Summary
Grain/seed yield and plant stress tolerance are two major traits that determine the yield potential of many crops. In cereals, grain size is one of the key factors affecting grain yield. Here, we identify and characterize a newly discovered gene Rice Big Grain 1 (RBG1) that regulates grain and organ development, as well as abiotic stress tolerance. Ectopic expression of RBG1 leads to significant increases in the size of not only grains but also other major organs such as roots, shoots and panicles. Increased grain size is primarily due to elevated cell numbers rather than cell enlargement. RBG1 is preferentially expressed in meristematic and proliferating tissues. Ectopic expression of RBG1 promotes cell division, and RBG1 co‐localizes with microtubules known to be involved in cell division, which may account for the increase in organ size. Ectopic expression of RBG1 also increases auxin accumulation and sensitivity, which facilitates root development, particularly crown roots. Moreover, overexpression of RBG1 up‐regulated a large number of heat‐shock proteins, leading to enhanced tolerance to heat, osmotic and salt stresses, as well as rapid recovery from water‐deficit stress. Ectopic expression of RBG1 regulated by a specific constitutive promoter, GOS2, enhanced harvest index and grain yield in rice. Taken together, we have discovered that RBG1 regulates two distinct and important traits in rice, namely grain yield and stress tolerance, via its effects on cell division, auxin and stress protein induction.
Keywords: rice, grain size, cell division, auxin, microtubule, root, abiotic stress
Introduction
Rice is the main staple food in many regions of the world, and it is also a good model for gene function analysis in monocots. In rice, grain yield per plant is determined by four traits: grain weight or size, panicle number per plant, grain number per panicle and the percentage of ripened grains (Hoshikawa, 1989; Xing and Zhang, 2010). Among the more than 400 quantitative trait loci (QTLs) associated with grain traits, several major QTLs governing grain size have been characterized (Huang et al., 2013; Zuo and Li, 2014). However, the identity and function of genes in most of these QTLs remain unclear (Huang et al., 2013; Ikeda et al., 2013). Investigations of genes regulating grain size, and their modes of action, are of great significance for breeding rice varieties with increased grain yield.
Recent rice functional genomics studies have identified several genes controlling grain size, including genes for grain length such as GS3 (Fan et al., 2006), GL3.1 (Qi et al., 2012), An‐1 (Luo et al., 2013), GS2 (Hu et al., 2015) and GL2 (Che et al., 2015); genes for grain width such as GW2 (Song et al., 2007), GW5 (Liu et al., 2017), GS5 (Li et al., 2011b) and GW7 (Wang et al., 2015); and genes for grain weight such as GIF1 (Wang et al., 2008a), TGW6 (Ishimaru et al., 2013), BG1 (Liu et al., 2015) and XIAO (Jiang et al., 2012). Some genes not only control grain size, but also affect the development of other organs such as panicles. For example, SHORT GRAIN1 (SG1) encodes an unknown protein, and its overexpression decreases grain size and internodes in rachis branches by decreasing BR responses and cell proliferation, thereby reducing organ elongation (Nakagawa et al., 2012). DENSE and ERECT PANICLE 2 (DEP2) encodes a plant‐specific protein with no known functional domains. A dep2 mutant exhibits a dense and erect panicle phenotype, as well as decreased panicle length that is caused by a defect in cell proliferation during the exponential elongation phase of panicle growth (Li et al., 2010).
Auxin also controls grain development and size. During organogenesis, auxin acts as a permissive signal for cell division, which is necessary for entering the G1/S transition in the cell cycle (Perrot‐Rechenmann, 2010). Auxin can affect cytoskeleton formation by regulating the microtubule‐associated protein complex (MAP) that controls the direction of cell division and elongation (Ruan and Wasteneys, 2014). AUXIN‐REGULATED GENE INVOLVED IN ORGAN SIZE (ARGOS) has been proposed to transduce auxin signals to regulate cell proliferation and organ growth through AINTEGUMENTA (ANT) during organogenesis in Arabidopsis (Hu et al., 2003). In both rice and Arabidopsis, ectopic expression of BIG GRAIN 1 (BG1), which encodes a positive regulator of auxin responses and transport, also enhances grain size (Liu et al., 2015). Similar to dicots, auxin also acts as a common integrator for many endogenous and environmental signals regulating almost every aspect of rice root development. It is well established that auxin biosynthesis, metabolism, transport and signalling have profound impacts on root development in rice (Overvoorde et al., 2010; Yu et al., 2016) (Chen et al., 2015).
In this study, we identified and characterized a novel gene RICE BIG GRAIN 1 (RBG1) that was identified from a T‐DNA activation‐tagged RBG1Act rice mutant based on its dominant large grain phenotype. Ectopic expression of RBG1 promotes cell proliferation as well as auxin accumulation and sensitivity in transgenic rice. Native RBG1 is preferentially expressed in shoot and root meristematic tissues and embryos, so overexpression of RBG1 not only increases crown root (CR) initiation and development but also resumes rapid growth of shoots following damage caused by osmotic, dehydration or heat stresses in transgenic rice. Importantly, RBG1‐overexpressing plants have a significantly higher grain yield than wild type when the gene is regulated by a specific constitutive promoter.
Results
Identification of RBG1 that promotes grain and organ size
We initially identified a T‐DNA‐tagged mutant, M35973 (herein designated RBG1Act), with a big grain phenotype by a forward genetics screen of the Taiwan Rice Insertional Mutant (TRIM) population (Hsing et al., 2007; Lo et al., 2016). The big grain phenotype was observed in both heterozygous and homozygous lines of RBG1Act, but not in wild type (WT) and segregated wild type (sWT) (Figure 1a). The T‐DNA was inserted 2.6 kb upstream of LOC_Os11g30430 (RBG1) on chromosome 11 of the RBG1 Act mutant, which activated the expression of RBG1 (Figure 1b,c). The big grain morphology was also observed in three other RBG1Act allelic mutants – M37341 (A1), M37342 (A2) and M82594 (A3) – in which the T‐DNA is inserted within 27 kb downstream of RBG1 and the expression of RBG1 is also activated (Figure 1b,c). Big grain morphology and RBG1 activation were not detected in another mutant (M44256) in which the T‐DNA is inserted 46 kb downstream of RBG1 (Figure 1b,c).
Figure 1.
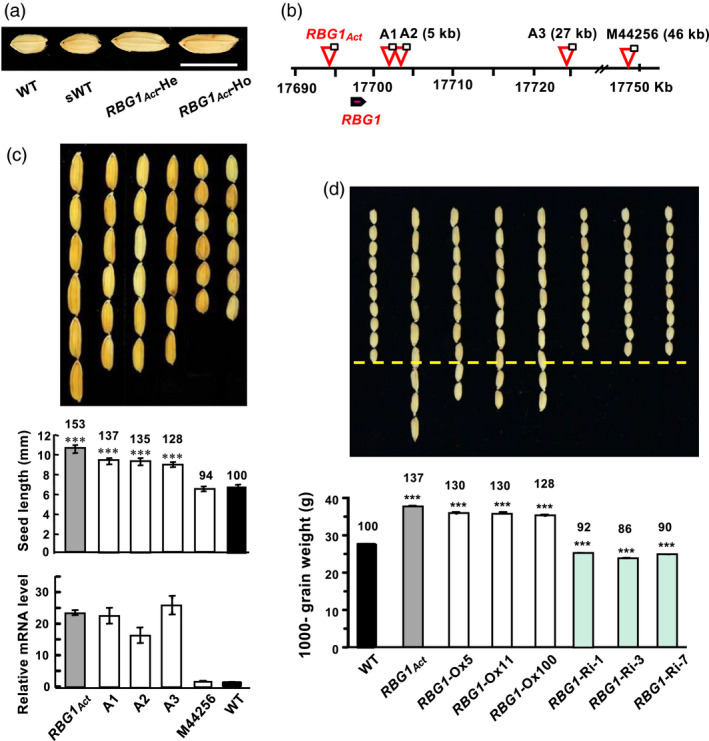
RBG1 activation rice mutants display the big grain phenotype. (a) Comparison of seed morphology among segregated WT/sWT, heterozygous (RBG1Act‐He) and homozygous (RBG1Act‐Ho) lines of the RBG1 activation mutant M35973. (b) Relative location of insertion sites of the RBG1Act mutant and its three allelic mutants, M37341 (A1), M37342 (A2) and M82594 (A3), and the non‐allelic mutant M44256. The numbers in parentheses indicate the distances of the CaMV35S enhancers within the T‐DNA from the translation initiation codon ATG of RBG1. The scale in Kb marks the location on rice chromosome 11. (c) Four RBG1Act allelic mutants display big grain phenotypes, as compared with WT, which correlates with the overexpression of RBG1 in the mutant seedlings. n = 30 for each line. (d) Overexpression of RBG1 under the control of the Ubi promoter (RBG1‐Ox lines) enhances grain size, while underexpression by RNA interference (RBG1‐Ri lines) slightly reduces grain size. The yellow dashed line indicates WT grain length. Grain morphology and 1000‐seed weight were compared with those of WT. n = 18 for each line. The numbers above bars are % relative to the value in WT.
A total of 39 transgenic rice lines overexpressing RBG1 under the control of the Ubi promoter (referred to as RBG1‐Ox lines) (Figure S1a) were generated, and they all displayed the big grain morphology (Figure 1d, Figures S1 and S2a,c). Nine of these lines were randomly selected for further characterizations, and statistical data indicated that they have longer grain length and heavier grain weight than those of WT (Figure S1b–d). Therefore, it was confirmed that ectopic expression of RBG1 leads to grain size increase. Herein, we have chosen three of these nine lines, that is Ubi:RBG1‐Ox‐5, Ubi:RBG1‐Ox‐11 and Ubi:RBG1‐Ox‐100 for further analyses in this study.
To further explore the function of RBG1, we generated RBG1 knockdown transgenic rice (referred to as RBG1‐Ri lines) by RNA interference technology. Immature panicles of RBG1‐Ri lines exhibited reduced RBG1 transcript levels (Figure S2b). Seed length was decreased in these lines, causing a reduction in 1000‐grain weight (Figure 1d and Figure S2c) but an increase in total grain number per plant, even though total grain yield per plant was not significantly different from WT (Figure S2c). Seed width and thickness in the RBG1Act, RBG1‐Ox and RBG1‐Ri lines are similar.
Overexpression of RBG1 also increased the lengths of immature spikelets (Figure 2a) and mature panicles and peduncles (Figure 2b,e) in RBG1Act and RBG1‐Ox plants, as well as of roots and shoots in 3‐week‐old RBG1Act and RBG1‐Ox seedlings (Figure 2c,d). However, internode length was unaltered (Figure 2e). These results indicate that RBG1 regulates the growth and development of other organs/tissues in addition to seeds.
Figure 2.
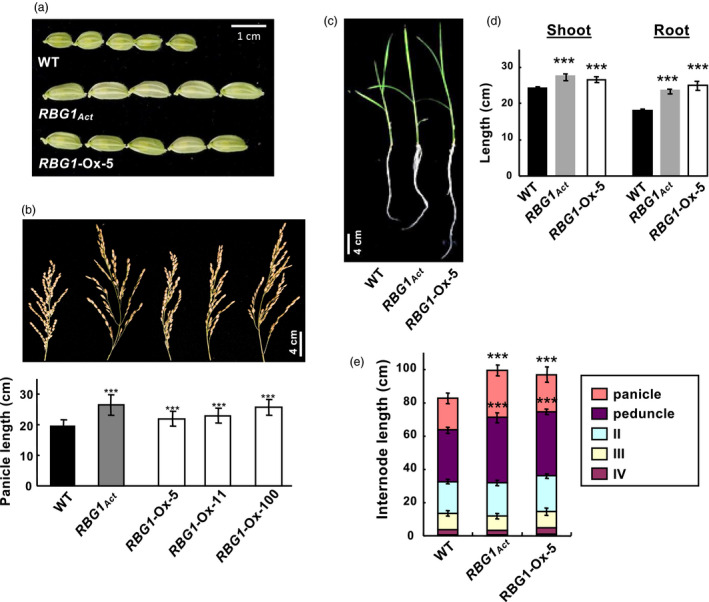
RBG1 overexpression induces increased length in various rice organs. (a) Immature spikelets from 15‐ to 20‐cm panicles. (b) Morphology (upper panel) and length (lower panel) of panicles. n = 130 for each line. (c) Morphology of shoots and roots at 21 DAI. (d) Length of shoots and roots at 21 DAI. n = 22, 14 and 12 for WT, RBG1Act and RBG1‐Ox‐5 lines, respectively. (e) Length of panicles, peduncles and three internode regions (II, III and IV with peduncle as the first internode). n = 20, 18 and 18 for WT, RBG1Act and RBG1‐Ox‐5 lines, respectively.
RBG1 is preferentially expressed in meristematic and proliferating tissues
We initially determined the spatial and temporal expression patterns of RBG1 in WT rice through RT‐PCR analysis (Figure 3a). RBG1 was expressed in embryos of germinated seeds from 1 to 5 days after imbibition (DAI), but was barely detected in shoots and roots up to 30 DAI. We also assessed β‐glucuronidase (GUS) activity in transgenic rice carrying the construct RBG1:GUS. At the vegetative stage, the RBG1 promoter was active in the radicle and plumule of germinated seeds at 1 DAI (Figure 3b), in the scutellum and shoot apical meristem at 5 DAI (Figure 3c), as well as in the cell‐dividing region of embryonic calli (Figure 3d), collar regions (lamina joints) of leaves (Figure 3e), parenchyma tissue between the xylem and phloem of vascular bundles in leaves and sheaths (Figure 3f,g), and in the root tips and shoot apical meristems (Figure 3h,i). At the reproductive stage, the RBG1 promoter was active in panicles throughout their development (Figure 3j). In developing flowers, the RBG1 promoter was active in anthers and pollen before pollination (Figure 3k) and in the basal region of ovaries after pollination (Figure 3l).
Figure 3.
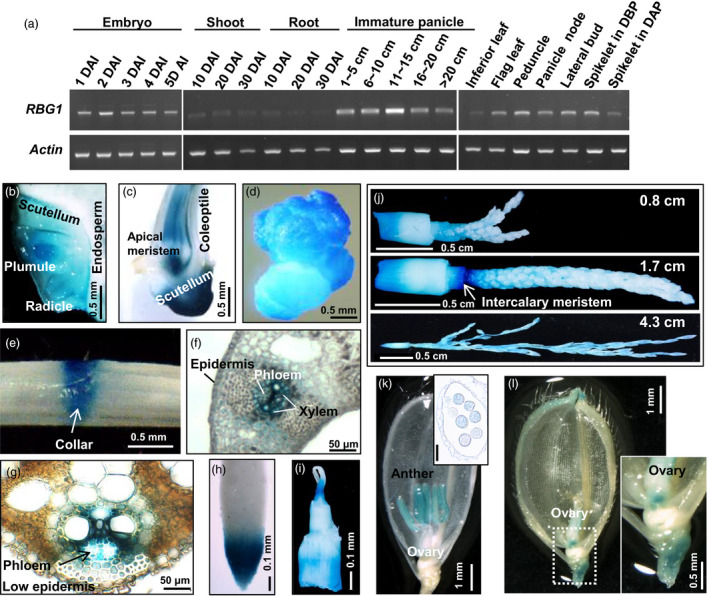
RBG1 is expressed preferentially in rice tissues with cell division activities. (a) Total RNAs were extracted from various tissues at different developmental stages of WT rice, and subjected to RT‐PCR analyses using gene‐specific primers (Table S4). DAI, day after imbibition; DBP, day before pollination; DAP, day after pollination. (b–l) Various tissues from transgenic rice carrying RBG1:GUS were sectioned and stained for GUS activity. (b) Longitudinal section of embryo at 1 DAI. (c) Longitudinal section of shoot at 5 DAI. (d) Cultured callus. (e) Leaf sheath showing collar at 15 DAI. (f) Cross section of leaf sheath showing vascular bundle at 20 DAI. (g) Vascular bundle at 30 DAI. (h) Root tip at 20 DAI. (i) Shoot apical meristem at 20 DAI. (j) Immature panicles at different developmental stages, that is 0.8, 1.7 and 4.3 cm in length, and intercalary meristem of panicle node. (k) Spikelet before pollination. The inset image shows pollen in a cross‐sectioned anther. Scale bar in inset: 50 µm. (l) Spikelet after fertilization. The inset image shows an enlarged ovary. Scale bar in inset: 500 µm.
RBG1 promotes cell division
Examination of abaxial epidermis cells of the hull lemma by scanning electron microscopy showed that cell lengths of RBG1Act, RBG1‐Ox and WT lines were similar, but the number of cells per unit seed length in RBG1Act and RBG1‐Ox lines was significantly increased compared to WT (Figure 4a). This result suggests that an increase in cell number or cell division is primarily responsible for the increase in seed size in RBG1Act and RBG1‐Ox lines. We confirmed this supposition by using an EdU (5‐ethynyl‐2’‐deoxyuridine)‐based assay to assess S‐phase cell cycle progression in rice root tips (Kotogany et al., 2010). EdU is a terminal alkyne‐containing nucleoside analog of thymine that is incorporated into newly synthesized DNA (Salic and Mitchison, 2008). We found that the EdU signal was greater in root tips of RBG1‐Ox and RBG1Act lines than in WT (Figure 4b). Quantification of the 3D volumes of fluorescence intensity indicated greater DNA synthesis in the RBG1‐Ox and RBG1Act lines compared to WT (Figure 4c).
Figure 4.
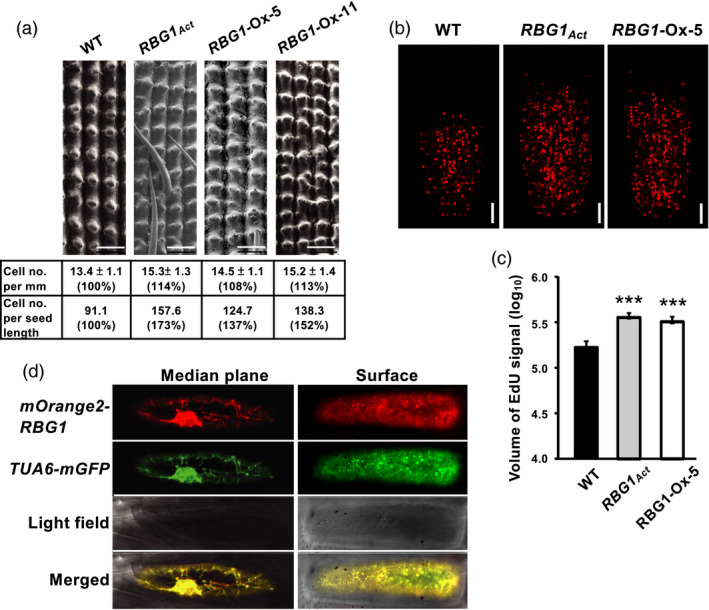
An increase in cell number caused by increased cell division is responsible for the increase in seed size in RBG1 overexpression lines. (a) Examination of abaxial epidermal cells of hull lemma by scanning electron microscopy revealed a significantly greater number of cells per unit length in RBG1 overexpression lines than in WT. Scale bar = 100 μm. (b) Roots of seedlings at 8 DAI were stained with 2 µm EdU and visualized by Z‐stack serial sections using confocal microscopy. Single optical sections of 6 μm (optical depth) on the median plane of rice root tips were captured. Scale bar = 50 μm. (c) Quantification of average EdU signal intensity of the meristem region presented on a logarithmic scale. n = 9, 20 and 18 for WT, RBG1Act and RBG1‐Ox‐5 lines, respectively. (d) Onion epidermal cells were co‐transfected with the constructs Ubi:mOrgange2‐RBG1 and Ubi:TUA6‐eGFP by particle bombardment. Left and right panels show the middle plane and the surface of the same cell, respectively.
RBG1 protein is co‐localized with microtubules
We investigated the subcellular localization of RBG1 in plant cells by transfection of onion epidermis cells with the construct CaMV35S:mOrange2‐RBG1. RBG1 was primarily localized to a filamentous structure distributed from the nucleus to the plasma membrane (Figure 4d and Figure S3a). We then co‐transfected CaMV35S:mOrange2‐RBG1 with the CaMV35S:TUA6‐mGFP construct that expresses a tubulin marker protein (TUA6, tubulin α‐6 chain) fused with mGFP. The fluorescence generated by RBG1‐mOrange2 overlapped with that of TUA6‐mGFP, indicating that RBG1 was co‐localized with microtubules (Figure 4d and Figure S3a). RBG1‐mOrange2 did not co‐localize with any other organelle (e.g. mitochondria, endoplasmic reticulum, Golgi) marker proteins fused to mGFP.
To confirm co‐localization of RBG1 and microtubules, we applied a microtubule depolymerization agent oryzalin, which dismantles the filament‐like microtubule structures, for 20 min to onion epidermis cells transfected with a Ubi:eGFP‐RBG1 construct. The filamentous distribution of eGFP‐RBG1 disappeared in cells treated with oryzalin, instead forming a punctate pattern. However, oryzalin treatment did not affect eGFP distribution in control cells transfected with the Ubi:eGFP construct that only expresses eGFP (Figure S3b, note that GFP alone was distributed only in cytoplasm), indicating that oryzalin did not cause general cytotoxicity in onion epidermal cells. These results suggest that RBG1 may be a microtubule‐associated protein regulating cell division. However, ectopic expression of RBG1 did not appear to affect the overall amount or orientation of microtubules (Figure S3c).
RBG1 overexpression enhances auxin accumulation and sensitivity
Since auxin is known as a key hormone regulating cell division, we measured the indole‐3‐acetic acid (IAA) level in coleoptiles and in adult plants. The IAA concentration in coleoptiles was increased by 70% in an RBG1Act line and by 250 and 350% in two RBG1‐Ox lines compared to WT (Figure 5a and Figure S4). The IAA concentration in stems of adult plants of one RBG1‐Ox line remained higher by 50% compared to WT, although at much lower absolute level than in coleoptiles (Figure 5b).
Figure 5.
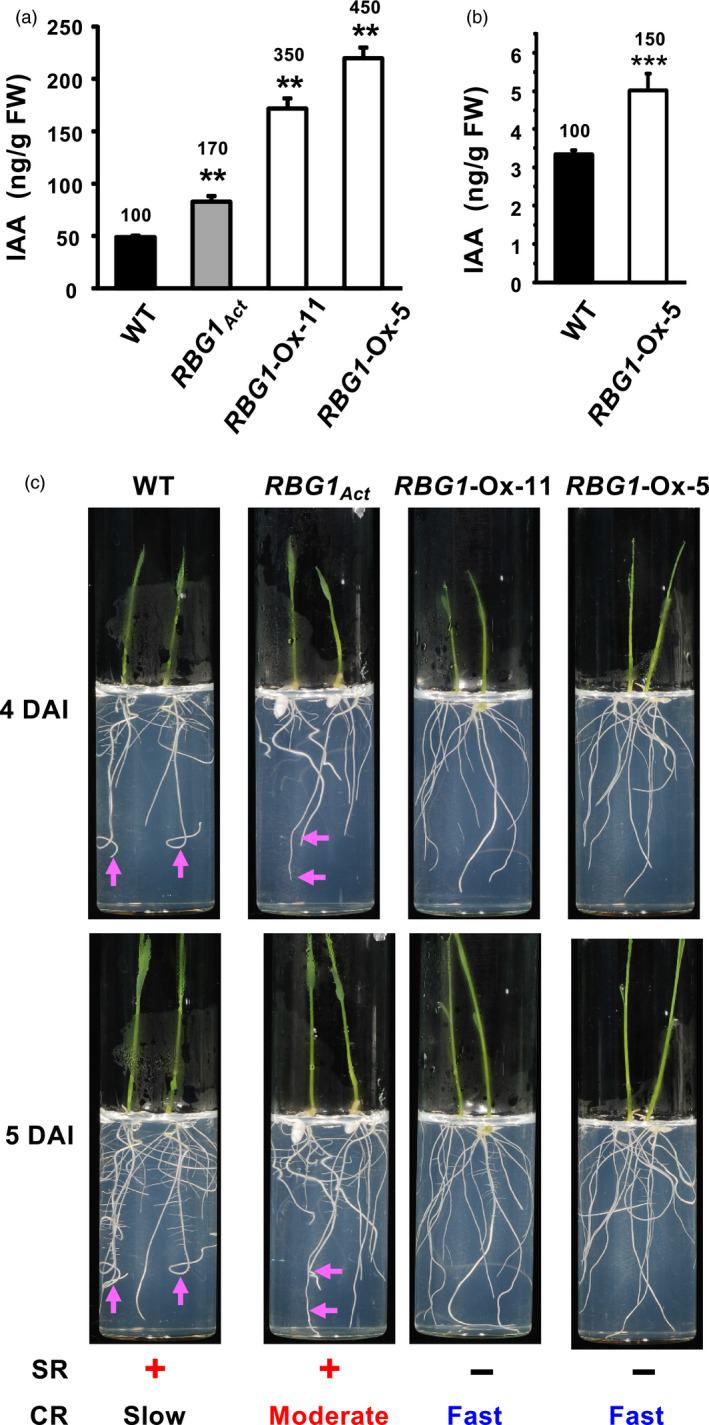
RBG1 promotes the initiation and elongation of crown roots that correlates with the increase in endogenous auxin levels. (a) IAA levels in embryos and coleoptiles at 2 DAI. (b) IAA levels in stems at 37 DAI. (c) Seedlings of WT, RBG1Act and two RBG1‐Ox lines grown in ½ MS medium for 4 and 5 DAI. Arrows indicate the same seminal roots at 4 and 5 DAI. SR: presence (+) or absence (−) of seminal roots; CR: slow, moderate or fast growth speed of crown roots.
Since auxin is a major regulator of root development, we also examined the root architecture. The growth of seminal root (SR) and lateral root (LR) was normal in the WT and the RBG1Act lines but was inhibited in RBG1‐Ox lines. In contrast, growth of CR was relatively slow in WT, moderate in the RBG1Act line, but rapid and extensive in RBG1‐Ox lines (Figure 5c). These results demonstrate that high auxin concentrations preferentially promote CR growth in rice. The auxin concentration in the RBG1Act line lies between those of the WT and RBG1‐Ox lines, which correlates with the intermediate SR and CR development phenotype of the RBG1Act line.
We also investigated the effect of auxin on cell division and expansion using cultured cells. Rice calli were induced from embryos and maintained as suspension cell cultures in medium containing 2,4‐dicholorophenoxyacetic acid (2,4‐D). Compared to WT, we found that RBG1Act and RBG1‐Ox suspension cells grew more slowly in the presence of 2 mg/L 2,4‐d, that is, the concentration normally used for rice suspension culture, but grew slightly faster at a much lower concentration of 0.04 mg/L 2,4‐d (Figure 6a). The growth rate of RBG1‐Ri suspension cells was similar to that of WT. These findings suggest that elevated RBG1 expression leads to hypersensitivity to 2,4‐d in suspension cells.
Figure 6.
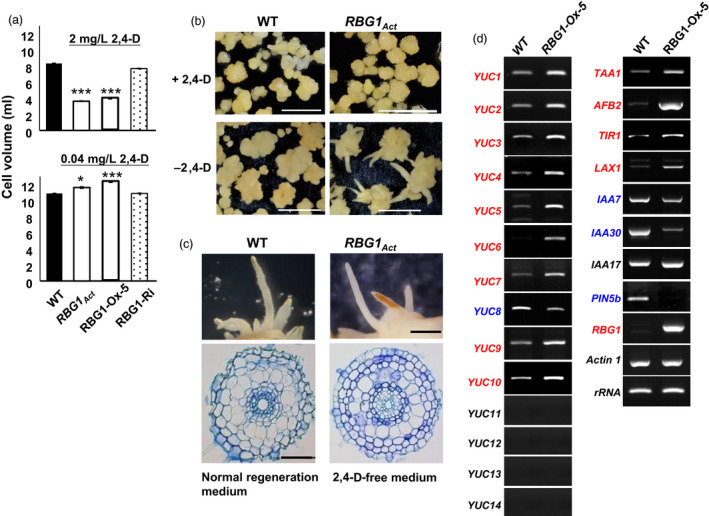
RBG1 overexpression confers auxin hypersensitivity in cultured cells and promotes root development without exogenously applied auxin. (a) Suspension cells of WT, RBG1Act and RBG1‐Ox lines were cultured in MS medium containing 2 mg/L (9 µm) and 0.04 mg/L (0.18 µm) of 2,4‐D. Cell volumes in 50 mL suspension cultures were measured after 14 days. Error bars indicate means ± SE for three independent repeats. (b) Morphology of suspension cells cultured in medium with (+) or without (−) 2,4‐D. (c) The root‐like structures regenerated from the RBG1Act line grown in medium without 2,4‐D were sectioned and compared with the roots of WT regenerated in medium containing auxin (NAA at 2.69 µm). Scale bar = 50 μm. (d) Overexpression of RBG1 up‐regulates a large numbers of genes involved in auxin biosynthesis and signalling. Total RNA was extracted from shoots of plants at 35 DAI and subjected to RT‐PCR analysis. Red and blue fonts indicate up‐ and down‐regulation, respectively.
Moreover, we observed that root‐like structures were regenerated from RBG1Act and RBG1‐Ox calli, but not from WT calli, in 2,4‐d‐free medium (Figure 6b). Cross sections of these root‐like tissues regenerated from RBG1Act calli in 2,4‐d‐free medium exhibited a similar morphology to those of regular roots regenerated from WT calli grown in normal seedling regeneration medium containing the synthetic auxin, 1‐naphthaleneacetic acid (NAA) (Figure 6c).
Gene expression profile analysis revealed that ectopic expression of RBG1 altered the expression of genes involved in auxin biosynthesis and signalling (Figure S5a). The major indole‐3‐acetic acid (IAA) biosynthesis pathway is catalysed by TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) and the YUCCA flavin monooxygenase (YUC) (Mashiguchi et al., 2011; Wang et al., 2018). In rice, the TAA gene (TAA1) and nine YUC genes were up‐regulated by ectopic expression of RBG1 (Figure 6d). Transcripts of the auxin receptor and signalling molecule TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F‐BOX 2 (TIR1/AFB2) and an auxin influx carrier (LAX1/AUX1) (Zhao et al., 2015) were elevated, but that of transcriptional repressors (IAA7 and IAA30) and an auxin efflux carrier (PIN5b) (Lu et al., 2015) were reduced (Figure 6d).
RBG1 encodes an unusual protein with six conserved sequences separated by stretches of amino acid homo‐oligomers
RBG1 encodes a novel protein composed of 315 amino acids with no known functional domains. Bioinformatics analyses of the rice genome identified an RBG1 homolog that was designated as RBG1‐like (RBG1‐L), as well as a total of 18 RBG1 and 9 RBG1‐L homologous proteins from various plant species (Table S1). Phylogenetic analysis of their amino acid sequences indicated that all plant RBG1 homologs could be classified into either a monocot or dicot clade, with the monocot clade being further divided into RBG1 and RBG1‐L clusters (Figure S6). Amino acid alignment revealed that five highly conserved sequences are shared among most members of the RBG1 and RBG1‐L clades (Figure S7). All of these conserved sequences with the exception of conserved sequence 3 are crucial for RBG1 regulation of seed size (Figure S8b). Interestingly, despite extensive searches, we could not identify any apparent RBG1 or RBG1–L homologs among gymnosperms, ferns, bryophytes or algae.
The seed lengths of two T‐DNA‐tagged RBG1‐L activation lines, M55932 and M56557 (Figure S9a), were longer than those of WT seeds (Figure S9b,c), but were still significantly less than those of RBG1 activation or overexpression lines (Figure 1). These differences in seed length could be due to a lack of motif 6 in RBG1‐L (Figure S7) in RBG1‐LAct mutant. Nevertheless, redundancy of RBG1 and RBG1‐L function may account for our observation that RBG1‐Ri lines displayed only a small yet significant reduction in grain size compared to the WT (Figure 1d). Overexpression of rice RBG1 under the control of the CaMV35S promoter did not change seed size in transgenic Arabidopsis (Figure S9d–f), suggesting that dicots have a different downstream system responding to RBG1.
RBG1 overexpression leads to osmotic, salt and heat tolerance, as well as faster recovery from water‐deficiency stress
Gene expression profile analysis revealed that at least 31 members of the heat‐shock protein (HSP) family were up‐regulated in RBG1‐Ox compared to WT (Table S2 and Figure S5b). To test whether RBG1‐overexpressing transgenic rice is more tolerant to abiotic stresses, we subjected seedlings of RBG1Act, RBG1‐Ox and RBG1‐Ri lines to heat (42 °C, 4 days), osmotic (30% PEG 6000, 18 h) or salt (250 mm NaCl, 4 days) stress. After release from osmotic stress, older leaves remained wilted in all lines, but only in RBG1Act and RBG1‐Ox, plants did new leaves emerge quickly and continue to grow (Figure 7a,b). The survival rates of RBG1Act and RBG1‐Ox plants were significantly higher than WT after recovery from all stresses, whereas that of RBG1‐Ri was about the same or slightly lower than WT (Figure 7c). These results indicate that ectopic expression of RBG1 confers enhanced tolerance to multiple abiotic stresses. Quite a few low molecular weight HSPs with sizes ranging from 17 to 28 kDa (Vierling, 1991) were up‐regulated in RBG1‐Ox lines (Figure 7d and Figure S5b).
Figure 7.
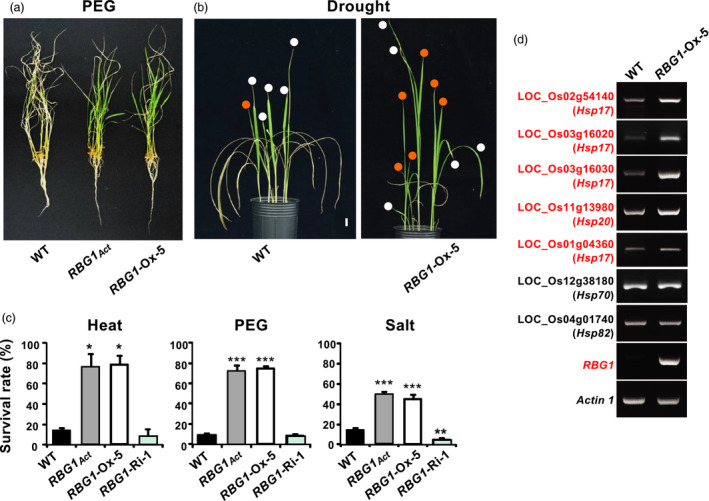
RBG1 overexpression confers abiotic stress tolerance in rice. Rice seedlings at 21 DAI were treated with stress conditions, allowed to recover in 0.5 X Kimura solution for 7 days, and the survival rate (%, number of plants surviving divided by the total number of plants x 100) was determined. (a) Only in the RBG1‐Ox line did new leaves rapidly emerge after recovery from PEG 6000 treatment. (b) Seedlings recovered from drought stress (water withheld for 14 days). Red dot indicates newly grown leaves, and white dot indicates leaves recovered from wilting. (c) Survival rates of seedlings after recovery from heat (42 °C, 4 days), PEG (30% PEG 6000, 18 h) and salt (250 mm NaCl, 4 days) treatments. (d) Overexpression of RBG1 up‐regulates HSPs. RNA samples were extracted from shoots of plants at 35 DAI. Red fonts indicate up‐regulated genes.
Ectopic expression of RBG1 regulated by the GOS2 promoter increases harvest index and grain yield
Overexpression of RBG1 driven by the strong constitutive promoter Ubi generated a big grain phenotype, yet the overall grain yield of the transgenic plants was lower than that of WT. Overexpression of RBG1 under the control of its native promoter (referred to as PRBG1:RBG1‐Ox lines) also increased grain size, but the total grain yield in most transgenic lines was also lower than or similar to the WT (Figure S2d). However, when expressed under the control of the GOS2 promoter (de Pater et al., 1992a), RBG1 enhanced several growth‐ and yield‐related traits in both japonica and indica rice varieties (Figure 8a and Table S3). In this set of experiments, all plant growth traits were measured by image analysis using the TraitMill technology (Reuzeau et al., 2010). Traits such as grain yield, size and filling rate, and number of tillers were markedly increased in japonica rice. Furthermore, the harvest index was increased (average 23.4%) relative to WT in all transgenic lines (Figure 8a). We also observed a consistent enhanced effect (average 34.4%) of RBG1 overexpression (mediated by the GOS2 promoter) on total weight of seeds per plant in the glasshouse. Increased seed size (average 8.4%) and increased tiller number (average 6.8%) over WT were also noted among transgenic lines. In indica rice, the effects of RBG1 overexpression on seed size were less pronounced, but we still observed an average increase of 4.6% in all transgenic events (Figure 8b). Notably, grain yield was increased by an average of 32.1% in indica rice. Increased tiller number was also correlated with an increase in total green biomass, with a general increase (9.2 and 6.6%, respectively) in plant biomass in both japonica rice and indica rice.
Figure 8.
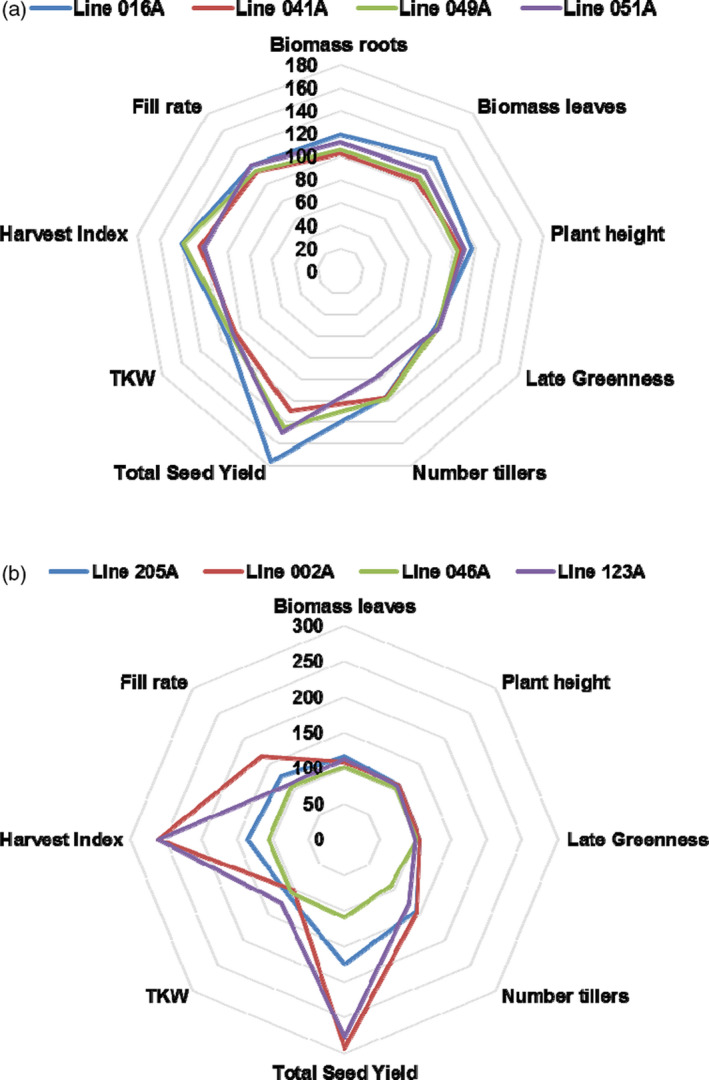
Ectopic expression of RBG1 driven by the GOS2 promoter enhances harvest index and grain yield in rice. Overexpression of RBG1 driven by the GOS2 promoter in (a) japonica rice and (b) indica rice. TraitMill glasshouse experiments were performed in 2012 for (a) and in 2014 for (b). All values in the spider net plots represent % relative to WT control. TKW, thousand kernel weight.
Discussion
The TRIM mutant population appears to be an excellent resource for studies of rice functional genomics, especially for the purpose of identifying unique genes responsible for desirable phenotypes. The presence of four copies of the CaMV35S enhancer in the T‐DNA allows activation of genes flanking either side of the T‐DNA insertion site in the rice genome. The expression level of genes in these flanking regions can be analysed to ascertain whether overexpression of a specific gene is correlated with manifestation of a phenotype of interest. Among the interesting mutants that we have observed in the TRIM activation‐tagged population was the “big grain” phenotype. In recapitulation experiments, RBG1‐overexpressing transgenic rice displayed the same big grain phenotype as the original mutant. Knocking down the expression of RBG1 via RNAi partially reduced the transcript level of RBG1 but did not totally suppress the expression of RBG1 (Figure S2b). Both the RBG1 knockdown and RBG1/RBG1‐L double knockdown transgenic lines exhibited reduced grain weights compared to that of WT, but the level of reduction in transcripts and grain weights was even greater for the double knockdown lines (Figure S10). Thus, these results support the notion that RBG1 is one of major genes regulating seed size in rice.
RBG1 is a unique protein with a role in enhancing cell division
RBG1 homologs from different plant species share six conserved conserved sequences, but these conserved sequences reveal no significant similarity with sequences of known functions. We noted that RBG1 overexpression not only causes big grain, but also increases the size of many parts of the rice plant (Figure 2). The major effect of RBG1 appears to act through cell division, which is supported by five lines of evidence. First, seed length increases but individual cell size along the long axis of multiple cell files in seed abaxial epidermis do not change appreciably in RBG1Act and RBG1‐Ox lines (Figure 4a). Since seed lengths increased in RBG1Act and RBG1‐Ox lines, we deduced that the number of cells in these lines increased by 37–73% over the WT control (Figure 4a). Second, RBG1 is primarily expressed in tissues exhibiting meristematic activities (Figure 3). RBG1 is also expressed in the vascular region between the xylem and phloem in leaf sheaths (Figure 3f,g). Although monocots do not have an easily recognizable vascular cambium, it has been suggested that certain cells in vascular regions retain cell division activities (Sakaguchi and Fukuda, 2008). Third, DNA synthesis is enhanced in the root meristem region in RBG1Act and RBG1‐Ox lines, as shown by EdU incorporation (Figure 4b,c). Similar to the incorporation of 3H‐thymidine and the thymidine analog 5‐bromo‐2'‐deoxyuridine (BrdU), EdU incorporation is a direct measurement of DNA replication, reflecting the ability of a cell to undergo cell division. Future experiments will include EdU staining at multiple times of grain development in WT and RBG1‐overexpressing plants to further elucidate the mode of action of RBG1 in controlling cell division. Lastly, RBG1 is primarily associated with microtubules (Figure 4d) that function in cell division. The mitotic spindles that are responsible for segregating newly replicated chromosomes in a parental cell equally into two daughter cells are primarily made of microtubules and many associated proteins (McIntosh, 2016).
Ectopic expression of RBG1 enhances auxin accumulation and modulates the auxin signalling pathway
The exact molecular function of RBG1 is still unclear, but we found that ectopic expression of RBG1 increased the concentration of endogenous IAA via up‐regulation of two key enzymes in its biosynthesis pathway, TAA1 and YUC (Figure 6d), which consequently impacts several aspects of rice plant growth and development. Auxin levels increase with time throughout the entire process of seed formation in both Arabidopsis and maize, indicating its important roles in seed development (Locascio et al., 2014). Auxin has also been implicated as playing important roles in regulating grain size. TGW6 is a major QTL controlling rice grain weight and filling, which encodes an IAA‐glucose hydrolase that plays an important role in auxin homoeostasis during endosperm development (Ishimaru et al., 2013). It has been reported that maize YUC1 regulates grain weight (Bernardi et al., 2012). The sizes of immature spikelets of RBG1Act and RBG1‐Ox lines were significantly larger than for WT (Figure 2a). It is plausible that the hypersensitivity to endogenous auxin or increased concentrations of auxin also promotes cell division during spikelet development in RBG1Act and RBG1‐Ox lines.
We observed that the growth of cultured cells from RBG1Act and RBG1‐Ox lines was inhibited when treated with high concentrations of an auxin mimic (2,4‐d) (Figure 6a), which supports the notion that a high dosage of auxin, or hypersensitivity to auxin, actually inhibits cell growth (Thimann, 1938). Additionally, callus cultures derived from RBG1Act lines responded to endogenous auxin (i.e. without added auxin) more readily by producing more biomass and differentiating into roots (Figure 6a–c). A similar phenomenon has also been found in transgenic rice overexpressing YUC1, for example, increased IAA levels, decreased callus growth rate and increased adventitious root development from callus and stem (Yamamoto et al., 2007). These observations indicate that overexpression of RBG1 may increase endogenous IAA levels. The concentration of auxin required for maximal root growth is several orders of magnitude less than those required for the growth of buds and stems (Thimann, 1938).
We also found that SRs were extended in WT and RBG1Act lines that produced less IAAs but were inhibited in RBG1‐Ox lines that produced much higher levels of IAAs. In contrast, CRs were extended more rapidly in RBG1‐Ox lines and more slowly in WT and RBG1Act lines (Figure 5). SR primordia are initiated and emerge from embryos, whereas CR primordia are initiated and emerge from shoots after germination (Coudert et al., 2010). It appears that the optimal concentration of auxin for inducing root primordia initiation differs between SRs and CRs, with CRs favouring higher concentrations of auxin.
The notion of auxin hypersensitivity is supported by the observation that RBG1 overexpression increased the expression of auxin receptor/signalling molecules (TIR1 and AFB2) (Leyser, 2018) and reduced the expression of transcriptional repressors (AUX/IAA7 and AUX/IAA30) of auxin response factors (ARFs) (Figure 6d). The possibility of altered auxin homoeostasis and/or transport by overexpression of RBG1 cannot be ruled out, as the expression of the auxin influx transporter gene (LAX1) is enhanced, whereas the expression of the auxin efflux transporter gene (PIN5b) is reduced. Overexpression of LAX1 increases, whereas knockdown reduces, lateral root initiation, auxin levels and the expression of a few cell cycle genes in rice (Zhao et al., 2015). PIN5b is an endoplasmic reticulum (ER)‐localized auxin efflux carrier. Overexpression of PIN5b reduces, but underexpression enhances, tiller number, root growth, panicle length and grain yield in rice (Lu et al., 2015). Our observations suggest that RBG1 regulates auxin‐mediated cell and organ growth in rice (Figure 2).
Indirect interactions between auxin level/action and microtubules have also been reported by other researchers. Ambrose et al. (2013a,b) have shown that a microtubule‐associated protein, CLASP, interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis. Schopfer and Palme (2016) have commented on the inhibition of cell expansion by rapid ABP1‐mediated auxin effect on microtubules. Since homoeostasis exists for most plant hormones including auxin, it is conceivable that auxin biosynthesis can be impacted by MT due to its interactions with auxin transporters such as PIN2. Therefore, we suggest that auxin and MT probably affect each other indirectly via auxin transporters as well as other signalling molecules.
Taken together, the role of RBG1 is most likely related to enhanced cell division in meristematic tissues by interacting with microtubules in an auxin‐dependent manner. Although microtubules and auxin have also been suggested to be involved in cell expansion, our observations with both RBG1Act and RBG1‐Ox lines indicate that cell enlargement is not the causal factor for the big grain phenotype. However, it should be cautioned that most of the experiments reported in this work were carried out with ectopic expression of RBG1, there is a remote possibility that the observations do not truly reflect the function of this gene. Future experiments with CRISPR‐based gene editing technology would be needed to further elucidate the function of RBG1.
Relationship of RBG1 to other genes regulating seed size
It has been suggested that seed size in rice is determined by a combination of different sets of genes regulating three different characteristics: grain length, grain width and grain filling (Ikeda et al., 2013; Xing and Zhang, 2010; Yan et al., 2011). Thus, it appears that many independent genetic pathways involved in cell proliferation and cell elongation regulate grain size (Zuo and Li, 2014). RBG1 most likely operates in the regulatory pathway controlling grain length, as RBG1Act and RBG1‐Ox lines produce longer but not wider grains. Increased seed weight in RBG1Act and RBG1‐Ox lines is a consequence of having longer grains. Many genes are involved in the regulation of grain length (Huang et al., 2013), but overexpression of RBG1 does not affect the expression of any of these genes (Figure S11). Ectopic expression of the auxin‐inducible BG1, which regulates auxin transport, also confers auxin hypersensitivity and enhances grain size in rice (Liu et al., 2015). However, the expression of BG1 was unaltered in our RBG1Act and RBG1‐Ox lines (Figure S11). Therefore, the action of RBG1 seems to be independent of the currently identified genes regulating grain size.
Ectopic expression of RBG1 enhances tolerance to abiotic stress
In our study, RBG1Act and RBG1‐Ox lines have elevated tolerance for abiotic stresses, including heat, osmotic stress and high salt (Figure 7). It is striking that a large number (31) of HSP family members are up‐regulated in the RBG1‐Ox‐5 line, with at least 13 of these HSP genes up‐regulated eightfold or higher (Figure 7d and Figure S5b and Table S2). In rice, overexpression of a small HSP leads to enhanced tolerance to drought stress (Sato and Yokoya, 2008). It has been suggested that heat stress transcription factors (HSFs) are in fact involved in responses to abiotic stresses in general (Guo et al., 2016). Furthermore, auxin has been linked to abiotic stress tolerance. In barley and Arabidopsis, high temperatures suppress the expression of the YUC auxin biosynthesis genes, leading to a reduction in endogenous auxin that causes male sterility, and application of auxin completely reversed male sterility in both plant species (Sakata et al., 2010). Therefore, it is plausible that the large number of HSPs up‐regulated by RBG1 overexpression is responsible for the elevated tolerance to drought, salt and heat stresses. We also suggest that the faster recovery rate for RBG1Act and RBG1‐Ox lines compared to the WT after exposure to osmotic stress is most likely related to the maintenance, as well as enhancement, of cell division in meristematic tissues. This finding is reminiscent of the rapid recovery of stressed resurrection plants after desiccation.
RBG1 actions are multifaceted, involving auxin accumulation and hypersensitivity, HSP production and interactions with microtubules
The action of RBG1 appears to be complex. Ectopic expression of RBG1 either by T‐DNA activation or driven by a ubiquitous promoter not only enhances organ size including grains, but also confers tolerance to environmental stresses. We also observed multiple intriguing intermediate events, that is increased auxin concentration, increased auxin sensitivity, elevated HSP expression and association of RBG1 with microtubules. These effects could actually be interrelated. HSPs have been shown to stabilize the auxin receptor TIR1 (Watanabe et al., 2017), and, conversely, some auxin signalling molecules are postulated to regulate HSP synthesis (Carranco et al., 2010). Auxin has also been shown to affect microtubules in regulating cell expansion (Chen et al., 2014), as well as affecting microtubule‐associated proteins (MAP) to control the direction of cell division and elongation (Ruan and Wasteneys, 2014). HSP90 protects tubulin from thermos‐denaturation (Weis et al., 2010). Although it is unclear how ectopic expression of RBG1 triggers these intermediate events, it is conceivable that they operate synergistically, leading to manifestation of the downstream phenotypes, that is, big grains and enhanced stress tolerance. We present a model explaining this multifaceted role of RBG1 in Figure 9.
Figure 9.
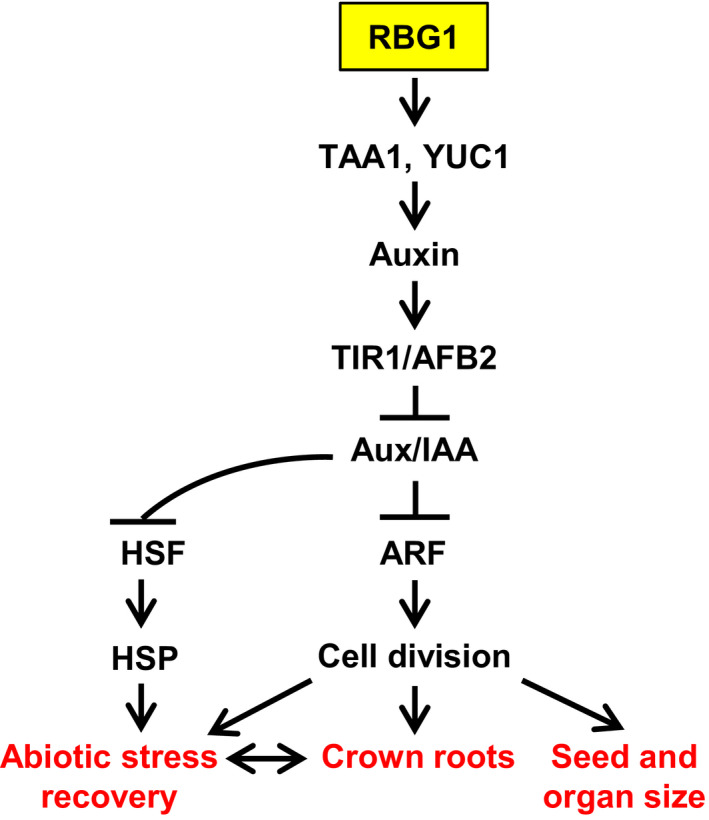
Proposed function of RBG1 in the regulation of the auxin biosynthesis and signalling pathways to promote seed and root development and stress recovery in rice.
RBG1 can be used for crop yield improvements
Ectopic expression of RBG1, either by activation tagging or by linking RBG1 to a strong constitutive promoter (i.e. the Ubi promoter), produced big grains, but the yield was decreased relativ to WT (Figure S2). The low yield was due to a low fertility rate. The total amount of photosynthates that a plant can produce is fixed, and the RBG1Act and RBG1‐Ox lines probably consume more energy to grow vegetative tissues, such as the longer leaves, peduncles and panicles and the bigger hulls compared to WT plants. Usually, grain number and grain weight are negatively correlated (Zuo and Li, 2014), but both parameters can increase concomitantly in some scenarios (Li et al., 2011a; Li et al., 2013; Liu et al., 2015). To achieve a balance between grain size and yield, proper selection of promoters to drive the expression of RBG1 at the time of seed development, but not during vegetative developmental stages, is crucial. For example, ectopic expression of the GRAIN INCOMPLETE FILLING 1 (GIF1) gene, which encodes a cell‐wall invertase required for carbon partitioning during early grain‐filling stages, with the CaMV35S or rice Waxy promoter resulted in smaller grains, whereas overexpression of GIF1 driven by its native promoter increased grain production (Wang et al., 2008a).
The use of a moderately constitutive rice GOS2 promoter significantly increased seed yield, moderately affected total biomass and had a significantly positive effect on panicle number, seed size and harvest index in another japonica variety (Figure 8a and Table S3). It has been reported that the GOS2 promoter is active in many tissues in monocots, but there could be a unique functional ASF‐1 binding site in vivo, which determines the expression level in roots, whereas other unknown motifs in the promoter may act in concert with the ASF‐1 binding site to regulate expression in other organs (de Pater et al., 1992a). Furthermore, Redillas et al (Redillas et al., 2012) used different promoters to regulate the expression of a transcription factor OsNAC9, leading to different phenotypic traits. These reports and our findings reveal that tissue specificity and promoter strength are crucial for translating the effect of ectopic expression of RBG1 into a desirable phenotype. In addition, a similar construct expressed in an indica variety led to similar positive effects on seed yield (Figure 8b and Table S3), but more moderate effects in other individual traits, thus corroborating the idea that seed shape and seed yield might be controlled by different gene modules. It is intriguing that harvest index has been affected by the ectopic expression of RBG1. Harvest index in rice and maize has been shown to be affected by availability of water (Yang and Zhang, 2010) and growth regulator applications (Hütsch and Schubert, 2017). Therefore, it is conceivable that elevated level of auxin in RBG1‐Ox lines, which are more tolerant to water deficit, could indirectly affect harvest index. Hughes et al. (Hughes et al., 2008) have also reported that an auxin signalling molecule, ARF2, regulates growth of integument, thus affecting yield in seed crops. Also, since the ectopic expression of RBG1 leads to bigger grains and more total grain yield, the change in harvest index could be just a consequence of this effect on grain development. The function of RBG1 in cell division might also be dependent on the tissue type and relative levels of RBG1 and partner proteins. These results indicate a likely independency of individual traits and an independent parallel signalling route for RBG1 to modify seed‐related traits. A similar situation has been observed in transgenic rice plants in which PGL1 (POSITIVE REGULATOR OF GRAIN LENGTH 1) interacts with APG (ANTAGONIST OF PGL1), yet transcription of two other known grain length‐related genes, GS3 and SRS3 (SMALL AND ROUND SEED 3), is largely unaffected (Heang and Sassa, 2012). It should also not be ruled out that the function of RBG1 is primarily related to stress tolerance and/or enhanced CR development, which could indirectly lead to higher grain yield.
Experimental procedures
Plant materials
The rice cultivar Oryza sativa cv Tainung 67 was used throughout this study. WT and mutant seeds were surface sterilized in 2.5% sodium hypochlorite and germinated on half‐strength MS agar medium (Murashige and Skoog Basal Medium with Vitamins; Phyto Technology Laboratories) at 28 ºC with 16‐h light and 8‐h darkness for 10–14 days.
EdU staining of root tips
Nuclei of dividing cells in rice root tips were stained with EdU as described (Kotogany et al., 2010), with slight modifications. De‐hulled seeds were germinated in water for 3 days and transferred to fresh 0.5× Kimura solution (Yoshida and Institute, 1976) 2 days for a total of 5 days. On the fifth day, seedlings were incubated in 0.5× Kimura solution containing 2 µm of EdU for 2 h. Seminal root tips 1 cm in length were cut, fixed in 4% paraformaldehyde in PBS buffer (2.7 mm KCl, 1.47 mm KH2PO4, 137 mm NaCl and 8 mm Na2HPO4, pH7.4) containing 0.1% (v/v) Triton X‐100, incubated for 30 min and then washed three times in PBS (10 min each time). Nuclei in root tips were then stained with EdU conjugated with the Alexa 555 fluorescence dye following the manufacturer's instructions (Invitrogen, Boston, MA, USA). The EdU signal was captured by Z‐stack serial sections under Zeiss LSM510 Meta confocal microscopy. EdU signal volume was calculated with Imaris 8.1.2 software (BitPlane, Zurich, Switzerland) with the default parameter settings for Surface items.
Subcellular localization of RBG1
Plasmid containing the Ubi:TUA6‐mGFP‐Nos construct was a generous gift from Dr. Ram Dixit, Biology Department, Washington University, St. Louis, MO, USA. Onion epidermis was co‐transfected with CaMV35S:mOrange2‐RBG1 and Ubi:TUA6‐mGFP constructs as described (Chou et al., 2014). Onion epidermis transfected with the Ubi:eGFP‐RBG1 construct was treated with oryzalin as described (Chuong et al., 2005). Barley aleurone layers were transfected with the Ubi:eGFP‐RBG1 construct as described (Lin et al., 2014).
Phenotypic evaluations of plants
For yield evaluation of Ubi:RBG1‐Ox transgenic plants, 3‐week‐old seedlings were transplanted to the open GM‐field at National Chung‐Hsing University and grown under natural conditions. At least two repeated blocks were used for each transgenic and WT lines, 24 plants in each block with a layout of 3 rows x 8 columns, and the space for each plant was 25 x 25 cm. Plant height, panicle number and total yield data were collected from 18 plants, excluding plants in the two marginal columns, in each block. General growth characteristics and flowering time were not noticeably different from the non‐transgenic WT.
For evaluations of GOS2:RBG1‐Ox transgenic plants, we selected six individual transformation events for which the T1 progeny segregated 3:1 for presence/absence of the transgene. For each event, approximately 10 T1 seedlings each containing the transgene (hetero‐ and homo‐zygotes) or lacking the transgene (nullizygotes) were selected by monitoring visual marker expression. Glasshouse conditions were short days (12 h of light), 28 °C in the light and 22 °C in the dark, and relative humidity of 70%. For all plants tested in the glasshouse, we used pots of 10 cm diameter but with no extra spacing between pots. General growth characteristics and flowering time were not noticeably different from the non‐transgenic WT.
The TraitMill technology for phenotypic evaluation was conducted as described (Reuzeau et al., 2010),(Lejeune et al., 2010). From the stages of sowing until maturity, individual rice plants grown in pots were passed several times through a digital imaging cabinet. At each time‐point, digital images (2048 x 1536 pixels, 16 million colours) were taken of each plant from at least six different angles as described (Lejeune et al., 2010). These measurements were used to determine different parameters.
We assessed green biomass, root biomass and time to flower of the plants using a previously described method (Lejeune and Leyns, 2007). We define the harvest index (HI) as the ratio between the total seed weight and the above ground area (mm2), multiplied by a factor of 106. The number of flowers per panicle is the ratio between the total numbers of seeds over the number of mature primary panicles. The ‘seed fill rate’ is the proportion (expressed as a %) of the number of filled seeds over the total number of seeds (i.e. total number of florets).
A two‐factor ANOVA (analysis of variance) was used as a statistical model for the overall evaluation of plant phenotypic characteristics. An F‐test was carried on all the parameters measured for all the plants over all events to check for an effect of the gene across all the transformed events and to verify for an overall effect of the gene, also known as a global gene effect. The threshold for significance for a true global gene effect was set at a 5% probability level for the F‐test. A significant F‐test value points to a global gene effect, meaning that it is not only the mere presence or position of the gene that causes differences in phenotype.
Analysis of endogenous auxin content by mass spectrometry
Samples were extracted in cold 50 mm sodium phosphate buffer (pH 7.0) containing [13C6]‐IAA (10 ng/sample), and extract was purified with the Oasis HLB (10 mg) SPE column as described (Novak et al., 2012). For the LC/MS analysis, an ACQUITY UPLC HSS T3 Column (2.1 x 100 mm, 1.8 µm, waters) was used for separation. The flow rate was 0.3 mL/min, the injection volume was 10 µL, and the column temperature was 30 °C. The composition of mobile phase A was deionized water containing 0.1% acetic acid, and for phase B, it was methanol containing 0.1% acetic acid. The gradient was as follows: 90% A at 0 min, 60% A at 1 min, 50% A at 3 min, 40% A at 5 min, 0% A between 7 and 8 min, 99.5% A between 9.5 and 12 min, 90% A between 12.5 and 15 min (Novak et al., 2012). Samples were then analysed using a Xevo TQ‐S tandem quadrupole mass spectrometer (Milford, MA). Data acquisition and processing were performed using MassLynx version 4.1 and TargetLynx software (Waters Corp, Milford, MA) (Cha et al., 2015). Characteristic MS transitions were monitored using the positive multiple reaction monitoring (MRM) mode for endogenous auxin (m/z, 176 > 130), 13C6‐IAA (m/z, 182 > 136), IAA‐Glu (m/z, 350 > 130) and IAA‐Asp (m/z, 291 > 130). At least three biological repeats were performed in this set of experiments.
Accession numbers
RBG1: NM_001189625/LOC_Os11g30430; RBG1‐L: NM_001051767/LOC_Os01g69290 are available in the NCBI/ MSU Rice Genome Annotation Project Database and Resource.
Conflict of interest
All authors declare that there are no conflicts of interest related to publishing this paper in Plant Biotechnology Journal.
Author contributions
THDH, SMY and CR conceived the original idea and designed the overall project. SFL and MLC performed most of the experiments. CR managed the agronomy trait analyses. YIH was responsible for the bioinformatic aspect of TRIM mutant population. YSC performed the root development analysis. KWL performed the experiments related to auxin hypersensitivity. YFH, YH and ASH performed experiments related to tissue‐specific expression and cellular localization of RBG1. PJC, LIW and NCC took care of the propagation and analysis of transgenic plants. THDH, SMY, CR and SFL interpreted all experimental results and collectively drafted the manuscript.
Supporting information
Figure S1 Large grain morphology was recapitulated by overexpression of RBG1 gene.
Figure S2 Ectopic expression of RBG1 driven by the Ubi promoter enhances grain size but reduces grain number and yield in transgenic rice.
Figure S3 RBG1 is co‐localized with microtubules.
Figure S4 RBG1 enhances the accumulation of endogenous auxin levels.
Figure S5 Heat maps indicate that the expression of auxin‐ and HSP‐related genes are significantly up‐regulated or down‐regulated by RBG1.
Figure S6 Phylogenetic analysis of RBG1 homologous proteins in plants.
Figure S7 Six conserved sequences are present in RBG1 and its homologous proteins from different plant species.
Figure S8 Conserved sequence 3 of RBG1 is not essential for its function.
Figure S9 Overexpression of RBG1‐L induces the big grain phenotype, but overexpression of RBG1 in Arabidopsis does not induce a bigger grain phenotype.
Figure S10 Correlations between level of RBG1 and RBG1‐L expression and the grain size.
Figure S11 RBG1 overexpression or underexpression does not affect the expression of genes known to regulate grain size in rice.
Figure S12 RBG1 is located within QTLs controlling seed length, soil stress tolerance and drought stress tolerance in rice.
Table S1 List of RBG1 and RBG1‐L homologous proteins from different plant species.
Table S2 Overexpression of RBG1 elevates the expression of stress tolerance‐related genes.
Table S3 Effect of RBG1 overexpression driven by the GOS2 promoter on agronomic traits in japonica rice (J) and in indica rice (I).
Table S4 List of primers.
Table S5 Detailed information on the QTLs on chromosome 11 that regulate seed length and abiotic stresses in rice.
Acknowledgements
We thank Dr. John O'Brien for critical review of the manuscript, Dr. Wei‐Che Hsu for assistance in the microarray data analysis, and Dr. Chih‐Yu Lin and Ms. Ting‐Hsiang Chang for auxin measurement. This work was supported by grants from Academia Sinica, the Ministry of Science and Technology (MOST 104‐2321‐B‐001‐054, MOST 105‐2321‐B‐001‐035 and MOST 106‐2321‐B‐001‐023), and in part by the Advanced Plant Biotechnology Center from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Contributor Information
Christophe Reuzeau, Email: christophe.reuzeau@cropdesign.com.
Tuan‐Hua David Ho, Email: ho@biology.wustl.edu.
Su‐May Yu, Email: ho@biology.wustl.edu.
References
- Ambrose, C. , Yuan, Y. , Gardiner, J. , Tamblyn, L.M. , Catching, A. , Kirk, V. , Marc, J. et al (2013a) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana . Dev Cell, 25, 649–659. [DOI] [PubMed] [Google Scholar]
- Ambrose, C. , Ruan, Y. , Gardiner, J. , Tamblyn, L.M. , Catching, A. , Kirik, V. , Marc, J. et al (2013b) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana . Dev. Cell, 24, 649–659. [DOI] [PubMed] [Google Scholar]
- Bernardi, J. , Lanubile, A. , Li, Q.B. , Kumar, D. , Kladnik, A. , Cook, S.D. , Ross, J.J. et al (2012) Impaired auxin biosynthesis in the defective endosperm18 mutant is due to mutational loss of expression in the ZmYuc1 gene encoding endosperm‐specific YUCCA1 protein in maize. Plant Physiol. 160, 1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranco, R. , Espinosa, J.M. , Prieto‐Dapena, P. , Almoguera, C. and Jordano, J. (2010) Repression by an auxin/indole acetic acid protein connects auxin signaling with heat shock factor‐mediated seed longevity. Proc. Natl. Acad. Sci. USA, 107, 21908–21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, J.Y. , Kim, W.Y. , Kang, S.B. , Kim, J.I. , Baek, D. , Jung, I.J. , Kim, M.R. et al (2015) A novel thiol‐reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat. Commun. 6, 8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, R. , Tong, H. , Shi, B. , Liu, Y. , Fang, S. , Liu, D. , Xiao, Y. et al (2015) Control of grain size and rice yield by GL2‐mediated brassinosteroid responses. Nat. Plants, 2, 15195. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Grandont, L. , Li, H. , Hauschild, R. , Paque, S. , Abuzeineh, A. , Rakusova, H. et al (2014) Inhibition of cell expansion by rapid ABP1‐mediated auxin effect on microtubules. Nature, 516, 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.S. , Lo, S.F. , Sun, P.K. , Lu, C.A. , Ho, T.H. and Yu, S.M. (2015) A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotechnol. J. 13, 105–116. [DOI] [PubMed] [Google Scholar]
- Chou, W.L. , Huang, L.F. , Fang, J.C. , Yeh, C.H. , Hong, C.Y. , Wu, S.J. and Lu, C.A. (2014) Divergence of the expression and subcellular localization of CCR4‐associated factor 1 (CAF1) deadenylase proteins in Oryza sativa . Plant Mol. Biol. 85, 443–458. [DOI] [PubMed] [Google Scholar]
- Chuong, S.D. , Park, N.I. , Freeman, M.C. , Mullen, R.T. and Muench, D.G. (2005) The peroxisomal multifunctional protein interacts with cortical microtubules in plant cells. BMC Cell Biol. 6, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert, Y. , Perin, C. , Courtois, B. , Khong, N.G. and Gantet, P. (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci. 15, 219–226. [DOI] [PubMed] [Google Scholar]
- Fan, C. , Xing, Y. , Mao, H. , Lu, T. , Han, B. , Xu, C. , Li, X. et al (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Liu, J.H. , Ma, X. , Luo, D.X. , Gong, Z.H. and Lu, M.H. (2016) The plant Heat Stress Transcription Factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 7, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heang, D. and Sassa, H. (2012) Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice. PLoS ONE, 7, e31325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa, K. (1989) Ripening and yield In The Growing Rice Plant – An Anatomical Monograph. p. 281 Tokyo: Nosan Gyoson unka Kyokai. [Google Scholar]
- Hsing, Y.I. , Chern, C.G. , Fan, M.J. , Lu, P.C. , Chen, K.T. , Lo, S.F. , Sun, P.K. et al (2007) A rice gene activation/knockout mutant resource for high throughput functional genomics. Plant Mol. Biol. 63, 351–364. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Xie, Q. and Chua, N.H. (2003) The Arabidopsis auxin‐inducible gene ARGOS controls lateral organ size. Plant Cell, 15, 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , Wang, Y. , Fang, Y. , Zeng, L. , Xu, J. , Yu, H. , Shi, Z. et al(2015) A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant, 8, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Huang, R. , Jiang, L. , Zheng, J. , Wang, T. , Wang, H. , Huang, Y. and Hong, Z. (2013) Genetic bases of rice grain shape: so many genes, so little known. Trends. Plant Sci. 18, 218–226. [DOI] [PubMed] [Google Scholar]
- Hughes, R. , Spielman, M. , Schruff, M.C. , Larson, T.R. , Graham, I.A. and Scott, R.J. (2008) Yield assessment of integument‐led seed growth following targeted repair of auxin response factor 2. Plant Biotechnol. J. 6, 758–769. [DOI] [PubMed] [Google Scholar]
- Hütsch, B.W. and Schubert, S. .(2017) Harvest index of maize (Zea mays L.): are there possibilities for improvement? In Advances in Agronomy (Sparks D.L., ed), pp. 37–82. Cambridge: Academic Press. [Google Scholar]
- Ikeda, M. , Miura, K. , Aya, K. , Kitano, H. and Matsuoka, M. (2013) Genes offering the potential for designing yield‐related traits in rice. Curr. Opin. Plant Biol. 16, 213–220. [DOI] [PubMed] [Google Scholar]
- Ishimaru, K. , Hirotsu, N. , Madoka, Y. , Murakami, N. , Hara, N. , Onodera, H. , Kashiwagi, T. et al (2013) Loss of function of the IAA‐glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707–711. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. , Bao, L. , Jeong, S.Y. , Kim, S.K. , Xu, C. , Li, X. and Zhang, Q. (2012) XIAO is involved in the control of organ size by contributing to the regulation of signaling and homeostasis of brassinosteroids and cell cycling in rice. Plant J. 70, 398–408. [DOI] [PubMed] [Google Scholar]
- Kotogany, E. , Dudits, D. , Horvath, G.V. and Ayaydin, F. (2010) A rapid and robust assay for detection of S‐phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods, 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune, P. and Leyns, F. (2007) Method and apparatus to determine the start of flowering in plants WO Patents. [Google Scholar]
- Lejeune, P. , Leyns, F. , Vandaele, C. and Van, C.W. (2010) Method for improved plant breeding. WO Patents. [Google Scholar]
- Leyser, O. (2018) Auxin signaling. Plant Physiol. 176, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Liu, W. , Tang, J. , Chen, J. , Tong, H. , Hu, B. , Li, C. et al (2010) Rice DENSE AND ERECT PANICLE 2 is essential for determining panicle outgrowth and elongation. Cell Res. 20, 838–849. [DOI] [PubMed] [Google Scholar]
- Li, M. , Tang, D. , Wang, K. , Wu, X. , Lu, L. , Yu, H. , Gu, M. et al (2011a) Mutations in the F‐box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice. Plant Biotechnol. J. 9, 1002–1013. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Fan, C. , Xing, Y. , Jiang, Y. , Luo, L. , Sun, L. , Shao, D. et al (2011b) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269. [DOI] [PubMed] [Google Scholar]
- Li, S. , Zhao, B. , Yuan, D. , Duan, M. , Qian, Q. , Tang, L. , Wang, B. et al (2013) Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA, 110, 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.R. , Lee, K.W. , Chen, C.Y. , Hong, Y.F. , Chen, J.L. , Lu, C.A. , Chen, K.T. et al (2014) SnRK1A‐interacting negative regulators modulate the nutrient starvation signaling sensor SnRK1 in source‐sink communication in cereal seedlings under abiotic stress. Plant Cell, 26, 808–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Tong, H. , Xiao, Y. , Che, R. , Xu, F. , Hu, B. , Liang, C. et al (2015) Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl. Acad. Sci. USA, 112, 11102–11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Chen, J. , Zheng, X. , Wu, F. , Lin, Q. , Heng, Y. , Tian, P. et al (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants, 3, 17043. [DOI] [PubMed] [Google Scholar]
- Lo, S.F. , Fan, M.J. , Hsing, Y.I. , Chen, L.J. , Chen, S. , Wen, I.C. , Liu, Y.L. et al (2016) Genetic resources offer efficient tools for rice functional genomics research. Plant Cell Environ. 39, 998–1013. [DOI] [PubMed] [Google Scholar]
- Locascio, A. , Roig‐Villanova, I. , Bernardi, J. and Varotto, S. (2014) Current perspectives on the hormonal control of seed development in Arabidopsis and maize: a focus on auxin. Front. Plant Sci. 5, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, G. , Coneva, V. , Casaretto, J.A. , Ying, S. , Mahmood, K. , Liu, F. , Nambara, E. et al (2015) OsPIN5b modulates rice (Oryza sativa) plant architecture and yield by changing auxin homeostasis, transport and distribution. Plant J. 83, 913–925. [DOI] [PubMed] [Google Scholar]
- Luo, J. , Liu, H. , Zhou, T. , Gu, B. , Huang, X. , Shangguan, Y. , Zhu, J. et al (2013) An‐1 encodes a basic helix‐loop‐helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell, 25, 3360–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi, K. , Tanaka, K. , Sakai, T. , Sugawara, S. , Kawaide, H. , Natsume, M. , Hanada, A. et al (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA, 108, 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, J.R. (2016) Mitosis. Cold Spring Harbor Perspect. Biol. 8, a023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, H. , Tanaka, A. , Tanabata, T. , Ohtake, M. , Fujioka, S. , Nakamura, H. , Ichikawa, H. et al (2012) Short grain1 decreases organ elongation and brassinosteroid response in rice. Plant Physiol. 158, 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, O. , Henykova, E. , Sairanen, I. , Kowalczyk, M. , Pospisil, T. and Ljung, K. (2012) Tissue‐specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J. 72, 523–536. [DOI] [PubMed] [Google Scholar]
- Overvoorde, P. , Fukaki, H. and Beeckman, T. (2010) Auxin control of root development. Cold Spring Harbor Perspect. Biol. 2, a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater, B.S. , van der Mark, F. , Rueb, S. , Katagiri, F. , Chua, N.H. , Schilperoort, R.A. and Hensgens, L.A. (1992a) The promoter of the rice gene GOS2 is active in various different monocot tissues and binds rice nuclear factor ASF‐1. Plant J. 2, 837–844. [DOI] [PubMed] [Google Scholar]
- Perrot‐Rechenmann, C. (2010) Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspect. Biol. 2, a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, P. , Lin, Y.S. , Song, X.J. , Shen, J.B. , Huang, W. , Shan, J.X. , Zhu, M.Z. et al (2012) The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin‐T1;3. Cell Res. 22, 1666–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redillas, M.C. , Jeong, J.S. , Kim, Y.S. , Jung, H. , Bang, S.W. , Choi, Y.D. , Ha, S.H. et al (2012) The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10, 792–805. [DOI] [PubMed] [Google Scholar]
- Reuzeau, C. , Pen, J. , Frankard, V. , De Wolf, J. , Peerbolte, R. , Broekaert, W. and Van Camp, W. (2010) TraitMill: a discovery engine for identifying yield‐enhancement genes in cereals. Plant Gene Trait 1. [Google Scholar]
- Ruan, Y. and Wasteneys, G.O. (2014) CLASP: a microtubule‐based integrator of the hormone‐mediated transitions from cell division to elongation. Curr. Opin. Plant Biol. 22C, 149–158. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, J. and Fukuda, H. (2008) Cell differentiation in the longitudinal veins and formation of commissural veins in rice (Oryza sativa) and maize (Zea mays). J. Plant Res. 121, 593–602. [DOI] [PubMed] [Google Scholar]
- Sakata, T. , Yagihashi, N. and Higashitani, A. (2010) Tissue‐specific auxin signaling in response to temperature fluctuation. Plant Signal Behav. 5, 1510–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic, A. and Mitchison, T.J. (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA, 105, 2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y. and Yokoya, S. (2008) Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat‐shock protein, sHSP17.7. Plant Cell Rep. 27, 329–334. [DOI] [PubMed] [Google Scholar]
- Schopfer, P. and Palme, K. (2016) Inhibition of cell expansion by rapid ABP1‐mediated auxin effect on microtubules? A critical comment. Plant Physiol. 170, 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X.J. , Huang, W. , Shi, M. , Zhu, M.Z. and Lin, H.X. (2007) A QTL for rice grain width and weight encodes a previously unknown RING‐type E3 ubiquitin ligase. Nat. Genet. 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Thimann, K.V. (1938) Hormones and the analysis of growth. Plant Physiol. 13, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling, E. (1991) The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 579–620. [Google Scholar]
- Wang, E. , Wang, J. , Zhu, X. , Hao, W. , Wang, L. , Li, Q. , Zhang, L. et al (2008a) Control of rice grain‐filling and yield by a gene with a potential signature of domestication. Nat. Genet. 40, 1370–1374. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Li, S. , Liu, Q. , Wu, K. , Zhang, J. , Wang, S. , Wang, Y. et al (2015) The OsSPL16‐GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47, 949–954. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, T. , Wang, R. and Zhao, Y. (2018) Recent advances in auxin research in rice and their implications for crop improvement. J. Exp. Bot. 69, 255–263. [DOI] [PubMed] [Google Scholar]
- Watanabe, E. , Mano, S. , Hara‐Nishimura, I. , Nishimura, M. and Yamada, K. (2017) HSP90 stabilizes auxin receptor TIR1 and ensures plasticity of auxin responses. Plant Signal Behav. 12, e1311439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis, F. , Moullintraffort, L. , Heichette, C. , Chretien, D. and Garnier, C. (2010) The 90‐kDa heat shock protein HSP90 protects tubulin against thermal denaturation. J. Biol. Chem. 285, 9525–9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y. and Zhang, Q. (2010) Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 61, 421–442. [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y. , Kamiya, N. , Morinaka, Y. , Matsuoka, M. and Sazuka, T. (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 143, 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, S. , Zou, G. , Li, S. , Wang, H. , Liu, H. , Zhai, G. , Guo, P. et al (2011) Seed size is determined by the combinations of the genes controlling different seed characteristics in rice. Theor. Appl. Genet. 123, 1173–1181. [DOI] [PubMed] [Google Scholar]
- Yang, J. and Zhang, J. (2010) Crop management techniques to enhance harvest index in rice. J. Exp. Bot. 61, 3177–3189. [DOI] [PubMed] [Google Scholar]
- Yoshida, S. and Institute, I.R.R. (1976) Laboratory Manual for Physiological Studies of Rice. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- Yu, P. , Gutjahr, C. , Li, C. and Hochholdinger, F. (2016) Genetic control of lateral root formation in cereals. Trends Plant Sci. 21, 951–961. [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Ma, T. , Wang, X. , Deng, Y. , Ma, H. , Zhang, R. and Zhao, J. (2015) OsAUX1 controls lateral root initiation in rice (Oryza sativa L.). Plant Cell Environ. 38, 2208–2222. [DOI] [PubMed] [Google Scholar]
- Zuo, J. and Li, J. (2014) Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 48, 99–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Large grain morphology was recapitulated by overexpression of RBG1 gene.
Figure S2 Ectopic expression of RBG1 driven by the Ubi promoter enhances grain size but reduces grain number and yield in transgenic rice.
Figure S3 RBG1 is co‐localized with microtubules.
Figure S4 RBG1 enhances the accumulation of endogenous auxin levels.
Figure S5 Heat maps indicate that the expression of auxin‐ and HSP‐related genes are significantly up‐regulated or down‐regulated by RBG1.
Figure S6 Phylogenetic analysis of RBG1 homologous proteins in plants.
Figure S7 Six conserved sequences are present in RBG1 and its homologous proteins from different plant species.
Figure S8 Conserved sequence 3 of RBG1 is not essential for its function.
Figure S9 Overexpression of RBG1‐L induces the big grain phenotype, but overexpression of RBG1 in Arabidopsis does not induce a bigger grain phenotype.
Figure S10 Correlations between level of RBG1 and RBG1‐L expression and the grain size.
Figure S11 RBG1 overexpression or underexpression does not affect the expression of genes known to regulate grain size in rice.
Figure S12 RBG1 is located within QTLs controlling seed length, soil stress tolerance and drought stress tolerance in rice.
Table S1 List of RBG1 and RBG1‐L homologous proteins from different plant species.
Table S2 Overexpression of RBG1 elevates the expression of stress tolerance‐related genes.
Table S3 Effect of RBG1 overexpression driven by the GOS2 promoter on agronomic traits in japonica rice (J) and in indica rice (I).
Table S4 List of primers.
Table S5 Detailed information on the QTLs on chromosome 11 that regulate seed length and abiotic stresses in rice.


