Abstract
Purpose
To investigate how macular thickness varies with intermediate age-related macular degeneration (iAMD) severity and the presence of subretinal drusenoid deposits (SDDs).
Methods
A longitudinal prospective study of 143 participants >50 years of age with no to intermediate AMD who were followed with multimodal imaging and functional testing. Participants were stratified by iAMD severity according to imaging features. Macular thicknesses measurements over the central circles with 1-mm, 3-mm, and 6-mm diameters obtained from ocular coherence tomography imaging were compared across severity categories using cross-sectional (143 eyes) and longitudinal (subset of 77 eyes followed for 4 years) multivariate analyses.
Results
Compared with control eyes without large drusen or SDDs (Group 0), central maculas of lower risk eyes with unilateral large drusen (Group 1) were thicker (P = 0.014), whereas higher risk eyes with SDDs (Group SDD) were thinner (P = 0.02) in cross-sectional multivariate analyses. In longitudinal analyses, maculas with SDDs thinned more rapidly over 4 years relative to control eyes (P = 0.0058), which did not show significant thinning. More rapid central macular thinning was associated with worse baseline best-corrected visual acuity (BCVA) (P = 0.016) and more rapid BCVA decline (P = 0.0059).
Conclusions
Macular thickness in iAMD varies with disease severity, showing small increases in eyes with large drusen and decreases in eyes with SDDs. Active processes possibly related to neuroinflammation and neurodegeneration may be contributory. Longitudinal central macular thickness evaluation is an accessible outcome measure relevant to functional measures and is potentially useful for iAMD interventional studies.
Keywords: reticular pseudodrusen, subretinal drusenoid deposits, intermediate age-related macular degeneration, optical coherence tomography, outcome measures, macular thickness
Age-related macular degeneration (AMD), a progressive retinal degenerative disease, is the leading cause of blindness in developed countries.1–3 Drusen deposits, in the form of large soft drusen, located beneath the retinal pigment epithelium (RPE), and reticular pseudodrusen or subretinal drusenoid deposits (SDDs), located in the subretinal space, are important phenotypes within the stage of intermediate AMD (iAMD), conferring increased risk for progression to late disease.4–7 However, it is yet unclear if and how the long-term presence of drusenoid deposits induces structural changes in the retina and if these changes influence visual function.
Efforts to develop novel interventions for iAMD have been challenged in part by a paucity of clinical endpoints that can characterize AMD progression within the intermediate stage.8,9 Also unclear is the nature of biological changes occurring in the retina that unfold during iAMD to pathogenetically drive progression to late AMD. As current animal models do not fully recapitulate the stepwise progression of AMD,10,11 detailed anatomical observations made in clinical studies can help provide critical insights into biological changes during iAMD.
Here, we performed a prospective longitudinal study that enrolled a cohort of patients with iAMD and age-matched controls and evaluated their retinal structure using spectral domain optical coherence tomography (SD-OCT) imaging over a 4-year period. To examine progressive changes in iAMD eyes, we stratified our study population by disease severity, utilizing fundus features corresponding to increased risk for the progression to late AMD. We incorporated SDDs into the stratification scheme, due to their recent identification as a risk-conferring feature separate from large drusen.12,13 We examined how retina structure in eyes with increasing severity categories of iAMD compared with control eyes without AMD when considered cross-sectionally and longitudinally, with the goal of understanding how retinal structure changes during iAMD. A characterization of structural changes can provide critical insights into the pathobiology of AMD and be leveraged to generate outcome measures helpful in testing interventions that slow iAMD progression.
Methods
Study Population
The study participants consisted of patients recruited from the eye clinic at the National Eye Institute, National Institutes of Health, Bethesda, MD, and enrolled in a prospective longitudinal study of dark adaptation in AMD (identifier NCT01352975, www.clinicaltrials.gov).14 The inclusion and exclusion criteria have been described previously.14 Patients 50 years and older with no to iAMD in at least one eye were enrolled, excluding patients with bilateral late AMD (the presence of choroidal neovascularization and/or central geographic atrophy as defined in the Age-Related Eye Disease Study [AREDS]).15 Eyes developed late AMD after the baseline visit were excluded from the 4-year longitudinal analysis. Two eyes had missing data for 4 years but had 5-year data which were included. The study was approved by the Institutional Review Board of the National Institutes of Health and followed the tenets of the Declaration of Helsinki. This research complied with the Health Insurance Portability and Accessibility Act. All participants provided written informed consent after the nature and possible consequences of the study were explained.
Categorization of AMD Severity
Eligible participants were grouped into severity categories based on their macular features, using two separate severity grading scales. In both scales, participants with SDDs in the study eye were assigned a single separate group (Group SDD), regardless of other macular features. The first severity grading scale, referred to as the modified simplified severity scale, was adapted from the AREDS simplified severity scale.15 Based on this scale, participants without SDDs were stratified into three groups: a control group (Group 0), consisting of participants with an AREDS simplified severity scale score of 0, characterized by the absence of large drusen (diameter ≥125 µm) or late AMD in either eye; Group 1, consisting of participants with an AREDS simplified severity scale score of 1, characterized by large drusen and no late AMD in the study eye and no large drusen or late AMD in the fellow eye; and Group 2, consisting of participants with an AREDS simplified severity scale score ranging from 2 to 4, characterized by large drusen in the study eye and either large drusen or late AMD in the fellow eye (Table 1).
Table 1.
Patient Demographics and Ocular Characteristics of Study Eyes (N = 143) at Study Baseline Grouped by AMD Severity Using the Modified AMD Simplified Severity Scale
| Group 0 | Group 1 | Group 2 | Group SDD | All Eyes | ||||
|---|---|---|---|---|---|---|---|---|
| N | 35 | 27 | 61 | 20 | 143 | |||
| Patient demographics | ||||||||
| Age at baseline (y), mean (SD) | 73.04 (9.26) | 69.14 (9.29) | P = 0.27† | 71.13 (9.52) | P = 0.69† | 78.40 (7.08) | P = 0.11† | 72.24 (9.45) |
| Sex (% male) | 37.14 | 62.9 | P = 0.13† | 44.26 | P = 0.87† | 35.00 | P > 0.99† | 44.75 |
| Ever smoked (%) | 45.71 | 40.74 | P = 0.97† | 54.10 | P = 0.82† | 50.00 | P = 0.99† | 48.95 |
| OD (%) | 60.00 | 48.14 | P = 0.73† | 40.98 | P = 0.21† | 45.00 | P = 0.64† | 47.55 |
| Ocular characteristics of study eyes | ||||||||
| BCVA (letters), mean (SD) | 85.37 (5.30) | 83.48 (4.89) | P = 0.58† | 80.77 (7.48) | P = 0.0027 † | 80.30 (6.20) | P = 0.016 † | 82.34 (6.64) |
| Axial length (mm), mean (SD)‡ | 24.36 (1.57) | 24.24 (1.20) | P = 0.98† | 24.07 (1.05) | P = 0.75† | 24.04 (1.13) | P = 0.81† | 24.16 (1.20) |
| Pseudophakia (%) | 34.29 | 22.22 | P = 0.64† | 19.60 | P = 0.32† | 60.00 | P = 0.11† | 29.37 |
OD is the percentage of study eyes that were right eyes.
Group 0 No large drusen (drusen >125 µm in diameter) or late AMD in either eye; Group 1 - Presence of large drusen in the study eye; fellow eye without large drusen or late AMD; Group 2 - presence of large drusen in study eye; presence of large drusen and/or late AMD in the fellow eye; Group SDD - presence of SDD in the study eye.
One-way ANOVA compared to Group 0, adjusted for multiple comparison using Šidák correction.
Axial length was obtained in 101 out of 143 study eyes (21, 21, 44, and 15 for Groups 0, 1, 2, and SDD, respectively).
The second scale, referred to as the AREDS-based scale (ABS), was derived from the nine-step AREDS severity scale.16 It categorized participants according to features found in the study eye alone, without reference to the fellow eye. Study eyes were divided into ABS Group 1 (AREDS step 1), ABS Group 2 (AREDS steps 2 and 3), ABS Group 3 (AREDS steps 4 and 5), and ABS Group 4 (AREDS steps 6–9). As before, eyes with SDDs were placed in a separate group (Group SDD) regardless of other macular features.
Study Assessments
Participants underwent measurement of best-corrected visual acuity (BCVA), using the Early Treatment Diabetic Retinopathy Study chart; ophthalmoscopic examination; and retinal imaging during both baseline and 4-year follow-up visits that took place at least 48 months after the initial visit. Color fundus photographs and fundus autofluorescence images were acquired with the TRC-50DX retinal camera (Topcon Medical Systems, Oakland, NJ, USA). Infrared reflectance and fundus autofluorescence images were acquired with the Spectralis system (Heidelberg Engineering, Heidelberg, Germany). Choroidal assessments were performed using enhanced depth imaging (EDI) SD-OCT scans acquired with the Spectralis instrument as a single horizontal B-scan (scan length of 8.7 mm comprised of 768 A-scans averaged over 100 individual repeated B-scans) centered at the fovea. Retinal thickness assessments were acquired with the Cirrus HD-OCT instrument (Carl Zeiss Meditec, Jena, Germany) using a 512 × 128 volume scan pattern with a 6 × 6-mm area centered on the fovea. All scans were manually assessed by two readers to achieve adequate quality and positioning of the retina that would allow accurate measurement of retinal thickness.
Measurement of Retinal Thickness
Total retinal thickness (TRT), defined by the Cirrus HD-OCT (Carl Zeiss Meditec) segmentation software as the distance from the inner border of the inner limiting membrane (ILM) to the inner one-third of the RPE layer, was computed from Cirrus HD-OCT scans employing Cirrus software version 9.5.0.11469. Automated retinal segmentation in volume scans was audited and manually corrected as needed, with attention to excluding drusen deposits external to the RPE layer from retinal thickness computations. Total retinal thickness measurements were averaged over three concentric circles that were 1 mm (subfoveal), 3 mm, and 6 mm in diameter, as adapted from the ETDRS grid.
Automated segmentation of retinal layers into the inner and outer retina was performed using the software package Orion version 3.0.6997 (Voxelron, Pleasanton, CA, USA). This algorithm was used to perform automated segmentation of the posterior boundary of the outer plexiform layer (OPL) line.17 The distance between the ILM and the OPL segmentation lines was defined as the inner retinal thickness (IRT), and the distance between the OPL and RPE segmentation lines was defined as the outer retinal thickness (ORT). Longitudinal comparisons of retinal thickness were performed by semi-automated spatial alignment of separately captured follow-up OCT scans using the Cirrus or Voxelron software. These were audited for accuracy and subjected to manual correction if necessary.
Measurement of Choroidal Parameters
EDI-OCT horizontal line scans obtained through the fovea were exported from Heidelberg Eye Explorer (HEYEX) software, version 1.9.13.0, into ImageJ software (National Institutes of Health, Bethesda, MD, USA). Measurements of choroidal parameters of total choroidal thickness and choroid vascularity index (CVI) were performed as described previously.18 These parameters were assessed in the central 6-mm and 3-mm regions (i.e., 3 mm and 1.5 mm, respectively, on either side of the foveal center). Choroidal thickness was measured from the outer border of the hyperreflective band representing the RPE/Bruch's membrane complex to the inner border of the hyporeflective line representing the sclera/choroid junction or suprachoroidal layer. The CVI was assessed as the ratio of the summed area of total cross-sectional intraluminal spaces within the choroid to the entire cross-sectional area of the choroid and was expressed as a percentage.
Statistical Analysis
A cross-sectional analysis of average retinal thickness measured in the each of the defined macular areas was performed at study baseline; comparisons between the control group (Group 0) and each AMD severity group were performed for each area using the ANOVA test, with Šidák correction. Longitudinal analysis was performed by computing changes in retinal thickness between the 4-year visit and the baseline visit (as percentage change from baseline). Comparisons between the control group (Group 0) and each AMD severity group were again performed for each macular area using a corrected ANOVA test. Univariate analyses compared retinal thickness at baseline and percentage changes in each retinal subfield (1-mm, 3-mm, and 6-mm circles) to age, sex, smoking status, phakic status, axial length, and BCVA, using non-parametric statistics (Mann–Whitney U test and Spearman's rank-order correlation). Multivariate analyses of retinal thickness percentage changes were performed with all variables except axial length. Axial length was excluded, as it was recorded in only a subset (101/143) of study eyes. Using the appropriate subsets of study eyes, we also conducted separate multivariate analyses of (1) retinal thickness at baseline using all variables including axial length, and (2) retinal thickness change over 4 years in all eyes, excluding eyes that developed central geographic atrophy and excluding eyes that developed either central or non-central graphic atrophy during the 4-year period. These analyses were conducted using mixed models for cross-sectional analyses of retinal thickness and generalized least squares for retinal thickness changes. Statistical significance was set at P < 0.05. All data analysis was conducted using SAS 9.3 software (SAS Institute, Cary, NC, USA).
Results
Participant Demographics
A total of 154 participants were enrolled in the study, with each contributing a study eye. Eleven participants were excluded from further analysis due to the following features in the study eye: presence of epiretinal membrane confounded measures (n = 6), inability to completely image choroidal structures using OCT because of increased choroidal thickness (n = 2), history of central serous chorioretinopathy (n = 1), presence of vitreomacular traction (n = 1), and history of complicated phacoemulsification (n = 1). The demographic features, ocular characteristics, and classification on the modified simplified severity scale of the 143 remaining participants analyzed are summarized in Table 1. Compared with control Group 0, study eyes in Groups 1, 2, and SDD were not statistically distinct in terms of age, sex, smoking, phakic status, and axial length, but Groups 2 and SDD demonstrated significantly decreased BCVA (P = 0.005 and P = 0.027, respectively).
Of the 143 study eyes analyzed at the baseline visit, 77 eyes in the study at the 4-year visit were available for longitudinal analysis. The demographics features and ocular characteristics in this subset are summarized in Supplementary Table S1. Among the 77 eyes, six eyes developed non-central geographic atrophy (GA) during the 4-year period. These six eyes are included in the main analysis, but a separate multivariate analysis excluding these eyes was also conducted (n = 71; Supplementary Table S3).
Cross-Sectional Comparison of Retinal Thickness of Eyes in Different AMD Severity Groups
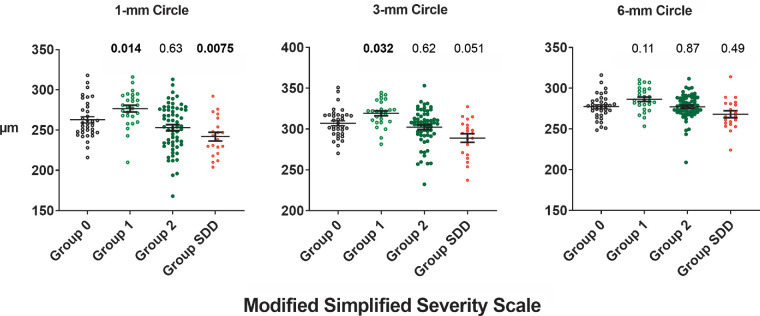
Total retinal thicknesses in the central 1-mm, 3-mm, and 6-mm circles are compared among study eyes (n = 143) of different AMD severity categories in a cross-sectional manner at the study baseline in Figure 1. Multivariate analyses (Table 2) determined that, relative to Group 0 control eyes, the TRT of Group 1 eyes was significantly greater in the central 1-mm circle (P = 0.014) and 3-mm circle (P = 0.032), but this increase did not reach statistical significance in the 6-mm circle (P = 0.11). Conversely, the TRT of Group SDD eyes was significantly lower in the central 1-mm circle (P = 0.02) and 3-mm circle (P = 0.032) but not in the 6-mm circle. Other significant associations found for TRT included sex, with TRT being greater in males in the 1-mm circle (P = 0.004) and 3-mm circle (P = 0.039), and BCVA, with TRT being greater with higher BCVA in the 1-mm circle (P < 0.0001) and 3-mm circle (P = 0.0009). None of the comparisons involving TRT in the 6-mm circle reached significance for any parameter examined, suggesting that intergroup differences in macular thicknesses are more prominent in the central 3-mm region of the macula than in regions of the outer macula. In a separate multivariate analysis of 101 eyes with axial length data, axial length was only associated negatively with retinal thickness in the 6-mm circle (P = 0.025) and not with the retinal thickness of the subfoveal or 3-mm circle (data not shown).
Figure 1.
Comparison of total retinal thickness (TRT) in eyes with iAMD across severity categories as determined using the modified AREDS simplified severity scale. TRT measurements were obtained in study eyes (n = 143) at baseline over the central circles 1 mm, 3 mm, and 6 mm in diameter. TRT distributions for eyes in each intermediate AMD severity group (Groups 1, 2, and SDD) on the modified AMD simplified severity scale were compared with the distribution in the control group (Group 0). The horizontal lines and error bars represent the means and standard errors of the distribution, respectively. The P values shown above each group were obtained from multivariate analyses; significant values are highlighted in bold.
Table 2.
Multivariate Analysis of Parameters Associated with TRT in the 1-mm (Subfoveal), 3-mm, and 6-mm Circles at Study Baseline (N = 143)
| Total Retinal Thickness (µm) | ||||||
|---|---|---|---|---|---|---|
| Parameter | 1-mm Circle | P | 3-mm Circle | P | 6-mm Circle | P |
| Age (y) | 0.025 (−0.49, 0.54) | 0.92 | −0.24 (−0.66, 0.18) | 0.26 | 0.17 (−0.55, 0.13) | 0.23 |
| Male (%) | 12.00 (3.88, 20.12) | 0.004 | 6.98 (0.36, 13.59) | 0.039 | 2.69 (−2.63, 8.00) | 0.32 |
| Smoking status | −3.93 (−11.78, 3.91) | 0.32 | −2.23 (−8.61, 4.16) | 0.49 | −1.47 (−6.60, 3.65) | 0.57 |
| BCVA (letters) | 1.66 (0.97, 2.36) | <0.0001 | 0.97 (0.41, 1.54) | 0.0009 | 0.43 (−0.025, 0.88) | 0.064 |
| Pseudophakia | −1.79 (−11.60, 8.03) | 0.72 | −5.27 (−13.26, 2.72) | 0.19 | −5.40 (−11.83, 1.02) | 0.098 |
| Group 1 vs. Group 0 | 15.56 (3.16, 27.96) | 0.014 | 2.17 (1.001, 21.19) | 0.032 | 6.67 (−1.44, 14.79) | 0.11 |
| Group 2 vs. Group 0 | −2.57 (−13.06, 7.92) | 0.63 | −2.13 (−10.67, 6.40) | 0.62 | −0.55 (−7.42, 6.31) | 0.87 |
| Group SDD vs. Group 0 | −16.46 (−30.27, −2.64) | 0.020 | −11.21 (−22.46, 0.035) | 0.051 | −3.13 (−12.06, 5.80) | 0.49 |
| Mean choroid thickness* | −0.063 (−0.11, −0.017) | 0.0075 | −0.025 (−0.062, 0.012) | 0.19 | −0.011 (−0.045, 0.023) | 0.52 |
| Mean choroidal vascularity index* | 18.98 (−53.85, 91.80) | 0.61 | 25.93 (−33.36, 85.21) | 0.39 | 52.17 (−7.67, 112.01) | 0.087 |
Values represent estimates with 95% confidence intervals in parentheses. Significant estimates are highlighted in bold.
Choroidal features were assessed for total retina thickness (TRT) in single horizontal scans traversing the fovea across the 1-mm, 3-mm, and 6-mm regions centered on the fovea.
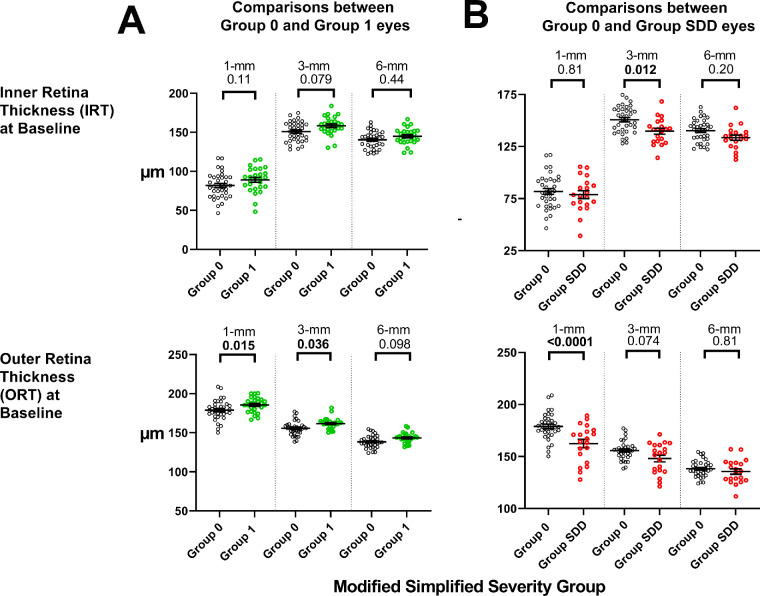
To evaluate the relative contributions of the inner and outer retinal layers to the observed differences in TRT, the SD-OCT images of the study eyes were subjected to segmentation analysis to obtain outer retinal and inner retinal thickness measurements. This sub-segmentation analysis indicated that the greater TRT measurements in Group 1 versus Group 0 eyes were detectable as slightly greater mean IRT and ORT in all three macular regions (Fig. 2A). However, the differences reached significance only for ORT in the 1-mm and 3-mm areas, suggesting a more prominent outer retinal contribution to this retinal thickness difference. Sub-segmentation analysis in Group SDD and Group 0 eyes similarly showed that both mean IRT and ORT were lower in Group SDD in all three macular regions, with significant differences found for IRT (at 3-mm) and ORT (at 1-mm) comparisons, indicating that decreases in both the inner and outer retina may contribute to lower TRT in Group SDD eyes.
Figure 2.
Analysis of inner and outer retinal contributions to differences in TRT observed among AMD severity categories. Total retinal thickness (TRT) measurements obtained at study baseline were segmented into IRT and ORT measurements for the 1-mm, 3-mm, and 6-mm retinal areas. (A) Comparisons of IRT and ORT measurements in Group 0 (n = 35) and Group 1 (n = 27) eyes. (B) Comparisons of IRT and ORT measurements in Group 0 (n = 35) and Group SDD (n = 20) eyes. Statistical comparisons were performed using one-way ANOVA with Šidák correction; comparisons with significant P values (P < 0.05) are highlighted in bold.
To support the above findings of differences in retinal thickness among severity groups defined by the modified simplified severity scale (which categorizes AMD severity from features observed in both eyes of the same participant), all 143 study eyes were categorized using an alternative AREDS severity scale (ABS), which employs features in the study eye alone without consideration of the fellow eye (Supplementary Table S2). We found that, relative to ABS Group 1 controls, ABS Group 2 and Group 3, which represent earlier stages of iAMD, demonstrated a trend toward increased TRT, particularly in the central 1-mm circle. Conversely, Group 4 and Group SDD, which represent later stages of iAMD, demonstrated a trend toward decreased TRT in the 1-mm and 3-mm circles (Supplementary Figure S1). These trends in this alternative severity staging scheme corroborate the observations that earlier stage iAMD eyes tend to show increased TRT, whereas later stage iAMD eyes, particularly those with SDDs, tend to show decreased TRT.
Longitudinal Analysis of Retinal Thickness Changes in Eyes in Different AMD Severity Groups
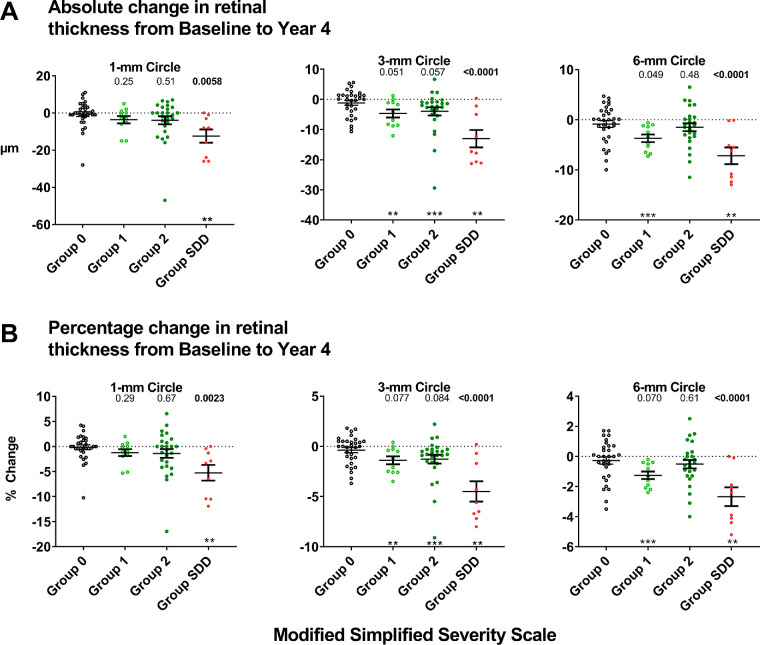
The intergroup differences detected in the above cross-sectional analyses suggest that eyes with iAMD develop progressive intraretinal changes even in the absence of late AMD. To investigate longitudinal changes in retinal thickness in study eyes with iAMD, we analyzed changes in TRT in a subset of study eyes that were followed over 4 years (n = 77). These eyes were categorized into the same AMD severity categories based on their baseline features, and comparisons of longitudinal changes in each group were made with the control group (Group 0). We found that Group 0 eyes demonstrated no significant changes from baseline TRT measurements, with the mean absolute and percentage change over 4 years being close to zero for all macular areas (1-mm, 3-mm, and 6-mm macular circles) (Fig. 3). Group SDD eyes demonstrated the largest and most significant changes over 4 years, comprised of significant TRT decreases from baseline values for all macular areas. Group 1 and Group 2 eyes showed some decrease in TRT over 4 years in all macular areas, but these decreases were smaller in magnitude than those observed in Group SDD.
Figure 3.
Analysis of longitudinal 4-year changes in total retinal thickness (TRT) in eyes with intermediate AMD across severity categories as determined using the modified AREDS simplified severity scale. Changes in TRT measurements over 4 years were computed for a subset of study eyes that did not progress to late AMD from the baseline to the year 4 study visits (n = 77). Absolute (A) and percentage (B) changes in TRT were computed for each severity group. The distribution for each group was compared to the horizontal (i.e., y = 0, representing no interval change); distributions significantly different from zero are indicated as *P < 0.05, **P < 0.01, or ***P < 0.001 (using the Wilcoxon signed-rank test). The distributions for Groups 1, 2, and SDD were compared with that for Group 0 using multivariate analysis; P values that are significant are highlighted in bold.
Multivariate analyses confirmed that, in intergroup comparisons, 4-year thickness changes in Group SDD eyes were significantly greater than those found in Group 0 in all macular areas, whereas comparisons involving Group 1 and Group 2 eyes with Group 0 eyes did not reach statistical significance (Table 3). We also found a similar pattern of significant decreases of TRT over 4 years in Group SDD eyes after excluding eyes that developed non-central GA over the 4-year period (Supplementary Table S3). Together, these findings indicate that, over 4 years, Group 0 eyes showed stability in retinal thickness and Group SDD eyes showed significant loss of retinal thickness over much of the macula, with Group 1 and Group 2 eyes showing less prominent changes. Other variables associated with greater longitudinal decreases in TRT in the 1-mm circle were older age (P = 0.021), worse BCVA at baseline (P = 0.016), and decreases in BCVA over 4 years (P = 0.0059). Baseline choroidal parameters (choroid thickness and CVI) were not significantly associated with longitudinal changes in TRT.
Table 3.
Multivariate Analysis of Parameters Associated with Absolute Changes in TRL Over 4 Years in the 1-mm (Subfoveal), 3-mm, and 6-mm Circles (N = 77)
| Total Retinal Thickness (µm) | ||||||
|---|---|---|---|---|---|---|
| Parameter | 1-mm Circle | P | 3-mm Circle | P | 6-mm Circle | P |
| Age at baseline (y) | 0.35 (0.055, 0.65) | 0.021 | 0.12 (−0.073, 0.31) | 0.22 | 0.008 (−0.12, 0.14) | 0.90 |
| Male (%) | −1.33 (−5.84, 3.18) | 0.56 | −1.81 (−4.70, 1.07) | 0.21 | −1.22 (−3.22, 0.79) | 0.23 |
| Smoking status | 2.07 (−2.32, 6.46) | 0.35 | 1.24 (−1.56, 4.05) | 0.38 | 0.36 (−1.54, 2.25) | 0.71 |
| BCVA baseline | 0.66 (0.13, 1.20) | 0.016 | 0.16 (−0.18, 0.50) | 0.36 | 0.020 (−0.21, 0.25) | 0.86 |
| Change in BCVA (letters) over 4 y | 0.65 (0.17, 0.99) | 0.0059 | 0.30 (0.25, 0.61) | 0.027 | 0.069 (−0.11, 0.25) | 0.44 |
| Pseudophakia | −4.55 (−10.20, 1.088) | 0.11 | −3.93 (−7.53, −0.33) | 0.030 | −1.48 (−3.94, 0.98) | 0.23 |
| Group 1 vs. Group 0 | −3.88 (−10.52, 2.75) | 0.25 | −4.22 (−8.46, −0.013) | 0.051 | −2.92 (−5.84, −0.0074) | 0.049 |
| Group 2 vs. Group 0 | −1.90 (−7.65, 3.86) | 0.51 | −3.57 (−7.24, 0.11) | 0.057 | −0.89 (−3.39, 1.62) | 0.48 |
| Group SDD vs. Group 0 | −10.82 (−18.38, −3.25) | 0.0058 | −11.88 (−16.77, −6.90) | <0.0001 | −6.74 (−9.96, 3.51) | <0.0001 |
| Choroid thickness at baseline* | −0.0011 (−0.043, 0.020) | 0.48 | −0.0016 (−0.022, 0.019) | 0.98 | −0.00076 (−0.017, 0.015) | 0.93 |
| Choroid vascularity index at baseline* | 21.43 (−24.49, 67.35) | 0.35 | 3.21 (−26.12, 32.54) | 0.83 | −4.19 (−29.32, 20.94) | 0.74 |
Values represent estimates with 95% confidence intervals in parentheses. Significant estimates are highlighted in bold.
Choroidal features were assessed for TRT in single horizontal scans traversing the fovea across the 1-mm, 3-mm, and 6-mm regions centered on the fovea.
To evaluate the relative contributions of the inner and outer retinal layers to the observed longitudinal decreases in TRT in Group SDD eyes, TRT changes in Group SDD (n = 27) and Group 0 (n = 35) were segmented into longitudinal IRT and ORT changes over 4 years and compared (Fig. 4). In Group 0 control eyes, both IRT and ORT were unchanged over 4 years. In Group SDD eyes, changes in IRT were not statistically different from those in Group 0 eyes, whereas changes in ORT demonstrated significant thinning compared to Group 0 eyes over all 1-mm, 3-mm, and 6-mm areas, indicating a predominant outer retinal thinning.
Figure 4.
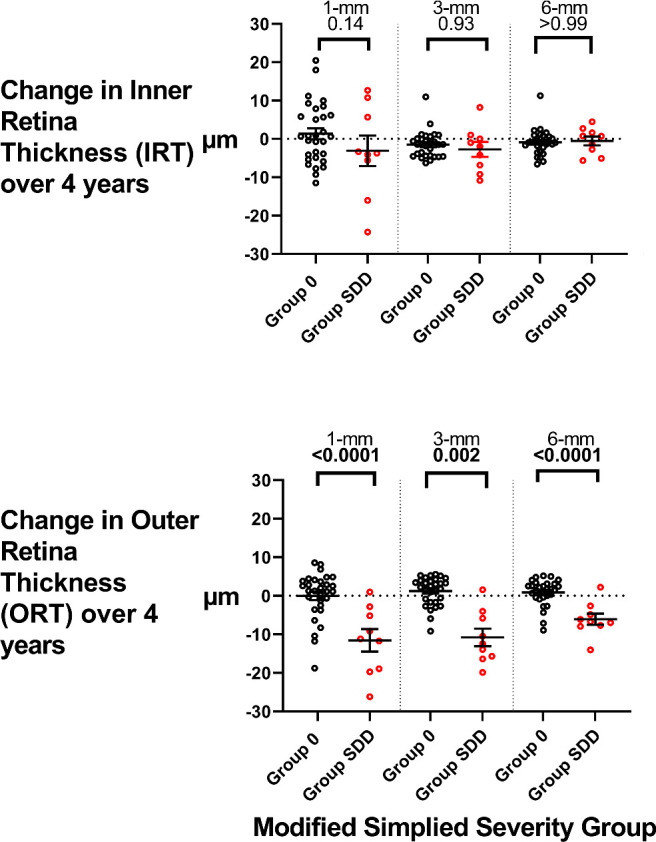
Analysis of longitudinal inner and outer retinal changes in Group 0 and Group SDD eyes over 4 years. TRT measurements obtained at study baseline and year 4 were segmented into IRT and ORT measurements for the 1-mm, 3-mm, and 6-mm retinal areas. Longitudinal changes were computed for Group 0 (n = 35) and Group SDD (n = 27) eyes. Statistical comparisons were performed using one-way ANOVA with Šidák correction; comparisons with significant P values (P < 0.05) are highlighted in bold.
Discussion
In this study, we evaluated how retinal thicknesses in eyes with iAMD of varying severity are different from eyes without AMD and how they may undergo longitudinal change, even without overt progression to late AMD. A quantitative characterization of the dynamic alterations in retinal anatomy during intermediate stages, as revealed by OCT, can potentially reflect intraretinal processes involving neurons and glia that are induced by the evolution and persistence of large drusen and SDDs, key phenotypes of iAMD. We approached this question of examining structural changes in eyes with iAMD in separate severity categories, stratified based on grading scales that relate to the 5-year risk of progression to late AMD.15 Statistical comparisons were made using a multivariate analysis, taking into account the contributions of other ocular and demographic factors so as to more specifically discern the impact of iAMD severity stage on retinal OCT anatomy.
Interestingly, we found that eyes in the earlier phase of iAMD (i.e., with large drusen in the study eye but not in the fellow eye, Group 1) demonstrated a small increase TRT in the central macula relative to Group 0 control eyes, with both the inner and outer layers of the retina apparently contributing to this increase. Other studies of retinal thickness in iAMD, performed using different approaches, have obtained varying results in this regard. In a cross-sectional study of 13 iAMD eyes, the foveal ORT was found to exceed that in 63 control eyes,19 consistent with our results here. Another cross-sectional comparative study of 71 early and intermediate AMD eyes found lower thicknesses in the inner retinal layers but higher thicknesses in the outer retinal layers20 compared with 31 non-AMD control eyes. In another study examining local retinal thickness in the region of drusen, retinal thicknesses immediately above large drusen were found to be locally decreased, whereas those in adjacent drusen-free regions were increased, relative to location-matched regions in control eyes.21,22 In contrast, a study with a similar design found a converse outer retinal thinning in loci both overlying drusen and within drusen-free regions, compared with location-matched measurements in normal control eyes lacking drusen.23
In the current study, we addressed this question more rigorously by instituting measures not performed in previous cross-sectional investigations, specifically (1) stratifying iAMD study eyes into lower and higher risk severity categories, and (2) performing multivariate analyses correcting for other influential patient and ocular factors. Our findings indicated that eyes with lower severity iAMD showed a small generalized increase in macular thickness in the central 3-mm macula area. The etiology of this change is unclear but may arise from early para-inflammatory responses to the presence of drusen.24 Large soft drusen contain multiple immunologically active molecules25 that can lead to the accumulation of activated innate immune cells near drusen and in the outer retina.26–28 These can trigger neuroinflammatory cellular changes, including gliotic and hypertrophic changes in Müller glia29,30 and the activation of proinflammatory microglia cells,31 that together might culminate in subtle non-cystic retinal edema and increased thickness.
The inconsistencies in previously published observations of retinal thickening versus thinning observed in iAMD may have arisen from opposite changes in the earlier versus later stages of iAMD. By stratifying iAMD eyes according to severity, we observed that retinal thickness, which is increased in less severe eyes, begins to trend toward lower values in eyes with more severe AMD, particularly those with SDDs. These data indicate that, although earlier responses to large drusen may induce subtle retinal swelling, more chronic retinal inflammation may give rise to degenerative changes that culminate in detectable retinal thinning. Other studies that did not perform stratification of iAMD eyes by severity may have combined eyes of varying severity, giving rise to varied comparisons. From both our observations and those in previous studies, it should be noted that the magnitude of macular thickening in early iAMD is small in all cases; as such, the establishment of its definite presence should be further investigated in a larger cohort with rigorous severity stratification. Clarification of the phenomenon, as well as the nature of drusen deposits that accompany it, can be insightful to understanding the influence that sub-RPE drusen formation can exert on the neuroretina. Furthermore, we utilized an automated method (Orion software) with manual verification for segmentation of retina to measure IRT and ORT. Past studies have used other segmentation methods such as the Spectralis OCT built-in software.20,32 Given the small magnitude of the changes in retinal thickness, more studies are necessary to compare these methods to understand if our different findings can be attributed partially to differences in segmentation methods.
These progressive atrophic changes occurring in the context of iAMD with the prolonged presence of drusen are also reflected in our 4-year longitudinal analysis of control versus AMD eyes. We observed that, although aged retinas without large drusen or SDDs demonstrated stability in thickness, retinas with more severe stages of iAMD and with SDDs demonstrated the most significant decrements in thickness across this relatively short time window. These decrements occurred not only over the specific regions immediately over drusen,21,23,33 which may have arisen sterically from the local deformations of retinal tissue from raised drusen, but also more generally across the central macula. These decrements in retinal thickness are particularly marked and rapid in retinas with SDDs, with decrements of approximately 5% in the central macula over 4 years. These decrements in SDD eyes also persisted even after we excluded the eyes that developed non-central GA over 4 years. These findings provide support for previous reports of decreased retinal thickness in iAMD eyes with SDDs12,32,34–36 and corroborate associations of SDDs with outer retinal thinning in the context of a preserved RPE, a description referred to as cORA (for complete outer retinal atrophy) in the OCT-based staging of AMD.13,37
Although progression to the late forms of AMD, including GA38 and neovascular AMD,39 has long been associated with marked decrements in central visual function, the functional consequences of changes in retinal thickness in the context of iAMD is less well characterized. In our dataset, the majority of eyes in the study demonstrated good baseline central function (mean BCVA > 80 for all severity groups), but we were still able to detect that lower baseline central macular thickness (1-mm and 3-mm circles) was significantly associated with lower baseline BCVA (Table 2), and that greater decreases in central macular thickness were also associated with greater 4-year decreases in BCVA (Table 3). These associations indicate that decrements in retinal thickness in iAMD can contribute modestly but significantly to decreased central visual function. Decreased retinal thickness in early and intermediate AMD and decreased visual function have also been correlated with decreased macular sensitivity on visual field testing40 and delays in dark adaptation.41 These data argue that longer term decrements in retinal thickness in iAMD parallel negative effects on visual function and that longitudinal changes in retinal thickness and BCVA may serve as useful outcome measures in interventional trials for iAMD.
The cellular mechanisms that connect the prolonged presence of soft drusen and SDDs to retinal thickness loss in iAMD have been speculated on, but causal relationships are yet unconfirmed. One hypothesis raised postulates that vascular insufficiency in either the retinal or the choroidal vasculature in iAMD eyes may drive retinal tissue loss. Studies of retinal vasculature using OCT angiography (OCT-A) have found that eyes with large drusen and at high risk for progressing to geographic atrophy have decreased parafoveal superficial vascular plexus flow density relative to non-AMD controls.42 Other OCT-A studies also detected decreased central retinal vessel density in the superficial and deep capillary plexuses in eyes with SDDs.34,36 With respect to the choroidal circulation, the presence of SDDs has been associated with decreases in choroidal thickness,43,44 as well as retinal thinning,36 suggesting that choroidal vascular compromise may drive retinal degeneration in SDD eyes. In our study population, although we found that iAMD eyes with SDDs had decreased choroidal thickness and CVI relative to normal controls,18 these parameters were not associated with decreased retinal thickness at baseline or accelerated retinal thinning over 4 years. Dissection of these relationships in future in vivo models of drusen formation may be required to establish causative mechanisms.
The limitations of our study include a relatively circumscribed number of prospectively followed eyes in each severity category and a lack of OCT-A and macular sensitivity characterizations. The AMD grading scales employed in this study stratify eyes into only four groups based on the presence of large drusen, pigmentary changes, the presence of SDD, and the presence of advanced AMD in the fellow eye. Although these incorporate key anatomic risk factors of AMD progression, additional AMD grading scales that incorporate drusen area and volume may yield more insights into the features that underlie retinal structural change.
In conclusion, we show here in a prospective and longitudinal study of non-advanced AMD eyes that biphasic changes in central macular thickness may occur in the course of iAMD. Increased macular thickness, albeit of small magnitude, was associated with the less severe categories of iAMD, and decreased thickness was associated with the more severe categories involving higher risk eyes and eyes with SDDs. Decreases in retinal thicknesses were detectable over a time course of 4 years and appear to be of functional importance, as they were associated with lower baseline visual acuity and more rapid decreases in visual acuity. These findings highlight the presence of dynamic biological changes occurring in the retina during the course of iAMD, prior to advancement to late AMD, and underscore the potential of longitudinal macular thickness measurements as an outcome measure for interventional studies targeting iAMD.
Supplementary Material
Acknowledgments
Supported by funds from the National Eye Institute Intramural Research Program, National Institutes of Health and through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Disclosure: T.T.-K. Chiang, None; T.D. Keenan, None; E. Agrón, None; J. Liao, None; B. Klein, None; E.Y. Chew, None; C.A. Cukras, None; W.T. Wong, None
References
- 1. Klein R, Klein BE, Cruickshanks KJ. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res. 1999; 18: 371–389. [DOI] [PubMed] [Google Scholar]
- 2. Kawasaki R, Yasuda M, Song SJ, et al.. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010; 117: 921–927. [DOI] [PubMed] [Google Scholar]
- 3. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018; 392: 1147–1159. [DOI] [PubMed] [Google Scholar]
- 5. Sivaprasad S, Bird A, Nitiahpapand R, et al.. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016; 61: 521–537. [DOI] [PubMed] [Google Scholar]
- 6. Finger RP, Chong E, McGuinness MB, et al.. Reticular pseudodrusen and their association with age-related macular degeneration: the Melbourne Collaborative Cohort Study. Ophthalmology. 2016; 123: 599–608. [DOI] [PubMed] [Google Scholar]
- 7. Gil JQ, Marques JP, Hogg R, et al.. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye (Lond). 2017; 31: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finger RP, Schmitz-Valckenberg S, Schmid M, et al.. MACUSTAR: development and clinical validation of functional, structural, and patient-reported endpoints in intermediate age-related macular degeneration. Ophthalmologica. 2019; 241: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic clinical trial endpoints for nonexudative age-related macular degeneration. Ophthalmology. 2016; 123: 1060–1079. [DOI] [PubMed] [Google Scholar]
- 10. Ding JD, Kelly U, Groelle M, Christenbury JG, Zhang W, Bowes Rickman C. The role of complement dysregulation in AMD mouse models. Adv Exp Med Biol. 2014; 801: 213–219. [DOI] [PubMed] [Google Scholar]
- 11. Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med. 2012; 33: 487–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spaide R. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013; 33: 1800–1808. [DOI] [PubMed] [Google Scholar]
- 13. Spaide RF. Improving the age-related macular degeneration construct: a new classification system. Retina. 2018; 38: 891–899. [DOI] [PubMed] [Google Scholar]
- 14. Flamendorf J, Agron E, Wong WT, et al.. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015; 122: 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferris FL, Davis MD, Clemons TE, et al.. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005; 123: 1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis MD, Gangnon RE, Lee LY, et al.. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005; 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behbehani R, Abu Al-Hassan A, Al-Salahat A, Sriraman D, Oakley JD, Alroughani R. Optical coherence tomography segmentation analysis in relapsing remitting versus progressive multiple sclerosis. PLoS One. 2017; 12: e0172120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keenan TD, Klein B, Agron E, Chew EY, Cukras CA, Wong WT. Choroidal thickness and vascularity vary with disease severity and subretinal drusenoid deposit presence in nonadvanced age-related macular degeneration. Retina. 2020; 40: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nusinowitz S, Wang Y, Kim P, et al.. Retinal structure in pre-clinical age-related macular degeneration. Curr Eye Res. 2018; 43: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamin A, Oakley JD, Dubis AM, Russakoff DB, Sivaprasad S. Changes in volume of various retinal layers over time in early and intermediate age-related macular degeneration. Eye (Lond). 2019; 33: 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadigh S, Cideciyan AV, Sumaroka A, et al.. Abnormal thickening as well as thinning of the photoreceptor layer in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 22. Sadigh S, Luo X, Cideciyan AV, et al.. Drusen and photoreceptor abnormalities in African-Americans with intermediate non-neovascular age-related macular degeneration. Curr Eye Res. 2015; 40: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogala J, Zangerl B, Assaad N, Fletcher EL, Kalloniatis M, Nivison-Smith L. In vivo quantification of retinal changes associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 1689–1700. [DOI] [PubMed] [Google Scholar]
- 24. Chen M, Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. J Leukoc Biol. 2015; 98: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crabb JW, Miyagi M, Gu X, et al.. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002; 99: 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010; 94: 918–925. [DOI] [PubMed] [Google Scholar]
- 27. Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003; 76: 463–471. [DOI] [PubMed] [Google Scholar]
- 28. Sennlaub F, Auvynet C, Calippe B, et al.. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013; 5: 1775–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards MM, McLeod DS, Bhutto IA, Villalonga MB, Seddon JM, Lutty GA. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp Eye Res. 2016; 150: 44–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sullivan R, Penfold P, Pow DV. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Invest Ophthalmol Vis Sci. 2003; 44: 856–865. [DOI] [PubMed] [Google Scholar]
- 31. Penfold PL, Liew SC, Madigan MC, Provis JM. Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997; 38: 2125–2133. [PubMed] [Google Scholar]
- 32. Abdolrahimzadeh S, Parisi F, Marcelli M, Giustolisi R, Gharbiya M. Optical coherence tomography evidence of macular ganglion cell-inner plexiform layer thinning in eyes with subretinal drusenoid deposits. Eye (Lond). 2019; 33: 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009; 116: 488–496.e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cicinelli MV, Rabiolo A, Sacconi R, et al.. Retinal vascular alterations in reticular pseudodrusen with and without outer retinal atrophy assessed by optical coherence tomography angiography. Br J Ophthalmol. 2018; 102: 1192–1198. [DOI] [PubMed] [Google Scholar]
- 35. Ramon C, Cardona G, Biarnes M, Ferraro LL, Mones J. Longitudinal changes in outer nuclear layer thickness in soft drusen and reticular pseudodrusen. Clin Exp Optom. 2019; 102: 601–610. [DOI] [PubMed] [Google Scholar]
- 36. Ahn SM, Lee SY, Hwang SY, Kim SW, Oh J, Yun C. Retinal vascular flow and choroidal thickness in eyes with early age-related macular degeneration with reticular pseudodrusen. BMC Ophthalmol. 2018; 18: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sadda SR, Guymer R, Holz FG, et al.. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018; 125: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999; 5: 25. [PubMed] [Google Scholar]
- 39. Wong TY, Chakravarthy U, Klein R, et al.. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008; 115: 116–126. [DOI] [PubMed] [Google Scholar]
- 40. Acton JH, Smith RT, Greenberg JP, Greenstein VC. Comparison between MP-1 and Humphrey visual field defects in glaucoma and retinitis pigmentosa. Optom Vis Sci. 2012; 89: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clark ME, McGwin G Jr, Neely D, et al.. Association between retinal thickness measured by spectral-domain optical coherence tomography (OCT) and rod-mediated dark adaptation in non-exudative age-related maculopathy. Br J Ophthalmol. 2011; 95: 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toto L, Borrelli E, Mastropasqua R, et al.. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: an optical coherence tomography angiography study. Br J Ophthalmol. 2017; 101: 774–779. [DOI] [PubMed] [Google Scholar]
- 43. Garg A, Oll M, Yzer S, et al.. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013; 54: 7075–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Querques G, Querques L, Forte R, Massamba N, Coscas F, Souied EH. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012; 53: 1258–1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.