Abstract
Matrix-assisted pulsed laser evaporation (MAPLE) has many benefits over conventional methods (e.g., dip-coating, spin coating, and Langmuir–Blodgett dip-coating) for manufacturing coatings containing pharmacologic agents on medical devices. In particular, the thickness of the coating that is applied to the surface of the medical device can be tightly controlled. In this study, MAPLE was used to deposit rapamycin-polyvinylpyrrolidone (rapamycin-PVP) thin films onto silicon and borosilicate optical glass substrates. Alamar Blue and PicoGreen studies were used to measure the metabolic health and DNA content of L929 mouse fibroblasts as measures of viability and proliferation, respectively. The cells on the MAPLE-deposited rapamycin-PVP surfaces exhibited 70.6% viability and 53.7% proliferation compared to a borosilicate glass control. These data indicate that the antiproliferative properties of rapamycin were maintained after MAPLE deposition.
Keywords: Rapamycin, Drug delivery, Matrix-assisted pulsed laser evaporation, Thin film
1 Introduction
Rapamycin is a water-insoluble macrocyclic triene with antiproliferative properties used in multiple applications[1]. For example, rapamycin has been coated on the surfaces of endovascular stents to prevent neointimal hyperplasia, in which the proliferation of smooth muscle cells causes a reduction in the lumen of the vessel[2,3]. Another application of interest to the medical community is delivering rapamycin to the eye to prevent cell proliferation for the treatment of Sjögren’s syndrome, neovascular age-related macular degeneration, diabetic macular edema, and prevention of corneal allograft rejection[4-7]. Shah et al. showed that rapamycin eye drops were able to increase tear secretion and affect genes associated with Sjogren’s syndrome in male non-obese diabetic mice[8]. However, the use of eye drop solutions is associated with low bioavailability for delivery of the pharmacologic agent to the ocular tissue due to several issues such as (a) blinking, (b) low corneal membrane permeability, (c) nasolacrimal drainage, (d) non-productive absorption through the conjunctiva, and (e) tearing[4]. Due to the aforementioned issues, the use of eye drop solutions is associated with a tear film residence time of 1–3 min and low bioavailability (1–3%)[4]. This limitation can be addressed with frequent dosing; however, frequent dosing is associated with low patient compliance and a high incidence of side effects[9]. Alternatively, rapamycin can be loaded onto contact lenses to improve drug bioavailability. The most straightforward mechanism to load a pharmacologic agent into contact lenses is by soaking a preformed contact lens in a solution containing the pharmacologic agent before use[10,11]. However, it is difficult to load high-molecular-weight pharmacologic agents within the bulk material of the contact lens by soaking. A coating approach that enables contact lenses to receive a uniform and ultrathin coating of a pharmacologic agent would enable therapeutic contact lenses to be efficiently produced.
Matrix-assisted pulsed laser evaporation (MAPLE) has many benefits over conventional methods for manufacturing coatings containing rapamycin or other pharmacologic agent-containing coatings (e.g., dip-coating, spin coating, and Langmuir–Blodgett dip-coating) such as the facile control of film thickness, roughness and uniformity, the deposition of thin films in a single “step”[12], and the maintenance of pharmacologic agent integrity. For example, poly(D, L) lactic acid/dexamethasone bilayer coatings deposited using MAPLE significantly reduced nitric oxide production in microglial cell cultures demonstrating that the biological function of dexamethasone is retained after the MAPLE process[13]. In addition, MAPLE was previously utilized for the coverage of non-planar substrates[14,15].
The MAPLE process involves laser evaporation of a frozen solution containing the pharmacologic agent in a matrix composed of a volatile solvent (Figure 1). Solvents used with MAPLE have a high vapor pressure and preferentially absorb the energy associated with laser wavelength[13]; the absorption of laser energy by the solvent matrix instead of the pharmacologic agent protects the agent from photodegradation. The laser ablation of the solvent matrix carries molecules of the pharmacologic agent into the vacuum where the high vapor pressure solvent is vaporized and removed. Thus, the pharmacologic agent forms most of the molecules deposited onto the substrate.
Figure 1.
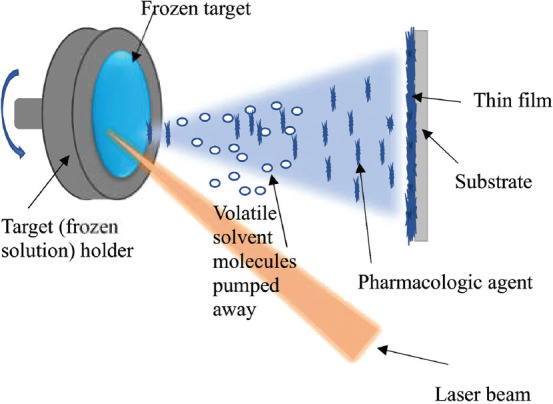
Schematic of the MAPLE process.
In this study, MAPLE was used to deposit antiproliferative rapamycin-PVP thin films on glass and silicon (Si) substrates. Alamar Blue and PicoGreen assays were performed to measure the metabolic health and DNA content of L929 mouse fibroblasts as a measure of viability and proliferation, respectively. The results of this study indicate that the MAPLE-deposited rapamycin-PVP thin films successfully reduced cell viability and proliferation.
2 Materials and methods
Homogenous solutions consisting of polyvinylpyrrolidone (PVP), rapamycin, and dimethyl sulfoxide (DMSO) were prepared at room temperature by dissolving 40 mg of PVP in 4 mL DMSO, and then adding 1 mg rapamycin. Before each laser deposition, 3.5 mL of the freshly prepared solution was dropped using a syringe in a copper holder and frozen in liquid nitrogen (77 K) for 30 min. The cryogenic process was accomplished by plunging the target in a Dewar vessel, which was filled with liquid nitrogen. After freezing, the target holder was rapidly mounted inside the deposition chamber.
All MAPLE thin films were fabricated using a 248 nm wavelength KrF* excimer laser source (25 ns pulse duration, 10 Hz repetition rate). The laser was operated at the fluence of 200 mJ/cm2 per pulse for 101,000 laser pulses. Both the target and substrate were rotated at a rate of 50 Hz during the coating process, while the laser beam was swept over the entire target surface at an incident angle of 45°. The target was maintained at cryogenic temperatures using direct contact with a cooling device connected to a liquid nitrogen reservoir by copper heat pipes. A laser beam homogenizer was used to produce a top-hat energy distribution and increase the deposition area on the substrate. All depositions were performed at a 10−1 Pa background pressure; a 5 cm substrate-to-target separation distance was used to deposit the MAPLE coatings.
One-side polished Si <100> and 10 × 10 mm2 borosilicate optical glass pieces were used as substrates in this study. The substrates were ultrasonically cleaned using ethanol, air dried, then sterilized by exposing to germicidal UV-C radiation from a VL-115UV UV lamp (Vilber, Marne-la-Vallée, France) before deposition.
Rapamycin-PVP thin films were characterized using atomic force microscopy (AFM) and attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy. The AFM micrographs were collected in tapping mode using a hard tapping tip (~300 kHz Fo, k ~ 40 N/m) using an Asylum Research MFP-3D instrument (Goleta, CA). Thirty-two scans with a resolution of 4 cm−1 were collected using Thermo Fisher Nicolet iS50 FTIR spectroscopy bench with built-in diamond attenuated total reflection attachment.
Cell viability and proliferation were measured using Alamar Blue (Thermo Scientific, Grand Island, NY) and PicoGreen (Thermo Scientific, Grand Island, NY) assays, respectively. All reagents were purchased from Thermo Scientific unless otherwise noted. L929 mouse fibroblasts (ATCC, Manassas, VA) were cultured using RPMI media with 10% v/v fetal bovine serum in T-150 flasks. The medium used in the plate assays contained an additional 100 IU/mL penicillin and streptomycin (ATCC, Manassas, VA); antibiotics are necessary since UV sterilization would damage the rapamycin-PVP thin films.
Three each of the unsterilized MAPLE-deposited rapamycin-PVP-coated glass samples and 10-mm square glass coverslips (Ted Pella, Redding, CA) were placed into a 24-well plate and seeded with 3.1 × 104 cells/cm2 using 0.5 mL of cell suspension. The plate was centrifuged at 50 g for 1 min then incubated overnight at 37°C, 5.0% CO2, 95% RH for cell attachment.
Samples and control coverslips were transferred to a fresh 24-plate and 0.5 mL of 10% v/v Alamar Blue in-media was added; wells containing only 10% v/v Alamar Blue in-media were used as the assay blank. The plate was then incubated for 3 h. 100 μl of solution from each well were added to a 96-well plate in triplicate and read in a fluorescence plate reader (SpectraMAX Gemini EM, Molecular Devices, San Jose, CA) using 570 nm excitation and 585 nm emission wavelengths. The remaining dye solution was aspirated from the 24-well plate, replaced with 0.5 mL of the lysis buffer (0.2% v/v Triton X-100 in TE buffer), and then placed on a microplate shaker for 30 min. Plates were then stored at -70°C overnight to complete cell lysis. Cell lysates in the treatment well and control wells were transferred in triplicate to a 96-well plate. PicoGreen dye solution was prepared according to manufacturer instructions and 100 μL of the dye solution was added to each well. After 30 min of staining, the double-stranded DNA signal was read on a fluorescence plate reader at 460 nm excitation and 540 emission wavelengths. Measurements from each sample or blank were obtained in triplicate and averaged. Reported data are the averages and standard deviations of three independent replicate experiments. The results were deemed statistically significant for P < 0.05 using the two-tailed Student’s t-test.
3 Results and discussion
AFM analysis showed a root-mean-square roughness of 23.150 nm; this value represents the overall mean magnitude of surface variations (Figure 2). The maximum and minimum measurements were 137.112 and −43.012 nm, respectively. Skewness that is associated with the lack of symmetry around the data point distribution curve was 1.71. Kurtosis, a measure of whether the data is more peaked (positive) or flat (negative) compared to a normal curve shape, was 4.28.
Figure 2.
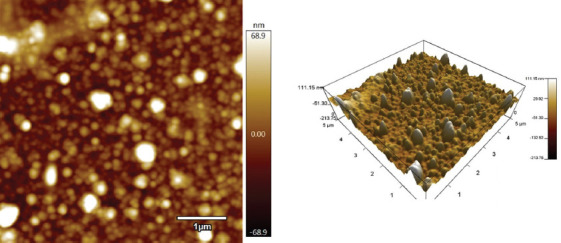
Representative atomic force micrograph of MAPLE-deposited rapamycin-PVP thin films on Si <100>.
The roughness of the MAPLE-deposited rapamycin-PVP thin films onto silicon was much higher than that of uncoated Si[16]. In general, thin films with <600 nm thickness[17] tend to closely follow the topography of the substrate. The MAPLE-deposited rapamycin-PVP thin films show a morphology similar to that of other MAPLE-deposited structures[18].
The characteristic absorption bands of rapamycin are visible in both the MAPLE-coated rapamycin-PVP thin films and dropcast control coatings (Figure 3). This result confirms that the MAPLE deposition method has not significantly altered the chemical structure of rapamycin-PVP starting material. There is a close resemblance between the peaks found in the spectrum of MAPLE-deposited rapamycin-PVP thin film and dropcast coating. The bands at 2862 and 2945 cm−1 are attributed to C-H stretching vibrations from the macrocyclic groups in rapamycin[19]. The band centered at 1660 cm−1 that corresponds to the carbonyl groups of rapamycin is also present[20]. The bands centered at 1430 and 1366 cm−1 are assigned to the stretching and bending vibration of methylene; these bands are ascribed to methylene groups in rapamycin[21]. The broad peaks at 915 cm−1[22,23] and 769 cm−1 are attributed to the borosilicate glass substrate[24].
Figure 3.
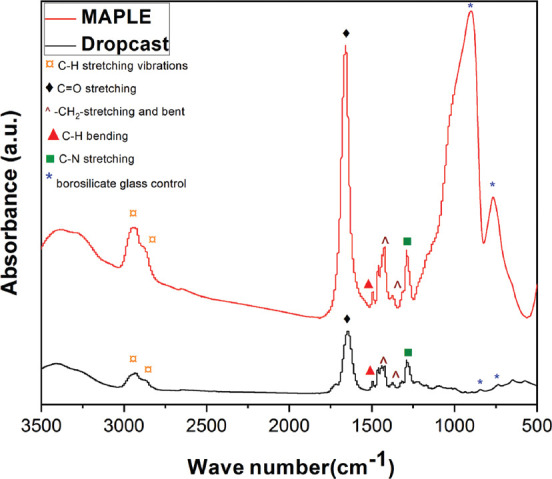
Typical Fourier transform infrared spectrum of MAPLE-deposited rapamycin-PVP thin film (red spectrum) and dropcast coating (black spectrum), respectively.
Alamar Blue and PicoGreen assays were used to measure metabolic health and DNA content as a measure of viability and proliferation, respectively (Figure 4). MAPLE-deposited rapamycin-PVP glass chips were found to have 70.6% viability and 53.7% proliferation versus a similarly sized borosilicate glass control. Alamar Blue and PicoGreen experiments demonstrated reduced cell viability and proliferation, respectively, when grown on MAPLE-deposited rapamycin-PVP surfaces compared to borosilicate glass control ones.
Figure 4.
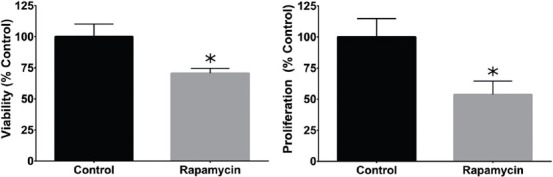
Reductions in proliferation (left) and cell viability (right) were observed in L929 fibroblasts cultured on borosilicate glass coated with rapamycin using MAPLE (rapamycin-PVP) versus untreated borosilicate glass (control). Values represent of x̄ ± SD N = 3 independent replicate experiments. Bars with asterisks are significantly different from the negative control (P < 0.05).
4 Conclusions
Highly uniform rapamycin-PVP thin films were successfully deposited onto the surfaces of silicon and borosilicate optical glass substrates using MAPLE. The antiproliferative properties of rapamycin were maintained after the MAPLE process; L929 mouse fibroblasts grown on MAPLE-deposited rapamycin-PVP thin films exhibited lower cell viability and proliferation rates than cells grown on borosilicate glass control surfaces. The MAPLE-fabricated thin films may have the potential use for preventing undesirable cell proliferation on the surfaces of medical devices such as therapeutic contact lenses. Further studies are underway to understand the in vivo attributes that may allow novel rapamycin release profiles from MAPLE-deposited thin films.
Acknowledgments
This work was supported by a grant of the Ministry of Research and Innovation, CNCS – UEFISCDI, Project Number PNIII-P4-ID-PCE-2016-0884 within PNCDI III and US National Science Foundation Award #1762202.
Disclaimer
The findings and conclusions in this paper have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
References
- 1.National Center for Biotechnology Information. PubChem Database. [Last accessed on 2019 Dec 24];Sirolimus, CID=5284616. Available from:https://www.pubchem.ncbi.nlm.nih.gov/compound/Sirolimus .
- 2.Stefanini GG, Byrne RA, Windecker S, et al. State of the Art:Coronary Artery Stents Past, Present and Future. Eurointervention. 2017;13:706–16. doi: 10.4244/EIJ-D-17-00557. DOI:10.4244/eij-d-17-00557. [DOI] [PubMed] [Google Scholar]
- 3.Byrne RA, Stone GW, Ormiston J, et al. Coronary Balloon Angioplasty, Stents, and Scaffolds. Lancet. 2017;390:781–92. doi: 10.1016/S0140-6736(17)31927-X. DOI:10.1016/s0140-6736(17)31927-x. [DOI] [PubMed] [Google Scholar]
- 4.Shah M, Edman MC, Janga SR, et al. Rapamycin Eye Drops Suppress Lacrimal Gland Inflammation in a Murine Model of Sjögren's Syndrome. Invest Ophthalmol Vis Sci. 2017;58:372–85. doi: 10.1167/iovs.16-19159. DOI:10.1167/iovs.16-19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yagasaki R, Nakahara T, Ushikubo H, et al. Anti-angiogenic Effects of Mammalian Target of Rapamycin Inhibitors in a Mouse Model of Oxygen-induced Retinopathy. Biol Pharm Bull. 2014;37:1838–42. doi: 10.1248/bpb.b14-00487. DOI:10.1248/bpb.b14-00487. [DOI] [PubMed] [Google Scholar]
- 6.Krishnadev N, Forooghian F, Cukras C, et al. Subconjunctival Sirolimus in the Treatment of Diabetic Macular Edema. Graefe's Arch Clin Exp Ophthalmol. 2011;249:1627–33. doi: 10.1007/s00417-011-1694-9. DOI:10.1007/s00417-011-1694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen TW, Benegas NM, Joplin AC, et al. Rapamycin Inhibits Corneal Allograft Rejection and Neovascularization. Arch Ophthalmol. 1994;112:1471–5. doi: 10.1001/archopht.1994.01090230085026. DOI:10.1001/archopht1994.01090230085026. [DOI] [PubMed] [Google Scholar]
- 8.Yan ZC, Bai YJ, Tian Z, et al. Anti-proliferation Effects of Sirolimus Sustained Delivery Film in Rabbit Glaucoma Filtration Surgery. Mol Vis. 2011;17:2495–506. [PMC free article] [PubMed] [Google Scholar]
- 9.Maulvi FA, Soni TG, Shah DO. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016;23:3017–26. doi: 10.3109/10717544.2016.1138342. DOI:10.3109/10717544.2016.1138342. [DOI] [PubMed] [Google Scholar]
- 10.Phan CM, Subbaraman L, Jones L. Contact Lenses for Antifungal Ocular Drug Delivery:A Review. Expert Opin Drug Deliv. 2014;11:537–46. doi: 10.1517/17425247.2014.882315. DOI:10.1517/17425247.2014.882315. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho IM, Marques CS, Oliveira RS, et al. Sustained Drug Release by Contact Lenses for Glaucoma Treatment A Review. J Controlled Release. 2015;202:76–82. doi: 10.1016/j.jconrel.2015.01.023. DOI:10.1016/j.jconrel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Chrisey DB, McGill RA, Horwitz JS, et al. Novel Laser-based Deposition of Active Protein Thin Films. Chem Rev. 2003;103:553–76. doi: 10.1021/cr010428w. [DOI] [PubMed] [Google Scholar]
- 13.Patz TM, Doraiswamy A, Narayan RJ, et al. Matrix Assisted Pulsed Laser Evaporation of Biomaterial Thin Films. Mater Sci Eng C. 2007;27:514–22. DOI:10.1016/j.msec.2006.05.039. [Google Scholar]
- 14.Cristescu R, Mihailescu IN, Jelinek M, et al. Functionalized Thin Films and Structures Obtained by Novel Laser Processing Issues. Kassing R, Petkov P, Kulisch W, et al., editors. Functionalized Properties of Nanostructured Materials. NATO Science Series by Springer, Series II:Mathematics. 2006:211–26. DOI:10.1007/1-4020-4594-8_15. [Google Scholar]
- 15.Sachan R, Jaipan P, Zhang J, et al. Printing Amphotericin B on Microneedles Using Matrix-assisted Pulsed Laser Evaporation. Int J Bioprinting. 2017;3(2):147–57. doi: 10.18063/IJB.2017.02.004. DOI:10.18063/ijb.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey K, Pandey PM. Chemically Assisted Polishing of Monocrystalline Silicon Wafer Si (100) by DDMAF. Procedia Eng. 2017;184:178–84. DOI:10.1016/j.proeng.2017.04.083. [Google Scholar]
- 17.Caio F, Moreau C. Influence of Substrate Shape and Roughness on Coating Microstructure in Suspension Plasma Spray. Coatings. 2019;9:746. DOI:10.3390/coatings9110746. [Google Scholar]
- 18.Popescu-Pelin G, Fufă O, Popescu RC, et al. Lincomycin-embedded PANI-based Coatings for Biomedical Applications. Appl Surf Sci. 2018;455:653–66. DOI:10.1016/j.apsusc.2018.06.016. [Google Scholar]
- 19.Stead SO, McInnes SJP, Kireta S, et al. Manipulating Human Dendritic Cell Phenotype and Function with Targeted Porous Silicon Nanoparticles. Biomaterials. 2018;155:92–102. doi: 10.1016/j.biomaterials.2017.11.017. DOI:10.1016/j.biomaterials.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi PJ, Murthy ZVP, Pati RK. Optimization of Process Parameters by Taguchi Robust Design Method for the Development of Nano-crystals of Sirolimus Using Sonication Based Crystallization. Cryst Res Technol. 2011;47(1):53–72. DOI:10.1002/crat.201100329. [Google Scholar]
- 21.Othman R, VladisavljevićG T, Nagy ZK, et al. Encapsulation and Controlled Release of Rapamycin from Polycaprolactone Nanoparticles Prepared by Membrane Micromixing Combined with Antisolvent Precipitation. Langmuir. 2016;32(41):10685–93. doi: 10.1021/acs.langmuir.6b03178. DOI:10.1021/acs.langmuir. 6b03178. [DOI] [PubMed] [Google Scholar]
- 22.Singh PK, Sah P, Meher JG, et al. Macrophage-targeted Chitosan Anchored PLGA Nanoparticles Bearing Doxorubicin and Amphotericin B Against Visceral Leishmaniasis. RSC Adv. 2016;6:71705–18. DOI:10.1039/c6ra06007b. [Google Scholar]
- 23.Jovanović Ž, Radosavljević A, Šiljegović M, et al. Structural and Optical Characteristics of Silver/poly(N–vinyl–2–pyrrolidone) Nanosystems Synthesized by γ–irradiation. Radiat Phys Chem. 2012;81:1720–8. DOI:10.1016/j.radphyschem.2012.05.019. [Google Scholar]
- 24.Nolan M, Perova TS, Moore RA, et al. Spectroscopic Investigations of Borosilicate Glass and its Application as a Dopant Source for Shallow Junctions. J Electrochem Soc. 2000;147(8):3100–5. DOI:10.1149/1.1393863. [Google Scholar]


