Abstract
Cancer is a leading cause of mortality and morbidity worldwide. Despite major improvements in current therapeutic methods, ideal therapeutic strategies for improved tumor elimination are still lacking. Recently, immunotherapy has attracted much attention, and many immune-active agents have been approved for clinical use alone or in combination with other cancer drugs. However, some patients have a poor response to these agents. New agents and strategies are needed to overcome such deficiencies. Phosphatidylserine (PS) is an essential component of bilayer cell membranes and is normally present in the inner leaflet. In the physiological state, PS exposure on the external leaflet not only acts as an engulfment signal for phagocytosis in apoptotic cells but also participates in blood coagulation, myoblast fusion and immune regulation in nonapoptotic cells. In the tumor microenvironment, PS exposure is significantly increased on the surface of tumor cells or tumor cell-derived microvesicles, which have innate immunosuppressive properties and facilitate tumor growth and metastasis. To date, agents targeting PS have been developed, some of which are under investigation in clinical trials as combination drugs for various cancers. However, controversial results are emerging in laboratory research as well as in clinical trials, and the efficiency of PS-targeting agents remains uncertain. In this review, we summarize recent progress in our understanding of the physiological and pathological roles of PS, with a focus on immune suppressive features. In addition, we discuss current drug developments that are based on PS-targeting strategies in both experimental and clinical studies. We hope to provide a future research direction for the development of new agents for cancer therapy.
Keywords: phosphatidylserine, cancer, T lymphocytes, immunotherapy, bavituximab
Introduction
Cancer is a leading cause of mortality and morbidity worldwide 1. Currently, the main treatments for cancer are surgery, chemotherapy and radiation therapy, which are designed to eliminate tumor cells by directly removing or killing them 2-4. With extensive research based on tumor biology, various genes and proteins have demonstrated potential inhibitory effects on tumor growth and proliferation, such as noncoding RNA, certain transcription factors and apoptotic pathway activators associated with tumor growth 5-8. Moreover, a new therapeutic strategy, which eliminates tumor cells by enhancing the immunity of patients, called immunotherapy, is becoming a popular therapeutic method for mono- or polytherapy of malignant tumors 9, 10. Immunotherapy fights cancer by helping the immune system recognize and target tumor cells. Until now, several types of immunotherapy have been used for cancer treatment, including T cell transfer therapy, immune checkpoint inhibitors, monoclonal antibodies and vaccines. For instance, chimeric antigen receptor T-cell immunotherapy (CAR-T), a T cell transfer therapy in which T cells from patients are modified and reinfused to better recognize tumor antigens, has been successfully used in some hematologic malignancies 11, 12. Additionally, some agents targeting the immune system have been developed for cancer treatment. Nivolumab and pembrolizumab are immune checkpoint inhibitors that enhance T cell immunity by blocking programmed cell death protein 1 (PD-1). Atezoizumab and avelumab are PD-1 ligand (PD-L1) inhibitors that were approved by the US Food and Drug Administration (FDA) in 2014 for certain cancers 13-16. Alemtuzumab, an antibody that binds to CD52 antigen on lymphocytes and attracts immune cells to destroy these cells, is used in chronic lymphocytic leukemia therapy 17, 18. Nonetheless, antibodies targeting CD47 19, OX40 20, CD20 21 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) all show immune activation effects. However, many patients in the clinic do not respond well 22, 23. For example, some patients have poor responses to PD-L1 inhibitors, which may be due to impaired T cell infiltration via upregulation of PD-L1, indoleamine-2,3-dioxygenase (IDO), and FoxP3(+) regulatory T cells (Tregs), termed primary resistance 24 or due to loss of T cell function via expression of different immune checkpoint proteins 25, defects in interferon signaling and antigen presentation, termed acquired resistance 26. Thus, new immunostimulatory targets are urgently needed to compensate for the deficiencies in cancer therapy.
Phospholipids compose the asymmetric bilayer membrane in eukaryotic cells 27. Among all the phospholipids, phosphatidylserine (PS) is a negatively charged amino-phospholipid and is predominately localized in the inner membrane leaflet 28. PS exposed on the outer leaflet of the plasma membrane responds to various stimuli; alternatively, PS present in certain vesicle membranes during vesicle generation participates in the progression of various diseases 29-31. In tumor microenvironments, PS exposure on tumor cells and immune cells leads to immune suppression and the promotion of tumor growth. PS exposure on blood cells, microparticles and neutrophil extracellular traps affects procoagulant activity in pancreatic cancer patients 32. Therefore, the location of PS on membranes is important for cell survival, growth, proliferation and cancer-related symptoms 33, 34. In this review, we summarize recent research on the roles of PS in physical and cancer biology, as well as related current clinical pharmacological trials, and we hope to provide new insights into future applications of PS in cancer therapy.
PS biology
PS synthesis
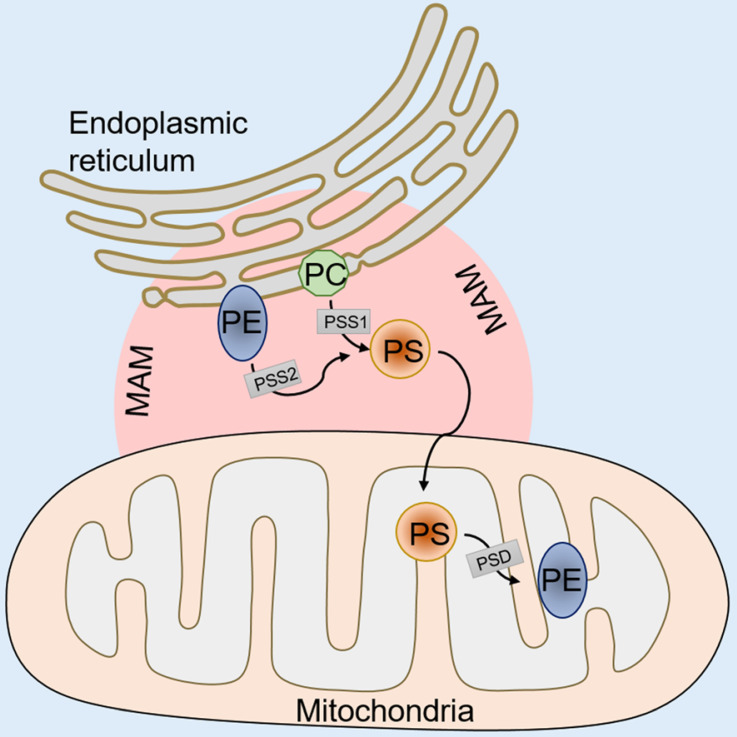
In mammalian cells, PS is synthesized in a specific domain of the endoplasmic reticulum called the mitochondria-associated membrane (MAM). The MAM facilitates the molecular exchange between the endoplasmic reticulum and mitochondria, and it plays a pivotal role in maintaining cellular health 35-37. PS synthesis in the MAM is from either phosphatidylcholine (PC) or phosphatidylethanolamine (PE) by phosphatidylserine synthase-1 (PSS-1) (from PC) or phosphatidylserine synthase-2 (PSS-2) (from PE) via a base-exchange reaction with serine (Figure 1). After synthesis, some of the PS is transported into the mitochondria by physical contact between the MAM and mitochondrial outer membranes 38, 39. Then, the PS in the mitochondria is decarboxylated and PE is synthesized by phosphatidylserine decarboxylase (PSD), an enzyme restricted to the mitochondrial inner membranes (Figure 1). This PE synthesis pathway from PS in the mitochondria is essential for the maintenance of mitochondrial integrity and cell growth, and a deficiency in the PSD gene results in embryonic lethality in mice 40, 41. The remaining synthesized PS in the MAM is transported to other organelles, such as the plasma membrane and the Golgi (Figure 1). The transportation mechanism is mostly through nonspontaneous diffusion mechanisms, including soluble transport proteins or vesicles 42. The proportion of synthesized PS that enters the mitochondria versus other organelles remains elusive. Previous phospholipid composition analysis of different organelles has shown that the highest PS content can be found in the plasma membrane and the lowest content is in mitochondria in cells of the rat liver and kidney 43, 44. However, the steady state levels of PS may not reflect actual synthesized PS transport because the mitochondrial PS would be rapidly converted to PE by PSD after entering the mitochondria. Additionally, recent studies have shown that the disruption of PS transport to the plasma membrane by knockdown gradient required protein rescue of the mitochondrial deficiency induced by PSS knockdown 45, suggesting a coordinated regulation of PS distribution from the ER into the plasma membrane or mitochondria.
Figure 1.
An illustration of PS synthesis. PS synthesis in the MAM from PC by PSS-1 or PE by PSS-2. After synthesis, some of the PS transported into mitochondria is decarboxylated to PE by PSD. The remaining synthesized PS in the MAM is transported to other organelles, such as the plasma membrane and the Golgi. MAM, mitochondria associated membranes; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PSS-1/2, phosphatidylserine synthase 1/2; PSD, phosphatidylserine decarboxylase.
PS exposure regulation
Flippases and scramblases coordinate the exposure of PS 46. The type IV subfamily of P-type ATPases (P4-ATPase) are eukaryotic flippases that translocate phospholipids from the outer leaflet to the inner leaflet of biological membranes by ATP-dependent active transport 47. The human gene encodes five classes of P4-ATPases: Class 1a (ATP8A1, ATP8A2); Class 1b (ATP8B1, ATP8B2, ATP8B3, ATP8B4); Class 2 (ATP9A, ATP9B); Class 5 (ATP10A, ATP10B, ATP10D); and Class 6 (ATP11A, ATP11B, ATP11C). ATP8A1, ATP8A2, ATP11A and ATP11C are known to flip PS from the exoplasmic to the cytoplasmic leaflet 48-50. Mechanistic studies have shown that the activities of ATP11A and ATP11C are affected by the cytosolic Ca2+ flux. In normal growing cells, ATP11A and ATP11C persistently transport PS from outer to inner membranes. When cytosolic Ca2+ increases due to various stimuli, such as platelet activation, ATP11A and ATP11C are inactivated, which enables exposure of PS on the cell surface. In addition, CDC50 family proteins (CDC50A, CDC50B, and CDC50C) complexed with P4-ATPase are required for normal flippase activity 51. CDC50A deficiency results in increasing PS exposure in cell membranes. It is worth noting that different variants of ATP11C are not functionally equivalent. ATP11C-a participates in PKC-mediated endocytosis, but not ATP11C-b or ATP11C-c, while ATP11C-b regulates the PS distribution in distinct regions of the polarized cell plasma membrane 52.
Scramblases catalyze another kind of phospholipid movement, which normally occurs in apoptotic cells or activated platelets 53. Scramblases mediate nonspecific, bidirectional, and ATP-independent phospholipid movement. TMEM16 and Xk-related protein 8 (Xkr8) are two proteins that have been identified as scramblases for PS transport. TMEM16 mediates Ca2+-activated scrambling activity and catalyzes PS exposure on the platelet surface. Xkr8 has been shown to increase PS exposure in response to apoptotic stimuli such as DNA degradation and oxidative stress. The molecular mechanism of Xkr8 activation depends on either a caspase-dependent signaling pathway 54, 55 or regulation via phosphorylation 56. The PS exposure mediated by Xkr8 is slow and irreversible, thus facilitating phagocytosis.
Apoptotic function of PS
The cells in our body constantly renew each day 57; thus, the “replaced” cells must be cleared out efficiently to prevent inflammation or autoimmunity 58. PS provides a recognizable and distinguished signal for cell phagocytic processes, also known as “apoptosis” 59, 60. Apoptotic cell death can be triggered by the intrinsic (via mitochondria) pathway or the extrinsic (via cell death factor) pathway. Caspase activation is the common signature of these two apoptotic pathways. Cytochrome C activates caspase 3 or caspase 7, which are released from mitochondria and induce the intrinsic apoptotic pathway. Alternatively, caspase 8 is activated by death factors such as tumor necrosis factor (TNF) and Fas ligand (FasL), subsequently activating caspase 3 and inducing apoptosis via the extrinsic pathway 61. PS exposure on the cell surface is a hallmark of apoptosis activation, presenting a recognition signal for macrophage recruitment and cell engulfment 62. Caspase 3 and caspase 7 directly activate Xkr8 by cleavage and execute scrambling activity. Mouse and human cancer cells with repressed Xkr8 expression via hypermethylation fail to expose PS during apoptosis 63. In addition to Xkr8, Xkr4 and Xkr9 possess a caspase recognition site and aid in PS exposure during apoptosis 64. Additional studies have shown that a complex consisting of Xkr8 with basigin (BSG) and neuroplastin (NPTN), which are type I membrane proteins of the Ig superfamily, is essential for PS exposure. Cells with a knockdown of BSG and NPTN failed to expose PS in response to apoptotic stimuli, though Xkr8 localized intracellularly 65, suggesting the involvement of a more complicated mechanism in caspase-dependent phospholipid scrambling activity. Conversely, caspases also recognize ATP11C sites and inactivated ATP11C on cell membranes. Mutation of the caspase recognition site of ATP11C leads to no exposure of PS during apoptosis and no engulfment by macrophages, indicating that apoptosis-related PS exposure is coordinated by both scramblase and flippase activity.
Non-apoptotic functions of PS
Coagulation
Blood coagulation consists of initiation, amplification and propagation phases 66. Formation of the prothrombin activator complex initiates coagulation. The process involves the activation of several coagulation cascades, including tissue factor (TF), activated factor VII (FVIIa), activated factors IX and X (FIXa and FXa) and the cofactor activated factor V (FVa) 67. PS present in the exofacial leaflet facilitates assembly of the complex and promotes thrombin formation 68, 69. Specifically, PS interacts with the 9-12-carboxyglutamic acid (Gla) domain at the NH2-terminus of the coagulation cascade (FVII, FIX, FX, FII) via Ca2+ 70, 71. PS exposure on platelets is mediated by TMEM16F activation. TMEM16F exerts its scrambling activity in response to Ca2+ and inactivates ATP11A and ATP11C, thereby exposing PS on limited regions of the cell surface 55, 72. However, this exposure of PS is transient, as the PS distribution will resume when the Ca2+ concentration returns to a normal level. This may explain why under physiological conditions, the constant flipping of PS prevents cells from being engulfed by macrophages 73.
Myoblast fusion
Myotube formation by myoblast fusion is essential for skeletal muscle development and regeneration. PS exposure is an initial fusion signal in myoblasts 74. Studies have shown that ATP11A and CDC50A-mediated PS exposure is required for myotube formation by myoblasts. In addition, the activity of a mechanosensitive Ca2+ channel that is predominantly expressed during myotube formation, PIEZO1, is affected by flippase-mediated inward translocation of PS from the cell surface. Ca2+ influx via PIEZO1 is impaired in CDC50A-deficient or ATP11A-deficient C2C12 cells, while this impairment can be recovered by exogenous expression of CDC50A or a PS flippase 75.
Apoptotic myoblasts also expose PS. The differences between the myoblast fusion-related PS exposure and myoblast apoptosis-related PS exposure are still elusive. One report has shown that differentiating muscle cells appear normal in terms of mitochondria potential and negative caspase 3 protein expression. Additionally, myotube formation and exposure of PS cannot be blocked by the caspase inhibitor zVAD(OMe)-FMK 74. However, other reports show that apoptosis blocked by a caspase inhibitor impaired myoblast fusion. They also found that a fraction of the myoblasts underwent apoptosis during myoblast fusion and that this small fraction induced PS exposure to promote myoblast fusion in the presence of caspase inhibitors 76, 77. How apoptotic myoblasts affect healthy myoblast fusion is still under investigation. Xkr8 may play a central role in myoblast differentiation. Studies have shown that Xkr8 knockdown myoblasts exhibit impaired differentiation and more apoptotic cells during differentiation. Moreover, Xkr8 accelerates myoblast differentiation via a mechanism unrelated to PS exposure and caspase-dependent cleavage 78.
T lymphocyte activation
Immunotherapies rely on boosting the preexisting or inducing a new tumor-resident T cell pool to eliminate tumor cells. CD4+ and CD8+ T cells are two types of T cells that are closely related to cancer immunotherapies. CD8+ T cells are cytotoxic T cells that are considered major drivers of antitumor immunity, while CD4+ T cells, including Th1, Th2, Th17, and Treg cells, play a prominent role in controlling tumor growth 79. PS exposure on T lymphocytes is normally associated with dead cell clearance. However, Elliott et al. reported that a subpopulation of activated/memory CD4+ T cells, which express low levels of the RB isoform of CD45 (CD4+CD45RBlo), express high levels of PS but are not apoptotic. In this study, CD45 expression levels inversely correlated with the proportion of T cells that exposed PS following P2X7 stimulation induced by benzoyl ATP (BzATP). This specific PS exposure in a subpopulation of T lymphocytes affected T cell migration, infiltration and rapid inflammation responses 80. Furthermore, human CD8+ cytotoxic T lymphocytes (CTLs) with antigen (Ag) recognition-induced PS exposure are also not apoptotic. The exposed PS on Ag-specific T cells is concentrated at the immunologic synapse, and a blockade of PS exposure by the annexin V protein during Ag recognition diminishes cytokine secretion 81, indicating that PS exposure on CTLs is related to cell-cell communication and T cell activation. The mechanism of PS exposure on lymphoma cells is related to TMEM16F activation, and this exposure level is comparable to that observed in apoptotic cells 82, again indicating that PS exposure alone is not sufficient to initiate phagocytosis.
PS receptors
PS receptors function as mediators to invoke immune suppression 83. Multiple proteins and protein families have been identified as PS receptors, including brain angiogenesis inhibitor 1 (BAI1), stabilin-1/2, integrin, TIM family proteins (T cell/transmembrane, immunoglobulin, and mucin), and TAM family proteins (Tyro3, AXL, and the MerTK receptor tyrosine kinase family). BAI1 84, stabilin-1/2 85 and the TIM family proteins 86 directly bind to PS, while TAM and integrin receptors indirectly bind to PS via ligand proteins. TAM receptors bind PS via the vitamin K-dependent proteins growth arrest-specific 6 (Gas6) and protein S (Pros1) 87 and integrin receptors bind to PS via Mfge8 88. Here, we briefly introduce the most studied PS receptors and their expression on immune cells. For more details on each PS receptor, please see other published reviews 89-92.
Stabilin-1/2
Stabilin-1 and stabilin-2 are widely expressed in endothelial cells in different organs, such as the liver, spleen, lymph nodes, bone marrow (stabilin-2), and adrenal cortex (stabilin-1) 92, 93. In apoptotic cells, stabilin-1 and stabilin-2 directly bind to PS on the cell surface and initiate cell engulfment by activation of Rac family small GTPase 1 (Rac1) via a phosphotyrosine-binding domain-containing engulfment adaptor protein (Gulp1)-dependent mechanism or a Gulp1-independent pathway 92. On immune cells, stabilin-1 is expressed in alternatively activated macrophages, also known as M2-like macrophages. M2 macrophages usually activate responses for healing or repair and provide a promoting environment for cancer growth 94, 95. In macrophages cocultured with apoptotic cells, stabilin-1 is recruited to sites of recognition and mediates clearance of dead cell in a PS-dependent manner 85. Additionally, stabilin-1-deficient macrophages in mice show reduced growth of primary tumors compared with controls. Anti-stabilin-1 antibody treatment leads to diminished numbers of immunosuppressive leukocytes in tumors 95, indicating that stabilin-1 on macrophages participates in tumor-related immune responses. Stabilin-2 has also been shown to be expressed on macrophages and to participate in TGF-β production. TGF-β is a key immune suppressive cytokine, which controls the adaptive and innate immune systems by regulating the function and generation of immune cells 96, 97 (Figure 2).
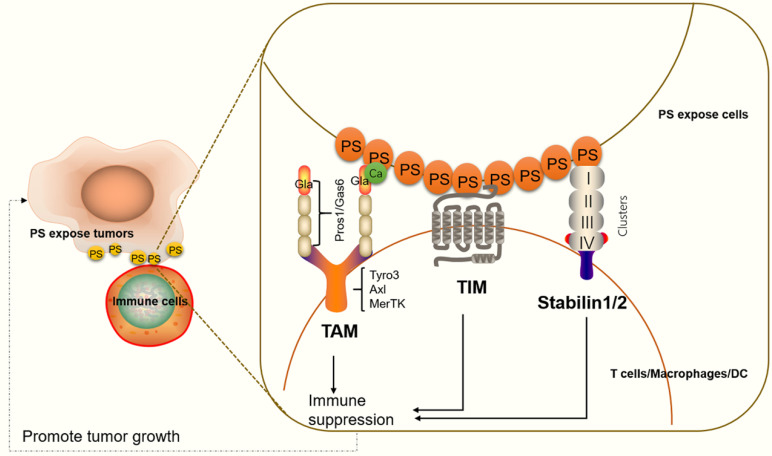
Figure 2.
An illustration of PS receptors and their functions in immune suppression. TAM interacts with PS through Gla domain-containing proteins, Gas6 or Pros1. Ca2+ also participates in effective PS binding and receptor activation. PS binds to TAM to regulate the feedback inhibition of the innate immune response in immune cells. The TIM protein forms a narrow cavity or pocket that PS binds to and plays a critical role in regulating immune responses. Stabilin-1/2 interacts with PS through four clusters (each cluster includes an EGF-like domain, an atypical EGF-like domain, a FAS domain and/or a link domain), which in turn activate a series of signals that lead to immune suppression.
TIM family
TIM proteins are type I cell surface glycoproteins and share common structural features, including Ig-like, mucin, transmembrane, and cytoplasmic domains 98. Structural studies have shown that PS binds to a narrow cavity formed by the CC and FG loops of IgV domains 99. TIM-1, TIM-3, and TIM-4 belong to the human TIM family and have been identified as PS receptors 100. TIM proteins are expressed on various immune cells and play critical roles in regulating immune responses and viral infections 101, 102. TIM-1 is expressed on CD4+ T cells 103, mast cells 104 and regulatory B cells 105. TIM-1 on Th2 cells functions as a potent costimulatory molecule for T cell activation 99. TIM-1-deficient B cells promote Th1 and Th17 responses but inhibit the generation of regulatory T cells 105. Moreover, TIM-1 is a binding site for Ebola virus on T lymphocytes and blocking TIM1-PS interactions reduces viral binding, T-cell activation, and cytokine production 106. TIM-4 is highly expressed on dendritic cells and macrophages, and TIM-4 on macrophages participates in inflammatory responses 107. TIM-3 is not expressed on naïve T cells, but it is expressed on fully differentiated Th1 cells 108. TIM-3 is also expressed on cytotoxic CD8+ T cells, Th2 cells, Th17 cells and regulatory T cells 109, 110, as well as on dendritic cells (DCs) and a subpopulation of macrophages 111. Targeting TIM-3 is known to suppress tumor growth (Figure 2). We will discuss these details in the section “Targeting PS receptors and cancer therapy”.
TAM family
The TAM family members indirectly bind to PS via the Gla domain of Gas6 or Pros1 ligand. Previous research has shown that Gas6 binds to and activates all three TAM receptors (Tyro3, AXL, and MerTK), while Pros1 is a ligand only for Tyro3 and MerTK 112. However, recent evidence has shown that Pros1 does bind to and activate AXL in glioma sphere cultures 113, and it modulates AXL expression in oral squamous cell carcinoma cell lines 114, suggesting that Pros1 induces receptor activity function differently in different tumor microenvironments. Of all three receptors, MerTK is the most studied receptor regarding immune responses. MerTK is expressed on dendritic cells, nature killer cells, B cells, and macrophages 115, 116. Regarding T cells, MerTK is expressed both on CD4+ T cells and CD8+ T cells. MerTK on dendritic cells competes for Pros1 interaction with MerTK in CD4+ T cells to control T cell activation 117 (Figure 2).
PS as an immune therapy target for cancer
PS exposure on tumor cells and induction of immune suppression
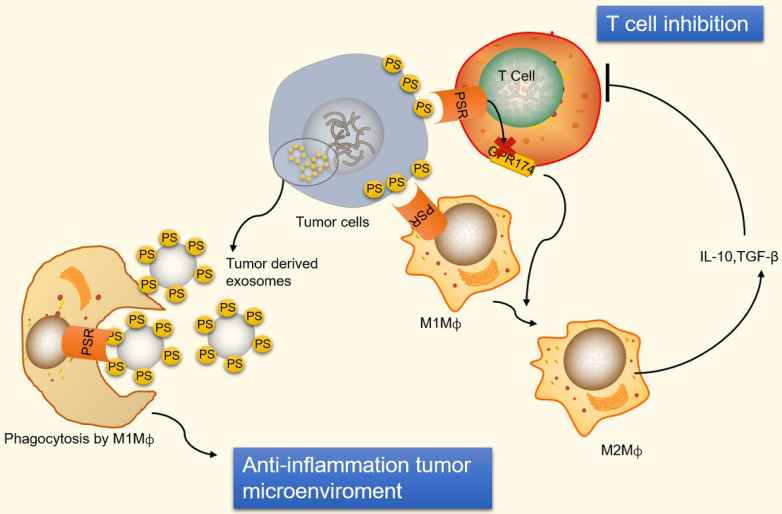
Immunosuppression and inflammation contribute to the creation of an environment that facilitates tumor growth and proliferation 118-120. Tumor cells can escape immune system surveillance by disrupting any step of T cell activation, or some tumor cells escape immune elimination by recruiting immunosuppressive leukocytes and orchestrating an antitumor microenvironment 121. PS is naturally exposed on tumor cells, the immature tumor vasculature and tumor-derived microvesicles 122-124. In addition, radiation therapy also causes an increase in the expression of PS on the surface of viable immune infiltrates in mouse B16 melanoma 125. The PS exposure on the surface of tumor cells prevents immune reaction by ligation of PS to receptors present on dendritic cells, macrophages and T cells 126. The ligation of PS to receptors on macrophages promotes macrophage polarization from a proinflammatory M1-like phenotype towards a protumor M2-like phenotype, allowing the secretion of the anti-inflammatory cytokines interleukin-10 (IL-10) and TGF-β 127. IL-10 and TGF-β are immunosuppressive cytokines that inhibit T cell activation by inhibiting tumor antigen presentation by dendritic cells and inducing regulatory T cells 128, 129 (Figure 3). Additionally, ligation of PS to receptors on T cells inhibits T cell activation via G protein-coupled receptor 174 (GPR174). GPR174-deficient Treg cells also promote the polarization of macrophages towards M2 and elevated expression of IL-10 130 (Figure 3).
Figure 3.
An illustration of PS exposure on tumor cells and vesicles inducing immune suppression. PS exposure on tumor cells induces immune suppression by ligation of PS to receptors on macrophages and T cells. PS binds to PSR on macrophages to promote maturation of M2-like macrophages, which are able to secrete the anti-inflammatory cytokines IL-10 and TGF-β. IL-10 and TGF-β are immunosuppressive cytokines that inhibit T cell activation. Additionally, ligation of PS to receptors on T cells inhibits T cell activation via GPR174-mediated M2 macrophage maturation. Conversely, PS exposed on tumor-derived microvesicles (externalized PS on microvesicles) increase the removal of apoptotic cells via phagocytes to prevent an undesirable inflammatory response and maintain an anti-inflammatory status in tumor microenvironments. GPR174, G protein-coupled receptor 174; M1Mф, M1-like macrophages; M2Mф, M2-like macrophages.
Conversely, PS is exposed on tumor-derived microvesicles, and microvesicles externalizing PS show increased removal of apoptotic cells via phagocytes, preventing an undesirable inflammatory response and maintaining an anti-inflammatory status in tumor microenvironments (Figure 3). Indeed, PS exposure on microvesicles (exosomes) derived from patient tumor samples has been shown to suppress the activation of T cell responses 131. However, PS exposure on tumor cells also favors antitumor effects by mediating long-lived inflammation. A recent study has shown that the exposure of PS on tumor cells is necessary for IFNγ and IL-12 binding and the conversion of transient cytokine stimuli into long-lived inflammation, thus mitigating immunosuppressive functions 132. Therefore, a complex mechanism underlies the immune response to PS exposure and its immune suppressive effects in tumor microenvironments.
PS and PS species as an imaging tool and biomarkers for cancer, respectively
Tumor cells expose PS on their surface, and therefore, methods for labeling PS are useful for tumor imaging 133. A liposomal nanoprobe PGN-L-IO/DiR, which binds specifically to PS and is subsequently internalized into cells, was shown to be a good imaging contrast agent for mice bearing MDA-MB231 breast tumors 134. Similarly, using a cyanine dye, indocyanine green, bound to PS antibodies helped to track and image apoptosis in triple-negative breast cancer cells 135, facilitating an effective treatment plan. In addition, PS-recognizing peptide 1 (PSP1) selectively binds to apoptosis-induced tumors in a radiation dose-dependent manner, which allows it to be used as an index probe to determine whether to continue radiation therapy in colorectal cancer 136.
Moreover, PS species can be biomarkers for cancer diagnosis. A clinical study conducted in 15 prostate cancer patients and 13 healthy controls examined the 36 most abundant lipid species. The results showed that a certain species of PS, PS (18:1/18:1), showed high significance between control and prostate cancer patients; furthermore, combinations of PS (18:1/18:1), lactosylceramide (d18:1/16:0) and PS (18:0/18:2) distinguished the two groups with 93% sensitivity and 100% specificity 137. More recently, a study compared lipids in surgical aerosols between tumor and adjacent normal tissues in lung cancer patients. Overexpression of PS (34:2), phosphatidylcholine (36:4), and triacylglycerol (46:2) and decreased phosphatidylcholine (34:3) were observed in cancerous aerosols 138, indicating that PS combined with other indices could be potential diagnostic biomarkers for lung cancer. In addition, species of PS may correlate with cancer proliferation and progression. The fatty acyl chain of PS has been shown to determine signal transduction efficiency. Studies have shown that nanoclusters of K-Ras, an important transduction signal, colocalize with markers of PS 139, and these nanoclusters are associated with PS species with one saturated and one unsaturated fatty acyl group (PS 18:0/18:1 or PS 16:0/18:1) 140. K-Ras is an isoform of Ras GTPase, in which mutations are commonly found in many cancers.
PS binding molecules and cancer therapy
As we discussed above, PS exposure on tumor and immune cells regulates immune responses in tumor microenvironments. Blocking PS on tumor cells restricts phagocytosis and T cell-mediated killing. Thus, targeting PS is considered a promising strategy for cancer therapy. Both preclinical and early phase clinical trials using PS targeting agents, including monoclonal antibodies, antibody-drug conjugations, liposomal carriers and natural products, have shown potential antitumor activities. Synergistic effects of PS targeting antibodies in combination with traditional cancer drugs have been observed in clinical trials. The therapeutic strategy of blocking PS is based mainly on two methods: 1) Disrupting PS on tumor cells. 2) Disrupting receptor signaling by targeting PS receptors.
Disrupting direct PS binding in cancer therapy
2aG4 and bavituximab
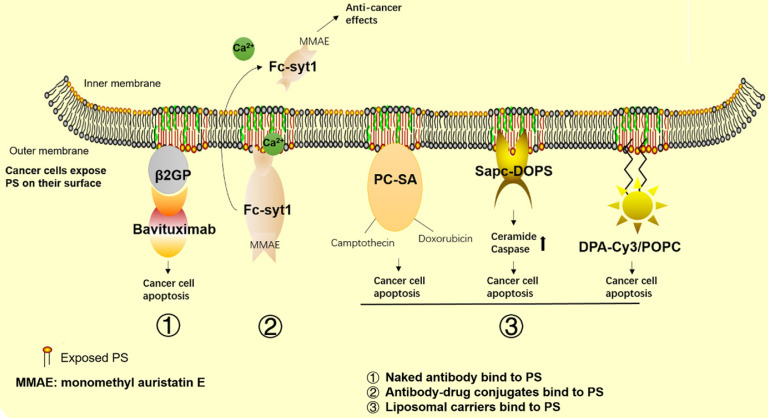
In recent years, a number of agents targeting PS have been developed for cancer therapy. Among these agents, bavituximab, a human-mouse chimeric monoclonal antibody that binds to PS indirectly by linking to β2GP1 with high affinity (Figure 4), has been the most studied. Mechanistic studies have shown that bavituximab blocks PS and exerts its antitumor immunity effects by promoting M1 macrophage maturation, as well as by inducing cellular cytotoxicity of tumor-associated endothelial cells. In experimental studies, 2aG4, the murine version of bavituximab, has been shown to exert a superior therapeutic effect in combination with the hepatocellular carcinoma therapy drug sorafenib compared with its use alone 141. Further mechanistic studies have shown increased PS exposure in response to sorafenib treatment in the tumor vasculature, while 2aG4 targets PS and significantly increases the amount of M1 macrophages, exerting its antitumor effects by secreting TNF-α, IL-12 and promoting the Th1 response. These findings indicate that 2aG4 targets PS to regulate the immune function of cells. In addition, the effects of transforming M2 to M1 macrophages are observed in prostate tumor-bearing mice by treatment with 2aG4 in combination with docetaxel 142. A recent study also showed that a manmade immunocytokine, 2aG4-IL2, which genetically links IL-2 to 2aG4, blocks PS induced immunosuppression in lung cancer. The vaccine was generated by coating tumor cells with 2aG4-IL2, and it reduced the incidence and number of spontaneous lung metastases 143. In addition, the combination of 2aG4 and APR-246, which is a small molecule drug that restores p53 function, effectively inhibited tumor growth in advanced hormone-dependent breast cancer 144.
Figure 4.
Cancer therapeutics related to PS. The graph shows the approved and promising drugs designed to target PS for cancer therapy. 1) Naked antibodies bind to PS. 2) Antibody-drug conjugates bind to PS. 3) Liposomal carriers bind to PS.
In clinical trials, bavituximab is used as a single agent or in combination with other traditional therapies in the treatment of lung cancer, hepatocellular carcinoma, breast cancer, pancreatic cancer, and solid tumors. By summarizing all the clinical results (Table 1), we found that as a monotherapy or in combination with chemotherapy or radiation therapy, bavituximab shows minimal side effects in the treatment of patients with lung cancer, hepatocellular carcinoma, breast cancer, solid tumors and rectal adenocarcinoma 141, 145-148. In phase Ⅰb and Ⅱ clinical trials, as a front line drug, bavituximab showed an overall response rate (ORR) of 28% and a progression-free survival rate (PFR) of 4.8% in combination with carboplatin and pemetrexed, and an ORR of 40.8% and PFR of 6.0% in combination with carboplatin and paclitaxel in non-small cell lung cancer therapy 149, 150. As a second-line drug, bavituximab also showed good ORRs and PFRs in combination with docetaxel in non-small cell lung cancer treatment 151. Similar results were observed in phase I and II clinical trials in the treatment of metastatic breast cancer; as a front-line drug, bavituximab combined with paclitaxel showed an ORR of 85% and a PFR of 4.8% in HER2-negative breast cancer 147. As a second-line drug, bavituximab combined with docetaxel showed an ORR of 60.9% and a PFR of 7.4%. In other phase II trials, bavituximab also showed good therapeutic results in the treatment of hepatocellular carcinoma in combination with sorafenib 152 and in the treatment of pancreatic cancers in combination with gemcitabine 153. However, a recent phase III clinical trial in non-small cell lung cancer patients showed that bavituximab was not sufficient to improve overall survival compared to treatment with docetaxel alone 154. This result is frustrating for the pharmaceutical company developing bavituximab; however, a retrospective case study analysis showed that this failure was due to poor quality data in phase II clinical trials and commercial consideration, so the phase III clinical trials should have been stopped earlier 155. To date, there are no other phase III clinical trial results for bavituximab in other cancer treatments, and whether this drug can be used in cancer treatments outside of non-small cell lung cancer or should be abandoned is still unknown. Concurrently, other strategies targeting PS are in development for tumor suppression.
Table 1.
Summary of clinical trials evaluating the combination of PS-targeting antibodies with chemotherapy or radiation
| Clinical trial phase | Tumor type | Drug name targeting PS | N | Duration (months) | Chemotherapy orradiation combination | Tumor growth inhibition | Side effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Phase Ib | Front line-advanced non-small-cell lung cancer | Bavituximab (0.3, 1 or 3 mg/kg) |
26 | Once every 3 weeks for 6 cycles | Carboplatin pemetrexed |
RR, 28% PFS, 4.8 OS, 12.2 |
Well tolerate | 149 |
| Phase II | Front line-Advanced non-small-cell lung cancer | Bavituximab (3 mg/kg) |
49 | Once every 3 weeks for 6 cycles then monotherapy | Carboplatin Paclitaxel | RR, 40.8% PFS, 6.0 OS,12.4 |
40.8% | 150 |
| Phase II | Second line-Advanced nonsquanous non-small-cell lung cancer | Bavituximab (1 or 3 mg/kg) or placebo |
121 | Once every 3 weeks for 6 cycles | Docetaxel | 1mg-RR,11.3%; PFS, 4.5 3mg-RR,17.1%; PFS, 4.5 |
Well tolerate | 151 |
| Phase III | Second line-Advanced non-small-cell lung cancer | Bavituximab (3 mg/kg) or placebo |
597 | Once a week | Docetaxel | Not superior to Docetaxel monotherapy | Well tolerate | 154 |
| Phase I | Front line-HER-2 negative metastatic breast cancer | Bavituximab (3 mg/kg) |
14 | Once every 4 weeks for 4 cycles | Paclitaxel | RR, 85%; PFS, 7.3 | -- | 147 |
| Phase II | Second line-advanced or metastatic breast cancer. | Bavituximab (3 mg/kg) |
46 | Once a week for first 3 weeks in a 28-day cycle of 6 cycles | Docetaxel | RR, 60.9%; PFR, 7.4 | Well tolerate | 194 |
| Phase I | Preoperative treatment of Rectal adenocarcinoma | Bavituximab (0.3, 1 or 3 mg/kg) |
14 | Once a week for 8 weeks | Radiation and capecitabine |
-- | Well tolerate | 146 |
| Phase I | Hepatocellular carcinoma | Bavituximab (0.3, 1 or 3 mg/kg) |
9 | Once a week for 8 weeks | Sorafenib or monotherapy |
-- | Well tolerate | 195 |
| Phase II | Front line-advanced hepatocellular carcinoma | Bavituximab (3 mg/kg) |
38 | Once a week | Sorafenib | PFS, 6.7; OS, 6.2 | 152 | |
| Phase I | Advanced solid tumors | Bavituximab (0.3, 1 or 3 mg/kg) |
26 | Once a week for 8 weeks | -- | -- | Well tolerate | 145 |
| Phase Ib | Front line-advanced solid tumors | Bavituximab (3 mg/kg) |
14 | Once a week for 8 weeks | Docetaxel or gemcitabine or paclitaxel plus carboplatin | -- | Well tolerate | 148 |
| Phase II | Front line-Stage IV Pancreatic Cancer | Bavituximab (3 mg/kg) vs Gemcitabine alone |
70 | Once a week for first 3 weeks in a 28-day cycle | Gemcitabine | ORR, 28.1 vs 12.9%; OS, 5.6 vs 5.2 months |
Well tolerate | 153 |
| Phase II | advanced gastric or gastroesophageal junction cancer | Bavituximab | 80 | Pembrolizumab | Still in progress |
OS, median overall survival time; ORR, overall response rate; PFR, Progression-free survival rate.
Antibody-drug conjugates
Antibody-drug conjugates are another branch of developing PS-targeting drugs. Antibody-drug conjugates combine a PS-targeting antibody to a cytotoxic drug to exert tumor killing effects. Experimental studies have shown that some antibody-drug conjugates have better tumor suppressive effects than “naked” antibodies. For example, Fc-Syt1 is a PS-targeting agent generated by fusing PS-binding domains to a human IgG1-derived Fc fragment C2A. Use of a Fc-Syt1 conjugated to monomethyl auristatin E, a cytotoxic drug, showed good antitumor effects in mouse breast and prostate cancer models 156. In addition, recent researchers have developed a new method in which a PS binding peptide, PSBP-6, is conjugated to pH-sensitive mixed micelles (PEG-PDLLA and PEG-PHIS) and then loaded onto the chemotherapeutic agent paclitaxel (PTX) in prepared micelles. These pH sensitive micelles represent an acid triggered drug release system that is suitable for the acidic tumor environment. An in vivo study showed that the conjugated agents had improved cytotoxicity and uptake by tumor cells, as well as accumulation at tumor sites 157.
Moreover, fusion proteins consisting of L-methionase linked to human Annexin-V, an antibody with high affinity for PS, have shown good effects in tumor cell killing compared with L-methionase with no fusion protein present 158. Moreover, the fusion protein shows almost no effects on normal cells, indicating that this strategy is a promising approach for new drug development.
Liposomal carriers
Liposomes are used as drug carriers because of their drug protecting and specific targeting characteristics. Targeting PS is a main antitumor mechanism of some liposomal carriers. Those liposomal carriers bind to PS on tumor cells and exert antitumor effects either by metabolic interference or synergistic effects with drug loading.
SapC-DOPS is a protein/lipid nanovesicle composed of the sphingolipid-activating protein Saposin C (SapC), which functions to catabolize glycosphingolipids and dioleoylphosphatidylserine (DOPS). SapC-DOPS selectively binds to PS on cancer cells and induces cell apoptosis via ceramide accumulation and caspase activation 159 (Figure 4). Research groups have used SapC-DOPS to treat different cancer cells, such as brain tumor cells 160, skin cancer cell lines 161 and pancreatic cancer cells 162. The results showed that SapC-DOPS has good antitumor effects in a number of primary and metastatic tumors. The same group later found that during irradiation therapy, cells with high levels of surface PS had a higher survival rate, which inversely correlated with sensitivity to radiation therapy and some chemotherapy. However, these cells are sensitive to SapC-DOPS treatment, suggesting that SapC-DOPS can be used as a combination therapy for cancer cells with high PS exposure during radiation therapy 163. A recent review focusing on cancer therapy treatments with SapC-DOPS is available 164.
In addition, phosphatidylcholine-stearylamine (PC-SA) is a cationic liposomal carrier that specifically binds to PS on cancer cells and tumors. A preclinical study showed that PC-SA has anticancer effects as a single agent and has higher efficacy when loaded with traditional antitumor drugs (Figure 4). In this study, using PC-SA alone or the anticancer drugs camptothecin and/or doxorubicin entrapped in PC-SA liposomes inhibited tumor growth and decreased tumor microvasculature formation in three tumor models. PC-SA enhances the half-life of antitumor drugs and shows no signs of obvious toxicity to other organs, suggesting its potential as a new drug or drug carrier for cancer combination therapy 165.
DPA-Cy3 is a lipid-soluble zinc(II)-bis-dipicolylamine derivative that contains the fluorophore cyanine 3 (Cy3) and two 22-carbon chains that can be anchored into liposomal membrane bilayers. Use of DPA-CY3 and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) liposomes resulted in selective binding to PS-enriched cancer cells. That study demonstrated that DPA-CY3/POPC could exert antitumor effects without any drugs loaded. DPA-CY3/POPC prefers to bind to the surface of breast cancer cells (MCF-7) versus noncancer cells (MCF-12A) due to the different levels of PS exposure. Additionally, internalization of DPA-CY3/POPC by MCF-7 is more stable than that by MCF-12A, and thus, the cytotoxic effects to tumor cells are more robust in tumor cells compared to normal cells 166.
Natural plant extracts targeting PS
It is worth noting that some natural products also show anticancer effects by targeting PS. For example, Chalepin is a compound extracted from the plant Ruta angustifolia that has been shown to exert cytotoxic activity against breast cancer cells but not normal cells. Mechanistic studies have shown that the induction of apoptosis by Chalepin is associated with PS externalization 167.
Targeting PS receptors and cancer therapy
As discussed in the “PS receptors” section, previous research has shown that PS receptors participate in the progression of tumor cells and control immune responses in the tumor microenvironment. Agents targeting TIM and TAM receptors have been developed for cancer treatment. TIM-3 antibody suppresses tumor growth via T cell regulation, especially in combination with anti-PD-1 drugs, in the treatment of fibrosarcomas 168. Blockade of TIM-3 enhances the antitumor immune response in head and neck cancer 169. Not surprisingly, a few pharmaceutical companies are now developing anti-TIM-3 antibodies as new antitumor agents, such as TSR-022 (NCT02817633; Tesaro), MBG543 (NCT02608268; Novartis), BMS-986258 (NCT03446040; MBS), and LY3321367 (NCT03099109; Eli Lilly and Company), which are all in phase I/II clinical trials 170-172. Most of those trials have not yet provided results. However, some results from the TSR-022 phase I clinical trial were released at the 2018 annual meeting of the Society for Immunotherapy of Cancer (SITC). The results showed that the combination of TSR-022 with PD-1 antibody showed good tolerance in both non-small cell lung cancer and melanoma patients, and a high dose of TSR-022 (300 mg) showed observed activity, with an ORR of 15% (3/20) and 40% stable disease (8/20) 173. The results showed the promise of anti-TIM-3 drugs in cancer treatment.
Agents that target TAM have also attracted much attention. TAM is abnormally expressed in various cancer cells, including acute myeloid leukemia, gastric and colorectal adenocarcinomas, non-small cell lung, breast and prostate cancers, and it is considered an oncogene. In this case, PS receptor inhibitors were developed to help enhance the innate immune response and kill cancer cells. Multiple drugs are being developed to selectively or nonselectively target TAM receptors. For example, Sitravatinib is a multikinase inhibitor that targets all three TAM receptors, and the combination of Sitravatinib with the PD-1 inhibitor Nivolumab is currently in phase Ⅱ (NCT02954991) and phase Ⅲ (NCT03906071) clinical trials for non-small cell lung cancer treatment. Additionally, the use of Sitravatinib for other cancer treatments, such as in combination with Tislelizumab for advanced or metastatic hepatocellular carcinoma (HCC) or gastric/gastroesophageal junction cancer (GC/GEJC) (NCT03941873), for advanced solid tumors (NCT03666143), and in combination with Nivolumab for advanced or metastatic kidney cancer (NCT03015740), urothelial carcinoma (NCT03606174) and clear cell renal cell carcinoma (NCT03680521), are in the recruiting phase for phase I or phase II clinical trials. Additionally, some agents that were initially not designed for TAM targeting were found to suppress TAM concurrently with their main targets. Those drugs, such as bosutinib 175, 176, crizotinib 177, 178, and cabozantinib 179, 180, have been approved for clinical use 174 as nonselective drugs. Moreover, many drugs have been developed to selectively target one of the TAM receptors, including selective AXL inhibitors (R428, TP0903, BMS777607 and NPS1304), selective MerTk inhibitors (MRX2843, UNC2025, UNC3133 and ONO7475) and Tyro3 inhibitors (Pfizer compounds 11, 12, 19, 21 and KRCT-6j) 91. Most of these agents have demonstrated synergistic effects to overcome the resistance of classical antitumor drugs. BMS777607 recovers Sunitinib sensitivity in advanced renal cell carcinoma 181, and NPS1034 restores gefitinib or erlotinib sensitivity in an EGFR-resistant lung cancer cell model 182. These selective TAM receptor drugs are designed to lower the side effects of nonselective TAM receptor drugs. Most of these agents do not specifically target TAM but also suppress other kinase families. For example, BMS777607 suppresses c-MET and AXL, and MRX2843 suppresses MerTK and Flit.
It should be noted that targeting TIM does not fully represent inhibition of PS because TIM can bind to other proteins; for example, TIM-3 has been identified to interact with galectin-9 (gal-9) and high-mobility group protein box 1 (HMGB-1) 183, 184, which also participate in immune responses. Moreover, conflicting results were reported in studies of TAM receptor targeting. MerTK-deficient tumors show increase leukocyte proliferation and higher infiltration of CD8+ T lymphocytes in a mouse model 185. Additionally, MerTK deficiency shows better tumor control after radiotherapy in colorectal and pancreatic adenocarcinoma mice 186. However, another study showed that MerTK and AXL-deficient mice exhibit exacerbated tumor growth and inflammation associated cancer 186. In addition, a recent study showed that MerTK expression on CD8+ T cells improves tumor-infiltrating lymphocyte expansion 187. The discrepancy between different studies may result from the different tumor types and PS exposure levels studied, but these results provide a hint that blindly inhibiting TAM, at least in the case of MerTK inhibitory agents, should be used with caution due to its potential for inducing inhibition of tumor killing.
PS exposure and CD47 for cancer therapy
PS exposure on the cell surface provides a phagocytic signal for macrophages, while CD47 expression on cells inhibits phagocytosis 124. CD47 is widely expressed on all cells but has high expression levels on various tumor cells 188. CD47 is a ligand of signal regulatory protein-α (SIRPα), a protein expressed on macrophages and dendritic cells. Upon binding CD47, SIRPα initiates a signaling cascade that results in the inhibition of phagocytosis 189, through which tumor cells can escape from immune surveillance of macrophages and T cells 190. In erythrocytes, CD47 ligation induces PS expression as part of a death pathway 191, suggesting that CD47 could affect PS exposure. Indeed, knockout of CDC50A, a subunit of ATP11C that participates in PS flipping, increases tumor-associated macrophages and enhances the effect of anti-CD47 blockade on limiting tumor growth 192. Knockout of CDC50A also increases PS exposure on Jurkat cells, which may affect T cell function, as we mentioned earlier. Thus, blockade of CD47 inhibits phagocytosis, however, PS exposure has been utilized to target tumor cells for macrophage clearance. In clinical trials, the combination of anti-CD47 agents has shown synergistic antitumor effects. The anti-CD47 inhibitor 5F9 combined with rituximab (anti-CD20 antibody) has shown a good response with minimal side effects in patients with aggressive and indolent lymphoma 193.
Perspective and Conclusion
Currently, immunotherapy that enhances T cell immunity has become a hot research area. PS exposure is known to be an immune suppressor in tumor microenvironments. In general, agents that directly or indirectly target PS rescue immune suppression, enhance antitumor drug activity and are accompanied by good tolerance, suggesting that PS is promising as a new drug for mono- or polytherapy for cancer. However, we notice that some risks arise during treatment with this kind of drug. First, inhibition of PS receptors, such as MerTk on T cells, may reduce tumor killing effects. Second, PS exposure on the cell surface provides a phagocytic signal for macrophages, and some immune therapies have utilized this mechanism to target tumor cells for macrophage clearance, such as CD47 inhibitors; thus, using anti-PS agents may modulate those drug therapy effects. Third, anti-PS therapy is mainly utilized in combination with other cancer drugs, and the safety and unknown side effects, such as the risk of developing thromboembolic events, is largely unknown. Therefore, although targeting PS shows promise in cancer therapy, many obstacles must be overcome for its successful application in the clinic. Ideally, agents that specifically target PS on tumor cells but do not affect PS on normal cells, and agents that sense the quantity of PS exposure when in combination with other cancer drugs, merit further investigation. Future research could focus on these obstacles and challenges to develop new kinds of drugs.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (81700704, 81622005), and the Natural Science Foundation of Shandong province (ZR2017BH087).
Author contributions
W Chang, Hongge Fa and Dandan Xiao collected all of the data, and W Chang and J Wang drafted and wrote the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Takeyama H, Beppu T, Higashi T, Kaida T, Arima K, Taki K. et al. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surg Today. 2018;48(4):431–8. doi: 10.1007/s00595-017-1603-x. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C. et al. Radiation therapy for the whole breast: Executive summary of an american society for radiation oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–52. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Ding W, Ge H, Ponnusamy M, Wang Q, Hao X. et al. Foxk transcription factors: Regulation and critical role in cancer. Cancer Lett. 2019;458:1–12. doi: 10.1016/j.canlet.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X. et al. Critical role of foxo3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W. et al. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019;23(8):4900–12. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Lin Z, He Y, Pang X, Wang Y, Ponnusamy M. et al. The long noncoding RNA d63785 regulates chemotherapy sensitivity in human gastric cancer by targeting mir-422a. Mol Ther Nucleic Acids. 2018;12:405–19. doi: 10.1016/j.omtn.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamboa L, Zamat AH, Kwong GA. Synthetic immunity by remote control. Theranostics. 2020;10(8):3652–67. doi: 10.7150/thno.41305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong L, Li J, Li Q, Wang X, Medikonda R, Zhao T. et al. Act001 reduces the expression of PD-L1 by inhibiting the phosphorylation of stat3 in glioblastoma. Theranostics. 2020;10(13):5943–56. doi: 10.7150/thno.41498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golubovskaya V. Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel) 2016;8(3):36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 13.Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA. et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019;6(9):e480–8. doi: 10.1016/S2352-3026(19)30114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion SV, Gonzalez R, Grob JJ, Rutkowski P, Lao CD. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 15.Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM. et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (keynote-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e448–58. doi: 10.1016/S2352-3026(19)30109-7. [DOI] [PubMed] [Google Scholar]
- 16.Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H. et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019;25(6):941–6. doi: 10.1038/s41591-019-0448-9. [DOI] [PubMed] [Google Scholar]
- 17.Sellar RS, Mehra V, Fox TA, Grigg A, Kulasekararaj A, Sarma A. et al. Comparative analysis of melphalan versus busulphan T-cell deplete conditioning using alemtuzumab in unrelated donor stem cell transplantation for acute myeloid leukaemia. Br J Haematol. 2019;187(1):e20–4. doi: 10.1111/bjh.16136. [DOI] [PubMed] [Google Scholar]
- 18.Ishizawa K, Fukuhara N, Nakaseko C, Chiba S, Ogura M, Okamoto A. et al. Safety, efficacy and pharmacokinetics of humanized anti-CD52 monoclonal antibody alemtuzumab in Japanese patients with relapsed or refractory B-cell chronic lymphocytic leukemia. Jpn J Clin Oncol. 2017;47(1):54–60. doi: 10.1093/jjco/hyw146. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H. et al. Advances in anti-tumor treatments targeting the cd47/sirpalpha axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Casey M, Oey H, Vari F, Stagg J, Gandhi MK. et al. Targeting an adenosine-mediated "don't eat me signal" augments anti-lymphoma immunity by anti-CD20 monoclonal antibody. Leukemia. 2020 doi: 10.1038/s41375-020-0811-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooradian MJ. Sullivan RJ. What to do when anti-PD-1 therapy fails in patients with melanoma. Oncology (Williston Park) 2019;33(4):141–8. [PubMed] [Google Scholar]
- 24.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT. et al. Up-regulation of PD-L1, Ido, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236(61):11–2. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 28.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319(5860):210–3. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 29.Ale A, Siebenhaar F, Kosanke K, Aichler M, Radrich K, Heydrich S. et al. Cardioprotective c-kit(+) bone marrow cells attenuate apoptosis after acute myocardial infarction in mice- in-vivo assessment with fluorescence molecular imaging. Theranostics. 2013;3(11):903–13. doi: 10.7150/thno.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Zhao X, Sui Z, Niu H, Chen L, Hu C. et al. A multi-omics investigation of the molecular characteristics and classification of six metabolic syndrome relevant diseases. Theranostics. 2020;10(5):2029–46. doi: 10.7150/thno.41106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Yang J, Liu R, Qiao C, Lu Z, Shi Y. et al. Dual-targeting theranostic system with mimicking apoptosis to promote myocardial infarction repair via modulation of macrophages. Theranostics. 2017;7(17):4149–67. doi: 10.7150/thno.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M, Li T, Li B, Liu Y, Wang L, Zhang J. et al. Phosphatidylserine-exposing blood cells, microparticles and neutrophil extracellular traps increase procoagulant activity in patients with pancreatic cancer. Thromb Res. 2020;188:5–16. doi: 10.1016/j.thromres.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA. et al. Phosphatidylserine externalization, "necroptotic bodies" release, and phagocytosis during necroptosis. PLoS Biol. 2017;15(6):e2002711. doi: 10.1371/journal.pbio.2002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vance JE. Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta. 2013;1831(3):543–54. doi: 10.1016/j.bbalip.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Annunziata I, Sano R, D'Azzo A. Mitochondria-associated ER membranes (MAMs) and lysosomal storage diseases. Cell Death Dis. 2018;9(3):328. doi: 10.1038/s41419-017-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M. et al. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018;9(3):329. doi: 10.1038/s41419-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacchiotti A. Exploring cellular stress response and chaperones. Cells. 2019;8(5):408. doi: 10.3390/cells8050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kainu V, Hermansson M, Hanninen S, Hokynar K, Somerharju P. Import of phosphatidylserine to and export of phosphatidylethanolamine molecular species from mitochondria. Biochim Biophys Acta. 2013;1831(2):429–37. doi: 10.1016/j.bbalip.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16(1):1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 40.Selathurai A, Kowalski GM, Mason SA, Callahan DL, Foletta VC, Della Gatta PA. et al. Phosphatidylserine decarboxylase is critical for the maintenance of skeletal muscle mitochondrial integrity and muscle mass. Mol Metab. 2019;27:33–46. doi: 10.1016/j.molmet.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem. 2005;280(48):40032–40. doi: 10.1074/jbc.M506510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W. et al. Intracellular transport. Phosphatidylserine transport by ORP/OSH proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349(6246):432–6. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero FM, Landriscina C, Gnoni GV, Quagliariello E. Lipid composition of liver mitochondria and microsomes in hyperthyroid rats. Lipids. 1984;19(3):171–8. doi: 10.1007/BF02534794. [DOI] [PubMed] [Google Scholar]
- 44.Zambrano F, Fleischer S, Fleischer B. Lipid composition of the golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim Biophys Acta. 1975;380(3):357–69. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Liang J, Ding L, Li X, Lam SM, Shui G. et al. Phosphatidylserine synthase regulates cellular homeostasis through distinct metabolic mechanisms. PLoS Genet. 2019;15(12):e1008548. doi: 10.1371/journal.pgen.1008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kay JG. Fairn GD. Distribution, dynamics and functional roles of phosphatidylserine within the cell. Cell Commun Signal. 2019;17(1):126. doi: 10.1186/s12964-019-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata S, Sakuragi T, Segawa K. Flippase and scramblase for phosphatidylserine exposure. Curr Opin Immunol. 2020;62:31–8. doi: 10.1016/j.coi.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the p4-ATPase ATP8A2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284(47):32670–9. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Uchida Y, Wang J, Matsudaira T, Nakagawa T, Kishimoto T. et al. Transport through recycling endosomes requires ehd1 recruitment by a phosphatidylserine translocase. EMBO J. 2015;34(5):669–88. doi: 10.15252/embj.201489703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takatsu H, Tanaka G, Segawa K, Suzuki J, Nagata S, Nakayama K. et al. Phospholipid flippase activities and substrate specificities of human type iv p-type atpases localized to the plasma membrane. J Biol Chem. 2014;289(48):33543–56. doi: 10.1074/jbc.M114.593012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coleman JA. Molday RS. Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the p4-ATPase ATP8A2. J Biol Chem. 2011;286(19):17205–16. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takayama M, Takatsu H, Hamamoto A, Inoue H, Naito T, Nakayama K. et al. The cytoplasmic c-terminal region of the ATP11c variant determines its localization at the polarized plasma membrane. J Cell Sci. 2019;132(17):jcs231720. doi: 10.1242/jcs.231720. [DOI] [PubMed] [Google Scholar]
- 53.Williamson P. Phospholipid scramblases. Lipid Insights. 2015;8(Suppl 1):41–4. doi: 10.4137/LPI.S31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivagnanam U, Palanirajan SK, Gummadi SN. The role of human phospholipid scramblases in apoptosis: An overview. Biochim Biophys Acta Mol Cell Res. 2017;1864(12):2261–71. doi: 10.1016/j.bbamcr.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Bevers EM. Williamson PL. Getting to the outer leaflet: Physiology of phosphatidylserine exposure at the plasma membrane. Physiol Rev. 2016;96(2):605–45. doi: 10.1152/physrev.00020.2015. [DOI] [PubMed] [Google Scholar]
- 56.Sakuragi T, Kosako H, Nagata S. Phosphorylation-mediated activation of mouse xkr8 scramblase for phosphatidylserine exposure. Proc Natl Acad Sci U S A. 2019;116(8):2907–12. doi: 10.1073/pnas.1820499116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L. et al. Cell death modalities: Classification and pathophysiological implications. Cell Death Differ. 2007;14(7):1237–43. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 58.Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: Lessons from the nervous system. Science. 1993;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 59.Shlomovitz I, Speir M, Gerlic M. Flipping the dogma-phosphatidylserine in non-apoptotic cell death. Cell Commun Signal. 2019;17(1):139. doi: 10.1186/s12964-019-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua BA, Ngo JA, Situ K, Morizono K. Roles of phosphatidylserine exposed on the viral envelope and cell membrane in hiv-1 replication. Cell Commun Signal. 2019;17(1):132. doi: 10.1186/s12964-019-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green DR. Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12):a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes SC, James Wilcox CR, Forbes White HS, Vanicek N. The effects of robot assisted gait training on temporal-spatial characteristics of people with spinal cord injuries: A systematic review. J Spinal Cord Med. 2018;41(5):529–43. doi: 10.1080/10790268.2018.1426236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and ced-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341(6144):403–6. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki J, Imanishi E, Nagata S. Exposure of phosphatidylserine by xk-related protein family members during apoptosis. J Biol Chem. 2014;289(44):30257–67. doi: 10.1074/jbc.M114.583419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki J, Imanishi E, Nagata S. Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc Natl Acad Sci U S A. 2016;113(34):9509–14. doi: 10.1073/pnas.1610403113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ten Cate H, Hackeng TM, Garcia de Frutos P. Coagulation factor and protease pathways in thrombosis and cardiovascular disease. Thromb Haemost. 2017;117(7):1265–71. doi: 10.1160/TH17-02-0079. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka KA, Key NS, Levy JH. Blood coagulation: Hemostasis and thrombin regulation. Anesth Analg. 2009;108(5):1433–46. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 68.Hussain JF. Mahaut-Smith MP. Reversible and irreversible intracellular Ca2+ spiking in single isolated human platelets. J Physiol. 1999;514( Pt 3):713–8. doi: 10.1111/j.1469-7793.1999.713ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991;71(1):129–53. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- 70.Srivasatava KR, Majumder R, Kane WH, Quinn-Allen MA, Lentz BR. Phosphatidylserine and Fva regulate Fxa structure. Biochem J. 2014;459(1):229–39. doi: 10.1042/BJ20131099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AK. et al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun. 2013;4:2367. doi: 10.1038/ncomms3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muratori C, Pakhomov AG, Gianulis E, Meads J, Casciola M, Mollica PA. et al. Activation of the phospholipid scramblase TMEM16F by nanosecond pulsed electric fields (NSPEF) facilitates its diverse cytophysiological effects. J Biol Chem. 2017;292(47):19381–91. doi: 10.1074/jbc.M117.803049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagata S, Suzuki J, Segawa K, Fujii T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016;23(6):952–61. doi: 10.1038/cdd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van den Eijnde SM, Van den Hoff MJ, Reutelingsperger CP, Van Heerde WL, Henfling ME, Vermeij-Keers C. et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114(Pt 20):3631–42. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchiya M, Hara Y, Okuda M, Itoh K, Nishioka R, Shiomi A. et al. Cell surface flip-flop of phosphatidylserine is critical for piezo1-mediated myotube formation. Nat Commun. 2018;9(1):2049. doi: 10.1038/s41467-018-04436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA. et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497(7448):263–7. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hochreiter-Hufford AE, Arandjelovic S, Ravichandran KS. Using phosphatidylserine exposure on apoptotic cells to stimulate myoblast fusion. Methods Mol Biol. 2015;1313:141–8. doi: 10.1007/978-1-4939-2703-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim GW, Nam GH, Kim IS, Park SY. Xk-related protein 8 regulates myoblast differentiation and survival. FEBS J. 2017;284(21):3575–88. doi: 10.1111/febs.14261. [DOI] [PubMed] [Google Scholar]
- 79.Van der Leun AM, Thommen DS, Schumacher TN. Cd8(+) T cell states in human cancer: Insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218–32. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C. et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7(8):808–16. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 81.Fischer K, Voelkl S, Berger J, Andreesen R, Pomorski T, Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108(13):4094–101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- 82.Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A. 2011;108(48):19246–51. doi: 10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dayoub AS. Brekken RA. TIMs, TAMs, and PS-antibody targeting: Implications for cancer immunotherapy. Cell Commun Signal. 2020;18(1):29. doi: 10.1186/s12964-020-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the elmo/dock180/rac module. Nature. 2007;450(7168):430–4. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 85.Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V. et al. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci. 2009;122(Pt 18):3365–73. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 86.Schweigert O, Dewitz C, Moller-Hackbarth K, Trad A, Garbers C, Rose-John S. et al. Soluble T cell immunoglobulin and mucin domain (TIM)-1 and -4 generated by a disintegrin and metalloprotease (adam)-10 and -17 bind to phosphatidylserine. Biochim Biophys Acta. 2014;1843(2):275–87. doi: 10.1016/j.bbamcr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 87.Geng K, Kumar S, Kimani SG, Kholodovych V, Kasikara C, Mizuno K. et al. Requirement of gamma-carboxyglutamic acid modification and phosphatidylserine binding for the activation of Tyro3, Axl, and Mertk receptors by growth arrest-specific 6. Front Immunol. 2017;8:1521. doi: 10.3389/fimmu.2017.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML. et al. The opsonin mfg-e8 is a ligand for the alphavbeta5 integrin and triggers dock180-dependent rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292(2):403–16. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D. et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23(6):962–78. doi: 10.1038/cdd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerr D, Tietjen GT, Gong Z, Tajkhorshid E, Adams EJ, Lee KYC. Sensitivity of peripheral membrane proteins to the membrane context: A case study of phosphatidylserine and the tim proteins. Biochim Biophys Acta Biomembr. 2018;1860(10):2126–33. doi: 10.1016/j.bbamem.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park M. Kang KW. Phosphatidylserine receptor-targeting therapies for the treatment of cancer. Arch Pharm Res. 2019;42(7):617–28. doi: 10.1007/s12272-019-01167-4. [DOI] [PubMed] [Google Scholar]
- 92.Park SY. Kim IS. Stabilin receptors: Role as phosphatidylserine receptors. Biomolecules. 2019;9(8):387. doi: 10.3390/biom9080387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou B, McGary CT, Weigel JA, Saxena A, Weigel PH. Purification and molecular identification of the human hyaluronan receptor for endocytosis. Glycobiology. 2003;13(5):339–49. doi: 10.1093/glycob/cwg029. [DOI] [PubMed] [Google Scholar]
- 94.Mills CD. Anatomy of a discovery: M1 and M2 macrophages. Front Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmen M, Kelkka T. et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res. 2014;20(24):6452–64. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 96.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH. et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15(1):192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 97.Batlle E. Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH. et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918–30. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. Tim genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–89. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS. et al. Identification of tapr (an airway hyperreactivity regulatory locus) and the linked TIM gene family. Nat Immunol. 2001;2(12):1109–16. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 101.Li Z, Ju Z, Frieri M. The T-cell immunoglobulin and mucin domain (TIM) gene family in asthma, allergy, and autoimmunity. Allergy Asthma Proc. 2013;34(1):e21–6. doi: 10.2500/aap.2013.34.3646. [DOI] [PubMed] [Google Scholar]
- 102.Evans JP. Liu SL. Multifaceted roles of TIM-family proteins in virus-host interactions. Trends Microbiol. 2020;28(3):224–35. doi: 10.1016/j.tim.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S, Zhu X, Xu Y, Zhang D, Li Y, Tao Y. et al. Programmed cell death-1 (PD-1) and T-cell immunoglobulin mucin-3 (TIM-3) regulate CD4+ T cells to induce type 2 helper T cell (Th2) bias at the maternal-fetal interface. Hum Reprod. 2016;31(4):700–11. doi: 10.1093/humrep/dew019. [DOI] [PubMed] [Google Scholar]
- 104.Corredera E, Phong BL, Moore JA, Kane LP, Lee SE. Tim-3-expressing mast cells are present in chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 2018;159(3):581–6. doi: 10.1177/0194599818774560. [DOI] [PubMed] [Google Scholar]
- 105.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. TIM-1 is essential for induction and maintenance of IL-10 in regulatory b cells and their regulation of tissue inflammation. J Immunol. 2015;194(4):1602–8. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Younan P, Iampietro M, Nishida A, Ramanathan P, Santos RI, Dutta M. et al. Ebola virus binding to TIM-1 on T lymphocytes induces a cytokine storm. mBio. 2017;8(5):e00845–17. doi: 10.1128/mBio.00845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu W, Bai F, Wang H, Liang Y, Du X, Liu C. et al. TIM-4 inhibits NLRP3 inflammasome via the LKB1/AMPKalpha pathway in macrophages. J Immunol. 2019;203(4):990–1000. doi: 10.4049/jimmunol.1900117. [DOI] [PubMed] [Google Scholar]
- 108.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T. et al. Th1-specific cell surface protein TIM-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 109.Sakhdari A, Mujib S, Vali B, Yue FY, MacParland S, Clayton K. et al. TIM-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS One. 2012;7(7):e40146. doi: 10.1371/journal.pone.0040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu R, Li L, Hu J, Wang Y, Tao J, Liu H. et al. Elevated TIM3 expression of T helper cells affects immune system in patients with myelodysplastic syndrome. J Investig Med. 2019;67(8):1125–30. doi: 10.1136/jim-2019-001059. [DOI] [PubMed] [Google Scholar]
- 111.Das M, Zhu C, Kuchroo VK. TIM-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsou WI, Nguyen KQ, Calarese DA, Garforth SJ, Antes AL, Smirnov SV. et al. Receptor tyrosine kinases, Tyro3, Axl, and Mer, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem. 2014;289(37):25750–63. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sadahiro H, Kang KD, Gibson JT, Minata M, Yu H, Shi J. et al. Activation of the receptor tyrosine kinase Axl regulates the immune microenvironment in glioblastoma. Cancer Res. 2018;78(11):3002–13. doi: 10.1158/0008-5472.CAN-17-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abboud-Jarrous G, Priya S, Maimon A, Fischman S, Cohen-Elisha M, Czerninski R. et al. Protein S drives oral squamous cell carcinoma tumorigenicity through regulation of Axl. Oncotarget. 2017;8(8):13986–4002. doi: 10.18632/oncotarget.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178(9):5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 116.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S. et al. The E3 ligase CBL-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–12. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cabezon R, Carrera-Silva EA, Florez-Grau G, Errasti AE, Calderon-Gomez E, Lozano JJ. et al. Mertk as negative regulator of human T cell activation. J Leukoc Biol. 2015;97(4):751–60. doi: 10.1189/jlb.3A0714-334R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang F, Li B, Fu P, Li Q, Zheng H, Lao X. Effects on tumor growth and immunosuppression of a modified talpha1 peptide along with its circular dichroism spectroscopy data. Data Brief. 2018;20:126–31. doi: 10.1016/j.dib.2018.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farhad M, Rolig AS, Redmond WL. The role of galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology. 2018;7(6):e1434467. doi: 10.1080/2162402X.2018.1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rajan R, Sabnani MK, Mavinkurve V, Shmeeda H, Mansouri H, Bonkoungou S. et al. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J Control Release. 2018;271:139–48. doi: 10.1016/j.jconrel.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beatty GL. Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–92. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009;283(2):168–75. doi: 10.1016/j.canlet.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 123.Ran S. Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1479–84. doi: 10.1016/s0360-3016(02)03928-7. [DOI] [PubMed] [Google Scholar]
- 124.Sharma B. Kanwar SS. Phosphatidylserine: A cancer cell targeting biomarker. Semin Cancer Biol. 2018;52(Pt 1):17–25. doi: 10.1016/j.semcancer.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 125.Budhu SaG, Rachel G, Aditi Z, Roberta S, Sara HC, Daniel C, Targeting phosphatidylserine enhances the anti-tumor response to tumor-directed radiation therapy in a preclinical model of melanoma. Cell Rep. 2019. D-19-02869. [DOI] [PMC free article] [PubMed]
- 126.Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and Mertk (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18(1):94. doi: 10.1186/s12943-019-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tse E. Kwong YL. T-cell lymphoma: Microenvironment-related biomarkers. Semin Cancer Biol. 2015;34:46–51. doi: 10.1016/j.semcancer.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14(6):1423–31. doi: 10.1080/21645515.2018.1431598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology. 2006;117(4):433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiu D, Chu X, Hua L, Yang Y, Li K, Han Y. et al. GPR174-deficient regulatory T cells decrease cytokine storm in septic mice. Cell Death Dis. 2019;10(3):233. doi: 10.1038/s41419-019-1462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelleher RJ Jr, Balu-Iyer S, Loyall J, Sacca AJ, Shenoy GN, Peng P. et al. Extracellular vesicles present in human ovarian tumor microenvironments induce a phosphatidylserine-dependent arrest in the T-cell signaling cascade. Cancer Immunol Res. 2015;3(11):1269–78. doi: 10.1158/2326-6066.CIR-15-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oyler-Yaniv J, Oyler-Yaniv A, Shakiba M, Min NK, Chen YH, Cheng SY. et al. Catch and release of cytokines mediated by tumor phosphatidylserine converts transient exposure into long-lived inflammation. Mol Cell. 2017;66(5):635–47. doi: 10.1016/j.molcel.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kawakami T, Takeuchi S, Soma Y. Elevated levels of serum Igm anti-phosphatidylserine-prothrombin complex antibodies in patients with cancer-associated vasculitis. Int J Dermatol. 2017;56(10):e203–4. doi: 10.1111/ijd.13689. [DOI] [PubMed] [Google Scholar]
- 134.Zhang L, Zhou H, Belzile O, Thorpe P, Zhao D. Phosphatidylserine-targeted bimodal liposomal nanoparticles for in vivo imaging of breast cancer in mice. J Control Release. 2014;183:114–23. doi: 10.1016/j.jconrel.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 135.Kannadorai RK, Udumala SK, Sidney YW. Noninvasive in vivo multispectral optoacoustic imaging of apoptosis in triple negative breast cancer using indocyanine green conjugated phosphatidylserine monoclonal antibody. J Biomed Opt. 2016;21(12):126002. doi: 10.1117/1.JBO.21.12.126002. [DOI] [PubMed] [Google Scholar]
- 136.Bae SM, Park SJ, Choi M, Song M, Cho YE, Do EJ. et al. Psp1, a phosphatidylserine-recognizing peptide, is useful for visualizing radiation-induced apoptosis in colorectal cancer in vitro and in vivo. Transl Oncol. 2018;11(4):1044–52. doi: 10.1016/j.tranon.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V. et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–32. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J, Zheng Q, Zhang W, Wang N, Xu J, Cheng X. et al. Lipids in surgical aerosol as diagnosis biomarkers for discrimination of lung cancer. Cancer Manag Res. 2019;11:5537–43. doi: 10.2147/CMAR.S190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou Y. Hancock JF. Deciphering lipid codes: K-RAS as a paradigm. Traffic. 2018;19(3):157–65. doi: 10.1111/tra.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Prakash P, Liang H, Cho KJ, Gorfe AA, Hancock JF. Lipid-sorting specificity encoded in K-RAS membrane anchor regulates signal output. Cell. 2017;168(1-2):239–51. doi: 10.1016/j.cell.2016.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng X, Li L, Thorpe PE, Yopp AC, Brekken RA, Huang X. Antibody-mediated blockade of phosphatidylserine enhances the antitumor effect of sorafenib in hepatocellular carcinomas xenografts. Ann Surg Oncol. 2016;23(Suppl 5):S583–91. doi: 10.1245/s10434-016-5107-5. [DOI] [PubMed] [Google Scholar]
- 142.Yin Y, Huang X, Lynn KD, Thorpe PE. Phosphatidylserine-targeting antibody induces M1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol Res. 2013;1(4):256–68. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 143.Huang X, Ye D, Thorpe PE. Enhancing the potency of a whole-cell breast cancer vaccine in mice with an antibody-IL-2 immunocytokine that targets exposed phosphatidylserine. Vaccine. 2011;29(29-30):4785–93. doi: 10.1016/j.vaccine.2011.04.082. [DOI] [PubMed] [Google Scholar]
- 144.Liang Y, Mafuvadze B, Besch-Williford C, Hyder SM. A combination of p53-activating APR-246 and phosphatidylserine-targeting antibody potently inhibits tumor development in hormone-dependent mutant p53-expressing breast cancer xenografts. Breast Cancer (Dove Med Press) 2018;10:53–67. doi: 10.2147/BCTT.S156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerber DE, Stopeck AT, Wong L, Rosen LS, Thorpe PE, Shan JS. et al. Phase I safety and pharmacokinetic study of bavituximab, a chimeric phosphatidylserine-targeting monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(21):6888–96. doi: 10.1158/1078-0432.CCR-11-1074. [DOI] [PubMed] [Google Scholar]
- 146.Meyer J, Arriaga Y, Anandam J, Karri S, Syed S, Verma U. et al. A phase I clinical trial of the phosphatidylserine-targeting antibody bavituximab in combination with radiation therapy and capecitabine in the preoperative treatment of rectal adenocarcinoma. Am J Clin Oncol. 2018;41(10):972–6. doi: 10.1097/COC.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 147.Chalasani P, Marron M, Roe D, Clarke K, Iannone M, Livingston RB. et al. A phase I clinical trial of bavituximab and paclitaxel in patients with Her2 negative metastatic breast cancer. Cancer Med. 2015;4(7):1051–9. doi: 10.1002/cam4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Digumarti R, Bapsy PP, Shan JS. A phase Ib safety and pharmacokinetic study of bavituximab plus chemotherapy in patients with refractory advanced solid tumor malignancies. J Clin Oncol. 2008;26(Suppl 15):S3038. [Google Scholar]
- 149.Grilley-Olson JE, Weiss J, Ivanova A, Villaruz LC, Moore DT, Stinchcombe TE. et al. Phase Ib study of bavituximab with carboplatin and pemetrexed in chemotherapy-naive advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19(4):e481–7. doi: 10.1016/j.cllc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 150.Digumarti R, Bapsy PP, Suresh AV, Bhattacharyya GS, Dasappa L, Shan JS. et al. Bavituximab plus paclitaxel and carboplatin for the treatment of advanced non-small-cell lung cancer. Lung Cancer. 2014;86(2):231–6. doi: 10.1016/j.lungcan.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 151.Gerber DE, Spigel DR, Giorgadze D, Shtivelband M, Ponomarova OV, Shan JS. et al. Docetaxel combined with bavituximab in previously treated, advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2016;17(3):169–76. doi: 10.1016/j.cllc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Yopp AC, Singal AG, Arriaga YE, Verma UN, Shan J, Kallinteris N. et al. A phase II study of bavituximab and sorafenib in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2015;33(Suppl 3):S345. [Google Scholar]
- 153.Pandya SS, Wong L, Bullock AJ, Grabelsky SA, Shum MK, Shan J. et al. Randomized, open-label, phase II trial of gemcitabine with or without bavituximab in patients with nonresectable stage IV pancreatic adenocarcinoma. J Clin Oncol. 2013;31(Suppl 15):S4054. [Google Scholar]
- 154.Gerber DE, Horn L, Boyer M, Sanborn R, Natale R, Palmero R. et al. Randomized phase III study of docetaxel plus bavituximab in previously treated advanced non-squamous non-small-cell lung cancer. Ann Oncol. 2018;29(7):1548–53. doi: 10.1093/annonc/mdy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun A. Benet LZ. Late-stage failures of monoclonal antibody drugs: A retrospective case study analysis. Pharmacology. 2020;105(3-4):145–63. doi: 10.1159/000505379. [DOI] [PubMed] [Google Scholar]
- 156.Li R, Chiguru S, Li L, Kim D, Velmurugan R, Kim D. et al. Targeting phosphatidylserine with calcium-dependent protein-drug conjugates for the treatment of cancer. Mol Cancer Ther. 2018;17(1):169–82. doi: 10.1158/1535-7163.MCT-17-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guan S, Zhang Q, Bao J, Duan T, Hu R, Czech T. et al. Phosphatidylserine targeting peptide-functionalized ph sensitive mixed micelles for enhanced anti-tumor drug delivery. Eur J Pharm Biopharm. 2020;147:87–101. doi: 10.1016/j.ejpb.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 158.Van Rite BD, Lazrak YA, Pagnon ML, Palwai NR, Neves LF, McFetridge PS. et al. Enzyme prodrug therapy designed to target L-methioninase to the tumor vasculature. Cancer Lett. 2011;301(2):177–84. doi: 10.1016/j.canlet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 159.Blanco VM, Curry R, Qi X. Sapc-dops nanovesicles: A novel targeted agent for the imaging and treatment of glioblastoma. Oncoscience. 2015;2(2):102–10. doi: 10.18632/oncoscience.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wojton J, Chu Z, Mathsyaraja H, Meisen WH, Denton N, Kwon CH. et al. Systemic delivery of Sapc-dops has antiangiogenic and antitumor effects against glioblastoma. Mol Ther. 2013;21(8):1517–25. doi: 10.1038/mt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abu-Baker S, Chu Z, Stevens AM, Li J, Qi X. Cytotoxicity and selectivity in skin cancer by Sapc-dops nanovesicles. J Cancer Ther. 2012;3(4):321–6. doi: 10.4236/jct.2012.34041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blanco VM, Latif T, Chu Z, Qi X. Imaging and therapy of pancreatic cancer with phosphatidylserine-targeted nanovesicles. Transl Oncol. 2015;8(3):196–203. doi: 10.1016/j.tranon.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davis HW, Vallabhapurapu SD, Chu Z, Vallabhapurapu SL, Franco RS, Mierzwa M. et al. Enhanced phosphatidylserine-selective cancer therapy with irradiation and Sapc-dops nanovesicles. Oncotarget. 2019;10(8):856–68. doi: 10.18632/oncotarget.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.N'Guessan KF, Patel PH, Qi X. Sapc-dops-a phosphatidylserine-targeted nanovesicle for selective cancer therapy. Cell Commun Signal. 2020;18(1):6. doi: 10.1186/s12964-019-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De M, Ghosh S, Sen T, Shadab M, Banerjee I, Basu S. et al. A novel therapeutic strategy for cancer using phosphatidylserine targeting stearylamine-bearing cationic liposomes. Mol Ther Nucleic Acids. 2018;10:9–27. doi: 10.1016/j.omtn.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ayesa U, Gray BD, Pak KY, Chong PL. Liposomes containing lipid-soluble Zn(II)-bis-dipicolylamine derivatives show potential to be targeted to phosphatidylserine on the surface of cancer cells. Mol Pharm. 2017;14(1):147–56. doi: 10.1021/acs.molpharmaceut.6b00760. [DOI] [PubMed] [Google Scholar]
- 167.Schuck-Paim C, Taylor RJ, Alonso WJ, Weinberger DM, Simonsen L. Effect of pneumococcal conjugate vaccine introduction on childhood pneumonia mortality in Brazil: A retrospective observational study. Lancet Glob Health. 2019;7(2):e249–56. doi: 10.1016/S2214-109X(18)30455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 169.Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H. et al. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res. 2018;37(1):44. doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Greenberger PA, Saltoun CA, Grammer LC. Northwestern university allergy-immunology syllabus. Allergy Asthma Proc. 2019;40(6):361. doi: 10.2500/aap.2019.40.4246. [DOI] [PubMed] [Google Scholar]
- 171.Murtaza A, Laken H, Da Silva Correia J, McNeeley P, Altobell L, Zhang J. et al. Discovery of TSR-022, a novel, potent anti-human TIM-3 therapeutic antibody. Eur J Cancer. 2016;69:S102. [Google Scholar]
- 172.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29(1):71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 173.Chen X, Song X, Li K, Zhang T. Fcgammar-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. 2019;10:292. doi: 10.3389/fimmu.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang S. et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer. 2018;17(1):20. doi: 10.1186/s12943-018-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rabbani SA, Valentino ML, Arakelian A, Ali S, Boschelli F. SKI-606 (bosutinib) blocks prostate cancer invasion, growth, and metastasis in vitro and in vivo through regulation of genes involved in cancer growth and skeletal metastasis. Mol Cancer Ther. 2010;9(5):1147–57. doi: 10.1158/1535-7163.MCT-09-0962. [DOI] [PubMed] [Google Scholar]
- 176.Hebbard L, Cecena G, Golas J, Sawada J, Ellies LG, Charbono A. et al. Control of mammary tumor differentiation by SKI-606 (bosutinib) Oncogene. 2011;30(3):301–12. doi: 10.1038/onc.2010.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu C, Yu H, Long Q, Chen H, Li Y, Zhao W. et al. Real world experience of crizotinib in 104 patients with ALK rearrangement non-small-cell lung cancer in a single chinese cancer center. Front Oncol. 2019;9:1116. doi: 10.3389/fonc.2019.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mattar MS, Chang J, Benayed R, Halpenny D, Powers A, Kleiner DE. et al. Complete pathological response to crizotinib in a patient with ALK-rearranged lung adenocarcinoma. Clin Lung Cancer. 2020;21(1e):25–9. doi: 10.1016/j.cllc.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R. et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–6. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choueiri TK, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J. et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;25(8):1603–8. doi: 10.1093/annonc/mdu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y. et al. Exosome-transmitted lncarsr promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–68. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 182.Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK, Yoon SJ. et al. Met and Axl inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of Met or Axl activation. Cancer Res. 2014;74(1):253–62. doi: 10.1158/0008-5472.CAN-13-1103. [DOI] [PubMed] [Google Scholar]
- 183.Tang D. Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat Immunol. 2012;13(9):808–10. doi: 10.1038/ni.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Y, Zhao E, Zhang Z, Zhao G, Cao H. Association between TIM3 and Gal9 expression and gastric cancer prognosis. Oncol Rep. 2018;40(4):2115–26. doi: 10.3892/or.2018.6627. [DOI] [PubMed] [Google Scholar]
- 185.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL. et al. Mertk inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest. 2013;123(8):3231–42. doi: 10.1172/JCI67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tormoen GW, Blair TC, Bambina S, Kramer G, Baird J, Rahmani R. et al. Targeting mertk enhances adaptive immune responses after radiation therapy. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peeters MJW, Dulkeviciute D, Draghi A, Ritter C, Rahbech A, Skadborg SK. et al. Mertk acts as a costimulatory receptor on human CD8(+) T cells. Cancer Immunol Res. 2019;7(9):1472–84. doi: 10.1158/2326-6066.CIR-18-0841. [DOI] [PubMed] [Google Scholar]
- 188.Oldenborg PA. CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013;2013:614619. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS. et al. The CD47-signal regulatory protein alpha (SIRPα) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Unanue ER. Perspectives on anti-CD47 antibody treatment for experimental cancer. Proc Natl Acad Sci U S A. 2013;110(27):10886–7. doi: 10.1073/pnas.1308463110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haubelt H, Anders C, Vogt A, Hoerdt P, Seyfert UT, Hellstern P. Variables influencing platelet function analyzer-100 closure times in healthy individuals. Br J Haematol. 2005;130(5):759–67. doi: 10.1111/j.1365-2141.2005.05680.x. [DOI] [PubMed] [Google Scholar]
- 192.Ennishi D, Healy S, Bashashati A, Saberi S, Hother C, Mottok A. et al. TMEM30A loss-of-function mutations drive lymphomagenesis and confer therapeutically exploitable vulnerability in B-cell lymphoma. Nat Med. 2020;26(4):577–88. doi: 10.1038/s41591-020-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N. et al. CD47 blockade by Hu5f9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med. 2018;379(18):1711–21. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tabagari D, Nemsadze G, Janjalia M, Jincharadze M, Shan J. Phase Ⅱ study of bavituximab plus docetaxel in locally advanced or metastatic breast cancer. J Clin Oncol. 2010;28(Suppl 15):S1042. [Google Scholar]
- 195.Bonar EE, Goldstick JE, Collins RL, Cranford JA, Cunningham RM, Chermack ST. et al. Daily associations between cannabis motives and consumption in emerging adults. Drug Alcohol Depend. 2017;178:136–42. doi: 10.1016/j.drugalcdep.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]