Abstract
An additive manufacturing technology based on projection light, digital light processing (DLP), three-dimensional (3D) printing, has been widely applied in the field of medical products production and development. The precision projection light, reflected by a digital micromirror device of million pixels instead of one focused point, provides this technology both printing accuracy and printing speed. In particular, this printing technology provides a relatively mild condition to cells due to its non-direct contact. This review introduces the DLP-based 3D printing technology and its applications in medicine, including precise medical devices, functionalized artificial tissues, and specific drug delivery systems. The products are particularly discussed for their significance in medicine. This review indicates that the DLP-based 3D printing technology provides a potential tool for biological research and clinical medicine. While, it is faced to the challenges of scale-up of its usage and waiting period of regulatory approval.
Keywords: Digital light processing, Three-dimensional print, Medical devices, Tissue engineering, Pharmacy
1 Introduction
Three-dimensional (3D) printing, a rapidly emerging technology, can construct products with complex geometric features by depositing materials according to the digital files[1-3]. This neoteric technology provides a rapid manufacturing method to fabricate medical products that are usually complex and urgent. In addition, 3D printing can fabricate customized products with complex accurate structure at comparatively low costs, especially in personalized medical application. This has important implications for a unique patient[4]. Therefore, 3D printing technology has profoundly impacted biomedicine for fabricating various complex and personalized medical products. In the meanwhile, the application of these products continues to grow rapidly. The latest forecast is that the global market for medical products manufactured through 3D printing will reach nearly 26 billion dollars by 2022, while the market related to bio-printing will exceed 1.3 billion dollars[3].
Digital light processing (DLP)-based 3D printing is utilizing projection light to polymerize materials to obtain the pre-designed structures[5]. Compared with other 3D printing methods, such as extrusion-based 3D printing technology and inkjet-based 3D printing technology, this technology has significant advantages in printing resolution, efficiency, and working condition. Hence, it can give many good features to the products. The medical models have fine shape and physical property for simulating human body. Additionally, favorable structures can be integrated into implants to promote tissue regeneration. Besides, artificial tissues and organs are built with precise bionic structure and high cell viability. Last but not least, customized drug delivery system can provide a nuanced solution to controlled release, accurate drug dosages, and minimally invasive delivery[6-10].
This review introduces the DLP-based 3D printing technology and its applications in medical field. The principle and characteristics of DLP printing technology are introduced. Then, the main applications demonstrate the influence of DLP-based 3D printing to medicine (Figure 1). Finally, this review culminates with the limitations of existing techniques and future research directions.
Figure 1.
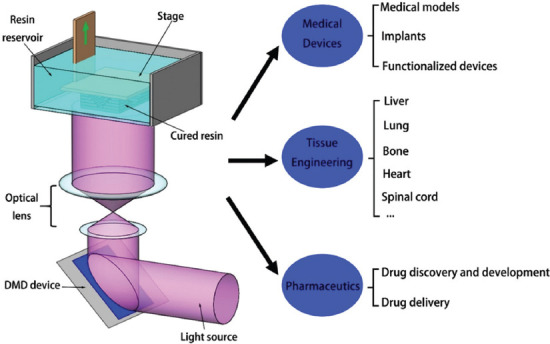
This schematic diagram shows the working principle and related application fields related to, DLP based 3D printing technology.
2 DLP-based 3D printing
2.1 DLP-based 3D printing technology
The concept of 3D printing is first described in 1986 by Charles[11]. The method, named stereolithography, sequentially print many layers by ultraviolet light to create 3D structures. With the development of additive manufacturing technology and material science, various 3D printing methods have emerged. There are two common types of 3D printing methods: The nozzle-based and the light-based 3D printing. The nozzle-based 3D printing includes extrusion printing and inkjet printing. In these printing methods, the printed materials are extruded or jetted and deposited onto the platform. The light-based 3D printing includes DLP printing, laser assisted printing, selective laser melting (SLM), and selective laser sintering (SLS). The DLP and laser assisted printing methods apply photopolymerization reactions. During the process of SLM and SLS, material powders are melted and reshaped at the high temperature created by laser. These 3D printing platforms have significant differences in printing mechanism, speed, material selection, and resolution[12,13]. Table 1 compares the DLP printing with other 3D techniques used in the medical field.
Table 1.
| Properties | Materials | Printing process | Resolution (μm) | Speed | Cell viability |
|---|---|---|---|---|---|
| Nozzle based printing | |||||
| Extrusion | Thermoplastic polymer | Serial (line by line) | 50 | Slow (μm/s) | 40-80% |
| Inkjet | Thermoplastic polymer | Serial (drop by drop) | 50 | Medium (mm/s) | >85% |
| Light based printing | |||||
| DLP | Photosensitive polymer | Continue Plane (layer by layer) | 6 | Fast (mm³/s) | 85-95% |
| Laser assisted | Photosensitive polymer | Serial (dot by dot) | ~1 | Medium (mm/s) | >85% |
| SLM/SLS | Metals and alloys powder, ceramic and polymer | Serial (dot by dot) | 80 | slow (μm/s) | — |
DLP-based 3D printing technology comes from the image projection technology developed by Texas Instruments in the 1980s[5]. This method uses a set of chipsets based on optical micro-electromechanical technology to process working light sources to photosensitive materials (Figure 1). The main functional part is a digital micro mirror device (DMD) which consists of a group of micron-sized, controllable mirrors. The mirrors rotate to control the path of light and then project it onto the photosensitive resin during working. The ordinary arrays have a large number of mirrors, from nearly a million mirrors to more than 2 million. On the other hand, the pixel spacing of the micromirror is only a few microns or a dozen microns. The resolution of the DLP-based 3D printing depends on the projection plane adjusted by DMD and lens. Thus, the DLP printing technique has a relatively high resolution, which is usually at the micron scale[12]. Furthermore, the printing conditions are mild for cells. In general, the printing process without high temperature, pressure, and shear stress caused by the nozzle is suitable for printing living tissues or organs with little cell damage. When light is projected onto the resin using DLP technology, instead of being restricted to a spot like the laser-assisting 3D printing, the entire layer is printed immediately. Hence, this technology allows fast printing[5,12].
2.2 The advancements of DLP-based 3D printing
During the past decade, the printing accuracy which is, a key indicator of 3D printing technology, has improved greatly. The accuracy improvement is related to the printing equipment, materials, and process parameters. When DLP 3D printing technology was first invented in 2006, the researchers manufactured constructs that have 20 μm microstructure[12]. In 2013, Yi et al. fabricated constructs with various topologies. In fact, the resolution of DLP 3D printing partially depends on the material chosen. When the 3D constructs are printed using polyethylene glycol diacrylate (PEGDA MW = 700 da) solely, the XY resolution of the constructs can reach nearly 6 by 6 μm. Meanwhile, the resolution is about 17 μm with bioink containing 10% gelatin-methacrylate (GelMA) and 3 × 106 cells/mL[16]. Dai et al. used a high precision DMD (each micromirror is 10.8 μm) to build the projection-based 3D printing platform. This platform can fabricate multiscale vascular channels, ranging from the main trunk channel (> 1100 μm wide) to the relatively small branch channel (up to 17 μm wide)[17]. Saha et al. combined the advantages of two-photon lithography (TPL) and DLP printing and developed a femtosecond projection TPL (FP-TPL) technique. By this method, complex 3D structures maintaining sub-500-nm features can be printed rapidly[18].
Besides accuracy, many methods were applied to improve printing speed. These methods greatly improve printing efficiency and ensure cell viability during tissue construction[19]. In 2015, John et al. modified the technique and created a continuous liquid interface fabrication method. The continuous liquid interface in this study is realized through the oxygen-permeating region under the printing platform, which forms a persistent liquid interface between the oxygen-permeating region and the photopolymerization region of the material, in which the material cannot conduct photopolymerization. When the average thickness of each layer is set at 50–100 μm, this method can manufacture a 3D structure with 5 cm high in <10 min[20]. Recently, a faster DLP 3D printing method, computed axial lithography, has been developed[21]. In this manufacturing system, light is reflected onto the photosensitive material in the form of a 2D image. When different plane images are projected onto the material from different angles, the energy generated by the multi-angle superposition exposure will cause the material to converge into the originally designed 3D structure. This technology can be scaled up to a relatively large additive manufacturing volume and is order of magnitude faster than conventional 3D printing methods.
To design products with ideal performance, the selection of appropriate materials has become an important requirement. Materials for DLP printing are photosensitive polymers that can polymerize from liquid into solid when exposed to light. The physical and chemical properties of traditional photosensitive polymers limit their applications. Most of them are incompatible with cells. What is more, the monomers and its vapors are also harmful to humans. Hence, researchers have manifolded the materials used for DLP-based 3D printing. Due to the biocompatibility and good polymerization effect, PEGDA and GelMA are widely used inks for bioprinting. Soon et al. modified silk fibroin with glycidyl methacrylate to get SF-based bioink-methyl acrylate (SiL-MA). This polymerized SiL-MA hydrogel has the characteristics of high tensile strength, which is available to suture. In addition, SiL-MA hydrogels produced by DLP 3D printing have remarkable biocompatibility, which gives the hydrogel a broad application prospect in the field of tissue engineering[22]. Many components can be mixed in the liquid printed materials to obtain functionalized constructs. The addition of piezoelectric BaTiO3 nanoparticles and electric silver nanoparticles to ink can give the products the functions of piezoelectricity and electroconductibility, respectively. Ceramic and glass products can be sintered with printing inks combining ceramic fillers or silicate[23,24]. These improvements made this manufacturing method more competitive in fabricating medical products.
Given the favorable properties of printing speed, accuracy, and materials, DLP 3D printing has great value in the rapid fabrication of precision personalized products.
3 Medical devices
A medical device is an important tool that can benefit the patient by helping health-care providers diagnose and treat patients, assisting patients to overcome sickness or disease, and improving their quality of life[25]. The 3D printing technology has been widely applied in fabricating specific medical devices based on patients’ disease. According to the statistics, the U.S. Food and Drug Administration (FDA) has approved at least 85 3D printed medical devices up to 2015[26]. These products including medical models, personalized implants, and functionalized devices.
3.1 Medical models
The solid 3D model can remedy the limitation of medical imaging technology that can only transform the information of patients’ lesions into visual graphics and display them to doctors and patients. These 3D models are tangible objects that can be operated by hand and can directly reflect patients’ conditions, providing more intuitive and convenient means for clinical medical teaching, diagnosis, and surgical planning. The 3D printing technology provides a method that can use the graphic information to create patient-specific anatomical 3D medical models[27]. So far, a series of medical models have been manufactured by various 3D printing technologies, such as skull, heart, pelvis, blood vessel, and tumor, which are beneficial to surgery assistance, disease analysis, and teaching demonstration[28,29].
Due to the fast printing speed of DLP-based 3D printing, doctors no longer have to wait for long hours to prepare 3D models of patients’ pathological organs. In the clinical treatment of various acute diseases, such as cerebral hemorrhage, cerebral infarction, and myocardial infarction, the effective treatment window period is only a few hours, requiring the doctor to prepare the patient’s surgical plan within a few minutes. On the premise of ensuring printing accuracy (~100 μm), polymeric models, up to tens of centimeters, in size can be produced in minutes[20]. This characteristic can meet the time requirement of clinical treatments of the above acute disease models. Compared with other 3D printing technologies, the DLP printing platform can flexibly set a series of manufacturing parameters, including printing time, light intensity, and even the wavelength of light. Kuang et al. designed a special grayscale light DLP 3D printing technology. This method utilizes a grayscale light to print the material that can be two-stage curing into a 3D structure that can be printed with relatively high precision and has remarkable changes in mechanical strength gradient. A bionic 3D structure with both soft muscle and hard bone was created by this printing technique (Figure 2A). When pressed lightly, the muscles are easily squeezed out of shape. However, in the process of pressing the muscles, the hard bones remain stable[6]. An other group fabricated spine-shaped phantom for stereotactic body radiation therapy and oral cavity model for orthodontics (Figure 2B and C)[30,31].
Figure 2.
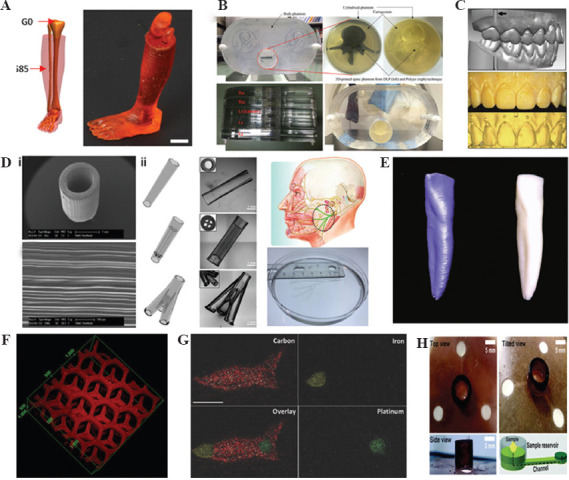
Digital light processing 3D printed medical devices: (A) A limb with soft “muscle” and stiff “bone”[6], (B) spine-shaped phantom[32]. (C) Oral cavity model[33], (D) (i) nerve conduit with microchannels[7]; (ii) a range of nerve conduits[34], (E) zirconia dental implant[35], (F) detoxification device[8], (G) toxin sensor[36], (H) blood senor for measuring glucose, cholesterol, and triglycerides[37].
In addition to the benefits of surgical assistance and disease analysis, DLP 3D printed disease model is also applicable to the teaching of clinical medicine, especially the basic subjects of medical education such as human body and pathological anatomy. For example, this technology can quickly print personalized zirconia material dental teaching model, this personalized teaching model has sufficient dimensional accuracy, and maintains clinical-pathological oral deformity so that students can get an intuitive impression of oral malformation disease[32,33].
3.2 Implants
The field of in vivo medical devices that can be printed with DLP-based 3D printers mainly includes biodegradable or non-degradable human implants made of various materials, such as metals, ceramics, and polymers. These implants are used to replace or repair the injuries in vivo. First, the shapes of the implants are well-matched with the injury parts. Then, the printed microstructure can guide the regeneration of injured tissues.
3.2.1 Biodegradable implants
Biodegradable implants refer to a series of medical implants that can be gradually degraded by the human body with the change of time and other conditions after implantation and finally replaced by human original tissues. Many biodegradable materials, such as alginate, cellulose, extracellular matrix (ECM), and collagen, have been developed and applied in DLP 3D printing. Christopher et al. used the DMD device that can print in micron scale to obtain nerve conduits with parallel microchannels (Figure 2D i). The light is reflected by the 20 μm size mirrors of the device. This translates the 2D image into a 3D microstructure that is identical to the micron array of mirrors. The microstructure can guide Schwann cell-directed migration. Tao et al. created similar nerve conduits encapsulating nanoparticles. The drugs loaded in nanoparticles can further improve the regeneration of injured nerve[38]. For treating different injury sites, Zhu et al. have utilized this technique to create a series of nerve conduits, such as conduits with microchannels and even bionic conduits that are beneficial to nerve repair in humans (Figure 2D ii)[34].
3.2.2 Non-biodegradable implants
DLP 3D printed implants play an important role in stomatology, brain surgery, ent (ear, nose and throat), thoracic surgery, and other surgical operations. Reham et al. reported that they used DLP technology to fabricate a customized zirconia implant and evaluated its related physical properties (Figure 2E). In this study, they evaluated the precision size, surface morphology, and physical properties of printed zirconia implants[39]. The results show that this printed custom implant has a precision size similar to the reference model and competitive bending strength (943 MPa), which can be compared with grinding zirconia (800–1000 MPa). In addition, DLP technology can also quickly print photosensitive resin materials. These resin materials have been widely used as a filling materials in stomatology due to the characteristics of low viscosity, curing and shrinking, fast processing rate, small swelling, and high wet strength[35].
3.3 Functionalized devices
Besides satisfying shapes and microstructures of DLP printed 3D models and implants, it can add specific functions to medical devices. These devices take the fixability to combine materials and structures to achieve their functions. Due to the efficiency and high accuracy of DLP printing, it only takes a short time to fabricate medical devices with powerful functions.
In 2014, Gou et al. loaded the printed hydrogel with polydiacetylene (PDA) nanoparticles to construct a detoxification device (Figure 2F). The lobule-like microstructures allow efficient contact between toxins and the 3D devices. At the same time, detoxification particles in the hydrogel can trap and attract toxins while sensing them. It turns out that the printed detoxification hydrogel device can effectively remove certain toxins from aqueous solutions[8]. The combination of PDA nanoparticles and 3D printed hydrogels not only act as a detoxification device but also a toxin sensor in solutions. When the nanoparticles bind to the toxin, the ordered π-conjugated chain structure on the particle surface is disrupted, triggering a fluorescent radiation reaction that makes it easy to detect the presence of the toxin. Zhu et al. printed swimming microfish encapsulated PDA, iron oxide, and platinum nanoparticles (Figure 2G). The microfish can be chemically powered and magnetically guided. They took the fluorescence intensity change of PDA as an indicator to evaluate the detoxification efficiency of hydrogels and the concentration of toxins in solution[36]. Recently, a 3D paper-based microfluidics analysis device (3D-μPAD) has been reported for in vitro diagnosis (Figure 2H). The 3D-μPAD is produced by a DLP 3D printer using a material called photoluminescent liquid resin, which is printed on both sides of the paper in one go, with no additional assembly required. This 3D-μPAD could be used to measure glucose, cholesterol, and triglycerides in human blood[40].
4. Tissue engineering
Tissue engineering aims at improving or replacing biological functions to improve clinical procedures for repairing damaged tissues and organs[41]. The 3D bioprinting allows cells, materials, and biological factors reasonably distributed in the construct. It provides a new method for building artificial tissue[2]. DLP-based 3D printing has a great advantage in bioprinting for its mild printing condition. It causes little damage to functional components, including cells, biological factors, and biomaterials[42]. The DLP 3D bioprinting technology has been applied to construct a range of tissues and organs, such as liver, cardiac, vascularized, and cancer models. In the following part, some typical applications are discussed in detail[8,43-45].
4.1 Liver
The liver is the largest internal organ and gland in the human body. Many important physiological functions are performed by it, which include bile secretion, detoxification, drug metabolism, and producing serum proteins. So, hepatic diseases have significant influence on patient. Actually, the liver has a certain degree of self-regeneration ability. However, many severe diseases, such as hepatocellular carcinoma and cirrhosis, can reduce or even eliminate the regenerative ability of the liver. For these patients, a common treatment is transplanting a new healthy liver to replace the old one. There is forecast predicting that the demand for liver transplantation will increase by 23% in the next two decades. Aside from the high cost of surgery, donors and their availabilities are very limited[46]. Thus, the artificial liver has wide application prospects.
In the past decades, people have been trying to obtain artificial liver tissue. However, due to the complex structure and various cell types, it is hard to construct an artificial liver with multiple functions. The bioprinting technology has promoted the development of engineered liver tissue. It can fabricate the interwoven networks such as bile duct, lymphatic, and vessels in the natural liver. DLP-based 3D printing technology has the potential of accurately patterning cells and biomaterials and maintaining high activity. Taking advantage of this technology, engineered livers’ constructs are more precise with increasing structural and functional complexity[47].
Ma et al. used a DLP-based 3D printer to obtain a 3D triculture hepatic model. The model consists of many micro hexagonal structures and encapsulated three kinds of cells, including human induced pluripotent stem cells (hiPSC)-hematopoietic progenitor cells (HPCs), human umbilical vein endothelial cells (HUVECs), and adipose-derived stem cells. (Figure 3A i)[48]. By characterizing the cell morphology, the liver-specific gene expression levels, metabolic product, and cytochrome P450 (CYP) induction, they found that the hiPSC-HPCs in the hepatic models are very similar to hepatic cells in human body. The results indicated that the microstructure and supporting cells can promote the maturation of hiPSC-HPCs and maintain their functions. Zhu et al. fabricated a relatively simple model that encapsulated HUVECs and HepG2 (Figure 3A ii)[47]. Grigoryan et al. used DLP printing technique to fabricate artificial liver with functional intravascular topologies[9]. Compared with tissues containing single hepatocyte, the vascularized liver containing hepatocyte aggregates showed 60 times activity of the albumin promoter. Furthermore, the DLP 3D printed vascularized liver tissues appeared better integration with host after implanted in vivo.
Figure 3.
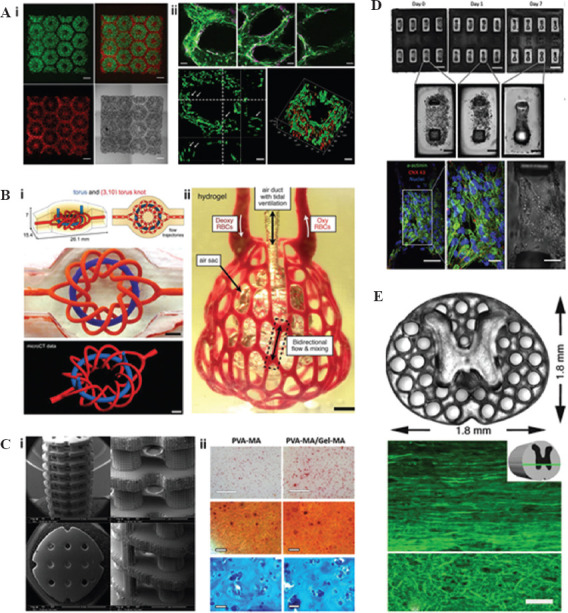
Digital light processing 3D printing of artificial tissues: (A) (i) Hepatic structure containing human induced pluripotent stem cells (hiPSC)-hematopoietic progenitor cells (green) and supporting cells (red)[48]; (ii) endothelial network of prevascularized hepatic tissue[47], (B) (i) multivascular network; (ii) vascularized lung tissue[9], (C) (i) bone tissue engineering scaffold[51]; (ii) staining of cells encapsulated in bone tissue engineering hydrogel[52], (D) hiPSC-derived cardiac tissue on force gauge[53], (E) spinal cord and its horizontal section[54].
4.2 Lung
Lung is one of the main functional organs of the respiratory system. The function of lung is to extract and transfer oxygen into human blood and release carbon dioxide to the air. However, human lungs have no regenerative capacity. Constructing artificial lung to replace diseased lung is the only alternative to treat patients with severe lung diseases[49]. They are faced with the same problems as liver disease patients. Therefore, the construction of artificial lung is one of the best solutions.
The lung contacts air and blood at the same time. Thus, it takes huge challenges to construct a satisfying artificial lung. The 3D printing has helped fabricate lung tissue analog[49]. Lenke et al. designed a lung model to simulate air-blood barrier architecture (Figure 3B). The model consisted of two layers: The endothelial layer containing HUVEC and A549 cells and thin Matrigel layer[50]. They have not evaluated the functional of the printed membrane model. However, natural lungs are 3D and can “breathe”. Grigoryan et al. added a nontoxic light blocker to improve the z-resolution of DLP 3D printing and fabricated vascularized alveolar model topologies[9]. The alveolar model showed two major functions of lung tissues that are blood flowing and air exchanging. Circulating moist 10 kPa, 0.5 Hz oxygen gas, the distension of the structures, and the shape changes of the concave airway regions could be found. In the meantime, they perfuse the deoxygenated red blood cells (RBCs) at the blood vessel inlet. Then, they saw the RBCs became clear after passing through the vessels adjacent to airway. In this work, the high precision of DLP 3D printing lent them the ability to fabricate 3D entangled networks in organs.
4.3 Bone
Bone is a dynamic vascularized tissue that can repair and remodel itself without leaving scars[55]. However, for critical size bone defects, bone replacement or surgical intervention is usually required[56]. Besides the autograft and allografts, the implant made by biocompatible metal or ceramic is an alternative to repair the injured bone. However, the inert implant will slowly break down with time. Therefore, active bone tissue is considered as an ideal implant to replace the injured part[14,57]. The 3D bioprinting allows precise bone scaffolds to be fabricated and a variety of cells arranged in the scaffolds to form bionic bone constructs.
The bone tissue engineering scaffolds are required to fit the defect site, allow transport of nutrient and growth factor, and degrade over time. DLP 3D printing has demonstrated a very important prospect to construct these scaffolds. Dean et al. took advantage of the high accuracy of DLP 3D printing to fabricate tissue-engineered bone scaffolds (Figure 3C i) [51]. The printed microstructure improved the adhesion, proliferation, and maturation of the cells. The degradation velocity of the scaffolds can also be adjusted by the microstructure. Recently, Lim et al. encapsulated mesenchymal stem cells (MSCs) in hydrogels to obtain bone or cartilage tissues[52]. They developed a new bioink by combining methacrylate polyvinyl alcohol (PVA-MA) and GelMA. Using this bioink and a DLP 3D printer, they printed some biologically relevant scaffolds with precise microstructures. The MSCs encapsulated in printed hydrogels maintain high viability (≈90%) after 21 days of culture. Through staining analysis, osteogenic differentiation, and cartilage-specific ECM were observed in the 3D printed hydrogel (Figure 3C ii). The result indicated potential applications of DLP 3D printing and the bioink in bone tissue engineering.
4.4 Heart
Cardiovascular diseases are the foremost cause of death over the world. The heart-valve replacement is a common treatment for valvular heart diseases. There are two kinds of artificial heart valves, including mechanical valves and biological valves (autograft and allograft). The mechanical valves lead to the risk of thrombus. The autograft implantation is a complex surgery that requires cutting the other part of the patient. On the other hand, the allograft always causes an immune response. In addition, the biological valves may fail more than 10 years after implantation[58,59]. Thus, a new strategy is necessary to be developed to fabricate artificial myocardium replacements.
Some studies have been carried out to develop 3D cardiac tissue in vitro. However, the cardiomyocyte is usually seeded on or encapsulated in a simple 3D scaffold in these studies[60]. It is very easy to culture cardiomyocyte and maintains its viability in vitro. However, the key point of fabricating artificial cardiac tissue is obtaining biomimetic structures and promoting cardiomyocyte orientation alignment. Liu et al. blended human embryonic stem cell-derived cardiomyocytes (hESC-CMs) into 3D pattern hydrogel constructs by a DLP-based 3D printer (Figure 3D)[61]. The DLP-based 3D printing technology allowed hESC-CMs to mimic the multilayered aligned myocardium. Meanwhile, they printed a customizable cantilever-based force detector to measure the force from the artificial cardiac model. Further to this, they encapsulated a specific hESC line that is sensitive to calcium. Then, they can detect the calcium transient of the cardiac model. Recently, this group used DLP-based 3D printing technology to obtain artificial cardiac tissue and detect the expression of mature cardiac marker genes. The work will promote the development of artificial cardiac tissue[53].
4.5 Spinal cord
The spinal cord, together with the brain, is called the central nervous system (CNS). It is a cylindrical structure and consists of nerve fibers and associated tissues. In USA, there are more than 50,000 people who are suffering from the diseases of spinal cord injury (SCI). Due to the importance of CNS, SCI causes significant influence on patients and is hard to cure. Recently, the artificial spinal cord shows great potential in assisting SCI repair[54,62,63].
The 3D printing technology allows the fabrication of personalized scaffolds that are matched with the patients’ injured sites. The microstructure can promote SCI repair by stimulating, guiding, and aligning axon. However, due to the complex inner structure of the spinal cord, it is difficult to construct a spinal cord structure in detail[62]. The technology of DLP 3D printing provides a method to manufacture such complex and precise structures. Using this rapid 3D printing technology, Koffler et al. printed 3D biomimetic hydrogel scaffolds suitable for rodent spinal cord size in several seconds (Figure 3E)[54]. The printed scaffolds can encapsulate the neuronal progenitor cells (NPCs) and promote axonal regeneration. After implanting the bionic scaffold to replace the injured site, the damaged axons regenerated and entered to the scaffold and synapsed with NPCs. The NPCs extended to and synapsed with the host spinal cord below the injury. The scaffold formed a new “contact” across the entire spinal cord and improved the recovery of the function of spinal cord. The artificial spinal cord tissue can be extended to human spinal cord size and fit to any shapes.
The 3D bioprinting has been widely used in fabricating various tissues and organs. Inkjet and extrusion bioprinters are good methods to solve part of the problems in tissue engineering. However, these two methods also have limitations. First, they are nozzle-based 3D printing technology. Due to the small aperture of the nozzle, used to deliver bio-ink, cells will be stressed and damaged during passing through the nozzle. Next, the printing resolution is limited by the aperture of the nozzle, which is usually bigger than 50 μm. Finally, the printing speed of these methods is not very high. Cells would subside to the bottom of the printing ink in the process of printing[16,64,65]. DLP 3D bioprinting is a rapid, precise and mild printing method. The printing speed is approximately ~1000× times faster than the traditional nozzle printers[16]. The X- and Y-resolutions can reach 6 μm. Thus, DLP 3D bioprinting has advantages in fabricating living tissues with complex and miniscule structure, such as liver, lung and other vascularized tissues.
5 Pharmacy
The 3D printing technology can be used in all phases of pharmacy research, including drug discovery, development, and delivery[66]. Despite the continuous improvement of detection equipment and technology, achievement in discovery and development require a long time and high cost, which are mainly reflected in screening drugs from a large number of candidates and researching their absorption, metabolization, toxicity, etc. As described in the previous section, the DLP 3D printed tissue or organ or disease model can simulate the real situation of human body. It can be used to discover and develop drugs in vitro and replace part animal experiments[42]. High-accuracy 3D printing allows the fabrication of pharmaceutical preparations with fine structures, and control the position and dose of drugs precisely.
5.1 Drug discovery and development
In the early stage of drug development, 3D printing products have important applications in early and high throughput drug screening due to their complex bionic structure and good repeatability[67]. It is difficult to get a satisfying result by applying traditional 2D and 3D disease models for drug screening. These disease models lack bionic structures, vascular networks, and complex microenvironments. In the section of tissue engineering, it has been discussed that DLP-based 3D printing can build complicated vascularized tissues, which can also be used in fabricating disease models correspond to those in the human body. If these models were applied in drug screening, the results might be more accurate. On the cover glass, an alginate loaded gel chip loaded with Escherichia coli was printed on the cover glass as a platform for high-throughput screening of micro drugs[68]. Three drops of the antibiotic mixture (penicillin/streptomycin, antifungal agent, and kanamycin sulfate) were added to evaluate the bioactivity, function, and antibacterial activity of antibiotics. Thus, DLP-based 3D printing provides a rapid method for disease modeling. Especially for precision medicine, high throughput drug screening is very important. The disease model based on patient’s cell can get an accurate screening result. Besides drug screening, the researches of cancer models can provide new solutions for cancer therapy.
Developing a drug, it is required to study its absorption, metabolization, excretion, optimal dosage, and toxicity. Most measurements are carried out in the traditional 2D monolayer culture system, which cannot simulate the natural 3D tissue microenvironment. Hence, the results are greatly different between the in vivo and in vitro tests[69]. Artificial tissue fabricated by 3D printing can simulate the state of the tissue in vivo more effectively and can quickly and cheaply carry out pharmacological, toxicological, and pharmacokinetic studies, so as to reduce the losses caused by pharmacodynamic, and toxicity problems found only in animal or clinical trials[14,70]. The 3D printed liver and kidney tissues have been applied to testing drug metabolism. For example, Ma et al. studied drug metabolism by their printed hepatic model. Treating the biomimetic hepatic model with an inducer, they characterized the expression levels of CYP which includes several key enzymes for drug metabolism. They found significant increases in three key enzymes. The positive response indicated that the inducer can be cleared by liver.
5.2 Drug delivery
As a manufacturing technology, DLP-based 3D printing technology can also be used in the preparation of the drug delivery system with customized dosages, shapes, sizes, and release ways. In 2015, the FDA approved a 3D-printed drug to accurately control the structure and dosage of the drug. Through 3D printing technology, the tablet is constructed in microporous structures, which will dissolve immediately after oral administration. Patients who have difficulties in swallowing can easily take the tablet[71]. This technology also encourages the development of 3D printed drug delivery system. The delivery system can also mix or print multi-drug layers to control drug release. Adding drugs at fixed points on drug delivery vectors can control the time of drug release to achieve the purpose of accelerating or delaying drug release. Printing different drug layers can make drugs release at intervals according to their structural characteristics. Changing 3D printing can make drug release at intervals according to their structural characteristics[72]. The filling degree of the printed preparation can accurately customize personalized drugs containing different dosages. Liu et al. fabricated microhydrogels with predesigned shapes and sizes to carry drugs. The microhydrogels are small enough to be injected through a 1-mL syringe in a noninvasive way[10]. Tao et al. encapsulated PDA nanoparticles into the microhydrogels (Figure 4A). Then, the microhydrogels are injected into the location of bacterial infection to improve tissue recovery[73]. The 3D printed implants can act as drug delivery systems. The 3D printed post-operative filler of drug-loaded gene drugs for patients with glioma, which can continuously release drugs to eliminate residual tumor cells and avoid recurrence of tumors[74]. Xu et al. encapsulated RGFP966 nanoparticle into 3D printed nerve conduits to improve nerve regeneration and repair (Figure 4B)[75]. Taking theophylline as the model drug, Hossam et al. used PEGDA at various concentrations to fabricate tablets (Figure 4C). Then, they studied the release of theophylline from the printed tablets. The DLP-based 3D printing is flexible in fabricating personalized drug delivery systems by changing printed structure, polymer concentration, and printer parameters, including light intensity and wavelength and exposure time. Therefore, this printing technology has great potential in fabricating personalized drugs[44].
Figure 4.
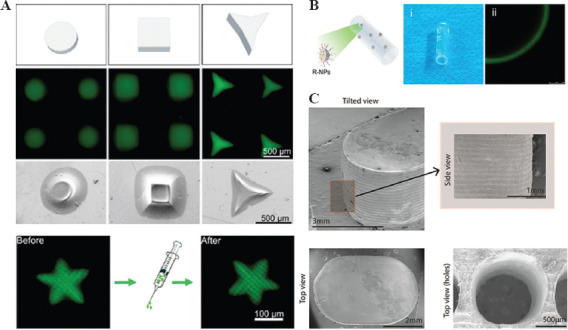
DLP 3D printing of drug delivery system: (A) A range of injectable microhydrogels encapsulating drugs[73], (B) functionalized nerve conduits[75], (C) microstructure of 3D printed tablets[44].
Taking advantage of DLP-based 3D printing technology, printed products can significantly save the drug discovery time and cost and accelerate the accuracy of drug screening. Meanwhile, the printed tissues or cancer models formed by the patient’s own cells could be used for precision medicine. Last but not least, the DLP-based 3D printed drugs or drug carriers can be designed with specific structures, precise dosages, and personalized release ways.
6 Challenges and future perspectives
As one of the 3D printing techniques, DLP-based 3D printing inherits the advantages of fabricating complex personalized productions. Meanwhile, this technique has its own characteristics of high precision, fast speed, and mild condition. Thus, on the basis of conventional medical applications, DLP-based 3D printing can improve the accuracy and cell livability of the fabricated products. As a result, the medical models manufactured by DLP 3D printing can better simulate the real states or locate implants. The devices with precision structures are more sensitive. The microstructures of tissues can be accurately built to obtain engineered tissues and organs for organ transplantation, disease research, and drug development. Furthermore, DLP 3D printing can manufacture predesigned microstructures of pharmaceutical and accurately control the position and doses of drugs.
The printing equipment and materials are the foundation of applications in medicine, which are increasingly developed. Some breakthroughs have been made. Continuous liquid interface printing improved the resolution and speed of DLP 3D printing[20]. Using tomographic reconstruction can fabricate a volumetric construct at once[21]. Metal, glass, and ceramic products can be obtained through printing and sintering. More and more materials are developed in DLP-based 3D printing[76]. For example, the materials include functionalized materials, initiator-free materials, and non-toxic initiators. Although DLP 3D printing technology has made advancements, it still takes some deficiencies[12]. The materials choice is limited to only photosensitive polymers. Many excellent materials cannot be printed directly. They are required to be combined with photosensitive polymer or chemical modified by photosensitive groups. To accelerate the photopolymerization, photoinitiator, mostly having toxicity, is required to be added in the mixed inks. When the light is projected from top-down in DLP-based 3D printer, the materials are not extruded by nozzles, but pre-filled the container. It may raise the concerns of materials wastage and cost increases. With the development of 3D printing technology, there will be more equipment and materials emerging in the future. There is forced onto the cells as well when the printing platform is lowered or the platform is separated from the printed surface. The repeated lifting of printing platform should be avoided or reduced during printing. More studies are required to reduce the separating force between the platform and the printed surface. Meanwhile, more clinical researches and improved law systems are required to regulate the use of 3D printed products. The clinical researches would provide a good foundation for making the rules. These, the rules could guide doctors or researchers to use 3D printing technology for medical applications.
Author contributions
J. Z. and Q. H. are co-first authors, who contributed equally to this work. S. W., J. T. and M.G. gave advice and discussion. M. G. supervised the project. All authors read and approved the final manuscript.
Conflict of interest
No conflict of interest was reported by the authors.
Acknowledgments
This work was supported by the Key Research and Development Projects of People’s Liberation Army (BWS17J036), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18017) and the Science and Technology Project of Chengdu (2018-CY02-00041-GX).
References
- 1.Kang HW, Lee SJ, Ko IK, et al. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat Biotechnol. 2016;34(3):312–9. doi: 10.1038/nbt.3413. DOI:10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32(8):773–85. doi: 10.1038/nbt.2958. DOI:10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 3.Ricles JCC, Di Prima M, Oh SS. Regulating 3D-Printed Medical Products. Sci Transl Med. 2018;10(461):6. doi: 10.1126/scitranslmed.aan6521. [DOI] [PubMed] [Google Scholar]
- 4.Rybicki FJ. Medical 3D Printing and the Physician-Artist. Lancet. 2018;391(10121):651–2. doi: 10.1016/S0140-6736(18)30212-5. DOI:10.1016/s0140-6736(18)30212-5. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Mapili G, Suhali G, et al. A Digital Micro-Mirror Device-Based System for the Microfabrication of Complex, Spatially Patterned Tissue Engineering Scaffolds. J Biomed Mater Res A. 2006;77(2):396–405. doi: 10.1002/jbm.a.30601. DOI:10.1002/jbm.a.30601. [DOI] [PubMed] [Google Scholar]
- 6.Kuang X, Wu J, Chen K, et al. Grayscale Digital Light Processing 3D Printing for Highly Functionally Graded Materials. Sci Adv. 2019;5(5):eaav5790. doi: 10.1126/sciadv.aav5790. DOI:10.1126/sciadv.aav5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pateman CJ, Harding AJ, Glen A, et al. Nerve Guides Manufactured from Photocurable Polymers to Aid Peripheral Nerve Repair. Biomaterials. 2015;49:13. doi: 10.1016/j.biomaterials.2015.01.055. DOI:10.1016/j.biomaterials.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Gou M, Qu X, Zhu W, et al. Bio-Inspired Detoxification Using 3D-Printed Hydrogel Nanocomposites. Nat Commun. 2014;5:3774. doi: 10.1038/ncomms4774. DOI:10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular Networks and Functional Intravascular Topologies Within Biocompatible Hydrogels. Science. 2019;364:8. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Tao J, Liu J, et al. 3D Printing Enabled Customization of Functional Microgels. ACS Appl Mater Interfaces. 2019;11(13):12209–15. doi: 10.1021/acsami.8b18701. DOI:10.1021/acsami.8b1⇽. [DOI] [PubMed] [Google Scholar]
- 11.Hull CW, Spence ST, Lewis CW, et al. Stereolithographic Curl Reduction US, US 5772947 A 1998 [Google Scholar]
- 12.Ngo TD, Kashani A, Imbalzano G, et al. Additive Manufacturing (3D Printing):A Review of Materials, Methods, Applications and Challenges. Compos Part B. 2018;143:172–96. DOI:10.1016/j.compositesb.2018.02.012. [Google Scholar]
- 13.Ligon SC, Liska R, Stampfl J, et al. Polymers for 3D Printing and Customized Additive Manufacturing. Chem Rev. 2017;117(15):10212–90. doi: 10.1021/acs.chemrev.7b00074. DOI:10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayavenkataraman S, Yan WC, Lu WF, et al. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv Drug Deliv Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. DOI:10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhu W, Ma X, Gou M, et al. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr Opin Biotechnol. 2016;40:103–12. doi: 10.1016/j.copbio.2016.03.014. DOI:10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Soman P, Chung PH, Zhang AP, et al. Digital Microfabrication of User-Defined 3D Microstructures in Cell-Laden Hydrogels. Biotechnol Bioeng. 2013;110(11):3038–47. doi: 10.1002/bit.24957. DOI:10.1002/bit.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue D, Wang Y, Zhang J, et al. Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Appl Mater Interfaces. 2018;10(23):19428–35. doi: 10.1021/acsami.8b03867. DOI:10.1021/acsami.8b03867. 1. 18. [DOI] [PubMed] [Google Scholar]
- 18.Saha SK, Wang D, Nguyen VH, et al. Scalable Submicrometer Additive Manufacturing. Science. 2019;366(6461):105–109. doi: 10.1126/science.aax8760. DOI:10.1126/science.aax∸. [DOI] [PubMed] [Google Scholar]
- 19.Bernal PN, Delrot P, Loterie D, et al. Volumetric Bioprinting of Complex Living-Tissue Constructs Within Seconds. Adv Mater. 2019;31:e1904209. doi: 10.1002/adma.201904209. DOI:10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 20.Tumbleston JR, Shirvanyants D, Ermoshkin N, et al. Continuous Liquid Interface Production of3D Objects. Science. 2015;347:5. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 21.Kelly BE, Bhattacharya I, Heidari H, et al. Volumetric Additive Manufacturing Via Tomographic Reconstruction. Science. 2019;8(363):1075–9. doi: 10.1126/science.aau7114. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Yeon YK, Lee JM, et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat Commun. 2018;9(1):1620. doi: 10.1038/s41467-018-03759-y. DOI:10.1038/s41467-018-03759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K, Zhu W, Qu X, et al. 3D Optical Printing of Piezoelectric Nanoparticle-Polymer Composite Materials. ACS Nano. 2014;8:8. doi: 10.1021/nn503268f. [DOI] [PubMed] [Google Scholar]
- 24.Fantino E, Chiappone A, Roppolo I, et al. 3D Printing of Conductive Complex Structures with In Situ Generation of Silver Nanoparticles. Adv Mater. 2016;28(19):3712–7. doi: 10.1002/adma.201505109. DOI:10.1002/adma.201505109. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration, is the Product A Medical Device? Available from:https://www.fda.gov/medical-devices/classify-your-medical-device/product-medical-device . [Google Scholar]
- 26.Rindelaub JD, Baird Z, Lindner BA, et al. Identifying Extractable Profiles from 3D Printed Medical Devices. PLoS One. 2019;14(5):e0217137. doi: 10.1371/journal.pone.0217137. DOI:10.1371/journal.pone.0217137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pashuck ET, Stevens M. From Clinical Imaging to Implantation of 3D Printed Tissues. Nat Biotechnol. 2016;34(3):295–6. doi: 10.1038/nbt.3503. DOI:10.1038/nbt.3503. [DOI] [PubMed] [Google Scholar]
- 28.Tack P, Victor J, Gemmel P, et al. 3D-Printing Techniques in a Medical Setting:A Systematic Literature Review. Biomed Eng Online. 2016;15(1):115. doi: 10.1186/s12938-016-0236-4. DOI:10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul GM, Amin R, Wen P, et al. Medical Applications for 3D Printing:Recent Developments. Mo Med. 2018;115(1):75–81. [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MJ, Lee SR, Lee MY, et al. Characterization of 3D Printing Techniques:Toward Patient Specific Quality Assurance Spine-Shaped Phantom for Stereotactic Body Radiation Therapy. PLoS One. 2017;12(5):e0176227. doi: 10.1371/journal.pone.0176227. DOI:10.1371/journal.pone.0176227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SY, Shin YS, Jung HD, et al. Precision and Trueness of Dental Models Manufactured with Different 3-Dimensional Printing Techniques. Am J Orthod Dentofacial Orthop. 2018;153(1):144–53. doi: 10.1016/j.ajodo.2017.05.025. DOI:10.1016/j.ajodo.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Hohne C, Schmitter M. 3D Printed Teeth for the Preclinical Education of Dental Students. J Dent Educ. 2019;83:1100–6. doi: 10.21815/JDE.019.103. DOI:10.21815/jde.019.103. [DOI] [PubMed] [Google Scholar]
- 33.Brown GB, Currier GF, Kadioglu O, et al. Accuracy of 3-Dimensional Printed Dental Models Reconstructed from Digital Intraoral Impressions. Am J Orthod Dentofacial Orthop. 2018;154(5):733–9. doi: 10.1016/j.ajodo.2018.06.009. DOI:10.1016/j.ajodo.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, Tringale KR, Woller SA, et al. Rapid Continuous 3D Printing of Customizable Peripheral Nerve Guidance Conduits. Mater Today. 2018;21(9):951–9. doi: 10.1016/j.mattod.2018.04.001. DOI:10.1016/j.mattod.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moin DA, Wismeijer DH. A Novel Approach for Custom Three-Dimensional Printing of a Zirconia Root Analogue Implant by Digital Light Processing. Clin Oral Implants Res. 2017;28(6):668–70. doi: 10.1111/clr.12859. DOI:10.1111/clr.12859. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W, Li J, Leong YJ, et al. 3D-Printed Artificial Microfish. Adv Mater. 2015;27(30):4411–7. doi: 10.1002/adma.201501372. DOI:10.1002/adma.201501372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu X, Xia B, Ji B, et al. Flow Controllable Three-Dimensional Paper-Based Microfluidic Analytical Devices Fabricated by 3D Printing Technology. Anal Chim Acta. 20191065:64–70. doi: 10.1016/j.aca.2019.02.046. DOI:10.1016/j.aca.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 38.Tao J, Zhang J, Du T, et al. Rapid 3D Printing of Functional Nanoparticle-Enhanced Conduits for Effective Nerve Repair. Acta Biomater. 2019;90:49–59. doi: 10.1016/j.actbio.2019.03.047. DOI:10.1016/j.actbio.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 39.Osman RB, van der Veen AJ, Huiberts D, et al. 3D-Printing Zirconia Implants;A Dream or a Reality?An in vitro Study Evaluating the Dimensional Accuracy, Surface Topography and Mechanical Properties of Printed Zirconia Implant and Discs. J Mech Behav Biomed Mater. 2017;75:521–8. doi: 10.1016/j.jmbbm.2017.08.018. DOI:10.1016/j.jmbbm.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Park C, Han YD, Kim HV, et al. Double-Sided 3D Printing on Paper Towards Mass Production of Three-Dimensional Paper-Based Microfluidic Analytical Devices (3D-muPADs) Lab Chip. 2018;18(11):1533–8. doi: 10.1039/c8lc00367j. DOI:10.1039/c8lc00367j. [DOI] [PubMed] [Google Scholar]
- 41.Bianco P, Robey PG. Stem Cells in Tissue Engineering. Nature. 2001;414:4. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Liu J, Zhu W, et al. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in vitro Disease Modeling. Adv Drug Deliv Rev. 2018;132:235–51. doi: 10.1016/j.addr.2018.06.011. DOI:10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anwar S, Singh GK, Miller J, et al. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl Sci. 2018;3(2):294–312. doi: 10.1016/j.jacbts.2017.10.003. DOI:10.1016/j.jacbts.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadry H, Wadnap S, Xu C, et al. Digital Light Processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur J Pharm Sci. 2019;135:60–7. doi: 10.1016/j.ejps.2019.05.008. DOI:10.1016/j.ejps.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Soman P, Kelber JA, Lee JW, et al. Cancer Cell Migration Within 3D Layer-By-Layer Microfabricated Photocrosslinked PEG Scaffolds with Tunable Stiffness. Biomaterials. 2012;33(29):7064–70. doi: 10.1016/j.biomaterials.2012.06.012. DOI:10.1016/j.biomaterials.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States:Update 2018. Gastroenterology. 2019;156(1):254–72.e211. doi: 10.1053/j.gastro.2018.08.063. DOI:10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu W, Qu X, Zhu J, et al. Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials. 2017;124:106–15. doi: 10.1016/j.biomaterials.2017.01.042. DOI:10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, Qu X, Zhu W, et al. Deterministically Patterned Biomimetic Human iPSC-Derived Hepatic Model Via Rapid 3D Bioprinting. Proc Natl Acad Sci U S A. 2016;113(8):2206–11. doi: 10.1073/pnas.1524510113. DOI:10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen TH, Calle EA, Zhao L, et al. Tissue-Engineered Lungs for in vivo Implantation. Science. 2010;329:5. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horvath L, Umehara Y, Jud C, et al. Engineering an in vitro Air-Blood Barrier by 3D Bioprinting. Sci Rep. 2015;5:7974. doi: 10.1038/srep07974. DOI:10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dean D, Jonathan W, Siblani A, et al. Continuous Digital Light Processing (cDLP):Highly Accurate Additive Manufacturing of Tissue Engineered Bone Scaffolds. Virtual Phys Prototyp. 2012;7(1):13–24. doi: 10.1080/17452759.2012.673152. DOI:10.1080/17452759.2012.673152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim KS, Levato R, Costa PF, et al. Bio-Resin for High Resolution Lithography-Based Biofabrication of Complex Cell-Laden Constructs. Biofabrication. 2018;10(3):034101. doi: 10.1088/1758-5090/aac00c. DOI:10.1088/1758-5090/aac00c. [DOI] [PubMed] [Google Scholar]
- 53.Ma X, Dewan S, Liu J, et al. 3D Printed Micro-Scale Force Gauge Arrays to Improve Human Cardiac Tissue Maturation and Enable High Throughput Drug Testing. Acta Biomater. 2019;95:319–27. doi: 10.1016/j.actbio.2018.12.026. DOI:10.1016/j.actbio.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury Repair. Nat Med. 2019;25(2):263–9. doi: 10.1038/s41591-018-0296-z. DOI:10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mourino V, Boccaccini AR. Bone Tissue Engineering Therapeutics:Controlled Drug Delivery in Three-Dimensional Scaffolds. J R Soc Interface. 2010;7(43):209–27. doi: 10.1098/rsif.2009.0379. DOI:10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seitz H, Rieder W, Irsen S, et al. Three-Dimensional Printing of Porous Ceramic Scaffolds for Bone Tissue Engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782–8. doi: 10.1002/jbm.b.30291. DOI:10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- 57.Gao G, Cui X. Three-Dimensional Bioprinting in Tissue Engineering and Regenerative Medicine. Biotechnol Lett. 2016;38(2):203–11. doi: 10.1007/s10529-015-1975-1. DOI:10.1007/s10529-015-1975-1. [DOI] [PubMed] [Google Scholar]
- 58.Scaglione MS, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States A Population-based Study. Orig Artic. 2015;49:7. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 59.Cheung DY, Duan B, Butcher JT. Current Progress in Tissue Engineering of Heart Valves:Multiscale Problems, Multiscale Solutions. Expert Opin Biol Ther. 2015;15(8):18. doi: 10.1517/14712598.2015.1051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen AH, Marsh P, Schmiess-Heine L, et al. Cardiac Tissue Engineering:State-of-the-art Methods and Outlook. J Biol Eng. 2019;13:57. doi: 10.1186/s13036-019-0185-0. DOI:10.1186/s13036-019-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, He J, Liu J, et al. Rapid 3D Bioprinting of in vitro Cardiac Tissue Models Using Human Embryonic Stem Cell-Derived Cardiomyocytes. Bioprinting. 2019;13:e00040. doi: 10.1016/j.bprint.2019.e00040. DOI:10.1016/j.bprint.2019.e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma ZLY, Yang Y, Wang J, et al. Research Progress and Prospects of Tissue Engineering Scaffolds for Spinal Cord Injury Repair and Protection. Regen Med. 2019;14(9):887–98. doi: 10.2217/rme-2018-0156. [DOI] [PubMed] [Google Scholar]
- 63.Ashammakhi N, Kim H, Ehsanipour A, et al. Regenerative Therapy for Spinal Cord Injury. Tissue Eng Part B Rev. 2019 doi: 10.1089/ten.teb.2019.0182. DOI:10.1089/ten.TEB.2019.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duan B, Hockaday LA, Kang KH, et al. 3D Bioprinting of Heterogeneous Aortic Valve Conduits with Alginate/Gelatin Hydrogels. J Biomed Mater Res A. 2013;101(5):1255–64. doi: 10.1002/jbm.a.34420. DOI:10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedde RD, Mirani B, Navaei A, et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv Mater. 2017;29(19):e1606061. doi: 10.1002/adma.201606061. DOI:10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- 66.Amir-Aslani A, Mangematin V. The Future of Drug Discovery and Development:Shifting Emphasis Towards Personalized Medicine. Technol Forecast Soc Change. 2010;77(2):203–17. DOI:10.1016/j.techfore.2009.09.005. [Google Scholar]
- 76.Trenfield SJ, Awad A, Goyanes A, et al. 3D Printing Pharmaceuticals:Drug Development to Frontline Care. Trends Pharmacol Sci. 2018;39(5):440–51. doi: 10.1016/j.tips.2018.02.006. DOI:10.1016/j.tips.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Mateen R, Ali MM, Hoare T. A Printable Hydrogel Microarray for Drug Screening Avoids False Positives Associated with Promiscuous Aggregating Inhibitors. Nat Commun. 2018;9(1):602. doi: 10.1038/s41467-018-02956-z. DOI:10.1038/s41467-018-02956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan Y, Nguyen DT, Akay Y, et al. Engineering a Brain Cancer Chip for High-throughput Drug Screening. Sci Rep. 2016;6:25062. doi: 10.1038/srep25062. DOI:10.1038/srep25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang YS, Yue K, Aleman J, et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng. 2017;45(1):148–63. doi: 10.1007/s10439-016-1612-8. DOI:10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.2015, First 3D-Printed Pill. Nat Biotechnol. 1014;33(10) doi: 10.1038/nbt1015-1014a. DOI:10.1038/nbt1015-1014a. [DOI] [PubMed] [Google Scholar]
- 72.Economidou SN, Lamprou DA, Douroumis D. 3D Printing Applications for Transdermal Drug Delivery. Int J Pharm. 2018;544(2):415–24. doi: 10.1016/j.ijpharm.2018.01.031. DOI:10.1016/j.ijpharm.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 73.Tao J, Xu X, Wang S, et al. Polydiacetylene-Nanoparticle-Functionalized Microgels for Topical Bacterial Infection Treatment. ACS Macro Lett. 2019;8:563–8. doi: 10.1021/acsmacrolett.9b00196. DOI:10.1021/acsmacrolett.9b00196. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Du T, Zhang J, et al. A 3D-Engineered Conformal Implant Releases DNA Nanocomplexs for Eradicating the Postsurgery Residual Glioblastoma. Adv Sci (Weinh) 2017;4(8):1600491. doi: 10.1002/advs.201600491. DOI:10.1002/advs.201600491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X, Tao J, Wang S, et al. 3D Printing of Nerve Conduits with Nanoparticle-Encapsulated RGFP966. Appl Mater Today. 2019;16:247–56. DOI:10.1016/j.apmt.2019.05.014. [Google Scholar]
- 76.Kotz F, Arnold K, Bauer W, et al. Three-Dimensional Printing of Transparent Fused Silica Glass. Nature. 2017;544(7650):337–9. doi: 10.1038/nature22061. DOI:10.1038/nature22061. [DOI] [PubMed] [Google Scholar]


