Summary
Seed vigour is an important trait for direct seeding in rice. In this study, indole‐3‐acetate beta‐glucosyltransferase OsIAGLU was cloned in rice, and its roles on seed vigour were mainly investigated. Disruption of OsIAGLU resulted in low seed vigour in rice. Quantitative RT‐PCR analysis showed that the expressions of OsIAGLU were relatively higher in the late developing and the early germinating seeds and were significantly induced by indole‐3‐acetic acid (IAA) and abscisic acid (ABA). Transcriptome analysis revealed that the IAA‐ and ABA‐related genes were involved in the OsIAGLU regulation of seed vigour in rice. The higher levels of free IAA and ABA were identified in germinating seeds of osiaglu mutants compared to wild‐type (WT) plants. When treated with exogenous IAA and ABA, the osiaglu mutants and WT plants showed sensitivity to ABA while not IAA, but the exogenous IAA amplified ABA‐induced reduction of seed vigour in rice. The continuously higher expressions of ABA‐INSENSITIVE 3 (OsABI3) and OsABI5 occurred in germinating seeds of osiaglu mutants compared to WT plants. The regulation of seed vigour by OsIAGLU might be through modulating IAA and ABA levels to alert OsABIs expression in germinating seeds in rice. Based on analysis of single‐nucleotide polymorphism data of rice accessions, two haplotypes of OsIAGLU that positively correlated with seed vigour were identified in indica accessions. This study provides important insights into the roles of OsIAGLU on seed vigour and facilitates the practical use of OsIAGLU in rice breeding.
Keywords: rice, seed vigour, hormone glucosyltransferases, indole‐3‐acetic acid, abscisic acid
Introduction
Rice is one of the oldest cultivated crops in China. In recent years, the direct seeding method of rice has been widely applied due to its advantages of cost‐ and labour‐saving (Liu et al., 2015a). Seed germination and seedling establishment as key events in plant life activities are the important factors influencing the yield of direct seeding in rice (Gommers and Monte, 2018). Seeds with high vigour will behave the characteristics of speed and uniform germination and vigorous seedling growth in fields, which will lead to better weed competitiveness and reduce yield losses (Chen et al., 2019). The varieties with high seed vigour are required for the direct seeding of rice (Mahender et al., 2015). The mining of key genes regulated seed vigour and illuminating their mechanisms are important objectives of rice breeding for direct seeding production.
Abscisic acid (ABA) as the major endogenous factor has been widely investigated involving in the inhibition of seed germination (Penfield, 2017). The ABA level in plants is maintained in strict homeostasis, including biosynthesis, degradation and conjugation, during various physiological processes (Palaniyandi et al., 2015; Seiler et al., 2011). The increase of ABA level in seeds is controlled by ABA biosynthesis genes zeaxanthin epoxidase (ZEP) and 9‐cis‐epoxycarotenoid dioxygenases (NCEDs; Eiji et al., 2010). The decrease of ABA level is regulated by ABA catabolism CYP707A family genes (Kushiro et al., 2004). Moreover, the conjugation of ABA with glucose catalysed by ABA‐specific glucosyltransferases represents another ABA inactivation mechanism. For example, the Arabidopsis UGT71B6 exhibits glucosylation activity towards ABA for the conversion of free ABA to inactive form ABA‐glucose (Priest et al., 2006). It is well known that ABA acts through the PYR/RCAR‐PP2C‐SnRK2 signalling cascade (Cutler et al., 2010). ABSCISIC ACID‐INSENSITIVE 3 (ABI3) is a major downstream component of ABA signalling, and it has been reported as a major regulator of seed dormancy and ABA inhibition of seed germination (Liu et al., 2013).
Auxin recently has also been reported to play essential roles in the regulation of seed dormancy in plants. In Arabidopsis, auxin controls seed dormancy through the stimulation of ABA signalling (Liu et al., 2013). When auxin signalling activated, auxin binds to its receptor transport inhibitor resistant 1/auxin signalling F‐box (TIR1/AFB) proteins and promotes the degradation of auxin/indole‐acetic acid (AUX/IAA; Dharmasiri et al., 2005). This degradation releases the activity of auxin response factor (ARF10/16) and maintains the expression of ABI3, which inhibits seed germination and maintains seed dormancy (Liu et al., 2013). The active auxin level is tightly controlled by the processes of synthesis, inactivation and transport (Korasick et al., 2013). In which, cellular auxin level can be altered by auxin glycosyltransferases usually through catalysing the transfer of activated sugars to auxin in plants. For example, the maize UDP glucosyltransferase IAGLU can conjugate IAA to glucose (Szerszen et al., 1994). The Arabidopsis UDP glucosyltransferase UGT84B1 has the glycosylation activities against IAA and indole‐3‐butyric acid (IBA; Jackson et al., 2001), and the glycosylation activities of Arabidopsis UGT74E2 and UGT75D1 are prominent towards IBA (Tognetti et al., 2010; Zhang et al., 2016).
Glycosylation of hormones plays important roles in plant development and growth (Palaniyandi et al., 2015; Shang et al., 2016; Zhang et al., 2016). Several studies have reported that auxin glycosyltransferases involve in plant development and stress tolerance in Arabidopsis. For example, the overexpression lines of auxin glycosyltransferase UGT84B1 and UGT74D1 behave plant dwarfing and leaf curl phenotype in Arabidopsis (Jackson et al., 2002; Jin et al., 2013). The IBA and IAA homeostasis is perturbed in the overexpression lines of UGT74E in Arabidopsis, that causes the increase of shoot branching, the alteration of rosette shape and the improvement of stress tolerance (Tognetti et al., 2010). Plants overexpressing UGT75D1 increased seed germination under stress conditions might be through modulating ARF16‐ABI3 signalling in Arabidopsis (Zhang et al., 2016). The phenotypes of enlarged leaf angle, dwarf plants and shorter panicles are observed in transgenic rice overexpressing OsIAAGLU (Yu et al., 2019). However, the regulation roles of auxin glycosyltransferase on seed vigour are few reported in rice.
Our previous RNA‐Seq data showed that the expression of indole‐3‐acetate beta‐glucosyltransferase OsIAGLU was significantly induced at the early seed imbibition stage in rice, suggesting it might be involved in the regulation of seed vigour. In this study, rice OsIAGLU was cloned and its regulatory functions on seed vigour were mainly investigated. Disruption of OsIAGLU resulted in low seed vigour in rice. The higher free IAA and ABA levels were observed in germinating seeds of osiaglu mutants compared with wild‐type (WT) plants in rice. The possible mechanisms of OsIAGLU involving in the regulation of seed vigour might be through modulating OsABIs expressions under the interactions of IAA and ABA in germinating seeds of rice. The application of OsIAGLU might be useful to genetic improvements of seed vigour for direct seeding of rice in the future.
Results
Disruption of OsIAGLU resulted in low seed vigour
Rice OsIAGLU was located on Chromosome 11, which was predicted to contain approximately 504 amino acids (Figure S1a). The phylogenetic tree indicated that the closer genetic relationships of IGAU exist among rice and maize (Figure S1b). To further confirm OsIAGLU function, we employed the CRISPR/Cas9 system to generate mutants, which were named osiaglu‐1, osiaglu‐2 and osiaglu‐3 (Figure 1a). The osiaglu‐1, osiaglu‐2 and osiaglu‐3 mutant plants contained a 59‐, 58‐ and 5‐bp deletion, respectively, in the exon of OsIAGLU (Figure 1b). The amino acid sequence of OsIAGLU, predicted based on these nucleotide sequences, contains only 39, 100 and 57 amino acids in osiaglu‐1, osiaglu‐2 and osiaglu‐3, respectively, and is caused by premature termination (Figure S2). These results indicate that the osiaglu‐1, osiaglu‐2 and osiaglu‐3 mutant lines lacked OsIAGLU. The progeny of these homozygous mutants were used in subsequent experiments.
Figure 1.
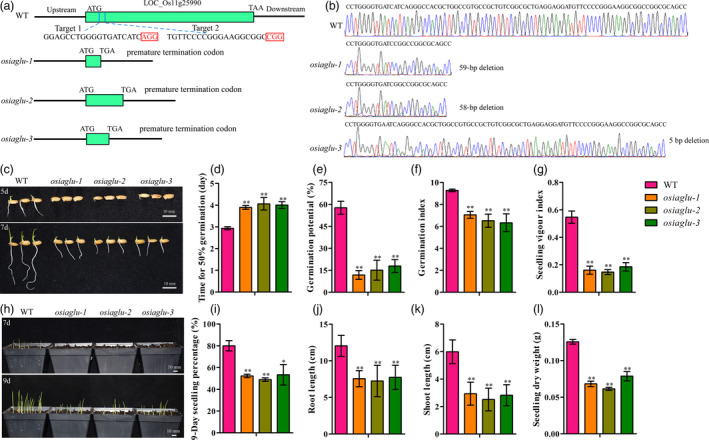
Comparison of seed vigour between WT and osiaglu mutants in rice. (a) Gene structures of WT and osiaglu mutants. Green box represents the OsIAGLU gene region. (b) A total of 59‐, 58‐ and 5‐bp nucleotides were deleted in the osiaglu‐1, osiaglu‐2 and osiaglu‐3, respectively. (c) Seed germination of WT and osiaglu mutants after 5 and 7 days. Bars = 10 mm. (d) Time to 50% germination percentage; (e) germination potential; (f) germination index; (g) seedling vigour index. (h) Seedling establishment of WT and osiaglu mutants after 7 and 9 days under direct seeding conditions. Bars = 10 mm. (i) seedling percentage; (j) root length; (k) shoot length; (l) seedling dry weight. Each column represents the means ± SD. * and ** indicate the significant difference compared to WT at 5% and 1% levels, respectively, according to Student’s t‐test.
Seed quality and seed dormancy established during seed development will influence the performances of seed vigour during seed germination. Our data showed that there were no significant differences of grain traits, including grain length, width, thickness and grain weight, between three osiaglu mutants and wild‐type (WT) (Figure S3). There were no seed dormancy occurred in three osiaglu mutants and WT with more than 90% germination percentage after 10 days of germination by dormancy breaking treatments in this study (Figure S4). Then, the seeds of three osiaglu mutants and WT were subsequently used for the evaluation of seed vigour. Phenotype evaluation demonstrated that the disruption of OsIAGLU resulted in low seed vigour, mainly including low germination speed and seedling growth, under various conditions in rice. The times for 50% germination of osiaglu‐1, osiaglu‐2 and osiaglu‐3 were significantly increased compared to those of wild‐type (WT) plants, while the germination potential, germination index and seedling vigour index were significantly reduced under normal conditions (Figure 1c–g). Moreover, the seedling percentage and seedling growth, including root length, shoot length and seedling dry weight, were significantly decreased in osiaglu‐1, osiaglu‐2 and osiaglu‐3 lines compared to those in WT plants under the direct seeding method in soils (Figure 1h–l). All these results suggest that OsIAGLU might play important roles on the regulation of seed vigour in rice.
Expression pattern of OsIAGLU and subcellular localization
To expand our understanding of the physiological function of OsIAGLU, the expression patterns of OsIAGLU during seed development and seed germination were further analysed using quantitative RT‐PCR approach. Transcripts of OsIAGLU were dynamically increased in the filling grain from 0 to 28 days after flowering (DAF) and sharply increased after the 28 DAF (Figure 2a). Additionally, the transcript of OsIAGLU was strongly detected at 4‐h imbibition time‐point during the early seed germination stage (Figure 2a). We also investigated whether the expression of OsIAGLU was induced by IAA, ABA and GA during seed germination. The results indicated that the expression of OsIAGLU could be obviously induced by both IAA and ABA while not GA within different duration of treatments from 12 to 72 h, with a stronger induction by IAA and ABA than GA (Figure 2b).
Figure 2.
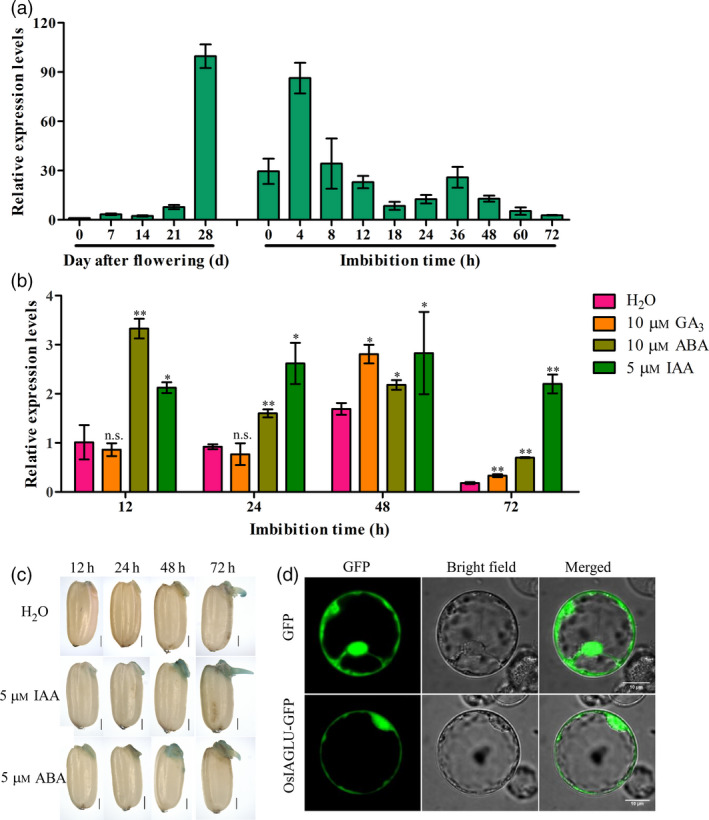
Expression patterns of OsIAGLU and subcellular localization in rice. (a) Expression pattern of OsIAGLU in various developmental and germination stages in rice using quantitative RT‐PCR approach. (b) Transcription levels of OsIAGLU response to GA3, ABA and IAA treatments in germinating seeds conducted using quantitative RT‐PCR approach. The expression of OsIAGLU was normalized to that of OsActin gene control. The relative expression levels were represented by fold change relative to the expression level of OsIAGLU at 0 day after flowering or 12‐h imbibition stage under H2O treatment. Each column represents the means ± SD. * and ** indicate the significant difference compared to WT at 5% and 1% levels, respectively, according to Student’s t‐test. n.s. represents not significant. (c) Histochemical staining for GUS activity of OsIAGLU during seed germination under normal, IAA and ABA treatment conditions in rice. (d) Subcellular localization of OsIAGLU tagged at the C‐terminus with GFP in rice protoplasts.
To investigate the tissue‐specific expression of OsIAGLU, histochemical staining for GUS activity of the OsIAGLU promoter::GUS transgenic lines was carried out. It showed that OsIAGLU was strongly expressed in embryos and shoots of germinating seeds during 12–72 h under normal, IAA and ABA treatment conditions (Figure 2c). To determine the subcellular localization of OsIAGLU, we constructed a recombinant OsIAGLU tagged at the C‐terminus with GFP and expressed it transiently in rice protoplasts. The GFP‐tagged OsIAGLU was found to distribute throughout the cytoplasm (Figure 2d), suggesting the catalytic activity of OsIAGLU is present in the cytoplasm. The specific expression of OsIAGLU in germinating seeds suggested that it may play a role in modulating seed vigour in rice.
OsIAGLU regulates IAA and ABA levels during seed germination
To further understand the OsIAGLU function, genome‐wide transcriptional levels were compared between osiaglu‐2 and WT at the 72‐h imbibition stage. By comparison, the data of RNA sequencing were high consistent (Pearson correlation >0.997) between samples in this study, and then the mean FPKM of three biological repeats was used for further analysis. A total of 9121 differentially expressed genes (DEGs) were identified between osiaglu‐2 and WT (Table S1). Of those, 3148 and 5973 genes were significantly up‐regulated and down‐regulated, respectively, in osiaglu‐2 compared to WT (Figure 3a). Through MapMan analysis, 222 differentially expressed genes were significantly enriched in hormone metabolism. In which, the majority of hormone‐related DEGs were involved in IAA (77), ABA (23), jasmonic acid (JA) (22) and ethylene (ETH) (60) metabolism (Figure 3b).
Figure 3.
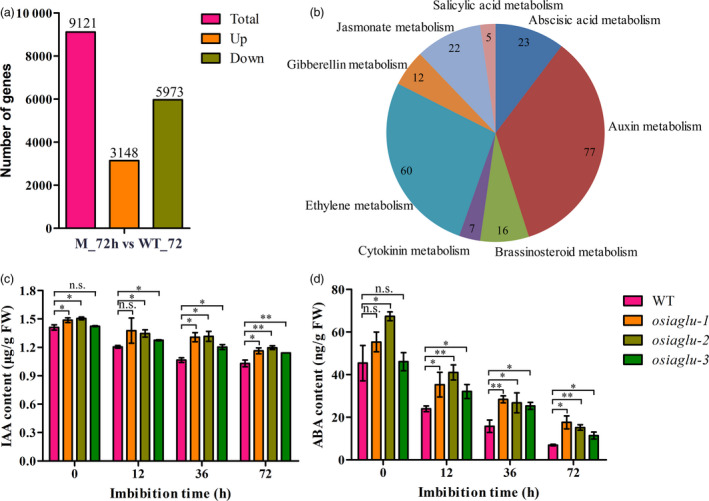
OsIAGLU altering the contents of IAA and ABA in germinating seeds in rice. (a) Differentially expressed genes (DEGs) with p < 0.001 between WT and osiaglu‐2 in germinating seeds (72 h) in rice. (b) DEGs involved in hormone metabolism. (c‐d) The contents of IAA and ABA in germinating seeds of WT and osiaglu mutants in rice. Each column represents the means ± SD. * and ** indicate the significant difference compared to WT at 5% and 1% levels, respectively, according to Student’s t‐test. n.s. represents not significant.
It has been reported that glycosyltransferases usually involved in IAA and ABA homeostasis in plants (Priest et al., 2006; Yu et al., 2019). Our above RNA‐Seq data indicated that OsIAGLU might involve in the regulation of IAA and ABA metabolism during seed germination in rice. We therefore speculated that OsIAGLU might regulate IAA and ABA levels during seed germination in rice. To confirm this presumption, the levels of endogenous IAA and ABA in mature and germinating seeds of osiaglu mutants and WT plants were quantified using an ultra‐performance liquid chromatography–tandem mass spectrometry (LC‐MS/MS) approach. By comparison, the levels of IAA and ABA were significantly increased in germinating seeds of osiaglu mutants compared to those in WT plants during 12–72 h after germination (Figure 3c‐d). Meanwhile, the increases of IAA and ABA levels were generally observed in osiaglu mutants compared to WT plants in mature seeds. Overall, the levels of endogenous IAA and ABA regulated by OsIAGLU in germinating seeds were confirmed.
OsIAGLU regulates seed vigour associated with the interaction of ABA and IAA
As descripted above, rice OsIAGLU can influence the free IAA and ABA levels during seed germination, and thus, we predicted that the higher accumulation of free IAA and ABA in osiaglu mutants might cause low seed vigour. Previous study showed that the ABA‐sensitive phenotypes during seed germination were observed at 5 to 50 μm ABA treatments in rice (Liu et al., 2015b), while no IAA‐sensitive phenotypes were observed at 5 to 50 μm IAA treatments according to our previous data. To further confirm the above prediction, the impacts of 5 μm and 10 μm exogenous IAA and ABA on osiaglu mutants and WT plants were analysed (Figure 4a). In the presence of exogenous IAA, there were no significant differences in seed vigour, including times for 50% germination, germination percentage and germination index, between control and treatments among osiaglu mutants and WT plants (Figure 4b‐d). However, in the presence of exogenous ABA, the seed vigour of osiaglu mutants and WT plants was significantly reduced by treatments (Figure 4e–g). Moreover, osiaglu plants exhibited more ABA‐sensitive phenotype with lower seed vigour compared with WT plants.
Figure 4.
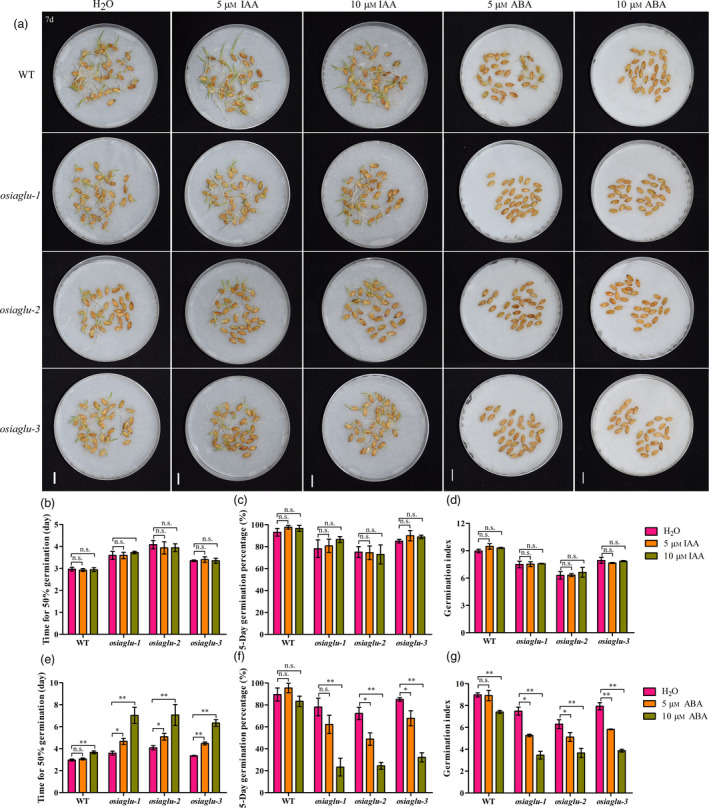
Effects of IAA and ABA treatments on seed vigour among WT and osiaglu mutants in rice. (a) Seed germination of WT and osiaglu mutants under IAA and ABA treatments for 7 days. Bars = 10 mm. (b‐d) Comparison of time to 50% germination percentage, germination potential, germination index between normal and IAA treatments in WT and osiaglu mutants. (e‐g) Comparison of seed vigour including time to 50% germination percentage, germination potential, germination index between normal and ABA treatments in WT and osiaglu mutants. Each column represents the means ± SD. * and ** indicate the significant difference compared to normal condition at 5% and 1% levels, respectively, according to Student’s t‐test. n.s. represents not significant.
Previous study indicated that auxin‐mediated seed dormancy is dependent on ABA in Arabidopsis (Liu et al., 2013). We, therefore, examined the interaction of IAA and ABA on the regulation of seed vigour in rice. The seed vigour of WT plants was tested under various concentrations of exogenous IAA (5 μm and 10 μm) treatments in the presence of exogenous ABA (5 μm; Figure 5). Our results indicated that the exogenous IAA can enhance the ABA‐mediated reduction of seed vigour in rice. Seed vigour was significantly reduced by IAA + ABA treatments compared with ABA treatments. Similarly, the exogenous IAA enhanced the ABA‐sensitive phenotype of osiaglu plants with lower seed vigour compared with WT plants (Figure S5). These results suggest that knockout of OsIAGLU results in low seed vigour because of excessive ABA and IAA accumulation and their interaction in rice.
Figure 5.
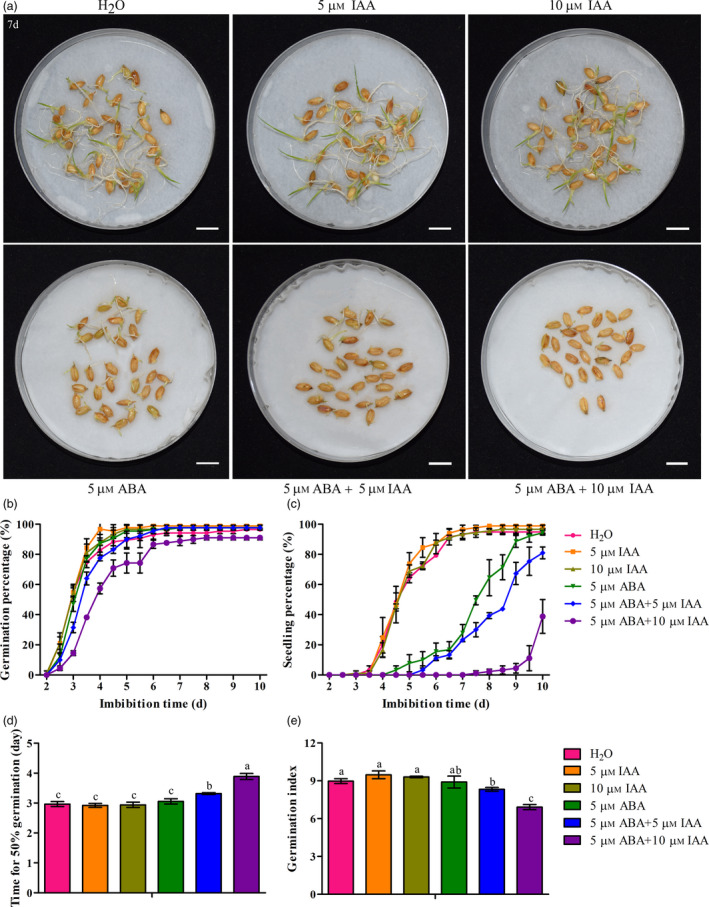
Interactions of IAA and ABA on seed vigour in rice. (a) Seed germination of WT under normal, IAA, ABA and IAA + ABA treatments for 7 days. Bars = 10 mm. (b‐c) Comparison of dynamic changes of germination percentage and seedling percentage in WT among normal, IAA, ABA and IAA + ABA treatments during seed germination. Each point represents the means ± SD. (e‐g) Comparison of time to 50% germination percentage and germination index in WT among normal, IAA, ABA and IAA + ABA treatments. Each column represents the means ± SD. Different lowercase letters represent significant difference at 5% level according to least significant difference (LSD) test.
OsIAGLU regulates seed vigour involved in OsABIs expression
How the OsIAGLU regulated seed vigour though influencing the IAA and ABA levels during seed germination? Our RNA‐Seq also showed that the majority of IAA‐related DEGs, including TIR1 (1), AUX/IAA (15) and ARFs (9), were involved in IAA signalling processes (Figure 6a), and the majority of ABA‐related DEGs, including ABA synthesis/degradation (10), ABA signal transduction (5) and ABA‐responsive gene (8), were involved in ABA signalling processes (Figure 6b). The consistent change trends were observed in the majority of IAA‐ and ABA‐related DEGs between RNA‐Seq and quantitative RT‐PCR approaches in germinating seeds of mutants compared to WT seeds (Figure S6; Figure S7). Interestingly, the transcripts of several identified ARFs (OsARF1, OsARF8 and OsARF10) and ABA signalling‐related genes (OsABI3, OsABI5, OsZIP23, OsTRAB1 and OsABL1) were significantly increased in osiaglu plants compared with those of WT plants at the 72‐h imbibition stage (Figure 6a,b). These results suggested that the regulation of OsIAGLU on seed vigour might involve in IAA and ABA signalling during seed germination in rice.
Figure 6.
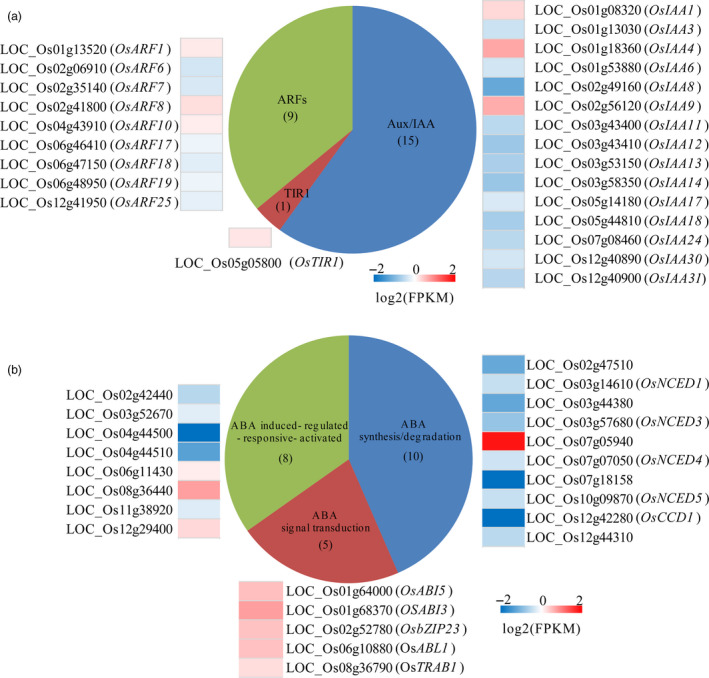
Rice OsIAGLU altering expressions of genes involved in IAA and ABA signalling during seed germination. Differentially expressed genes (DEGs) with p < 0.001 between WT and osiaglu‐2 mutant involved in IAA (a) and ABA (b) signalling in germinating seeds (72 h) of rice. Red, up‐regulation; grey, no change; blue, down‐regulation.
It has been well studied that the auxin and ABA acted synergistically to inhibit seed germination is largely dependent on the ABI3 function in Arabidopsis (Liu et al., 2013). ABIs are the downstream factors of ABA signalling pathway, which play the key roles on seed germination. Thus, the expressions of OsABI3 and OsABI5 regulated by OsIAGLU were focused on detection during seed germination in rice. We observed that the expressions of OsABI3 and OsABI5 were significantly induced by IAA and ABA treatments during seed germination in rice (Figure 7a‐d). As descripted above, the higher levels of IAA and ABA were accumulated in osiaglu mutants than WT plants in rice. We therefore speculated that the higher expressions of OsABI3 and OsABI5 will be occurred in osiaglu mutants compared with WT plants during seed germination in rice. It was confirmed by our quantitative RT‐PCR analysis (Figure 7e,f). Firstly, we observed that the expressions of OsABI3 and OsABI5 were generally down‐regulated in WT plants during seed germination, while those of osiaglu mutants exhibited similar higher expression patterns especially at 12‐ to 48‐h imbibition stages. Moreover, the significant higher expressions of OsABI3 and OsABI5 were observed in osiaglu mutants compared with WT plants during seed germination especially at 36‐ to 72‐h imbibition stages. These results indicated that the continuously higher expressions of OsABI3 and OsABI5 in germinating seeds caused low seed vigour in osiaglu mutant plants in rice.
Figure 7.
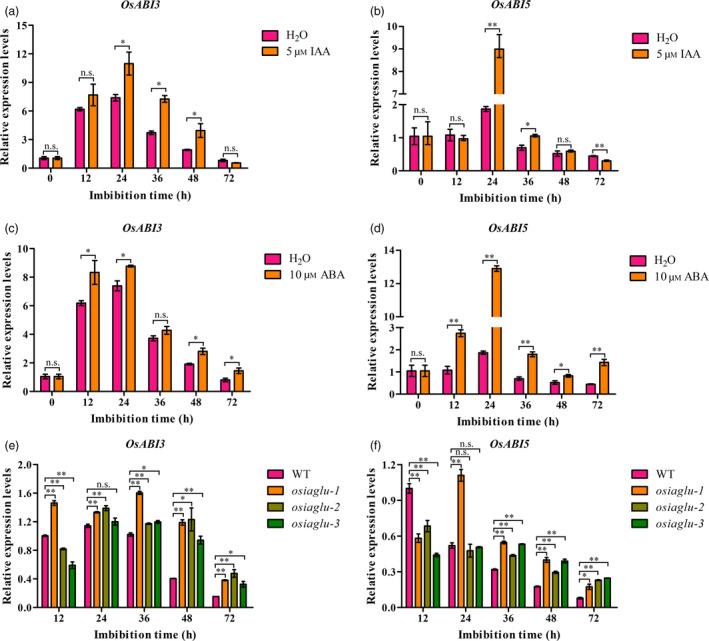
Rice OsIAGLU altering expressions of ABA signalling genes OsABIs during seed germination. Expressions of OsABI3 and OsABI5 in germinating seeds induced by various IAA (a‐b) and ABA (c‐d) concentrations using quantitative RT‐PCR approach. Comparison of OsABI3 (e) and OsABI5 (f) expression in germinating seeds between WT and osiaglu mutants during seed germination. The expression of genes was normalized to that of OsActin gene control. The relative expression levels were represented by fold change relative to the expression levels of H2O condition or WT. Each column represents the means ± SD. * and ** indicate the significant difference compared to normal condition at 5% and 1% levels, respectively, according to Student’s t‐test. n.s. represents not significant.
Association of OsIAGLU haplotypes with seed vigour
In general, indica accessions have stronger seed vigour than japonica accessions surveyed in this study (Figure 8a,b; Table S2). Therefore, to investigate whether the variation of OsIAGLU allele was associated with the difference of seed vigour between two subspecies, SNPs in the region from ~ 2 kb upstream to ~ 1 kb downstream of OsIAGLU were analysed using the SNP data of rice accessions (McCouch et al., 2016). Five haplotypes of OsIAGLU were identified among these accessions (Figure 8c). The elite haplotypes Hap 1 and Hap 2 associated with high seed vigour were mainly existed in the indica accessions, while the haplotypes Hap 3 and Hap 5 mainly existed in the japonica accessions and Hap 4 existed in both indica and japonica accessions were associated with low seed vigour (Figure 8c‐e). Several elite indica accessions with high seed vigour (seed germination percentage after 2 days more than 80%) were identified harbouring Hap 1 or Hap 2 (Table S3). We speculated that the SNPs of OsIAGLU affect its expression during seed germination to regulate seed vigour in rice. Thus, the expressions of OsIAGLU in accessions harbouring different haplotypes were detected during seed germination in rice. The significant higher expressions of OsIAGLU were observed in accessions harbouring Hap 1 or Hap 2 haplotypes (high seed vigour) compared to those of accessions harbouring Hap 3, Hap 4 or Hap 5 haplotypes (low seed vigour) in rice (Figure 8f–g; Table S4). Taken together, the variations in OsIAGLU seem to be associated with the differential seed vigour between japonica and indica subspecies in rice.
Figure 8.
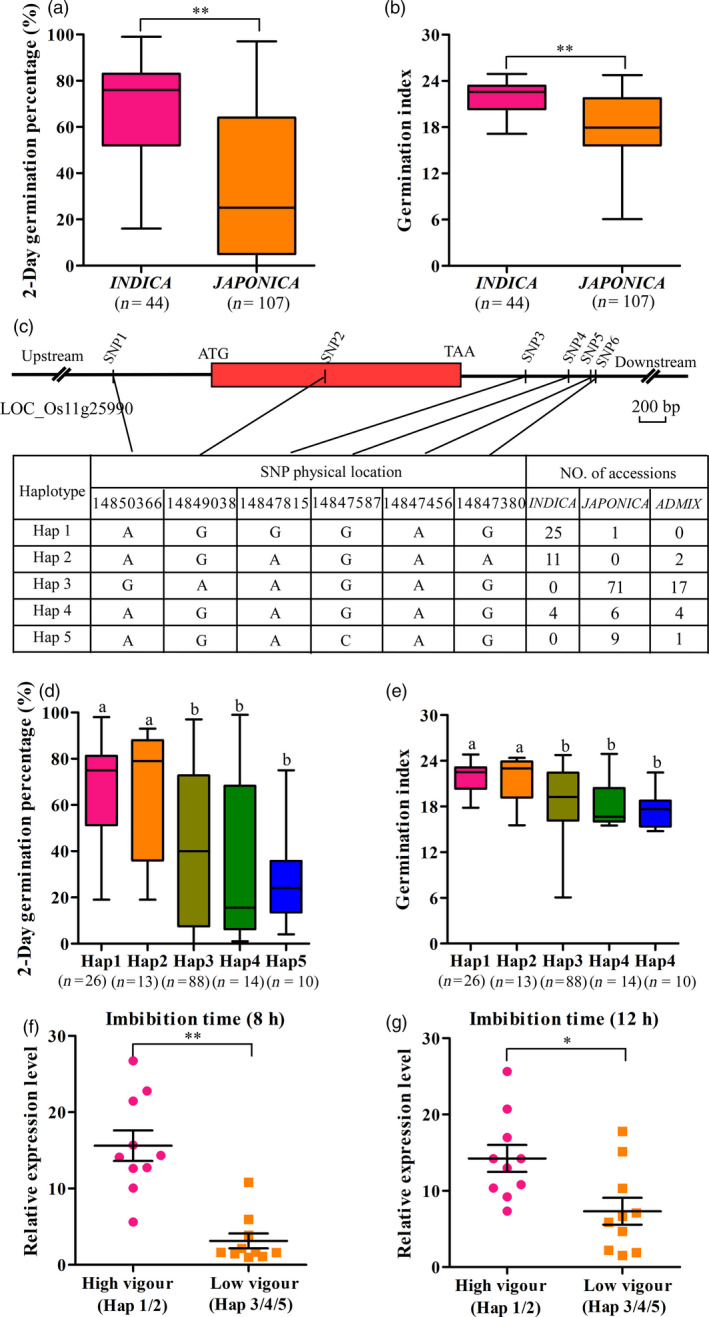
Haplotypes of OsIAGLU associated with seed vigour in rice. Comparison of seed germination percentage (a) and germination index (b) between indica and japonica accessions. (c) Haplotypes of OsIAGLU identified in the region from ~2 kb upstream to ~1 kb downstream of the gene. Box represents the OsIAGLU gene region. (d‐e) Germination phenotype of accessions harbouring different haplotypes. Number of rice accessions listed in brackets. (f‐g) Relative expression levels of OsIAGLU during seed germination in rice accessions harbouring different haplotypes identified by quantitative RT‐PCR approach. Each column represents the means ± SD. * and ** indicate the significant difference compared to normal condition at 5% and 1% levels, respectively, according to Student’s t‐test. Different lowercase letters represent significant difference at 5% level according to least significant difference (LSD) test.
Discussion
Seed vigour, including speed germination and vigorous seedling growth, is an important agronomic trait for direct seeding in rice. Glycosylation is thought to be an important process in the regulation of hormone homeostasis in plants (Palaniyandi et al., 2015). Previously, the plants overexpressing IAA glucosyltransferase OsIAAGLU exhibited typical phenotypes associated with a reduction in free IAA content with enlarged leaf angle, dwarf plants and shorter panicles in rice (Yu et al., 2019). However, the regulation of seed vigour by IAA glucosyltransferase was not investigated previously in rice. In this study, we observed that the disruption of OsIAGLU caused low seed vigour, including reducing germination speed and seedling growth, in rice. To the best of our knowledge, this is the first report on the regulation of seed vigour by OsIAGLU in rice. The mechanism underpinning the regulation of seed vigour by OsIAGLU was investigated in this study.
It has been reported that auxin glucosyltransferase was involved in seed germination in Arabidopsis. Ectopic expression of Arabidopsis UGT75D1 modulates cotyledon development and stress tolerance in seed germination, and its expression was strongly induced in germinating seeds and cotyledons during 3–14 days after germination (Zhang et al., 2016). In this study, our results showed that the OsIAGLU exhibited especially relative higher expression at the late mature stage (28 DAF) and the early germination (4‐h imbibition) stage. Further GUS staining for OsIAGLU in the present study was detected in germinating seeds and young seedlings. It suggested that OsIAGLU regulated seed vigour might be through influencing seed development and seed germination in rice. Here, the influence of seed germination by OsIAGLU was focused on analysis and that of seed development needs further investigation in the future. Moreover, the expression of OsIAGLU were up‐regulated in response to hormones, such as GA, IAA and ABA, especially by IAA and ABA during whole germination stages, which are known to involve in seed germination. These results indicated that the regulation of seed vigour by OsIAGLU might be involved in IAA‐ and ABA‐mediated processes.
It has been reported that glycosyltransferases usually involved in IAA and ABA homeostasis in plants (Tognetti et al., 2010; Yu et al., 2019). Meanwhile, our RNA‐Seq data also showed that OsIAGLU regulated seed vigour might involve in IAA‐ and ABA‐mediated processes. Thus, the regulation of IAA and ABA contents by OsIAGLU was firstly focused to study its regulatory mechanism on seed vigour in this study. We observed that the free IAA and ABA contents in the germinating seeds of osiaglu mutants were significantly higher than those of WT plants. It suggested that OsIAGLU plays important roles in IAA and ABA homeostasis in germinating seeds of rice. The biochemical study of OsIAGLU glucosylating activity towards different hormones, such as IAA, IBA, ABA and jasmonic acid (JA), is on progress. The actions of IAA and ABA are highly dependent on their concentrations, and high concentrations of IAA and ABA generally inhibit seed germination. It was, therefore, hypothesized that the low seed vigour in osiaglu mutants might be due to the higher accumulation of free IAA and ABA in mutants compared to WT plants. However, our further results showed that the reductions of seed vigour among osiaglu mutants and WT plants were only observed under ABA while not IAA treatments. We therefore preliminarily speculated that the increased ABA levels while not IAA in osiaglu mutants caused low seed vigour in this study.
Several reports indicated that the crosstalk of auxin and ABA is essential for the regulation of seed germination (Brady et al., 2003; Chen et al., 2014; Liu et al., 2013; Zhang et al., 2016). Auxin and ABA act synergistically to inhibit seed germination in Arabidopsis (Liu et al., 2013). Thus, we speculated that the effects of OsIAGLU on seed vigour might be tightly associated with the crosstalk between IAA and ABA in this study. This hypothesis was confirmed by the results that the exogenous IAA can amplify ABA‐induced reduction of seed vigour in this study. How is the regulation of OsIAGLU on seed vigour involved in the IAA‐ and ABA‐mediated processes in this study? Our global analysis of transcripts in imbibed seeds indicated that the IAA‐ and ABA signalling‐related genes, such as OsAFRs and OsABIs, were significantly induced in osiaglu mutants compared to those of WT plants in rice. Previously, it has been reported that the auxin controls seed dormancy through stimulation of ABA signalling by inducing ARF mediated ABI3 activation in Arabidopsis (Liu et al., 2013). Therefore, we supposed that OsIAGLU regulated seed vigour might be depending on OsABIs functions in this study. This hypothesis was confirmed by our following analyses of gene expressions. Firstly, the expressions of OsABI3 and OsABI5 in germinating seeds were significantly induced by exogenous IAA and ABA treatments in rice. The responses of OsABI3 and OsABI5 to IAA might be depending on the up‐regulation of OsARFs in this study (Liu et al., 2013). Moreover, the continuously higher expressions of OsABI3 and OsABI5 were observed in osiaglu mutants compared to those of WT plants during seed germination in rice. Thus, we confirmed that the higher endogenous IAA and ABA in germinating seeds of osiaglu mutants will induce higher expressions of OsABI3 and OsABI5, which leading to the decreases of seed vigour in mutants.
Previous studies have shown that the indica varieties have higher seed vigour than japonica varieties (Lai et al., 2016; Liu et al., 2014; Wang et al., 2010). Similar results were observed in this study. To reveal whether OsIAGLU contributing to the differences of seed vigour between indica and japonica rice, the allelic diversity of OsIAGLU was analysed using randomly selected 180 accessions, including indica and japonica accessions, in rice (McCouch et al., 2016). After analysing the SNP data of rice accessions, the Hap 1 and Hap 2 haplotypes of OsIAGLU were identified as positively correlated with seed vigour in rice. Interestingly, we observed that the elite Hap 1 and Hap 2 haplotypes mainly existed in indica accessions while not in japonica accessions. Meanwhile, the significant higher expressions of OsIAGLU were observed in accessions harbouring Hap 1 or Hap 2 haplotypes during seed germination compared with accessions harbouring other three haplotypes. It suggested that the higher OsIAGLU expressions during seed germination might contribute to seed vigour in rice. However, how the SNPs of OsIAGLU affecting its expression needs to be further investigated. In addition, the proper expression of OsIAGLU contributing to seed vigour needs to be further investigated in the future. The determination of OsIAGLU allelic diversity with a focus on newly identified elite rice accession is of interest. Several accessions from China that harbouring Hap 1 or Hap 2, that is Ai‐Chiao‐Hong, Pao‐Tou‐Hung, TeQing, Chang Ch'Sang Hsu Tao and Zhenshan 97B, were identified as elite accessions in this study. Additionally, we observed that the OsIAGLU may not involve in the regulation of grain yield in rice. It suggested that the OsIAGLU is a potential candidate gene for increasing seed vigour without influencing grain yield in rice. The identified elite haplotypes and accessions might be useful for improving seed vigour in rice. We speculated that the seed vigour of japonica could be improved by introducing Hap 1 or Hap 2 from indica into japonica rice. The confirmation of this hypothesis is now in progress.
In summary, our results provide new insights into the regulation of OsIAGLU on seed vigour (Figure 9). The phenotype of low seed vigour in osiaglu mutants in this study implies that OsIAGLU may play a positive role in modulating seed vigour in rice. The expressions of OsABIs will be reduced in germinating seeds under the low levels of endogenous free IAA and ABA conditions regulated by OsIAGLU, thus leading to an enhance of seed vigour in rice. However, what other factors regulating OsIAGLU expression in germinating seeds and how OsIAGLU influencing the expressions of OsABI3 and OsABI5 need to be further investigated. The expression of OsIAGLU during seed development was also observed in this study, but its functions on modulating the establishment of seed dormancy or seed vigour during seed development warrant investigation in the future.
Figure 9.
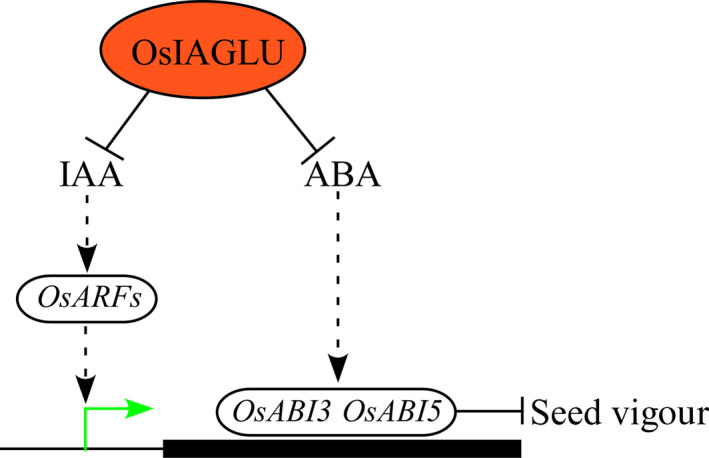
Hypothetical model of the OsIAGLU roles on seed vigour in rice. The expression of OsIAGLU in germinating seeds reduces the free IAA and ABA levels during seed germination. The decreases of free IAA and ABA accumulation in germinating seeds regulated by OsIAGLU will inhibit the expressions of OsARFs and OsABIs, which contributes to the enhancement of seed vigour. Arrows and lines with slanted dashes indicate positive and negative effects, respectively, and the dotted lines indicate indirect regulation. The black box indicates the OsABI3/OsABI5 gene region.
Materials and methods
Plant materials
The osiaglu mutants were generated using the CRISPR/Cas9 system in the japonica Nipponbare background. The generation and identification of mutants were conducted according to He et al. (2019a) using specific primers (Table S5). Three independent homozygous mutants, osiaglu‐1, osiaglu‐2 and osiaglu‐3, were identified in rice. All plants were grown in an experimental field at the South China Agricultural University. All seeds were harvested at their maturity stage and dried at 42◦C for 7 days to break seed dormancy (He et al., 2019b).
Seed germination
Thirty seeds per replicate of osiaglu mutants and wild‐type (WT) Nipponbare were imbibed in 9‐cm‐diameter Petri dishes with 10 mL distilled water at 25 ± 1ºC for 10 days. Meanwhile, thirty seeds per replicate were sowed in about 1‐cm deep soils under natural conditions (25–30ºC) for 10 days. Seed germination of osiaglu mutants and WT was also conducted under various concentrations of IAA and ABA (5 μm and 10 μm) treatments. The traits of seed vigour, including germination potential, time for 50% of the germination, germination index and seedling vigour index, were tested according to Fu et al. (2019) and He et al. (2019a). Three replications were performed.
Expression analysis
Total RNA was extracted from the developing grains (0, 7, 14, 21 and 28 days after flowering; DAF) and germinating seeds (0‐, 4‐, 8‐, 12‐, 18‐, 24‐, 36‐, 48‐, 60‐ and 72‐h imbibition) of Nipponbare, using the HP Plant RNA Kit (Omega, Atlanta, USA), according to the manufacturer’s instructions. The first‐strand cDNA was synthesized, and quantitative RT‐PCR was carried out according to He et al. (2019a). The quantitative RT‐qPCRs were performed in a CFX96 Real‐Time System (Bio‐Rad, USA) with the rice OsActin gene as internal control. The PCR conditions were as follows: 95 ◦C for 25 min, followed by 40 cycles of 95 ◦C for 5 s and 60 ◦C for 10 s. Primers used for quantitative RT‐PCR are listed in Table S5. Normalized transcript levels were calculated using the comparative CT method (Livak and Schmittgen, 2001). Three biological replications were performed. Meanwhile, transgenic plants carrying the OsIAGLU promoter‐GUS fusion construct in Nipponbare were used for a GUS staining assay. GUS staining of tissues from the positive transgenic plants was performed as previously described (Fujino et al., 2008).
Hormone quantification
Approximately 1.0 g of each sample was rapidly frozen in liquid nitrogen and homogenized into a powder. The extraction and quantification of endogenous IAA and ABA were conducted according to the manufacturer’s instructions (Wuhan Metware Biotechnology Co., Ltd., Wuhan, China). The quantification of IAA and ABA was conducted using an ultra‐performance liquid chromatography–tandem mass spectrometry (LC‐MS/MS) system (UPLC, Shim‐pack UFLC SHIMADZU CBM30A system, Kyoto, Japan; MS, Applied Biosystems, Foster City, CA). The content of IAA and ABA was determined using the external standard method and is expressed as μg/g fresh weight (FW) and ng/g FW, respectively. Three biological replications were performed.
Differentially expressed gene analysis
RNA‐Seq approach and differentially expressed gene analysis were conducted according to He et al. (2019a). Total RNA was extracted from approximately 80–100 mg powder of WT and osiaglu‐2 seeds after 72‐h imbibition using the HP Plant RNA Kit (Omega, Atlanta, GA) according to the manufacturer’s instructions. Construction of cDNA libraries and BGISEQ‐500RS sequencing were performed at BGI‐Wuhan Co., Ltd., Wuhan, China. Levels of gene expression were quantified in terms of FPKM (fragments per kilo base of exon per million) using RSEM version 1.1.11 (Li and Dewey, 2011). The differentially expressed genes (DEGs) with a P‐adj (P‐adjusted) < 0.001 were selected for further analysis. A metabolic pathway overview was depicted by MapMan version 3.6.0RC1 (http://mapman.gabipd.org) and shown using colour intensity (Usadel et al., 2009). Three biological replications were performed.
Haplotype analyses
To determine the haplotypes of OsIAGLU, 700,000 SNP markers of rice accessions listed at https://ricediversity.org/data/index.cfm were used (McCouch et al., 2016). Seed germination was conducted in 9‐cm‐diameter Petri dishes with 10 mL distilled water using randomly selected 180 accessions as descripted above for 10 days (Table S2). Haplotypes represented at least ten investigated accessions that were previously used for a comparative analysis of phenotypes.
Data analysis
Experimental data were analysed using the SAS software (Cary, NC), and significant differences among samples were compared using Student’s t‐test or Fisher’s least significant difference (LSD) test at the 5% and 1% levels of probability.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Zhoufei Wang and Yongqi He planned the research. Yongqi He and Jia Zhao performed all important experiments. Bin Yang performed haplotype analyses. Shan Sun and Liling Peng performed seed germination experiments. Zhoufei Wang, Yongqi He and Jia Zhao analysed the data and wrote the paper.
Supporting information
Figure S1 Characterization of OsIAGLU in rice.
Figure S2 Comparison of amino acid sequences between WT and osiaglu mutants in rice.
Figure S3 Comparison of grain traits between WT and osiaglu mutants in rice.
Figure S4 Dynamic changes of seed germination after dormancy breaking treatments among WT and osiaglu mutants in rice.
Figure S5 Comparison of IAA and ABA interactions on seed vigor between WT and osiaglu mutants in rice.
Figure S6 The expression confirmation of IAA‐related differently expression genes in 72 h‐imbibed seeds using quantitative RT‐PCR approach in rice.
Figure S7 The expression confirmation of ABA‐related differently expression genes in 72 h‐imbibed seeds using quantitative RT‐PCR approach in rice.
Table S1 Differently expression genes (DEGs) between WT and osiaglu‐2 in 72 h‐imbibed seeds in rice.
Table S2 Information of 180 accessions used for haplotype analyses in rice.
Table S3 Information of the elite accessions harboring Hap 1 and Hap 2 of OsIAGLU with high seed vigor in rice.
Table S4 Information of the accessions harboring different haplotypes used for the analysis of OsIAGLU expression in rice.
Table S5 The primer pairs used in this study.
Acknowledgements
The authors would like to thank USDA‐ARS for seeds of the Rice Diversity Panel. This work was supported by the National Key Research and Development Plan (Grant No. 2018YFD0100901), the National Natural Science Foundation of China (Grant Nos. 31901601, 31971995 and 31771889), the Postdoctoral Science Foundation of China (Grant No. 2019M650203) and the Guangdong Province Key Research and Development Program (Grant No. 2018B020202012).
References
- Brady, S.M. , Sarkar, S.F. , Bonetta, D. and McCourt, P. (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis . Plant J. 34, 67–75. [DOI] [PubMed] [Google Scholar]
- Chen, C. , Letnik, I. , Hacham, Y. , Dobrev, P. , Ben‐Daniel, B.H. , Vankova, R. , Amir, R. et al. (2014) Ascorbate peroxidase 6 protects Arabidopsis thaliana desiccating and germinating seeds from stress and mediates crosstalk between ROS. ABA and auxin. Plant Physiol. 166, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Zhang, Q. , Wang, C.C. , Liu, Z.X. , Jiang, Y.J. , Zhai, L.Y. , Zheng, T.Q. et al. (2019) Genetic dissection of seedling vigor in a diverse panel from the 3,000 Rice (Oryza sativa L.). Genome Project Sci. Rep. 9, 4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S.R. , Rodriguez, P.L. , Finkelstein, R.R. and Abrams, S.R. (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol. 61, 651–679. [DOI] [PubMed] [Google Scholar]
- Dharmasiri, N. , Dharmasiri, S. and Estelle, M. (2005) The F‐box protein TIR1 is an auxin receptor. Nature, 435, 441–445. [DOI] [PubMed] [Google Scholar]
- Eiji, N. , Masanori, O. , Kiyoshi, T. , Ryoichi, Y. , Mitsunori, S. and Yuji, K. (2010) Abscisic acid and the control of seed dormancy and germination. Seed Sci Res. 20, 55–67. [Google Scholar]
- Fu, Y. , Gu, Q. , Dong, Q. , Zhang, Z. , Lin, C. , Hu, W. , Pan, R. et al. (2019) Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 24, pii: E1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, K. , Sekiguchi, H. , Matsuda, Y. , Sugimoto, K. , Ono, K. and Yano, M. (2008) Molecular identification of a major quantitative trait locus, qLTG3–1, controlling low‐temperature germinability in rice. Proc. Natl. Acad. Sci. U. S. A. 105, 12623–12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommers, C.M.M. and Monte, E. (2018) Seedling establishment: a dimmer switch‐regulated process between dark and light signaling. Plant Physiol. 176, 1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Cheng, J. , He, Y. , Yang, B. , Cheng, Y. , Yang, C. , Zhang, H. et al. (2019a) Influence of isopropylmalate synthase OsIPMS1 on seed vigor associated with amino acid and energy metabolism in rice. Plant Biotechnol J. 17, 322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Yang, B. , He, Y. , Zhan, C. , Cheng, Y. , Zhang, J. , Zhang, H. et al. (2019b) A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 97, 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, R.G. , Lim, E.K. , Li, Y. , Kowalczyk, M. , Sandberg, G. , Hoggett, J. , Ashford, D.A. et al. (2001) Identification and biochemical characterization of an Arabidopsis indole‐3‐acetic acid glucosyltransferase. J. Biol Chem. 276, 4350–4356. [DOI] [PubMed] [Google Scholar]
- Jackson, R.G. , Kowalczyk, M. , Li, Y. , Higgins, G. , Ross, J. , Sandberg, G. and Bowles, D.J. (2002) Overexpression of an Arabidopsis gene encoding a glucosyltransferase of indole‐3‐acetic acid: phenotypic characterization of transgenic lines. Plant J. 32, 573–583. [DOI] [PubMed] [Google Scholar]
- Jin, S.H. , Ma, X.M. , Han, P. , Wang, B. , Sun, Y.G. , Zhang, G.Z. , Li, Y.J. et al. (2013) UGT74D1 is a novel auxin glycosyltransferase from Arabidopsis thaliana . PLoS ONE, 8, e61705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick, D.A. , Enders, T.A. and Strader, L.C. (2013) Auxin biosynthesis and storage forms. J. Exp. Bot. 64, 2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro, T. , Okamoto, M. , Nakabayashi, K. , Yamagishi, K. , Kitamura, S. , Asami, T. , Hirai, N. et al. (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8'‐hydroxylases: key enzymes in ABA catabolism. EMBO J. 23, 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Y. , Cheng, J. , He, Y. , Yang, B. , Wang, Z. and Zhang, H. (2016) Identification of QTLs with additive, epistatic, and QTL × seed maturity interaction effects for seed vigor in rice. Plant Mol. Biol. Rep. 34, 160–171. [Google Scholar]
- Li, B. and Dewey, C.N. (2011) RSEM: accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics 12, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.D. , Zhang, H. , Zhao, Y. , Feng, Z. , Li, Q. , Yang, H.Q. , Luan, S. et al. (2013) Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF mediated ABI3 activation in Arabidopsis . Proc. Natl. Acad. Sci. USA 110, 15485–15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Lai, Y. , Cheng, J. , Wang, L. , Du, W. , Wang, Z. and Zhang, H. (2014) Dynamic quantitative trait locus analysis of seed vigor at three maturity stages in rice. PLoS ONE 9, e115732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Hussain, S. , Zheng, M. , Peng, S. , Huang, J. , Cui, K. and Nie, L. (2015a) Dry direct‐seeded rice as an alternative to transplanted‐flooded rice in Central China. Agron Sustain Dev. 35, 285–294. [Google Scholar]
- Liu, S.J. , Xu, H.H. , Wang, W.Q. , Li, N. , Wang, W.P. , Møller, I.M. and Song, S.Q. (2015b) A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment. Physiol. Plant. 154, 142–161. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐DDC(T) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mahender, A. , Anandan, A. and Pradhan, S.K. (2015) Early seedling vigor, an imperative trait for direct‐seeded rice: an overview on physio‐morphological parameters and molecular markers. Planta 241, 1027–1050. [DOI] [PubMed] [Google Scholar]
- McCouch, S.R. , Wright, M.H. , Tung, C.W. , Maron, L.G. , McNally, K.L. , Fitzgerald, M. , Singh, N. et al. (2016) Open access resources for genome‐wide association mapping in rice. Nat. Commun. 7, 10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyandi, S.A. , Chung, G. , Kim, S.H. and Yang, S.H. (2015) Molecular cloning and characterization of the ABA‐specific glucosyltransferase gene from bean (Phaseolus vulgaris L.). J. Plant Physiol. 178, 1–9. [DOI] [PubMed] [Google Scholar]
- Penfield, S. (2017) Seed dormancy and germination. Curr. Biol. 27, R874–R878. [DOI] [PubMed] [Google Scholar]
- Priest, D.M. , Ambrose, S.J. , Vaistij, F.E. , Elias, L. , Higgins, G.S. , Ross, A.R. , Abrams, S.R. et al. (2006) Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana . Plant J. 46, 492–502. [DOI] [PubMed] [Google Scholar]
- Seiler, C. , Harshavardhan, V.T. , Rajesh, K. , Reddy, P.S. , Strickert, M. , Rolletschek, H. , Scholz, U. et al. (2011) ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought‐stress conditions. J. Exp. Bot. 62, 2615–2632. [DOI] [PubMed] [Google Scholar]
- Shang, X.L. , Xie, R.R. , Tian, H. , Wang, Q.L. and Guo, F.Q. (2016) Putative zeatin O‐glucosyltransferase OscZOG1 regulates root and shoot development and formation of agronomic traits in rice. J. Integr. Plant Biol. 58, 627–641. [DOI] [PubMed] [Google Scholar]
- Szerszen, J.B. , Szczyglowski, K. and Bandurski, R.S. (1994) iaglu, a gene from Zea mays involved in conjugation of growth hormone indole‐3‐acetic acid. Science, 265, 1699–1701. [DOI] [PubMed] [Google Scholar]
- Tognetti, V.B. , Van Aken, O. , Morreel, K. , Vandenbroucke, K. , van de Cotte, B. , De Clercq, I. , Chiwocha, S. et al. (2010) Perturbation of indole‐3‐butyric acid homeostasis by the UDP‐glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell, 22, 2660–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel, B. , Poree, F. , Nagel, A. , Lohse, M. , Czedik‐Eysenberg, A. and Stitt, M. (2009) A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant Cell Environ 32, 1211–1229. [DOI] [PubMed] [Google Scholar]
- Wang, Z.F. , Wang, J.F. , Bao, Y.M. , Wang, F.H. and Zhang, H.S. (2010) Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. B 11, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X.L. , Wang, H.Y. , Leung, D.W.M. , He, Z.D. , Zhang, J.J. , Peng, X.X. and Liu, E.E. (2019) Overexpression of OsIAAGLU reveals a role for IAA‐glucose conjugation in modulating rice plant architecture. Plant Cell Rep. 38, 731–739. [DOI] [PubMed] [Google Scholar]
- Zhang, G.Z. , Jin, S.H. , Jiang, X.Y. , Dong, R.R. , Li, P. , Li, Y.J. and Hou, B.K. (2016) Ectopic expression of UGT75D1, a glycosyltransferase preferring indole‐3‐butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana . Plant Mol. Biol. 90, 77–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Characterization of OsIAGLU in rice.
Figure S2 Comparison of amino acid sequences between WT and osiaglu mutants in rice.
Figure S3 Comparison of grain traits between WT and osiaglu mutants in rice.
Figure S4 Dynamic changes of seed germination after dormancy breaking treatments among WT and osiaglu mutants in rice.
Figure S5 Comparison of IAA and ABA interactions on seed vigor between WT and osiaglu mutants in rice.
Figure S6 The expression confirmation of IAA‐related differently expression genes in 72 h‐imbibed seeds using quantitative RT‐PCR approach in rice.
Figure S7 The expression confirmation of ABA‐related differently expression genes in 72 h‐imbibed seeds using quantitative RT‐PCR approach in rice.
Table S1 Differently expression genes (DEGs) between WT and osiaglu‐2 in 72 h‐imbibed seeds in rice.
Table S2 Information of 180 accessions used for haplotype analyses in rice.
Table S3 Information of the elite accessions harboring Hap 1 and Hap 2 of OsIAGLU with high seed vigor in rice.
Table S4 Information of the accessions harboring different haplotypes used for the analysis of OsIAGLU expression in rice.
Table S5 The primer pairs used in this study.


