Abstract
Background
SARS-CoV-2 enters cells by binding of its spike protein to angiotensin-converting enzyme 2 (ACE2). Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) have been reported to increase ACE2 expression in animal models, and worse outcomes are reported in patients with co-morbidities commonly treated with these agents, leading to controversy during the COVID-19 pandemic over whether these drugs might be helpful or harmful.
Methods
: Animal, in vitro and clinical data relevant to the biology of the renin-angiotensin system (RAS), its interaction with the kallikrein-kinin system (KKS) and SARS-CoV-2, and clinical studies were reviewed.
Findings and Interpretation
SARS-CoV-2 hijacks ACE2to invade and damage cells, downregulating ACE2, reducing its protective effects and exacerbating injurious Ang II effects. However, retrospective observational studies do not show higher risk of infection with ACEI or ARB use. Nevertheless, study of the RAS and KKS in the setting of coronaviral infection may yield therapeutic targets.
Keywords: COVID-19, ACE2, ACE inhibitors, ARBs, SARS-CoV-2, Kallikrein-kinin system, Renin-angiotensin system
1. Introduction
In the unprecedented crisis of the COVID-19 pandemic, we must define the epidemiology, predictors of complications and mortality, and potential modifiable risk factors that might prevent or decrease the severity of the disease. Recently there has been controversy over whether use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) might be harmful in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with cardiovascular disease, hypertension, or diabetes mellitus under treatment with these agents. In contrast, it has been suggested that ARBs could be protective in the setting of SARS-CoV-2 infection.
SARS-CoV-2, the coronavirus causing COVID-19, enters host cells via binding of the virus spike protein to angiotensin-converting enzyme 2 (ACE2). ACEIs or ARBs have been reported to increase expression of ACE2 in animal models [1]. This has led to speculation that use of ACEIs or ARBs might contribute to a higher risk of contracting the infection and worse outcomes of COVID-19 in patients with cardiovascular diseases, hypertension and diabetes [2], as these drugs are commonly used in these conditions. Moreover, these comorbid conditions are increased with age, which is itself also associated with worse outcomes. As we await evidence from and plan clinical studies, it is essential to understand the biology of the renin-angiotensin system (RAS) and its modulation by the SARS-CoV-2 virus.
2. Brief primer on ACE, ACE2 and the renin-angiotensin system (Fig. 1)
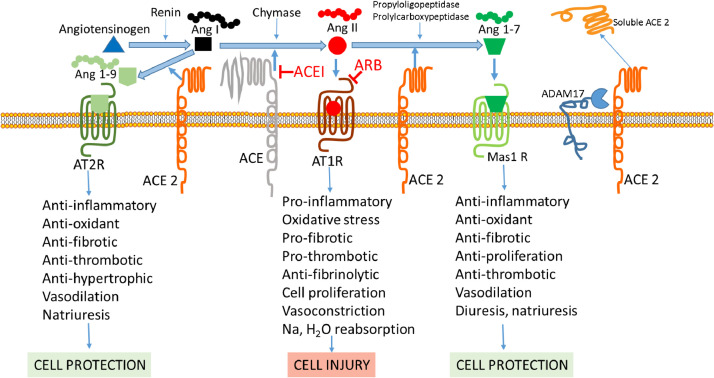
Fig. 1.
ACE and ACE 2 pathways. Renin from the kidneys converts angiotensinogen to Ang I. ACE, and to a lesser extent secreted proteases, such as chymase, catalyzes the conversion of Ang I to Ang II. Ang II binds to the angiotensin II receptor AT1R, leading to vasoconstriction and cellular injury pathways. ACEIs inhibit the conversion of Ang I to Ang II, reducing Ang II production, while ARBs block the Ang II receptor, AT1R. The ACE-Ang II-AT1R pathway is balanced by ACE2, which degrades Ang II and Ang I to produce Ang 1–7 and Ang I to Ang 1–9. Ang1–7 and Ang 1–9 pathways exert protective effects via the receptors Mas1 and AT2R, respectively. Downregulation of ACE2 is associated with an increase in Ang II and activation of the Ang II / AT1R pathway. Upregulation of ACE2 degrades Ang I, limiting the substrate for ACE, degrades Ang II, limiting its adverse effects, and generates Ang 1–7 and Ang 1–9, leading to protective effects. ACE2 is shed from the cell surface by the action of ADAM 17, which is dispensable, but which releases a soluble active form of ACE2 and reduces membrane-bound ACE2. Ang I - angiotensin I. Ang II - angiotensin II. Ang 1–7 - angiotensin 1–7. ACE - angiotensin converting enzyme. ACE 2 - angiotensin converting enzyme 2. AT1 R - angiotensin 1 receptor. AT2 R - angiotensin 2 receptor. Mas1 R - mitochondrial assembly receptor. ACEI - angiotensin converting enzyme inhibitor. ARB - angiotensin 1 receptor blocker.
Angiotensin converting enzyme (ACE) catalyzes the removal of two residues from the decapeptide angiotensin I (Ang I) to form the octapeptide angiotensin II (Ang II). Secreted proteases, including mast cell chymase (human heart chymase), play a minor role in forming Ang II, but are more active when patients are on long-term ACEI therapy. Ang II binding to the type 1 angiotensin II receptor (AT1R) activates pro-inflammatory, vasoconstrictor, pro-oxidant, pro-thrombotic, anti-fibrinolytic, and pro-fibrotic signaling pathways. ACEIs inhibit conversion of Ang I to Ang II, thereby reducing Ang II production, while ARBs block the downstream effects of Ang II by blocking its receptor, AT1R. The functions of ACE are balanced by its homologue ACE2, which catalyzes the removal of one residue from Ang II to form Ang 1–7 and one residue from Ang I to form Ang 1–9. In doing so ACE2 reduces Ang II and its effects, while enabling the Ang1–7 and Ang 1–9 pathways that protect against Ang II effects by promoting vasodilation, as well as anti-inflammatory, anti-oxidant, anti-thrombotic, and anti-fibrotic activity via the receptors Mas1 (a G-protein coupled receptor [GPCR]) and AT2R, respectively [3]. Ang1–7 activated Mas1 receptors trigger downstream signaling pathways, including arachidonic acid release and activation of phospholipase A1, NOS, PI3K/Akt, MAP kinases, RhoA, and cAMP/PKA [4]. The Mas1 receptor also acts as an antagonist of the AT1R, and Ang1–7/Mas1 signaling suppresses Ang II effects, including Ang II-induced reactive oxygen species (ROS) overproduction, apoptosis, phosphorylation of MAPKs and c-Src, leading to TGF-β1 and collagen production, ICAM-1, VCAM-1, and MCP-1 expression [4]. Additionally, if expressed in molar excess to the AT1R, AT2R will directly bind Ang II, leading to its protective activity.
Supporting a complex balance of the ACE/ACE2 pathways in vivo, downregulation of ACE2 is associated with an increase in Ang II and activation of the Ang II / AT1R pathway. However, ACE2 upregulation increases Ang I degradation limiting the substrate for ACE, increases Ang II degradation limiting its adverse effects, and increases the production of Ang 1–7 and Ang 1–9, leading to their protective effects (Fig. 1) [5]. The mechanism of the reported increase of ACE2 expression by ACEIs and ARBs is not well understood, but may be mediated by increased Ang II metabolism by ACE2 and Ang1–7 mediated mitogen-activated (MAP) kinase activity [1]., [6], [7] ACE2 is shed from the cell surface by the action of a disintegrin and metalloproteinase (ADAM) 17, also called tumor necrosis factor-alpha converting enzyme, and ADAM10, which leads to release of a soluble active form of ACE2 and reduced membrane-bound ACE2 [3], [8]; elevated plasma ACE2 activity in heart failure is associated with worse prognosis [9].
3. ACE, ACE2, and Bradykinin (Fig. 2)
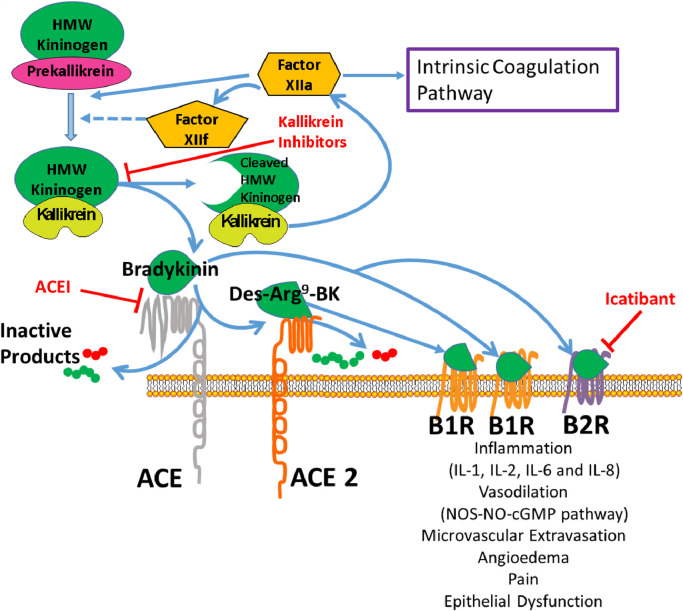
Fig. 2.
Interactions between the renin-angiotensin system and kallikrein-kinin pathways. Factor XII (FXII) autoactivates and then cleaves plasma prekallikrein to activate plasma kallikrein. FXII and plasma kallikrein continue to activate each other, augmenting their activity. Plasma kallikrein cleaves high molecular weight (HMW) kininogen to form bradykinin (BK). The renin-angiotensin system angiotensin converting enzyme (ACE) or kininase enzymes are involved in kinin metabolism. BK is hydrolyzed by ACE into des-Arg9-BK and inactive products. ACE2 breaks down des-Arg9-BK into inactive peptides. BK and des-Arg9-BK interact with two G-protein-coupled bradykinin receptors (B1R and B2R) at the cellular membrane to cause inflammation with release of IL-1, IL-2, IL-6, and IL-8 cytokines; vasodilation via the nitric oxide synthase (NOS), nitric oxide (NO) and cyclic GMP pathway; contraction of non-vascular smooth muscle; increased vascular permeability; and fluid extravasation. Plasma kallikrein inhibitors and B2 receptor antagonists (e.g. icatibant) are two categories of medications used to treat hereditary angioedema, a rare genetic condition.
ACE and ACE2 also have intimate roles with the plasma kallikrein-kinin system (KKS), a hormonal pathway that modulates the intrinsic blood coagulation system, endothelial cell growth and angiogenesis, the complement pathway and RAS. The KKS consists of plasma and tissue kallikreins, plasma high (HK) and low (LK) molecular weight kininogens, their derivative kinin peptides, including bradykinin (BK) and des-Arg9-BK, and two G-protein-coupled bradykinin receptors (B2R and B1R) [10]. Plasma prekallikrein (PK) is activated by blood coagulation factor XII or an endothelial cell serine protease, prolylcarboxypeptidase, to preferentially cleave high molecular HK to liberate BK; the residual cleaved kininogen (cHK) is stable in plasma and may be used as a biomarker of KKS activation [11]. BK binds to its receptor B2R, which is constitutively expressed in the intravascular compartment, and des-Arg9-BK binds to the B1R, which arises in inflammatory states. Separately, tissue kallikreins have preference to cleave LK, releasing Lys-BK, which when acted on by several carboxypeptidases, generates des-Arg9-Lys-BK, which also activates B1R receptor.
The vasodilatory effects of BK are predominantly mediated through B2R, which is abundant in vascular endothelium and constitutively expressed in most tissues. B2R activation causes a cascade involving nitric oxide synthase (NOS), leading to synthesis of nitric oxide (NO) and cGMP [12]. BK and its active metabolite des-Arg9-bradykinin also agonise B1R, which is minimally expressed in healthy tissue, but induced by tissue injury and inflammatory stimuli, playing a role in chronic pain and inflammation [13,14]. Activation of both the B1 and B2 receptors mediate massive vascular permeability and inflammation, causing marked increases in the levels of inflammatory cytokines, such as IL-1, IL-2, IL-6, IL-8, and TNF-alpha that have been implicated in the cytokine storm observed with SARS-CoV-2 ARDS [15,16].
The crosstalk between the RAS and the KKS is profound [17]. Plasma kallikrein converts prorenin to renin. ACE is the major intravascular peptidase of BK, producing des-Arg9-BK and several inactive intermediates, including BK1-5. ACE2 inactivates des-Arg9-BK by cleaving its C-terminal residue, but has no effect on BK [18]. BK receptors are also known to heterodimerise with angiotensin receptors AT1R, AT2R, and Mas that may augment or diminish their activity [19], [20], [21]. Benefits of ACE inhibition can also be attributed to an intracellular signaling cascade that prevents B2R desensitisation. ACEIs inhibit BK and des-Arg9-BK degradation, potentiating their effects [22]. Prolylcarboxypeptidase, an enzyme that also produces Ang 1–7 from Ang II is a PK activator. Finally, in relation to influence of thrombosis risk mediated in part through regulation of vessel wall tissue factor, the B2R, AT2R, and Mas work in concert to counterbalance the prothrombotic influence of the AT1R [23,24].
4. ACE2 in lung injury models
Animal models of acute lung injury, sepsis or inflammation (including acid aspiration, lipopolysaccaride challenge, cecal ligation and perforation sepsis) cause increased lung vascular permeability reminiscent of SARS-induced acute respiratory distress syndrome (ARDS). In these models lung ACE2 is decreased and ACE-dependent Ang II production is increased, implicating loss of ACE2 protective effects. Indeed, chemically induced lung injury leads to severe pathology in Ace2 knockout mice, which is rescued by treatment with the ARB losartan [25]. Dual genetic knockdown of Ace2 and Ace also attenuates lung injury and is associated with decreased Ang II, suggesting that the beneficial effects of ACE2 in this system are mediated through modulation of ACE effects. Over-expression of ACE2 or administration of recombinant catalytically active ACE2 in lung injury models has been associated with partial attenuation of injury indices [26,27]. Animal ARDS models also report increased ACE, high Ang II, decreased ACE2 levels[25] and Ang II/AT1R pathway mediated apoptosis and activation of NF-κB and JAK2/STAT pathways that may be ameliorated by the ARB losartan or ACEI captopril[28]. In ARDS models studying the ACE2/Ang1–7/MasR axis, decrease in lung injury with supplemental Ang1–7 and rhACE2 have also been reported [29]. Ang1–7/MasR reduces apoptosis and cytokine secretion by inhibiting phosphorylation of JNK-NF-κB. Treatment with Compound 21 (C21), an AT2R agonist, also reduced fibrosis, inflammatory cytokines, macrophage infiltration, TNF-alpha and IL-6 in pulmonary hypertension or lung injury models [29]. These studies suggest a protective effect of ACE2 in the lung, as well as an adverse effect of Ang II. While it appears here that accumulation of excessive Ang II is deleterious, Ang II and AT1R also have vital and life-preserving roles, for example in maintaining adequate blood pressure and water-electrolyte balance. AT1R knock-out is lethal, and ACEIs and ARBs are beneficial as they restore more normal RAS homeostasis.
Involvement of the KKS, particularly B1R activity, in pulmonary injury and ARDS has been under study for decades. Components of the system have been found to be activated irrespective of etiology of lung injury [16,30]. Bronchoalveolar lavage (BAL) fluid in patients with ARDS have been found to have increased levels of activated factor XII (FXII), prekallikrein (PK) and high molecular weight kininogen (HK) along with plasminogen and complement proteins [31]. HK activity but not antigen has been found to be significantly reduced in patients with both sepsis and trauma-induced ARDS [30]. In vitro studies indicate that BK stimulates IL-1, IL-2, IL-6 and IL-8 production by lung parenchyma [16]. BK is also known to stimulate Type II pneumocytes to release neutrophil and monocyte chemotactic molecules [32]. A decrease in ACE2 in lung injury would reduce metabolism of des-Arg9-BK, potentially increasing its effect via the B1R to increase vascular permeability and fluid extravasation. B1R antagonism attenuates lipopolysaccharide-induced neutrophil influx in murine models of acute lung injury [33].
5. Lessons from SARS-CoV
SARS-CoV was the coronavirus causing the SARS outbreak in 2003. The highly glycosylated viral spike proteins form club-shaped projections extending from the surface of the virions, giving the defining appearance of the "corona" around all CoVs, including SARS-CoV and SARS-CoV-2, the causative agent of COVID-19. The spike protein is a key determinant for virus attachment and entry into target cells. Animal studies confirm ACE2 as the important receptor for the SARS-CoV spike protein. In Ace2 knockout mice, only a very small amount of virus could be recovered from lung tissue, supporting the importance of ACE2 as the SARS-CoV receptor [34]. Infection of wild type mice with SARS-CoV reduces ACE2 expression [34]. SARS spike protein bound to ACE2 induces shedding of ACE2 with downregulation of ACE2 (Fig. 3) [35]. Intraperitoneal injection of a SARS-CoV Spike-Fc fusion protein into mice with acute acid-induced lung injury worsens acute lung failure that is attenuated by the AT1 receptor blocker losartan [34]. Combining the infection and lung injury studies, the data suggest that both cell surface and released ACE2 catalytic activity producing Ang 1–7 is protective against lung injury. As SARS-CoV binding to ACE2 is associated with shedding and downregulation of ACE2 that may worsen injury, loss of Ang 1–7 protective effects and increased Ang II and des-Arg9-BK as a result of diminished ACE2 activity may also lead to deleterious effects. Injury in these models was attenuated with AT1 receptor blockade. ACE2 expression is lower in rat lung tissues with age [36], kidney tissues in type 2 diabetes with renal disease[37] and post-mortem brain tissue in Alzheimer's disease [38].
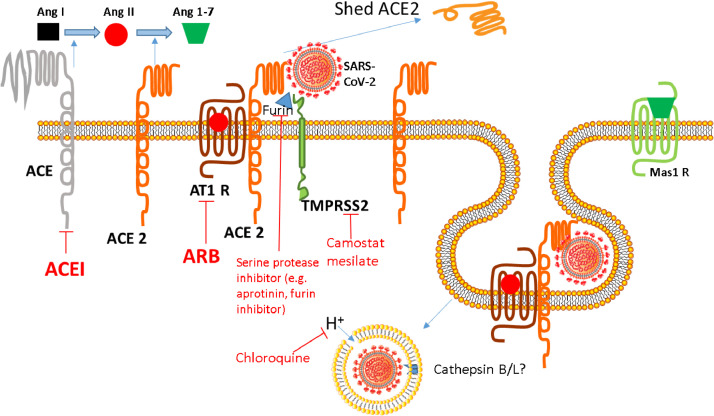
Fig. 3.
SARS-CoV-2 interaction with ACE 2 and TMPRSS 2. The spike protein around SARS-CoV-2 binds to its receptor, ACE2, driving fusion of viral and host cell membranes. Viral entry is also dependent on spike protein priming at its S1/S2 cleavage site (e.g. by furin) and then at its S2′ site by TMPRSS2, a process inhibited by camostat mesilate and serine protease / furin inhibition. Although SARS-CoV-2 fusion is thought to occur in the endosomes of target cells, the requirement of cathepsins B and L for optimal membrane fusion efficiency in vivo remains unclear. Chloroquine increases the pH of lysosomes and is thought to inhibit the activity of proteases that promote membrane fusion and viral release into the cell. Ang I - angiotensin I. Ang II - angiotensin II. Ang 1–7 - angiotensin 1–7. ACE - angiotensin converting enzyme. ACE 2 - angiotensin converting enzyme 2. AT1 R - angiotensin 1 receptor. Mas1 R - mitochondrial assembly receptor. ACEI - angiotensin converting enzyme inhibitor. ARB - angiotensin 1 receptor blocker. TMPRSS2 - transmembrane protein serine protease 2.
6. The SARS-CoV-2 spike protein and ACE2
Given the novel emergence of SARS-CoV-2, studies on cellular and animal models are just emerging. Similar to SARS-CoV, the receptor for SARS CoV-2 is ACE2. The early availability of sequence information of virus isolates facilitated structural studies confirming the binding of SARS CoV-2 spike protein to ACE2. The SARS-CoV-2 spike protein has significant structural homology to the spike protein of SARS-CoV. Both spike proteins bind to ACE2, but SARS-CoV-2 spike protein has been reported to bind with tighter affinity than SARS-CoV [39], so the lessons from SARS-CoV are expected to apply to SARS-CoV-2, perhaps to an even higher degree. The contribution of the enhanced binding to ACE2 to the infectivity of SARS-CoV-2 is not well understood, and binding affinity may reflect genetic variation in ACE2, but the distribution of ACE2 in lung alveolar cells, mouth, intestines, heart, endothelium, kidneys, testes, and brain may explain effects on lung injury, gastrointestinal symptoms, cardiac damage, acute kidney injury, and reports of late potentially neurally mediated cardiorespiratory depression (Fig. 4). Like SARS-CoV, SARS-CoV-2 spike protein requires priming by the serine protease TMPRSS2 for optimal cell entry [40]. Lung and intestines show ACE2 and TMPRSS2 expression and are primary sites of viral entry. The heart shows high levels of ACE2, but low levels of TMPRSS2 expression, which calls into question the mechanism of injury and myocarditis observed in severe cases of COVID-19. However, a polybasic furin cleavage site has been recently identified in the SARS-CoV-2 spike protein [41]; furin-like proteases that may contribute to SARS-CoV-2 spike protein processing are more ubiquitously expressed and may explain an expanded cell and tissue tropism of SARS-CoV-2 compared to SARS-CoV, which lacks this site [41], [42], [43].
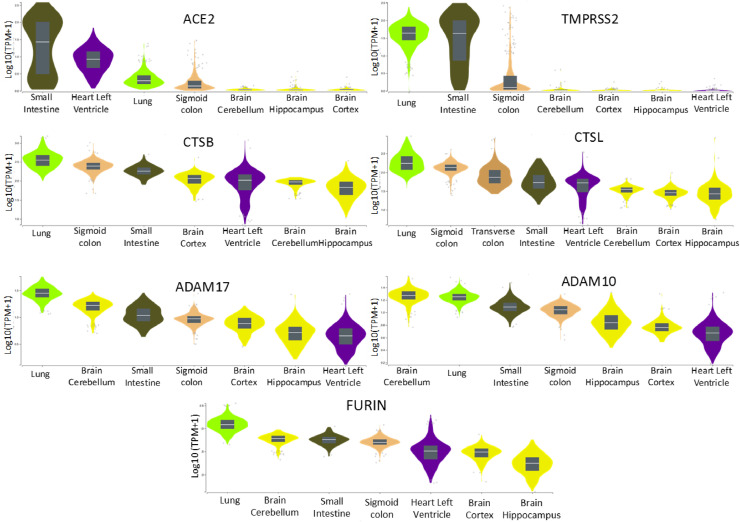
Fig. 4.
Gene expression by tissue of potential genes encoding proteins that may interact with SARS-CoV from GTEx. The lung and intestines express high levels of ACE2 and TMPRSS2, whereas the heart left ventricle expresses ACE2 at high levels, but TMPRSS2 at low levels. Brain tissues show low level expression of ACE2 and TMPRSS2. CTSB and CTSL (encoding cathepsins B and L, respectively), ADAM17, ADAM10, and FURIN show expression in all tissues shown. Obtained from the Genotype-Tissue Expression (GTEx) Portal, accessed on 04/06/2020 and 05/06/2020.
We now know that SARS-CoV-2 cell entry involves two spike protein subunits, which mediate distinct functions. The S1 subunit mediates ACE2 attachment through the receptor binding domain, whereas the S2 subunit, containing the fusion peptide and transmembrane domains, drives fusion of viral and host cell membranes. In addition to attachment, viral entry is determined by spike protein cleavage at two proteolytic cleavage sites, termed S1/S2 and S2′ subunits. Unlike SARS-CoV, the S1/S2 site of the SARS-CoV-2 spike protein is processed by the cellular protease furin [44]. Subsequently, processing of the S2’ site by the cellular serine protease TMPRSS2 (transmembrane protein serine protease 2) occurs, and both furin and TMPRSS2 are required for entry into human lung cells [45]. Spike protein priming by TMPRSS2 was also shown to be essential for spread of SARS-CoV in infected mouse models [46], [47], [48]. Although SARS-CoV-2 fusion is thought to occur in the endosomes of target cells, the requirement of cathepsins B and L in acidic lysosomes for optimal membrane fusion efficiency in vivo remains unclear (Fig. 3) [40]. SARS-CoV-2 induced shedding of ACE2 may also reduce its ability to metabolise des-Arg-BK, which could contribute to pulmonary inflammation, vascular permeability, and cytokine generation via B1R, which is increased in the setting of tissue injury.
7. ACE2, platelets and thrombosis
Thrombotic disorders, including MI and stroke, are common features in patients with SARS-CoV-2 infection [49]. SARS-CoV-2 virus has been found in endothelium and leads to vessel apoptosis, a risk factor for thrombosis [50]. The coagulopathy associated with COVID-19 is like disseminated intravascular coagulation (DIC) with elevated d-dimer, but high fibrinogens and, in the majority of the patients in the USA, lacking strict criteria for DIC. The cross-talk between the KKS and coagulation system via the activation of Factor XII by kallikrein may contribute to the pro-coagulant state. Kallkrein has also been shown to stimulate activation of the complement system through C3 activation, which likely contributes to the associated coagulopathy [51]. In experimental models of thrombosis, ACE2 expression was detected in thrombus extract raising the possibility that ACE2 may play a role in the regulation of both thrombotic and hemostatic functions of circulating platelets [52]. Activation of the ACE2/Ang1–7/Mas pathway and/or reduction of Ang II by use of an ACE2 activator (XNT) demonstrated antithrombotic activity in an animal model [52]. SARS-CoV-2 replicates in lung tissue, and the lung is a major site for extra-medullary thrombopoiesis [53]. Single-stranded RNA (SSRNA) viruses, including influenza, were recently demonstrated to augment platelet activation and platelet-to-leukocyte recruitment through the platelet toll-like receptor 7 (TLR7) [54]. Since SARS-CoV-2, like influenza, is also a SSRNA virus, the possibility exists that SARS-CoV-2 may promote dysregulated platelet activity directly through surface receptor-mediated pathways or indirectly by secreting platelet-derived molecules that regulate the coagulation cascade. Lastly, angiotensin receptors are expressed on the surface of platelets, and medications inhibiting the RAS attenuate platelet activation [55,56]. Therefore, the impact of anti-platelet medications and ACEIs/ARBs on platelet function and thrombotic events in patients with SARS-CoV-2 needs further investigation.
8. ACEIs and ARBs
Helpful or harmful? Though ARBs and ACEIs may be associated with an increase in ACE2 expression, which theoretically may enhance viral infection, their inhibition of the RAS with increase in ACE2 expression, reduction of Ang II or Ang II effects, and increase in Ang1–7 and Ang 1–9 may have protective effects. ARBs may increase Ang II by competing with binding to AT1R, but this may create more available substrate for ACE2 and formation of Ang1–7 with its downstream protective effects. Binding of substrates to ACE2 may induce conformational changes in ACE2; it is unknown whether these interactions would enable or reduce SARS-CoV-2 spike protein binding. Specific effects of ACEIs and ARBs on this process are not known. Genetic factors, including genetic variability in ACE2 polymorphisms, may also determine functional roles of ACE2 for ACEIs and ARBs, as well as in its interaction with the CoV-2 spike protein, and will be important to dissect in the future [57]. Finally, ACEI could reduce metabolism of BK, leading to B1R- and B2R-mediated inflammatory, vasodilatory, vascular permeability and fluid extravasation effects.
9. Clinical studies
Initial clinical data showing conflicting outcomes in COVID-19 associated with ARB or ACEI use were confounded by lack of adjustment for co-morbidities (Table). In a study of 187 COVID-19 patients from Wuhan, China, ACEI or ARB use was higher in patients with myocardial injury and elevated troponin T (TnT) levels (21•1%) compared to patients with normal TnT (5•9%), p = 0•002 [58]. In 42 patients with COVID-19 on antihypertensive therapy, severe disease was observed in 23•5% on and 48% not on an ACEI or ARB, but this was not significant due to the small sample size [59]. A pre-publication report of 78 COVID-19 + patients with hypertension reported ARB use (n = 10) was associated with lower occurrence of severe disease (OR 0•343, 95% CI 0•128–0•916, p = 0•025). In a study of 362 hospitalized COVID-19 patients with hypertension, ACEI and/or ARB use (n = 115) was not significantly different between patients with severe vs. non-severe illness or in non-survivors vs survivors [60].
Table.
Studies of ACEIs and/or ARBs in COVID-19 patients.
| Study | Population | N | Design | Outcome | P value |
|---|---|---|---|---|---|
| Guo, et al.[58] | COVID-19+ (Wuhan, China) |
187 | Retrospective; unadjusted | TnT on ACEI/ARB Normal 8/135 (5•9%) Elevated 11/52 (21•1%) Mortality ACEI/ARB + 7/19 (36•8%) - 43/168 (25•6%) |
0•002 |
| Meng, et al.[59] | COVID-19+ on antihypertensive therapy (Shenzhen, China) |
42 | Retrospective; unadjusted | Severe disease ACEI/ARB + 4/17 (23•5%) - 12/25 (48%) |
NS |
| Liu, et al.[74]* | COVID-19+ hypertension (Shenshen, Wuhan, Beijing, China) |
78 HTN 10 ARB 2 ACEI |
Meta-analysis unadjusted |
Severe disease (Whole cohort NS) age>65 (N = 46) ARB OR 0•343 95% CI 0•128–0•916 ACEI 0•571 0•139–2•342 |
0•025 0•378 |
| Li, et al.[60] | COVID-19+ hypertension Hospitalized (Wuhan, China) |
362 115 ACEI/ARB |
Retrospective, unadjusted | Severe illness Non-severe illness ACEI use 9•2% 10•1% ARB use 24•9% 21•2% ACEI/ARB use 32•9% 30•7% Non-survivors Survivors ACEI use 9•1% 9•8% ARB use 19•5% 23•9% ACEI/ARB use 27•3% 33•0% No differences in ACEI/ARB use outcomes in patients with HTN and comorbidities |
0•80 0•40 0•65 0•85 0•42 0•34 |
| Bean, et al.[61] | UK Acute Hospital Trust acute inpatients (London, UK) |
399 | Retrospective; adjusted | Death or transfer to critical care unit for organ support within 21 days of symptom onset (n = 127) ACEI/ARB adjusted OR 0•63 95% CI 0•47–0•84# |
<0•01 |
| Rentsch, et al.[62]* | Veterans Administration Birth Cohort; veterans (USA) | 3789 tested 585 COVID+ 40•5% ACEI/ARB |
Retrospective cohort study; adjusted | Multivariable OR (95% CI) ARB/ACEI COVID+ test 0•98 (0•41–0•83) hospitalization 1•15 (0•71–1•87) ICU 1•66 (0•94–2•93) |
NS NS NS |
| Zhang, et al.[75] | COVID-19+ hypertension (Hubei, China) |
1128 HTN 188 ACEI/ARB (31 ACEI, 157 ARB) |
Retrospective, adjusted and propensity score | Propensity score matched (1:2) ACEI/ARB (n = 174) vs non-ACEI/ARB (n = 522) HR 95% CI All-cause mortality 0•37 0•15–0•89 Septic shock 0•32 0•13–0•80 ARDS 0•65 0•41–1•04 AKI 0•78 0•37–1•65 Acute heart injury 0•76 0•44–1•32 |
0•03 0•01 0•07 0•52 0•33 |
| Mehta, et al.[63] | Patients undergoing testing for COVID-19 (Ohio, Florida) |
18,472 tested ACEI/ARB 2285 ACEI 1322 ARB 982 1735 positive ACEI 116 ARB 98 |
Retrospective cohort study; overlap propensity score weighted mean or proportion | SARS-CoV-2 test positivity: OR 95% CI ACEI 0•89 0•72–1•10 ARB 1•09 0•87–1•37 ACEI or ARB 0•97 0•81–1•15 Test positive patients ACEI ARB ACEI or ARB Admitted to hospital 1•84 (1•22–2•79) 1•61 (1•04–2•50) 1•93 (1•38–2•71) Admitted to ICU 1•77 (1•07–2•92) 1•16 (0•67–2•02) 1•64 (1•07–2•51) Mechanical ventilation 1•35 (0•74–2•47) 1•12 (0•59–2•12) 1•32 (0•80–2•18) |
|
| Mancia, et al.[64] | Patients tested for SARS-CoV-2 vs Regional Health Service controls (Italy) | 6272 SARS-CoV-2+ 30,759 controls Age ≥40 yrs |
Population-based case-control, conditional logistic regression multivariate analysis | Adjusted OR, 95% CI cases vs. matched controls ARB ACEI Covid-19+ 0•95 (0•86–1•05) 0•96 (0•87–1•07) Mild to moderate disease 0•96 (0•87–1•07) 0•97 (0•88–1•07) Critical or fatal disease 0•83 (0•63–1•10) 0•91 (0•69–1•21) No significant differences in COVID-19 positivity or severity of disease |
|
| Reynolds, et al.[65] | Patients tested for Covid-19 (New York City) |
12,594 tested 5894 positive ACEI 1044 ARB 1328 |
Propensity score matched, Bayesian methods | All Matched patients Drug Covid-19 test positivity + - Median difference% (95% CI) ACEI 60•1% 62•5% −2•5 (−6•7 - 1•6) ARB 58•4% 56•2% 2•2 (−1•9 - 6•3) ACEI/ARB 58•1% 57•7% −0•5 (−2•6 - 3•6) Severe Covid-19 ACEI 23•9% 25•9% 1•9 (−6•6 - 2•8) ARB 24•4% 25•8% −1•4 (−6•1 - 3•3) ACEI/ARB 24•8% 24•9% −0•1 (−3•7 - 3•5) No differences in test positivity or severe disease with ARB and/or ACEI use Similar findings in matched patients with hypertension |
|
| Gao, et al.[66] | Patients admitted with COVID-19 (Wuhan, China) | 2877 850 HTN RAASi 183 Non RAASi 527 |
Propensity score adjusted | Mortality adjusted HR 95% CI HTN 3•45 1•39–8•55 RAASi 0•93 0•31–2•84 RAASi – Severity of disease, mechanical ventilation - NS |
0•008 0•901 |
| Fosbal, et al.[67] | COVID-19 positive patients Danish national administrative registries |
4480 with COVID-19 895 ACEI/ARB users 3585 nonusers |
Cox regression model with nested case-control framework | ACEI/ARB users Nonusers Fully adjusted HR (95% CI) 30-day mortality 20.2% 8.3% 0.83 (0.67–1.03) Death or severe disease 32.6% 14.7% 1.04 (0.89–1.23) Severe disease 22.6% 10.4% 1.15 (0.95–1.41) Nested case control with HTN COVID-19 Controls Adjusted HR (95%CI) ACEI/ARB use 86.5% 85.4% 1.05 (0.08–136) |
0.09 0.61 0.15 0.67 |
ACEI = angiotensin converting enzyme inhibitor; AKI = acute kidney injury; ARB = angiotensin receptor blocker; ARDS = acute respiratory distress syndrome; HTN = hypertension; ICU = intensive care unit; OR = odds ratio; CI = confidence interval; NS = not significant; RAASi = renin-angiotensin-aldosterone system inhibitor.
non-peer reviewed pre-print.
Adjusted for age, sex, hypertension, diabetes mellitus, chronic kidney disease, ischaemic heart disease, heart failure.
In a report of 399 acute inpatients from the UK Acute Hospital Trust, 53 died or were transferred to a critical care unit within 21 days of symptom onset. A lower rate of this endpoint occurred in patients on an ACEI or ARB (OR 0•63, CI 0•47–0•84, p < 0•01), adjusting for age, gender, hypertension, diabetes mellitus, chronic kidney disease, ischaemic heart disease and heart failure [61]. In a preprint from the Veterans Administration Birth Cohort, 585 patients tested positive for COVID-19 among 3789 tested, and 40•5% were on an ACEI or ARB; in adjusted analyses there were no differences in COVID-19 test positivity, hospitalisation, or intensive care in patients receiving or not receiving an ACEI or ARB [62].
Recently 6 studies using adjusted and/or propensity-score adjusted or matched analyses have been published (Table). Three tested associations of ACEI and/or ARB use on COVID-19 test positivity and found no significant differences. Disease severity and hospital outcomes were assessed in 4 studies. In a study of 1128 patients with hypertension, 188 were on an ACEI or ARB; propensity score matched cohorts showed a lower risk of all-cause mortality (HR 0•37, 95% CI 0•15–0•89, p = 0•03) and septic shock in patients on an ACEI or ARB. In a study of 18,472 patients tested, 1735 were positive; ACEI and/or ARB use was associated with a higher rate of hospital admission with ACEI and ACEI or ARB use was associated with a higher risk of admission to the ICU, but with no significant difference in need for mechanical ventilation [63]. In a study of 6272 COVID-19 positive patients, ARB or ACEI use was not associated with degree of disease [64]. Another study of 12,594 tested patients, including 5894 who tested positive, ACEI, ARB, or either were not associated with test positivity or severe COVID-19 disease [65]. In a study of 2877 hospitalized patients with COVID-19, 850 had hypertension of which 183 were treated with renin-angiotensin-aldosterone system inhibitors (RAASi) and 527 were not; RAASi use was not associated with severity of disease or mortality [66]. Lastly, in a study of 4480 patients with COVID-19 in Danish national administrative registries, prior ACEI or ARB use was not associated with death or severe COVID-19; in a nested case-control study of patients with hypertension, ACEI/ARB use was not significantly associated with COVID-19 diagnosis [67]. Taken together, it is now consistent clinical evidence that ACEI or ARB use does not appear to predispose to SARS-CoV-2 infection, which was the main concern raised due to postulated effects of ACEIs or ARBs in raising ACE2 expression. Recent studies have shown no association of ACEI or ARB use with SARS-CoV-2 test positivity. Studies do not indicate harm from ACEI or ARB use in terms of severity of disease but with some conflicting results regarding the benefit vs. risk of ACEIs. However, the overall the balance appears to be in favor of no significant harm from ACEI or ARB use in COVID-19, though larger studies are needed to assess the relative effects of ACEIs versus ARBs, whether continuation or withdrawal of these agents impact outcomes, or if ACEI/ARB use may actually be beneficial in alleviating lung or other organ injury in patients with COVID-19.
10. Recommendations regarding ACEIs and ARBs
Given the current reassuring data showing no significant association of ACEI or ARB use with test positivity, lack of consistent or convincing evidence as to the risk or benefit of an ACEI or ARB, as well as the potential harm that may occur with withdrawing of ACEIs or ARBs in patients with cardiovascular and other diseases [68], findings support the European Society of Cardiology, American Heart Association, American College of Cardiology, and the Heart Failure Society of America recommendations that patients on these therapies should be continued as clinically indicated. The recent data show consistent lack of an association with SARS-CoV-2 positivity. However, there remains a need to further assess impact of ACEIs and ARBs on severity of disease, potentially through larger or randomized studies.
11. Implications for novel and repurposable therapeutics
The spike protein is a target for drug discovery and vaccine development. Blocking the spike protein-ACE2 interaction sites may be targetable with antibodies or small molecules, and use of soluble ACE2 may competitively bind to the spike protein [69]. Strategies to increase ACE2 shedding from cells may be protective against viral infection [70]. Furin inhibitors or other serine protease inhibitors may inhibit SARS-CoV-2 replication via the S1/S2 cleavage site [45]. TMPRSS2 is dispensable for homeostatic function and blocked by the serine protease inhibitor camostat mesilate, a drug approved in Japan for unrelated conditions [40]. Our recent systems biology study suggested several repurposable drugs for potential treatment of COVID-19, including melatonin and ARBs (i.e., irbesartan) [71]. Melatonin regulates expression of several cellular targets of human CoV, including ACE2, Ang II and AT1R [72]. Hydroxychloroquine and chloroquine have been commonly tried for treatment of COVID-19. Besides inhibiting viral-endosome fusion and release of viral particles to the cell by reducing endosomal acidification, chloroquine impairs terminal glycosylation of ACE2, which may have effects on binding affinity between ACE2 and Co-V spike protein [73]. However, efficacy remains to be established, and randomized trials are ongoing. Therapeutic application of Ang1–7 and Ang1–9 is limited because of the short half-life of these peptides and unavailability of FDA-approved drugs that can substitute for the potential benefits attributed to these peptides. Interventions directed at blocking BK and the pathways leading to its formation may also be of benefit. Hereditary angioedema, a rare genetic disorder causing predisposition to attacks of angioedema, is treated with medications to suppress activity in the KKS. These medications largely consist of direct kallikrein inhibitors, B2R antagonists, and replacement with C1 inhibitor. BK's role in COVID-19 is under investigation, and use of these suppressive medications is being explored. Until such time when there is a highly effective antiviral or a vaccine, these adjunctive approaches need to be developed.
12. Summary
In clinical practice the protective effects of ARBs and ACEIs are thought to be associated with an increase in ACE2 expression and their inhibition of the overactive renin-angiotensin system through reduction of Ang II effects. Coronavirus infection hijacks ACE2 expression to invade cells and spread infection-associated damage, downregulating ACE2 expression, reducing its protective effects and exacerbating the injurious Ang II effects. Retrospective observational studies do not show associations with higher risk of infection for persons receiving ACEIs or ARBs. However, controlled clinical trials would be needed to determine the risks or benefits of these agents in treating COVID-19. Studies in SARS-CoV-2 models and clinical retrospective and prospective studies in patients might further clarify these important questions. Such studies may also identify plausible therapeutic agents for targets within the RAS and KKS in the setting of coronaviral infection.
13. Outstanding Questions
Important questions remaining for future research include whether drugs targeting components of the RAS or the KKS might be helpful in the treatment of patients with COVID-19. Prospective controlled clinical trials are needed. Basic research on mechanisms to determine if ACE2 expression affects viral infectivity in vitro and expression of components of the RAS and KKS in infected tissues are needed to help clarify the role of cell-bound or shed ACE2 in COVID-19 pathophysiology. Investigations into specific cell types vulnerable to SARS-CoV-2 infection may help focus targeting of therapies.
Search Strategy and Selection Criteria. Data for this Review were identified by searches of PubMed with search terms including combinations of ACE inhibitors, ARBs, COVID-19, SARS-CoV-2, renin-angiotensin system, kallikrein-kinin system.
Authors' contributions
Mina K. Chung, MD - writing, revisions, literature search, figures, responsibility for the manuscript
Sadashiva Karnik, PhD - RAS expertise, critical writing and revisions, figures
Joshua Saef, MD - KKS expertise, critical writing and revisions, figure
Cornelia Bergmann, PhD - coronavirus expertise, critical writing and revisions
John Barnard, PhD - GTex query/data, figure
Michael M. Lederman, MD - virology, infectious disease expertise, critical writing and revisions
John Tilton, MD - virology expertise, critical writing and revisions
Feixiong Cheng, PhD - expertise in COVID-19 drug repurposing, writing/revisions
Clifford, V. Harding, III, MD, PhD - cell biology and immunology expertise, critical revisions
James B. Young, MD - ACEI/ARB, heart failure/cardiology expertise, critical comments/revisions
Neil Mehta, MD - ACEI/ARB expertise, contributed data/studies on ACE/ARBs
Scott J. Cameron MD, PhD - expertise in platelets and thrombosis in COVID-19, critical writing, revisions
Keith R. McCrae, MD - expertise in thrombosis, critical revisions
Alvin H. Schmaier, MD - expertise in thrombosis, KKS, critical revisions
Jonathan D. Smith, PhD - revisions, insights in ACE2 shedding
Ankur Kalra, MD - ACEI/ARB expertise in COVID-19, review and suggestions for manuscript
Surafel K. Gebreselassie, MD - RAS revisions/suggestions for manuscript
George Thomas, MD - RAS revisions/suggestions for manuscript
Edward S. Hawkins, MD - RAS revisions/suggestions for manuscript
Lars G. Svensson, MD, PhD - critical comments, revisions
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R01 HL111314 (MKC, JB, JS); R01 NS110700, R01 NS091183 (CCB); R01 AI140847 (JCT); U01 HL143402 (KRM); K08 HL128856 (SJC); R01 HL132351 and HL142091 (SK); the NIH National Center for Research Resources for Case Western Reserve University and Cleveland Clinic Clinical and Translational Science Award UL1-RR024989; and the Cleveland Clinic Center of Excellence for Cardiovascular Translational Functional Genomics, funded by the Cleveland Clinic Heart, Vascular and Thoracic Institute and Lerner Research Institute philanthropy funds. The funders had no role in paper design, data collection, data analysis, interpretation, or writing of the paper.
The authors gratefully acknowledge the graphics support provided by Mary Ann Citraro and secretarial support provided by Anastasia Harris.
References
- 1.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnik S.S., Singh K.D., Tirupula K., Unal H. Significance of angiotensin 1-7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22. Br J Pharmacol. 2017;174(9):737–753. doi: 10.1111/bph.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher P.E., Ferrario C.M., Tallant E.A. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295(5):C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varagic J., Ahmad S., Nagata S., Ferrario C.M. ACE2: angiotensin II/angiotensin- (1-7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16(3):420. doi: 10.1007/s11906-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol. 2008;52(9):750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marceau F., Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3(10):845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- 11.Schmaier A.H. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 12.Paz Ocaranza M., Riquelme J.A., Garcia L., Jalil J.E., Chiong M., Santos R.A.S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17(2):116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEachern A.E., Shelton E.R., Bhakta S., Obernolte R., Bach C., Zuppan P. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci USA. 1991;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke J.G., Borkowski J.A., Bierilo K.K., MacNeil T., Derrick A.W., Schneck K.A. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994;269(34):21583–21586. [PubMed] [Google Scholar]
- 15.Roche J.A., Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020;34(6):7265–7269. doi: 10.1096/fj.202000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paegelow I., Werner H., Vietinghoff G., Wartner U. Release of cytokines from isolated lung strips by bradykinin. Inflamm Res. 1995;44(7):306–311. doi: 10.1007/BF02032574. [DOI] [PubMed] [Google Scholar]
- 17.Schmaier A.H. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R1–13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 18.Busse P.J., Christiansen S.C. Hereditary Angioedema. N Engl J Med. 2020;382(12):1136–1148. doi: 10.1056/NEJMra1808012. [DOI] [PubMed] [Google Scholar]
- 19.Abadir P.M., Periasamy A., Carey R.M., Siragy H.M. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48(2):316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 20.AbdAlla S., Lother H., Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407(6800):94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 21.Quitterer U., Fu X., Pohl A., Bayoumy K.M., Langer A., AbdAlla S. Beta-Arrestin1 Prevents Preeclampsia by Downregulation of Mechanosensitive AT1-B2 Receptor Heteromers. Cell. 2019;176(1–2) doi: 10.1016/j.cell.2018.10.050. 318-33 e19. [DOI] [PubMed] [Google Scholar]
- 22.Blais C., Jr., Rouleau J.L., Brown N.J., Lepage Y., Spence D., Munoz C. Serum metabolism of bradykinin and des-Arg9-bradykinin in patients with angiotensin-converting enzyme inhibitor-associated angioedema. Immunopharmacology. 1999;43(2–3):293–302. doi: 10.1016/s0162-3109(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 23.Fang C., Stavrou E., Schmaier A.A., Grobe N., Morris M., Chen A. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavrou E.X., Fang C., Merkulova A., Alhalabi O., Grobe N., Antoniak S. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125(4):710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2020;113 doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Zeng Z., Cao Y., Liu Y., Ping F., Liang M. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-kappaB signaling pathways. Sci Rep. 2016;6:27911. doi: 10.1038/srep27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C., Bo L., Li P., Lu X., Li W., Pan L. Losartan, a selective antagonist of AT1 receptor, attenuates seawater inhalation induced lung injury via modulating JAK2/STATs and apoptosis in rat. Pulm Pharmacol Ther. 2017;45:69–79. doi: 10.1016/j.pupt.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Wang D., Chai X.Q., Magnussen C.G., Zosky G.R., Shu S.H., Wei X. Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulm Pharmacol Ther. 2019;58 doi: 10.1016/j.pupt.2019.101833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho A.C., DeMarinis S., Scott C.F., Silver L.D., Schmaier A.H., Colman R.W. Activation of the contact system of plasma proteolysis in the adult respiratory distress syndrome. J Lab Clin Med. 1988;112(2):270–277. [PubMed] [Google Scholar]
- 31.McGuire W.W., Spragg R.G., Cohen A.B., Cochrane C.G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama S., Sato E., Nomura H., Kubo K., Miura M., Yamashita T. Bradykinin stimulates type II alveolar cells to release neutrophil and monocyte chemotactic activity and inflammatory cytokines. Am J Pathol. 1998;153(6):1885–1893. doi: 10.1016/S0002-9440(10)65702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J.H. Blocking of Kinin B1 receptor: a promising way for the treatment of acute lung injury. Crit Care Med. 2015;43(11):2520–2522. doi: 10.1097/CCM.0000000000001317. [DOI] [PubMed] [Google Scholar]
- 34.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie X., Chen J., Wang X., Zhang F., Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51(4):613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Kehoe P.G., Wong S., Al Mulhim N., Palmer L.E., Miners J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer's disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res Ther. 2016;8(1):50. doi: 10.1186/s13195-016-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. 281-92 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X., Yang W., McVey D.G., Zhao G., Hu J., Poston R.N. FURIN expression in vascular endothelial cells is modulated by a coronary artery disease-associated genetic variant and influences monocyte transendothelial migration. J Am Heart Assoc. 2020;9(4) doi: 10.1161/JAHA.119.014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupays L., Towers N., Wood S., David A., Stuckey D.J., Mohun T. Furin, a transcriptional target of NKX2-5, has an essential role in heart development and function. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0212992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–784. doi: 10.1016/j.molcel.2020.04.022. .e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., et al. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 46.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6) doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Jr., Nunneley J.W. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merkulova A., Mitchell S.C., Stavrou E.X., Forbes G.L., Schmaier A.H. Ponatinib treatment promotes arterial thrombosis and hyperactive platelets. Blood Adv. 2019;3(15):2312–2316. doi: 10.1182/bloodadvances.2019000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irmscher S., Doring N., Halder L.D., Jo E.A.H., Kopka I., Dunker C. Kallikrein cleaves C3 and activates complement. J Innate Immun. 2018;10(2):94–105. doi: 10.1159/000484257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraga-Silva R.A., Sorg B.S., Wankhede M., Dedeugd C., Jun J.Y., Baker M.B. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16(5–6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefrancais E., Ortiz-Munoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koupenova M., Corkrey H.A., Vitseva O., Manni G., Pang C.J., Clancy L. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10(1):1780. doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalinowski L., Matys T., Chabielska E., Buczko W., Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension. 2002;40(4):521–527. doi: 10.1161/01.hyp.0000034745.98129.ec. [DOI] [PubMed] [Google Scholar]
- 56.Li P., Fukuhara M., Diz D.I., Ferrario C.M., Brosnihan K.B. Novel angiotensin II AT (1) receptor antagonist irbesartan prevents thromboxane A (2)-induced vasoconstriction in canine coronary arteries and human platelet aggregation. J Pharmacol Exp Ther. 2000;292(1):238–246. [PubMed] [Google Scholar]
- 57.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bean D.M., Kraljevic Z., Searle T., Bendayan R., O’Gallagher K., Pickles A., Folarin A. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. European Journal of Heart failure. 2020 doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rentsch C.T., Kidwai-Khan F., Tate J.P., Park L.S., King J.T., Skanderson M., et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States Veterans Aged 54-75 Years. medRxiv. 2020.
- 63.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. e201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA. 2020 doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hosseinzadeh A., Javad-Moosavi S.A., Reiter R.J., Hemati K., Ghaznavi H., Mehrzadi S. Idiopathic pulmonary fibrosis (IPF) signaling pathways and protective roles of melatonin. Life Sci. 2018;201:17–29. doi: 10.1016/j.lfs.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 73.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020
- 75.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]