Summary
Drought stress is the major limiting factor in agriculture. Wheat, which is the most widely grown crop in the world, is predominantly cultivated in drought‐prone rainfed environments. Since roots play a critical role in water uptake, root response to water limitations is an important component for enhancing wheat adaptation. In an effort to discover novel genetic sources for improving wheat adaptation, we characterized a wheat translocation line with a chromosomal segment from Agropyron elongatum, a wild relative of wheat, which unlike common wheat maintains root growth under limited‐water conditions. By exploring the root transcriptome data, we found that reduced transcript level of LATERAL ROOT DENSITY (LRD) gene under limited water in the Agropyron translocation line confers it the ability to maintain root growth. The Agropyron allele of LRD is down‐regulated in response to water limitation in contrast with the wheat LRD allele, which is up‐regulated by water deficit stress. Suppression of LRD expression in wheat RNAi plants confers the ability to maintain root growth under water limitation. We show that exogenous gibberellic acid (GA) promotes lateral root growth and present evidence for the role of GA in mediating the differential regulation of LRD between the common wheat and the Agropyron alleles under water stress. Suppression of LRD also had a positive pleiotropic effect on grain size and number under optimal growth conditions. Collectively, our findings suggest that LRD can be potentially useful for improving wheat response to water stress and altering yield components.
Keywords: lateral root density, KNAT genes, wheat, root, drought, GA
Introduction
Major staple crops of the world, wheat (Triticum aestivum), rice (Oryza sativa) and maize (Zea mays) are predicted to suffer substantial yield losses based on climatic predictions with serious implications on global food security (Iizumi et al., 2013; Rosenzweig et al., 2014). Wheat is the most widely grown crop in the world (FAO, 2013). Drought stress and heat waves are the main yield‐limiting factors in most wheat‐growing regions (Asseng et al., 2015; Lobell et al., 2011; Ray et al., 2015; Zhang et al., 2017). The impact of drought stress on wheat yield is dependent on several factors such as the intensity and duration of drought stress and also the developmental stage at which the plants experience water limitations. Wheat breeding efforts in past, including the green revolution, have mainly focused on improving shoot architecture under high yielding conditions (Borlaug, 2008; Hedden, 2003; Waines and Ehdaie, 2007). However, genetic selections that primarily focused on shoot traits may have unintentionally impacted critical root traits in some modern high yielding cultivars (Mac Key et al., 1973; Waines and Ehdaie, 2007). An important and emerging aspect of improving drought adaptation is to optimize root architecture traits (Li et al., 2019; Lopes and Reynolds, 2010; Wasson et al., 2012).
Elucidating the phenotypic plasticity in root architecture and the underlying genetic basis of these responses under variable soil environments is essential for incorporating root‐related traits to enhance drought adaptability (Manschadi et al., 2006). Currently, targeted efforts to incorporate root‐associated traits in wheat breeding programmes are limited as there are no wheat genes known with well‐defined role in regulating root architecture in response to water limitation. This is partly due to the technical challenges involved in phenotyping roots in a physiologically relevant experimental set‐up. This has created a knowledge gap between optimal above ground and below ground traits under varying field environments in wheat (Hu et al., 2018). In wheat, some of the root traits that have been studied include root growth rate, rooting depth, vascular architecture (xylem number, diameter, thickness, etc.), root angle and root branching (Hurd, 1968; Nakamoto and Oyanagi, 1994; O’Brien, 1979; Placido et al., 2013; Richards and Passioura, 1989). Root:shoot biomass ratio is another important trait for drought tolerance and overall productivity (Siddique et al., 1990). However, wheat genes regulating these traits remain to be discovered.
In monocots, root system is primarily composed of coarse and fine roots. While primary and seminal roots provide axial transport pathway from root to shoot, fine roots (lateral roots and root hairs) are more effective in extracting water and nutrients from the soil due higher surface area (Ahmed et al., 2016). Lateral roots provide high plasticity to root systems because small changes in root microenvironment can alter the length and density of lateral roots (Bao et al., 2014). While the genetic control of lateral root development is well studied in Arabidopsis, especially in relation to auxin (Benková and Bielach, 2010), limited progress has been made in rice and wheat. Rice mutants lacking lateral roots were shown to have altered auxin sensitivity (Chhun et al., 2003; Cho et al., 2013; Meng et al., 2019; Wang et al., 2006). Elucidating the genetic and molecular basis of lateral root plasticity in cereals is important for understanding the root dynamics under water stress. However, spatial and temporal plasticity of lateral root development itself present substantial challenges to study such traits. Further, it is not feasible to extract fine root structure from field studies with varying soil environment due to breakage and root loss. Consequently, very few genes or allelic variants have been identified for lateral root development in monocots (Chen et al., 2013; Yu et al., 2016).
Bread wheat is a hexaploid with seven homoeologous groups of chromosomes, derived from A, B and D diploid progenitor genomes (Sears, 1969). These genomes can differ in their contribution to various traits. For example, the D genome is a major contributor to variation in lateral root number (Wang et al., 2018). The hexaploid status also makes wheat quite receptive to chromosome manipulation, including incorporation of numerous stress tolerance genes from alien species (Gill et al., 2004). For example, an introgression from Agropyron elongatum in Pavon 76 (P76) was used to improve leaf rust resistance (Sarma and Knott, 1966). This translocation line (TL) with the leaf rust resistance gene is homozygous for the Agropyron segment located on the long arm of wheat chromosome 7D, which replaces the syntenic wheat 7DL segment (Sarma and Knott, 1966). Previously, we showed that the 7DL Agropyron translocation conferred the ability to maintain root growth under limited‐water conditions at seedling and pre‐tillering stages (Placido et al., 2013). While the parental background line (Pavon 76) exhibited a decrease in lateral roots under limited‐water conditions, the TL plants maintained lateral root formation at a rate similar to the well‐watered TL plants. At later developmental stages, this increased lateral root response manifests itself as higher root biomass under water stress. It is noteworthy that lateral root density in seedlings and root biomass in pre‐tillering plants is not significantly different between the TL and P76 under well‐watered conditions. Therefore, the effect of the translocation on the root system of the TL plants is an emergent trait under water‐limiting conditions.
To elucidate the genetic basis of this root biomass trait, we previously performed root transcriptome analysis and identified a candidate gene mapping to the wheat 7DL chromosome (Placido et al., 2013). The homologous gene for this candidate in Arabidopsis is KNAT3 which belongs to the class II KNOTTED1‐like homeodomain gene family (Truernit and Haseloff, 2007). Members of this class II KNAT family are associated with root development and KNAT6 is involved in lateral root formation (Dean et al., 2004). KNAT3 is expressed in mature roots and is repressed by exogenous cytokinin treatment without changing root morphology (Truernit et al., 2006). However, plants overexpressing KNAT3 and knock‐out mutants did not exhibit a root phenotype, possibly due to gene redundancy in Arabidopsis (Truernit and Haseloff, 2007). Here, we show that the differential regulation of the wheat homolog of KNAT3, named LRD, is the genetic basis of the lateral root growth difference observed between the Agropyron introgression line (TL) and the common wheat under water‐limiting conditions. We also present evidence for the role of GA in regulating lateral root growth in wheat and regulation of LRD expression in roots.
Results
Wheat LRD, a KNAT3 homolog, negatively regulates root growth during water stress
The expression of LRD, a KNAT3 homolog of Arabidopsis, is down‐regulated in the TL but up‐regulated in the P76 in response to water stress (Placido et al., 2013). Based on this contrasting expression‐level response to water stress, we hypothesized that LRD regulates lateral root growth in wheat under limited‐water conditions. To directly test this hypothesis, we generated and characterized wheat transgenic RNAi lines. We obtained three independent transgenic events with reduced LRD expression relative to wild‐type CBO37 (Figure S1). We also generated three independent lines overexpressing (OE) LRD in wheat and included these in all our experiments (Figure S1). We used the wheat allele of LRD (LRD Ta) sequence for generation of the RNAi and OE wheat lines. We first focused on characterizing the root phenotypes at seedling stage using the cigar roll method that was used to identify early root growth differences between P76 and the TL (Placido et al., 2013). We compared the root and shoot phenotypes of the wild type (WT) with the transgenic seedlings under well‐watered and limited‐water conditions (Figure 1a–d; Figure S2). Six‐day‐old RNAi seedlings maintained the lateral root number and density under limited‐water condition compared with well‐watered plants (Figure 1a,b). In contrast, in CBO37 and OE seedlings, these two parameters were reduced by nearly 40% under limited water relative to well‐watered controls (Figure 1a,b). Under limited water, RNAi plants exhibited significantly higher lateral root density than WT and OE plants (Figure 1b). No significant differences were observed in primary or seminal root lengths or shoot length for all three genotypes under well‐watered and limited‐water conditions (Figure 1c,d, Figure S2). Thus, constitutive suppression of wheat LRD in the RNAi plants mirrored the effects of the LRD Ag in TL by maintaining lateral root production under limited‐water conditions. This result supported our hypothesis that LRD plays a role in regulating root growth in wheat under water‐limiting conditions. It is noteworthy that the lateral root phenotype in the RNAi lines is only observed under water stress. It also indicates that the root response of TL under limited water is due to differential regulation of LRD expression.
Figure 1.
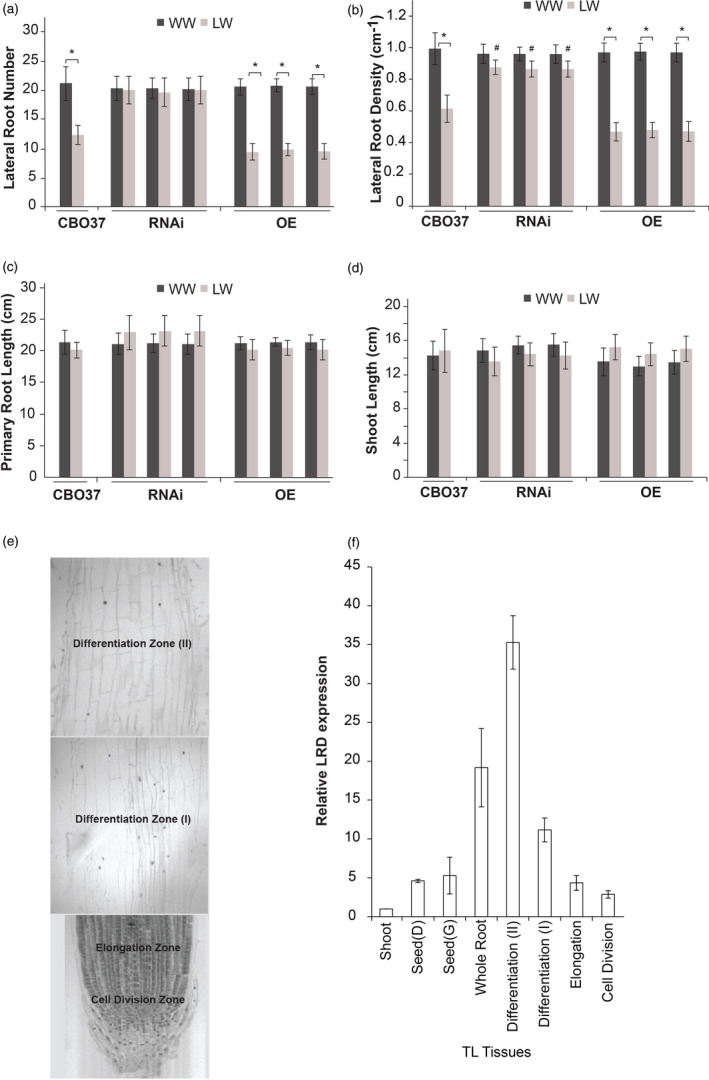
Wheat LRD a KNAT3 homolog, negatively regulates lateral root growth during water stress. (a–d) Six‐day‐old seedlings of wild‐type CBO37, three RNAi and three overexpression mutants (OE) for wheat LRD Ta grown in cigar rolls under well‐watered (WW) and limited‐water (LW) conditions were assayed for (a) lateral root number on the primary root, (b) lateral root density on the primary root, (c) primary root length and (d) shoot length. Bar graphs represent mean ± SD; n = 30. ‘*’indicates significant difference between the WW and LW treatment within each genotype at P < 0.05 (two‐tailed Student’s t‐test) and ‘#’indicates significant difference between CBO37 and the RNAi lines under LW at P < 0.05 (two‐tailed Student’s t‐test). (e) Wheat root longitudinal section representing different root zones. (f) Relative expression of LRD in shoot (6 days), developing seed (Seed D; 48 HAP), germinating seed (Seed G; 3 days), and different root zones (6 days). Transcript abundance was measured relative to expression in shoot. HAP: hours after pollination.
In Arabidopsis, KNAT3 has higher expression in mature regions of roots. We analysed the expression of LRD in different wheat tissues and root zones of 6‐day‐old seedlings to determine whether it correlates with regions of lateral root emergence (Figure 1e,f). The highest expression levels were detected in fully differentiated region or mature parts of the root. We found that expression of LRD is reduced in cell division/meristematic region of the roots (Figure 1f). This is consistent with reduced KNAT3 activity in meristematic zone of Arabidopsis roots (Truernit et al., 2006).
We next examined whether the impact of reduced LRD expression during seedling stage also persisted at a later vegetative phase using a different experimental set‐up in greenhouse conditions. For this, we measured several morphological and physiological parameters for the wild‐type CBO37 and the OE and RNAi transgenic plants that were exposed to a longer duration (20 days) of water stress initiated 7 days after germination (Figure 2a–c, Figure S3a–c). We observed that the LRD RNAi lines at pre‐tillering stage had higher root (Figure 2c) and shoot (Figure 2b) biomass compared with CBO37 and the OE plants under limited water. Both CBO37 and OE lines had significantly reduced root and shoot biomass and length in response to water stress, while the smaller decline for the RNAi lines was not significant (Figure 2b,c, Figure S3b,c). The RNAi lines also exhibited a longer root system in comparison with the CBO37 and OE lines under limited‐water treatment (Figure 2a, Figure S3b). It is pertinent to mention that lateral root density measurement is intractable in the greenhouse experiments as the root system for pre‐tillering plants is elaborate and not feasible to characterize beyond root length and biomass. This observation of longer/larger root system is consistent with the previously observed pre‐tillering stage phenotypic difference between P76 and TL under comparable water‐limiting conditions, wherein TL plants with reduced LRD expression had bigger roots and shoots (Placido et al., 2013). Overall, the root phenotyping using two different experimental set‐ups at seedling and pre‐tillering stages confirm that suppression of LRD enables wheat plants to sustain root growth during water deficit, unlike wild‐type wheat that maintains high LRD transcript and reduces growth under the same conditions.
Figure 2.
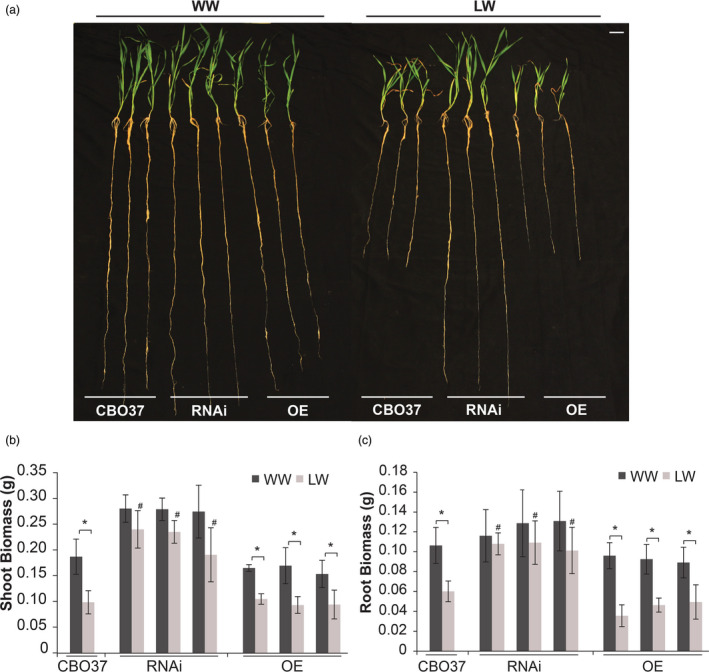
LRD regulates root and shoot traits under water stress at pre‐tillering vegetative stage. (a–c) Shoot and root traits of pre‐tillering stage CBO37, RNAi and OE plants grown in greenhouse under WW or LW conditions. Water stress initiated at 7 days after transplanting was maintained for 20 days. (a) Representative pre‐tillering stage CBO37, RNAi and OE plants under 20 days of LW stress. (b) Shoot biomass, and (c) root biomass of CBO37, RNAi, and OE pre‐tillering stage plants under LW. The plants were sampled at 27 days after transplanting. Data are represented as mean ± SD; n = 9. Means were compared using two‐tailed Student’s t‐test. ‘*’indicates significant difference between WW and LW at P < 0.05, and ‘#’indicates a significant difference between CBO37 and the RNAi lines under LW at P < 0.05. White scale bar = 20 mm.
LRD RNAi mutants maintain stomatal conductance under water stress
To obtain insight into the physiological status of the pre‐tillering plants and evaluate genotypic differences, we measured the stomatal conductance and net photosynthetic rate for the wild‐type and transgenic lines under well‐watered and water‐limiting conditions. These measurements were recorded on days 21, 24 and 27 of the pre‐tillering experiment conducted under greenhouse conditions. The water stress was initiated 7 days after transplanting, and sand moisture status showed a gradual decline with the progression of the stress treatment (Figure S3a). We observed a ~50% decline in stomatal conductance in CBO37 and OE plants under limited water at all three time points, but no significant change in the stomatal conductance of the RNAi plants under limited water (Figure 3a–c). It is notable that under water‐limited conditions, RNAi plants had significantly higher stomatal conductance than WT and OE plants (Figure 3a–c). Further, a drop in the photosynthetic rate (Pn) from well‐watered to limited‐water condition was greater in CBO37 and the OE plants compared with the RNAi plants, although the latter still exhibited significant reduction (Figure 3d–f). These results suggest that greater root biomass for the RNAi lines under limited water conditions result in higher gas exchange and transpiration compared with WT. Collectively, these results show that wheat LRD expression during water limitation negatively impacts vegetative growth.
Figure 3.
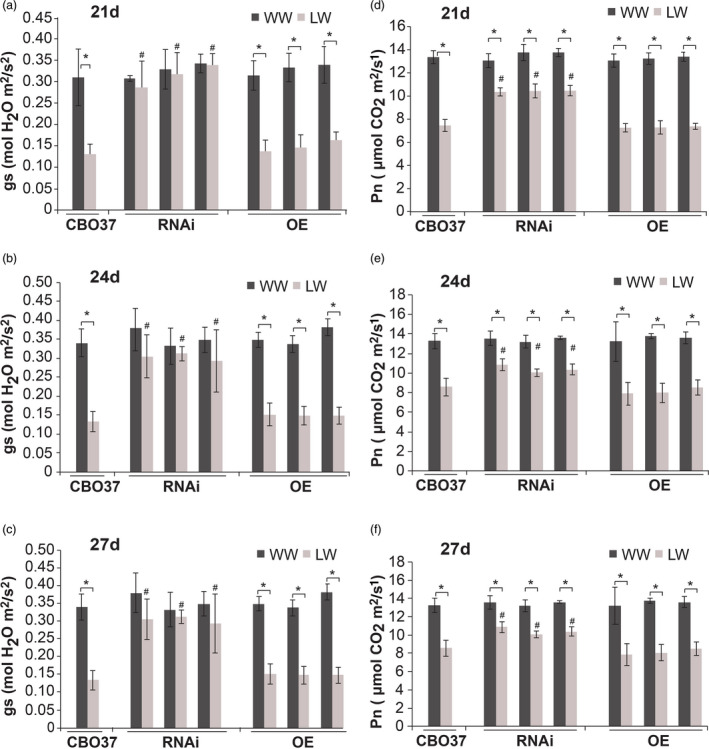
Pre‐tillering stage LRD RNAi mutants maintain stomatal conductance under limited water. (a–c) Stomatal conductance (gs) and (d–f) photosynthetic rate (Pn) of CBO37, RNAi and OE plants at (a, d) 21; (b, e) 24; and (c, f) 27 days after transplanting. ‘*’indicates significant difference between WW and LW within each genotype at P < 0.05, and ‘#’ indicates significant (P < 0.05) difference between RNAi or OE and CBO37 under LW. Data are represented as mean ± SD; n = 9, and means were compared using two‐tailed Student’s t‐test.
GA promotes lateral root growth in wheat under water stress
In addition to screening for differential expression of genes mapping to the 7DL chromosome arm, we mined the root transcriptomic data from 6‐day‐old seedlings of P76 and TL to identify differentially regulated genes and pathway(s) that could potentially be linked to root growth (Placido et al., 2013). We reasoned that a subset of differentially expressed genes could either be upstream of LRD regulation or are regulated differently as a consequence of differences in the LRD expression between P76 and TL. These genes could provide insight into the lateral root density phenotype and possible correlation with LRD expression. We found several genes associated with the hormone gibberellic acid (GA) to be differentially regulated between these two genotypes under limited‐water treatment. Because GA plays an important role in plant growth and development, we examined the effect of treating TL and P76 seedlings with 1 μm GA3 under well‐watered and limited‐water conditions. GA had no effect on roots of either genotype under well‐watered conditions, but under limited water GA restored lateral root number and density to the well‐watered levels in P76 (Table 1). Notably, GA increased the TL lateral root number and density under limited water to above the untreated values for both water conditions and also above the GA‐treated level for well‐watered conditions (Table 1). However, GA treatment of P76 seedlings had a positive effect on lateral root density only under limited‐water conditions. To further confirm that GA regulates lateral root growth, we treated the P76 and TL seedlings under well‐watered and limited‐water conditions with GA biosynthesis inhibitor paclobutrazol (PAC). PAC treatment reduced lateral root density but did not change the root length in both genotypes, indicating the specific role of GA levels is on lateral roots (Table S1).
Table 1.
The effect of gibberellin A3 (GA3) on lateral root density
| Treatment | Primary root length (cm) | Lateral root number | Lateral root density (cm−1) |
|---|---|---|---|
| P76, WW | 25.9 ± 2.6 | 20 ± 5 | 0.86 ± 0.2 |
| P76, WW (+GA3) | 25.5 ± 2.8 | 24 ± 4 | 0.96 ± 0.1 |
| P76, LW | 18.8 ± 2.2* | 10 ± 3* | 0.53 ± 0.1* |
| P76, LW (+GA3) | 23.9 ± 2.2† | 23 ± 3† | 0.96 ± 0.1† |
| TL, WW | 26.6 ± 2.3 | 23 ± 4 | 0.86 ± 0.1 |
| TL, WW (+GA3) | 27.2 ± 2.2 | 24 ± 5 | 0.89 ± 0.2 |
| TL, LW | 27.3 ± 2.7 | 28 ± 4 | 1.01 ± 0.1 |
| TL, LW (+GA3) | 26.4 ± 2.4 | 38 ± 4† | 1.44 ± 0.1† |
GA, gibberellic acid; TL, translocation line.
Significant difference between water‐watered (WW) and limited‐water (LW) treatments for a given genotype and GA treatment (P < 0.05).
Significant difference between untreated control and GA3 within genotype and water treatment at P < 0.05 (mean ± SD; n = 30). Number of replicates = 3. The later root number and density were measured on primary root.
GA levels decline in P76 but not in TL under limited‐water conditions
Given the positive impact of exogenous GA on lateral roots, we measured the endogenous GA levels in seedling roots from P76 and TL. We found that GA1 decreased in P76 under water limitation (Figure 4a). However, no significant difference was observed in GA1 levels in TL in response to water limitation (Figure 4a). Our results suggest that unlike P76, TL plants maintain GA levels, which allows them to initiate and grow lateral roots under limited‐water conditions. We next examined whether there is a direct relationship between LRD expression and GA levels in roots in the context of genotypic differences between P76 and TL. For this, we sequenced the coding regions of LRD from the P76, TL and an A. elongatum accession with the goal of developing allele‐specific LRD primers for accurately measuring expression‐level differences. The sequence alignment from the three genotypes indicated that there are multiple polymorphisms in the conserved KNOX2, ELK and the homeobox domains as well as non‐conserved regions of LRD among the genotypes. These polymorphic sites between the wheat (LRD Ta) and LRD Ag alleles were used to develop and validate allele‐specific expression in the P76 and TL roots (Figure S1a, Table S2). To test the allele‐specific regulation of LRD, under limited water we performed qRT‐PCR on TL and P76 roots under well‐watered and limited‐water conditions (Figure 4b). In contrast to the normal wheat allele (LRD Ta), which is up‐regulated, the LRD Ag allele is down‐regulated in response to water stress in TL (Figure 4b). Because TL contains only the Agropyron allele, the lateral root response in TL is correlated with reduced LRD transcript under limited‐water conditions.
Figure 4.
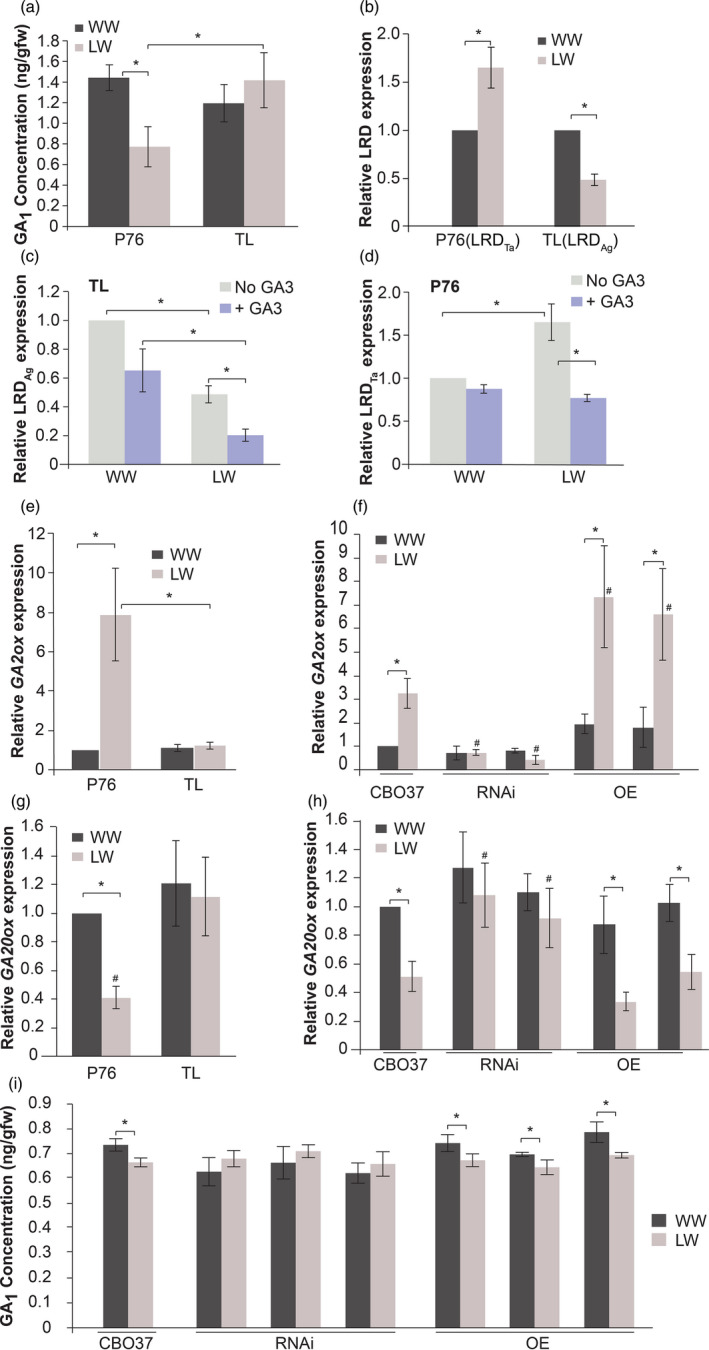
Water stress causes GA level reduction in P76 but not in TL by regulating GA‐related genes. (a) GA1 levels in P76 and TL seedling roots (6 days) under WW and LW conditions (mean ± SD n = 30). (b–d) Allele‐specific expression of LRD in 6‐day‐old seedling roots. LRD Ta denotes the wheat allele and LRDAg denotes the Agropyron allele. (b) Expression of LRD under LW for each genotype is relative to respective WW condition (mean ± SD from three replicates). (c, d) LRD levels in (c) TL (LRDAg ) and (d) P76 (LRD Ta) roots (6 days) under WW and LW conditions, with and without GA3 treatment. Transcript abundance was measured relative to WW, non‐GA3 treated sample within each genotype (mean ± SD n = 3). (e–h) Transcript abundance of GA metabolic genes in 6‐day‐old roots of different genotypes. (e, f) GA catabolic; GA2ox, and (g, h) GA biosynthesis; GA20ox gene, in 6‐day‐old roots under WW and LW conditions (mean ± SD; n = 3). Transcript abundance (e, g) in P76/TL was measured relative to WW P76 sample, and (f, h) in CBO37/RNAi/OE was measured relative to WW CBO37 roots. ‘#’ indicates significant difference between genotypes within a water treatment (p < .05). (i) GA1 levels in CBO37, RNAi and OE in 6‐day‐old roots under WW and LW conditions (mean ± SD; n = 30). ‘*’represents significant difference (P < 0.05) using two‐tailed Student’s t‐test.
Using these validated allele‐specific primers, we measured the expression of LRD in TL and P76 seedling roots under well‐watered and limited‐water conditions, with and without GA treatment (1 μm). The abundance of the Agropyron LRD Ag transcripts in roots of GA‐treated TL seedlings under limited water was reduced about twofold compared with its untreated counterpart, representing a fivefold reduction relative to the untreated well‐watered control (Figure 4c). The measured value for GA treatment in the TL under well‐watered conditions was also modestly lower, but the difference was not statistically significant. GA also decreased the wheat LRD Ta transcript in P76 under limited water, but not below the level seen under well‐watered conditions (Figure 4d). These data suggest that the LRD Ag allele is more sensitive to GA, hence repressed more than the wheat LRD Ta allele under limited‐water conditions. The higher expression‐level GA sensitivity of LRDis clearly observed only under limited‐water conditions.
To further understand the mechanistic relationship between GA levels and LRD expression, we quantified the expression of genes involved in GA biosynthesis and catabolism. GA2ox, a gene encoding a putative GA catabolic enzyme, was differentially regulated between P76 and TL roots under limited‐water treatment in our transcriptome analysis (Placido et al., 2013). We validated this result using qRT‐PCR assay in P76 and TL seedling roots. GA2ox is induced by eightfold in P76 in response to water stress but is not differentially expressed in the TL (Figure 4e). To determine whether the induction of GA2ox is influenced by LRD expression, we compared its expression in CBO37 with the RNAi and OE lines. GA2ox is up‐regulated by water stress in CBO37 and OE but is not induced in the RNAi lines (Figure 4f). Under limited water, the expression of GA2ox in RNAi plants is significantly lower while in OE plants it is significantly higher than CBO37. The expression of GA2ox is up‐regulated by more than sixfold in the OE lines compared with a threefold induction in CBO37 in response to water stress. Next, we checked whether the GA20ox biosynthesis gene is differentially regulated between P76 and TL genotypes. Interestingly, GA20ox in P76 is significantly reduced under water limitation but is maintained in TL (Figure 4g). Similarly, GA20ox is reduced by water stress in both CBO37 and OE mutant plants but is maintained in RNAi mutants (Figure 4h). We also measured the level of GA1 in CBO37, RNAi and OE roots under well‐watered and water‐limited conditions. The GA1 levels in the RNAi roots were maintained under low water condition but CBO37 and OE both exhibit a slight but significant decline in the GA in response to water stress (Figure 4i). Collectively, our data suggest that wheat lines (TL and the RNAi lines) with reduced expression of LRD do not exhibit water stress‐mediated induction of the GA catabolic gene (GA2ox), maintain expression of a biosynthesis gene (GA20ox) and consequently are able to maintain GA levels under water stress.
Auxin has been shown to mediate the GA‐regulated root growth in Arabidopsis and has a well‐established role in lateral root initiation (Fu and Harberd, 2003). Therefore, we next asked whether the roots from P76 and TL have different auxin levels that may explain differences in lateral root initiation and consequently lateral root density. We measured the auxin levels in the roots obtained from seedlings grown under well‐watered and limited‐water conditions (Figure S4). Our data indicate that the decreased lateral root density in P76 under limited‐water conditions was not due to reduced auxin levels in P76 relative to TL under limited water. In fact, P76 exhibits increased auxin levels in response to water stress. These data further implicate a GA‐dependent mechanism(s) either independent of auxin levels or overriding the auxin signal for maintaining lateral root growth in the TL during water stress (Figure 4a; Figure S4).
Pleiotropic effect of LRD on yield components
We observed that mature RNAi plants grown under optimal greenhouse conditions produced larger grains than CBO37. In contrast, seeds from OE lines were smaller than CBO37 (Figure 5a). To further confirm this observation, we quantified the grain size parameters for RNAi, CBO37 and OE plants grown under well‐watered conditions in greenhouse. We found a significant increase in grain length, width and thickness of RNAi grains compared with CBO37 (Figure 5b). In contrast, OE grains exhibited significant decreases in these parameters (Figure 5b). To evaluate whether grain size difference is being compensated by total number of grains, we counted grain number per plant for CBO37, RNAi and OE lines. RNAi plants produced more grains than CBO37 while OE plants had significantly lower number of grains (Figure 5d). To further test the impact of constitutive LRD suppression on grain weight, we measured the thousand‐grain weight from plants grown in small‐scale plots in field condition. A significant 6%–12% increase in the thousand‐grain weight was observed from the three RNAi plots relative to CBO37 (Figure 5c). Next, we evaluated whether increase in yield components is an auxiliary outcome of an advantageous root system of RNAi plants or whether LRD has a more direct role in grain development. In this context, we quantified the expression pattern of LRD in young wheat grains during early grain development. The RNA pools of developing grains of cultivar Redland grown under well‐watered conditions were used for this assay (Begcy and Walia, 2015). Transcript abundance of LRD peaked at 48 h after fertilization (Figure 5e). It is noteworthy that this peak corresponds to the development window when wheat grains are undergoing rapid mitotic divisions and enlargement (Figure 5f). This suggests that altered LRD levels in transgenic lines may have an pleiotropic effect on yield associated traits.
Figure 5.
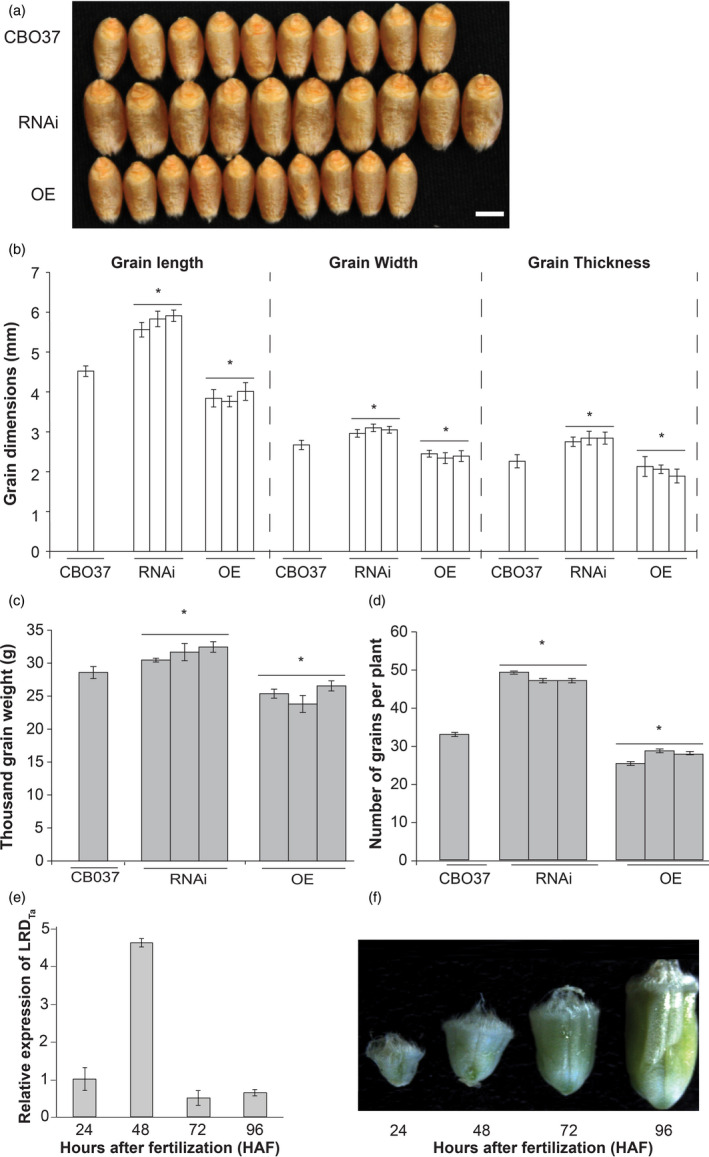
Pleiotropic effects of LRD on wheat yield components. (a) Representative grains of CBO37, LRD RNAi and OE shown by aligning 10 seeds from a pool of 10 plants per genotype. (b) Mature grain length, width and thickness of CBO37, RNAi and OE grains collected from plants grown in well‐watered, optimal greenhouse conditions. (c) Wheat 1000‐grain weight of CBO37, LRD RNAi and OE plants grown in field conditions. Data from three independent transgenic events, 10 plants per genotype are shown. (d) Number of grains per wheat plant for each genotype. Plants were grown to maturity in WW greenhouse conditions (mean ± SD; n = 10). ‘*’indicates a significant difference between CBO37 and RNAi or OE events at P < 0.05. (e) The expression of LRDTa peaks at 48 h after fertilization (HAF), which corresponds to the syncytial stage of wheat endosperm. Expression of LRDTa was measured during early seed development using qRT‐PCR (mean ± SD n = 3). The 24 HAF seeds were used as a control to measure relative expression. (f) Representative developing wheat seeds 24, 48, 72 and 96 HAF exhibit a rapid increase in seed size after pollination.
Discussion
Our knowledge of wheat genes that regulate root traits and play a role during water stress is limited. This is hindering our ability to precisely target root traits for wheat improvement. This study elucidates the genetic basis of a novel root water response trait observed in a wild introgression wheat (TL) that is missing in common wheat (P76). Here, we used the LRD RNAi and OE mutants in a spring wheat background (CBO37) to show that (a) LRD is the major gene conferring the ability of the Agropyron TL to maintain root growth under limited‐water conditions. (b) GA regulates lateral root emergence and growth under limited‐water conditions in wheat seedlings. The ability of the TL to maintain lateral root growth under limited water is associated with higher root GA levels (relative to P76) during water stress and is likely mediated via the down‐regulation of the LRD allele of Agropyron. (c) Suppression of LRD in wheat RNAi lines has a pleiotropic effect of increased seed size and seed weight per plant.
We propose that the Agropyron allele of LRD in the translocation wheat genotype, TL, confers the ability to maintain the root growth under water stress. This is supported by reduced lateral root density in seedlings stage and reduced root biomass at pre‐tillering stage of OE and WT (CBO37) genotypes. These results are consistent with previously reported phenotypic response of P76, a common wheat, wherein induction of LRD gene is associated with reduced growth under water stress (Placido et al., 2013). On contrary, RNAi lines with suppressed LRD maintain the lateral root density and root biomass at seedling and pre‐tillering stage, respectively. This observation is in accordance with previously shown ability of TL to maintain the root growth under water stress, wherein LRD allele is down‐regulated (Placido et al., 2013). It is likely that the higher root biomass and longer roots of the LRD RNAi and TL at pre‐tillering is due to its ability to maintain lateral root growth during early growth stages, which enables higher water uptake, stomatal conductance and carbon fixation resulting in overall biomass enhancement observed as larger roots and shoots compared with wild‐type and OE lines. This is further supported by ability of RNAi plants to maintain their stomatal conductance at pre‐tillering stage under water stress. In wheat, early seedling stage root growth traits are not always correlated with the adult root system (Watt et al., 2013). Therefore, it is possible that the LRD RNAi lines undergo a similar change in root growth habit with changing phenology. However, accurately determining the lateral root traits at pre‐tillering stage possesses substantial challenges as wheat has a very complex root system. Therefore, potential direct role of LRD gene in regulating root growth throughout the growth cycle still remains technically challenging.
Based on our results, a plausible hypothesis for the greater tolerance to water limitation in the TL in contrast to P76 is the following: In P76, LRD Ta is induced by water limitation, which directly or indirectly promotes the induction of GA catabolic gene GA2ox and suppression of GA biosynthesis gene G20ox.. This reduces GA levels under limited‐water conditions. Since exogenous GA promotes lateral root growth in wheat seedlings under limited‐water conditions, lower GA levels in P76 under limited water apparently represses lateral root growth, resulting in reduced lateral root density. In contrast, the expression of LRD Ag in TL roots is repressed by water stress. Since both GA2ox and GA20ox are maintained at the well‐watered level under water stress, root GA levels are maintained and consequently the TL lateral root density does not change in response to water limitation. The negative role of LRD in regulating GA‐related genes was further supported by water stress‐mediated induction of GA2ox and reduction of GA20ox in CBO37 and OE plants; wherein LRD is induced by water stress and is constitutively overexpressed, respectively. In contrast, RNAi plants are able to retain normal expression of these genes under water stress, thus maintaining GA levels similar to TL plants and lateral root density does not change. Notably, in maize a class I KNOTTED1‐like homeobox gene that is required for maintaining shoot meristematic activity regulates GA levels by directly binding a GA2ox gene (Bolduc and Hake, 2009). Whether a similar direct mechanistic link exists in wheat between LRD and the GA2ox in the roots to determine the lateral root density remains to be explored.
The root GA levels and LRD expression under limited‐water conditions are negatively associated. For instance, exogenous application of GA suppressed LRD Ta and LRD Ag under limited‐water conditions. The results presented do not address why the promotion of lateral roots by GA treatment is observed only under limited‐water conditions. Similarly, the lateral root density of the RNAi lines is comparable to wild‐type CBO37 under well‐watered conditions but higher only under limited‐water conditions. It is possible that GA‐promoted lateral root growth is repressed by another, yet to be determined factor, which itself is down‐regulated by water stress thus releasing the GA‐promoted lateral root formation pathway. In P76, because GA levels decrease in response to water limitation, the lateral root density decreases and can be compensated by exogenous GA. In TL water‐stressed roots, even though GA levels and lateral root density are maintained at levels similar to well‐watered conditions, the plants still respond to exogenous GA application under limited‐water conditions by further increasing lateral root density. Therefore, the proposed hypothesis of an undetermined lateral root repressor that is down‐regulated under limited‐water conditions is plausible. Although auxin is well‐known determinant of lateral root initiation, we did not find an obvious difference in auxin level at whole root level. However, a role of auxin cannot be ruled out solely based on total auxin levels the in LRD‐GA mediated lateral root development under water stress, as spatial distribution of auxin is a critical factor in initiating lateral root primordia (Casimiro et al., 2001).
At pre‐tillering stage, we observed that the RNAi lines under non‐stressed conditions had higher shoot biomass compared with wild‐type plants but there were no significant differences in plant height. Pavon 76, the TL and the CBO37 are semi‐dwarf wheat genotypes. In the semi‐dwarf wheat genotypes that were developed during the ‘green revolution’ in 1960s, plant height is reduced by a series of Rht genes/alleles linked to GA insensitivity (Peng et al., 1999). These semi‐dwarfing genes reduced plant height but did not impact the root length in two genetic backgrounds in multiple experimental set‐ups at the seedling stage (Wojciechowski et al., 2009). However, the impact of GA insensitivity in semi‐dwarf wheat backgrounds and its impact on early root growth is not fully explored under water‐limiting conditions.
Higher lateral root density and larger roots due to increased root number and/or deeper roots can potentially impact drought tolerance for plants under field conditions. However, there are no optimal drought‐tolerant root architecture traits that can prove advantageous in all environments. Importantly, LRD RNAi did not alter root development in well‐watered conditions, so it might not produce a growth penalty when water is available. The crop performance in water‐limiting environments is also determined by a range of factors, such as soil type, soil heterogeneity, agricultural environment (rainfed or irrigated) and development stage of the plants when drought occurs and the duration of drought stress. If the root response of the LRD RNAi lines translates under field conditions, it could potentially enable the RNAi plants to harness the moisture from the topsoil during seedling stage through greater lateral root density and then develop a larger and deeper root system for mining subsoil water during reproductive stage compared to wild‐type plants. However, it is also possible that wheat genotypes with reduced LRD expression could overspend resources on roots under water stress, leading to underperformance in environments experiencing prolonged water stress or where the soils lack sufficient moisture even in the deeper root zone. Extracting water from subsoil during reproductive stage is critical for wheat, maize, rice and sorghum yields in environments with terminal drought (Hammer et al., 2009; Hund et al., 2009; Manschadi et al., 2006; Steele et al., 2006; Uga et al., 2011; Vadez et al., 2011; Vadez et al., 2011; Wasson et al., 2012). Therefore, the discovery of a novel allelic variant that may enhance water extraction from deeper root zones could be potentially useful for these major crops.
Deeper and more efficient root systems have been associated with higher grain yields in wheat (El Hassouni et al., 2018; Fang et al., 2017; Kirkegaard et al., 2007), rice (Arai‐Sanoh et al., 2014) and sorghum (Mace et al., 2012). In this study, we observed a striking pleiotropic effect of altered LRD expression in transgenic lines on grain yield components under well‐watered conditions, including larger grains and more grains per plant. This observation is notable given the correlation between better/more adapted root systems and higher yields observed in cereals. Since LRD is expressed during early stages of grain development, it is possible that the increase in grain size is a consequence of direct role of LRD in determining the grain sink capacity. Sink capacity is primarily determined during early stages of grain development, typically characterized by rapid expansion of the caryopsis. Alternatively, the increase in grain size and grain number in the LRD RNAi plants could be an indirect consequence of a larger root system, which enhances source capacity. Our results suggest that LRD may be the first gene discovered in wheat that regulates root architecture under water stress and also improves multiple yield components under optimum conditions. In rice, DRO1 has been shown to regulate root depth and yield stability under drought stress conditions (Uga et al., 2011, 2013). Interestingly, (Voss‐Fels et al., 2018) reported that one of the key flowering time genes, VERNALIZATION 1 also influences root architecture in wheat. This further supports the genetic association between root growth and reproductive development. It is possible that genetic regulation of sink capacity during vegetative development (i.e. size of roots) and reproductive development (grain size and number) has some shared components. LRD could be one such gene that influences sink capacity during the life cycle of a wheat plant. Future research will focus on exploring the direct and indirect role of LRD gene and interacting molecular pathways in controlling yield and roots traits under longer water stress conditions.
Collectively, we show that LRD is the gene that regulates root plasticity in the Agropyron TL during water stress. The results from RNAi plants indicate that the lateral root growth trait is transferable to common wheat independent of the Agropyron translocation. Currently, there are not many functionally validated genes in wheat that directly regulate root architecture under any condition (Hu et al., 2018). This is a major limitation for breeding wheat cultivars that have root architecture tailored to specific environments. Therefore, discovery of LRD, with direct validation of root traits in wheat is potentially very useful for breeding efforts. The impact of LRD on grain yield components could also be a valuable trait for improving wheat productivity and needs to be examined further. Since wheat is widely grown in rainfed environments, our findings could potentially contribute towards the knowledge base needed for developing more resilient future wheat cultivars.
Methods
Overexpression of LRD Ta gene in spring wheat genotype CBO37
The LRD ORF amplified from spring wheat cultivar CBO37 was excised from an intermediate vector pGEM‐LRD as a EcoRI and SpeI fragment. The ORF was subcloned between the sugarcane UBi4 promoter and the 35S CaMV polyadenylation sequence (Wei et al., 2003). The expression cassette was introduced in the binary plasmid pPZP212 and the final vector designated pPTN1250 (Hajdukiewicz et al., 1994). The binary plasmid was mobilized into Agrobacterium tumefaciens strain C58C1/pMP90 via tri‐parental mating (Koncz and Schell, 1986), and the derived transconjugant was used for transformation of the spring wheat genotype CBO37 as previously described (Clemente and Mitra, 2005).
RNAi‐mediated suppression of LRD Ta in spring wheat genotype CBO37
Genetic element derived from LRD Ta was designated Kor369 and was amplified using primers enlisted in Table S3. To assist the cloning, BamHI and Xba I restriction sites were at the ends of the LRD Ta element. A base‐plasmid designated pUC18RNAi‐intron (developed by H. Cerutti, University of Nebraska) harbours the second intron of the Arabidopsis small nuclear protein, which is delineated by compatible restriction sites to facilitate hairpin assembly of the LRD Ta PCR product. The derived inverted repeat was subsequently subcloned between the maize ubiquitin 1 promoter coupled with its first intron (Christensen et al., 1992) and the 35S CaMV polyadenylation sequence. The silencing cassette was then cloned into the binary vector pPZP212 and the resulting plasmid designated pPTN1272 for the Kor369 RNAi element. Introduction into A. tumefaciens and subsequent spring wheat CBO37 transformations were conducted as outlined in (Clemente and Mitra, 2005). Expression level of LRD Ta in three independent OE and RNAi lines was confirmed using qRT‐PCR (Figure S1).
Seedling stage root screening
For the seedling stage root screening, we used the cigar roll method as described in (Placido et al., 2013; Zhu et al., 2006). For making each cigar roll, five seeds were placed at equidistant spacing between two sheets of germination paper, rolled and placed vertically in 1 L beaker in a growth chamber. Uniformly germinated thirty seeds per genotype per treatment were grown in cigar rolls under well‐watered (WW) or limited‐water (LW) conditions. For WW treatments, the beakers were filled with 200 mL of 1/10th strength Hoagland solution, while 50 mL water was supplied for LW treatment. Solution was added to each beaker daily to maintain the volume of 50 or 200 mL. All measurements were taken on day 6 after germination. Primary and secondary root lengths were measured from the root‐tip to the root‐shoot junction. Shoot length was measured from the tip of the longest leaf to the root‐shoot junction. Lateral root density was calculated by dividing the number of lateral roots on the primary root with the length of the root. For gene expression and hormone analysis experiments, tissue was collected at 6 days after germination.
Pre‐tillering stage limited water experiments
Uniformly germinated seeds from CBO37, RNAi and OE lines were transplanted to PVC pipes (1 m long × 15.5 cm diameter) lined with clear poly plastic bags and filled with approximately 9 kg of fine silica sand. For the first week after transplant, each tube received 100 mL water daily. After the first week, the WW controls were supplied with 50 mL of tap water and 50 mL of half‐strength Hoagland solution twice weekly. For the LW treatment, each tube received 50 mL of half‐strength Hoagland solution twice weekly. Each treatment comprised nine replicates per genotype/event with one plant per tube/replicate. Roots and shoots were harvested 27 days after transplanting. The sand moisture content for each representative treatment was collected on days 21, 24 and 27 using the ECH2O EC‐5 sensor (Decagon Devices, Pullman, WA, USA) at a depth of 10 cm (Figure S3a). Roots were removed from the sand column by pulling the plastic sleeve and cleaned gently before measuring root and shoot lengths. Root and shoot dry weights were determined after drying the tissue for 5 days at 60 °C.
Photosynthesis measurements
Photosynthesis data were collected from the Pre‐tillering stage limited water experiment. Photosynthetic rate and stomatal conductance were measured for CBO37 and the transgenic lines on the youngest fully expanded leaf at pre‐tillering stage using a LI‐COR 6400 instrument (LI‐COR Biosciences, Lincoln, NE, USA; n = 9 per genotype per treatment). Measurements were recorded on 21‐, 24‐ and 27‐day‐old seedlings for WW and LW treatment described above. The following settings were used: photosynthetic photon flux density of 800 mmol/m2, chamber CO2 concentration at 400 µmol CO2/mol, leaf temperature at 20 °C and chamber vapour concentration at 20 mmol H2O/mol. Measurements were recorded between 09:00 and 13:00 h. Reference CO2 levels were maintained at 400 µmol/s and chamber flow was set at 300 µmol.
GA treatment at seedling stage
All GA treatment experiments with wild‐type, transgenic and translocation genotypes were performed at the seedling stage using the cigar roll set‐up described above. P76, a spring wheat genotype and TL, a 7DL TL in P76 background reported in Placido et al. (2013) were used in GA treatments. The GA3 was obtained from Sigma‐Aldrich Corp., St. Louis, MO (Gibberellic acid potassium salt, G1025) for this assay. The GA treatment was carried out by adding GA3 dissolved in ethanol to the 1/10th strength Hoagland solution in the beakers at the start of the experiment. Same amount of ethanol was added to no‐GA‐treated seedlings. We applied a series of GA3 concentrations (0.01, 0.1, 1 and 10 µm) to determine the GA sensitivity and response of the P76 and TL roots (Table S4). Based on the GA3 dosage root response, we used a GA3 concentration of 1 µm for subsequent limited‐water experiment Table S4. The seedlings were treated with 1uM of GA3 under limited and well‐watered conditions. The root measurements were recorded on day 6 after germination. The root tissues for gene expression analysis were also collected at 6 days after germination.
Quantification of root GA and IAA content
Gibberellic acid was quantified essentially as described previously, except that the initial extraction step was scaled up fivefold for 250 mg of root tissue (Pan et al., 2010). Hormonal analysis was performed on three biological replicates per genotype per treatment. Each biological replicate consisted of roots from three to five plants. Quantification of IAA was done by GC/MS using negative chemical ionization essentially as previously described (Suza and Staswick, 2008), with the following changes. [13C6]‐IAA (Cambridge Isotope Laboratories, Tewksbury, MA, USA) was the internal standard, 30–60 mg of tissue was extracted, ion exchange was performed with a 0.25‐mL bed volume of DEAE resin in a 1‐cm‐diameter 4‐mL disposable column, resin‐bound sample was washed with 2 mL methanol and then eluted with 2 mL 6% (v/v) acetic acid in methanol. Ethyl acetate was substituted for chloroform for the organic extraction, and the GC oven temperature ramp was 150–250 °C at 15 °C/min.
Root tissue collection, RNA extraction and gene expression analysis using qRT‐PCR
For gene expression analysis, root tissue was collected in liquid nitrogen from 6‐day‐old seedlings grown in cigar rolls. Root RNA isolation and purification steps were performed as described in Placido et al. (2013). The iScript cDNA synthesis kit was used for real‐time quantitative PCR (qRT‐PCR) following the manufacturer's protocol (BIO‐RAD, Hercules, CA, USA). 0.5 µg of RNA was used per 10 µL reaction mixture. For the qPCR reaction, 4 µL of the diluted cDNA (1:10) was used per 15 µL reaction mixture. In the qPCR reaction volume, 7.5 µL of iQ SYBR Green Supermix was used (Bio‐Rad Laboratories). The qRT‐PCR was run using Roche Light Cycle 480 II with the following parameter settings (Roche Applied Science, Indianapolis, IN, USA): 95 °C pre‐incubation for 10 min, amplification was done for 45 cycles at 95 °C for 10 s and 60 °C for 10 s; the melting curve was set‐up for 95, 65 and 97 °C; and cooling was set‐up at 40 °C for 30 s. The melting curve data were collected for all samples and genes to ensure a single peak, indicating amplification of specific region by a pair of primers. Each qPCR run was performed with three independent tissue samples with each sample comprised roots from three to five plants and had two technical replicates. Two genes that showed stable expression values in a large set of microarray experiments across treatments and tissue samples were used as internal controls. A description of the genes and primer sequences is provided in Table S2.
Small‐scale plot‐level experiments
Plants of wild‐type CBO37, three RNAi and three OE events were grown under field conditions in Mead, NE, USA (41.12°N, 96.44°W) between April and July 2014. The soil type is silt clay loam. We planted 100 seeds per event for the RNAi and OE events (T3 generation) and 200 seeds for CBO37. Seeding distance was 5 cm, and rows were 81 cm apart. The supplementary irrigation was stopped after the wheat seedlings were fully established. The plots were not irrigated for rest for growing season but received 351 mm of rainfall between sowing and harvest. Plants were grown without supplementary fertilizer or disease treatment. The 1000‐grain weight was determined separately for each transgenic event and CBO37 after harvest. Mature grains from each row (genotype) were pooled and 1000 grains were randomly selected for weighing. Thousand‐grain weight data were collected from five to seven different sets of 1000 grains for each genotype/event.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
DP and HW designed the experiments; DP and PS performed the experiments; TC, SS, NN and TQ performed wheat transformations; and DP, HW, JS and PS wrote the manuscript.
Accession numbers
The IWGSC RefSeq v1.1 ID for LRD is TraesCS7D02G501000.
Supporting information
Figure S1 (a) Multiple sequence alignment of LRDTL/Ag and LRDP76 coding sequences. (b) Confirmation of altered expression of LRD Ta in the RNAi and OE plants.
Figure S2 Maximum seminal root length of wild‐type (CBO37), 3 RNAi and 3 OE 6 days old seedlings grown in cigar‐rolls under well‐watered (WW) and limited water (LW) conditions.
Figure S3 The pre‐tillering water stress experiment for CBO37, RNAi and OE genotypes.
Figure S4 Auxin levels in P76 and TL root tips under WW and LW.
Table S1 Effect on GA biosynthesis inhibitor Paclobutrazol (PAC) on lateral root density.
Table S2 Primer pairs used for RT‐qPCR.
Table S3 Primer pairs used for the construction of the LRD RNAi lines in CBO37 background.
Table S4 Dosage dependent effects of GA on wheat root traits.
Acknowledgements
The authors thank A. Lukaszewski (UC Riverside) for sharing the Agropyron translocation line, R. Cahoon (UNL) for technical assistance with GA analysis, P. Tenopir (UNL) for assistance with field plot experiments and K. Begcy (UNL) for technical assistance. This research was supported by grants from Daugherty Water for Food Institute and Institute of Agriculture and Natural Resources, University of Nebraska.
References
- Ahmed, M.A. , Zarebanadkouki, M. , Kaestner, A. and Carminati, A. (2016) Measurements of water uptake of maize roots: the key function of lateral roots. Plant Soil, 398, 59–77. [Google Scholar]
- Arai‐Sanoh, Y. , Takai, T. , Yoshinaga, S. , Nakano, H. , Kojima, M. , Sakakibara, H. et al. (2014) Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 4. 10.1038/srep05563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseng, S. , Ewert, F. , Martre, P. , Rötter, R.P. , Lobell, D.B. , Cammarano, D. et al. (2015) Rising temperatures reduce global wheat production. Nat. Clim. Chang. 5, 143–147. [Google Scholar]
- Bao, Y. , Aggarwal, P. , Robbins, N.E. , Sturrock, C.J. , Thompson, M.C. , Tan, H.Q. et al. (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl. Acad. Sci. USA, 111, 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy, K. and Walia, H. (2015) Drought stress delays endosperm development and misregulates genes associated with cytoskeleton organization and grain quality proteins in developing wheat seeds. Plant Sci. 240, 109–119. [DOI] [PubMed] [Google Scholar]
- Benková, E. and Bielach, A. (2010) Lateral root organogenesis ‐ from cell to organ. Curr. Opin. Plant Biol. 13, 677–683. [DOI] [PubMed] [Google Scholar]
- Bolduc, N. and Hake, S. (2009) The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell, 21, 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, N.E. (2008) Feeding A world of 10 billion people: our 21st century challenge. In Perspectives in World Food and Agriculture 2004 ( Miranowski, J.A. and Scanes, C.G. , eds), pp. 31–56. Iowa: Iowa State Press. [Google Scholar]
- Casimiro, I. , Marchant, A. , Bhalerao, R.P. , Beeckman, T. , Dhooge, S. , Swarup, R. et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell, 13, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Shi, Jing , Hao, X. , Liu, H. , Shi, Jianghua , Wu, Y. et al. (2013) OsORC3 is required for lateral root development in rice. Plant J. 74, 339–350. [DOI] [PubMed] [Google Scholar]
- Chhun, T. , Taketa, S. , Tsurumi, S. and Ichii, M. (2003) The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice (Oryza sativa L.). Plant Growth Regul. 39, 161–170. [Google Scholar]
- Cho, S.‐H. , Yoo, S.‐C. , Zhang, H. , Pandeya, D. , Koh, H.‐J. , Hwang, J.‐Y. et al. (2013) The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL‐related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 198, 1071–1084. [DOI] [PubMed] [Google Scholar]
- Christensen, A.H. , Sharrock, R.A. and Quail, P.H. (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 18, 675–689. [DOI] [PubMed] [Google Scholar]
- Clemente, T. and Mitra, A. (2005) Genetic engineering of wheat: protocols and use to enhance stress tolerance. In Genetically Modified Crops: Their Development, Uses, and Risks ( Liang, G.H. and Skinner, D.Z. , eds), pp.131-163. New York, NY: Haworth Press. [Google Scholar]
- Dean, G. , Casson, S. and Lindsey, K. (2004) KNAT6 gene of Arabidopsis is expressed in roots and is required for correct lateral root formation. Plant Mol. Biol. 54, 71–84. [DOI] [PubMed] [Google Scholar]
- El Hassouni, K. , Alahmad, S. , Belkadi, B. , Filali‐Maltouf, A. , Hickey, L.T. and Bassi, F.M. (2018) Root system architecture and its association with yield under different water regimes in Durum wheat. Crop Sci. 58, 2331–2346. [Google Scholar]
- Fang, Y. , Du, Y. , Wang, J. , Wu, A. , Qiao, S. , Xu, B. et al. (2017) Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 8, 672. 10.3389/fpls.2017.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . (2013). FAO Statistical Yearbook, 2013. Part 3: Feeding the world. 130–133. Accessible at http://www.fao.org/docrep/018/i3107e/i3107e03.pdf.
- Fu, X. and Harberd, N.P. (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 421, 740–743. [DOI] [PubMed] [Google Scholar]
- Gill, B.S. , Appels, R. , Botha‐Oberholster, A.M. , Buell, C.R. , Bennetzen, J.L. , Chalhoub, B. et al. (2004) A workshop report on wheat genome sequencing: international genome research on wheat consortium. Genetics, 168, 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz, P. , Svab, Z. and Maliga, P. (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. [DOI] [PubMed] [Google Scholar]
- Hammer, G.L. , Dong, Z. , McLean, G. , Doherty, A. , Messina, C. , Schussler, J. et al. (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Sci. 49, 299–312. [Google Scholar]
- Hedden, P. (2003) The genes of the green revolution. Trends Genet. 19, 5–9. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Wang, R. , Zheng, M. , Liu, X. , Meng, F. , Wu, H. et al. (2018) TaWRKY51 promotes lateral root formation through negative regulation of ethylene biosynthesis in wheat (Triticum aestivum L.). Plant J. 96, 372–388. [DOI] [PubMed] [Google Scholar]
- Hund, A. , Ruta, N. and Liedgens, M. (2009) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant Soil, 318, 311–325. [Google Scholar]
- Hurd, E.A. (1968) Growth of roots of seven varieties of spring wheat at high and low moisture levels1. Agron. J. 60, 201. [Google Scholar]
- Iizumi, T. , Sakuma, H. , Yokozawa, M. , Luo, J.J. , Challinor, A.J. , Brown, M.E. et al. (2013) Prediction of seasonal climate‐induced variations in global food production. Nat. Clim. Chang. 3, 904–908. [Google Scholar]
- Kirkegaard, J.A. , Lilley, J.M. , Howe, G.N. and Graham, J.M. (2007) Impact of subsoil water use on wheat yield. Aust. J. Agric. Res. 58, 303–315. [Google Scholar]
- Koncz, C. and Schell, J. (1986) The promoter of TL‐DNA gene 5 controls the tissue‐specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204, 383–396. [Google Scholar]
- Li, X. , Ingvordsen, C. , Weiss, M. , Rebetzke, G.J. , Condon, A.G. , James, R.A. and Richards, R.A. (2019) Deeper roots associated with cooler canopies, higher NDVI and greater yield in three wheat populations grown on stored soil water. J. Exp. Bot. 24, 4963–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell, D.B. , Schlenker, W. and Costa‐Roberts, J. (2011) Climate trends and global crop production since 1980. Science (80‐.), 333, 616–620. [DOI] [PubMed] [Google Scholar]
- Lopes, M.S. and Reynolds, M.P. (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant Biol. 37, 147–156. [Google Scholar]
- Mac Key, J. , Sears, E. and Sears, L. (1973) The wheat root, Proceedings 4th International Wheat Genetics Symposium, pp. 827–842. Columbia, MO: University of Missouri. [Google Scholar]
- Mace, E.S. , Singh, V. , van Oosterom, E.J. , Hammer, G.L. , Hunt, C.H. and Jordan, D.R. (2012) QTL for nodal root angle in sorghum (Sorghum bicolor L. Moench) co‐locate with QTL for traits associated with drought adaptation. Theor. Appl. Genet. 124, 97–109. [DOI] [PubMed] [Google Scholar]
- Manschadi, A.M. , Christopher, J. , Devoil, P. and Hammer, G.L. (2006) The role of root architectural traits in adaptation of wheat to water‐limited environments. Funct. Plant Biol. 33, 823–837. [DOI] [PubMed] [Google Scholar]
- Meng, F. , Xiang, D. , Zhu, J. , Li, Y. and Mao, C. (2019) Molecular mechanisms of root development in rice. Rice, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto, T. and Oyanagi, A. (1994) The direction of growth of seminal roots of Triticum aestivum L. and experimental modification thereof. Ann. Bot. 73, 363–367. [Google Scholar]
- O’Brien, L. (1979) Genetic variability of root growth in wheat (Triticum aestivum L.). Aust. J. Agric. Res. 30, 587–595. [Google Scholar]
- Pan, X. , Welti, R. and Wang, X. (2010) Quantitative analysis of major plant hormones in crude plant extracts by high‐performance liquid chromatography‐mass spectrometry. Nat. Protoc. 5, 986–992. [DOI] [PubMed] [Google Scholar]
- Peng, J. , Richards, D.E. , Hartley, N.M. , Murphy, G.P. , Devos, K.M. , Flintham, J.E. et al. (1999) “Green revolution” genes encode mutant gibberellin response modulators. Nature, 400, 256–261. [DOI] [PubMed] [Google Scholar]
- Placido, D.F. , Campbell, M.T. , Folsom, J.J. , Cui, X. , Kruger, G.R. , Baenziger, P.S. and Walia, H. (2013) Introgression of novel traits from a wild wheat relative improves drought adaptation in wheat. Plant Physiol. 161, 1806–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, D.K. , Gerber, J.S. , MacDonald, G.K. and West, P.C. (2015) Climate variation explains a third of global crop yield variability. Nat. Commun. 6, 5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, R.A. and Passioura, J.B. (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain‐fed environments. Aust. J. Agric. Res. 40, 943–950. [Google Scholar]
- Rosenzweig, C. , Elliott, J. , Deryng, D. , Ruane, A.C. , Müller, C. , Arneth, A. et al. (2014) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA, 111, 3268–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma, D. and Knott, D.R. (1966) The transfer of leaf‐rust resistance from Agropyron to Triticum by irradiation. Can. J. Genet. Cytol. 8, 137–143. [Google Scholar]
- Sears, E.R. (1969) Wheat cytogenetics. Annu. Rev. Genet. 3, 451–468. [Google Scholar]
- Siddique, K.H.M. , Belford, R.K. and Tennant, D. (1990) Root:shoot ratios of old and modern, tall and semi‐dwarf wheats in a mediterranean environment. Plant Soil, 121, 89–98. [Google Scholar]
- Steele, K.A. , Price, A.H. , Shashidhar, H.E. and Witcombe, J.R. (2006) Marker‐assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. Appl. Genet. 112, 208–221. [DOI] [PubMed] [Google Scholar]
- Suza, W.P. and Staswick, P.E. (2008) The role of JAR1 in Jasmonoyl‐L: ‐isoleucine production during Arabidopsis wound response. Planta, 227, 1221–1232. [DOI] [PubMed] [Google Scholar]
- Truernit, E. and Haseloff, J. (2007) A role for KNAT class II genes in root development. Plant Signal. Behav. 2, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit, E. , Siemering, K.R. , Hodge, S. , Grbic, V. and Haseloff, J. (2006) A map of KNAT gene expression in the Arabidopsis root. Plant Mol. Biol. 60, 1–20. [DOI] [PubMed] [Google Scholar]
- Uga, Y. , Okuno, K. and Yano, M. (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 62, 2485–2494. [DOI] [PubMed] [Google Scholar]
- Uga, Y. , Sugimoto, K. , Ogawa, S. , Rane, J. , Ishitani, M. , Hara, N. et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Vadez, V , Deshpande, S.P. , Kholova, J. , Hammer, G.L. , Borrell, A.K. , Talwar, H.S. and Hash, C.T. (2011) Stay‐green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Funct. Plant Biol. 38, 553–566. [DOI] [PubMed] [Google Scholar]
- Vadez, V. , Krishnamurthy, L. , Hash, C.T. , Upadhyaya, H.D. and Borrell, A.K. (2011) Yield, transpiration efficiency, and water‐use variations and their interrelationships in the sorghum reference collection. Crop Pasture Sci. 62, 645–655. [Google Scholar]
- Voss‐Fels, K.P. , Robinson, H. , Mudge, S.R. , Richard, C. , Newman, S. , Wittkop, B. et al. (2018) VERNALIZATION1 modulates root system architecture in Wheat and Barley. Mol. Plant, 11, 226–229. [DOI] [PubMed] [Google Scholar]
- Waines, J.G. and Ehdaie, B. (2007) Domestication and crop physiology: roots of green‐revolution wheat. Ann. Bot. 100, 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Taketa, S. , Miyao, A. , Hirochika, H. and Ichii, M. (2006) Isolation of a novel lateral‐rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Sci. 170, 70–77. [Google Scholar]
- Wang, H. , Hu, Z. , Huang, K. , Han, Y. , Zhao, A. , Han, H. et al. (2018) Three genomes differentially contribute to the seedling lateral root number in allohexaploid wheat: evidence from phenotype evolution and gene expression. Plant J. 95, 976–987. [DOI] [PubMed] [Google Scholar]
- Wasson, A.P. , Richards, R.A. , Chatrath, R. , Misra, S.C. , Prasad, S.V.S. , Rebetzke, G.J. et al. (2012) Traits and selection strategies to improve root systems and water uptake in water‐limited wheat crops. J. Exp. Bot. 63, 3485–3498. [DOI] [PubMed] [Google Scholar]
- Watt, M. , Moosavi, S. , Cunningham, S.C. , Kirkegaard, J.A. , Rebetzke, G.J. and Richards, R.A. (2013) A rapid, controlled‐environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 112, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. , Wang, M.L. , Moore, P.H. and Albert, H.H. (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J. Plant Physiol. 160, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Wojciechowski, T. , Gooding, M.J. , Ramsay, L. and Gregory, P.J. (2009) The effects of dwarfing genes on seedling root growth of wheat. J. Exp. Bot. 60, 2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, P. , Gutjahr, C. , Li, C. and Hochholdinger, F. (2016) Genetic control of lateral root formation in cereals. Trends Plant Sci. 21, 951–961. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Obringer, R. , Wei, C. , Chen, N. and Niyogi, D. (2017) Droughts in India from 1981 to 2013 and implications to wheat production. Sci. Rep. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Mickelson, S.M. , Kaeppler, S.M. and Lynch, J.P. (2006) Detection of quantitative trait loci for seminal root traits in maize (Zea mays L.) seedlings grown under differential phosphorus levels. Theor. Appl. Genet. 113, 1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a) Multiple sequence alignment of LRDTL/Ag and LRDP76 coding sequences. (b) Confirmation of altered expression of LRD Ta in the RNAi and OE plants.
Figure S2 Maximum seminal root length of wild‐type (CBO37), 3 RNAi and 3 OE 6 days old seedlings grown in cigar‐rolls under well‐watered (WW) and limited water (LW) conditions.
Figure S3 The pre‐tillering water stress experiment for CBO37, RNAi and OE genotypes.
Figure S4 Auxin levels in P76 and TL root tips under WW and LW.
Table S1 Effect on GA biosynthesis inhibitor Paclobutrazol (PAC) on lateral root density.
Table S2 Primer pairs used for RT‐qPCR.
Table S3 Primer pairs used for the construction of the LRD RNAi lines in CBO37 background.
Table S4 Dosage dependent effects of GA on wheat root traits.


