Abstract
Purpose
To describe a case of corneal ulceration associated with dupilumab use for atopic dermatitis.
Observations
A patient developed an inflammatory corneal ulcer 3 weeks after starting bi-weekly intravenous dupilumab therapy. Symptoms resolved with topical prednisolone and discontinuation of the systemic therapy with dupilumab.
Conclusion and importance
Dupilumab is known to cause ocular surface inflammation, but we report a novel association between dupilumab use and potentially sight-threatening corneal ulceration.
Keywords: Dupilumab, Atopic dermatitis, Conjunctivitis, Corneal ulceration
1. Introduction
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling by blocking the shared receptor component, the IL-4α subunit, to reduce the T helper 2 (Th2) immune response implicated in allergic diseases such as asthma and atopic dermatitis.1 It was approved by the United States Food and Drug Administration (US-FDA) in 2017 as the first biologic intravenous treatment for moderate to severe atopic dermatitis in adult patients.2 Several publications have reported ocular surface complications associated with dupilumab use for atopic dermatitis. The most common associated disease was conjunctivitis as seen in the LIBERTY AD CAFÉ phase 3 clinical trial where 28% of patients in the group receiving dupilumab every 2 weeks developed conjunctivitis versus 11% in the group receiving placebo.3 Previous reports of dupilumab associated keratitis exist, but all corneal inflammation was reported to be confined to the anterior, superficial cornea.4, 5, 6 Herein, we report a novel case of corneal ulceration associated with dupilumab use. We look to emphasize the importance of ophthalmological evaluation for physicians prescribing dupilumab for atopic dermatitis.
2. Case report
A 50-year-old man presented to the Wilmer Johns Hopkins Cornea Service with irritation, redness, and constant foreign body sensation for the past week in the right eye (OD). His ophthalmic history was notable for keratoconus, for which he underwent a penetrating keratoplasty in his fellow, left eye (OS), 8 years prior to the current illness. The left eye also underwent cataract surgery approximately one year before he sought medical care for the right eye irritation. He also had a longstanding history of floppy eyelids and mild bilateral corneal exposure but had no previous episodes of corneal ulceration or conjunctivitis. The patient's medical history was notable for atopic dermatitis, well suppressed HIV infection, hypertension, chronic obstructive pulmonary disease, obstructive sleep apnea, and obesity. Ocular symptoms began 3 weeks after starting dupilumab treatment that consisted of a 1200mg initial dose and subsequent 300mg doses every 2 weeks. His other systemic medications included albuterol/fluticasone propionate/beclomethasone inhalers, ibuprofen, triamcinolone, ipratropium bromide/salbutamol, escitalopram oxalate, fluticasone nasal spray, prednisolone acetate, prednisone, and abacavir/dolutegravir/lamivudine. Before presentation he had tried naphazoline with only brief irritation relief.
At presentation, his best corrected visual acuity (BCVA) was 20/40 OD and 20/125 OS. His intraocular pressure (IOP) was 8 mm Hg OD and 6 mm Hg OS. On slit lamp examination, the patient exhibited floppy eyelids and mild exposure that were symmetrical in both eyes. No significant lid margin pathology was noted. Anterior segment findings included moderate to severe conjunctival injection more pronounced in his right eye. The right eye was notable for an inferior, paralimbal dense corneal infiltrate with a full epithelial defect and 30% corneal thinning (See Fig. 1). In his left eye, he had a decentered corneal graft with superficial scarring and inferonasal thinning up to 80% within the graft that were noted two years prior to the current episode which appeared stable. No active keratitis was noted in the left eye, nor was there any intraocular inflammation present. Corneal cultures were taken from the area of ulceration in the right eye. Dupilumab was stopped and the patient was started on moxifloxacin 0.5% every hour around the clock OD.
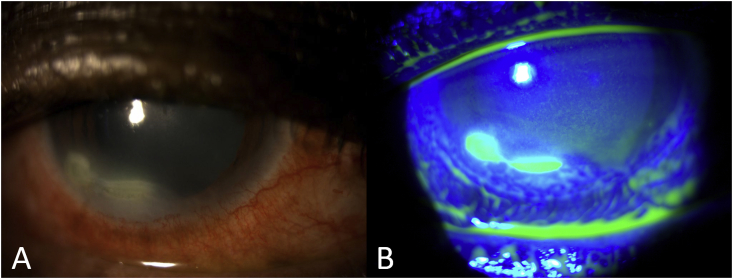
Fig. 1.
Slit lamp photographs of patient's right eye at presentation demonstrating dense corneal infiltrate (A); and an epithelial defect as shown by Fluorescein staining (B).
Upon follow-up 2 days later, his symptoms were stable with moxifloxacin but not improved. Because of the static nature of the corneal ulcer despite antibiotic treatment, and in the presence of negative corneal cultures, the ulcer was presumed sterile. Moxifloxacin 0.5% was reduced to every 2 hours while awake and prednisolone 1% was introduced at 4 times daily dose. Four days later the patient reported resolution of his ocular symptoms of eye pain, itching, or discomfort. Slit lamp exam revealed marked improvement in conjunctival injection, closure of the epithelial defect and resolution of the corneal infiltrate. The patient continued to improve on this treatment protocol, and was later switched to cyclosporine 1% 4 times daily and tacrolimus 0.03% skin ointment twice daily for control of his atopic keratoconjunctivitis. Prednisolone was slowly tapered down. The corneal ulcer was healed one month after initial presentation.
3. Discussion
Atopic dermatitis (AD) is a common chronic inflammatory skin disease that affects up to 10% of adults and 20% of children worldwide.7,8 Topical corticosteroids have been the main avenue for treatment. However, their efficacy is limited for the 20% of AD patients suffering from moderate-to-severe disease who require high potency agents due to severe side effects such as skin atrophy and HPA axis suppression associated with prolonged use.8 Many patients with moderate-to-severe AD require systemic treatment to effectively manage their disease. Dupilumab is a recently introduced systemic therapy for moderate to severe atopic dermatitis. Dupilumab blocks downstream signaling of IL-4 and IL-13 resulting in downregulation of inflammatory mediators and upregulation of structural proteins, lipid metabolism proteins, and epidermal barrier proteins. It has also been reported to significantly reduce serum levels of CCL17, a key regular of Th2-mediated immunity and a specific, objective biomarker of AD.1 Randomized, double-blind, placebo-controlled trials involving adults who had moderate-to-severe atopic dermatitis unresponsive to treatment with topical glucocorticoids and calcineurin inhibitors demonstrated that 85% of patients in the dupilumab group experienced a 50% reduction in their Eczema Area and Severity Index (EASI) scores compared to 35% of those in the placebo group after 12-weeks.9
Ocular adverse events have been documented in the clinical trials of dupilumab. Specifically, conjunctivitis was noted in 8–28% of patients receiving this drug.3,10, 11, 12 Most conjunctivitis events were mild to moderate and resolved by the end of the treatment period without necessitating discontinuation of dupilumab.13 More severe cases of conjunctivitis have been described: Levine et al. reported a case where a patient with severe AD developed cicatrizing blepharoconjunctivitis with subepithelial fibrosis and punctal stenosis after 8 weeks of dupilumab treatment.14 Lee et al. similarly reported cases of punctal stenosis with progression to punctal obstruction and cicatricial ectropion.7 Most recently, Liberman et al. presented a case of conjunctival cicatrization and symblepharon.15 Although keratitis was reported in association with dupilumab treatment, it tended to be superficial only, as in superficial punctate keratopathy, inferior punctate keratitis, or dry eye. A retrospective review of 77 AD patients treated with dupilumab revealed 3 instances of unspecified keratitis. The majority of adverse events in this review resolved after treatment with lubricating eye drops or with the addition of prednisolone acetate 1% ophthalmic suspension.16 Maudinet et al. reports 6 patients with mild conjunctivitis associated with inferior corneal epithelial abnormality and evaporative dry eye with tear break up time (TBUT) less than 10 seconds. Therapy included warm lid compresses and high viscosity artificial tears which were later switched to sodium hyaluronate, and then to trehalose/hyaluronate tear substitute. All patients improved with this regimen without recurrence.4 Only 1 patient out of 425 in the dupilumab treatment groups needed to discontinue treatment in the CHRONOS randomized, double-blind, placebo-controlled, phase 3 study due to allergic keratitis in one eye.12
Here we present a case of sight threatening corneal infiltration, ulceration, and thinning that began 3 weeks after commencing treatment with dupilumab. While the patient's history of mild corneal exposure may have contributed to his presenting symptoms, it was not until the patient was exposed to dupilumab that his otherwise healthy keratoconic cornea in the right eye developed ulceration. Naturally, a drug rechallenge with dupilumab was not performed, and yet the temporal relation of the ulcer onset relative to initiating dupilumab use is highly suspicious and leads us to believe the ulceration is a dupilumab-induced effect. The corneal ulceration resolved completely with removal of the inciting agent and treatment with topical anti-inflammatory medications. Corneal inflammation was also seen in the clinical trials.
The pathogenesis of dupilumab-associated ocular complications remains unclear. One hypothesis proposes that dupilumab inhibits goblet cell proliferation and mucin production which contribute to dry eye and corneal defects in clinical trials. This mechanism is supported by a rodent study revealing that application of IL-4 and IL-13 to conjunctival goblet cells increases cell proliferation. Additionally, IL-13 was also seen to stimulate mucin secretion.17 Interestingly, a human study revealed reduced tear protein levels of Muc5AC in patients treated with dupilumab.18 Dupilumab's blockade of IL-4 and IL-13 signaling may reduce goblet cell activity below baseline levels to produce tear film insufficiencies and subsequent corneal erosion. Interestingly, the increased incidence of conjunctivitis was observed only for AD treatment and not for dupilumab treatment of other allergic diseases such as asthma and chronic rhinosinusitis with nasal polyps.13,19 This suggests an inflammatory mechanism and AD-specific interaction rather than an inherent effect of dupilumab. There is an association between the incidence of conjunctivitis and baseline AD severity, prior history of conjunctivitis, and certain biomarkers (thymus and activation-regulated chemokine, IgE, eosinophils).13 No consensus has been reached regarding optimal management and treatment of dupilumab-induced conjunctivitis. Successful reported modalities of treatment include topical corticosteroids, antibiotics, tacrolimus, artificial tears, and antihistamines.19
As dupilumab use rises, we may see a similar increase in keratitis and corneal ulceration incidence. Our findings suggest that atopic dermatitis patients presenting with eye symptoms after initiation of treatment with dupilumab should be promptly evaluated by an ophthalmologist to rule out corneal ulceration. Ophthalmic evaluation prior to starting dupilumab therapy and the pretreatment of existing ocular disease may be valuable in minimizing ocular issues associated with dupilumab use. Additionally, there may be a role for prophylactic treatment in high risk patients, as determined in future randomized controlled trials addressing this issue. Dermatologists prescribing dupilumab should be vigilant for ocular symptoms such as redness, pain, discharge, and changes in vision.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Financial Support
No funding or grant support.
Authorship
All authors attest that they met the current ICMJE criteria for Authorship.
Declaration of competing interest
No conflicting relationship exists for any author.
Acknowledgements
None.
References
- 1.Gooderham M.J., Hong H.C., Eshtiaghi P., Papp K.A. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78:S28–S36. doi: 10.1016/j.jaad.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115–1121. doi: 10.1007/s40265-017-0768-3. [DOI] [PubMed] [Google Scholar]
- 3.de Bruin‐Weller M., Thaçi D., Smith C.H. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ) Br J Dermatol. 2018;178:1083–1101. doi: 10.1111/bjd.16156. [DOI] [PubMed] [Google Scholar]
- 4.Maudinet A., Law-Koune S., Duretz C., Lasek A., Modiano P., Tran T.H.C. Ocular surface diseases induced by dupilumab in severe atopic dermatitis. Ophthalmol Ther. 2019;8:485–490. doi: 10.1007/s40123-019-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivert L., Wahlgren C., Ivert L., Lundqvist M., Bradley M. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375–378. doi: 10.2340/00015555-3121. [DOI] [PubMed] [Google Scholar]
- 6.Yamane M.L.M., Belsito D.V., Glass L.R.D. Two differing presentations of periocular dermatitis as a side effect of dupilumab for atopic dermatitis. Orbit. 2019;38:390–394. doi: 10.1080/01676830.2018.1553190. [DOI] [PubMed] [Google Scholar]
- 7.Lee D.H., Cohen L.M., Yoon M.K., Tao J.P. Punctal stenosis associated with dupilumab therapy for atopic dermatitis. J Dermatol Treat. 2019:1–4. doi: 10.1080/09546634.2019.1711010. 0. [DOI] [PubMed] [Google Scholar]
- 8.Mayba J.N., Gooderham M.J. Review of atopic dermatitis and topical therapies. J Cutan Med Surg. 2017;21:227–236. doi: 10.1177/1203475416685077. [DOI] [PubMed] [Google Scholar]
- 9.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 10.Simpson E.L., Bieber T., Emma G.-Y. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med Boston. 2016;375:2335–2348. doi: 10.1056/nejmc1700366. [DOI] [PubMed] [Google Scholar]
- 11.Thaçi D., Simpson E.L., Beck L.A. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52. doi: 10.1016/S0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A., de Bruin-Weller M., Gooderham M. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 13.Akinlade B., Guttman‐Yassky E., de Bruin‐Weller M. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–473. doi: 10.1111/bjd.17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine R.M., Tattersall I.W., Gaudio P.A., King B.A. Cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018;154:1485–1486. doi: 10.1001/jamadermatol.2018.3427. [DOI] [PubMed] [Google Scholar]
- 15.Liberman P., Shifera A.S., Berkenstock M. Dupilumab-associated conjunctivitis in patients with atopic dermatitis. Cornea. 2020 doi: 10.1097/ICO.0000000000002262. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Kraus C.N., Patel K.G., Ganesan A.K., Grando S.A. Real-world experience of dupilumab treatment for atopic dermatitis in adults: a retrospective analysis of patients' records. Int J Dermatol. 2020;59:253–256. doi: 10.1111/ijd.14573. [DOI] [PubMed] [Google Scholar]
- 17.García-Posadas L., Hodges R.R., Diebold Y., Dartt D.A. Context-dependent regulation of conjunctival goblet cell function by allergic mediators. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-30002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett B.P., Afshari N.A. Dupilumab-associated mucin deficiency (DAMD) Transl Vis Sci Technol. 2020;9 doi: 10.1167/tvst.9.3.29. 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utine C.A., Li G., Asbell P., Pflugfelder S., Akpek E. Ocular surface disease associated with dupilumab treatment for atopic diseases. Ocul Surf. 2020 doi: 10.1016/j.jtos.2020.05.008. [DOI] [PubMed] [Google Scholar]