Abstract
Purpose
To retrospectively study the rate of visual field (VF) progression in patients with retinitis pigmentosa (RP) as it relates to different targets and inheritance patterns.
Methods
A total of 275 kinetic VF tests were collected from 52 subjects with RP over a period of up to 29 years (mean, 12 years). The VF areas of Goldmann targets V4e, III4e, and I4e were calculated using Photoshop. Differences in the rate of VF loss among different targets and inheritance patterns were compared.
Results
There was a significant interocular correlation in both visual acuity (VA) (R2 = 0.739, P < 0.001) and VF area (R2 = 0.815, P < 0.001). The annual rates of decline in VF area for V4e, III4e, and I4e targets were 7.5%, 10.7%, and 12.5%, respectively (all P < 0.001). All of the rates were significantly different from each other (P < 0.001). The mean rate of VF loss was 10.3% (P = 0.009) for autosomal recessive, 2.7% (P = 0.215) for autosomal dominant, and 7.2% (P = 0.009) for X-linked patterns of inheritance. However, the differences among them were not statistically significant (P > 0.05). Based on VF, survival analysis indicated that our patients failed the vision standard for driving and reached legal blindness at the median ages of 37 and 55 years, respectively.
Conclusions
The rate of VF loss varies among targets in patients with RP. Fifty percent of patients are not qualified to drive by the age of 37 and become legally blind by the age of 55. These results can be useful for counseling patients with RP as to their potential rate of VF decline.
Keywords: retinitis pigmentosa, visual field, V4e, III4e, I4e, inheritance pattern, Vision Standard for Driving
Retinitis pigmentosa (RP) is a group of heritable retinal disorders characterized by progressive degeneration of photoreceptors with a prevalence of approximately 1/4000 in a general population.1 The typical features of RP include night blindness, photophobia, progressive deterioration of visual fields (VFs), reduced to non-detectable electroretinogram amplitudes, and a decline of visual acuity. The classical presentation of long-term VF progression is patchy loss of peripheral VF evolving to a ring scotoma, tunnel vision, and eventually blindness.1
In RP, visual field loss not only has a great impact on a patient's quality of life but also is an important indicator of disease progression and efficacy of treatment. Goldmann kinetic perimetry has been the widely accepted clinical standard for recording visual field.2 In this method, V4e is the largest and most intense target that maps the farthest peripheral vision, III4e is a middle-sized target that is used in the definitions for driving standard and legal blindness in Canada, and I4e is the second smallest sized target and is more sensitive in detecting field loss in the central 30 degrees of vision.3–5 Hence, it is more informative to measure all three targets when evaluating the VF loss in RP. However, calculating the whole VF areas quantitatively (in mm2 or degree2) is a tedious and time-consuming process because the fields are measured and drawn manually on paper sheets if historic data are used. In addition, the nature of the visual field test (subjectivity and high variability) makes it even more difficult to evaluate the rate of visual field loss in RP.
Up to now, limited studies have assessed the rate of decline in Goldmann visual field (GVF) area in patients with RP, especially for the III4e and I4e targets. Most studies measured only one single target V4e,6–12 had limited sample sizes6,7,13–15 or short follow-up times,13,14,16 or evaluated GVF patterns/types17–20 or radius12 instead of the GVF areas. Here, we performed a retrospective study, based on all seeing areas in the GVF measured by three different targets (V4e, III4e, and I4e), with a relatively large sample size (275 visual fields) and long follow-up times (up to 29 years), to evaluate the long-term natural course of visual field loss in RP in our patient population. Our intention was to provide more specific advice to patients who commonly ask about the expected rate of vision loss.
Methods
Description of the Study Subjects
The study adhered to the tenets of the Declaration of Helsinki and was approved by the University of Alberta Human Research Ethics Board. Two ocular genetic databases—namely, the Eye Institute of Alberta and the University of Alberta Hospital—were used in the study. From the databases, more than 1300 medical records were reviewed from patients seen in our eye clinic for the past 40+ years. Patients with RP were included who had at least two Goldmann visual field tests. For these subjects, data were collected for medical history, age at onset, genotype, inheritance pattern, best-corrected visual acuity (BCVA), and kinetic VF. The VF tests marked by technicians as poor and/or lacking cooperation and/or reliability were all excluded. The clinical diagnosis of RP was confirmed by ophthalmological examinations, including BCVA, VF test, fundus examination, and electroretinography. Some patients also underwent genetic analysis.
Visual Acuity and Visual Field Measurement
Visual acuity was measured according to common standards using Snellen charts and was then converted to logMAR scores. Kinetic visual field data were collected. These fields were obtained as part of each patient's scheduled clinical examination. Three stimuli—V4e, III4e, and I4e—were evaluated. Visual field sheets were scanned into digital images and then the area of each stimulus was measured in degree2 (deg2) using Photoshop CC 2018 (Adobe, San Jose, CA, USA) with the method described by Zahid et al.21 All seeing areas, including central and all peripheral islands, were measured and included in the calculation of visual field area. Based on the definition of the vision standard for driving and legal blindness in Canada, the visual field length at the horizontal meridian for target III4e was also measured.
Although a cartographic distortion arises from plotting data generated in a perimeter bowl in a flat visual field chart and the projection of visual field onto the retina is nonlinear in a schematic eye,22 the majority of studies on visual field progression in retinitis pigmentosa have not applied corrections to their perimetry data.6,7,9–11,14,16,17,23,24 Therefore, in order to remain consistent and to be able to compare our results to previous ones, we chose to do the same.
Statistical Analysis
To describe disease progression, visual field areas were transformed to natural logarithms to reflect the exponential decay of photoreceptors and retinal function in patients with RP.25,26 A mixed-model linear regression (performed with SPSS Statistics 25; IBM, Armonk, NY, USA) was used to estimate the slope and calculate the mean rate of change for visual field area. This model enables a better analysis of repeated measurements and the use of data from both eyes. In this model, individuals were included as random factors on slope and intercept. Eye was also included as a random factor, and age, target, and inheritance patterns were set as fixed factors.
The interocular correlation was performed with Pearson correlation. The Kaplan–Meier method and Cox regression were used in the survival analysis for driving standards and legal blindness. Survival time was calculated in years using major endpoints based on the loss of visual field and visual acuity. Descriptive statistics, χ2 analysis, and ANOVA with Bonferroni post hoc test were also used. Statistical computations were performed using SPSS Statistics 25. Significance was accepted at the probability level of P < 0.05.
Results
Subject Characteristics and Interocular Correlation
A total of 275 VF tests were measured and analyzed. They were collected from 52 RP subjects (47 from the Eye Institute of Alberta, five from the University of Alberta Hospitals). The average follow-up time was 12 years (median, 10 years; range, 2–29 years). The average number of visual field tests per subject was 5.3 (median, 4; range, 2–18). Inheritance patterns included autosomal recessive (AR; n = 32), autosomal dominant (AD; n = 5), and X-linked (XL; n = 15).
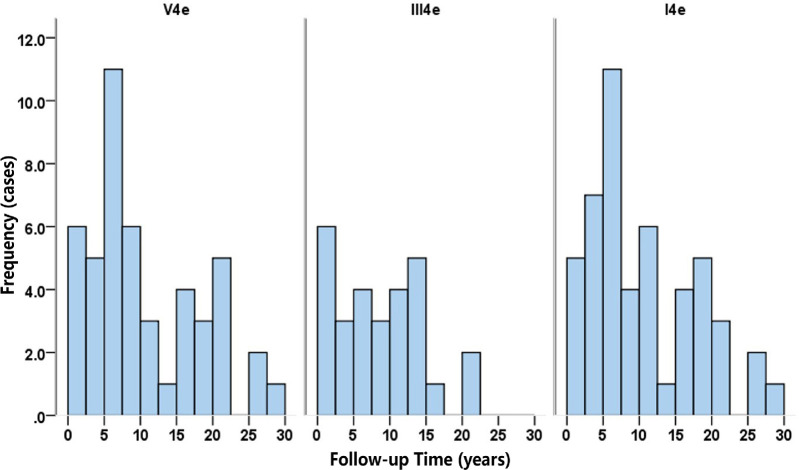
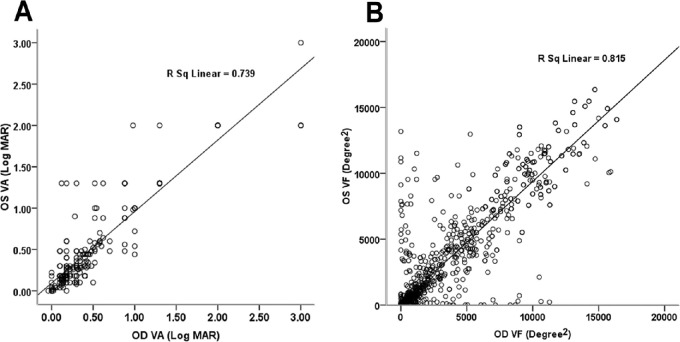
Figure 1 demonstrates the distribution of follow-up time for each stimulus. The median follow-up times were 10 years, 8 years, and 10 years for V4e, III4e, and I4e, respectively. Table 1 summarizes the age, gender, visual acuity, and visual field area (measured by the targets V4e, III4e, and I4e) of the subjects at baseline and their final visit. There were significant interocular correlations in both VA (R2 = 0.739, P < 0.001) and VF area (R2 = 0.815, P < 0.001), as demonstrated in Figure 2.
Figure 1.
Distribution of follow-up time for each stimulus (V4e, III4e, and I4e). The median follow-up times were 10 years, 8 years, and 10 years for V4e, III4e, and I4e, respectively.
Table 1.
Ocular Function of Subjects at Baseline and Final Visit
| Visual Field Area (deg2)* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visual Acuity (logMAR)* | V4e | III4e | I4e | |||||||
| Male/ | ||||||||||
| Time | Female (%) | Age (y)* | OD | OS | OD | OS | OD | OS | OD | OS |
| Baseline | 62/38 | 30.7 (2.2) | 0.25 (0.03) | 0.28 (0.04) | 8.61 (0.19) | 8.72 (0.17) | 7.79 (0.47) | 8.42 (0.29) | 6.31 (0.22) | 6.03 (0.24) |
| Final visit | 62/38 | 42.5 (2.1) | 0.56 (0.09) | 0.58 (0.08) | 7.64 (0.21) | 7.77 (0.23) | 7.13 (0.66) | 7.48 (0.66) | 5.29 (0.23) | 5.20 (0.26) |
Values are expressed as mean (SEM).
Figure 2.
Interocular correlation of best corrected VA (A) and VF area (B). There were significant interocular correlations in both VA (R2 = 0.739, P < 0.001) and VF area (R2 = 0.815, P < 0.001).
Annual Rate of Decline in Visual Field Area
Table 2 presents the estimated slopes and annual rates of decline in visual field area obtained by mixed-model linear regression. The average rates of decline were 7.5%, 10.7%, and 12.5% for V4e, III4e, and I4e, respectively (all P < 0.001). Multiple comparisons with Bonferroni correction revealed that these rates are all significantly different from each other (all P < 0.001). When we divided our subjects into different inheritance patterns, the mean rate of visual field loss was estimated to be 10.3% (P = 0.009) for autosomal recessive, 2.7% (P = 0.215) for autosomal dominant, and 7.2% (P = 0.009) for X-linked inheritance. However, the differences among inheritance patterns were not statistically significant (P > 0.05).
Table 2.
Estimated Slopes and Annual Rates of Decline in Visual Field Area
| Slope | ||||
|---|---|---|---|---|
| Stimulus/Inheritance Pattern | n * | Estimate (SE) | P | Annual Decline Rate (%) |
| V4e | 239 | –0.078 (0.020) | <0.001 | 7.5 |
| III4e | 120 | –0.114 (0.028) | <0.001 | 10.7 |
| I4e | 207 | –0.133 (0.025) | <0.001 | 12.5 |
| Autosomal recessive | 339 | –0.108 (0.039) | 0.009 | 10.3 |
| Autosomal dominant | 43 | –0.028 (0.019) | 0.215 | 2.7 |
| X-linked | 184 | –0.074 (0.025) | 0.009 | 7.2 |
Number of visual field tests.
Survival Analysis
The age distribution for driving standard and legal blindness was established by Kaplan–Meier survival analysis. Based on VF, our patients failed the driving standard and reached legal blindness at a median ages of 37 years (95% CI, 30–44) and 55 years (95% CI, 49–61), respectively, with a significant difference between these two survival distributions (P < 0.001) (Fig. 3, curves DSVF and LBVF). Regarding legal blindness, the mean age of survival defined by VA was 63 years, which was 11 years older than that defined by VF (52 years). Relatively high confidence can be placed in this difference (P = 0.05).
Figure 3.
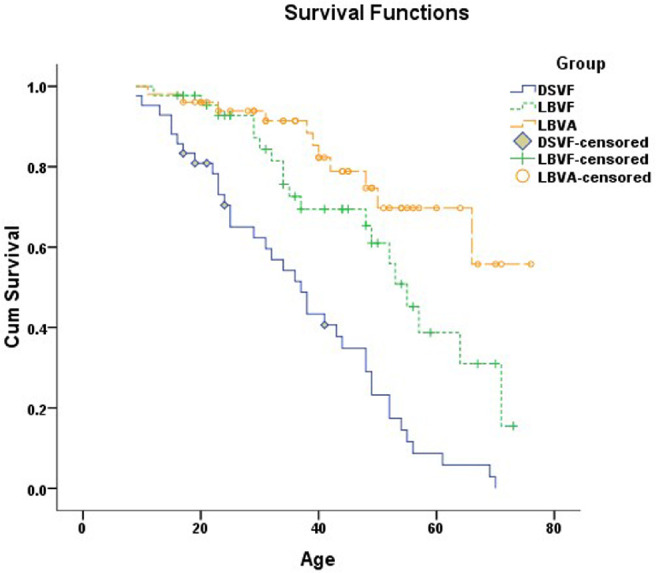
Kaplan–Meier survival analysis for driving standard and legal blindness. Based on VF, the median age for failing the driving standard was 37 years and for reaching legal blindness was 55 years. Based on VA, our patients became legally blind at an average age of 63 years. DSVF, driving standard based on visual field (endpoint, binocular horizontal VF < 120° measured by III4e); LBVF, legal blindness defined by visual field (endpoint, binocular horizontal VF < 20° measured by III4e); LBVA, legal blindness based on visual acuity (endpoint, binocular BCVA < 20/200).
Cox Regression
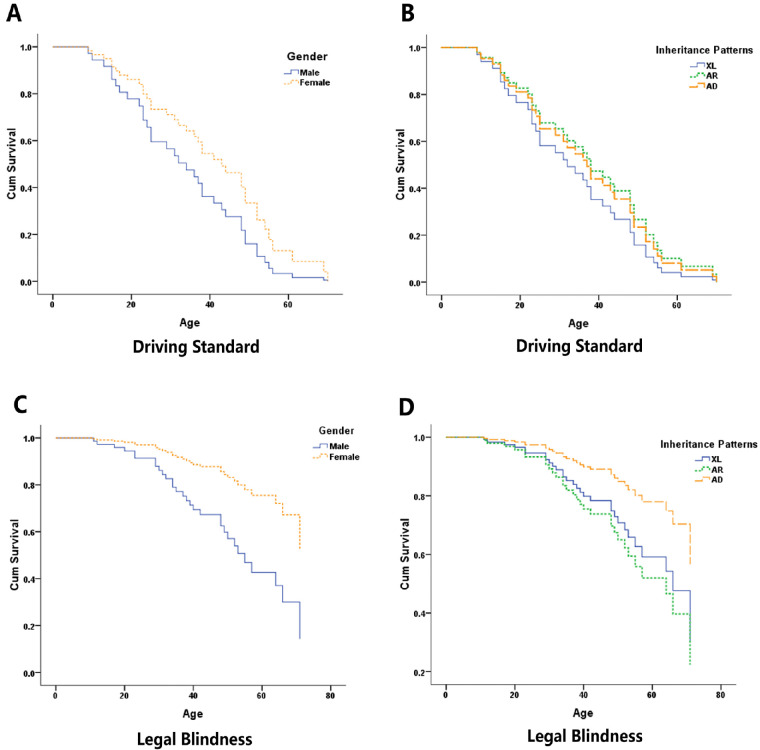
Cox regression was used to compare the effect of gender and inheritance patterns on the survival curves for driving and legal blindness. Figures 4A and 4B demonstrate that survival for the driving standard was similar between genders (P = 0.184) and indistinguishable among X-linked, autosomal recessive, and autosomal dominant groups (P = 0.706). For legal blindness, the effect of gender was statistically significant, with male gender showing a hazard ratio of 3.03 (95% CI, 1.18–7.82; P = 0.022) compared to female gender (Fig. 4C). In terms of genetic subtypes, the survival curve for autosomal dominant showed a tendency to shift to older ages compared to the curves for autosomal recessive and X-linked inheritance. However, the difference was not statistically significant (P = 0.865) (Fig. 4D).
Figure 4.
Cox regression survival analysis for driving standard and legal blindness by gender and inheritance patterns. (A, B) Driving standard survival based on visual field (endpoint: binocular horizontal VF < 120° measured by III4e). The survival distribution was similar between genders (P = 0.184) and among inheritance patterns (P = 0.706). (C, D) Legal blindness survival defined by visual field (endpoint: binocular horizontal VF < 20° measured by III4e). The effect of gender was significant (P = 0.022), but the effect of inheritance pattern was not (P = 0.865).
Discussion
In the current study, we assessed the rate of visual field loss from a total of 275 visual field tests over a period of up to 29 years (average, 12 years) from a group of 52 patients with RP. To our knowledge, this is among the largest studies that have evaluated the entire Goldmann visual field area of RP patients with comparisons among different target sizes (V4e, III4e, and I4e) and inheritance patterns.
Interocular Correlation and Annual Rate of Decline
We found a significant interocular correlation in both visual acuity and visual field area for targets V4e, III4e, and I4e. These results agree with a previous study by Massof and colleagues27 in which they reported a high degree of bilateral symmetry in visual acuity and visual field areas. Similarly, Talib et al.8 suggested that CRB1-associated retinal atrophies had symmetrical visual function between eyes in most patients.
In our study, the estimated annual rates of decline in visual field area were 7.5%, 10.7%, and 12.5% for targets V4e, III4e, and I4e respectively, all of which were significantly different from each other (P < 0.001). These results indicate that visual fields deteriorate at a faster rate for smaller targets than bigger ones, which agrees with our clinical observation that RP patients lose their ability to see the smaller targets earlier than their ability to see bigger targets. In terms of inheritance patterns, the average rates of field loss for autosomal recessive, autosomal dominant, and X-linked patterns were 10.3%, 2.7%, and 7.2%, respectively. Although not statistically significant, these results could suggest that autosomal dominant RP tends to have a slower rate of visual field loss than the other two genetic subtypes. This tendency was also demonstrated in previous studies by Sandberg et al.,9–11 who estimated the annual rate of decline to be 2.6% for autosomal dominant RP with rhodopsin mutations, 7.0% for autosomal recessive RP due to the USH2A gene, and 4.7% for X-linked RP due to RPGR gene mutations.
The mean annual rate of decline for the target V4e was reported to be 4.6% by Berson et al.,23 12% by Holopigian et al.,7 6.9% by Hafler et al.,6 and 5% by Talib et al.8 Our rate of 7.5% falls within the range of these values. Given the heterogeneity of phenotypes and genotypes in RP, this variability in the progression rate among different studies is not surprising. Another contributing factor could be the test–retest variability of Goldmann visual fields in patients with RP, which has been reported to be as high as 50%, especially in advanced cases with substantially reduced visual fields.28 In addition, whether or not there were corrections for cartographic distortions could affect the results. Among the above studies, the one reported by Talib et al.8 was the only one that used corrections, and the rate (5%) was similar to those of the other studies but on the smaller side.
Limited literature has been published regarding the rate of progression in Goldmann VF area for the targets III4e and I4e in RP. Nagy and colleagues24 estimated the annual rate of decline for III4e to be 14.5%, whereas the rate in our patients (10.7%) was slightly lower. Iannaccone et al.14 reported a half-life of 4 years for I4e (corresponding to 16% decline annually), and Nowomiejska et al.13 found a decline of 13% in I4e area during a 2-year period (corresponding to 6.7% decline per year). Our rate of 12.5% falls between the above-reported values; however, all of the three previous studies had small sample size (n = 16–23) and shorter follow-up times (range, 2–6 years). Hence, the results from our study may be more representative.
Survival Analysis
Patients with RP are involved in more care accidents than their age-matched controls.29 Visual field loss, especially the extent of the horizontal visual field, is the strongest predictor of automotive accidents.30,31 In this study, the median age of survival for the Canadian driving standard, based on visual field, was 37 years. To the best of our knowledge, this is the first report on survival for driving standards in RP. This information is highly valuable in counseling patients in terms of how much longer they can expect to hold their driver's licenses. Also, it highlights the need for healthcare professionals to perform early and regular screening regarding a patient's medical fitness to drive and to report or refer for a driving assessment as needed.
The median age of survival for legal blindness reported by other investigators has varied substantially, ranging from 44 to 77 years.8–11 Our result (55 years) falls in the lower third of that range. The main explanation for the discrepancies may be that some of the studies were based on a specific genetic subtype of RP. In addition, the methods used to analyze the data differed among studies. Furthermore, various sample sizes and follow-up times could have also contributed to the differences in the findings.
We found a significant effect of gender on the survival distribution for legal blindness. Male gender had a hazard ratio of 3.03 compared to female gender (P = 0.022); however, this effect was not shown on survival for the driving standard. This interesting finding might suggest that the visual field declines at a similar rate in both genders in the early stage of disease but more quickly in males at a later stage. Further study with a larger sample size would be helpful in confirming this phenomenon and providing better insight into the underlying mechanisms. Due to the limited number of patients with autosomal dominant or X-linked patterns, we did not find any statistically significant difference among various genetic subtypes in survival for the driving standard or for legal blindness. However, our results (Fig. 4D) show a tendency for the survival curve for autosomal dominant to shift to older ages compared to the curves for autosomal recessive and X-linked inheritance. This tendency is also reflected in our results on the annual change of VF area where the autosomal dominant genetic subtype showed a slower rate of decline than the other two subtypes. This finding is in consistent with previous studies by Sandberg et al.9–11
Limitations
Admittedly, our study has its limitations. First, the study is retrospective, and each patient was examined by different visual field technicians with various training and experience at baseline and follow-ups. These factors resulted in higher inter-visit and inter-examiner variability, thus reducing our ability to detect the difference in VF progression among different targets and inheritance patterns. Second, there were a limited number of patients with an autosomal dominant pattern of inheritance. A future study with a larger sample size would be helpful in further distinguishing the difference among genetic subtypes.
Conclusion
In summary, our data demonstrated that: the deterioration in visual acuity and visual field area is highly correlated between two eyes in patients with RP, the rate of VF loss varies among target sizes, and 50% of RP patients are not qualified to drive by the age of 37 and become legally blind by the age of 55. To our knowledge, this is the first study to estimate the median survival age of the vision standard for driving in patients with RP. It is also among the largest studies (if not the largest) that has followed up patients with RP for an extended period of time (up to 29 years) and evaluated all seeing areas in the Goldmann visual field with comparisons among three important target sizes (V4e, III4e, and I4e) and different inheritance patterns. Our results can be useful not only in counseling patients with RP as to their potential rate of visual field loss but also in guiding healthcare professionals on screening regarding patients’ medical fitness to drive.
Acknowledgments
The authors thank Stacey Stone for her help in the data collection.
Disclosure: M. Xu, None; Y. Zhai, None; I.M. MacDonald, None
References
- 1. Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006; 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bass SJ, Sherman J. Visual field testing in the low vision patient. In: Rosenthal BP, Cole RG, eds. Functional Assessment of Low Vision. St. Louis, MO: Mosby; 1996: 89–104. [Google Scholar]
- 3. Wong SH, Plant GT. How to interpret visual fields. Pract Neurol. 2015; 15: 374–381. [DOI] [PubMed] [Google Scholar]
- 4. Dersu I, Wiggins M, Luther A, Harper R, Chacko J. Understanding visual fields, Part I; Goldmann perimetry. J Ophthalmic Med Technol. 2006; 2: 438–440. [Google Scholar]
- 5. Wong AMF, Sharpe JA. A comparison of tangent screen, Goldmann, and Humphrey perimetry in the detection and localization of occipital lesions. Ophthalmology. 2000; 107: 527–544. [DOI] [PubMed] [Google Scholar]
- 6. Hafler BP, Comander J, Weigel DiFranco C, Place EM, Pierce EA. Course of ocular function in PRPF31 retinitis pigmentosa. Semin Ophthalmol. 2016; 31: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holopigian K, Greenstein V, Seiple W, Carr RE. Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology. 1996; 103: 398–405. [DOI] [PubMed] [Google Scholar]
- 8. Talib M, van Schooneveld MJ, van Genderen MM, et al.. Genotypic and phenotypic characteristics of CRB1-associated retinal dystrophies: a long-term follow-up study. Ophthalmology. 2017; 124: 884–895. [DOI] [PubMed] [Google Scholar]
- 9. Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007; 48: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 10. Sandberg MA, Rosner B, Weigel-DiFranco C, McGee TL, Dryja TP, Berson EL. Disease course in patients with autosomal recessive retinitis pigmentosa due to the USH2A gene. Invest Ophthalmol Vis Sci. 2008; 49: 5532–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002; 43: 3027–3036. [PubMed] [Google Scholar]
- 12. Pearlman JT. Mathematical models of retinitis pigmentosa: a study of the rate of progress in the different genetic forms. Trans Am Ophthalmol Soc. 1979; 77: 643–656. [PMC free article] [PubMed] [Google Scholar]
- 13. Nowomiejska K, Brzozowska A, Koss MJ, et al.. Quantification of the visual field loss in retinitis pigmentosa using semi-automated kinetic perimetry. Curr Eye Res. 2015; 41: 993–998. [DOI] [PubMed] [Google Scholar]
- 14. Iannaccone A, Kritchevsky SB, Ciccarelli ML, et al.. Kinetics of visual field loss in Usher syndrome Type II. Invest Ophthalmol Vis Sci. 2004; 45: 784–792. [DOI] [PubMed] [Google Scholar]
- 15. Sunga RN, Sloan LL. Pigmentary degeneration of the retina: early diagnosis and natural history. Invest Ophthalmol. 1967; 6: 309–325. [PubMed] [Google Scholar]
- 16. Edwards A, Fishman GA, Anderson RJ, Grover S, Derlacki DJ. Visual acuity and visual field impairment in usher syndrome. Arch Ophthalmol. 1998; 116: 165–168. [DOI] [PubMed] [Google Scholar]
- 17. Grover S, Fishman GA, Anderson RJ, Alexander KR, Derlacki DJ. Rate of visual field loss in retinitis pigmentosa. Ophthalmology. 1997; 104: 460–465. [DOI] [PubMed] [Google Scholar]
- 18. Grover S, Fishman GA, Brown J Jr. Patterns of visual field progression in patients with retinitis pigmentosa. Ophthalmology. 1998; 105: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 19. Sadeghi AM, Eriksson K, Kimberling WJ, Sjostrom A, Moller C. Longterm visual prognosis in Usher syndrome types 1 and 2. Acta Ophthalmol Scand. 2006; 84: 537–544. [DOI] [PubMed] [Google Scholar]
- 20. Fishman GA, Bozbeyoglu S, Massof RW, Kimberling W. Natural course of visual field loss in patients with type 2 Usher syndrome. Retina. 2007; 27: 601–608. [DOI] [PubMed] [Google Scholar]
- 21. Zahid S, Peeler C, Khan N, et al.. Digital quantification of Goldmann visual fields (GVFs) as a means for genotype-phenotype comparisons and detection of progression in retinal degenerations. Adv Exp Med Biol. 2014; 801: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dagnelie G. Technical note. conversion of planimetric visual field data into solid angles and retinal areas. Vis Res. 1990; 5: 95–100. [Google Scholar]
- 23. Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985; 99: 240–251. [DOI] [PubMed] [Google Scholar]
- 24. Nagy D, Schoönfisch B, Zrenner E, Jägle H. Long-term follow-up of retinitis pigmentosa patients with multifocal electroretinography. Invest Ophthalmol Vis Sci. 2008; 49: 4664–4671. [DOI] [PubMed] [Google Scholar]
- 25. Massof RW, Dagnelie G, Benzschawel T, Palmer RW, Finkelstein D. First order dynamics of visual field loss in retinitis pigmentosa. Clin Vis Sci. 1990; 5: 1–26. [Google Scholar]
- 26. Clarke G, Collins RA, Leavitt BR, et al.. A one-hit model of cell death in inherited neuronal degenerations. Nature. 2000; 406: 195–199. [DOI] [PubMed] [Google Scholar]
- 27. Massof RW, Finkelstein D, Starr SJ, et al.. Bilateral symmetry of vision disorders in typical retinitis pigmentosa. Br J Ophthalmol. 1979; 63: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bittner AK, Iftikhar MH, Dagnelie G. Test-retest, within-visit variability of Goldmann visual fields in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011; 52: 8042–8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fishman GA, Anderson RJ, Stinson L, Haque A. Driving performance of retinitis pigmentosa patients. Br J Ophthalmol. 1981; 65: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szlyk JP, Alexander KR, Severing K, Fishman GA. Assessment of driving performance in patients with retinitis pigmentosa. Arch Ophthalmol. 1992; 110: 1709–1713. [DOI] [PubMed] [Google Scholar]
- 31. Szlyk JP, Fishman GA, Master SP, Alexander KR. Peripheral vision screening for driving in retinitis pigmentosa patients. Ophthalmology. 1991; 98: 612–618. [DOI] [PubMed] [Google Scholar]