Abstract
This study explored the potential of three-dimensional printing (3DP) technology in producing a three-dimensional (3D) medication label for blind and visually impaired (BVI) patients to ease their drug administration. Different variations of label wordings, dosing instructions, and medication identifiers were designed with reference to guidelines by the American Foundation for the Blind. Shapes and symbols were used as dosing instructions and medication identifiers to the patient’s medical conditions. Prototype designs were created with common graphics computer-assisted drafting software and 3D-printed using acrylonitrile butadiene styrene as the polymer filament. Feedback was then obtained from five people with normal vision and four BVI persons. The initial prototype comprised four components, namely, medication name and strength, patient’s name, dosing instruction, and medication identifier. A revised label comprising the latter two components was developed after feedback by BVI persons. Words were in all uppercase and regular font type, with a 5-mm center-to-center letter spacing. Elevation heights of the letters alternated between 1 mm and 1.5 mm. A half sphere represented the medication dose unit, while vertical lines and a horizontal center line with alternating elevation of arrowheads represented the frequency of administration and the medication’s consumption in relation to food, respectively. Symbols based on target organs were used as medication identifiers. With rapid advancements in 3DP technologies, there is tremendous potential for producing 3D labels in patients’ medication management.
Keywords: Blind, Medication label, Pharmacy, Prescription label, Three-dimensional printing, Visually impaired
1 Introduction
According to the World Health Organization, there are ~2.2 billion people globally estimated to be blind and visually impaired (BVI), among which majority are 50 years and above[1]. In the United States, ~23.7 million American adults (61% women, 39% men) experience vision loss[2]. The average age of these BVI persons is 62 years[3], which is in sync with the global trend. The risk of vision loss increases exponentially with age due to chronic eye diseases and aging processes[4]. This is of concern as the proportion of the elderly is expected to increase from 617 million (8.5% of the world’s population) to nearly 1.6 billion (17%) by the year 2050[5].
While medication compliance is challenging for many patients, this is even more so for BVI patients, who face additional challenges. The World Health Organization defines low vision as acuity <6/18 and blindness as acuity <3/60[6]. When sight is limited or absent, these patients may not be able to read the text or identify and comprehend important information on conventional printed medication labels[7]. This visual limitation puts them in the same or higher risk than the general population for drug-related problems associated with improper use of medications[8], such as concomitant medication use and consuming wrong dosages[9]. The difficulties experienced by BVI patients are also compounded by other issues, such as similar container shapes and sizes, or being prescribed with multiple medications[6]. Studies have reported that blind Americans have an average of 3.3 health conditions[1]. This is particularly of concern for elderly BVI patients because they tend to have more co-morbidities and more medications because of age.
The most common approach that BVI patients use to read medication labels is having someone else (e.g., caregiver) read to them[10], which creates dependency and undermines self-determination and well-being. This is especially so for individuals who are used to being self-sufficient before suffering from their medical condition. They may feel more diminished and vulnerable as they play a less active role in managing their health[11]. Furthermore, communication challenges arise as information is transcribed from the pharmacist or physician to the patient through a third party[7]. In addition, patient confidentiality is compromised for those who do not wish to have their health information disclosed to others. Their rights to object to disclosure are diminished as they have no other meaningful alternative of reading the medication labels, other than relying on someone else[11].
Other approaches that visually-impaired patients use to manage their medications include the use of handheld magnifiers and reading glasses to enlarge their medication labels, and relying on memory to remember instructions provided during a face-to-face counseling session with their health-care provider, of which the former method is inapplicable for the blind[12]. These methods present risks for these patients, especially those who are prescribed with multiple medications, each with differing instructions.
Several auxiliary communication aids are available, including Braille embossers and Audible Prescription Labeling Systems (APLS). Braille embossers can print medication information and enable information accessibility at will[11]. However, only a small percentage of BVI patients can read Braille[13]. Furthermore, pharmacists are not formally trained to read the Braille printout, thus are unable to ensure information accuracy. Braille printouts are also often bulky and large, thereby challenging the ease of affixation on medications[11]. On the other hand, APLS aids rely on human voice recordings, where an electronic form of prescription information is stored in a microchip that is embedded in the medication label. BVI patients can then use a hand-held reader to decode the label information[11]. However, this is not scalable as reading and comprehension abilities vary among BVI patients[11]. To this end, it is necessary to develop an auxiliary aid that addresses the needs of BVI patients and the limitations of existing aids.
Recently, additive manufacturing, commonly known as three-dimensional printing (3DP) technology has been used widely in various fields, such as manufacturing for product prototyping[14], customization of hearing aids and dental crowns[15], and manufacture of volumetric tactile symbols for tactile maps to teach geography to BVI persons. By harnessing the potential of 3DP technologies, tactile aids may be developed to provide BVI patients with important information regarding their medications through their sense of touch.
Multiple 3DP technologies have been used for biological and medical applications, for example, stereolithography apparatus (SLA) and inkjet printing[16,17]. SLA uses liquid photopolymer resins and laser to create 3D structures. Hou et al. utilized SLA to generate a simplified artificial skin model useful for rapidly screening nanoparticles for their transdermal penetration capacity[18]. Inkjet bioprinters deliver a controlled amount of bioink to the desired printing surface, forcing the content to flow continuously or drop out from the nozzle[19]. Xu et al. created complex cellular structures by inkjet printing of primary embryonic hippocampal and cortical neurons[20].
On the other hand, fusion deposition modeling (FDM), also known as fused filament fabrication (FFF), using a temperature-controlled extrusion nozzle to deposit a viscous molten thermoplastic polymer into a 3D structure, is among the most widely used 3DP technologies due to its availability and affordability. Rimington et al. used skeletal muscle cells in multiple scaffolds printed by FDM with biopolymers to study cellular behaviors[21]. FDM has also been used to manufacture medical instruments[22].
Various FDM printable materials have been reported, for example, ceramics[23,24], metal[25], polylactic acid[26], and acrylonitrile butadiene styrene (ABS)[21,26]. ABS is a strong and durable material. Previously, we used ABS to print molds to make dental anesthetic patches[27] and oral capsules for controlled drug release[28]. Domingo-Espin et al. identified several parameters in the FDM printing process that affected the lifespan of ABS materials[29].
In this study, we used an FDM printer to produce 3D ABS medication labels for BVI patients. Our hypothesis is that 3D labels are useful as an aid for BVI patients to manage their medications. In addition, this project aims to identify the features that make a 3D medication label more patient-centric and user-friendly for BVI patients.
2 Materials and methods
2.1 3D label design and printing
Different variations of format parameters, inclusive of the label wordings, dosing instructions, and medication identifiers, were designed, 3D-printed, and visually inspected by the authors at the first instance. The label wordings were designed with reference to format styles recommended by American Foundation for the Blind (AFB) guidelines[10]. Variations in letter case, type style, center-to-center letter spacing, and elevation of letters were printed. For example, medication names were printed in regular and bold fonts, as well as using an all uppercase style compared to an upper- and lower-case combination. The center-to-center spacing of each letter in the word design was varied between 1 mm and 5 mm, using 1-mm intervals, while height variations of the words above 0.38 mm were also printed following previous studies[30]. As the words could not be printed intact if the elevations were <0.5 mm, subsequent word designs were printed in height increments of 0.5 mm, 1.0 mm, and 1.5 mm instead. Word designs with elevations of 0.5 mm, 1.0 mm, and 1.5 mm were printed with a center-to-center letter spacing of 4 mm and 5 mm, since there was residual material in the printed prototype when the letter spacing was <3 mm. For the medication names to be felt more distinctively by touch, letters with alternating height elevations were printed and compared against uniform elevations. For letters with alternating height elevations, different variations in height were also printed.
Shapes and symbols were used to design the dosing instructions of medications. Three parameters were considered. These included the dose units, frequency of administration per day, and how the medication would be taken with regards to food. For dose units, different shapes representing different dosage forms (e.g., half sphere and oval representing a tablet and capsule, respectively) and a universal half-sphere shape to represent all dosage forms were printed. For the frequency of administration, vertical and horizontal lines were printed. For medication timing with reference to food, left and right arrows would indicate that the medication has to be taken before and after food, respectively, while a double-pointing arrow would indicate that the medication could be taken without regard to food. Hence, a 3DP medication label with three half spheres, three vertical lines, and a right arrow would indicate an instruction to take three tablets 3 times a day after food (Figure 1).
Figure 1.
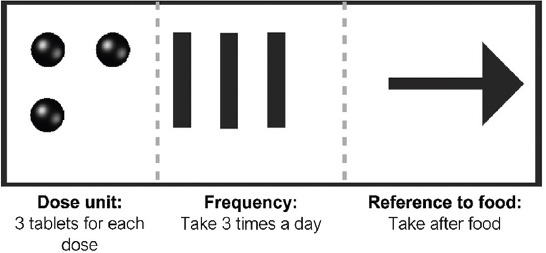
Illustration of directions for use design with the respective symbols’ representations.
In addition, symbols were also designed to serve as medication identifiers to the medical condition of the patient. The medication identifiers were classified based on common chronic diseases in Singapore[31] and the target organs of the medications (Table 1). All medication identifiers were printed in different sizes and elevation heights.
Table 1.
Formatting variations for the three-dimensional medication label prototypes. The preferred variations are italicized and underlined.
| Format parameters | Variations | Pictorial examples of format parameters |
|---|---|---|
| Letter case | All uppercase; Upper & lower |  |
| Type style | Regular; Bold |  |
| Letter spacing | 1,2,3,4,5 mm |  |
| Elevation of letters | Height – 0.38 to 1.5 mm. 1.0 & 1.5 mm
Elevation – Uniform; Alternating |
 |
 | ||
| Dose unit | Universal shape; Different shapes for different dosage forms |  |
| Frequency of administration | Vertical lines; Horizontal lines |  |
| Medication to be taken with regards to food | Centre line – Vertical; Horizontal |  |
| Type of identifier | Based on disease; Based on target orsan |
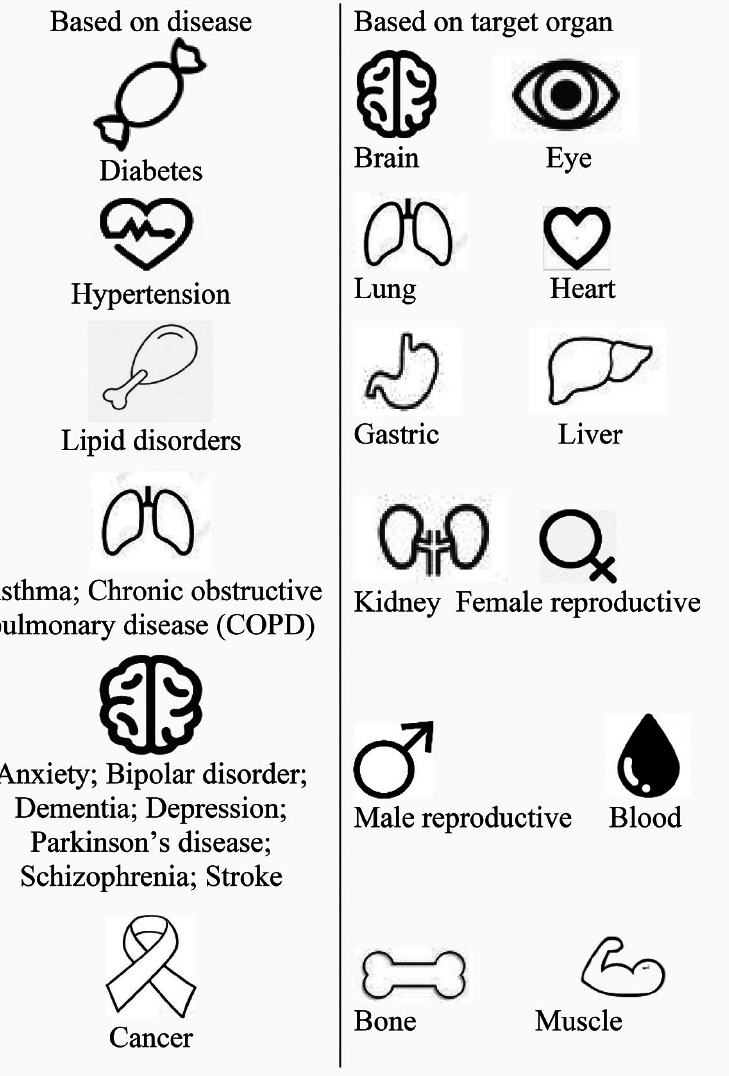 |
| Identifier size | 1.0cm ⨯ 1.0cm; 1.5cm ⨯ 1.5cm; 2.0cm ⨯ 2.0cm | |
| Elevation | 0.38 to 1.5 mm, 1.0mm | |
The printing of the labels followed a standard FDM process, in which the thermoplastic polymer ABS was deposited layer by layer to form the labels[32]. The 3D model of symbols was designed using AutoCAD 2015 (Autodesk Inc., CA, USA), while words were first typed out in Arial font at size 32 using Inkscape™ vector graphic editor (version 0.91, Sodipodi, USA), then exported as Drawing eXchange Format files (Figure 2). AutoCAD was used to create the 3D model of the words at a scale of 0.2 and 0.25 of the original font sizes. AutoCAD design files were exported as stereolithography format to be compatible with the XYZware printer software (version 1.4.1, Kinpo Group, CA, USA). The 3D printing process was carried out using the XYZ da Vinci 1.0 printer (XYZprinting Inc., Lake Forest, CA, USA). The 3D printer operated using FFF technology[29], in which a polymer strand (1.75 mm) was heated to semi-solid state and extruded through a small nozzle tip (0.4 mm) on a build platform (20 cm × 20 cm)[33], to form a layer that represented a cross-section of the object. Subsequent layers formed on top of each other in the direction of the z-axis[34]. The polymer filament used was ABS. The layer height of the extruded polymer filament was set to 0.1 (finest) and 3D density was set to high (50%).
Figure 2.

Process of creating the 3D-printed medication label.
2.2 Pilot testing by BVI patients
The variations of the format parameters were shown to five individuals with normal vision to obtain their preferences for the 3DP medication label. Their preferences for the parameters were combined to develop the initial 3DP label prototype, which was then shown to four target users to obtain their feedback and suggestions for improvement. These users were selected because of their close interactions with BVI patients or personal experiences as BVI persons. They comprised a geriatric pharmacist, an executive from the Dialogue in the Dark (DiD) Singapore[35], and two visually-impaired persons who worked at DiD Singapore as guides.
3 Results
The initial label prototype consisted of four components (medication name and strength, patient’s name, dosing instruction, and medication identifier) split into four rows (Figure 3). The latter two components were further divided into four columns represented by symbols. From the responses of the five individuals with normal vision, they preferred word designs of a 0.25 scale, in an all uppercase style and regular font type, with a 5-mm center-to-center letter spacing (Table 1). It was proposed that the 3DP label be of 10 cm × 6.5 cm × 0.15 cm (length × breadth × height), which meant that each row of the prototype could only comprise up to eight letters. The individuals also preferred that the letters in the word design be of elevation heights that alternated between 1 mm and 1.5 mm.
Figure 3.
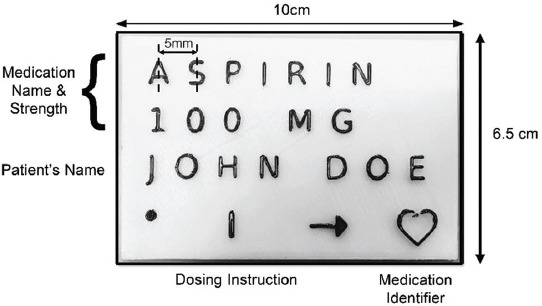
An example of the initial 3D-printed medication label prototype.
With regards to the dosing instruction, a universal shape of a half sphere was preferred by the individuals to represent the medication dose unit regardless of dosage form, and vertical lines were preferred over horizontal lines to convey the frequency of administration. A horizontal center line with an alternating elevation of arrowheads and center line was preferred to convey information regarding the medication’s consumption time in relation to food, and medication identifiers based on the target organs were preferred over identifiers based on diseases. The optimal size of the medication identifier was 1.5 cm × 1.5 cm and its preferred elevation height was 1 mm. The individuals also preferred a convex elevation for the label prototype.
When the initial 3DP medication label prototype was shown to the target users, their feedback was that the words were too small and could not be felt clearly. They suggested that font size and center-to-center letter spacing should be increased but were mindful that the resultant label might be too large to be affixed to the medication packaging if this was done. In terms of word design, short forms of medication names were suggested as some 3DP letters could be confused with each other. Furthermore, using alphabets for the words might not be useful to the elderly BVI population who were English illiterate.
The target users were supportive of the symbols in the 3DP label prototype as they currently used similar approaches to manage their medication regimens. Both the dosing instructions and medication identifiers were well received by the target group as this information was their top priority compared to medication names. However, they suggested that the symbols for the medication identifiers should be enlarged and more elevated to make it more distinctive for the blind and elderly BVI patients who had a poorer sense of touch. They also noted that if symbols were used in the 3DP medication label, the BVI patients would also need to learn and memorize the meaning of each symbol at the point of dispensing and counseling by the pharmacist.
Based on the feedback provided by the target users, a revised 3DP medication label design was developed. In the new design, medication names were removed since these would still be reflected on the conventional medication label provided at the point of dispensing. However, medication identifiers would be enlarged and more elevated while the dosing instructions would still retain their original format as the initial label prototype (Figure 4).
Figure 4.
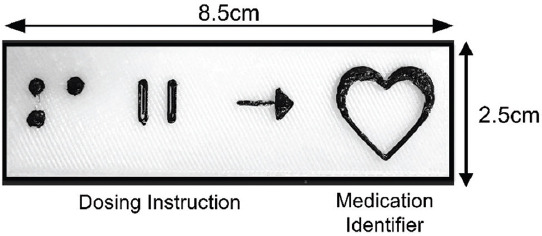
Revised design example of the 3D-printed medication label.
4 Discussion
In this study, a medication label prototype for BVI patients was designed and created through 3DP. From our knowledge, this is the first study that explores the potential of 3DP technology for medication labeling for BVI patients. Feedback from target users showed that 3DP labels would potentially be useful for BVI patients to manage their medications at home. The label could exist alongside the main conventional dispensed label for medications, which contains all legally required information about the patient’s prescription, such as the patient’s name, medication name and strength, and dosing instructions[36]. However, unlike the conventional label, the 3DP label would only consist of the dosing instructions and medication identifiers. This combination would allow for space optimization on the 3DP label, as well as enable it to be appropriately affixed to the medication packaging. If needed, additional 3DP labels containing other information about the medications (e.g., medication name and strength) could also be provided to patients.
The potential of 3DP technology was explored for medication labeling in this project as it is a rapidly advancing field and may potentially circumvent certain issues with auxiliary aids for communicating medical information to BVI patients. 3D printers cost as low as US$1000 and this is expected to decrease lower as it continues its evolution to be a desk-top commodity[37]. While Braille embossers have been used to print medication information[11], only a small percentage of BVI patients and health-care professionals can read Braille. Furthermore, the setting up of a multilingual APLS system can be useful to a multilingual society, but can be costly[38]. On the other hand, the ease of transmitting and editing designs in CAD files among users[37], such as hospitals and clinics, reduces cost and labor and can allow for standardization of 3DP labels across prescription and non-prescription medications. Moreover, a 3DP label can complement face-to-face counseling, as it allows users to access the medication information independently and at will. When complemented with appropriate training, the 3DP label can be understood by the vast majority of BVI patients and health-care professionals, and it can be affixed securely to medication packaging. In addition, the independence regained by BVI patients in terms of their own medication management can enhance their confidence in managing activities of daily living, strengthening person-centricity, and dignity.
Elevation of alphabets was used for the word design on the 3DP medication label as Braille would not be widely understood by majority of BVI patients. To keep the formatting of words familiar to BVI persons, the word design on the 3DP label was adapted from the format style guidelines by the AFB[10], such as using a sans serif font of more than 18-point size, non-italicized and not condensed, and using black letters on a contrasting white background. Interestingly, the preferred letter case format for the 3DP label was an all uppercase style, which differed from the AFB recommendation guidelines of a combination of upper- and lower-case letters. Our respondents suggested that an all uppercase style was preferred because the lowercase style of the letters “a,” “o,” and “e” could be confused with each other when relying purely on a sense of touch. However, our results were similar to another study by McDonald et al.[39], which also observed that their participants found it easier to interpret capitalized words through touch. There were concerns regarding the use of English words due to the low literacy levels among elderly BVI patients and those who lost their vision early in their lives. Furthermore, as tactile acuity is lower than the sense of vision[12], the words on the 3DP label could have been too complicated to be perceived by the sense of touch compared to simple tactile shapes.
The use of shapes and symbols for the dosing instructions and medication identifiers were received positively by the target users, probably because it would be more intuitive for those who were less literate[10], and thus could address the challenge of literacy levels in our patients. However, a universal half-sphere shape was preferred over different shapes representing different dosage forms, as it could be confusing to users and required them to remember the meanings of many symbols. It was also suggested that the symbols be enlarged and more elevated so as to improve its user-friendliness for the blind. Our results were similar to another study by Ramsamy-Iranah et al.[40], which suggested that a larger height contrast for 3D symbols was more easily differentiated from other components by the blind. These findings reflect the need for a solution that is able to provide comprehensive medication information to BVI patients, yet simple in delivery. Such a solution would require trade-offs, underpinned by harmony between information comprehensiveness and individual intuition. The trade-offs for 3DP medication labels to be implemented in clinical practice would be the size of the labels and the high initial costs of producing it, which would require a decision on its cost-benefit by target end-users and the health-care system.
There were several limitations in this project. As this was a proof-of-concept project, the 3D printer used was not a high-end product. As such, the print resolution by the FFF was limited by nozzle diameter and resulted in higher layer thickness; hence, only elevations of some increments could be printed, and this also led to a poorer resolution of the printed objects. In addition, the deformation of the 3DP products occurred, which led to some components not being produced in alignment with the design. This contributed at times to inadequate separation among letters and between letters and the background. However, the best variations of the 3DP labels were shown to the target users and it was also communicated that the labels were only a prototype and would be more aesthetically pleasing with improvements in technology with time. In addition, feedback could only be obtained from a small sample size. Future work could further identify the perceptions and preferred formatting parameters of the 3DP label in a larger patient cohort, and the role of 3DP labels in complementarity with the main dispensing labels and other auxiliary approaches for BVI patients to manage their medications could also be studied.
5 Conclusion
The label consists of dosing instructions and medication identifiers that are represented by shapes and symbols. In addition, several features that make the 3DP label more patient-centric and user-friendly for BVI patients have been identified. With future advancements in 3DP technologies, there is great potential to produce 3DP labels in patients’ medication management, so as to transform the last-mile delivery of health-care services by enabling patient independence and ownership against the backdrop of an aging population.
Acknowledgement
The authors thank the support provided by the Dialogue in the Dark (DiD) Singapore and School of Pharmacy, University of Sydney.
References
- 1.World Health Organization. Blindness and Vision Impairment. [[Last accessed on 2020 Apr 28]];2019 Available from:https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment .
- 2.American Foundation for the Blind. Facts and Figures on Adults with Vision Loss. American Foundation for the Blind, Arlington County. [Last accessed on 2020 Apr 19];2020 Available from:http://www.afb.org/info/blindness-statistics/adults/facts-and-figures/235.
- 3.Zuckerman DM. Blind Adults in America:Their Lives and Challenges. [[Last accessed on 2020 Apr 19]];2020 Available from:http://center4research.org/wp-content/uploads/2010/05/blind02041.pdf .
- 4.Orrico KB. Caring for Visually Impaired Patients. J Am Pharm Assoc. 2013;53:e142–50. doi: 10.1331/JAPhA.2013.13514. [DOI] [PubMed] [Google Scholar]
- 5.Cire B. World's Older Population Grows Dramatically. [Last accessed on 2020 Apr 19];2016 Available from:https://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically .
- 6.World Health Organization. Change the Definition of Blindness. [Last accessed on 2020 Apr 19];World Health Organization, Geneva. 2020 Available from:https://www.who.int/blindness/Change the Definition of Blindness.pdf .
- 7.Sansgiry SS, Pawaskar MD, Bhounsule P. Over-the-counter Medication Purchase and Use by Blind Consumers. J Health Care Poor Underserved. 2012;23:1048–57. doi: 10.1353/hpu.2012.0095. DOI:10.1353/hpu.2012.0095. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Chong EW, Wong WL, et al. Prevalence and Causes of Low Vision and Blindness in an Urban Malay Population:The Singapore Malay Eye Study. Arch Ophthalmol. 2008;126:1091–9. doi: 10.1001/archopht.126.8.1091. DOI:10.1001/archopht.126.8.1091. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Lavanya R, Wu R, et al. Prevalence and Causes of Visual Impairment and Blindness in an Urban Indian Population:The Singapore Indian Eye Study. Ophthalmology. 2011;118:1798–804. doi: 10.1016/j.ophtha.2011.02.014. DOI:10.1016/j.ophtha.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 10.American Foundation for the Blind. Guidelines for Prescription Labeling and Consumer Medication Information for People with Vision Loss. [Last accessed on 2020 Apr 19];American Foundation for the Blind, Arlington County. 2020 Available from:https://www.afb.org/blindness-and-low-vision/your-rights/rx-label-enable-campaign/guidelines-prescription-labeling .
- 11.Little J. Effective and Confidential Communication of Prescription Information:Accommodating the Blind and Visually-impaired. In: Mann WC, Helal AA, editors. Promoting Independence for Older Persons with Disabilities:Selected Papers from the 2006 International Conference on Aging. Disability and Independence; IOS Press,US: 2006. [Google Scholar]
- 12.McMahon JM, Curtis A. Methods of Reading Information on Labels of Prescription Medications by Persons Who Are Visually Impaired. J Vis Impair Blind. 2009;103(5):303–8. DOI:10.1177/0145482x0910300508. [Google Scholar]
- 13.Massof RW. The Role of Braille in the Literacy of Blind and Visually Impaired Children. Arch Ophthalmol. 2009;127(11):1530–1. doi: 10.1001/archophthalmol.2009.295. DOI:10.1001/archophthalmol.2009.295. [DOI] [PubMed] [Google Scholar]
- 14.Kaur S. How is Internet of the 3D Printed Products Going to Affect Our Lives?Pushing Frontiers with the First Lady of Emerging Technologies. IETE Tech Rev. 2012;29(5):360–4. DOI:10.4103/0256-4602.103164. [Google Scholar]
- 15.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D Printing Based on Imaging Data:Review of Medical Applications. Int J Comput Assist Radiol Surg. 2010;5(4):335–41. doi: 10.1007/s11548-010-0476-x. DOI:10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 16.Gu BK, Kwon K, Park S.J, et al. 3D Bioprinting Technologies for Tissue Engineering Applications, Advances in Experimental Medicine and Biology. In: Chun HP, Kwon I, Khang G, editors. Cutting-Edge Enabling Technologies for Regenerative Medicine. Vol. 1078. Springer, Berlin; 2018. DOI:10.1007/978-981-13-0950-2. [DOI] [PubMed] [Google Scholar]
- 17.Mehrban N, Teoh GZ, Birchall MA. 3D Bioprinting for Tissue Engineering:Stem Cells in Hydrogels. Int J Bioprint. 2016;2(1):20. DOI:10.18063/ijb.2016.01.006. [Google Scholar]
- 18.Hou X, Liu S, Wang M, et al. Layer-by-Layer 3D Constructs of Fibroblasts in Hydrogel for Examining Transdermal Penetration Capability of Nanoparticles. SLAS Technol. 2016;22(4):447–53. doi: 10.1177/2211068216655753. DOI:10.1177/2211068216655753. [DOI] [PubMed] [Google Scholar]
- 19.Hong N, Yang GH, Lee J, et al. 3D Bioprinting and itsIn VivoApplications. J Biomed Mater Res B Appl Biomater. 2018;106(1):444–59. doi: 10.1002/jbm.b.33826. [DOI] [PubMed] [Google Scholar]
- 20.Xu T, Gregory CA, Molnar P, et al. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials. 2006;27(19):3580–8. doi: 10.1016/j.biomaterials.2006.01.048. DOI:10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 21.Rimington RP, Capel AJ, Christie SD, et al. Biocompatible 3D Printed Polymers Via Fused Deposition Modelling Direct C2C12 Cellular PhenotypeIn Vitro. Lab Chip. 2017;17(17):2982–93. doi: 10.1039/c7lc00577f. DOI:10.1039/c7lc00577f. [DOI] [PubMed] [Google Scholar]
- 22.Culmone C, Gand S, Breedveld P. Additive Manufacturing of Medical Instruments:A State-of-the-Art Review. Addit Manuf. 2019;27:461–73. DOI:10.1016/j.addma.2019.03.015. [Google Scholar]
- 23.Swee LS, Wai YY, Florencia EW, et al. Direct Selective Laser Sintering and Melting of Ceramics:A Review. Rapid Prototyp J. 2017;23(3):611–23. [Google Scholar]
- 24.Galante R, Figueiredo-Pina C, Serro AP. Additive Manufacturing of Ceramics for Dental Applications:A Review. Dent Mater. 2019;35(6):825–46. doi: 10.1016/j.dental.2019.02.026. DOI:10.1016/j.dental.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Yu WH, Sing SL, Chua CK, et al. Particle-Reinforced Metal Matrix Nanocomposites Fabricated by Selective Laser Melting:A State of the Art Review. Prog Mater Sci. 2019;104:330–79. DOI:10.1016/j.pmatsci.2019.04.006. [Google Scholar]
- 26.Goh GD, Yap YL, Tan HK, et al. Process-Structure-Properties in Polymer Additive Manufacturing via Material Extrusion:A Review. Crit Rev Solid State Mate Sci. 2020;45(2):113–33. DOI:10.1080/10408436.2018.1549977. [Google Scholar]
- 27.Ou YH, Ou YH, Gu J, et al. Personalised Anaesthetic Patches for Dental Applications. Int J Bioprint. 2019;5:15. doi: 10.18063/ijb.v5i2.1.203. DOI:10.18063/ijb.v5i2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim SH, Chia SM, Kang L, et al. Three-Dimensional Printing of Carbamazepine Sustained-Release Scaffold. J Pharm Sci. 2016;2155;105(7) doi: 10.1016/j.xphs.2016.04.031. DOI:10.1016/j.xphs.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Domingo-Espin M, Travieso-Rodriguez JA, Jerez-Mesa R, et al. Fatigue Performance of ABS Specimens Obtained by Fused Filament Fabrication. Materials. 2018;11(12):2521. doi: 10.3390/ma11122521. DOI:10.3390/ma11122521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berla EP. Haptic Perception of Tangible Graphic Displays. In: Wand S, Foulke E, editors. Tactual Perception:A Sourcebook. Cambridge University Press; Cambridge: 1982. pp. 364–86. [Google Scholar]
- 31.Ministry of Health Singapore. Chronic Disease Management Programme (CDMP) [Last accessed on 2020 Apr 19];Ministry of Health Singapore, Singapore. 2020 Available from:https://www.moh.gov.sg/policies-and-legislation/chronic-disease-management-programme-(cdmp)
- 32.ASTM. [[Last accessed on 2020 Apr 19]];ISO/ASTM52900-15 Standard Terminology for Additive Manufacturing-General Principles-Terminology. 2015 Available from:https://www.astm.org/standards/isoastm52900.htm .
- 33.XYZPrinting. [Last accessed on 2020 Apr 19]];da Vinci 1.0. 2020 Available from:https://www.xyzprinting.com/en-us/product/da-vinci-1-0 .
- 34.Giannatsis Jand, Dedoussis V. Additive Fabrication Technologies Applied to Medicine and Health Care:A Review. Int J Adv Manuf Technol. 2009;40(1-2):116–27. DOI:10.1007/s00170-007-1308-1. [Google Scholar]
- 35.Ngee Ann Polytechnic. [Last accessed on 2020 Apr 19]];Dialogue in the Dark. 2020 Available from:http://www.dialogueinthedark.com.sg .
- 36.The United States Pharmacopeial Convention. Prescription Container Labeling. [Last accessed on 2020 Apr 19]];Pharmacopeia and National Formulary, United States. 2012 Available from:https://www.usp.org/sites/default/files/usp/webform/c17.pdf . DOI:10.4135/9781412963855.n1200.
- 37.Campbell T, Williams C, Ivanova O, et al. Could 3D Printing Change the World?Technologies, Potential, and Implications of Additive Manufacturing. [Last accessed on 2020 Apr 19]]; Available from:http://www.globaltrends.thedialogue.org/wp-content/uploads/2014/11/could-3d-printing-change-the-world-technologies-potential-and-implications-of-additive-manufacturing.pdf. DOI:10.1089/3dp.2014.1501.
- 38.Englehardt JB, Allnatt R, Mariano A, et al. An Evaluation of the Functionality and Acceptability of the Voice Prescription Label. 2001 DOI:10.1177/0145482X0109501108. [Google Scholar]
- 39.McDonald S, Dutterer J, Abdolrahmani A, et al. The 16th International ACM SIGACCESS Conference on Computers and Accessibility ACM; Rochester, New York, USA: 2014. Tactile Aids for Visually Impaired Graphical Design Education; pp. 275–6. DOI:10.1145/2661334.2661392. [Google Scholar]
- 40.Ramsamy-Iranah S, Maguire M, Gardner J, et al. A Comparison of Three Materials Used for Tactile Symbols to Communicate Colour to Children and Young People with Visual Impairments. Br J Vis Impair. 2016;34(1):54–71. DOI:10.1177/0264619615610161. [Google Scholar]


