Abstract
Electrically conducting hydrogels are gaining increasing attention due to their potential application in smart patches, biosensors, functional tissue engineering scaffolds, wound management, and implants. The current review focuses on these novel materials, their synthesis routes, and their composites. Special attention is paid to fabrication routes to produce functional composites with organic and inorganic components. The design of conductive hydrogels leads to inheritance of the advantages of each component and offers new features from the synergistic effects between the components, thus opening new application areas. The review also discusses the emerging role of 3D printing as an advanced approach toward new design, functionality, and material combination possibilities. The issue of lack of the spatial control with current techniques is highlighted, and possible new routes to solve it are discussed. The review will provide readers with knowledge tool to select appropriate methodology for designing desired hydrogel material composites.
Keywords: Conducting hydrogel, hydrogel composite, 3D printing, tissue engineering
1 Introduction
Biological functions are complex and replicating them requires understanding and transforming variety signals such as biochemical, electrical, and mechanical. A large number of materials have been developed as bioactive scaffolds to transmit such signals. Hydrogels have been at the forefront of the material development especially for tissue engineering. They possess ideal characteristics of extra-cellular matrix (ECM), cell support, biocompatibility, and Young’s modulus close to human tissue[1,2]. Hydrogels have spatially cross-linked chain network composed of natural and/or synthetic hydrophilic polymer chains that can absorb a large amount of water while maintaining 3D structure, which makes them highly compatible for biomedical applications[2].
Human body is a resident for electrical energy. Many research work has been focusing on understanding the effect of electrical signal on cells[3]. It is predicted that electrical stimulation can impact cells adhesion, differentiation, and growth, but the underlying phenomenon is not well understood. Recent developments in bioelectronics, bioionics, and neural interfaces have placed demands for electrically conducting scaffolds[4,5]. Although hydrogels have found niche application in tissue engineering, they are inherently insulating by nature. Recent research has shown that hydrogels not only possess necessary characteristics to support biological species but can also interface with electrical circuitry if modified[4,5]. Hence, research on conducting hydrogels have gained widespread interest for applications such as health recording electrodes, stimulating electrodes, biosensors, biomedical patches, implantable devices, and electronic skin (Figure 1).
Figure 1.
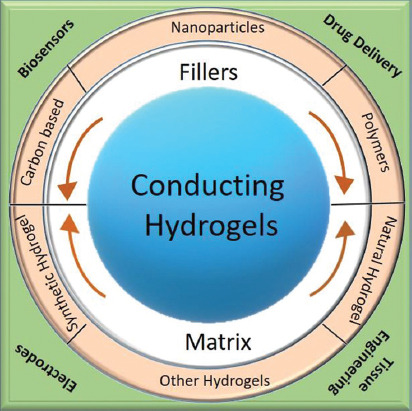
Schematic illustration of conducting hydrogels, their components and applications.
This review is dedicated to conducting hydrogels and their fabrication methods. The paper delves on modification aspects of hydrogels, to make them conducting through addition of metal nanoparticles, carbon-based materials, and conducting polymers. The conducting material may be physically or chemically attached to the hydrogel matrix. The electrical conductivity is influenced by the sizes of the nanoparticles, the presence of water bound by hydrogels, and the presence of any additional components that may affect the conductivity. Although copolymerization and mixing of materials are the standard approach to synthesize the conducting hydrogels, additive manufacturing has proven time-effective and added customization to the characteristics. The review will focus on discussing the work carried out on 3D printing of conducting hydrogels. The in-depth discussion of various 3D printing techniques and equipment is beyond the scope of this review. The review will highlight key research findings to generate electrically active, mechanical stiff, and multi-functional hydrogels.
2 Material Innovation
This section will discuss material synthesis for electrically conducting hydrogels. Most hydrogels are inherently insulating, and additives enhance their electrical behavior. The conductivity of most of the hydrogels is at or below tens mS.cm−1. It is to be noted that most of the time the conductivity reported in the literature refers to the ionic conductivity of the electrolyte that swells them. The contribution from additives materials to overall conductivity in such cases is small. However, recent research efforts in this direction have shown promise in inducing electrical conductivity from the additive materials. Table 1 summarizes the achieved electrical conductivities in various hydrogels.
Table 1.
Summary of the material composites and their electrical conductivities achieved to make conducting hydrogels.
| Hydrogel | Additive | Conductivity (mS.cm−1) | Reference |
|---|---|---|---|
| PANI | Cellulose | 70 | 39 |
| MWCNT | 1540 | 27 | |
| Graphene | 182 | 31 | |
| PPy | PEG | 4.3 | 43 |
| PAA | 5 | 47 | |
| Cellulose | 7.8 | 40 | |
| CuPcTs | 780 | 49 | |
| rGO | 480 | 32 | |
| PEDOT | PAA | 1.2 | 48 |
| PAAM | 2.6 | 46 | |
| PEG | 16.9 | 37 | |
| PU | 120 | 38 | |
| PAA | Silver | 572 | 13 |
| Gum | 33.5 | 88 | |
| Graphene | 10-3 | 28 | |
| PAAM | Graphene | 0.2 | 27 |
| Chitosan | Graphene | 1 | 26 |
| PNIPAM | GO | 285×104 | 25 |
| CNT | 0.7 | 25 | |
| Collagen | CNT | 10-7 | 20 |
| PEDOT:PSS | CNT | 105 | 24 |
| Gelatin methacrylate | CNT | 102 | 21 |
| PTAA | MAAG | 0.1 | 36 |
2.1 Conducting hydrogels with metal nanoparticles
Metal nanoparticles are well known to possess good electrical conductivity and are also easier to process. This makes them an obvious choice for reinforcement material in hydrogel matrix. The final composite is synergistic, unique and has desired properties that are not found in individual components. The final properties depend on the type of nanoparticles incorporated, which in turn determines the proposed application. Silver (Ag) and gold (Au) are the most commonly used conducting materials for hydrogels. Silver has an added antimicrobial property and for this reason, it is blended with hydrogels to make functional coatings[6]. A study done by Endo et al. revealed the relationship between silver ion concentration and swelling rate of hydrogel[7]. A higher concentration of silver ions results in better conductivity, but reduced the swelling ratio and vice versa. So far, silver nanoparticles have been added to many hydrogels, namely polyacrylamide (PAAM), polyacrylic acid (PAA), N-isopropylacrylamide (NIPAAm), methyl methacrylate, and polyvinyl alcohol (PVA)[8-12]. Silver nanoparticles added to PAA through in situ reduction and polymerization showed swelling dependent electrical conductivity, which could be varied from 13.6 to 572 mS.cm-1[13]. The use of gold for making conducting hydrogel remains limited due to its high cost. Gold nanoparticles were utilized to make conducting poly(3,4-ethylenedioxythiophene)/poly(acrylic acid) (PEDOT/PAA) hydrogel, which exhibited excellent catalytical activity for p-nitophenol[14]. It has been proven that by varying gold concentrations, it is possible to change the properties of the final composite. There are sporadic reports on employing metal nanoparticles for making conducting hydrogels. Platinum (Pt) nanoparticles have also been deployed in polyaniline (PANI) hydrogel to make heterostructures for a glucose sensor[15]. The porous structure of hydrogels favors trapping the nanoparticles and immobilizing enzymes for glucose sensing. In one of the early reports, Fuhrer et al. incorporated magnetic cobalt nanoparticles to hydrogel backbone[16]. The cobalt nanoparticles were encapsulated in stable carbon shells, which were covalently modified to have vinyl-termination groups. Copolymerization of carbon coated cobalt and 2-hydroxyethyl methacrylate was carried out to form a magnetically active hydrogel. Crosslinking cobalt nanomagnets into the hydrogel provided a safe way to reduce magnetic particle migration or loss. Iron oxide magnetic nanoparticles have been added in a hydrogel to provide remedy for methylene blue contaminated water[17].
Apart from 3D nanoparticles, 1D (one-dimensional) nanostructures have been used to enhance the electrical conductivity of hydrogels. This is motivated by the fact that 1D nanostructures have better electrical transport[18]. Stretchability is an additional property that can be achieved in the final hydrogel composite through 1D nanostructures, as they act as electrical connectors during bending, stretching, or flexing of the material[19]. A recent report by Lim et al. confirmed the theory where silver nanowires were chosen as fillers for alginate hydrogel[20]. A wearable antenna patch was fabricated using the silver nanowire alginate composite with excellent conductivity, tough structural integrity, and stretchability. The fabrication procedure did not involve formation of covalent bonds by polymerization, thus making it compatible to incorporate different inorganic and metallic nanomaterials.
2.2 Conducting hydrogels with carbon material
Carbon based materials such as carbon nanotubes (CNT), carbon black (CB), and graphene have played an important role in wide range of research fields due to their electrical, thermal, and mechanical properties. Their enhanced electrical properties are highly attractive, and this shows their potential to be used as reinforcement and additive material for composites. Hence, metal nanoparticles are being replaced by carbon-based materials such as carbon nanotubes, graphene, and carbon black to add more functionalities, especially because they have shown higher compatibility for biological species compared to metal counterparts. Better response to biological species comes from the fact that carbon-based materials can be trapped in synthetic and natural hydrogels. This immobilization leads to better biocompatibility. An early attempt was made by Cho et al. to encapsulate CNTs into collagen to electrically stimulate PC12 cells[21]. A loading of <1% CNTs in collagen led to enhanced conductivity and flexibility. It is argued that adding CNTs in collagen has many advantages compared to pure CNT or only collagen scaffolds. First, CNTs enhances the structural integrity of the fabricated scaffold. Second, such composites reduce the mismatch between Young’s modulus of human tissues and rigid electrodes. Third, collagen matrix is effective in confining CNTs and thus reducing harmful effect due to migration. Khademhosseini’s research group developed conducting cardiac patches by mixing CNT in gelatin methacrylate (GelMA) hydrogel[22]. The composite material not only had excellent mechanical integrity but also showed advanced electrophysiological functions. In a similar work, to construct heart patches, Pok et al. added CNTs to chitosan[23]. The resulting scaffold supported cardiomyocyte functionalization and speeded up conduction velocity to achieve beating heart functionality of a rat. CNTs have been added repeatedly to various hydrogels such as chitosan, poly(3,4-ethylenedioxythiophene) doped with poly(4-styrenesulfonate) (PEDOT:PSS), and poly (N-Isopropylacrylamide) (PNIPAM) for electrocatalysis, battery electrode, and fuel cell[24-26]. A different morphology of core-shell of hydrogel composite was synthesized using PANI and CNT[27].
CNTs were replaced with graphene in chitosan for tissue engineering application. It showed similar swelling mechanism and yielded higher mechanical strength of the final composite material[28]. Biological scaffold made of graphene oxide and PAAM composite hydrogel displayed muscle such as stiffness apart (Young’s modulus of approximately 50 kPa) from conductivity[29]. Enhanced proliferation and myogenic differentiation were observed in the scaffold. Graphene oxide shows good suspension in water but aggregates in acidic medium. In a modified approach, Alam et al. tailored the oxidation degree of graphene sheets by controlling the sonication time and acid concentration[30]. Graphene/polyacrylic acid hydrogel was formed using in situ polymerization process, where graphene sheets are mixed with acrylic acid in the presence of a cross-linker and an initiator. The synthesized composite demonstrated high mechanical performance with low percolation threshold of electrical conductivity at 0.4% with added feature of pH sensitivity[30]. Graphene was also added to PANI and PPy[31,32]. Most of the other work on graphene-based hydrogels was targeted toward electro-chemical and electrical applications[33,34].
2.3 Conducting hydrogels with polymers
Concerns on biocompatibility of metal nanoparticles and carbon-based materials have led to increased interest in replacing them with polymeric materials. Polymeric materials are used in various forms namely particles, core-shells, micelles, and dendrimers[35-38]. There are two common routes through which polymers are added into the hydrogels, (i) electro- or chemical polymerization of conducting monomer in prefabricated hydrogel and (ii) mixing the precursor monomer followed by polymerization. The main idea is to entrap the conducting polymer chains in the hydrogel matrix. Polyaniline (PANI) and cellulose composite hydrogel exhibited a continuous and linear crawling motion under a low applied electric field, apart from high compressive strength[39]. Cellulose was also mixed with polypyrrole (PPy) in an ionic liquid, giving rise to high electrical conductivity of 7.83 × 10−3 S.cm−1[40]. Gilmore et al. demonstrated the fabrication of hybrid composite composed of PPy and PAAM. PPy was directly electropolymerized on the hydrogel[41]. The work has since led to plethora of polymer-hydrogel combinations for various applications. Most common tissue engineering application is for cardiac tissues. Poly(triaryl amine) (PTAA) was homogeneously combined with methacrylate hydrogel achieving conductivity similar to myocardial tissues[42]. High-quality conductive composite hydrogels composed of single-walled carbon nanotubes (SWNTs), polypyrrole (PPy) and poly(ethylene glycol) diacrylate (PEGDA) hydrogel were successfully fabricated through interfacial polymerization (IP)[43]. PEDOT:PSS is a high conductivity polymer and heavily used in organic electronic applications. PEDOT:PSS was mixed with polyethylene glycol (PEG) to make electrically conductive scaffold for muscle and nerve tissues[44]. Sasaki et al. reported the fabrication of the hydrogel-based devices with high electrically conductivity using a combination of chemical polymerization and electropolymerization of PEDOT and polyurethane (PU)[45]. Mechanically strong conducting hydrogels composed of PAAM and PEDOT-PSS was synthesized through the construction of a special double-network (sDN) structure[46]. PAA has been polymerized with both PPy and PEDOT resulting in pH responsive and gel with high mechanical strength, respectively[47,48]. Experimenting with a different class of materials, copper phthalocyanine-3,4′,4″,4‴-tetrasulfonic acid tetrasodium salt (CuPcTs) was added to PPy through a supramolecular self-assembly approach[49]. The steric and electrostatic interactions between CuPcTs and PPy favored the self-assembly of PPy chains, which promotes the 1D growth of PPy and resulted in the formation of interconnected nanofibers.
3 Methods of Fabrication
3.1 Traditional approaches
Several approaches have been taken to synthesize conducting hydrogels depending on the nature of the additive and hydrogel matrix. The most common method for aqueous compatible conducting materials is to simply mix with the hydrogel components aided by ultrasonic energy or heating. However, there are five other main approaches that have been identified in the literature to synthesize conducting hydrogel composites with uniform distribution, as shown in Figure 2. These includes hydrogel monomers with cross-linkers and nanoparticles gelated together[50]; physically embedding nanoparticles into hydrogel matrix after gelation[51]; reactive nanoparticle formation aided by the hydrogel network where nanoparticle precursors are loaded in the gel[52]; cross-linking using nanoparticles to form hydrogels[53]; and hydrogel formation using nanoparticles, polymers, and other molecules[54]. Gold nanoparticles were added into the solution of monomer of N-isopropylacrylamide/acrylamide (NIPAAm/AAm) followed by addition of the gelation initiator ammonium persulfate (APS) and accelerator tetramethylethylenediamine (TMEDA) by Sershen et al. to form gold-hydrogel composite[51]. Gold nanoparticles tend to agglomerate under electric field and hence cannot be electropolymerized. To this end, gold nanoparticles were incorporated in PAAM hydrogel after the gel has been formed. The hydrogel was formed through a “breathing in” process which consists of (i) collapsing the gel by placing it in acetone and causing the water to be expelled; and (ii) placing the dehydrated gel in aqueous solution of gold nanoparticles. This step caused the hydrogel to swell up again, termed as breathing in; and (iii) washing the hydrogel with water to remove any weakly surface-adsorbed nanoparticles. The above steps were repeated many times to obtain the desired nanoparticle density in the final hydrogel composite. There are many reports available, which involve loading the nanoparticle precursors into the hydrogel matrix rather than adding the preformed nanoparticles. Although Langer’s group initiated the work in this direction[52], Saravanan et al. improved the methodology to form silver laden NIPAAm hydrogel[55]. The group carried out free-radical cross-linking polymerization of acrylamide monomer in an aqueous medium containing silver ions. Different reducing agent for nanoparticle ions and precursors has been reported. The benefit of this method is the formation of hydrogels with enhanced mechanical strength. In an alternate approach, the surface of nanoparticles was functionalized by adding cross-linking groups to bind with the hydrogel. Zhang et al. extensively studied several combination of semiconductor nanoparticle-hydrogel composites through self-initiated polymerization under light irradiation[56]. Silicon nanoparticles were added to a hydrogel framework by in situ polymerization to synthesize a well-connected 3D structure.
Figure 2.
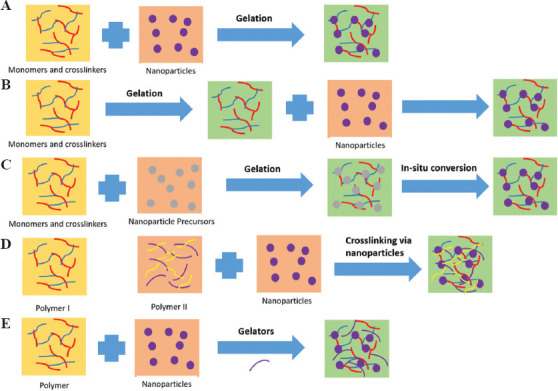
Schematic diagram depicting various approaches to synthesize conducting hydrogel: (A) hydrogel monomers with cross-linkers and nanoparticles gelated together; (B) physically embedding nanoparticles into hydrogel matrix after gelation; (C) reactive nanoparticle formation aided by the hydrogel network where nanoparticle precursors are loaded in the gel; (D) cross-linking using nanoparticles to form hydrogel; and (E) hydrogel formation using nanoparticles, polymers, and other molecules.
Fabricating electrically conducting hydrogels mostly employs traditional approaches. They may be well-established, but there are some limitations. One of the most common issues encountered is agglomeration and poor dispersion of the additives in the hydrogel matrix. As discussed above, this issue can be overcome to some extent by selecting suitable solvents for dispersion. Due to the use of metal nanoparticles, namely, silver and gold, the overall cost of the final material may be high. This prevents their use in commercial application. There are also some reports wherein the response time of devices fabricated using conducting hydrogels is slower. Much work needs to be done to improve the overall device performance in conducting hydrogels.
3.2 3D printing
Additive manufacturing, also called 3D printing, is defined as “process of joining materials to make parts from 3D model data, usually layer on layer, as opposed to subtractive manufacturing and formative manufacturing methodologies” according to the International Organization for Standardization (ISO)/American Society for Testing and Materials (ASTM) 52900:2015 standard. It has been a disruptive technology for fabricating complex shapes in various materials, thus overcoming the limitations of traditional manufacturing techniques[57,58]. 3D printing of metals is a mature technology and polymer printing is making its way to industries. This technology has forayed into electronic and biomaterials and opened doors for design and device innovation[59-61]. Conducting hydrogels have seen a surge in being processed using 3D printing technique due to ease of constructing complex shapes, customized constructs, and time efficient processing[61-64]. 3D printing is an umbrella term and mostly three different techniques have been applied to print conducting hydrogels namely 3D bioplotting, inkjet, and light-based technique[65-67]. In 3D bioplotting, the conducting hydrogel material is extruded from an orifice on to a substrate of choice (Figure 3A). The technique generally relies on the shear thinning behavior of the hydrogels, thus making them flow on applying pressure and allowing their deposition[65,68]. Inkjet technique uses a piezoelectric head for the orifice. A piezoelectric material deforms on applying voltage or current. Thus, the orifice opening can be controlled by varying the voltage applied to the printer head. Inkjet printing creates small droplets (sub-micron volume), which are deposited on the surface. Small volume of material deposition, as against large material ejection through extrusion, helps to print high-resolution constructs and scaffolds[66]. Stereolithography (SLA) and direct-laser writing (DLW) are light-based techniques, wherein either a material precursor is illuminated by light or a pattern is projected[67,69]. Curing of the material takes places in selected regions where the light hits it. In most printers, the printhead and platform move in X-Y directions relative to each other. However, here a Z-displacement of the precursor reservoir is performed to fabricate a 3D shape. Detailed discussion on operation and methodology of these techniques is beyond the scope of this review. Hydrogel to be processed through the printer needs to be converted into an ink format. Ink development is considered one of the most important aspects of 3D printing. Hydrogel inks need to have the right rheological properties to fulfill the physical and mechanical needs of the orienting process. Ink formulation, properties, and optimization are a widely researched[70-72] area and will not be discussed in this paper.
Figure 3.
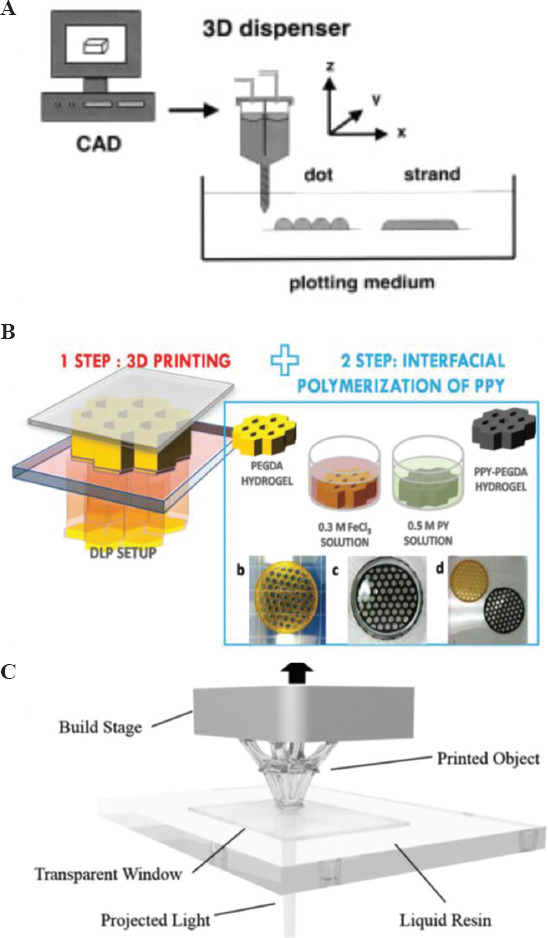
Sketch of (A) 3D bioplotting system (Reproduced with permission[65]) (B) digital light projector (DLP) 3D printing system to 3D print conducting hydrogel scaffolds (Reproduced with permission[74]), and (C) stereolithography process (Reproduced with permission[75]).
Sayyar et al. used extrusion based bioplotting system to print graphene-chitosan composite biocompatible scaffolds. Addition of 3% of graphene in the composite resulted in 200% enhancement of mechanical strength[28]. They further developed a new UV-crosslinkable conducting hydrogel based on the same materials[73]. Functionalized chitosan served as the host polymer and chemically converted graphene as the filler. The incorporation of graphene into chitosan resulted in cytocompatible matrix with enhanced mechanical, cell adhesion, and electrical properties. In creating a composite of two polymers, namely, poly(ethylene glycol)diacrylate and polypyrrole digital light processing 3D printing technique was combined with interfacial polymerization[74] (Figure 3B). The achieved composite material was electroactive and could be drawn into complex geometry. Silica nanoparticle has been used as additives for many hydrogels to impart novel functionalities. Odent et al. added silica nanoparticles to ammonium containing PAAM and processed through SLA machine[75] (Figure 3C). The resulting composite hydrogel was stretchable, mechanically tough and ionically conducting, and the enhanced properties were due to addition of anion charged sulfonated silica nanoparticles. It was found that the stiffness of the composite hydrogel increased with increasing amount of silica nanoparticles. In another report, a hybrid hydrogel of silica/alumina was modified by adding poly(N-isopropylacrylamide) (PNIPAm) microparticles, resulting in a printable material that responded to both heat and electrical stimuli[76]. The water molecules trapped in PNIPAm are released to the silica/alumina matrix on heating. PNIPm particles are dehydrated and act as light scattering centers leading to an optical change from opaque to transparent. The response of the material to electrical stimulation was exploited for fabricating an optical switch. Resin based SLA was also used to print all polymer-based composite based on the polyethylene glycol diacrylate (PEGDA). Poly(3,4-ethylenedioxythiophene) (PEDOT):polystyrene sulfonate (PSS) aqueous solution was freeze-dried and mixed with PEGDA to make it conducting[77]. Increasing the PEDOT:PSS concentration, enhanced the electrochemical characteristics of the printed platform, which was used to electrically stimulate cells for neuronal differentiation. 3D printing enables fabricating complex construct shapes with customization as anatomy of humans are very different from each-other.
Commercialization of 3D printing whether for conducting hydrogels or other biomaterials is still far away. This is due to many issues that need to be resolved before industries can take it up for mass-production. Hydrogels are considered soft materials and their lack of mechanical strength poses limitation in printing sturdy 3D constructs and shapes. These materials are unable to follow the original design models, as the printed construct does not retain the original shape. Achieving functional gradients and hierarchical properties have also been challenging and new design approaches are being developed to tackle them. 3D printing is heralded as a unique solution to print human organs off-the-shelf to counter their storage. However, precision printing in layer-by-layer fashion mimicking human tissues is a mammoth task. A prominent weakness is the lack of precise control on the cellular microenvironment, which governs cell attachment and proliferation. Various professional and off-the-shelve hobbyist 3D printers are available, but few are open-source which allows users to define desired toolpaths. Lack of such a capability does not allow the users to write customized codes or load their model files.
3.3 Fabrication enabling spatial distribution
Conducting hydrogels can now be processed into complex shapes and structures with the help of 3D printing. However, one critical challenge still plagues them. When conducting material is added to the hydrogel, they may be distributed homogeneously but they cannot be confined spatially in desired spots. There has been growing needs to confine conducting material in space within hydrogels and not distribute evenly. This can lead to building electrical circuits encompassed within hydrogels. Some emerging applications may require the conducting material not distributed in the entire hydrogel matrix but be restricted in specific spots to enable electrical stimulation. This section of the paper deals with latest research efforts directed to print soft electrical circuits on hydrogels. With the assistance of transfer techniques and 3D printing, it is now possible to lay circuits on hydrogel surfaces. Such platforms find application in wound management, drug delivery, on-skin patches, implantable, and bioelectronic devices. In one of the early reports, Sekine et al. used micropatterning to lay down PEDOT on agarose hydrogel in a predefined fashion[78]. The fabricated platform was organic, moist and served as flexible electrode to cultivate contractile myotubes. A similar peel and transfer process were applied to fabricate silver nanowire (AgNW)-based microelectrode on polydimethylsiloxane (PDMS) substrate[79] (Figure 4A). The desired microelectrode pattern was first fabricated on glass substrate using photolithography and later directly transferred to PDMS based hydrogel. Electrical conductivity could be control by varying the density of silver nanowires. Peel and transfer methods are neither reproducible nor up-scalable for commercial use. Shay et al. and their group created microfluidic channels through soft lithography in PDMS[80]. Combination of acrylamide (AAm) and acrylic acid (AA) was used as the hydrogel material to fill the holes, while liquid metal eutectic gallium indium (EGaIn) was injected into the channels to form flexible electrodes. Agarwala et al. demonstrated the usability of 3D printing by printing silver nanoparticle ink over gelatin methacryloyl (GelMA) hydrogel[81]. They printed interdigitated electrode and micro-heater design with the silver nanoparticle ink sandwiched between printed layers of hydrogel loaded with cells (C2C12 mouse cells and human fibroblast cells) (Figure 4B). Authors studied cell proliferation under electrical stimulation.
Figure 4.
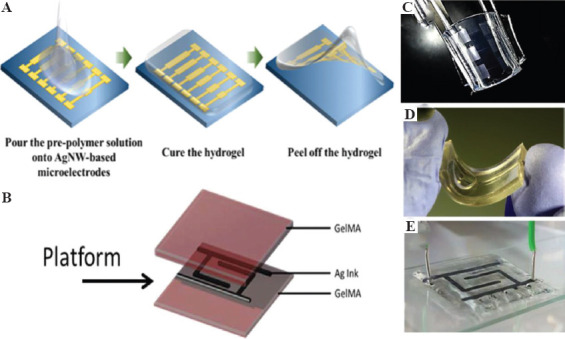
Schematic representation of (A) peel and transfer process (Reproduced with permission[79]), and (B) 3D printing microvalve process for spatially resolved microelectrode circuits on hydrogel substrates (Reproduced with permission[81]). Photograph of (C) bended AgNW-based microelectrodes on hydrogel (Reproduced with permission[79]), (D) liquid metal injected PDMS layers for ECG electrode (Reproduced with permission[80]), and (E) interdigitated electrode sandwiched between two hydrogel layers (Reproduced with permission[81]).
4 Biomedical Applications for Conducting Hydrogels
4.1 Biosensors
Biosensors have been at the forefront of the biomedical research in helping to collect clinically relevant data for medicinal activity, surgery, and intensive care[82]. Electrodes used for sensing biological functions or stimulating tissues usually require non-fouling surface, which are resistant to protein adsorption. Fouling can significantly reduce the performance of the electrodes by adsorption of biological materials such as proteins, cells, and oligonucleotides[83]. Implantable devices and electrodes have been found to give rise to inflammatory response due to exposure to body fluids. This leads to tissue damage and can be tackled through material – tissue interactions that include the electro-stimulated release of bioactive agents at the site of implantation. Non-conducting hydrogel coatings have shown high interfacial impedance and thus, conducting hydrogels are finding much use in such scenarios. Composites of poly(HEMA-co-PEGMA) and a cross-linker, tetraethylene glycol diacrylate (TEGDA), have been formulated for biosensing applications[84].
4.2 Drug delivery vehicles
Drug delivery vehicles are scaffolds and platforms that allow a pharmaceutical compound to be released on or within human body for therapeutic effects. Hydrogels are natural candidate for such application due to their porous network, which can be controlled by managing the density of cross-linkers. Electroactive hydrogels are the new generation of smart materials that allow the direct delivery of electrical signals to control the delivery of the drug. PAAM and PPy were used to make a cylindrical drug delivery device[85]. Controlled release of compounds was demonstrated for the treatment of schizophrenia and bipolar disorder. Some treatments may require delayed release of the medicinal compounds. To this end, poly(p-phenylenevinylene) (PPV) was used to create a hydrogel with PAAM, where the release profile was optimized using cathodic potential[86]. The release of salicylic acid was delayed from 3 h to 15 h after application of appropriate potential. Some other conducting hydrogels investigated for drug delivery are based on gum ghatti, vinyl monomers, and aniline[87-89]. The electro-stimulated devices made from these materials benefit from high loading capacity and low voltage actuation. There are, however, many limitations to the current system of drug delivery using conducting hydrogels. First, there are active and passive losses of the loaded drug through exchange with the environment and by diffusion, respectively. Second, many of these materials suffer from low diffusion coefficients of the drug resulting in poor release kinetics. Finally, many of these materials are affected from low drug loading capacity. Incorporating nanoparticles is not just impart electrical conductivity but also improves drug loading capacity through increase in surface area[90]. Graphene oxide (GO) has been widely researched to make conducting hydrogels, where the resulting material has shown no passive drug diffusion[91,92]. GO was combined with PPy and PEDOT to generate electrically active composite.
4.3 Tissue engineering
Hydrogels have been a success in tissue engineering and tissue regeneration for various human organs. Conducting hydrogels have been attractive for cell growth, adhesion, and proliferation of muscles, cardiovascular nerves, and bone tissue cells[78,93-95]. PTAA and methacrylate aminated gelatin were used in cardiac tissue engineering demonstrating a conductivity up to 10−1 m.S.cm−1, which is similar to the conductivity of myocardial tissue. This study demonstrated positive effects on cardiac differentiation efficiency[42]. A conducting hydrogel composed of PPy/graphene/chitosan composite good adhesion, proliferation, and viability toward fibroblast cells[28]. A conducting tissue engineering scaffold for muscle and nerve tissues was fabricated using PEDOT:PSS and PEG hydrogel mix[44]. In another variation, PEDOT and PU were mixed to fabricate a hydrogel-based device. The PEDOT/PU hydrogel exhibited high electrical conductivity of up to 120 S. cm−1 at 100% elongation[45].
5 Conclusion and Outlook
We have reviewed conducting hydrogel composites as the state-of-the-art and versatile class of materials that are gaining attention due to their suitability for various applications. The review summarizes synthesis methods and strategies to achieve electrical conduction in otherwise insulating hydrogels. Incorporation of nanoparticles, carbon-based materials, and polymers has been discussed, and this provides better understanding to the readers to design novel combination of materials for desired applications. Working with conducting hydrogels has its own limitations, especially at the hydrogel device interface. Adhesion of hydrogels layers and their dehydration over longer time span pose stability issues. In many reports, the biocompatibility tests are limited to in vitro screening, and further animal studies may be required. Conducting scaffolds can provide ideal platform for regenerative tissue engineering. However, role of electrical stimulation for cell growth is poorly understood. We have also delved into the area of 3D printing for conducting hydrogels. Much work is needed to determine most promising printing technique and functionalization approaches for 3D printed conducting hydrogels. We have also discussed on how 3D printing can lay down materials in desired locations within a hydrogel, thus creating flexible circuits instead of homogeneous conducting material. The area of conducting hydrogels is still full of unresolved technological challenges, and thus provides researchers with opportunity for development, as this field is growing fast beyond its early stage. Improvement in the conductivity of the hydrogels may be one research direction, while incorporating new functionalities such as biodegradability and mechanical strength can open new avenues for applications. Innovation is also required in fabrication methods to allow varied composition of hydrogels to be laid down in desired fashion.
Acknowledgments
The work was supported by the Department of Engineering, Aarhus University, Denmark.
References
- 1.Miyata T, Uragami T, Nakamae K. Biomolecule-sensitive Hydrogels. Adv Drug Deliv Rev. 2002;54:79–98. doi: 10.1016/s0169-409x(01)00241-1. DOI:10.1016/s0169-409x(01)00241-1. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman AS. Hydrogels for Biomedical Applications. Adv Drug Deliv Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Hardy JG, Villancio-Wolter MK, Sukhavasi RC. Electrical Stimulation of Human Mesenchymal Stem Cells on Conductive Nanofibers Enhances their Differentiation Toward Osteogenic Outcomes. Macromol Rapid Commun. 2015;36:1884–90. doi: 10.1002/marc.201500233. DOI:10.1002/marc.201500233. [DOI] [PubMed] [Google Scholar]
- 4.Guiseppi-Elie A. Electroconductive Hydrogels:Synthesis, Characterization and Biomedical Applications. Biomaterials. 2010;31:2701–16. doi: 10.1016/j.biomaterials.2009.12.052. DOI:10.1016/j.biomaterials.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Distler T, Boccaccini AR. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors-a Review. Acta Biomater. 2019;101:1–13. doi: 10.1016/j.actbio.2019.08.044. DOI:10.1016/j.actbio.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Nair LS, Laurencin CT. Silver Nanoparticles:Synthesis and Therapeutic Applications. J Biomed Nanotechnol. 2007;3:301–16. [Google Scholar]
- 7.Endo T, Ikeda R, Yanagida Y, et al. Stimuli-responsive Hydrogel-silver Nanoparticles Composite for Development of Localized Surface Plasmon Resonance-based Optical Biosensor. Anal Chim Acta. 2008;611:205–11. doi: 10.1016/j.aca.2008.01.078. DOI:10.1016/j.aca.2008.01.078. [DOI] [PubMed] [Google Scholar]
- 8.Vimala K, Sivudu KS, Mohan YM, et al. Controlled Silver Nanoparticles Synthesis in Semi-hydrogel Networks of Poly (Acrylamide) and Carbohydrates:A Rational Methodology for Antibacterial Application. Carbohydr Polym. 2009;75:463–71. DOI:10.1016/j.carbpol.2008.08.009. [Google Scholar]
- 9.Bardajee GR, Hooshyar Z, Rezanezhad H. A Novel and Green Biomaterial Based Silver Nanocomposite Hydrogel:Synthesis, Characterization and Antibacterial Effect. J Inorg Biochem. 2012;117:367–73. doi: 10.1016/j.jinorgbio.2012.06.012. DOI:10.1016/j.jinorgbio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Mohan YM, Lee K, Premkumar T, et al. Hydrogel Networks as Nanoreactors:A Novel Approach to Silver Nanoparticles for Antibacterial Applications. Polymer. 2007;48:158–64. DOI:10.1016/j.polymer.2006.10.045. [Google Scholar]
- 11.Wei QB, Fu F, Zhang YQ, et al. Preparation, Characterization, And Antibacterial Properties Of Ph-Responsive P(MMA-Co-MAA)/Silver Nanocomposite Hydrogels. J Polym Res. 2014;21(349) DOI:10.1007/s10965-013-0349-4. [Google Scholar]
- 12.Juby KA, Dwivedi C, Kumar M, et al. Silver Nanoparticle-loaded PVA/gum Acacia Hydrogel:Synthesis, Characterization and Antibacterial Study. Carbohydr Polym. 2012;89:906–13. doi: 10.1016/j.carbpol.2012.04.033. DOI:10.1016/j.carbpol.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Devaki SJ, Narayanan RK, Sarojam S. Electrically Conducting Silver Nanoparticle-polyacrylic Acid Hydrogel by In situ Reduction and Polymerization Approach. Mater Lett. 2014;116:135–8. DOI:10.1016/j.matlet.2013.10.110. [Google Scholar]
- 14.Xiao H, Xia Y, Cai C. Conducting Polymer Hydrogel Reactor for Synthesizing Au Nanoparticles and Its Use in the Reduction of P-nitrophenol. J Nanopart Res. 2013;1521;15 DOI:10.1007/s11051-013-1521-9. [Google Scholar]
- 15.Zhai D, Liu B, Shi Y. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano. 2013;7:3540–6. doi: 10.1021/nn400482d. DOI:10.1021/nn400482d. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrer R, Athanassiou EK, Luechinger NA, et al. Crosslinking Metal Nanoparticles into the Polymer Backbone of Hydrogels Enables Preparation of Soft, Magnetic Field-driven Actuators with Muscle-like Flexibility. Small. 2009;5(3):383–8. doi: 10.1002/smll.200801091. DOI:10.1002/smll.200801091. [DOI] [PubMed] [Google Scholar]
- 17.Mittal H, Ballav N, Mishra SB. Gum Ghatti and Fe3O4Magnetic Nanoparticles Based Nanocomposites for the Effective Adsorption of Methylene Blue from Aqueous Solution. J Ind Eng Chem. 2014;20:2184–92. DOI:10.1016/j.jiec.2013.09.049. [Google Scholar]
- 18.Yuan B, Cademartiri L. Flexible One-dimensional Nanostructures:A Review. J Mater Sci Technol. 2015;31:607–15. [Google Scholar]
- 19.Gong S, Cheng W. One?dimensional Nanomaterials for Soft Electronics. Adv Electron Mater. 2017;3:1600314. DOI:10.1002/aelm.201600314. [Google Scholar]
- 20.Lim C, Shin Y, Jung J. Stretchable Conductive Nanocomposite Based on Alginate Hydrogel and Silver Nanowires for Wearable Electronics. APL Mater. 2019;7:031502. DOI:10.1063/1.5063657. [Google Scholar]
- 21.Cho Y, Borgens RB. The Effect of An Electrically Conductive Carbon Nanotube/Collagen Composite on Neurite Outgrowth of PC12 Cells. J Biomed Mater Res A. 2010;95:510–7. doi: 10.1002/jbm.a.32841. DOI:10.1002/jbm.a.32841. [DOI] [PubMed] [Google Scholar]
- 22.Shin SR, Jung SM, Zalabany M, et al. Carbon-nanotube-embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano. 2013;7:2369–80. doi: 10.1021/nn305559j. DOI:10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pok S, Vitale F, Eichmann SL, et al. Biocompatible Carbon Nanotube Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano. 2014;8:9822–32. doi: 10.1021/nn503693h. DOI:10.1021/nn503693h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu XW, Huang YX, Sun XF, et al. Conductive Carbon Nanotube Hydrogel as a Bioanode for Enhanced Microbial Electrocatalysis. ACS Appl Mater Interfaces. 2014;6:8158?64. doi: 10.1021/am500624k. DOI:10.1021/am500624k. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, To JW, Wang C, et al. A Three-dimensionally Interconnected Carbon Nanotube-conducting Polymer Hydrogel Network for High-performance Flexible Battery Electrodes. Adv Energy Mater. 2014;4(1400207) DOI:10.1002/aenm.201400207. [Google Scholar]
- 26.Kumar GG, Hashmi S, Karthikeyan C, et al. Graphene Oxide/Carbon Nanotube Composite Hydrogels-Versatile Materials for Microbial Fuel Cell Applications. Macromol Rapid Commun. 2014;35:1861–5. doi: 10.1002/marc.201400332. DOI:10.1002/marc.201400332. [DOI] [PubMed] [Google Scholar]
- 27.Chen PY, Courchesne NM, Hyder MN, et al. Carbon Nanotube-polyaniline Core-shell Nanostructured Hydrogel for Electrochemical Energy Storage. RSC Adv. 2015;5:37970–9977. DOI:10.1039/c5ra02944a. [Google Scholar]
- 28.Sayyar S, Murray E, Thompson BC, et al. Processable Conducting Graphene/Chitosan Hydrogels for Tissue Engineering. J Mater Chem B. 2015;3:481–90. doi: 10.1039/c4tb01636j. [DOI] [PubMed] [Google Scholar]
- 29.Jo H, Sim M, Kim S, et al. Electrically Conductive Graphene/Polyacrylamide Hydrogels Produced by Mild Chemical Reduction for Enhanced Myoblast Growth and Differentiation. Acta Biomater. 2017;48:100–9. doi: 10.1016/j.actbio.2016.10.035. DOI:10.1016/j.actbio.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Alam A, Meng Q, Shi G, et al. Electrically Conductive, Mechanically Robust, pH-sensitive Graphene/Polymer Composite Hydrogels. Compos Sci Technol. 2016;127:119–26. DOI:10.1016/j.compscitech.2016.02.024. [Google Scholar]
- 31.Baniasadi H, Ramazani AS, Mashayekhan S. Fabrication and Characterization of Conductive Chitosan/Gelatin-Based Scaffolds for Nerve Tissue Engineering. Int J Biol Macromol. 2015;74:360–6. doi: 10.1016/j.ijbiomac.2014.12.014. DOI:10.1016/j.ijbiomac.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Li BY, Ni T, et al. 2015c, One-step Synthesis of Iodine Doped Polyaniline-Reduced Graphene Oxide Composite Hydrogel with High Capacitative Properties. Compos Sci Technol. 109:12–7. [Google Scholar]
- 33.Zhang L, Shi G. Preparation of Highly Conductive Graphene Hydrogels for Fabricating Supercapacitors with High Rate Capability. J Phys Chem C. 2011;115:17206–12. DOI:10.1021/jp204036a. [Google Scholar]
- 34.Xu Y, Lin Z, Huang X, et al. Flexible Solid-state Supercapacitors Based on Three-dimensional Graphene Hydrogel Films. ACS Nano. 2013;7:4042–9. doi: 10.1021/nn4000836. DOI:10.1021/nn4000836. [DOI] [PubMed] [Google Scholar]
- 35.Moughton AO, Hillmyer MA, Lodge TP. Multicompartment Block Polymer Micelles. Macromolecules. 2012;45:2–19. DOI:10.1021/ma201865s. [Google Scholar]
- 36.Li GL, Mohwald H, Shchukin DG. Precipitation Polymerization for Fabrication of Complex Core-shell Hybrid Particles and Hollow Structures. Chem Soc Rev. 2013;42:3628–46. doi: 10.1039/c3cs35517a. DOI:10.1039/c3cs35517a. [DOI] [PubMed] [Google Scholar]
- 37.Schlüter AD, Halperin A, Kröger M, et al. Dendronized Polymers:Molecular Objects Between Conventional Linear Polymers and Colloidal Particles. ACS Macro Lett. 2014;3:991–8. doi: 10.1021/mz500376e. DOI:10.1021/mz500376e. [DOI] [PubMed] [Google Scholar]
- 38.McLeish TC. Weinheim, Germany: Wiley-VCH; 2007. Macromolecular Engineering:Precise Synthesis, Materials Properties, Applications; p. 1605. [Google Scholar]
- 39.Shi XW, Hu YL, Tu K, et al. Electromechanical Polyaniline-cellulose Hydrogels with High Compressive Strength. Soft Matter. 2013;9:10129–34. DOI:10.1039/c3sm51490k. [Google Scholar]
- 40.Liang XT, Qu B, Li JR, et al. Preparation of Cellulose-based Conductive Hydrogels with Ionic Liquid. React Funct Polym. 2015;86:1–6. [Google Scholar]
- 41.Gilmore K, Hodgson AJ, Luan B, et al. Preparation of Hydrogel/conducting Polymer Composites. Polym Gels Netw. 1994;2:135–43. DOI:10.1016/0966-7822(94)90032-9. [Google Scholar]
- 42.Yang B, Yao F, Hao T, et al. Development of Electrically Conductive Double-Network Hydrogels via One-Step Facile Strategy for Cardiac Tissue Engineering. Adv Healthc Mater. 2016;5:474–88. doi: 10.1002/adhm.201500520. DOI:10.1002/adhm.201500520. [DOI] [PubMed] [Google Scholar]
- 43.Xiao YH, He L, Che JF. An Effective Approach for the Fabrication of Reinforced Composite Hydrogel Engineered with SWNTs, Polypyrrole and PEGDA Hydrogel. J Mater Chem. 2012;22:8076–82. DOI:10.1039/c2jm30601h. [Google Scholar]
- 44.Kim YS, Cho K, Lee HJ, et al. Highly Conductive and Hydrated PEG-Based Hydrogels for the Potential Application of a Tissue Engineering Scaffold. React Funct Polym. 2016;109:15–22. DOI:10.1016/j.reactfunctpolym.2016.09.003. [Google Scholar]
- 45.Sasaki M, Karikkineth BC, Nagamine K, et al. Highly Conductive Stretchable and Biocompatible Electrode-Hydrogel Hybrids for Advanced Tissue Engineering. Adv Healthc Mater. 2014;3:1919–27. doi: 10.1002/adhm.201400209. DOI:10.1002/adhm.201400209. [DOI] [PubMed] [Google Scholar]
- 46.Dai TY, Qing XT, Zhou H, et al. Mechanically Strong Conducting Hydrogels with Special Double-network Structure. Synth Met. 2010;160:791–6. DOI:10.1016/j.synthmet.2010.01.024. [Google Scholar]
- 47.Elyashevich GK, Smirnov MA. New pH-responsive and Electroactive Composite Systems Containing Hydrogels and Conductive Polymers on a Porous Matrix. Polym Sci A. 2012;54:900–8. DOI:10.1134/s0965545x12110028. [Google Scholar]
- 48.Dai TY, Qing XT, Xia YY. Conducting Hydrogels with Enhanced Mechanical Strength. Polymer. 2009;50:5236–41. DOI:10.1016/j.polymer.2009.09.025. [Google Scholar]
- 49.Wang YQ, Shi Y, Pan LJ, et al. Dopant-enabled Supramolecular Approach for Controlled Synthesis of Nanostructured Conductive Polymer Hydrogels. Nano Lett. 2015;15:7736–41. doi: 10.1021/acs.nanolett.5b03891. DOI:10.1021/acs.nanolett.5b03891. [DOI] [PubMed] [Google Scholar]
- 50.Sershen SR, Westcott SL, Halas NH, et al. Independent Optically Addressable Nanoparticle-polymer Optomechanical Composites. Appl Phys Lett. 2002;80:4609. DOI:10.1063/1.1481536. [Google Scholar]
- 51.Pardo-Yissar V, Gabai R, Shipway AN, et al. Gold Nanoparticle/Hydrogel Composites with Solvent?Switchable Electronic Properties. Adv Mater. 2001;13:1320–3. DOI:10.1002/1521-4095(200109)13:17<1320::aid-adma1320>3.0.co;2-8. [Google Scholar]
- 52.Wang C, Flynn NT, Langer R. Controlled Structure and Properties of Thermoresponsive Nanoparticle-hydrogel Composites. Adv Mater. 2004;16:1074–9. DOI:10.1002/adma.200306516. [Google Scholar]
- 53.Souza GR, Christianson DR, Staquicini FI, et al. Networks of Gold Nanoparticles and Bacteriophage as Biological Sensors and Cell-targeting Agents. Proc Natl Acad Sci USA. 2006;103:1215–20. doi: 10.1073/pnas.0509739103. DOI:10.1073/pnas.0509739103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, Yu G, Pan L, et al. Stable Li-ion Battery Anodes by In situ Polymerization of Conducting Hydrogel to Conformally Coat Silicon Nanoparticles. Nat Commun. 2013;4:1943. doi: 10.1038/ncomms2941. DOI:10.1038/ncomms2941. [DOI] [PubMed] [Google Scholar]
- 55.Saravanan P, Raju MP, Alam S. A Study on Synthesis and Properties of Ag Nanoparticles Immobilized Polyacrylamide Hydrogel Composites. Mater Chem Phys. 2007;103:278–82. DOI:10.1016/j.matchemphys.2007.02.025. [Google Scholar]
- 56.Zhang D, Yang J, Bao S, et al. Semiconductor Nanoparticle-based Hydrogels Prepared Via Self-initiated Polymerization Under Sunlight, Even Visible Light. Sci Rep. 2013;3:1399. doi: 10.1038/srep01399. DOI:10.1038/srep01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaezi M, Chianrabutra S, Mellor B, et al. Multiple Material Additive Manufacturing-Part 1:A Review. Virtual Phys Prototyp. 2013;8:19–50. DOI:10.1080/17452759.2013.778175. [Google Scholar]
- 58.Cheng K, Mukherjee P, Curthoys I. Development and Use of Augmented Reality and 3D Printing in Consulting Patient with Complex Skull Base Cholesteatoma. Virtual Phys Prototyp. 2017;12:241–8. DOI:10.1080/17452759.2017.1310050. [Google Scholar]
- 59.Espalin D, Muse DW, MacDonald E, et al. 3D Printing Multifunctionality:Structures with Electronics. Int J Adv Manuf Technol. 2014;72:963–78. DOI:10.1007/s00170-014-5717-7. [Google Scholar]
- 60.Zhao D, Liu T, Park JG. Conductivity Enhancement of Aerosol-jet Printed Electronics by Using Silver Nanoparticles Ink with Carbon Nanotubes. Microelectron Eng. 2012;96:71–5. DOI:10.1016/j.mee.2012.03.004. [Google Scholar]
- 61.Wang S, Lee JM, Yeong WY. Smart Hydrogels for 3D Bioprinting. Int J Bioprint. 2015;1:3–14. [Google Scholar]
- 62.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. DOI:10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 63.Cui H, Nowicki M, Fisher JP, et al. 3D Bioprinting for Organ Regeneration. Adv Healthc Mater. 2017;6(1601118) doi: 10.1002/adhm.201601118. DOI:10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JM, Ng WL, Yeong WY. Resolution and Shape in Bioprinting:Strategizing Towards Complex Tissue and Organ Printing. Appl Phys Rev. 2019;6(1):011307. DOI:10.1063/1.5053909. [Google Scholar]
- 65.Landers R, Mülhaupt R. Desktop Manufacturing of Complex Objects, Prototypes and Biomedical Scaffolds by Means of Computer-assisted Design Combined with Computer-guided 3D Plotting of Polymers and Reactive Oligomers. Macromol Mater Eng. 2000;282:17–21. DOI:10.1002/1439-2054(20001001)282:1<17::aid-mame17>3.0.co;2-8. [Google Scholar]
- 66.Sun J, Ng JH, Fuh YH, et al. Comparison of Micro-dispensing Performance Between Micro-valve and Piezoelectric Printhead. Microsyst Technol. 2009;15:1437–48. DOI:10.1007/s00542-009-0905-3. [Google Scholar]
- 67.Chua CK, Leong KF. 4th ed. Singapore: World Scientific Publishing; 2015. 3D Printing and Additive Manufacturing:Principles and Applications. [Google Scholar]
- 68.Luo Y, Lode A, Akkineni AR, et al. Concentrated Gelatin/alginate Composites for Fabrication of Predesigned Scaffolds with a Favorable Cell Response by 3D Plotting. RSC Adv. 2015;5:43480–8. DOI:10.1039/c5ra04308e. [Google Scholar]
- 69.Sun C, Fang N, Wu DM, et al. Projection Microstereolithography Using Digital Micro-mirror Dynamic Mask. Sens Actuators A Phys. 2005;121:113–20. DOI:10.1016/j.sna.2004.12.011. [Google Scholar]
- 70.Hölzl K, Lin S, Tytgat L, et al. Bioink Properties Before, During and after 3D Bioprinting. Biofabrication. 2016;8(032002) doi: 10.1088/1758-5090/8/3/032002. DOI:10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 71.Jang TS, Jung HD, Pan HM, et al. 3D Printing of Hydrogel Composite Systems:Recent Advances in Technology for Tissue Engineering. Int J Bioprint. 2017;4:1–28. doi: 10.18063/IJB.v4i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donderwinkel I, van Hest JC, Cameron NR. Bio-inks for 3D Bioprinting:Recent Advances and Future Prospects. Polym Chem. 2017;8:4451–71. DOI:10.1039/c7py00826k. [Google Scholar]
- 73.Sayyar S, Gambhir S, Chung J, et al. 3D Printable Conducting Hydrogels Containing Chemically Converted Graphene. Nanoscale. 2017;9:2038–50. doi: 10.1039/c6nr07516a. DOI:10.1039/c6nr07516a. [DOI] [PubMed] [Google Scholar]
- 74.Fantino E, Roppolo I, Zhang D, et al. 3D Printing/Interfacial Polymerization Coupling for the Fabrication of Conductive Hydrogel. Macromol Mater Eng. 2018;303(1700356) DOI:10.1002/mame.201700356. [Google Scholar]
- 75.Odent J, Wallin TJ, Pan W, et al. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv Funct Mater. 2017;27(1701807) DOI:10.1002/adfm.201701807. [Google Scholar]
- 76.Zhou Y, Layani M, Wang S, et al. Fully Printed Flexible Smart Hybrid Hydrogels. Adv Funct Mater. 2018;28(1705365) DOI:10.1002/adfm.201705365. [Google Scholar]
- 77.Heo DN, Lee SJ, Timsina R, et al. Development of 3D Printable Conductive Hydrogel with Crystallized PEDOT:PSS for Neural Tissue Engineering. Mater Sci Eng C Mater Biol Appl. 2019;99:582–90. doi: 10.1016/j.msec.2019.02.008. DOI:10.1016/j.msec.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Sekine S, Ido Y, Miyake T, et al. Conducting Polymer Electrodes Printed on Hydrogel. J Am Chem Soc. 2010;132:13174–5. doi: 10.1021/ja1062357. DOI:10.1021/ja1062357. [DOI] [PubMed] [Google Scholar]
- 79.Ahn Y, Lee H, Lee D, et al. Highly Conductive and Flexible Silver Nanowire-Based Microelectrodes on Biocompatible Hydrogel. ACS Appl Mater Interfaces. 2014;6:18401–7. doi: 10.1021/am504462f. DOI:10.1021/am504462f. [DOI] [PubMed] [Google Scholar]
- 80.Shay T, Velev OD, Dickey MD. Soft Electrodes Combining Hydrogel and Liquid Metal. Soft Matter. 2018;14:3296–303. doi: 10.1039/c8sm00337h. DOI:10.1039/c8sm00337h. [DOI] [PubMed] [Google Scholar]
- 81.Agarwala S, Lee JM, Ng WL, et al. A Novel 3D Bioprinted Flexible and Biocompatible Hydrogel Bioelectronic Platform. Biosens Bioelectron. 2018;102:365–71. doi: 10.1016/j.bios.2017.11.039. DOI:10.1016/j.bios.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 82.Jaffari SA, Turner AP. Recent Advances in Amperometric Glucose Biosensors for In vivo Monitoring. Physiol Meas. 1995;16:1–15. doi: 10.1088/0967-3334/16/1/001. DOI:10.1088/0967-3334/16/1/001. [DOI] [PubMed] [Google Scholar]
- 83.Wisniewski N, Moussy F, Reichert WM. Characterization of Implantable Biosensor Membrane Biofouling. Fresenius J Anal Chem. 2000;366:611–21. doi: 10.1007/s002160051556. DOI:10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 84.Abraham S, Brahim S, Ishihara K, et al. Molecularly Engineered p(HEMA)-Based Hydrogels for Implant Biochip Biocompatibility. Biomaterials. 2005;26:4767–78. doi: 10.1016/j.biomaterials.2005.01.031. DOI:10.1016/j.biomaterials.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 85.Saha S, Sarkar P, Sarkar M, et al. Electroconductive Smart Polyacrylamide-polypyrrole (PAC-PPY) Hydrogel:A Device for Controlled Release of Risperidone. RSC Adv. 2015;5:27665–73. DOI:10.1039/c5ra03535j. [Google Scholar]
- 86.Tao Y, Cheng G, Zhang M, et al. A General Route to 2D Nanoleaves and Nanoplates of Polyaniline. Russ J Phys Chem A. 2015;89:2267–70. [Google Scholar]
- 87.Sharma K, Kaith BS, Kumar V, et al. Gum Ghatti Based Novel Electrically Conductive Biomaterials:A Study of Conductivity and Surface Morphology. Express Polym Lett. 2014;8:267–81. DOI:10.3144/expresspolymlett.2014.30. [Google Scholar]
- 88.Sharma K, Kumar V, Kaith BS, et al. Evaluation Of Conducting Interpenetrating Network Based On Gum Ghatti-G-Poly (Acrylic Acid-Aniline) As Colon-Specific Delivery For Amoxicillin Trihydrate And Paracetamol. New J Chem. 2015;39:3021–34. DOI:10.1039/c4nj01982b. [Google Scholar]
- 89.Sharma K, Kumar V, Chaudhary B, et al. Application of Biodegradable Superabsorbent Hydrogel Composite Based on Gum Ghatti-co-poly (Acrylic Acid-aniline) for Controlled Drug Delivery. Polym Degrad Stab. 2016;124:101–11. DOI:10.1016/j.polymdegradstab.2015.12.021. [Google Scholar]
- 90.Tandon B, Magaz A, Balint R, et al. Electroactive Biomaterials:Vehicles for Controlled Delivery of Therapeutic Agents for Drug Delivery and Tissue Regeneration. Adv Drug Deliv Rev. 2018;129:148–68. doi: 10.1016/j.addr.2017.12.012. DOI:10.1016/j.addr.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Weaver CL, LaRosa JM, Luo X, et al. Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano. 2014;8:1834–43. doi: 10.1021/nn406223e. DOI:10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catt K, Li H, Hoang V, et al. Self-powered Therapeutic Release from Conducting Polymer/graphene Oxide Films on Magnesium. Nanomed Nanotechnol Biol Med. 2018;14:2495–503. doi: 10.1016/j.nano.2017.02.021. DOI:10.1016/j.navno.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balint R, Cassidy NJ, Cartmell SH. Conductive Polymers:Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014;10:2341–53. doi: 10.1016/j.actbio.2014.02.015. DOI:10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 94.Kawahara Y, Yamaoka K, Iwata M, et al. Novel Electrical Stimulation Sets the Cultured Myoblast Contractile Function to On. Pathobiology. 2006;73:288–94. doi: 10.1159/000099123. DOI:10.1159/000099123. [DOI] [PubMed] [Google Scholar]
- 95.Pedrotty DM, Koh J, Davis BH, et al. Engineering Skeletal Myoblasts:Roles of Three-dimensional Culture and Electrical Stimulation. Am J Physiol Heart Circ Physiol. 2005;288:H1620–6. doi: 10.1152/ajpheart.00610.2003. DOI:10.1152/ajpheart.00610.2003. [DOI] [PubMed] [Google Scholar]


