Abstract
Coronaviruses are a group of enveloped viruses with non-segmented, single-stranded, and positive-sense RNA genomes. In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan City, China. The World Health Organization (WHO) declared the coronavirus outbreak as a global pandemic in March 2020. Fever, dry cough and fatigue are found in the vast majority of all COVID-19 cases. Early diagnosis, treatment and future prevention are keys to COVID-19 management. Currently, the unmet need to develop cost-effective point-of-contact test kits and efficient laboratory techniques for confirmation of COVID-19 infection has powered a new frontier of diagnostic innovation. No proven effective therapies or vaccines for SARS-CoV-2 currently exist. The rapidly increasing research regarding COVID-19 virology provides a significant number of potential drug targets. Remdesivir may be the most promising therapy up till now. On May 1, 2020, Gilead Sciences, announced that the U.S. Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for the investigational Remdesivir as a potential antiviral for COVID-19 treatment. On May 7, 2020, Gilead Sciences, announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has granted regulatory approval of Veklury® (Remdesivir) as a treatment for SARS-CoV-2 infection, the virus that causes COVID-19 acute respiratory syndrome, under an exceptional approval pathway. Also, Corticosteroids are recommended for severe cases only to suppress the immune response and reduce symptoms, but not for mild and moderate patients where they are associated with a high-risk side effect. Based on the currently published evidence, we tried to highlight different diagnostic approaches, side effects and therapeutic agents that could help physicians in the frontlines.
Keywords: COVID-19, SARS-CoV-2, Remdesivir, Diagnosis, Epidemiology, Therapy
Introduction
In December 2019, a novel coronavirus, SARS-CoV-2, was identified as the pathogen causing coronavirus disease (COVID-19) in Wuhan, China. On March 11, 2020, the World Health Organization declared COVID-19 as a global pandemic (Whitworth, 2020[71]).
COVID-19 is an enveloped, positive-sense, single-stranded RNA virus that belongs to the beta-CoV genus, which also includes SARS-CoV and MERS-CoV. It shares 89 % nucleotide identity with bat SARS-like CoVZXC21 and 82 % identity with human SARS-CoV (Chan et al., 2020[15]).
COVID-19 is transmitted by inhalation or contact with infected droplets. The incubation period for COVID-19 is on average, 5-6 days, but can be up to 14 days. During this period, also known as the “presymptomatic” period, some infected persons can be contagious, from 1-3 days before symptom onset (Wei et al., 2020[69]). The clinical manifestations of COVID-19 varied from asymptomatic carrier status, acute respiratory disease (ARD) and pneumonia. The prevalence of asymptomatic cases is significant (20-86 % of all infections) and is defined as individuals with positive viral nucleic acid tests but without any COVID-19 symptoms. Most people with COVID-19 develop only mild (40 %) or moderate (40 %) disease, approximately 15 % develop a severe disease that requires hospitalization and oxygen support, and 5 % have a critical disease with complications such as respiratory failure, acute respiratory distress syndrome (ARDS), sepsis and septic shock, thromboembolism, and/or multiorgan failure, including acute kidney injury and cardiac injury (CDC, 2020[14]) Older age, co-morbidities such as diabetes, hypertension, cardiac disease, chronic lung disease, cancer and BMI > 40 kg/m2 have been reported as risk factors for severe disease and death (CDC, 2020[13]).
Common Signs and Symptoms
Wang and colleagues (2020[66]) reported that there are 6 common signs and symptoms that 30 % of the patients have felt including fever (98.5 %), fatigue (69.9 %), dry cough (59.4 %), anorexia (39.8 %), myalgia (34.8 %), dyspnea (31.1 %) and for the most common comorbidities are hypertension (31.1 %) and cardiovascular disease (14.5 %). Symptoms may develop 2 days to 2 weeks following exposure to the virus (CDC, 2020[14]). According to Wu and McGoogan (2020[74]), among 72,314 SARS-CoV-2 cases reported to the Chinese Center for Disease Control and Prevention (CCDC), 81 % were mild (mild or absent pneumonia), 14 % were severe (dyspnea, hypoxia, > 50 % lung involvement within 1-2 days), 5 % were critical (respiratory failure, shock, multiorgan dysfunction), and 2.3 % were fatal. Symptoms in children with infection appear to be uncommon, although some children with severe COVID-19 have been reported (CDC, 2020[13]). Based on currently available information and clinical expertise, risk factors for severe COVID-19 include older adults ≥ 65 years as well as people of all ages with chronic lung disease or moderate to severe asthma, serious heart conditions, diabetes, severe obesity, chronic kidney disease, liver disease and immunocompromised people (CDC, 2020[13]).
Suggested Infection Mechanism
Upon infection with COVID-19, it binds to the host cell's angiotensin-converting enzyme 2 (ACE2) receptors which commonly expressed on the epithelial cells of alveoli, trachea, bronchi, and bronchial serous glands of the respiratory tract. Then the virus enters and replicates in these cells (Liu et al., 2011[45]). The newly developed virions are then released and infect new target cells. Unfortunately, there is no specific antiviral treatment or vaccine recommended for COVID-19 that is currently available.
Current Epidemiological Situation
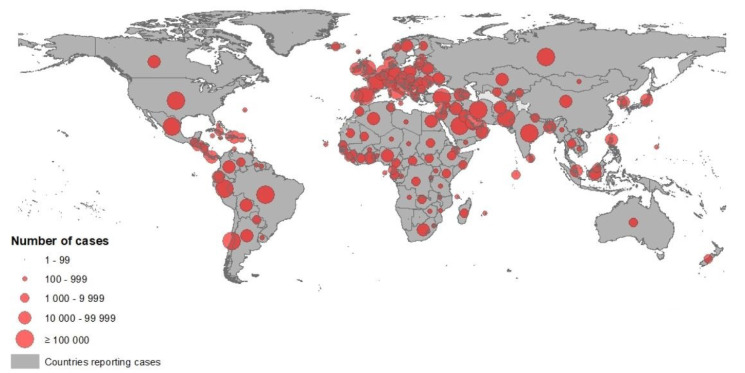
According to the European Centre for Disease Prevention and Control (ECDC), since 31 December 2019 and as of 14 June 2020, 7,759,691 cases of COVID-19 have been reported including, most cases in America (n = 3788548) were reported from the United States (2,074,526), Brazil (850,514) and Peru (225,132), followed by Europe (n = 2,170,600): most cases were reported in Russia (520,129), United Kingdom (294,375) and Spain (243,605), Asia (n = 1557541): most cases were in India (320,922), Iran (184,955) and Turkey (176,677), Africa (n = 233528): most cases were in South Africa (65,736), Egypt (42,980), Nigeria (15,682), Oceania (n = 8766): most cases were in Australia (7,290), New Zealand (1,154) and Guam (185) (Figure 1(Fig. 1)), including 430,127 deaths, most deaths in America (n = 201,874) were reported from the United States (115,436), Brazil (42,720) and Mexico (16,872), followed by Europe (n = 182674): most deaths were in United Kingdom (41,662), Italy (34,301) and France (29,398), Asia (n = 39147): most deaths were in India (9,195), Iran (8,730) and Turkey (4,792), Africa (n = 6294): most deaths were in Egypt (1,484), South Africa (1,423) and Algeria (760), Oceania (n = 131): most deaths were in Australia (102), New Zealand (22) and Guam (5) (ECDC, 2020[26]).
Figure 1. Novel coronavirus COVID-19 geographical distribution over the word 2020-05-09 (ECDC, 2020).
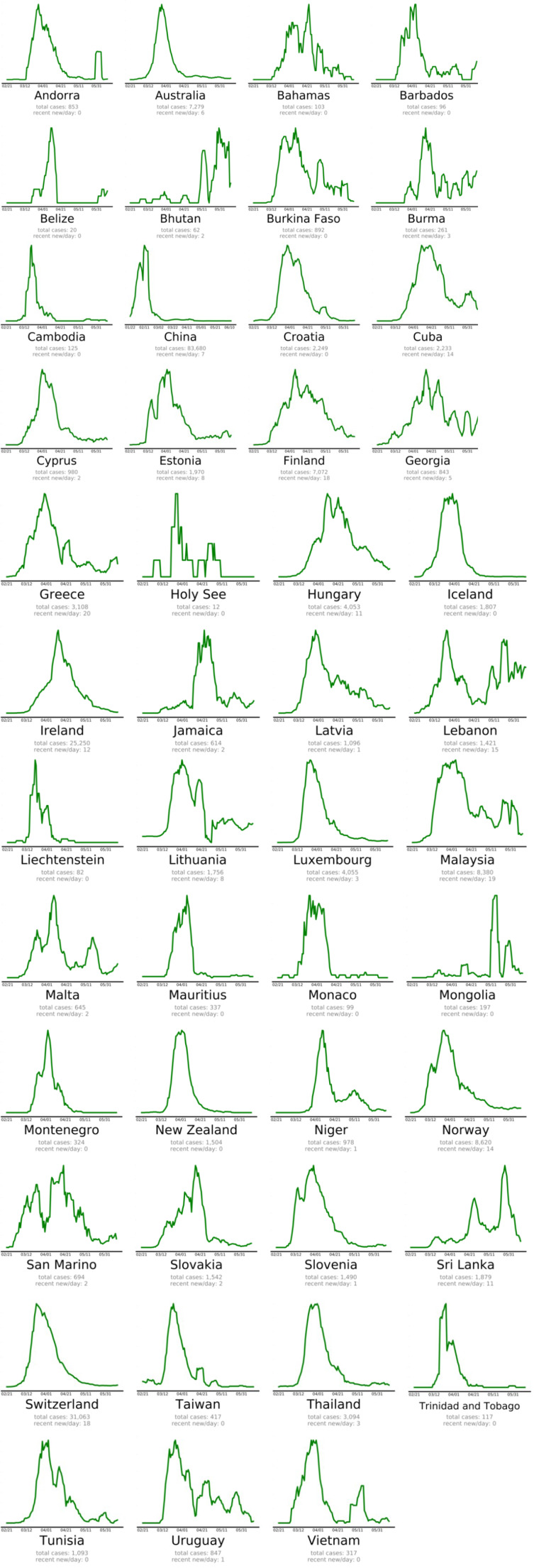
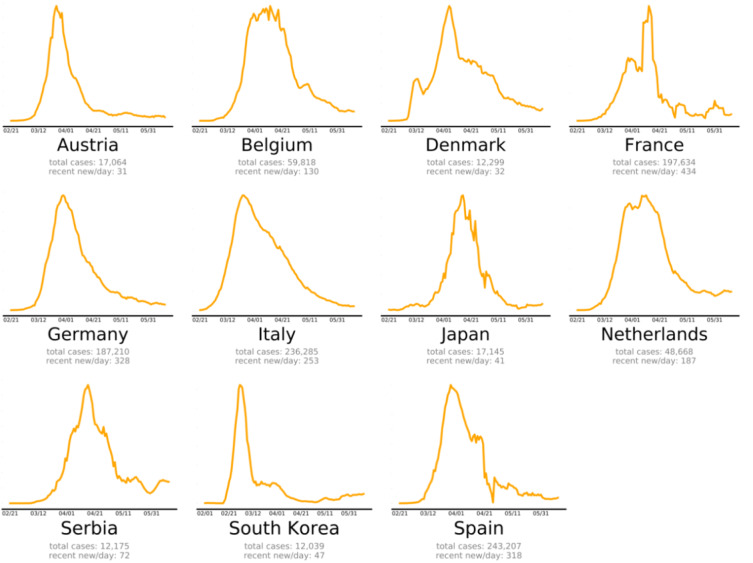
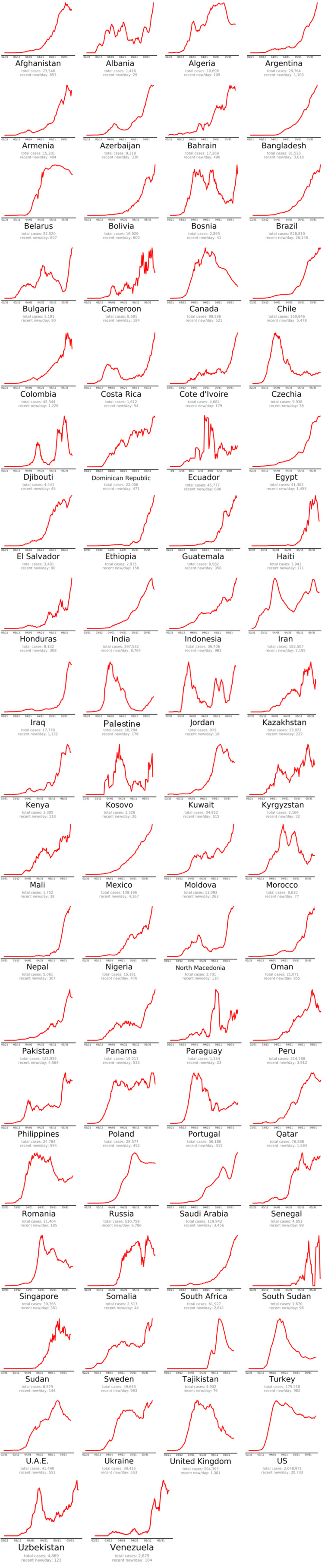
The countries that beat COVID-19 were divided into three groups as follows: countries beating COVID-19: green plots (Figure 2(Fig. 2)), countries that are nearly there: yellow plots (Figure 3(Fig. 3)) and countries that need to take action: red plots (Figure 4(Fig. 4)). These plots adjusted for each country to better show the data. The vertical axis is plotted in arbitrary units, to easily compare the shapes of the curves (EndCoronavirus, 2020[30]).
Figure 2. Countries beating COVID-19 in alphabetical order (EndCoronavirus, 2020).
Figure 3. Countries that are nearly there (EndCoronavirus, 2020).
Figure 4. Countries that need to do an action (EndCoronavirus, 2020).
SARS-CoV-2 Diagnosis
The diagnosis of COVID-19 mainly depends on the demonstration of the virus in respiratory secretions by special molecular tests. Common laboratory findings include normal/ low white cell counts with elevated C-reactive protein (CRP). The computerized tomographic chest scan is usually abnormal even in those with no symptoms or mild disease (Singhal, 2020[58]). In addition to laboratory testing capacity and reagent shortages, the rapidly growing SARS CoV 2 pandemic has encouraged many diagnostic manufacturers to develop and sell fast and easy-to-use equipment to facilitate testing outside the laboratory (WHO, 2020[72]).
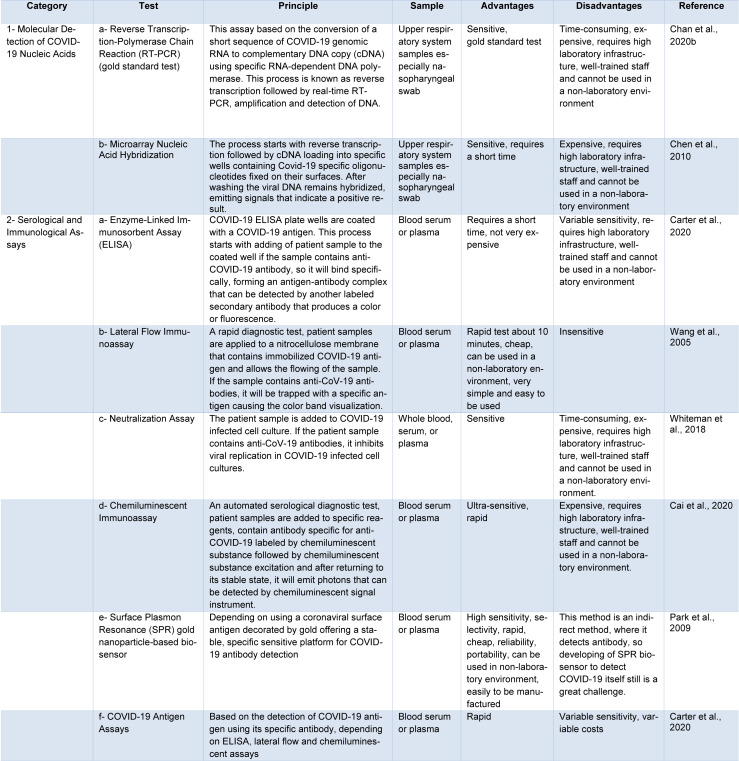
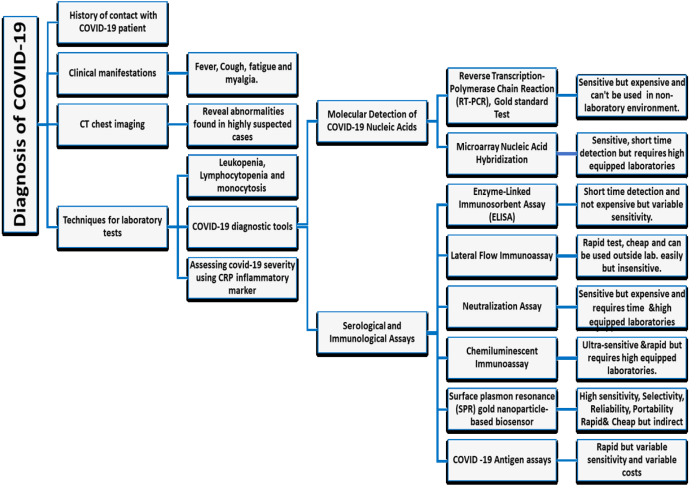
Currently, there are two main categories commercially available for COVID-19 tests. The first category includes molecular assays for detection of SARS-CoV-2 viral RNA using polymerase chain reaction (PCR)-based methods. The second category includes serological and immunological assays that largely depend on detecting antibodies produced by individuals as a result of exposure to the virus or on the detection of antigenic proteins in infected individuals. It is necessary to ensure that these two categories of tests serve overlapping purposes in the management of the SARS-CoV-2 pandemic (Carter et al., 2020[11]). Current COVID-19 diagnostic tools and techniques are shown in Table 1(Tab. 1) (References in Table 1: Cai et al., 2020[7]; Carter et al., 2020[11]; Chan et al., 2020[16]; Chen et al., 2010[19]; Park et al., 2009[52]; Wang et al., 2005[65]; Whiteman et al., 2018[70]) and a diagnostic model for COVID-19 in Figure 5(Fig. 5).
Table 1. Current SARS-CoV-2 diagnostic tools and techniques.
Figure 5. The Diagnostic Model for COVID-19.
SARS-CoV-2 Different Therapeutic Approaches
Symptomatic treatment and oxygen therapies represent the major treatment interventions for patients with severe infection. Mechanical ventilation may be necessary in cases of respiratory failure refractory to oxygen therapy, whereas hemodynamic support is essential for managing septic shock (Cascella et al., 2020[12]).
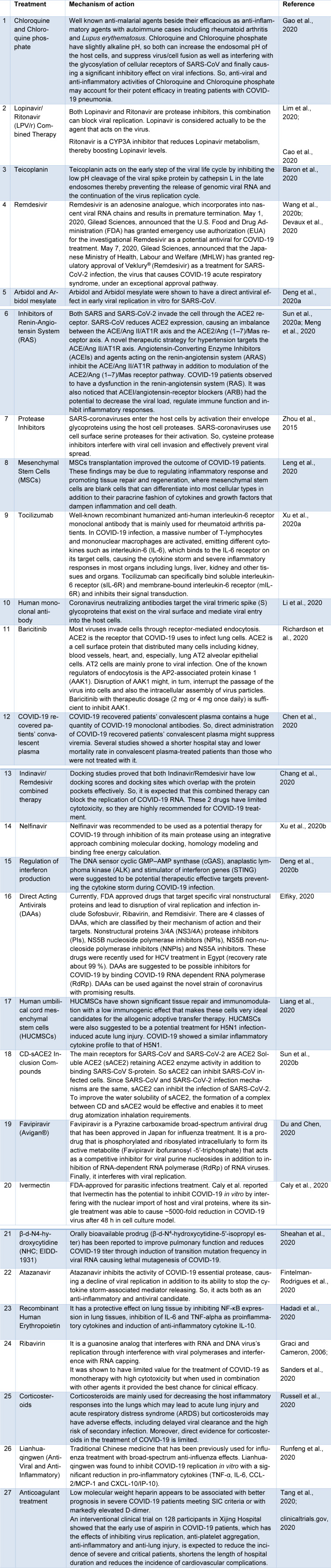
To the best of our knowledge, different therapeutic approaches have been evaluated against COVID-19 in vivo, vitro and in clinical trials. Many of these therapies had a great impact on clinical recovery. Current COVID-19 therapies are shown in Table 2(Tab. 2) (References in Table 2: Baron et al., 2020[3]; Caly et al., 2020[8]; Cao et al., 2020[10]; Chang et al., 2020[17]; Chen et al., 2020[18]; clinicaltrials.gov, 2020[20]; Deng et al., 2020[22][23]; Devaux et al., 2020[24]; Du and Chen, 2020[25]; Elfiky, 2020[28]; Fintelman-Rodrigues et al., 2020[32]; Gao et al., 2020[34]; Graci and Cameron, 2006[36]; Hadadi et al., 2020[37]; Leng et al., 2020[41]; Li et al., 2020[42]; Liang et al., 2020[43]; Lim et al., 2020[44]; Meng et al., 2020[48]; Richardson et al., 2020[53]; Runfeng et al., 2020[54]; Russell et al., 2020[55]; Sanders et al., 2020[56]; Sheahan et al., 2020[57]; Sun et al., 2020[59][60]; Tang et al., 2020[61]; Wang et al., 2020[67]; Xu et al., 2020[75][76]; Zhou et al., 2015[80]).
Table 2. Different SARS-CoV-2 therapeutic approaches and mechanisms.
SARS-CoV-2 Therapeutic Approaches - Side Effects
Despite the approved beneficial effects of these therapeutic approaches, recent studies concluded that most of these candidate's administration has a toxic effect in overdoses, causing common and severe adverse effects including nausea, pruritus, arrhythmias, hypoglycemia, anemia, jaundice, hyperlipidemia, electrolyte abnormalities, acute renal injury, hematological disorders, hyperuricemia, neuropsychiatric effects and various drug-drug interactions.
Chloroquine (CQ) interferes with ventricular repolarization that increases the risk of torsades de pointes (TdP) and may cause sudden cardiac death (Ursing et al., 2020[64]), also it causes neuropsychiatric manifestations including confusion agitation, psychosis, mania, hallucinations, paranoia, suicidal ideation, depression, insomnia and catatonia (Aneja et al., 2019[2]) as well as severe hypoglycemia (El-Solia et al., 2018[29]). Moreover, CQ has severe immunological mediated adverse effects including drug reaction with eosinophilia and systemic symptoms (DRESS) (Girijala et al., 2019[35]), Stevens-Johnson syndrome (Leckie and Rees, 2002[40]) and toxic epidermal necrolysis (Cameron et al., 2014[9]).
Lopinavir/Ritonavir (LPV/r) combination has been reported to have gastrointestinal disorders, so in some SARS-CoV-2 patients, the treatment was stopped due to these severe side events (Owa and Owa, 2020[51]). Notwithstanding the minimal side effects of Teicoplanin, it may cause thrombocytopenia in some treated cases (Terol et al., 1993[63]).
A recent clinical trial regarding Remdesivir with severe COVID-19 patients concluded that adverse events including hypokalemia, constipation, hypoalbuminemia, anemia, jaundice, hyperlipidemia, liver enzyme elevation and thrombocytopenia were reported (Wang et al., 2020[68]).
An exploratory randomized controlled trial assessing the efficacy and safety of Arbidol in COVID-19 patients reported that patients had adverse events including diarrhea, nausea and loss of appetite (Eikenberry et al., 2020[27]), also hypotension, acute renal injury, teratogenicity, hypersensitivity, electrolyte abnormalities, fatigue, diarrhea, weakness, anemia and chest pain are the most common risk factors during treatment of COVID-19 patients using inhibitors of the renin-angiotensin system (Ingraham et al., 2020[38]).
Zhang and colleagues (2020[79]) reported that intravenous transplantation of Wharton's jelly derived mesenchymal stem cells (hWJCs) was safe and effective especially, in COVID-19 critical severe cases. Regarding Tocilizumab that was used as a treatment for severe COVID-19 cases, it may cause serious adverse reactions, like intestinal perforation, candidiasis and lipid metabolism abnormalities (Tao et al., 2020[62]).
FDA has approved convalescent plasma therapy in COVID-19 critical patients, but up till now, only three studies with small sample size reported effectiveness and safety so more clinical trials are needed to ensure both safety and efficacy (Bloch et al., 2020[6]).
Otherwise Direct-acting antivirals (DAAs) demonstrated, a safe therapeutic approach with common side effects including fatigue, headache, nausea and neuropsychiatric symptoms (Medeiros et al., 2017[47]). Concerning using of Favipiravir (Avigan®) as a treatment for COVID-19 patients, it was reported that Favipiravir elevates plasma uric acid, so this finding should be considered in hyperuricemia, gout and kidney impairment patients (Mishima et al., 2020[49]).
Despite the beneficial effect of Corticosteroids with COVID-19 patients, they are associated with a high risk of death, side effects like bacterial infections and hypokalemia so they are not recommended for mild and moderate COVID-19 patients, but they should be used in severe cases only to suppress the immune response and reduce symptoms (Yang et al., 2020[77]).
Chloroquine Triggers Oxidation and Hemolytic Anemia in G6PD Deficient Cases & World Health Organization Discontinued its Treatment Trials
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one of the most common human enzymatic disorders affecting around 400 million people worldwide (Luzzatto and Arese, 2018[46]). Decreased G6PD production results in low levels of NADPH and reduced glutathione stimulating hemolytic anemia which is characterized by oxidative stress and red blood cell lysis (Francis et al., 2013[33]).
The risk of hemolytic anemia should be considered during Chloroquine/Hydroxy Chloroquine (CQ/HCQ) therapy of patients with G6PD deficiency (Mohammad et al., 2018[50]).
Beauverd and colleagues (2020[4]) reported that SARS-CoV-2 infection can enhance severe acute hemolysis in patients with G6PD‐deficiency, and CQ/HCQ can worsen this crisis. During the treatment of SARS-CoV-2, it is important to carefully monitor potential hemolytic effects of CQ/HCQ in G6PD deficiency cases. If a decline in hemoglobin levels during the first days of CQ/HCQ treatment is observed, the treatment should be stopped. Hemolysis usually is reversible after finishing therapy with CQ/HCQ (De Franceschi et al., 2020[21]). Also, Kapoor and Kapoor (2020[39]) warned of the use of CQ because of the risk of hematological disorders in patients with G6PD deficiency.
In contrast, both (Youngster et al. 2010[78]; Beutler 1994[5]) concluded that CQ or HCQ mono-therapies are safe also in G6PD deficient cases.
Afra and colleagues (2020[1]) reported that infections might be the most common causes of hemolysis in G6PD deficient patients. Thus, SARS‐CoV‐2 patients may show significant hemolysis even before CQ or HCQ administration.
Finally, SARS‐CoV‐2 treatment using CQ or HCQ, especially in areas with high G6PD deficiency prevalence, should alert medical staff to this possible harmful effect. The US Food and Drug Administration warned of cardiotoxicity caused by hydroxychloroquine and mentioned G6PD as a baseline test before the onset of hydroxychloroquine treatment (FDA, 2020[31]). Moreover, in July 2020 the WHO discontinued clinical trials with hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 (WHO, 2020[73]), where both therapies produced little and no reduction in the mortality of hospitalized SARS‐CoV‐2 cases when compared to standard of care.
Conclusion
Finally, COVID-19 pandemic is a highly infectious disease caused by the novel coronavirus SARS-CoV-2 that can be transmitted through droplets and close contact and represents a global public health crisis. Fever, fatigue and dry coughs are the most common signs and symptoms of COVID-19. Due to rapid transmission, countries around the world should increase attention to disease surveillance systems. SPR gold nanoparticle-based biosensors may be a promising diagnostic technique as it had high sensitivity, selectivity, reliability, portability, is rapid and cheap, but this method is an indirect method, where it detects antibody, so developing of SPR biosensor to detect COVID-19 itself still is a great challenge. No proven effective therapies or vaccines for SARS-CoV-2 currently exist. The most promising therapy up till now maybe Remdesivir, also we recommend Corticosteroids therapy for severe cases only to suppress the immune response and reduce symptoms, but not for mild and moderate patients where they are associated with high-risk side effects. G6PD should be considered as a baseline test for starting CQ or HCQ treatment protocol to avoid its possible hemolytic effect. We should further strive to develop specific medications, support the research and development of vaccines, and also decrease morbidity and death of SARS-CoV-2 to preserve the population.
Notes
Ahmed Nabil, Mitsuhiro Ebara (Research Center for Functional Materials, National Institute for Materials Science (NIMS), 1-1Namiki, Tsukuba, Ibaraki 305-0044, Japan; Tel: 008180-6661-5342, E-mail: EBARA.Mitsuhiro@nims.go.jp) and Gamal Shiha (Egyptian Liver Research Institute and Hospital (ELRIAH), Sherbin, El Mansoura, Egypt; Hepatology and Gastroenterology Unit, Internal Medicine Department, Faculty of Medicine, Mansoura University, Egypt; Tel: (+20)1223280501, E-mail: g_shiha@hotmail.com) equally contributed as corresponding authors.
Authors contribution
Ahmed Nabil: Resources, Conceptualization, Original draft writing, Supervision, Review & Editing. Koichiro Uto: Original draft writing, Review & Editing. Mohamed M. Elshemy: Original draft writing, Review, Editing & Resources. Reham Soliman: Writing, Review & Editing. Ayman A. Hassan: Writing & Editing. Mitsuhiro Ebara: Conceptualization, Resources, Original draft writing, Supervision, Review & Editing. Gamal Shiha: Conceptualization, Original draft writing, Review, Editing & Supervision.
Acknowledgement
All authors express their great gratitude for researchers, physicians, nurses, health care technicians and all co-workers in the frontlines in Egypt, Japan and any spot of the globe who spend their life during fighting this virus, hoping this work could help them in their mission.
Special thanks for Ebara Labo., NIMS, Japan research team & ELRIAH, El Mansoura, Egypt researchers, physicians, nurses and health care technicians.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Afra TP, Vasudevan Nampoothiri R, Razmi TM. Doubtful precipitation of hemolysis by hydroxychloroquine in glucose-6-phosphate dehydrogenase-deficient patient with COVID-19 infection. Eur J Haematol. 2020;epub ahead of print. doi: 10.1111/ejh.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aneja J, Goya D, Choudhary B. Psychosis consequent to antimalarial drug use in a young child. Family Med Prim Care. 2019;8:1781–1783. doi: 10.4103/jfmpc.jfmpc_225_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron SA, Devaux C, Colson P, Raoult D, Rolain JM. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents. 2020;55(4):105944. doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauverd Y, Adam Y, Assouline B, Samii K. COVID-19 infection and treatment with hydroxychloroquine cause severe haemolysis crisis in a patient with glucose-6-phosphate dehydrogenase deficiency. Eur J Haematol. 2020;epub ahead of print doi: 10.1111/ejh.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 6.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai X, Chen J, Hu J, Long Q, Deng H, Fan K, et al. A peptide-based magnetic chemiluminescence enzyme immunoassay for serological diagnosis of Corona Virus disease 2019 (COVID-19) J Infect Dis. 2020;222:189–193. doi: 10.1093/infdis/jiaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron MC, Word AP, Dominguez A. Hydroxychloroquine-induced fatal toxic epidermal necrolysis complicated by angioinvasive rhizopus. Dermatol Online J. 2014;20(11):25419748. [PubMed] [Google Scholar]
- 10.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19) Statpearls [internet]: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 13.CDC. People who are at higher risk for severe illness. [25 June 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html.
- 14.CDC. Symptoms of Coronavirus. [13 May 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html?CDC_AA_refVal=https %3A %2F %2Fwww.cdc.gov %2Fcoronavirus %2F2019-ncov %2Fabout %2Fsymptoms.html.
- 15.Chan JF-W, Kok K-H, Zhu Z, Chu H, To KK-W, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JF-W, Yip CC-Y, To KK-W, Tang TH-C, Wong SC-Y, Leung K-H, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR Assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58:e00310–e00320. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y-C, Tung Y-A, Lee K-H, Chen T-F, Hsiao Y-C, Chang H-C, et al. Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking. Preprints 2020. 2020;2020020242 doi: 10.20944/preprints202002.0242.v1.. doi: 10.20944/preprints202002.0242.v1.. Available from: [DOI] [Google Scholar]
- 18.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Li J, Deng Z, Xiong W, Wang Q, Hu Y-Q. Comprehensive detection and identification of seven animal coronaviruses and human respiratory coronavirus 229E with a microarray hybridization assay. Intervirology. 2010;53:95–104. doi: 10.1159/000264199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.clinicaltrials.gov. Protective effect of aspirin on COVID-19 patients (PEAC), NCT04365309. 2020.
- 21.De Franceschi L, Costa E, Dima F, Morandi M, Olivieri O. Acute hemolysis by hydroxycloroquine was observed in G6PD-deficient patient with severe COVD-19 related lung injury. Eur J Intern Med. 2020;77:136–137. doi: 10.1016/j.ejim.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng X, Yu X, Pei J. Regulation of interferon production as a potential strategy for COVID-19 treatment. arXiv. 2020;2003.00751. [Google Scholar]
- 24.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y-X, Chen X-P. Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020:online ahead of print. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 26.ECDC, European Centre for Disease Prevention and Control. COVID-19 situation update worldwide, as of 9 May 2020. [9 May 2020]. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
- 27.Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, et al. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Solia A, Al-Otaibi K, Ai-Hwiesh AK. Hydroxychloroquine-induced hypoglycaemia in non-diabetic renal patient on peritoneal dialysis. BMJ Case Rep. 2018;2018:bcr2017223639. doi: 10.1136/bcr-2017-223639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EndCoronavirus. Which countries do best in beating COVID-19? [31 May 2020]. Available from: https://www.endcoronavirus.org/countries.
- 31.FDA. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. [1 July 2020]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or.
- 32.Fintelman-Rodrigues N, Sacramento CQ, Lima CR, da Silva FS, Ferreira AC, Mattos M, et al. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. bioRxiv. 2020:2020.04.04.020925. [Google Scholar]
- 33.Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, Spitalnik SL. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–282. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 35.Girijala RL, Siddiqi I, Kwak Y, Wright D, Patel DB, Goldberg LH. Pustular DRESS syndrome secondary to hydroxychloroquine with EBV reactivation. J Drugs Dermatol. 2019;18:207–209. [PubMed] [Google Scholar]
- 36.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92:915–918. doi: 10.1002/jmv.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, et al. Understanding the renin-angiotensin-aldosterone-SARS-CoV-axis: A comprehensive review. Eur Respir J. 2020;2020:2000912. doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor KM, Kapoor A. Role of chloroquine and hydroxychloroquine in the treatment of COVID-19 infection- A systematic literature review. medRxiv. 2020:2020.03.24.20042366. [Google Scholar]
- 40.Leckie MJ, Rees RG. Stevens–Johnson syndrome in association with hydroxychloroquine treatment for rheumatoid arthritis. Rheumatology. 2002;41:473–474. doi: 10.1093/rheumatology/41.4.473. [DOI] [PubMed] [Google Scholar]
- 41.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Jin R, Peng Y, Wang C, Ren W, Lv F, et al. Generation of antibodies against COVID-19 virus for development of diagnostic tools. medRxiv. 2020:2020.02.20.20025999. [Google Scholar]
- 43.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. chinaXiv. 2020;2:v1. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: The application of Lopinavir/Ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 2018;378:60–71. doi: 10.1056/nejmra1708111. [DOI] [PubMed] [Google Scholar]
- 47.Medeiros T, Salviato CdM, do Rosário NF, Saraiva GdN, Esberard EBC, Almeida JR, et al. Adverse effects of direct acting antiviral-based regimens in chronic hepatitis C patients: A Brazilian experience. Int J Clin Pharm. 2017;39:1304–1311. doi: 10.1007/s11096-017-0552-1. [DOI] [PubMed] [Google Scholar]
- 48.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishima E, Anzai N, Miyazaki M, Abe T. Uric acid elevation by Favipiravir, an antiviral drug. Tohoku J Exp Med. 2020;251:87–90. doi: 10.1620/tjem.251.87. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad S, Clowse MEB, Eudy AM, Criscione-Schreiber LG. Examination of hydroxychloroquine use and hemolytic anemia in G6PDH-deficient patients. Arthritis Care Res (Hoboken) 2018;70:481–485. doi: 10.1002/acr.23296. [DOI] [PubMed] [Google Scholar]
- 51.Owa AB, Owa OT. Lopinavir/ritonavir use in Covid-19 infection: Is it completely non-beneficial? J Microbiol Immunol Infect. 2020;epub ahead of print doi: 10.1016/j.jmii.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park TJ, Hyun MS, Lee HJ, Lee SY, Ko S. A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome. Talanta. 2009;79:295–301. doi: 10.1016/j.talanta.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–ee1. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A Review. JAMA. 2020:epub ahead of print. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 57.Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singhal T. A review of Coronavirus Disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun ML, Yang JM, Sun YP, Su GH. [Inhibitors of RAS Might be a good choice for the therapy of COVID-19 pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chin J Tuberculosis Respir Dis. 2020;43:219–222. doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. (Ger). [DOI] [PubMed] [Google Scholar]
- 60.Sun P, Lu X, Xu C, Wang Y, Sun W, Xi J. CD-sACE2 inclusion compounds: An effective treatment for coronavirus disease 2019 (COVID-19) J Med Virol. 2020:epub ahead of print. doi: 10.1002/jmv.25804. [DOI] [PubMed] [Google Scholar]
- 61.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao Y, Tang LV, Hu Y. Treatments in the COVID-19 pandemic: an update on clinical trials. Exp Opin Emerg Drugs. 2020;25(2):81–88. doi: 10.1080/14728214.2020.1773431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terol MJ, Sierra J, Gatell JM, Rozman C. Thrombocytopenia due to use of teicoplanin. Clin Infect Dis. 1993;17:927. doi: 10.1093/clinids/17.5.927. [DOI] [PubMed] [Google Scholar]
- 64.Ursing J, Rombo L, Eksborg S, Larson L, Bruvoll A, Tarning J, et al. High-dose chloroquine for uncomplicated Plasmodium falciparum malaria is well tolerated and causes similar QT interval prolongation as standard-dose chloroquine in children. Antimicrob Agents Chemother. 2020;64:e01846–e01819. doi: 10.1128/AAC.01846-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang B, Potter SJ, Lin Y, Cunningham AL, Dwyer DE, Su Y, et al. Rapid and sensitive detection of severe acute respiratory syndrome coronavirus by rolling circle amplification. J Clin Microbiol. 2005;43:2339–2344. doi: 10.1128/JCM.43.5.2339-2344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAM. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJJM, et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whiteman MC, Bogardus L, Giacone DG, Rubinstein LJ, Antonello JM, Sun D, et al. Virus reduction neutralization test: A single-cell imaging high-throughput virus neutralization assay for dengue. Am J Trop Med Hyg. 2018;99:1430–1439. doi: 10.4269/ajtmh.17-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitworth J. COVID-19: a fast evolving pandemic. Trans R Soc Trop Med Hyg. 2020;114:241–248. doi: 10.1093/trstmh/traa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.WHO, World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19: Scientific brief. [8 April 2020]. Available from: https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19.
- 73.WHO, World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. [4 July 2020]. Available from: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
- 74.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 75.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Z, Peng C, Shi Y, Zhu Z, Mu K, Wang X, et al. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv. 2020:2020.01.27.921627. [Google Scholar]
- 77.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, et al. Medications and glucose-6-phosphate dehydrogenase deficiency. Drug Saf. 2010;33:713–726. doi: 10.2165/11536520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11(1):207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Jr, Nunneley JW, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]