Abstract
The field of three-dimensional (3D) bioprinting is rapidly emerging as an additive manufacturing method for tissue and organ fabrication. The demand for tissues and organ transplants is ever increasing, although donors are not as readily available. Consequently, tissue engineering is gaining much attention to alleviate this problem. The process of achieving well-structured 3D bioprinted constructs using hydrogel bioinks depends on symmetrical precision, regulated flow rates, and viability of cells. Even with the mentioned parameters optimized, the printed structures need additional refining by removing excessive liquids, as peptide hydrogel bioprints encapsulate water. However, it is challenging to eliminate the confined fluids without compromising the printing process. In this paper, we introduced a vacuum system to our 3D bioprinting robotic arm and thus optimized the printing quality for complex and refined 3D scaffolds. Moreover, the proposed vacuum system supports printing with cells. Our results show improved printing resolution which facilitates the printing of higher and more stable structures.
Keywords: Three-dimensional bioprinting, ultrashort peptides, biomaterials, bioinks, tissue engineering, vacuum system
1. Introduction
On average, 20 people die every day in the US alone while waiting for organ transplants[1]. As this number continues to grow and more patients are added to the waiting lists, biologists and biomedical engineers struggle to find a stable solution that can be cost-effective and suitable for tissue and organ fabrication. Research is rapidly growing in the field of tissue engineering as an alternative solution to tissue and organ transplantation, with a particular focus on three-dimensional (3D) bioprinting[1].
3D bioprinting is gaining much attention because of its potential to resolve issues that occur with classical tissue engineering[2]. Although tissue engineering has been effective for the regeneration of several types of organs, tissue-engineered scaffolds do not entirely mimic the native tissue and lack the intricate details of its structure. Furthermore, the use of organic solvents in the process of tissue fabrication may negatively affect cell growth[3].
Consequently, a valuable advantage of 3D bioprinting is the possibility of whole organ fabrication. With the help of computer-aided design techniques, models can be designed and generated to closely mimic organ structures[4]. Additional advantages include rapid prototyping, high precision, high resolution, and computer-automated control[5].
Inkjet bioprinting is quite similar to conventional paper printing. Bioinks are stored in cartridges and acoustic or thermal waves, generated by air bubbles or piezoelectric actuators, which are used for dispensation[3,5].
Extrusion-based bioprinting involves a linear moving extruder and stage unit which moves across the X-Y-Z axes. Bioinks are extruded through nozzles using microfluidic pumps, pneumatic pressure, or solenoid control[5].
Laser-assisted bioprinting uses laser beams to print at a cell resolution[5]. It is becoming widely popular due to its high precision. An additional advantage is that it does not require a nozzle, which eliminates issues of clogging[3].
However, for complex structures, recent research indicates the advantages in robotic 3D printing as compared to linear printing. This approach allows for a minimum of six degrees of freedom, providing much more precision, flexibility, and speed. It has the potential to provide scaffold-free printing, precise tissue dispensation, and better scalability of organ fabrication[6]. Our system adopts the approach of robotic 3D bioprinting to be more compact, versatile, and achieve a higher level of accuracy while being cost-effective.
However, several challenges need to be overcome before this technology will reach full implementation and commercialization. The complexity to merge tissue engineering processes with an automated printing mechanism involves multiple factors including print quality, vascularization, cell viability, mechanical strength of scaffolds, and surface topography[1,7].
Another area of concern is the durability of bioinks. Biomaterials are assessed based on printability, cell compatibility, and mechanical properties[7]. Natural and synthetic polymers are commonly used for bioprinting. Some natural polymers include alginate, collagen, and fibrin. Synthetic polymers, such as polyethylene glycol and poly(L-lactic acid), are also used as bioinks[3].
In our proposed robotic 3D bioprinting system, we investigate ultrashort self-assembling peptides which have proven to be promising biomaterials for tissue engineering applications. These peptides are composed of only four natural amino acids which can easily be synthesized by solid phase peptide synthesis. Due to their amphiphilic character and their innate tendency to self-assemble in water, they form rapidly nanofibrous scaffolds in an aqueous solution in forms of soft solid and transparent hydrogels. The natural but synthetic character of these self-assembling peptides renders them as appealing bioinks for bioprinting[8]. Peptides are generally known for their biocompatibility, biodegradability, and suitability for cell growth[9]. However, one challenge of using peptides as bioinks is their low viscosity. As peptide hydrogels retain high amounts of water, the extrusion system tends to accumulate water at the base of the construct while printing which weakens the printed structure and increases the chance of collapse over time[10].
Aiming to benefit from the biological properties of the ultrashort peptides and to combat its unstable mechanical properties, we propose introducing a vacuum system into the 3D bioprinting process. The vacuum system, placed under the print bed, will allow the excess water to be drained and leave the refined structure intact. Aspect biosystems have implemented a similar technology in their RX1 Bioprinter[5]. This paper will assess the effect of a vacuum mechanism in optimizing the robotic 3D bioprinter to achieve better printing results.
2. Materials and Methods
The components of the bioprinter system include the 3D bioprinting robotic arm, our custom-designed coaxial nozzle, three syringe pumps, and the vacuum mechanism. The experimental setup is shown in Figure 1a. A vacuum pump with a maximum pressure of −0.35 bar was fitted with tubings and attached to the hose barb of a vacuum flask. A 5-mm suction cup was placed on a rubber stopper. Then, a PET track-etched cell culture membrane with a pore size of 0.4 μm was placed on the suction cup to serve as the printing surface and to allow the excess of water to penetrate through the membrane and into the flask (Figure 1b).
Figure 1.
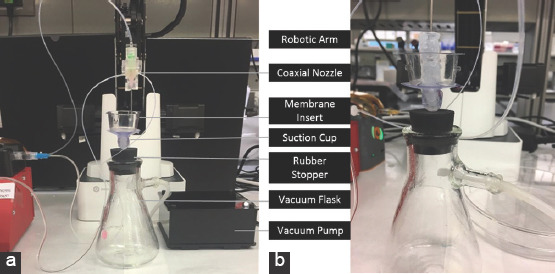
Setup of vacuum mechanism in three-dimensional bioprinting system. Elements of vacuum mechanism (a) A close up of peptide printing with vacuum mechanism (b).
For the bioprinting process, three fresh solutions were prepared which later made up the peptide hydrogel. A solution of 10 × phosphate buffer was loaded into syringe 1. Serum-free medium was loaded into syringe 2. The peptide powder was weighed out in a ratio of 15 mg/mL and loaded into syringe 3. The bioprinting process has been discussed in more detail in another publication (Rauf, 2018, submitted).
Cellular viability is an essential factor in the tissue engineering process. In this experiment, neonatal human dermal fibroblasts (HDFn) were used. The cells were first cultured to reach the desired cell number. After centrifugation, cell pellets were transferred into a tube of approximately 500 μm. The rate of the seeded cells ranged from 1.46 × 107 to 1.6 × 107 cells. The cells were then added into a 1 mL solution of serum-free medium. Subsequently, the mixture of cells and medium was loaded into a microfluidics tube which fed the liquid into the coaxial nozzle. Similarly, another 1 mL of serum-free medium was loaded into a syringe which helped to dispense the preloaded cells from the nozzle.
After completing the bioprinting process, the printed cylinder was submerged with complete medium and then incubated at 37°C, 95% humidity, and 5% CO2. Consequently, the cylinder was cultured for 2 weeks, to enable the cells to grow and to stretch in all directions (Figure 2a-d).
Figure 2.
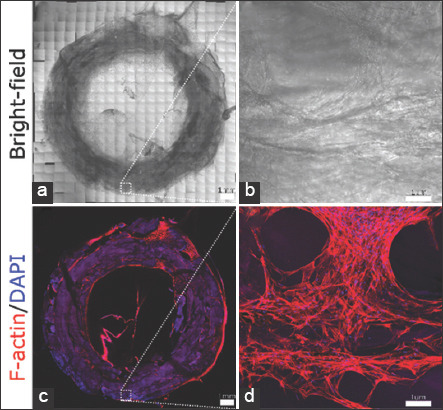
Three-dimensional (3D) bioprinted human dermal fibroblasts (HDFn) after 2 weeks of culture. Whole-mount tile scanning of the 3D bioprinted HDFn ring construct in bright-field (a) and fluorescent F-actin/DAPI staining (c). HDFn cells are distributed throughout the entire ring. Scale bars, 1 mm. Zoom-in of the square in bright-field (b) and fluorescent F-actin/DAPI staining (d). HDFn cells are stretched, interconnected, and grown in all directions. Scale bars are 100 μm.
A cylindrical structure was designed using the CAD software and then transformed into a g-code through a slicing software with dimensions of 10 mm × 40 mm. For comparison reasons, constructs were printed without the vacuum mechanism. Several tests were performed with and without the vacuum mechanism to confirm the observations.
3. Results
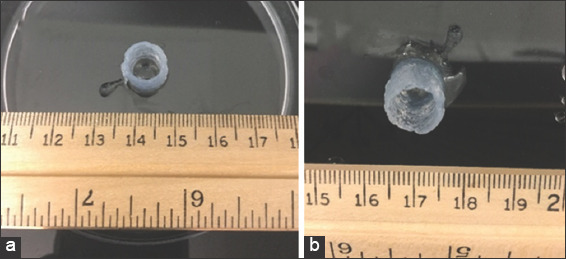
Two cylindrical constructs with dimensions of 10 mm × 10 mm × 10 mm were printed. Figure 3a shows how a sample is printed directly onto a Petri dish without applying the vacuum mechanism. Figure 3b shows how a scaffold is printed on a 0.4 μm membrane installed on the flask vacuum mechanism. The images clearly proof that the applied vacuum function significantly reduced the excess water which was pooling at the bottom of the construct (Figure 3a).
Figure 3.
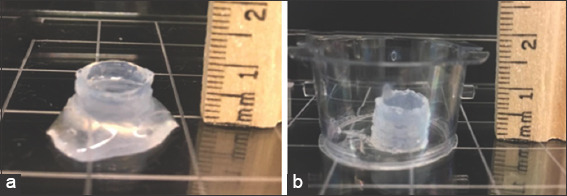
Cylindrical constructs printed with three-dimensional bioprinter. A cylindrical construct of height 10 mm printed without vacuum (a) A cylindrical construct with height 10 mm printed with vacuum (b).
The ability to remove excess water while printing with a vacuum system allowed us to print taller scaffold constructs of up to 40 mm, as shown in Figure 4.
Figure 4.
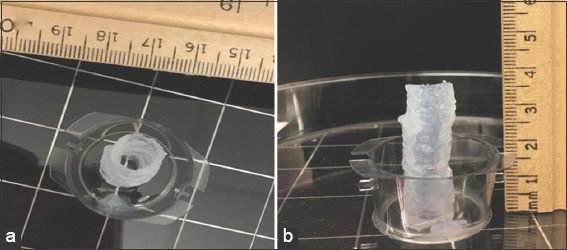
Cylindrical constructs of height 40 mm. Side view (a) Top view (b).
The 3D bioprinting system was also tested for cell viability. The HDFn cells were pumped into the nozzle and mixed with the peptide hydrogel on extrusion. Figure 2 shows the results of a 3D bioprinted ring construct after 2 weeks of cell culture. The cells were found to be distributed throughout the ring. They were stretched and were connected in all directions, indicating healthy growth and confirming cell viability during the bioprinting process.
4. Discussion
The experiments which we conducted to test the presence and function of a vacuum mechanism during the 3D bioprinting process were very successful in generating stably printed peptide scaffolds. Adding the vacuum mechanism increased the resolution of printed constructs and made the bioprinting process more facile and stable. Previously, the user would manually remove the excess of water during the printing process using tissue wipes. This step has now been completely eliminated by introducing the vacuum mechanism.
Furthermore, the ability to systematically remove excess water allowed us to print taller constructs. Without the vacuum system, the tallest constructs printed were about 20 mm. The vacuum mechanism allowed us to double the height of the cylindrical structure up to 40 mm, and it is anticipated to fabricate even higher structures. This optimization of the system will be crucial for the printing of more complex structures involving curvatures and structures with finer details.
Prior the newly introduced vacuum system, the printed structures encapsulated liquid between the layers, which created a challenge to remove the water without injuring the print. Over time, the water which had been entrapped by the fiber network would be released, which then weakened the integrity of the printed structure and resulted in an overall decrease in its size Figure 5. Thus, optimizing the process by incorporating the vacuum system allowed the printed construct to keep its shape over a much longer period of time (several months).
Figure 5.
(a) Top view of the printed structure on 1st day, (b) top view of the same print after 7 days.
5. Conclusion
Our investigation regarding the introduction of an additional vacuum system successfully improved the printability of scaffold when using the robotic 3D bioprinter. By incorporating a vacuum mechanism, the peptide hydrogel produced more refined shapes which is crucial for bioprinting precision. Our experiments were successful in printing 40-mm cylindrical structures with decreased water content, allowing the structure to hold firmly in place. Our results confirm that a vacuum system must be incorporated into the 3D bioprinting system to facilitate printing of more complex structures with a prolonged half-life.
Acknowledgments
The research was supported by funding from King Abdullah University for Science and Technology (KAUST).
Authors’ Contributions
C.A.E.H guided and supervised the project. S.R. designed and supervised the experiments. K.K. and Z.K conducted the experiments and wrote the manuscript. G.R.C did microscopic imaging and N. P. supported the experimental set-up.
Conflicts of Interest
The authors declare that they do not have any competing interests.
References
- 1.Chua C, Yeong W. New Jersey: World Scientific; Bioprinting; p. 2015. https://doi.org/10.1142/9193. [Google Scholar]
- 2.Organdonor.gov. Organ Donation Statistics. [Last accessed on 2018 Oct 30];Organ Donor. 2018 Available from:https://www.organdonor.gov/statistics-stories/statistics.html .
- 3.Sundaramurthi D, Rauf S, Hauser C. 3D bioprinting technology for regenerative medicine applications. Int J Bioprint. 2016;2:9–26. https://doi.org/10.18063/IJB.2016.02.010. [Google Scholar]
- 4.Fermeiro J, Calado M, Correia I. State of the Art and Challenges in Bioprinting Technologies, Contribution of the 3D Bioprinting in Tissue Engineering 2015. IEEE 4thPortuguese Meeting on Bioengineering 2015 [Google Scholar]
- 5.Choudhury D, Anand S, Naing M. The arrival of commercial bioprinters-towards 3D bioprinting revolution! Int J Bioprint. 2018;4:139. doi: 10.18063/IJB.v4i2.139. https://doi.org/10.18063/ijb.v4i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mironov V, Kasyanov V, Markwald R. Organ printing:From bioprinter to organ biofabrication line. Curr Opin Biotechnol. 2011;22:667–673. doi: 10.1016/j.copbio.2011.02.006. https://doi.org/10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering:A review of recent trends and advances. Bioact Mater. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. https://doi.org/10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arab W, Rauf S, Al-Harbi O, et al. Novel ultrashort self-assembling peptide bioinks for 3D culture of muscle myoblast cells. Int J Bioprint. 2018;4:129. doi: 10.18063/IJB.v4i2.129. https://doi.org/10.18063/ijb.v4i1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa R, Rauf S, Hauser C. Towards biologically relevant synthetic designer matrices in 3D bioprinting for tissue engineering and regenerative medicine. Curr Opin Biomed Eng. 2017;2:90–98. https://doi.org/10.1016/j.cobme.2017.05.001. [Google Scholar]
- 10.Hauser C, Deng R, Mishra A, et al. Natural tri- to hexapeptides self-assemble in water to amyloid-type fiber aggregates by unexpected-helical intermediate structures. Proc Natl Acad Sci. 2011;108:1361–1366. doi: 10.1073/pnas.1014796108. https://doi.org/10.1073/pnas.1014796108. [DOI] [PMC free article] [PubMed] [Google Scholar]


