Sendai virus (SeV) (34-37). To date, the SeV model appears similar to the response to human pathogens such as influenza A virus (IAV) and respiratory enterovirus (EV-D68) in mice (17, 37) and the type 2 immune response found in humans with lung disease due to asthma and chronic obstructive pulmonary disease (COPD) (34, 35, 38, 39). Together, the present approach offered opportunities not found in models that lack long-term disease and ILC2-specific targeting (40-42) and thereby provided for a series of unexpected findings to establish a distinct paradigm for ILC2 engagement in host defense and inflammatory disease in collaboration with neighboring niches of myeloid and epithelial cell populations.
Materials and methods
Mice
Male and female wild-type C57BL/6J mice (000664) mice were obtained from The Jackson Laboratory. The Il7rawt/Cre-Rorafl/fl mice were bred by crossing Il7rawt/Cre and Rorafl/fl generated as described previously (28, 29) with the Rorafl/fl mice kindly provided by Andrew McKenzie (Cambridge, UK) and the Il7ra-Cre mice by Hans-Reimer Rodewald (German Cancer Research Center, Heidelberg, GDR). The Il13wt/gfp mice were initially generated as described previously (7) and were also kindly provided by Andrew McKenzie. The op/opT mice were generated as described previously (43) and were kindly provided by Nandini Ghosh-Choudhury (University of Texas, San Antonio). These mice were crossed to wild-type mice to generate heterozygous wt/opT mice and littermate wt/wt control mice as described previously (36). The Il33cherry/cherry mice were generated with insertion of an mCherry-reporter cassette between exons 4 and 5 of the Il33 gene in ES cells from C57BL/6J mice. Levels of IL-33 expression were checked with goat anti-mouse IL-33 Ab (R&D Systems, AF3626) for western blotting of lung tissue samples as described previously (44). All mouse strains were maintained on a C57BL/6J genetic background and except where indicated were studied as the gene-targeted strain compared to littermate control mice housed under the same conditions.
Mouse model
All mice were maintained and co-housed in a barrier facility using cages fitted with micro-isolator lids. Animal husbandry and experimental procedures were approved by the Animal Studies Committees of Washington University School of Medicine in accordance with the guidelines from the National Institutes of Health. SeV was obtained from ATCC (Sendai/52 Fushimi strain, ATCC VR-105) and prepared and titered by plaque-forming assay and qPCR assay as described previously (34). Mice were infected with SeV (2.6 × 105 PFU) as described previously (37). Dosing was performed intranasally using SeV in 30 μl of PBS or an equivalent amount of UV-inactivated virus or PBS alone under ketamine/xylazine anesthesia at 6-9 wk of age. Results from male and female mice were pooled since no significant differences were found between sexes as reported initially (45) and confirmed recently (37) and in the present experiments (data not shown). Viral titers for stock solutions and lung infections were monitored by PCR assay using primers defined previously (37). All animals undergo daily cage side observation for clinical behavior and food consumption.
Flow cytometry
Single cell suspensions were generated from minced lung tissue that was subjected to collagenase (Liberase TM Research Grade, Roche), hyaluronidase (Sigma), DNAse I (Sigma), and Dispase II (Roche) digestion for 45 min at 37 °C and then treated with ACK buffer (Lonza) to remove red blood cells. Following FcR blockade, lung cell suspensions were incubated with labeled antibodies and were sorted using a Sony SY3200 Synergy high-speed cell sorter. The following antibodies were used: anti- mouse CD31 (clone MEC 13.3; BD Biosciences), anti-mouse CD45 (clone 30-F11; BD Biosciences), anti-mouse EpCAM (clone G8.8; BioLegend), anti-mouse F4/80 (Clone BM8; eBiosciences), anti-mouse Ly6G (Clone 1A8, BD Biosciences), anti-mouse CD11c (Clone HL3; BD Biosciences), anti-mouse Siglec F (Clone E50-2440; BD Biosciences), anti-mouse CD11b (Clone M1/70; BD Biosciences), Mouse Lineage Antibody cocktail (BD Biosciences), anti-mouse 90.2 (Clone 53-2.1; BD Biosciences), anti-mouse ST2 (Clone DIH9; BioLegend), anti-mouse CD25 (Clone PC61; BD Biosciences), anti-mouse CD117 (Clone 2B8, BD Biosciences), anti-mouse CD278 (Clone 7E.17G9, BD Biosciences), anti-mouse 6A/E (Clone E13-161.7, BD Biosciences), anti-mouse MHCII (Clone M5/114.15.2, eBiosciences), anti-mouse CD3e (Clone 145-2C11, BD Biosciences), anti-mouse NK1.1 (Clone PK136, BD Biosciences), and anti-GFP (Clone 1A12-6-18, BD Biosciences). Flow cytometry results were plotted and analyzed using FlowJo software (TreeStar).
Immunostaining
Cells were fixed and permeabilized, and lung sections were incubated with citrate-based antigen unmasking solution for antigen retrieval as described previously (38). Immunostaining was performed using the following antibodies: chicken anti-GFP (Abcam), rat anti-mouse F4/80 (clone CI:A3-1; Abcam), rabbit anti-human GATA-3 (clone D13C9, Cell Signaling Technology, goat anti-mouse IL-13 (R&D systems), and goat anti-mouse IL-33 (R&D systems). Antibody binding was visualized using Alexa 488 or 594 conjugated secondary antibody (Life Technologies). All slides were also counterstained with Prolong Gold with DAPI (Life Technologies) and then imaged by immunofluorescent microscopy using a Leica DM5000 B microscope. Staining was quantified in whole lung sections using a NanoZoomer S60 slide scanner (Hamamatsu) and ImageJ software.
Real-time quantitative PCR assay
RNA was purified from lung homogenates and cells using Trizol (Invitrogen) and was converted to cDNA using a High-Capacity cDNA Archive kit (Life Technologies). Target mRNA was quantified by real-time PCR assay using specific fluorogenic probes and primer sets and the Fast Universal PCR Master Mix system (Applied Biosystems). The forward and reverse primers and probes for SeV-NP were 5’- GGCGGTGGTGCAATTGAG-3’, 5’-CATGAGCTTCTGTTTCTAGGTCGAT-3’, 5’- AGCTCTAGACAATGCC-3’; for Il13 were 5’-GGTGCCAAGATCTGTGTCTC-3’, 5’- CCACACTCCATACCATGCTG-3’, 5’-AAGACCAGACTCCCCTGTGCAAC-3’; for Muc5ac were 5’- TACCACTCCCTGCTTCTGCAGCGTGTCA-3’, 5’-ATAGTAACAGTGGCCATCAAGGTCTGTCT-3’, 5’- TATACCCCTTGGGATCCATCATCTACA-3’; for Arg1 were 5’-AGTGTTGATGTCAGTGTGAGC-3’, 5’- GAATGGAAGAGTCAGTGTGGT-3’, 5’-ACAGTCTGGCAGTTGGAAGCATCT-3’; for Il33 were 5’- TCATGTTCACCATCAGCTTCT-3’, 5’-GTGCTACTACGCTACTATGAGTC-3’, 5’- ACCGTCGCCTGATTGACTTGCA-3’; for Il12b were 5’-TGTCCTCAGAAGCTAACCATC-3’, 5’- TCCAGTCCACCTCTACAACA-3’, 5’-ACGTCTTTCTCCAGCTCCCACATG-3’; for Gata3 were 5’- CCTTATCAAGCCCAAGCGAA-3’, 5’-GTCCCCATTAGCGTTCCTC-3’, 5’- TGTCCCTGCTCTCCTTGCTGC-3’; for Rora were 5’-TGGAGACAAATCGTCAGGAAT C-3’, 5’-GACAGGAGTAGGTGGCATTG-3’, 5’- TGG TGT CATTAC GTG TGA AGG CTG C-3’; for Il1rl1 were 5’- AAT CCT CCA TAC AAC CAC ACA A-3’, 5’- GACATCAGCCAAGAAGTGAGAG-3’, 5’- AAGTAT TGCCTGTTCAGCTTGCTTTGG-3’; for Areg were 5’-GTCACTATCTTTGTCTCTGCCA -3’, 5’- CCTCCTTCTTTCTTCTGTTTCTCC-3’, 5’-AGTATCGTTTCCAAAGGTGCACTGTGA-3’. Samples were assayed with the 7300HT or QuantStudio 6 Fast Real-Time PCR System and analyzed using Fast System Software (Applied Biosystems). All real-time PCR data was normalized to the level of GAPDH mRNA. Values were expressed as fold-change based on the delta-delta Ct method as described previously (35) with the exception of Fig. 3f where Il13 mRNA was quantified by copy number using an Il13-expressing plasmid as an internal standard (38).
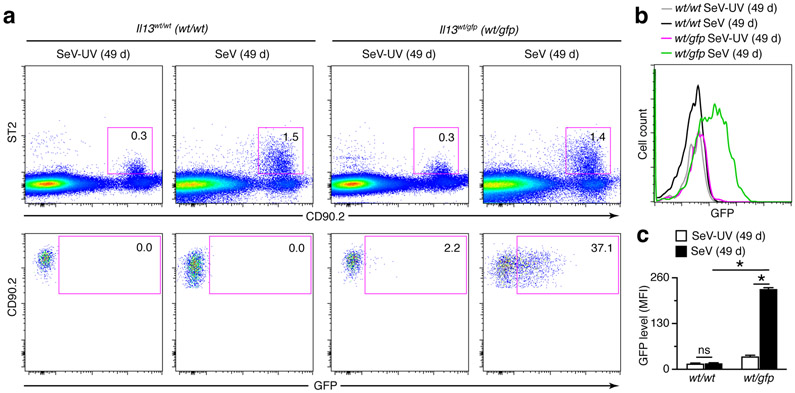
FIGURE 3.
ILC2s contribute to chronic IL-13 production after viral infection based on Il13 reporter gene expression. (a) Flow cytograms for ILC2s (Lin−CD90.2+ST2+GFP+) and GFP-expressing ILC2s (Lin−CD90.2+ST2+GFP+) in lungs of Il13wt/wt (wt/wt) and Il13wt/gfp (wt/gfp) mice at 49 d after SeV or SeV-UV. (b) Histograms for ILC2s (Lin−CD90.2+ST2+) in lungs of Il13wt/wt and Il13wt/gfp mice at 49 d after infection with SeV or SeV-UV based on GFP signal detection. (c) Levels of IL-13-GFP based on MFI for conditions in (b). All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
ELISA
For IL-33 production in lung tissues, mouse lungs were homogenized in RIPA buffer (Sigma) supplemented with protease inhibitor cocktail (Roche) and 1 mM EDTA. For IL-33 production in bronchoalveolar lavage (BAL), mouse lungs were washed twice with 1 ml of PBS on ice. The samples from each mouse were pooled, spun to remove cells, supplemented with protease inhibitor cocktail and 1 mM EDTA. Levels of IL-33 were determined using the Duoset ELISA kit (R&D Systems).
Statistical analysis
All data are presented as mean and s.e.m. and are representative of at least three experiments with at least 5 data points per experiment. Unpaired student’s t-test with Bonferroni correction as well as mixed-model repeated measures analysis of variance with Tukey correction for multiple comparisons were used to assess statistical significance between means. In all cases, significance threshold was set at P<0.05.
Results
ILC2s do not influence acute respiratory viral infection
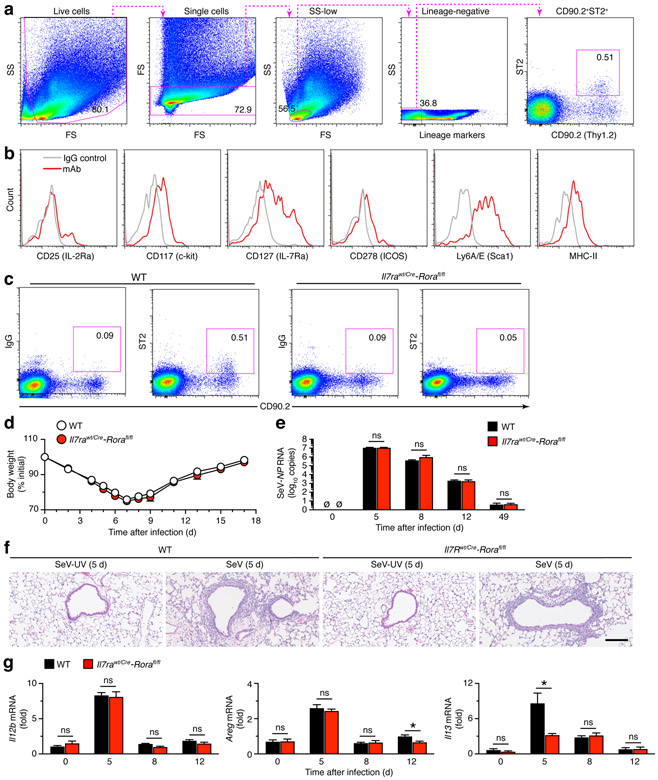
Initial experiments established the presence of ILC2s in the lung at baseline using flow cytometry for forward scatter (FS) and side scatter (SS) combined with absence of immune cell lineage markers (CD11c, NK1.1, CD3e, CD45R/B220, CD11b, TER-119, and Ly6G/6C) and presence of CD90.2 (alloantigen Thy-1.2) and ST2 (IL-33R subunit IL-1rl1) (Fig. 1a). This cell population also expressed CD25 (IL-2rα), CD117 (c-Kit), CD127 (IL-7rα), CD278 (ICOS), Lyc6A/E (Sca1), and MHC-II based on additional flow cytometry analysis (Fig. 1b). Together, this profile for ILC2s is similar to reports using the comparable methods (16, 29, 46, 47). Moreover, this ILC2 population was no longer detected in the lungs of Il7rawt/Cre-Rorafl/fl mice that were designed to specifically delete ILC2s (Fig. 1c). ILC2 deficiency was consistent with previous work using this genetic approach (28, 29).
FIGURE 1.
ILC2s do not influence acute illness after viral infection. (a) Flow cytometry scheme for analysis of ILC2s in lungs from wild-type C57BL/6J (WT) mice assessed at baseline without infection. Lineage markers are CD11c, NK1.1, CD3e, CD45R/B220, CD11b, TER-119, and Ly6G/6C. (b) Expression of biomarkers in lung ILC2 (CD90.2+ST2+) population from WT mice at baseline. (c) Flow cytograms for levels of ILC2s from lungs of WT and Il7rwt/Cre-Rorafl/fl mice at baseline. Values indicate percentage of cells in the corresponding gate. (d) Body weights at indicated times after infection with SeV in WT and Il7rwt/Cre-Rorafl/fl mice. (e) Corresponding viral loads in lungs for conditions in (d). (f) Hematoxylin and eosin staining of lung sections for indicated conditions. Scale bar, 500 μm. (g) Levels of mRNA expression in lungs from WT and Il7rwt/Cre-Rorafl/fl mice at indicated times after SeV infection. All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
These findings allowed us to next test the effect of ILC2 deficiency on the outcome from respiratory viral infection. As introduced above, we used SeV, as a natural mouse pathogen capable of replicating at high efficiency and causing acute infectious illness across outbred and inbred strains (including C57BL/6J). In this setting, we found no significant changes in the typical weight loss, viral RNA level and clearance, or immune cell accumulation in Il7rawt/Cre-Rorafl/fl mice compared to wild-type (WT) control mice (Fig. 1d-f). Similarly, we found no significant differences in ILC2-deficient mice in Il12b mRNA (Fig. 1g) as a validated marker of acute inflammation after SeV infection (48). In addition, we detected no significant differences in amphiregulin (Areg) mRNA level in ILC2-deficient mice except for a slight decrease at 12 d after infection (Fig. 1g). However, by this time, repair of epithelial injury and clearance of infectious virus are already complete (37, 49). Thus, the results are consistent with normal recovery in ILC2-deficient mice as noted above (Fig. 1d-f). In addition, we detected attenuation of the usual increase in lung levels of Il13 mRNA at the time of peak viral titer (5 d after infection) and not at other time points (Fig. 1g), consistent with a transient type 2 immune response found after viral infection and the role of ILC2s in IL-13 production during acute activation in gut and lung (4, 5, 7, 8, 10).
ILC2s contribute to chronic lung disease after viral infection
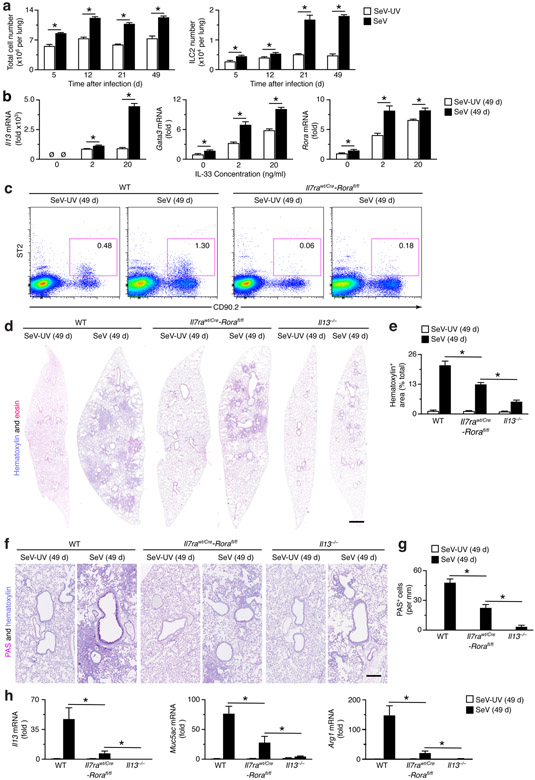
We next studied the behavior and influence of ILC2s during the development of chronic lung disease that develops after clearance of active viral infection. For SeV infection, chronic disease becomes detectable at 21 d and maximal at 49 d after infection as key time points for study (34, 35, 37, 50). This strategy showed that the levels of ILC2s in the lung were increased markedly at 21 and 49 d after SeV infection in WT mice and were more pronounced than the increases at 5 d after infection or the overall increases in total cell numbers that are typical of acute illness and chronic lung disease (Fig. 2a). In addition, ILC2s that were FACS-purified from the lung showed an increased induction of ILC2 biomarkers Il13, Gata3, and Rora mRNA in response to IL-33 stimulation at 49 d after SeV infection compared to SeV-UV controls (Fig. 2b). As expected, the increase in ILC2s found in WT mice was markedly attenuated in Il7rawt/Cre-Rorafl/fl mice based on flow cytograms at 49 d after infection (Fig. 2c). Together, these findings provided for ILC2 accumulation and activation in concert with the development and progression of chronic lung disease after viral infection.
FIGURE 2.
ILC2s do not fully account for chronic lung disease after viral infection. (a) Levels of total cells and ILC2s in the lungs of WT mice at indicated times after infection with SeV or Se-UV as determined by flow cytometry. (b) Levels of ILC2 markers (Il13, Gata3, and Rora) mRNA in ILC2s that were FACS-isolated from lung and treated with IL-33 (0-20 ng/ml). (c) Cytograms for ILC2s (Lin−CD90.2+ST2+) in WT and Il7rwt/Cre-Rorafl/fl mice at 49 d after SeV or SeV-UV. (d) Hematoxylin and eosin staining of full-lung sections from indicated mouse strains at 49 d after SeV or SeV-UV. (e) Quantitation of tissue staining in (d) using image-analysis software. (f) PAS and hematoxylin staining of lung sections for conditions in (d). (g) Quantitation of tissue staining in (f). (h) Levels of indicated mRNAs in lung tissue for conditions in (d). All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
To pursue any possible function of ILC2s in the development of chronic lung disease, we next compared WT to Il7rawt/Cre-Rorafl/fl mice for signs of this disease at 49 d after SeV infection. Consistent with ILC2 accumulation and activation with disease, we found significant attenuation of hematoxylin+ cell-staining (a sign of focal immune and epithelial cell accumulation) in lung sections from Il7rawt/Cre-Rorafl/fl compared to WT mice at 49 d after SeV infection (Fig. 2d,e). Similarly, we found attenuation of increased PAS+ staining (a sign of focal mucus production) in lung sections from Il7rawt/Cre-Rorafl/fl compared to WT mice (Fig. 2f,g). In concert with these observations, we also detected reduction of the usual increases in Il13, Muc5ac, and Arg1 mRNA levels (as signs of a type 2 immune response and mucus production) in the lungs of Il7rawt/Cre-Rorafl/fl compared to WT mice at 49 d after SeV infection (Fig. 2h). Together, the findings established a significant role for ILC2s in the development of chronic lung disease after viral infection. However, we also recognized that the degree of attenuation in Il7rawt/Cre-Rorafl/fl mice was incomplete in comparison to Il13−/− mice for all phenotypes, i.e., hematoxylin+ staining (Fig. 2d,e), PAS+ staining (Fig. 2f,g), and Il13, Muc5ac, and Arg1 mRNA levels (Fig. 2h). These results indicated that ILC2 deficiency alone cannot fully block the development of chronic lung disease after viral infection, and the remaining effect might be due at least in part to a separate cellular mechanism for IL-13 production in this setting.
ILC2s and macrophages contribute to IL-13 production
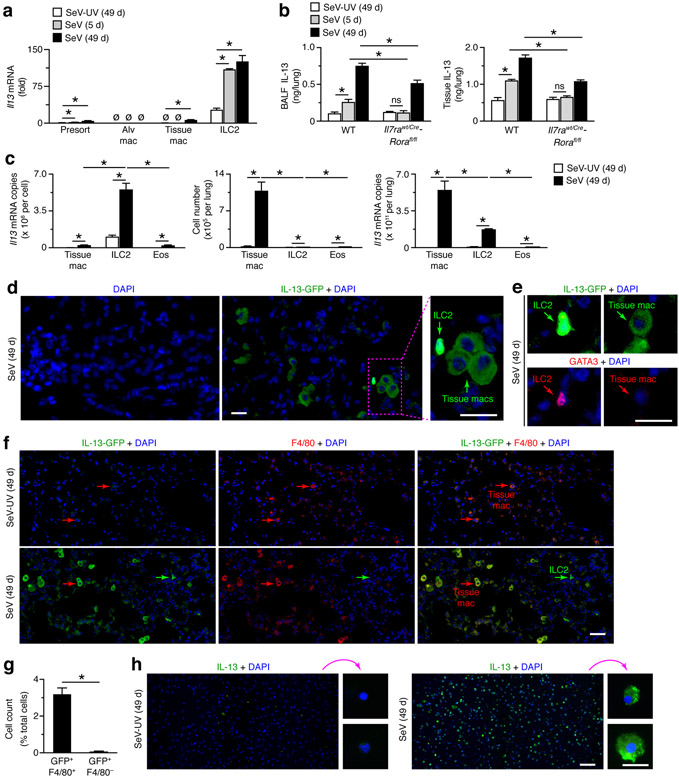
Our previous work indicated that lung macrophages (but not mast cells, basophils, neutrophils, dendritic cells, B cells, CD4+ T cells, CD8+ T cells, NK cells, or NKT cells) can also be a source of increased IL-13 production after viral infection based on PCR-assay for Il13 mRNA and immunostaining for IL-13, and thereby contribute to the development of chronic lung disease after SeV infection (34, 36). To more precisely define the cellular source of IL-13, here we studied IL-13-GFP reporter mice, using heterozygous Il13wt/gfp mice that manifest the same degree of acute illness and chronic lung disease as wild-type control (Il13wt/wt) mice after SeV infection (data not shown). Accordingly, we found a similarly increased percentage of lineage−ST2+CD90.2+ ILC2s in lungs of Il13wt/wt and Il13wt/gfp mice at 49 d after SeV infection compared to SeV-UV control based on flow cytograms from these conditions (Fig. 3a). In addition, we found a marked increase in GFP+ ILC2s at 49 d after SeV infection compared to SeV-UV in lungs of Il13wt/gfp mice, and both of these values were increased compared to background levels found in WT mice (Fig. 3a). Similarly, we found high-level IL-13 expression marked by GFP fluorescence in ILC2s from the lungs of Il13wt/gfp mice compared to Il13wt/wt mice, and this signal was significantly increased at 49 d after SeV compared to SeV-UV control based on mean fluorescence intensity (MFI) (Fig. 3b,c).
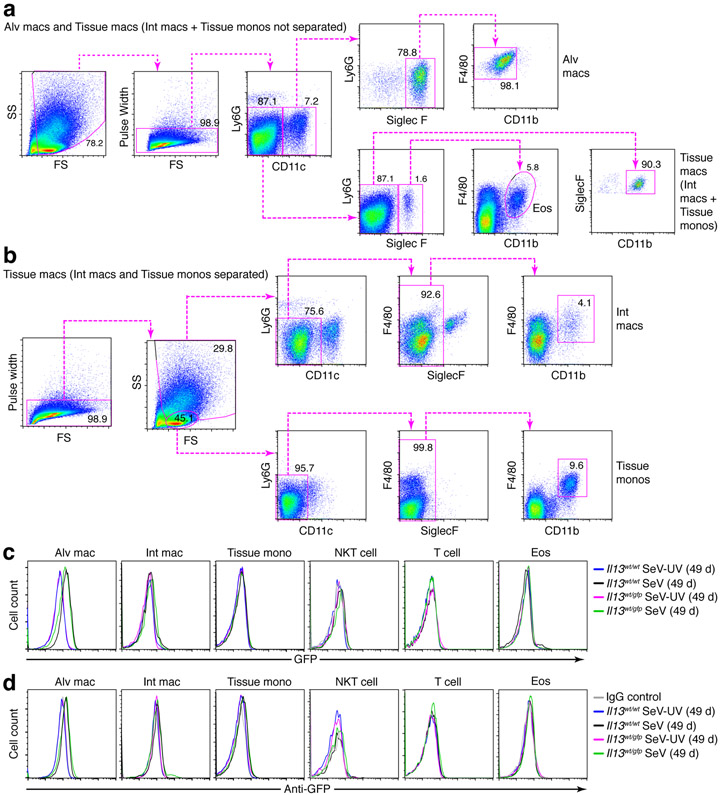
We also checked for GFP expression in lung macrophage populations based on flow cytometry schemes that separated these populations into alveolar macrophages (Ly6G−CD11c+Siglec-F+F4/80+CD11b−) and tissue macrophages (Ly6G−CD11c−Siglec-F−F4/80+CD11b+) and further separated tissue macrophages into interstitial macrophages and tissue monocytes based on differences in cell size (Fig. 4a,b). In contrast to results with ILC2s, we found no detectable GFP+ population of macrophages (alveolar or tissue subsets) at 49 d after SeV infection compared to SeV-UV control using detection of fluorescence directly from GFP or anti-GFP mAb (Fig. 4c,d). As expected from our previous work (34), we also found no detectable IL-13-GFP signal in NKT cells (SSlowCD3e+NK1.1+) or T cells (SSlowCD3e+) (Fig. 4c,d). Similarly, we found no significant IL-13-GFP signal in eosinophils (Ly6G−CD11c−Siglec-F+F4/80−CD11b+) (Fig. 4c,d) that contribute to IL-13 production in other models (51). Together, these findings again show that IL-13-expressing ILC2s were recruited and activated during the chronic lung disease that develops after SeV infection but did not as yet identify alternative cell sources of IL-13 production.
FIGURE 4.
IL-13-GFP reporter expression is not detectable on lung macrophage or non-ILC2 lymphoid populations after viral infection. (a) Cytograms for analysis of alveolar macrophages (Ly6G−CD11c+Siglec-F+F4/80+CD11b−), eosinophils (eos; Ly6G−CD11c−Siglec-F+F4/80CD11b+), and tissue macrophages (Ly6G−CD11c−Siglec-F−F4/80+CD11b+) in lung tissue from WT mice at 49 d after SeV infection. (b) Cytograms for analysis of tissue macrophages separately as interstitial macrophages (SShighLy6G−CD11c−F4/80+CD11b+) or tissue monocytes (SSlowLy6G−CD11c−F4/80+CD11b+) for conditions in (a). (c) Histograms for analysis of IL-13-GFP expression in indicated macrophage and lymphocyte populations (NKT cells as SSlowCD3e+NK1.1+ and T cells as SSlowCD3e+) and eosinophils from lungs of Il13wt/wt and Il13wt/gfp mice at 49 d after SeV or SeV-UV. Detection was based on GFP fluorescence signal. (d) Corresponding histograms for conditions in (c), except that detection was based on anti-GFP mAb. All data are representative of three separate experiments with 5 mice per condition in each experiment. *P<0.01.
To investigate this issue, we developed additional approaches that might better define any role for lung macrophages as a source of IL-13 production in this model (34, 36), especially relative to ILC2s and in relation to macrophage subsets. Accordingly, we combined FACS of alveolar and tissue macrophages with PCR-based assay for Il13 mRNA to establish cell-type sites of IL-13 expression. Similar to the results from the IL-13–GFP-reporter approach, we found that the level of Il13 mRNA per cell at baseline (without SeV infection) and after induction at 5 d and 49 d after SeV infection was highest in lung ILC2s compared to macrophages, where only low levels could be detected in tissue macrophages at 49 d after infection (Fig. 5a). To further define the site of IL-13 production, we also determined IL-13 protein levels in bronchoalveolar lavage fluid (BALF) and lung tissue. This approach demonstrated increases in IL-13 levels in both compartments at 5 d and 49 d after SeV compared to SeV-UV in WT mice, and these increases were attenuated in ILC2-deficient Il7rawt/Cre-Rorafl/fl mice (Fig. 5b). However, despite the selective increase in Il13 mRNA in ILC2s, the loss of ILC2s did not fully block IL-13 production in the lung or release into the airway/alveolar space after SeV infection. This finding again raised the possibility for another cellular source of IL-13 in the lung, with the tissue macrophage as a particular candidate.
FIGURE 5.
ILC2s need tissue macrophages to account for chronic IL-13 production after viral infection. (a) Levels of Il13 mRNA in FACS-isolated alveolar macrophage (Alv mac), tissue macrophage, ILC2, and lung epithelial cell (CD45−CD31−EpCAM+) populations from WT mice at 5 and 49 d after infection with SeV or SeV-UV. (b) Levels of IL-13 in bronchoalveolar lavage fluid (BALF) and lung tissue in WT and Il7rawt/Cre-Rorafl/fl mice for conditions in (a). (c) Levels of Il13 mRNA in tissue macrophage, ILC2, and eosinophil populations that were FACS-isolated from lungs of WT mice at 49 d after SeV or SeV-UV with results expressed as Il13 mRNA copies per cell and as Il13 mRNA copies per lung (copies per cell x number of cells per lung). (d) Immunostaining for IL-13-GFP alone and with DAPI counterstaining in lung sections from Il13wt/gfp mice at 49 d after SeV. Arrows indicate representative ILC2 and tissue macrophage morphology and staining intensity. Scale bar, 100 μm. (e) Immunostaining for IL-13-GFP and GATA-3 for conditions in (d). Scale bar, 50 μm. (f) Immunostaining for IL-13-GFP (using anti-GFP Ab) and F4/80 with DAPI counterstaining in lung sections from Il13wt/gfp mice at 49 d after SeV or SeV-UV. Arrows indicate examples of GFP− F4/80+ and GFP+F4/80+ tissue macrophages and GFP+F4/80− ILC2. Scale bar, 100 μm. (g) Quantitation of immunostaining in (f). (h) Immunostaining for IL-13 (using anti-IL-13 Ab) in tissue macrophages that were FACS-isolated from WT mice at 49 d after SeV or SeV-UV. All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
To address this issue, we compared Il13 mRNA levels in the chief cell sources (tissue macrophages and ILC2s) as mRNA copy number per cell and per lung. Using this comparison in our analysis of Il13 mRNA per cell, we again found a relatively low level of Il13 mRNA in tissue macrophages compared to ILC2s (Fig. 5c). However, when we expressed Il13 mRNA as levels per lung (where lung level = level per cell x number of cells per lung), we recognized that more abundant tissue macrophages are the main site of Il13 mRNA expression at 49 d after SeV infection (Fig. 5c). In fact, the fold-increase and overall level of Il13 mRNA in the lung at 49 d after SeV infection was significantly higher for tissue macrophages than ILC2s (Fig. 5c). The comparative analysis for eosinophils (gated as shown in Fig. 4a) showed Il13 mRNA levels similar to tissue macrophages, but eosinophil number was not sufficient to provide a substantial contribution to Il13 expression in the lung (Fig. 5c). As introduced above, the larger number of lung macrophages was also accompanied by increased post-viral induction of Il13 gene expression with 21-fold in tissue macrophages compared to 5.2-fold in ILC2s and 5.1-fold in eosinophils.
To further assign production of IL-13 to macrophages, we also performed immunostaining for IL-13 protein production in lung tissue and FACS-isolated cells. Tissue immunostaining for IL-13 itself might not discriminate sites of cell production versus cell binding or uptake. We overcame this difficulty by staining for GFP expression in Il13wt/gfp mice. This approach showed cells with tissue macrophage morphology and location and dim staining as well as cells with ILC2 morphology and bright staining for IL-13-GFP with no background signal with GFP only staining (Fig. 5d). Cells identified as ILC2s by GFP-staining intensity and morphology were also found to immunostain positive for GATA3 (Fig. 5e), as a marker of type 2 lymphocytes. Further, this approach showed that cells with macrophage morphology and location were not stained for GATA3 (Fig. 5e) but instead were co-stained for IL-13-GFP and F4/80 in lung sections from Il13wt/gfp mice at 49 d after SeV infection but only for F4/80 in SeV-UV control mice (Fig. 5f). These results were similar to macrophage immunostaining for IL-13 in previous work (34-36). Here again, we identified cells with lymphoid morphology that were stained even more brightly for IL-13-GFP but were not co-stained for F4/80 (Fig. 5f), consistent with identification as IL-13-expressing ILC2s. Image-analysis quantitation of tissue immunostaining indicated that GFP+F4/80+ cells (consistent with IL-13-expressing tissue macrophages) were far more abundant than GFP+F4/80− cells (consistent with IL-13-expressing ILC2s) (Fig. 5g). As introduced above, we also checked for IL-13 expression in FACS-isolated tissue macrophages, in this case by immunostaining with anti-mouse IL-13 Ab that can define IL-13 production in preparations of purified cells. Here again, we demonstrated that tissue macrophages defined by flow cytometry and morphology characteristics were immunostained for IL-13 when obtained from the lungs at 49 d after SeV but not after SeV-UV control (Fig. 5h). Together, these results provided evidence for marked induction of Il13 gene expression in tissue macrophages during chronic lung disease after viral infection and for this population along with ILC2s to represent prominent sites of IL-13 production. This combined contribution might therefore lead to an increased concentration-dependent effect of IL-13 to better explain continued IL-13-dependent lung disease despite ILC2-deficiency (Fig. 2d-h).
ILC2 activation depends on myeloid-macrophage input to ST2
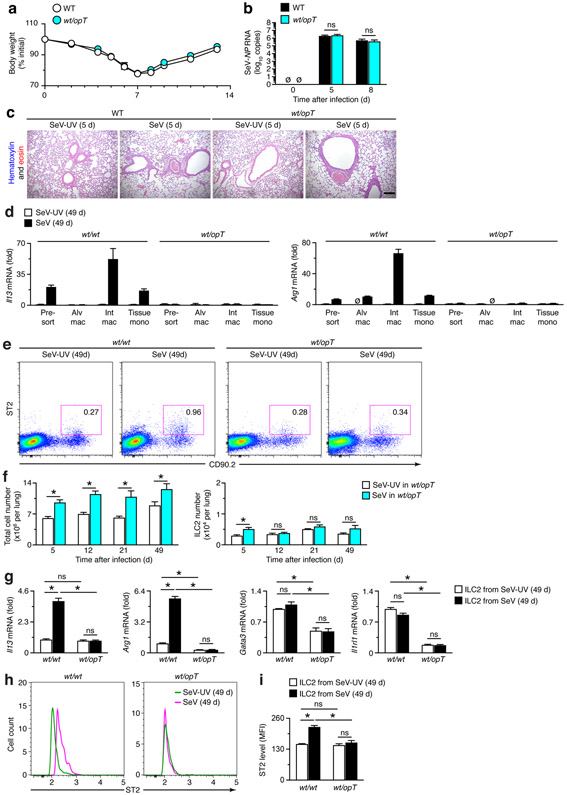
To further address macrophage contribution to IL-13 production, we next incorporated a genetic strategy for targeted Csf1-deficiency that would markedly down-regulate the myeloid-macrophage lineage and in turn tissue macrophage levels. For this approach, we started with Csf1op/opT (op/opT) mice that carry an osteocalcin-driven Csf1 transgene (T) that rescues the osteopetrosis but not the macrophage defect in Csf1op/op (op/op) mice (43). We then generated heterozygous Csf1wt/opT (wt/opT) mice that still manifest a significant decrease in the level of lung tissue macrophages (i.e., interstitial macrophages plus tissue monocytes) and attenuation of chronic lung disease (36). Comparison of wt/opT to littermate wild-type (wt/wt) mice can therefore provide an indication of tissue macrophage contribution to phenotype, including IL-13 expression. In this setting, we found no significant changes in the typical weight loss, viral RNA level and clearance, or immune cell accumulation in wt/opT mice compared to wt/wt mice (Fig. 6a-c). These results indicated that down-regulation of the myeloid-macrophage lineage did not significantly influence acute illness after viral infection, similar to the case for ILC2 deficiency.
FIGURE 6.
Tissue macrophages regulate ILC2 accumulation and activation in chronic lung disease after viral infection. (a) Body weights at indicated times after infection with SeV in WT and wt/opT mice. (b) Corresponding viral loads in lungs for conditions in (a). (c) Hematoxylin and eosin staining of lung sections for indicated conditions. Scale bar, 500 μm. (d) Levels of Il13 and Arg1 mRNA in FACS-isolated macrophage populations from lungs of wt/wt and wt/opT mice at 49 d after SeV or SeV-UV. (e) Flow cytograms for ILC2s from wt/wt and wt/opT mice at 49 d after SeV or SeV-UV. (f) Quantitation of total cell and ILC2 levels from lungs of wt/opT mice at 5-49 d after SeV or SeV-UV using flow cytometry conditions in (e). (g) Levels of Il13, Gata3, Arg1, and Il1rl1 mRNA in FACS-isolated ILC2s from wt/wt and wt/opT mice at 49 d after SeV or SeV-UV. (h) Flow histograms for ST2 expression in ILC2s from lungs of wt/wt and wt/opT mice at 49 d after SeV infection or SeV-UV. (i) Levels of ST2 based on MFI for conditions in (h). All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
In contrast to the results for acute illness, we detected informative differences in the development of chronic disease in wt/opT mice. In particular, we found that the increased levels of Il13 mRNA were localized selectively to both subsets of tissue macrophages and that Il13 mRNA induction was markedly down-regulated in wt/opT compared to wt/wt mice (Fig. 6d). The IL-13-target Arg1 mRNA was up-regulated in both alveolar and tissue macrophages in corresponding WT mice (i.e., wt/wt mice), and this effect was fully blocked in wt/opT mice (Fig. 6d). These results were consistent with our earlier observations that tissue macrophages were sensitive to Csf1-dependent down-regulation (36), in this case leading to decreases in Il13 and Il13-dependent gene expression.
To further define macrophage control of IL-13 production, we also determined the levels of ILC2 recruitment and activation in the lungs of wt/opT mice compared to wt/wt mice. Unexpectedly, we found first that the increased percentage of Lin−CD90.2+ST2+ ILC2s at 49 d after SeV in wt/wt mice was attenuated in wt/opT mice without a significant change in baseline percentages after SeV-UV (Fig. 6e). Quantitation of flow cytometry analysis showed the usual increase in total cell number after SeV infection in wt/opT mice (Fig. 6f) that was similar to wt/wt mice (Fig. 2a). We also detected an increase in ILC2 levels at 5 d after SeV infection in wt/opT (Fig. 6f), again similar to wt/wt control mice (Fig. 2a). However, the marked increases in ILC2 levels in the lung at 21 and 49 d after SeV infection found in wt/wt mice (Fig. 2a) were no longer significant in wt/opT mice (Fig. 6f), recognizing that ST2 expression can represent cell number and activation as contributions to cell function. We did not find a significant difference in ILC2 levels in wt/opT compared to wt/wt mice (Fig. 6f versus Fig. 2a) at any timepoint in SeV-UV controls, indicating that there was no defect in ILC2 development and migration to the lung tissue under baseline conditions.
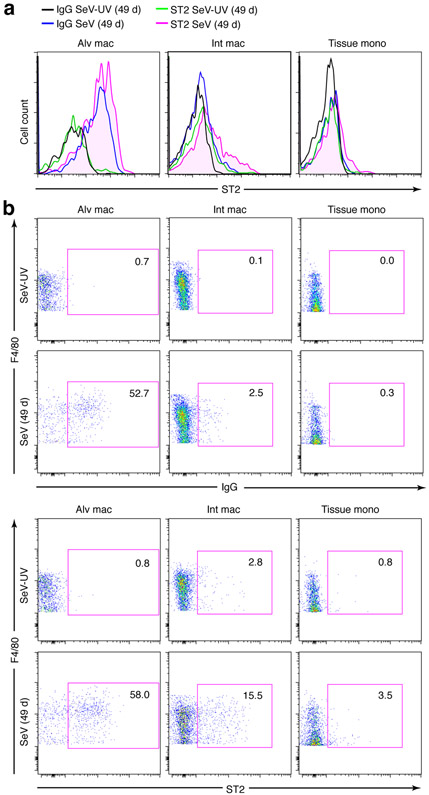
Similar to the results for ILC2 levels, we found that the usual induction of Il13 and Arg1 mRNA found in ILC2s from the lungs of wt/wt mice were fully blocked in wt/opT mice at 49 d after SeV (Fig. 6g). Further, the levels of Gata3 and Il1rl1 mRNA (the latter encoding for the IL-33 receptor designated ST2) were markedly down-regulated in ILC2s from wt/opT mice at 49 d after SeV or SeV-UV (Fig. 6g). In contrast to Il1rl1 mRNA, levels of ST2 protein on ILC2s were up-regulated at 49 d after SeV infection, and this effect was fully blocked in wt/opT mice based on cell counts (Fig. 6h) or MFI (Fig. 6i). Similar induction of ST2 expression after SeV infection was also found on tissue macrophages based on flow cytometry analysis by histogram and cytogram (Fig. 7a,b), albeit on a smaller subset of this cell population compared to broad expression across the ILC2 population (Fig 6h). Nonetheless, both findings suggested a basis for enhanced ST2-dependent activation of ILC2s and tissue macrophages for IL-13 production and for the unexpected role of Csf1-dependent myeloid cells in controlling ILC2 accumulation and activation as a distinct mechanism for chronic lung disease after viral infection. Moreover, the loss of myeloid cell input resulted in a targeted decrease in Il1rl1/ST2 expression and consequent down-regulation of the type 2 immune response as marked by Il1rl1, Gata3, and ST2 levels after viral infection in wt/opT mice.
FIGURE 7.
ST2 expression is detectable on a subset of tissue macrophages after viral infection. (a) Histograms for analysis of ST2 expression in indicated macrophage populations from lungs of WT mice at 49 d after SeV infection or SeV-UV. Detection was based on anti-ST2 mAb or IgG control. (b) Corresponding cytograms for conditions in (a). Data are representative of three separate experiments with 5 mice per condition in each experiment.
ILC2 activation via ST2 depends on IL-33 production
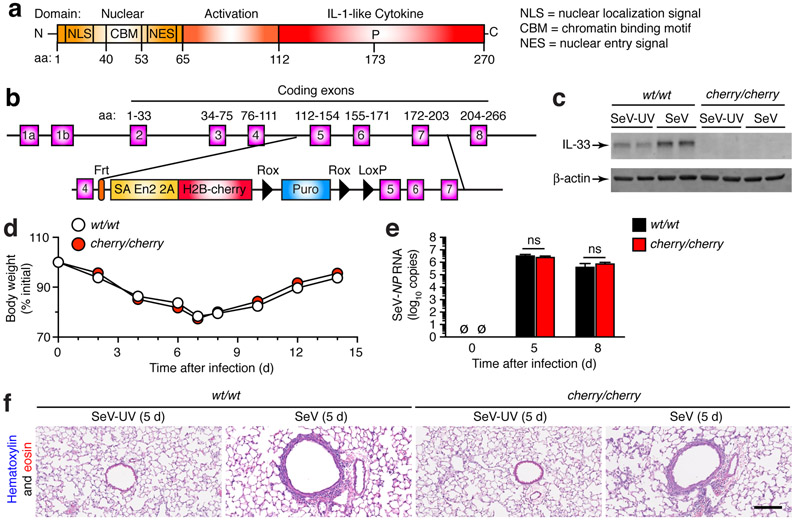
To further develop this new disease mechanism, we next sought to define the basis for increased ST2 expression and the impact on ST2 signaling function after viral infection. As introduced above, we recognized that ST2 signal transduction was required for induction of Il13 gene expression, and this signal depended on IL-33 as ST2-receptor ligand in the present and previous mouse models wherein mucus production is markedly attenuated by anti-ST2 mAb or Ilrl1 or Il33 gene targeting (35, 52). To better define this issue, we generated IL-33-deficient mice wherein a cherry-reporter gene cassette was inserted between exons 4 and 5 of the Il33 gene (Fig. 8a,b). Accordingly, homozygous Il33cherry/cherry mice were unable to generate any detectable IL-33 protein in the lung at 49 d after SeV infection or SeV-UV (Fig. 8c). Nonetheless, the acute illness after SeV infection was no different in Il33cherry/cherry mice compared to Il33wt/wt mice based on cage side observation, body weight loss, viral RNA level, and histopathology (Fig. 8d-f). These results are also similar to our previous findings using Il33Gt/Gt mice that show no differences from WT mice in these same parameters after SeV infection (35).
FIGURE 8.
Generation and phenotype of Il33-cherry transgenic reporter mice reveals no effect of Il33 gene expression on acute illness after viral infection. (a) Scheme for domain structure of mouse IL-33 protein. (b) Scheme for targeting construct for generating IL-33-cherry reporter mice (Il33cherry/cherry). (c) Western blot for IL-33 levels in lung tissue from Il33wt/wt (wt/wt) and Il33cherry/cherry (cherry/cherry) mice at 49 d after infection with SeV or SeV-UV. (d) Body weights at indicated times after infection with SeV in ll33wt/wt and ll33cherry/cherry mice. (e) Corresponding viral loads in lungs for conditions in (d). (f) Hematoxylin and eosin staining of lung sections for indicated conditions. Scale bar, 500 μm. All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment.
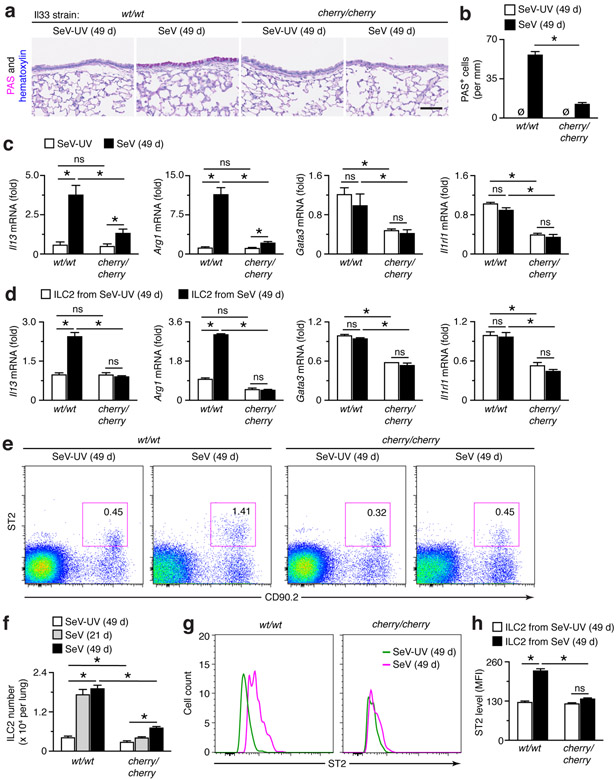
Despite similarities in acute illness, the development of chronic lung disease marked by the usual increases in inflammatory cells and PAS+ airway mucous cells in lung sections (Fig. 9a,b) and in levels of Il13 and Arg1 mRNA in lung tissue (Fig. 9c) were all significantly attenuated in Il33cherry/cherry mice compared to Il33wt/wt mice at 49 d after SeV infection. In addition, lung levels of Gata3 and Il1rl1 mRNA were decreased in Il33cherry/cherry mice after SeV and SeV-UV (Fig. 9c). The same pattern of down-regulated gene expression was found in ILC2s from Il33cherry/cherry mice (Fig. 9d), in concert with decreased levels found in ILC2s from wt/opT mice (Fig. 6g). In addition, we found that the increased percentage of ILC2s in the lungs of Il33wt/wt mice at 49 d after SeV infection was also significantly down-regulated in Il33cherry/cherry mice based on flow cytometry analysis for Lin−CD90.2+ ST2+ ILC2s (Fig. 9e). Quantitation of this analysis confirmed that the marked increases in the numbers of ILC2s in the lungs at 21 and 49 d after SeV infection in Il33wt/wt mice were significantly decreased in Il33cherry/cherry mice (Fig. 9f). This analysis also demonstrated a slight decrease in ILC2 levels in Il33cherry/cherry compared to Il33wt/w mice at baseline in SeV-UV controls (Fig. 9f), consistent with the relatively small effect of IL-33 on ILC2 development in the bone marrow and migration to the lung tissue under baseline conditions (53). In follow-up to the attenuation of ILC2 levels, we also observed that the increased levels of ST2 on ILC2s at 49 d after SeV infection in Il33wt/w mice were no longer found in Il33cherry/cherry mice based on cell counts (Fig. 9g) and MFI (Fig. 9h). Together, these findings indicated that IL-33 production was a suitable candidate for Csf1-dependent myeloid cell control of ILC2 accumulation and activation for chronic lung disease after viral infection.
FIGURE 9.
Phenotype of Il33-cherry transgenic reporter mice demonstrates Il33 gene function for ILC2 activation and accumulation during chronic post-viral lung disease. (a) PAS and hematoxylin staining of lung sections from wt/wt and cherry/cherry mice at 49 d after SeV or SeV-UV. Scale bar, 200 μm. (b) Quantitation of PAS+ cells for conditions in (a). (c) Levels of indicated mRNAs in lungs from wt/wt and cherry/cherry mice at 49 d after SeV or SeV-UV. (d) Levels of indicated mRNAs in ILC2s isolated from lungs of wt/wt and cherry/cherry mice at 49 d after SeV or SeV-UV. (e) Flow cytograms for ILC2s in wt/wt and cherry/cherry mice at 49 d after SeV or SeV-UV. (f) Quantitation of ILC2 levels in wt/wt and cherry/cherry at 21 and 49 d after SeV and at 49 d after SeV-UV using flow cytometry conditions in (e). (g) Flow histograms for ST2 expression in ILC2s from lungs of wt/wt and cherry/cherry mice at 49 d after SeV or SeV-UV. (h) Levels of ST2 based on MFI for conditions in (g). All data are representative of three separate experiments (mean and s.e.m.) with at least 5 mice per condition in each experiment. *P<0.01.
IL-33 production depends on myeloid-macrophage lineage input
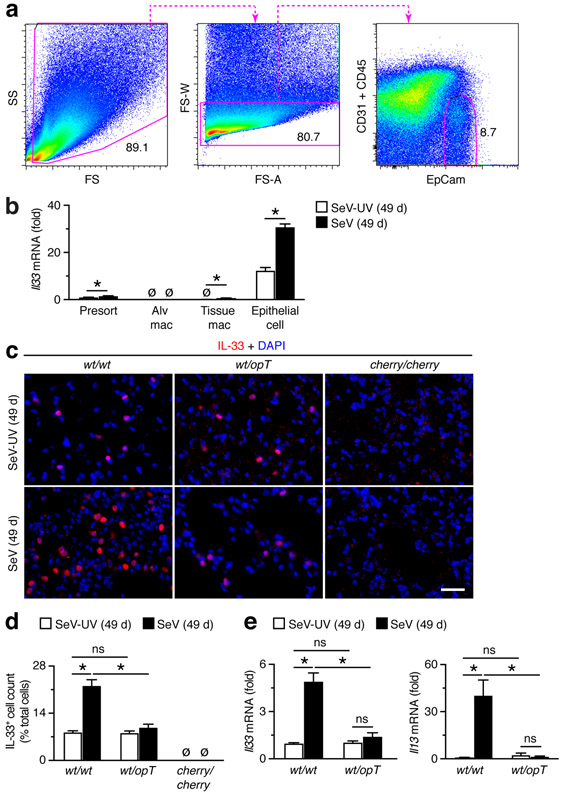
We next aimed to determine whether in fact myeloid cells could control IL-33 production as a mechanism to regulate the development of disease after viral infection. As a first step, we combined a flow cytometry scheme for separating CD31−CD45−EpCam+ lung epithelial cells (Fig. 10a) with our scheme for lung macrophages (Fig. 4a) to track quantitatively the cell site of IL-33 expression. This combined analysis showed that Il33 mRNA was almost entirely confined to lung epithelial cells at baseline levels without infection and at increased levels at 49 d after SeV infection (Fig. 10b). This finding was consistent with the predominant site of IL-33 expression in the mouse lung being localized to alveolar epithelial type 2 (AT2) cells based on cell and tissue morphology and co-expression with AT2 cell markers such as surfactant protein C (Sftpc) (35, 52, 54, 55). Indeed, we also found increases in IL-33+ immunostaining localized to cells with AT2 cell morphology and location in wt/wt mice at baseline (Fig. 10c). No such immunostaining was found in Il33-deficient cherry/cherry reporter mice as a sign of specificity for IL-33 detection. This approach also showed that IL-33+ immunostaining was increased in wt/wt mice at 49 d after SeV compared to SeV-UV, and this increase was attenuated in wt/opT mice (Fig. 10c). These findings were confirmed as significant with quantitation of IL-33+ cell levels in lung tissue (Fig. 10d). In addition, we found that the usual increase in Il33 mRNA and consequent increase in Il13 mRNA at 49 d after SeV infection in the lungs of wt/wt mice were no longer detectable in wt/opT mice (Fig. 10e). Together, these results are consistent with an effect of Csf1-dependent myeloid cells to promote epithelial cell expression of IL-33 (in lung epithelial cells) that in turn drives downstream IL-13 production and feed-forward ST2 expression (in ILC2s and tissue macrophages) that are all critical for the development of chronic lung disease after viral infection.
FIGURE 10.
Lung Csf1-dependent myeloid cells are required for induction of Il33 gene expression in lung epithelial cells after viral infection. (a) Cytograms for analysis of lung epithelial cells (CD45−CD31−EpCAM+) in lung tissue from WT mice at 49 d after SeV infection. (b) Levels of Il33 mRNA in FACS-isolated populations of macrophages and epithelial cells from lungs of wt/wt mice at 49 d after SeV or SeV-UV. (c) Immunostaining for IL-33 in lung sections from wt/wt, wt/opT, and control Il33-deficient cherry/cherry mice at 49 d after SeV or SeV-UV. Scale bar, 200 μm. (d) Quantitation of IL-33+ cells for conditions in (c). (e) Levels of Il33 and Il13 mRNA in lungs of wt/wt and wt/opT mice at 49 d after SeV or SeV-UV. Data are representative of three separate experiments with at least 5 mice per condition in each experiment. *P<0.01.
Discussion
Here we apply improved genetic technologies and a distinct mouse model to define the role of ILC2s in acute illness and chronic disease after respiratory viral infection. Relative to previous work, the present study provides a series of unexpected conceptual advances. In particular, we show that ILC2s are activated during respiratory viral infection with a natural mouse pathogen (SeV) but are not required to handle acute illness and recovery from this type of infection. In contrast, we find that this type of infection also activates ILC2s chronically for IL-13 production that is required for the development of long-term post-viral lung disease with features of asthma and COPD. Notably, however, ILC2s require critical activities of Csf1-dependent myeloid cells at two levels: first, at a downstream level for significant additional IL-13 production; and second, at an upstream level for enhanced IL-33 expression that is essential for ILC2 participation at all in the development of disease. Together, these findings provide a revised scheme (as depicted in Fig. 11) that appears distinct from the conventional view of an innate immune response engineered for short-term versus long-term activation and function. This alternative paradigm thereby provides a new framework for understanding and controlling the innate immune response to viral infection and likely other stimuli of the type 2 immune response. Here we discuss several of our observations that are critical to this issue.
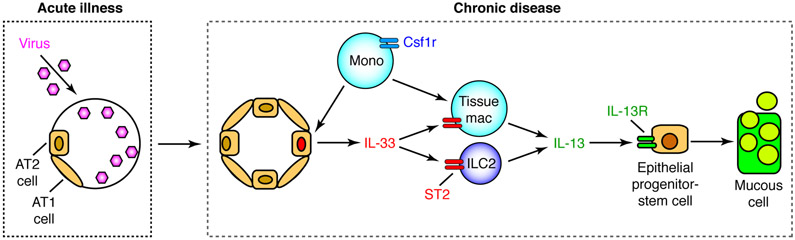
FIGURE 11.
Scheme for Csf1-dependent myeloid cell inputs to ILC2 function in chronic disease after respiratory viral infection. Sequential steps in the scheme include: (1) acute illness with damage to host cells, including progenitor AT2 cells and daughter alveolar epithelial type 1 (AT1) cells; (2) subsequent replacement of these populations including AT2 cells that provide increased IL-33 to activate ILC2 production of IL-13 and in turn IL-13R-dependent differentiation of epithelial stem cells into mucous cells. The Csf1-dependent myeloid cells act upstream for induction of Il33 gene expression and downstream to provide tissue macrophages for additional Il13 gene expression that is required for full development of chronic lung disease after viral infection.
First, we address the possible function of ILC2s in host defense against respiratory viral infection. As noted in the Introduction, ILC2s were reported to be responsible for clinical deterioration via poor epithelial repair after infection with IAV (PR8 strain) based on ILC2 depletion using anti-CD90.2 mAb in Rag−/− mice (16). More recently, another group found decreased survival after IAV (California/04/2009 strain) infection based on ILC2 depletion with Il7ra-Cre–Rora-flox mice (56). The combined studies of IAV implicate an IFNγ–IL-5–amphiregulin axis for the ILC2-dependent phenotype after IAV infection. Similarly, others find a role for IL-33–IL-33R signal transduction (that should activate ILC2s) in defense against IAV infection (16, 56). In contrast, we found no detectable effect of ILC2 depletion on recovery from acute illness (or induction of amphiregulin expression during tissue repair) and no effect of IL33 or IL-33R deficiency on acute illness in the present or previous work on SeV infection (35). Whether the low number and/or activation of ILC2s and consequent absence of function during acute illness found in our model is present in other conditions is uncertain. Nonetheless, there are no reported increases in susceptibility to severity of respiratory viral infections in humans treated with mAbs that block IL-5–IL-5R or IL-33-IL-33R signal transduction (57-59), and IFN-γ-deficient humans are limited to susceptibility to mycobacterial infection (60). Our results are also consistent with normal susceptibility to viral infection in ILC-deficient humans (61). Thus, it is possible that ILC2s might manifest a distinct role specific to IAV infection adapted to mice, recognizing that studies of this issue are ongoing.
Second, we address the key question of ILC2 function in chronic inflammatory disease. Here we took special advantage of the capacity of SeV infection to trigger long-term lung disease lasting the 1-2 year lifespan of mice (50), analogous to epidemiology data that links severe respiratory viral infection in infancy to lifelong lung disease in humans (62). Consistent with these observations, we find that ILC2s are activated persistently after viral infection, thereby providing a basis for contributing to chronic lung disease that develops long after infectious virus is cleared. This prolonged time course is distinct from reports of short-term ILC2 activation after other stimuli, e.g., allergen-challenge and infections with helminths and respiratory viruses in mice (4, 5, 7, 8, 10, 41, 42) and recently in humans (63). These studies often focused on the ILC2 population as a source of IL-13 production and therefore a driver for IL-13-dependent disease that is characteristic of a type 2 immune response. Here we also find that the ILC2s exhibit high-level IL-13 expression in concert with the later development and progression of lung disease after viral infection. However, we also discover two distinct features of the ILC2-based disease paradigm.
One of those elements is the observation that ILC2s cannot fully account for IL-13 production that develops during post-viral lung disease. This observation led us to identify tissue monocytes and interstitial macrophages (that together we designate as tissue macrophages) as an additional site for induction of Il13 gene expression during chronic lung disease after viral infection. The present approach incorporated ILC2-deficient mice to quantify the contribution to lung IL-13 levels. In addition, the strategy incorporated Il13-gfp transgene reporter mice to better localize the cell-type source of Il13 gene expression. Together, these strategies defined Il13 gene expression in ILC2s (at high levels per cell) and tissue macrophages (at relatively lower levels per cell as also found in eosinophils) using a combination of FACS with PCR-based assay and immunostaining for IL-13 expression and tissue immunostaining for GFP-reporter expression to enhance our previous analysis of lung macrophages (34-36). Together, the approach significantly extends evidence of IL-13 expression in mouse and human macrophages from our lab and other labs in the context of a type 2 immune response (34, 36, 64, 65). In particular, this full battery of assays provides a quantitative basis for the relative contributions of ILC2s versus tissue macrophages for the key IL-13 cytokine in the context of chronic disease. However, it is not yet possible to fully separate cytokine contributions of macrophages versus ILC2s because of another previously unrecognized control for ILC2 function.
This second feature of ILC2 function in chronic disease is the newly identified requirement for additional myeloid cell participation, separate from direct production of IL-13. Thus, we incorporated wt/opT to down-regulate the Csf1-dependent myeloid cells (particularly tissue macrophages) and thereby define the relative contributions to IL-13 production. As introduced above, this approach resulted in marked down-regulation of Il13 gene expression and IL-13 protein production and in turn complete attenuation of chronic asthma-like lung disease after viral infection. Unexpectedly, we also found that wt/opT mice additionally manifested a marked down-regulation of ILC2 expansion and activation. The decreased activation included attenuation of ST2 expression via transcriptional and post-transcriptional regulation (the former based on decreased mRNA levels in wt/opT mice and the latter based on an increase in ST2 protein but not corresponding mRNA in ILC2s from wild-type mice and blockade of this increase in wt/opT mice). Comparable albeit lower level ST2 expression was also found in tissue macrophages after SeV infection, suggesting similar regulation of ST2 levels in this cell population. In that regard, the present results were also similar to the appearance of Trem-2 on tissue macrophages after SeV infection, again based on increases in protein with no change in corresponding mRNA levels (36). Together, the findings continue to highlight the role of post-transcriptional control of the type 2 immune response after viral infection but left unchecked how this or post-transcriptional control might occur.
This question was also resolved with unexpected findings. Thus, we generated a new Il33-cherry transgenic reporter mice given the role of IL-33-dependent signal transduction in controlling IL-13 production (35). Based on study of this new mouse line, we learned that Il33 gene expression was required for ILC2 accumulation and activation perhaps as expected, but also that Il33 gene function was required for the transcriptional increase in Il1rl1 mRNA and post-transcriptional up-regulation of ST2 on the surface of ILC2s during the development of chronic lung disease after viral infection. Moreover, this essential Il33-based signal was lost in wt/opT mice, thereby establishing an unrecognized role for Csf1-dependent myeloid cells in controlling ILC2 participation in the disease. Given that the major site for induction of Il33 gene expression is localized to lung epithelial cells (particularly AT2 cells in this model and other models) (35, 52, 54, 55), the findings also implied that Csf1-dependent myeloid cell control of the epithelial cell-derived IL-33 was in turn responsible for ST2-dependent ILC2 activation. These findings are consistent with macrophage support of AT2 cell expansion found after helminth infection and other models of short-term tissue injury and repair (66-69). However, these previous models defined an effect of macrophages (often M2 macrophages) that is downstream of IL-13 production (67-69). Instead, in the present case, the Csf1-dependent myeloid cell effect is upstream of the epithelial cell to IL-33 production to ILC2 activation to IL-13 production axis that drives disease. We therefore propose that Csf1-dependent myeloid cells might represent an immune-cell niche for lung epithelial (likely AT2) cells. This aspect of regulating ILC2 function appears to have no clear precedent based on a review of previous work (70). Since this review was published, others have reported that Csf1-dependent macrophages are required for maintenance of Paneth cells and nearby stem cells in the intestinal epithelium (71) but full definition of the macrophage subset was not performed and no cytokine biology or disease model was studied. The present possibility for myeloid-lineage control of IL-33-expression in AT2 cells thereby significantly advances this concept.
Together, the present results provide a new framework as well as new questions for the development of progressive post-viral lung disease in particular and chronic inflammatory disease in general. Given the distinct nature of our findings, the myeloid cell effect on lung epithelial cells cannot yet be attributed to tissue macrophages versus another subset of the Csf1-dependent myeloid cell lineage. This issue still needs to be defined, most likely by identifying molecular factors that control Csf1-dependent myeloid cell activation and effector function in this setting and performing the corresponding loss-of-function and reconstitution experiments. Similarly, we still need to determine the precise epithelial cell target population and how it is regulated by myeloid-lineage cells during acute illness and chronic disease in the present model and in general. In that context, our study nonetheless provides a distinct cell and molecular axis from viruses to innate myeloid cells (a Csf1-dependent subset) to epithelial cells (likely an AT2 cell subset at least in mice) to innate lymphoid cells (an ILC2 subset) in the development of persistent inflammatory disease. In the present model, regulation of ST2 levels provides a key checkpoint for the development of long-term disease. It will therefore be of interest to revisit whether a similar molecular and/or cellular mechanism will also participate in the type 2 immune- response in related settings, e.g., allergy and parasitic infection. These comparisons will also define additional molecular interactions that drive this axis and thereby provide a therapeutic target for correcting the consequent inflammatory disease without interfering with host defense. Nonetheless, these findings already better explain the cellular context for ILC2 function and the specialized need for myeloid cell cooperation for the development of a long-lasting type 2 immune response that allows progression from acute infectious illness to long-term lung disease. This type of post-viral disease resembles immunopathologic features found in asthma and COPD as well late events in the course of severe respiratory virus infections in general. Thus, post-viral lung disease can develop after severe infections due to respiratory syncytial virus, influenza virus, respiratory enterovirus, and coronavirus (CoV) in humans and animal models, including CoV outbreaks causing severe acute respiratory syndrome (SARS) and coronavirus disease of 2019 (COVID-19) (17, 37, 63, 72, 73). Therefore, defining pathogenesis and consequent therapeutic targets for progressive post-viral lung disease is critical to present and major public health concerns.
Key points:
ILC2s are activated even after injury from respiratory viral infection is resolved.
ILC2s contribute to a chronic type 2 immune-response after viral infection.
ILC2s depend on myeloid-macrophage lineage cells to drive post-viral disease.
Acknowledgments
The authors thank Di Wu, Xiaohua Jin, and Rose Tidwell and the staff in the Siteman Flow Cytometry Core and Pulmonary Morphology Core for expert assistance.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01 AI130591), National Heart, Lung, and Blood Institute (R35 HL145242), and the Cystic Fibrosis Foundation.
Abbreviations used in this article:
- AT2 cell
alveolar epithelial type 2 cell
- ATCC
American Type Culture Collection
- PFU
plaque-forming unit
- ILC2
group 2 innate lymphoid cell
- NP
nucleoprotein
- PAS
periodic acid-Schiff
- qPCR
quantitative PCR
- MUC5AC
mucin 5AC
- SeV
Sendai virus
- ST2
suppressor of tumorigenicity 2
Footnotes
Conflict of Interest Disclosures
MJ Holtzman declares that he is a member of the Data Safety Monitoring Board for AstraZeneca and is the founder for NuPeak Therapeutics Inc. The other authors declare no competing financial interests.
References
- 1.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, and Spits H. 2018. Innate lymphoid cells: 10 years on. Cell 174: 1054–1066. [DOI] [PubMed] [Google Scholar]
- 2.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hurst SD, Zurawski G, Leach MW, Gorman DM, and Rennick DM. 2001. IL-25 induces IL-4, IL-5, and IL-13 pathologies and Th2-associated pathologies in vivo. Immunity 15: 985–995. [DOI] [PubMed] [Google Scholar]
- 3.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, and Coffman RL. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol 169: 443–453. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, and McKenzie ANJ. 2006. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth explusion. J. Exp. Med 203: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, and Koyasu S. 2010. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature 463: 540–544. [DOI] [PubMed] [Google Scholar]
- 6.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, and Artis D. 2010. IL-25 elicits a multi-potent progenitor cell population that promotes Th2 cytokine responses. Nature 464: 1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, and McKenzie ANJ. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, and Locksley RM. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. American Review of Respiratory Disease 107: 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, and Spits H. 2011. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol 12: 1055–1162. [DOI] [PubMed] [Google Scholar]
- 10.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, and McKenzie ANJ. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol 129: 191–198. [DOI] [PubMed] [Google Scholar]
- 11.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JTJ, Rollins DR, Gorentla B, Liu W, Gorska MM, Chu H, Martin RJ, and Alam R. 2015. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J. Allergy Clin. Immunol 136: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria J-P, O’Byrne PM, Gauvreau GM, Boulet L-P, Lemiere C, Martin JG, Nair P, and Sehmi R. 2015. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol 137: 75–86. [DOI] [PubMed] [Google Scholar]
- 13.Halim TYF, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie ANJ, and Takei F. 2014. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr., and Paul WE. 2015. Innate immunological function of TH2 cells in vivo. Nat. Immunol 16: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhariwal J, Cameron A, Trujillo-Torralbo M-B, del Rosario A, Bakhsoliani E, Paulsen M, Jackson DJ, Edwards MR, Rana BMJ, Cousins DJ, Hansel TT, Johnston SL, and Walton RP. 2017. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. Am. J. Respir. Crit. Care Med 195: 1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monticelli LA, Sonnenberg GF, Abt MC, Aleghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathallyawal T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, and Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol 12: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeler SP, Agapov EV, Hinojosa ME, Letvin AN, Wu K, and Holtzman MJ. 2018. Influenza A virus infection causes chronic lung disease linked to sites of active viral RNA remnants. J. Immunol 201: 2354–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang L-C, Johnson D, Scanlon ST, McKenzie ANJ, Fallon PG, and Ogg GS. 2013. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med 210: 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker JA, Oliphant CJ, Englezakis A, Yu Y, Clare S, Rodewald H-R, Belz G, Liu P, Fallon PG, and McKenzie ANJ. 2015. Bcl11b is essential for group 2 innate lymphoid cell development. J. Exp. Med 212: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoef PA, Constantinides MG, McDonald BD, Urban JF Jr., Sperling AI, and Bendelac A. 2015. Intrinsic functional defects of type 2 innate lymphoid cells impair innate allergic inflammation in promyelocytic leukemia zinc finger (PLZF)-deficient mice. J. Allergy Clin. Immunol 2016: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF Jr., and Paul WE. 2015. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol 16: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M-W, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, and Chawla A. 2015. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar A-L, Kabata H, Monticelli LA, Moriyama S, Garbes Putzel G, Rakhilin N, Shen X, Kostenis E, Konig GM, Senda T, Carpenter D, Farber DL, and Artis D. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, and Veiga-Fernandes H. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, Usher N, Zhu J, Urban JF Jr., Paul WE, and Germain RN. 2018. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monticelli LA, Buck MD, Flamar A-L, Saenz SA, Tait Wojno ED, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald H-R, Shah H, Cross JR, Diamond JM, Cantu E, Christie JD, Pearce EL, and Artis D. 2016. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol 17: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriyama S, Brestoff JR, Flamar A-L, Moeller JB, Klose CSN, Rankin LC, Yudanin NA, Monticelli LA, Putzel CG, Rodewald H-R, and Artis D. 2018. B2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 28.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon P, and McKenzie ANJ. 2014. MHCII-mediated dialog between group 2 innate lymphoid cells and CD(+) T cells potentiates type 2 immunity and promotes parasitic helminth explusion. Immunity 41: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halim TYF, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, and McKenzie ANJ. 2016. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat. Immunol 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, Jorg Fehling H, and Rodewald H-R. 2010. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32: 426–436. [DOI] [PubMed] [Google Scholar]
- 31.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, and McKenzie ANJ. 2012. Transcription factor RORa is critical for nuocyte development. Nat. Immunol 13: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halim TYF, MacLaren A, Romanish MT, Gold MJ, McNagny KM, and Takei F. 2012. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity 37: 463–474. [DOI] [PubMed] [Google Scholar]
- 33.Dussault I, Fawcett D, Matthyssen A, Bader J-A, and Giguere V. 1998. Orphan nuclear receptor Rora-deficient mice display the cerebellar defects of staggerer. Mechanisms of Dev 70: 147–153. [DOI] [PubMed] [Google Scholar]
- 34.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, and Holtzman MJ. 2008. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med 14: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy YG, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, and Holtzman MJ. 2013. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest 123: 3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu K, Byers DE, Jin X, Agapov E, Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober DL, Brett TJ, and Holtzman MJ. 2015. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med 212: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Mao D, Keeler SP, Wang X, Wu K, Gerovac BJ, Shornick LP, Agapov E, and Holtzman MJ. 2019. Respiratory enterovirus (like parainfluenza virus) can cause chronic lung disease if protection by airway epithelial STAT1 is lost. J. Immunol 202: 2332–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alevy Y, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, Miller CA, Heier RF, Byers DE, Brett TJ, and Holtzman MJ. 2012. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest 122: 4555–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byers DE, Wu K, Dang-Vu G, Jin X, Agapov E, Zhang X, Battaile JT, Schechtman KB, Yusen R, Pierce RA, and Holtzman MJ. 2018. Triggering receptor expressed on myeloid cells-2 (TREM-2) expression tracks with M2-like macrophage activity and disease severity in COPD. Chest 153: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Y, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, and Umetsu DT. 2011. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol 12: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajput C, Cui T, Han M, Lei J, Hinde JL, Wu Q, Kelley Bentley J, and Hershenson MB. 2017. Rora-dependent type 2 innate lymphoid cells are required and sufficient for mucous metaplasia in immature mice. Am. J. Physiol. Lung Cell Mol. Physiol 312: L983–L993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV, Zhou B, McKenzie AN, and Peebles RSJ. 2016. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoic cells thorugh thymic stromal lymphopoietin. J. Allergy Clin. Immunol 138: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abboud SL, Woodruff K, Liu C, Shen V, and Ghosh-Choudhury N. 2002. Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony-stimulating factor-1. Endocrinology 143: 1942–1949. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Mao D, Roswit WT, Jin X, Patel AC, Patel DA, Agapov E, Wang Z, Tidwell RM, Atkinson JJ, Huang G, McCarthy R, Yu J, Yun NE, Paessler SL, Lawson TG, Omattage NS, Brett TJ, and Holtzman MJ. 2015. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat. Immunol 16: 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Nunen MCJ, and van der Veen J. 1967. Experimental infection with Sendai virus in mice. Arch Gesamte Virusforsch 22: 388–397. [DOI] [PubMed] [Google Scholar]
- 46.Califano D, Cho JJ, Uddin MN, Lorentsen KJ, Yang Q, Bhandoola A, Li H, and Avram D. 2015. Transcription factor Bcl11b controls identity and function of mature type 2 innate lymphoid cells. Immunity 43: 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, and Locksley RM. 2018. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol 19: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter MJ, Kajiwara N, Karanja P, Castro M, and Holtzman MJ. 2001. IL-12 p40 production by barrier epithelial cells during airway inflammation. J. Exp. Med 193: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Look DC, Walter MJ, Williamson MR, Pang L, You Y, Sreshta JN, Johnson JE, Zander DS, and Brody SL. 2001. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am. J. Pathol 159: 2055–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walter MJ, Morton JD, Kajiwara N, Agapov E, and Holtzman MJ. 2002. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest 110: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsen EA, Helmers RA, Lee JJ, and Lee NA. 2012. The expanding role(s) of eosinophils in health an disease. Blood 120: 3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardman CS, Panova V, and McKenzie ANJ. 2012. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur. J. Immunol 43: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stier MT, Zhang J, Goleniewska K, Cephus JY, Rusznak M, Wu L, Van Kaer L, Zhou B, Newcomb DC, and Peebles RS Jr. 2019. IL-33 promotes the egress of group 2 innate lymphoid cells from bone marrow. J. Exp. Med 215: 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, and Girard J-P. 2012. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol 188: 3488–3495. [DOI] [PubMed] [Google Scholar]
- 55.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H-E, and Locksley RM. 2016. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal immunology 9: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Califano D, Furuya Y, Roberts S, Avram D, McKenzie ANJ, and Metzger DW. 2018. IFN-g increases susceptibility to influenza A infection through suppression of group II innate lymphoid cells. Mucosal immunology 11: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagnasco D, Caminati M, Ferrando M, Aloe T, Testino E, Canonica GW, and Passalacqua G. 2018. Anti-IL-5 and IL-5Ra: efficacy and safety of new therapeutic strategies in severe uncontrolled asthma. BioMed Research International 2018:5698212: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y-L, Gutowska-Owsiak D, Hardman CS, Westmoreland M, MacKenzie T, Cifuentes L, Waithe D, Lloyd-Lavery A, Marquette A, Londei M, and Ogg G. 2019. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med 11. [DOI] [PubMed] [Google Scholar]
- 59.Chinthrajah S, Cao S, Liu C, Lyu S-C, Sindher SB, Long A, Sampath V, Petroni D, Londei M, and Nadeau KC. 2019. Phase 2a randomized, placebo-controlled study of anti–IL-33 in peanut allergy. JCI Insight 4: e131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche M-C, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile J-F, Ducoulombier H, Edgar D, Clarke J, Oxelius V-A, Brai M, Novelli V, Heyne K, Fischer A, Holland SM, Kumararatne S, Schreiber RD, and Casanova J-L. 1999. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet 21: 370–378. [DOI] [PubMed] [Google Scholar]
- 61.Vely F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, Perchet T, Petit M, Yessaad N, Touzot F, Bruneau J, Mahlaoui N, Zucchini N, Farnarier C, Michel G, Moshous D, Blanche S, Dujardin A, Spits H, Distler JHW, Ramming A, Picard C, Golub R, Fischer A, and Vivier E. 2016. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol 17: 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Claverley PMA, Celli BR, Christenson SA, Crystal RG, Fageras M, Freeman CM, Groenke L, Hoffman EA, Kesimer M, Kostikas K, Paine R, Rafii S, Rennard SI, Segal LN, Shaykhiev R, Stevenson C, Tal-Singer R, Vestbo J, Woodruff PG, Curtis JL, and Wedzicha JA. 2018. At the root: defining and halting progression of early chronic obstuctive pulmonary disease. Am. J. Respir. Crit. Care Med 197: 1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vu LD, Siefker D, Jones TL, You D, Taylor R, DeVincenzo JP, and Cormier SA. 2019. Elevated levels of type 2 respiratory innate lymphoid cells in human infants with severe RSV bronchiolitis. Am. J. Respir. Crit. Care Med 200: 1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung Y, Hong JY, Lei J, Chen Q, Bentley JK, and Hershenson MB. 2015. Rhinovirus infection induces interleukin-13 production from CD11b-positive, M2-polarized exudative macrophages. Am. J. Respir. Cell Mol. Biol 52: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Grinchuk V, Urban JF Jr., Bohl J, Sun R, Notari L, Yan S, Ramalingam T, Keegan AD, Wynn TA, Shea-Donohue T, and Zhao A. 2013. Macrophages as IL-24/IL-33-responsive cells play an important role in the induction of type 2 immunity. PloS ONE 8: e59441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung L-Y, Sen D, Oniskey TK, Katzen J, Cohen NA, Vaughan AE, Nieves W, Urisman A, Beers MF, Krummel MF, and Herbert DR. 2018. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal immunology 12: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, and Rock JR. 2017. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21: 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, and Suzuki K. 2016. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Invest 126: 2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Y. K, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, and Rothlin CV. 2017. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gieseck RL, Wilson MS, and Wynn TA. 2017. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol in press. [DOI] [PubMed] [Google Scholar]
- 71.Sehgal A, Donaldson DS, Pridans C, Sauter KA, Hume DA, and Mabbott NA. 2018. The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat. Comm 9: 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Page C, Goicochea L, Matthews K, Zhang Y, Klover P, Holtzman MJ, Hennighausen L, and Frieman M. 2012. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respriatory syndrome coronavirus infection. J. Virol 86: 13334–13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barton LM, Duval EJ, Stroberg E, Ghosh S, and Mukhopadhyay S. 2020. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 153: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]