Abstract
Chromium-catechin complex was synthesized by reacting [Cr(H2O)6]2+ (hexa-aqua) with catechin as a ligand. Toxicity studies were carried out for the complex using bacterial models for safer application of this complex in the future as a drug. Chromium-catechin complex was characterized using ESI Mass spectrometry, electronic spectroscopy, FT-IR spectroscopy and cyclic voltammetry. The complex was found mildly inhibitory towards B. subtilis with the mode of action being oxidative damage, targeting cell membrane. The complex was supportive towards E. coli, which was evident from the growth profile and inhibition studies. SEM analysis supported the results of membrane integrity studies, where the bacterial liposomes upon treatment with the complex revealed slight morphological changes in the case of B. subtilis, without any change in the case of E. coli. The toxicity studies on chromium-catechin complex using bacterial model saves time, as well as resources by providing quick and reliable results, which could ease up the work to be done in future with higher group of organisms like animal model. Therefore, in the future, this complex can be used as an antidiabetic drug after performing toxicity studies with animal model.
Keywords: Environmental science, Microbiology, Toxicology, Environmental pollution, Environmental toxicology, Chromium-catechin complex, Hexa-aqua, Ecotoxicology, Biomarkers, Metals
Environmental science; Microbiology; Toxicology; Environmental pollution; Environmental toxicology; Chromium-catechin complex; Hexa-aqua; Ecotoxicology; Biomarkers; Metals.
1. Introduction
Chromium, a transition metal occurs in several oxidation states [Cr(II) – Cr(VI)], but the trivalent [Cr(III)] and the hexavalent [Cr(VI)] are the most stable forms [1]. Chromium is used in various industries such as leather processing, metallurgy, power plants, textiles, wood preservation, electroplating, in the manufacture of dyes, paints, jet aircrafts and magnetic tapes [2]. Cr(VI) is highly toxic and carcinogenic, synthesized by the oxidation of Cr(III), which is less toxic found in most of the foods substances and natural supplements. Cr(III) acts as a cofactor in sugar metabolism by interacting with the oligopeptide – LMWCr (Low molecular weight chromium binding substance) [3].
Catechin is a plant polyphenol commonly found in tea, coffee, cocoa beans, etc. Catechin is one representative of vegetable tannin, it plays an important role in stabilizing the skin protein collagen [4]. Catechin is a free radical scavenger with anti-bacterial and anti-inflammatory activity. Since catechin possesses antioxidant activity, the complexing of trivalent chromium with catechin at 1:2 ratio could possibly reduce the genotoxic side effects of Cr(III).
Metal complexes play a vital role in pharmaceuticals for designing drugs which may be a replacement for the commercial antibiotics that suffer the drawback of drug resistance [5]. Metal ions chelated with suitable ligand results in various applications in the field of catalysis, adsorption, storage, magnetism, molecular recognition, fluorescence sensors, etc [6, 7]. In coordination chemistry, enormous work has been done on the metal chromium because of its importance in biological processes, such as DNA damage [8], Plasmid cleavage, protein damage [9, 10]. As Cr(III) is required in trace amount for sugar and lipid metabolism, trivalent chromium has been complexed with natural Picolinic acid [11], Phenylalanine [12] and Glucosaminic acid [13] for treating obesity and type II diabetes. Due to some drawbacks of synthetic Cr(III) complexes such as Cr(Picolinate) which may shift the redox potential of chromium in the complex and generate hydroxyl radicals causing DNA damage, treatment of type II diabetes requires safe dietary supplements in the form of drugs. Therefore, a new complex has been synthesized using Cr(III) and catechin, and the toxicity was assessed first using bacterial models so that a drug for type II diabetes in the future could be developed. In this work chromium-catechin complex was synthesized and characterized by ESI mass spectroscopy, FT-IR spectroscopy and cyclic voltammetry. Further to check whether the chromium-catechin complex possesses any detrimental effect against bacterial models, growth profile and growth inhibition studies were carried out. Generation of Reactive Oxygen Species (ROS) was monitored as Cr is known for producing free radicals. Bacterial membrane damage upon exposure to the complex was studied by the formation of bacterial liposomes and analysis of liposomes before and after treatment with the complex using fluorescence spectroscopy and confocal microscopy was carried out. Production of Extra Cellular Proteins (ECPs) was estimated by the Bradford method and was analyzed by SDS-PAGE.
2. Results and discussion
2.1. Synthesis and characterization of chromium-catechin complex
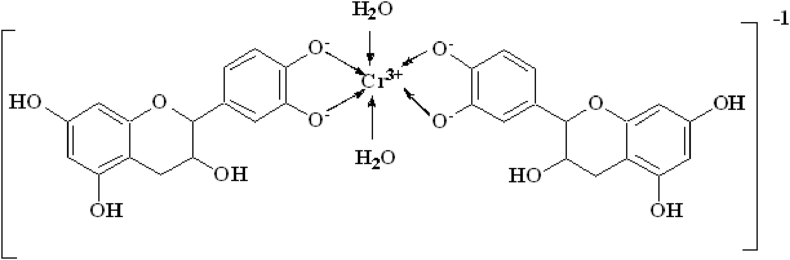
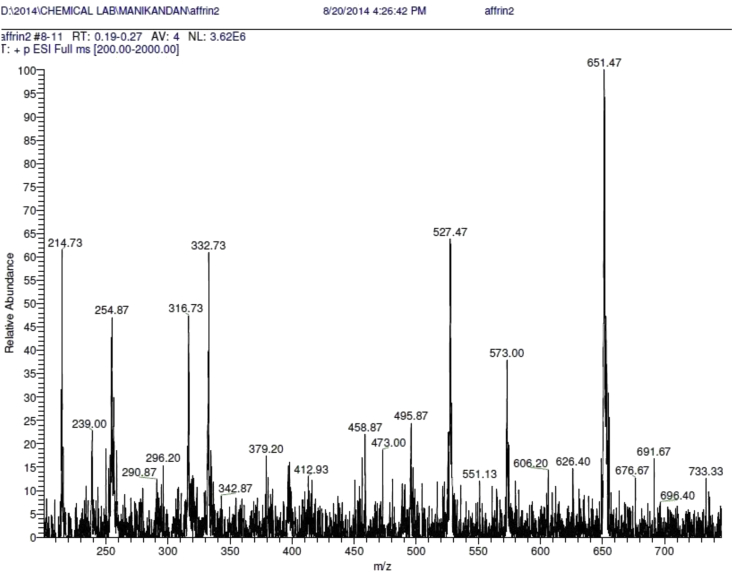
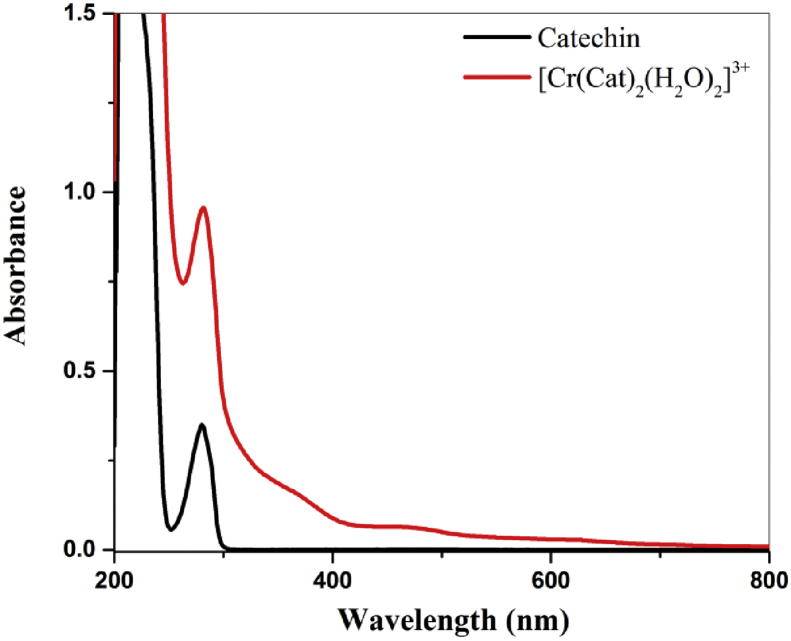
Chromium-catechin complex has been synthesized by reacting 2:1 mol ratio of catechin hydrate with [Cr(H2O)6]2+ solution in nitrogen atmosphere followed by aerial oxidation. The structure of the complex has been shown in Scheme 1. The formation of the complex was confirmed by ESI-MS spectrum of chromium-catechin complex (Figure 1) which shows a base peak at m/z 651 corresponding to the molecular ion ([Cr(catechin)2]− Na+). Two phenol hydroxide protons (Catechol unit) were deprotonated during complexation. UV-VIS analysis for free catechin and complex was carried out in DMSO solvent. The catechin ligand-centred absorbance band appears at 281 nm and other ligand field transitions appear as a shoulder at 371 and 471 nm. Electronic spectra of free catechin had an absorbance peak at 280 nm due to π-π∗transitions. Whereas the complex exhibited multiple absorbance peaks between 200 and 800 nm (Figure 2).
Scheme 1.
Structure of chromium-catechin complex in the present study.
Figure 1.
ESI-MS spectrum of Chromium-catechin complex.
Figure 2.
UV-Visible spectrum of Catechin and Chromium-catechin complex.
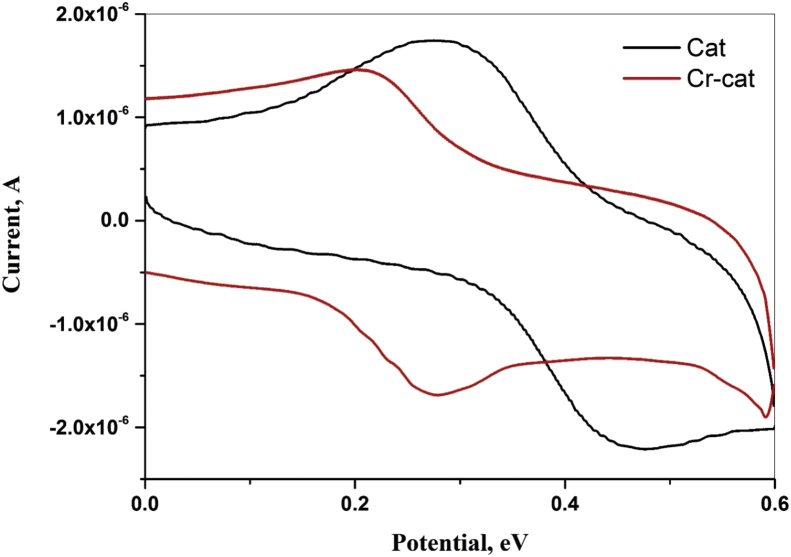
Redox properties of the synthesized complex have been studied using cyclic voltammetry. The cyclic voltammogram of free (+) catechin (Epc 0.477 V and Epa 0.276 V) and chromium-catechin complex (Epc 0.274 V and Epa 0.198 V) shows reversible redox peaks (Figure 3). In the case of complex, the oxidation peak observed was lower than the free catechin clearly suggesting complex formation. Broad IR peak at 3257–3582 cm−1 clearly shows hydrogen bonding interaction, in addition to this free OH groups from the A ring of catechin appear at 3743 cm−1. Perchlorate ion shows a peak at 1096 cm−1 which is not coordinated to the metal centre. The formation of weak bands at 386–426 cm−1 region could be attributed to ν(Cr–O), which confirms complexation.
Figure 3.
Cyclic Voltammogram of free ligand catechin (black) and complex (red) (1 mM) in pH 5.2 acetate buffer.
2.2. Growth profile and inhibition
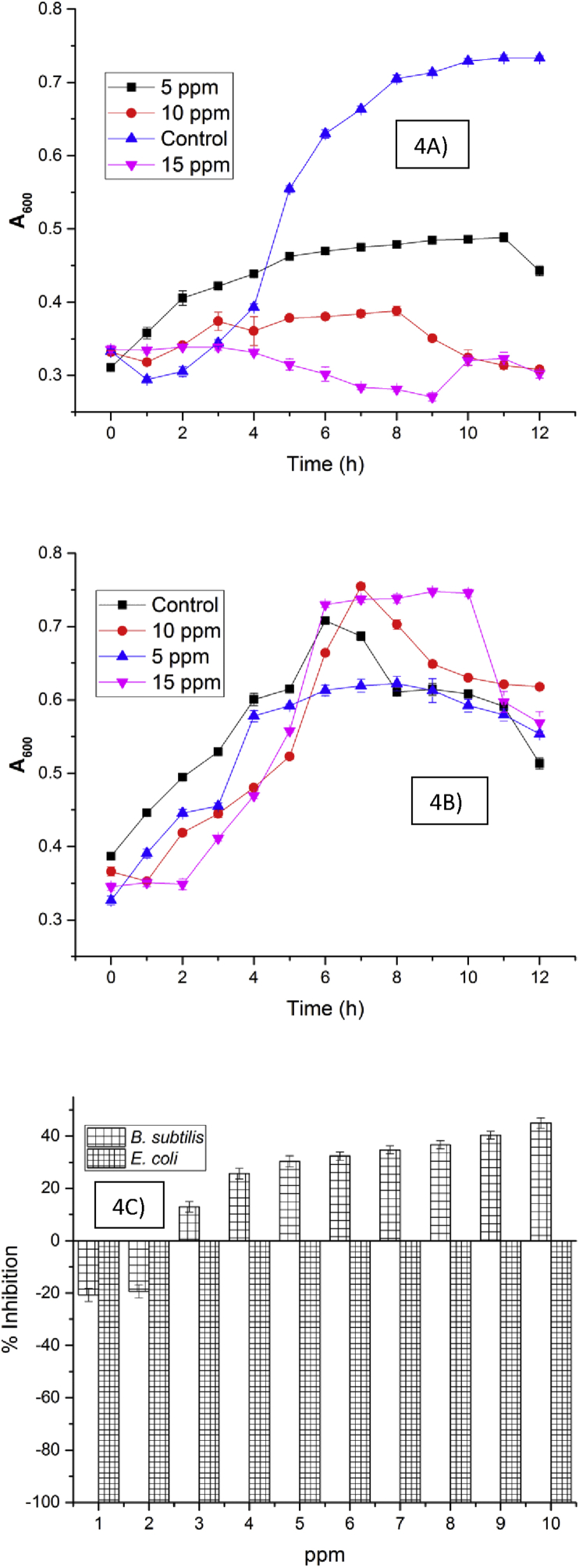
The complex was found to be growth supportive and non-toxic to the Gram negative bacterial, the growth profile of B. subtilis was found to be decreasing with an increase in the concentration of the complex (Figure 4A). The reason for the decrease in the growth profile and growth inhibition of B. subtilis with increasing concentration of the complex was due to the presence of the ligand, catechin which was found to be more toxic to B. subtilis than E. coli [21]. The complex seems to be supportive in the case of E. coli, since catechin being a plant polyphenol it might have acted as an additional nutrient at a specific concentration for promoting the growth of Gram negative bacteria (E. coli). The complex had an inhibitory effect on B. subtilis with an IC50 value of more than 10 ppm (Figure 4C). In the case of E. coli, the complex supported the growth upon an increase in its concentration ranging from 5–15 ppm (Figure 4B). There was no growth inhibition even at 10 ppm suggesting the supportive nature of the complex in the case of E. coli (Figure 4C).
Figure 4.
Growth profile and growth inhibition pattern of Bacillus subtilis and E. coli in the presence and absence of complex (A, B and C).
2.3. Oxidative damage
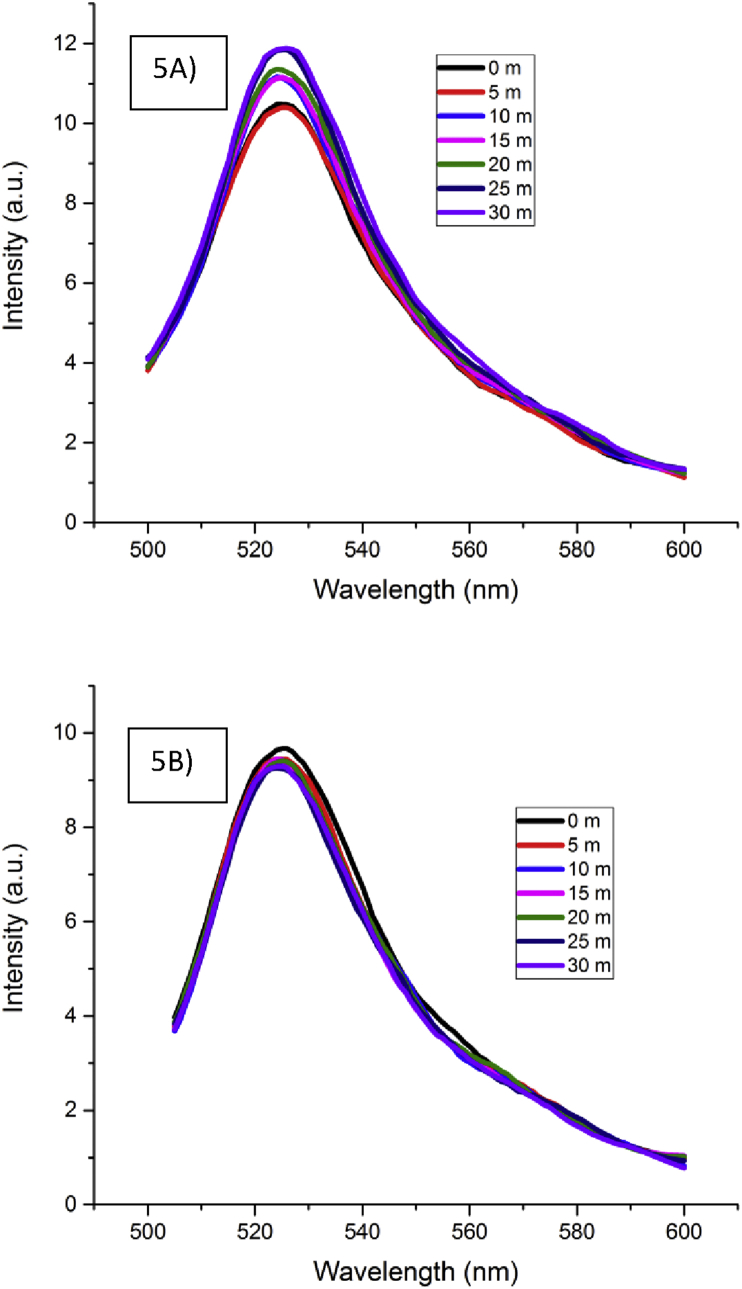
Oxidative damage influenced by the complex was analyzed by measuring the ROS generated upon damage using the probe DCFH-DA. The probe gets converted into a fluorescent product 2, 7 – dichlorofluorescein (DCF) when it encounters the ROS, as a result, this fluorescent product gets trapped inside the cell. The intensity of the product is measured and found to be proportional to the amount of damage. There is an increase in the fluorescence intensity upon increase in time in the case of B. subtilis (Figure 5A), and a decreasing fluorescence pattern could be seen in the case of complex treated E. coli (Figure 5B) suggesting less damage to the cells. An increase in the fluorescence intensity with increasing time, in the case of B. subtilis suggests the chromium-catechin complex to cause more damage. Once again the reason for this could be the presence of catechin as the ligand in the complex in 2:1 ratio, which has higher affinity to the peptidoglycan layer of gram positive bacteria leading to binding and absorption, thereby, causing membrane damage.
Figure 5.
Production of Reactive Oxygen Species (ROS) by complex in Bacillus subtilis and E. coli (A and B).
3. SOD activity
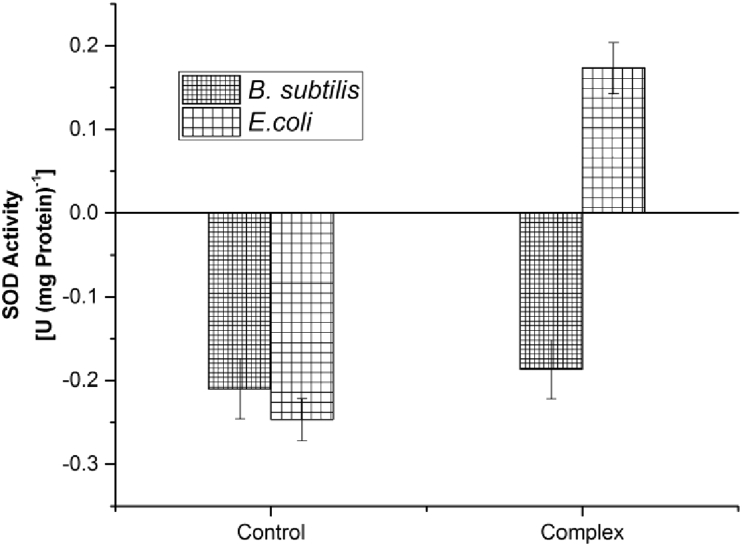
SOD activity in crude protein extracts of treated bacteria was analyzed to check the presence of superoxide dismutase activity upon exposure of bacteria to the chromium- catechin complex, assuming the enzyme to scavenge the ROS generated due to membrane damage. From the results, it becomes clear that in the case of complex treated E. coli cells the SOD activity was present (Figure 6) with a value of 0.2 U (mg protein)-1. The SOD activity was found to be absent with the negative values of -0.27, -0.2 and -0.19 U (mg protein)−1 for untreated E. coli cells, untreated and complex treated B. subtilis cells. Superoxide dismutase activity was monitored in order to understand the reason for damage caused upon the treatment of bacteria with the chromium-catechin complex, as it is known to sequester the ROS generated as a result of oxidative damage [21].
Figure 6.
Determination of SOD activity upon exposure to Chromium-catechin complex.
3.1. Integrity and permeabilization of the bacterial cell membrane
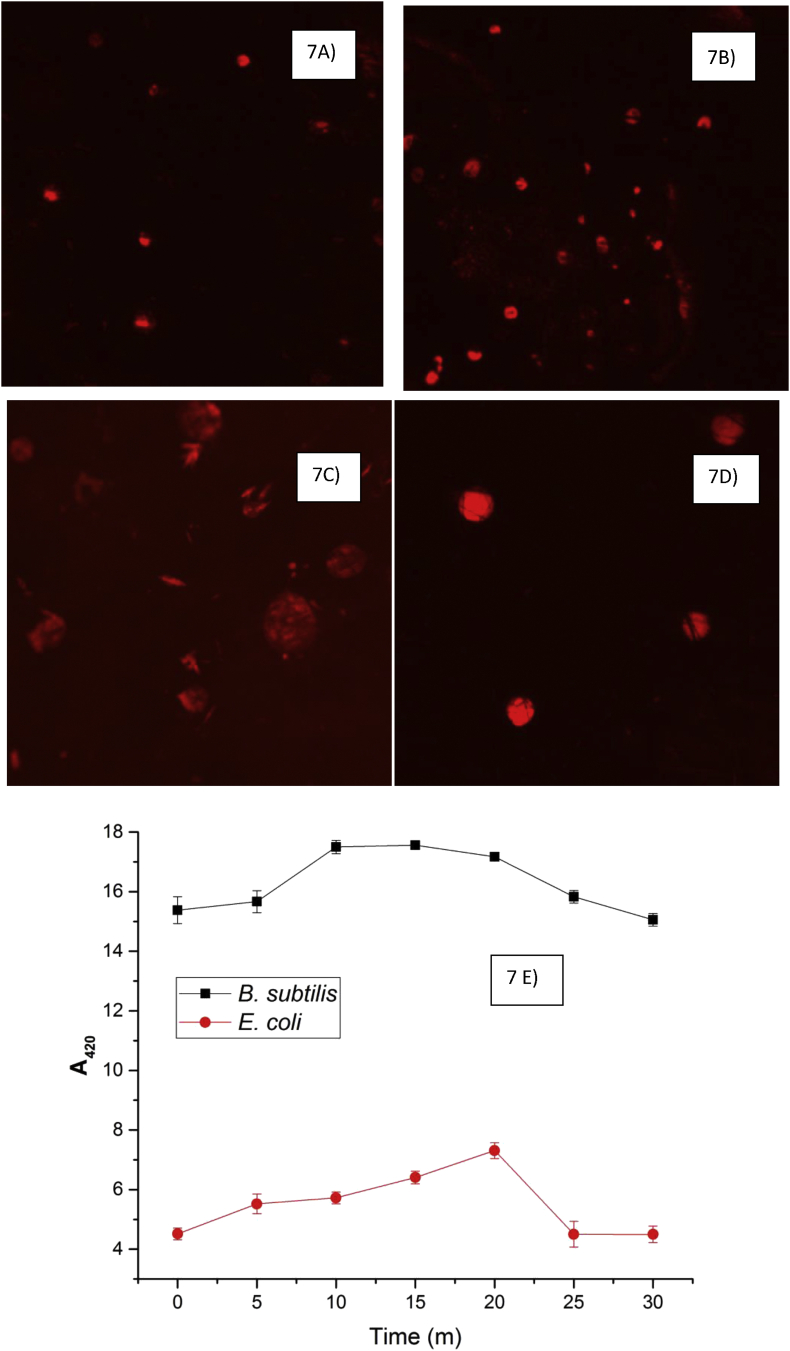
The integrity of the cell membrane and permeabilization was studied by the extraction of membrane lipids followed by liposome formation. The complex treated and untreated liposomes were visualized by confocal microscope in order to monitor changes in morphology. Complex treated liposomes of B. subtilis reveals clear membrane damage when compared with the control liposomes (Figure 7A and B). In the case of E. coli, complex treated liposomes showed less damage with their morphology being unaltered and resembling that of the control liposomes (Figure 7C and D). Further, the fluorescence intensity of the liposomes was recorded to understand the permeabilization mechanism, as the liposomes contain the fluorescent probe NPN, from the results it was found that the complex treated liposomes of B. subtilis showed an increase in the intensity when compared to the fluorescence intensity of treated E. coli liposomes (Figure 7E).
Figure 7.
Membrane integrity studies using optical and confocal microscopy A) Confocal image of untreated liposomes of B. subtilis, B) Confocal image of complex treated liposomes of B. subtilis, C) Confocal image of untreated liposomes of E. coli and D) Confocal image of complex treated liposomes of E. coli and E) Membrane permeabilization studies with 1-N-Phenylnaphthylamine (NPN) by Bacillus subtilis and E. coli after treatment with complex.
Visualization of bacterial liposomes with a confocal microscope reveals the changes in size and shape of the treated liposomes leading to membrane damage. The chromium-catechin complex treated liposomes of E. coli remained intact in size and shape revealing no or very little damage, whereas, the B. subtilis liposomes upon treatment appeared slightly distorted in shape and size suggesting a mild toxic effect of the complex. From the results, it becomes clear that upon treatment of the B. subtilis liposomes with chromium-catechin complex the fluorescence intensity was high due to an increase in penetration, which again was due to membrane damage caused by the complex. In the case of E. coli, the decrease in the fluorescence reveals the low permeabilization potential of the membrane and thereby suggesting no or less damage.
3.2. Extracellular proteins (ECP) – Bradford and SDS-PAGE
Bacterial culture supernatant was analyzed for ECP by the Bradford method. Table 1, shows the concentration of protein secreted by Cr(III), catechin and Complex treated and untreated bacteria. From the results, it becomes clear that the amount of ECP secreted by complex treated bacteria was found to be less than Cr(III) and catechin treated bacteria. The production of these new proteins apart from the control indicates the adaptation strategy of the bacteria towards stressful conditions [20].
Table 1.
Extracellular protein (ECP) produced by Bacillus subtilis and E.coli after treatment with Complex.
| Concentration (ppm) |
B. subtilis | E. coli |
|---|---|---|
| 0 | 3.8 ± 0.26 | 2.5 ± 0.4 |
| 10 | 5.2 ± 0.2 | 3.1 ± 0.13 |
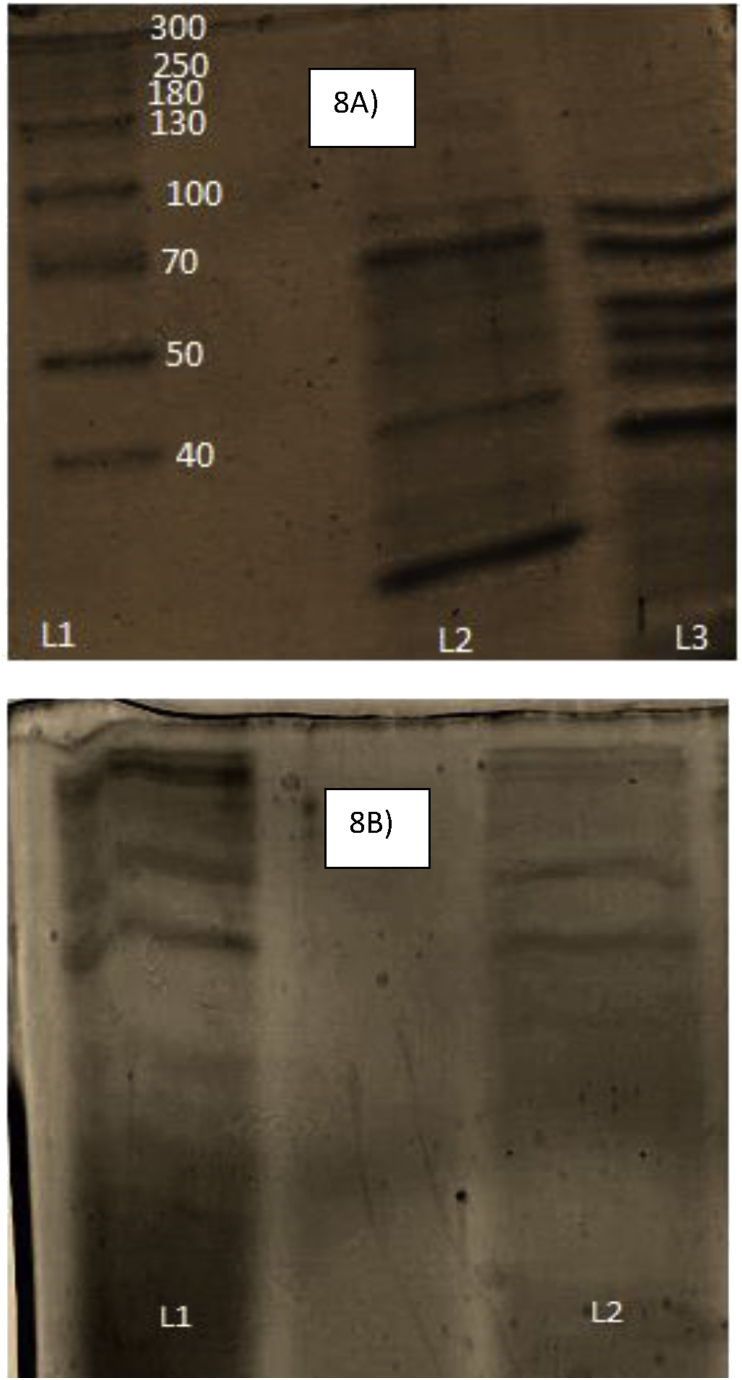
Upon loading of the same culture supernatant of the treated and untreated bacteria, unique protein bands were obtained. Figure 8A, Lane 1 – Marker, Lane 3 – ECP isolated from complex treated B. subtilis and Lane 2 – ECP isolated from untreated B. subtilis. On comparing the banding pattern of complex treated B. subtilis (Figure 8A, Lane 3)and E. coli(Figure 8B, Lane 2) it could be seen that more protein bands were present in the complex treated B. subtilis sample showing damage or stress caused by the complex when compared with E. coli. Lane 3 of Figure 8A, shows protein bands for treated B. subtilis cells revealing the expression of two different proteins seen as thick bands at 30 and 40 kDa. Further few proteins with the molecular weight around 45, 50, 55 and 75 KDa in the control cells were down-regulated in treated cells of B. subtilis. In the case of E. coli, the protein expression pattern of treated and control was identical revealing no damage or stress due to the complex (Figure 8B).
Figure 8.
SDS-PAGE analysis of ECPs isolated from A) B. subtilis – Lane 1 – Marker, Lane 2 – untreated and Lane 3 – complex treated B) E. coli – Lane 1 – untreated and L 2 – complex treated. See supplementary material for full image.
Extracellular protein expression helps in understanding the degree of membrane damage caused by the complex. The proteins which were present in treated B. subtilis cells were not found in the control cells as Polyphenols (catechin ligand in the complex) up-regulate stress proteins related to the defensive mechanism and downregulate metabolic and biosynthetic proteins, revealing a correlation between expression of stress proteins and damage [22].
3.3. Scanning electron microscopy (SEM)
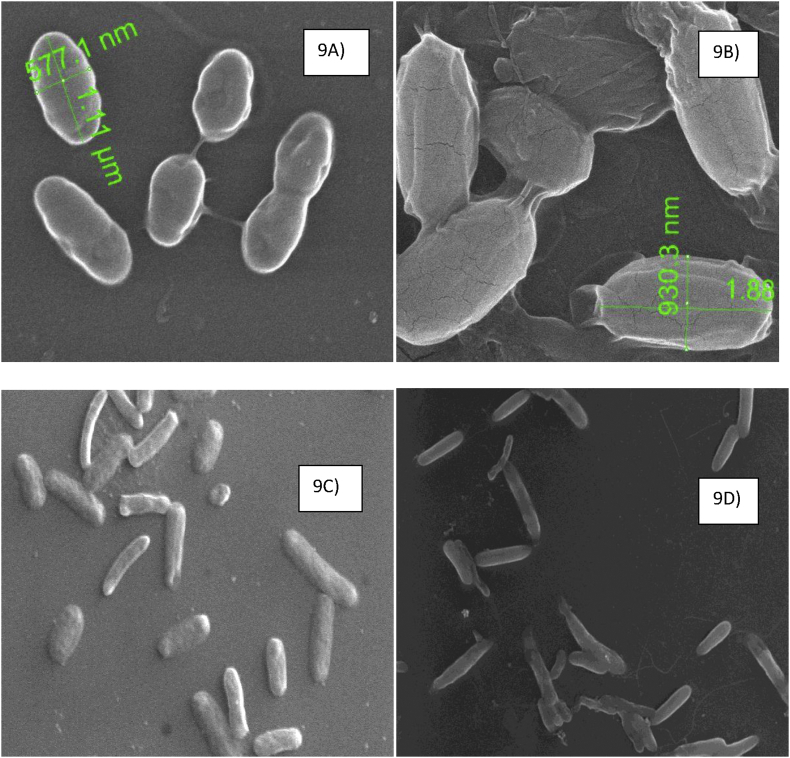
The morphology of the complex treated and untreated bacterial cells were analyzed using SEM. Figure 9A and B shows treated and control cells of B. subtilis, and from the figure, it becomes clear that upon treatment the smooth cell surface of control cells becomes rough with some changes in shape. In the case of E. coli, the control and the treated cells appear more or less similar in morphology suggesting no damage upon treatment (Figure 9C and D).
Figure 9.
SEM image of untreated B. subtilis cells (a), Complex treated B. subtilis cells (b), Untreated E. coli cells (c), Complex treated E. coli cells.
The morphological changes in the control and treated bacteria were observed using SEM analysis. The SEM images clearly revealed slight changes in the morphology of B. subtilis treated cells; also it was found that the morphology of the treated E. coli cells remained unaltered suggesting the complex as nontoxic. As Cr(III) acts as a cofactor in sugar metabolism the synthesized complex tested against bacterial models could be used in future for treating obesity and type II diabetes without any genotoxic complications, as the oxidative stress generated due to Cr(III) would be nullified by catechin present in the complex as a ligand which was tested using bacteria as a model organism.
3.4. Conclusions
Chromium-catechin complex was obtained by reacting [Cr(H2O)6](ClO4)2 and Catechin as ligand. The complex was growth supportive and non-toxic to Gram negative bacteria (E. coli) with a slight inhibitory effect on Gram positive bacteria (B. subtilis). As expected the treatment of bacterial liposomes with the complex showed mild morphological changes on the liposomes of B. subtilis without exerting any change in the case of E. coli.
4. Experimental materials
Gram positive bacterium, Bacillus subtilis 168 was obtained from the Biotechnology department, IITM (Indian Institute of Technology Madras) India and Gram negative bacterial culture E. coli MTCC 40 was procured from MTCC (Microbial type culture collection) Chandigarh, India. Bacterial growth media (Nutrient Agar and Nutrient Broth) was purchased from Hi-media, Mumbai, India. Catechin, Chromium Chloride, 1-N- phenyl naphthylamine (NPN) and 2,7-dichlorofluorescein diacetate (DCFH-DA) was purchased from Sigma Aldrich, India. All other chemicals and reagents were obtained from SD Fine chemicals Ltd, India.
Elemental analysis was performed using a EURO EA 3000–Single CHNS analyzer. ESI mass spectra of the complexes were recorded with Thermofinnigan LCQ-6000 Advantage Max ion trap mass spectrometer equipped with an electron spray source. The IR spectrum of the complexes was recorded using an FT-IR, ABB BOMEMMB 3000 spectrophotometer and the samples were prepared by using the KBr mull sampling technique. The electronic spectrum of a DMSO solution of the complex was taken in a Perkin–Elmer Lambda 35 double beam spectrophotometer. Cyclic voltammetry was performed in a three-electrode cell consisting of a glassy carbon electrode of 3 mm diameter as a working electrode, a platinum wire as a counter electrode, and saturated calomel as a reference electrode. The working electrode, before use, was polished over a micro cloth with alumina particles and washed with deionized water. The cyclic voltammetry experiment was carried out at room temperature and under the N2 atmosphere, using tetrabutylammonium perchlorate solution (100 mM) as a supporting electrolyte in DMSO solvent. CH electrochemical analyzer was used.
4.1. Synthesis of [Cr (H2O)6]2+ complex
A fresh stock solution of [Cr(H2O)6](ClO4)2 has been prepared by reduction of [Cr(H2O)6](ClO4)3 solution using amalgamated zinc as a reducing agent. The Cr2+ concentration measured spectrophotometrically as per the reported method and stored under an oxygen-free nitrogen atmosphere prior to use.
4.2. Synthesis of [Cr (Catechin-2H)2] (ClO4)3 Na
The chromium-catechin complex was synthesized by reacting [Cr(H2O)6](ClO4)2 and its corresponding ligand (catechin) under the nitrogen atmosphere followed by air oxidation. Two equivalent of catechin was dissolved in 10 ml of methanol and purged with nitrogen for 20 min. This has been transferred to the predetermined one equivalent of hexa-aqua chromium (II) solution using cannula strictly under the nitrogen atmosphere. The resulting brown solution was then bubbled with air for 3 h. The resultant orange-yellow compound was filtered off and recrystallized from a mixture of acetone/water (2:1). The recrystallized product was then filtered off, washed with diethyl ether, and dried under vacuum. Ana. Calcd for C30H28CrNaO26 (M/W 985.87): calcd. C 36.55, H 2.86; found C 35.96, H 2.32. ESI-MS: m/z 651 [Cr(Cat-2H)2]− Na+; FTIR 3743 (free OH stretch) 3257–3582 (OH stretch, H-bonded, broad); 3100–3200 (C–H stretch); 1446, 1520, 1615 Aromatic (-C=C- stretch), 1096 (ClO4−)
Caution! Although we did not face any difficulty during our work, perchlorate salts are potentially explosive! Utmost care should be exercised in handling them.
4.3. Bacterial growth profile and inhibition
Growth profile and growth inhibition experiments were carried out using minimal growth medium comprising of NaNO3 – 0.5 g L−1, K2HPO4 – 0.12 g L−1, KH2PO4 – 0.06 g L−1, yeast extract – 0.5 g L−1, and glucose – 5 g L−1 followed by autoclaving at 121 °C at 15 lbs pressure for 20 min. A stock solution of the complex of 1000 ppm was prepared using Dimethyl Sulphoxide (DMSO), sonicated for 10–30 min if any insoluble material appears, this can be stored at room temperature. To understand the growth profile and inhibition of the bacteria in the presence of complex of varying concentration (0, 5, 10 and 15 ppm for growth profile 0–10 ppm for growth inhibition), minimal growth medium with and without complex was inoculated using the overnight culture of the test bacterium B. subtilis and E. coli. The flasks and tubes were incubated at 37 °C for 18–24 h in an incubator shaker incubator. For growth profile studies, the sample was collected every hour and in the case of growth inhibition studies, the absorbance was recorded after 18–24 h of incubation followed by recording absorbance at 600 nm using UV-Visible spectrophotometer (UV – 1800, Shimadzu Spectrophotometer).
4.4. Detection of oxidative damage by estimation of Reactive Oxygen Species (ROS)
The production of ROS upon oxidative damage by complex was studied using the fluorescent probe 2, 7-dichlorofluorescein diacetate (DCFH-DA), a nonfluorescent cell permeable precursor of dichlorofluorescein (DCF). Once ROS were produced the colourless probe (DCFH-DA) gets converted to a fluorescent product 2, 7 – dichlorofluorescein (DCF) inside the cell making it fluorescent [13]. Production of ROS due to oxidative damage caused by complex was studied by harvesting overnight bacterial cells by centrifugation at 10000 rpm for 10 min followed by washing and resuspending in 0.1 M phosphate buffer saline (PBS, pH-7.2). The fluorescent probe DCFH-DA was added to the bacterial cell suspension at a ratio of 1: 2000, mixed well and incubated in a shaker incubator at 37 °C for 30 min. After incubation, the cells were washed twice with 0.1 M PBS to remove the coated DCFH-DA outside the cell. Then the cells were treated with a complex of 10 ppm concentration. An excitation wavelength of 488 nm and an emission wavelength of 535 nm was set to measure the fluorescent intensity using Varian Cary Eclipse fluorescence spectrophotometer.
5. Determination of SOD activity
Overnight bacterial culture (Control and treated) was centrifuged at 10000 rpm for 10 min, followed by careful aspiration of the supernatant. The supernatant was further concentrated using Concentrator (Eppendorf Plus). The concentration of extracellular proteins (ECPs) was estimated by Bradford method [14] to assess the SOD activity inhibition of photochemical reduction of nitro blue tetrazolium (NBT) was measured [15]by using 1 ml of the reaction mixture consisting of 50 mM potassium phosphate buffer (pH 8.5), 0.1 mM EDTA, 0.02 mM riboflavin, 13 mM methionine, 0.6 mM NBT and 45 μg of crude protein extract. The absorbance at 550 nm was recorded after illuminating the samples for 15 min using white light. A SOD unit was defined as the amount of enzyme causing 50 % inhibition of NBT reduction.
5.1. Effect of chromium-catechin complex on cell membrane integrity and permeabilization of bacterial liposomes
Bacterial lipids were extracted to form liposomes in order to study membrane integrity and permeabilization as the major composition of the bacterial cell membrane consists of lipids [17]. For membrane damage NPN treated bacterial liposomes with a complex of 10 ppm concentration was incubated. After incubation, the untreated and complex treated liposomes were observed under a confocal microscope (Nikon Eclipse E600 PDL with Leica EMMP). Outer membrane permeabilization was detected by measuring the fluorescence intensity of the NPN treated bacterial liposomes (control and test). Fluorescence was recorded at an excitation and an emission wavelength of 350 nm and 420 nm using Varian Cary Eclipse fluorescence spectrophotometer.
5.2. Estimation and detection of extracellular proteins (ECP) by Bradford assay and SDS-PAGE
Bacterial ECP's were isolated and studied from 24h old bacterial culture comprising of varying concentrations of complex (5, 10, 15 and 20 ppm). The complex treated and untreated bacterial culture was centrifuged at 10000 rpm for 10 min and the supernatant was carefully aspirated and lyophilized. The ECP's were quantified using the Bradford assay [14]. Expression of extracellular proteins, which was expected to be synthesized by the bacterium under unfavourable conditions was studied by SDS – PAGE using the lyophilized protein samples, electrophoresis was carried out at 180 V on a 12 % (w/v) polyacrylamide gel using a standard protocol [18].
5.3. Scanning electron microscopy (SEM)
Cell membrane integrity of complex treated and untreated bacterial cells was further visualized by SEM. Glass coverslips of 13 mm diameter was placed in each well of six well tissue culture plate. To the growth medium with and without complex of 10 ppm concentration, the overnight bacterial inoculum was added and the plate was incubated at 37 °C for 24 h. After incubation, the supernatant was carefully aspirated and discarded from the wells and the coverslips were carefully removed, washed twice with 0.1 M PBS. Then the coverslips were fixed with 2% glutaraldehyde for 30 min, followed by washing twice with 0.1 M PBS. Graded series of ethanol (60, 70, 80, 90 and 100%) were used to flood the coverslips to dehydrate the cells fixed onto the coverslips [19]. Finally, the coverslips were sputter-coated and examined using HR-SEM – FEI Quanta 200F at SAIF, IIT Madras, India.
Declarations
Author contribution statement
Aafreen Fathima: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Verasundaram Manickavasagar Manikandamathavan: Performed the experiments.
Jonnalagadda Raghava Rao: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Balachandran Unni Nair: Analyzed and interpreted the data.
Funding statement
This work was supported by CSIR who provided senior research fellowship (SRF), Grant No – 31/6(388)/2013-EMR-I to Aafreen Fathima.
Competing interest statement
The authors declare no conflict of interest
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Dr. Aruna Dhathathreyan, Emeritus Scientist, Biophysics department, CLRI for guiding to perform liposome work. CLRI Communication Number: A/2020/INO/CLRI-SRF/1400.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Material
References
- 1.Yadav S., Shukla O.P., Rai U.N. Chromium pollution and bioremediation. Arch. Environ. News. 2005;11:1. [Google Scholar]
- 2.Bai R.S., Abraham T.E. Biosorption of chromium(VI) from aqueous solution by Rhizopus nigricans. Bioresour. Technol. 2001;79:891. doi: 10.1016/s0960-8524(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 3.Cefalu W.T., Hu F.B. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 4.Madhan B., Subramanian V., Rao J.R., Nair B.U., Ramasami T. Stabilization of collagen using plant polyphenol: role of catechin. Int. J. Biol. Macromol. 2005;37:47. doi: 10.1016/j.ijbiomac.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Taylor P.W., Stapleton P.D., Luzio J.P. New ways to treat bacterial infections. Drug Discov. Today. 2002;7:1086. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh A.K., Mitra M., Fathima A., Yadav H., Choudhury A.R., Nair B.U., Ghosh R. Antibacterial and catecholase activities of Co(III) and Ni(II) Schiff base complexes. Polyhedron. 2016;107:1. [Google Scholar]
- 7.Zhang C., Wang W., Duan A., Zeng G., Huang D., Lai C., Tan X., Cheng M., Wang R., Zhou C., Xiong W., Yang Y. Adsorption behavior of engineered carbons and carbon nanomaterials for metal endocrine disruptors: Experiments and theoretical calculation. Chemosphere. 2019;222:184. doi: 10.1016/j.chemosphere.2019.01.128. [DOI] [PubMed] [Google Scholar]
- 8.Morse J.L., Luczak M.W., Zhitkovich A. Chromium(VI) causes interstrand DNA cross-linking in vitro but shows no hypersensitivity in cross-link repair-deficient human cells. Res. Toxicol. 2013;26:1591. doi: 10.1021/tx400293s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrivastava H.Y., Nair B.U. Chromium(III)-mediated structural modification of glycoprotein: impact of the ligand and the oxidants. Biochem. Biophys. Res. Commun. 2001;285:915. doi: 10.1006/bbrc.2001.5258. [DOI] [PubMed] [Google Scholar]
- 10.Mita Y., Ishihara K., Fukuchi Y., Fukuya Y., Yasumoto K. Supplementation with chromium picolinate recovers renal Cr concentration and improves carbohydrate metabolism and renal function in type 2 diabetic mice. Biol. Trace Elem. Res. 2005;105:229. doi: 10.1385/BTER:105:1-3:229. [DOI] [PubMed] [Google Scholar]
- 11.Yang X., Palanichamy K., Ontko A.C., Rao M.N., Fang C.X., Ren J., Sreejayan N.A. A newly synthetic chromium complex – chromium(phenylalanine)3improves insulin responsiveness and reduces whole bodyglucose tolerance. FEBS Lett. 2005;579:1458. doi: 10.1016/j.febslet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Ou S.J., Chen G., Lin Z.H., Bai Z.P., Duan C.H., Mao C.P. Chromium(III) complexes of D-glucosaminic acid and their effect on decreasing blood sugar in vivo. Arch. Pharm. Chem. Life. Sci. 2006;339:527. doi: 10.1002/ardp.200600053. [DOI] [PubMed] [Google Scholar]
- 13.Shoeb M., Singh B.R., Khan J.A., Khan W., Singh B.N., Singh H.B., Naqvi A.H. ROS-dependent anticandidal activity of zinc oxide nanoparticles synthesized by using egg albumen as a biotemplate. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013;4 [Google Scholar]
- 14.Fathima A., Rao J.R. Selective toxicity of Catechin—a natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016;100:6395. doi: 10.1007/s00253-016-7492-x. [DOI] [PubMed] [Google Scholar]
- 15.Bradford M.M. A dye binding assay for protein. Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497. [PubMed] [Google Scholar]
- 18.Karunakaran E., Biggs C.A. Mechanisms of Bacillus cereus biofilm formation: an investigation of the physicochemical characteristics of cell surfaces and extracellular proteins. Appl. Microbiol. Biotechnol. 2011;89:1161. doi: 10.1007/s00253-010-2919-2. [DOI] [PubMed] [Google Scholar]
- 19.Sun J.L., Zhang S.K., Chen X.X., Chen Y.J., Han B.Z. Growth properties of Staphylococcus aureus in biofilm formed on polystyrene plate. Afr. J. Microbiol. Res. 2012;6:3284. [Google Scholar]
- 20.Khan A.L., Ullah I., Hussain J., Kang S.M., Al-Harrasi A., Al-Rawahi A., Lee I.J. Regulations of essential amino acids and proteomics of bacterial endophytes Sphingomonas sp. Lk11 during cadmium uptake. Environ. Toxicol. 2014;31:887. doi: 10.1002/tox.22100. [DOI] [PubMed] [Google Scholar]
- 21.Ladomersky E., Petris M.J. Copper tolerance and virulence in bacteria. Metallomics. 2015;7:957. doi: 10.1039/c4mt00327f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material