Abstract
Introduction: Seeds of Securigera securidaca (L.) Degen & Dorfl are rich in flavonoids and phenolic acids which have potent biological effects. The current study was undertaken to evaluate the effects of hydroalcoholic extract of S. securidaca seeds (HESS) alone, and in combination with a standard drug, glibenclamide (GB) on paraoxonase1 (PON1) activity, lipid profile and peroxidation, and cardiovascular risk indices in streptozotocin (STZ) induced diabetic rats.
Methods: Forty-eight male Wistar rats were randomly divided into eight equal groups and orally treated with various doses of HESS (100, 200, 400 mg/kg) alone and in combination with GB (5 mg/kg) for 35 consecutive days. After blood sampling, lipid profile including triglyceride (TG), cholesterol, high, low and very low-density lipoprotein-cholesterol (HDL-C, LDL-C, and VLDL-C), as well as serum PON1 activity, were assessed. Malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) levels were also measured. Several indices of cardiovascular risk and the correlation between PON1 activity and these indices were calculated based on the obtained results from the lipid profile.
Results: Induction of diabetes could dramatically alter all of the parameters mentioned above, and the lower dose of HESS (100 mg/kg) was not effective in restoring the parameters. However, the higher doses (200 and 400 mg/kg) alone and in combination with GB could significantly improve lipid profile, restore PON1 activity, and decrease cardiovascular risk indices, MDA, as well. However, neither HESS nor GB could significantly reduce TNF-α and hs-CRP. A significant negative correlation also was detected between PON1 activity and cardiovascular risk indices.
Conclusion: conclusively, HESS can be considered as a potent antihyperlipidemic agent with remarkable cardioprotective effects and can potentiate the antidiabetic effects of GB.
Keywords: Diabetes, Securigera securidaca, Paraoxonase 1, Glibenclamide
Introduction
Diabetes mellitus is recognized as a common metabolic disorder in adult human populations. Unfortunately, since 1980, its prevalence has increased or at best remained unchanged, in every country.1 The disease grows worse over time and comes with major complications, particularly atherosclerosis, hyperlipidemia, and cardiovascular diseases (CVDs). For several years, researchers from all over the world are tightly working to treat and control diabetes. Because of the chronic nature of diabetes, the treatment usually requires long term administration of hypoglycemic and hypolipidemic agents, which unfortunately eventuates in serious side effects. As a result, the trend has been changed during the past decade, and researchers have focused on safer and healthier resources to minimize the undesired effects. A variety of chemical, synthetic, and natural agents have already been tested for possible curative effects. However, among them, herbal materials have attracted considerable interest due to numerous advantages, including safety, readily availability, cheapness, and more importantly, biocompatibility.
The seeds of S. securidaca a member of the Fabaceae family with common names of Gandeh Talkheh or Adasolmolk have been used in the traditional medicine of Iran, Egypt, and India since ancient time, as a remedy for diabetes, hypertension, and hyperlipidemia.2,3 The observed beneficial effects are probably due to the presence of numerous biologically active compounds, including phenolics, flavonoids, saponins, and tannins as well as 82.9% unsaturated fatty acids in the seed extract which have been detected by phytochemical analysis.4,5 Recent animal model studies have shown that herbal phenolics and polyphenols, in particular flavonoids, are effective agents in reducing triglyceride (TG), low-density lipoprotein-cholesterol, oxidative stress, and lipid peroxidation levels.3,6,7
Glibenclamide (GB) is the second-generation sulfonylureas, and its function is increasing insulin secretion mainly by binding to regulatory sulfonylurea receptor1 subunits of ATP-sensitive potassium channels in pancreatic beta cells8,9 The drug is mainly used in combination with other antidiabetic agents, including metformin or as a second line of therapy where the patient cannot tolerate metformin. However, during recent years, it has been tested in combination with natural agents, and promising results have been yielded.10,11
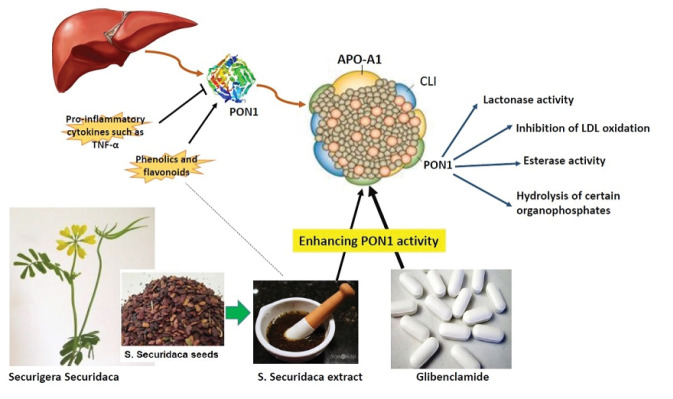
Paraoxonase1 (PON1) belongs to a family of enzymes containing three isomers (PON1, PON2, and PON3) with aryldialkylphosphatase activity. It primarily is released from the liver and binds to circulating high-density lipoprotein-cholesterol (HDL-C), especially HDL3-C via apolipoprotein A-I (apoA-I). Although the first described function was as an organophosphate hydrolase, the essential role of PON1 in the body is lipid peroxide hydrolysis, which prevents the accumulation of oxidized lipids in LDL-C and the formation of the atherosclerotic lesion. Lactonase is another PON1 activity, which hydrolyzes certain lactones/hydroxyl acids such as homocysteine thiolactone a potent atherogenic agent. Therefore, it is responsible for the antioxidant and anti-atherosclerotic functions of HDL-C.12,13 Numerous studies on hypercholesterolemia and diabetic disorders reported a decrease in the level of PON1 activity due to a reduction in apolipoprotein A-I levels.14-16 Such lower plasma PON1 activity and consequently increased pro-inflammatory and pro-atherogenic conditions are associated with elevated coronary artery disease risk.16 With this aspect, many researchers have tried to find predictable formulas to predict the likelihood of CVD.
Previously, to predict the risk of heart disease, indexes such as non-HDL-C levels and cholesterol ratios like total cholesterol (TC)/HDL-C and LDL-C/HDL-C named cardiovascular risk factors were used. Recently, the atherogenic index of plasma (AIP) has been suggested as a comprehensive lipid index compared to the classical ratio of LDL-C/HDL-C to predict the risk of atherosclerosis and CVD.17 AIP represents the relationship between pre- and anti-atherogenic lipoprotein particles and is associated with their size.18
Although several studies have confirmed the hypoglycemic and hypolipidemic effects of hydroalcoholic extract of S. securidaca seed (HESS), no study has been done on the effects of HESS on PON1 activity and atherogenic index in streptozotocin (STZ) -induced diabetic rats.3,6,7 The study was designed to evaluate the effect of HESS on the aforementioned parameters and compare the results with GB alone and in combination with two doses of HESS.
Materials and Methods
Materials
STZ (>98%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Folin-Ciocalteu reagent, quercetin, gallic acid, aluminum chloride (AlCl3), paraxone, NaOH, and NaHCO3 were purchased from Merck (Darmstadt, Germany). Glibenclamide (5 mg) tablets were obtained from Chemidarou (Tehran, Iran). TG, cholesterol, HDL-C, and LDL-C assay kits were obtained from PARSAZMUN (Tehran, Iran). Rat TNF-α ELISA kit was purchased from Diaclone (Besançon, France). Rat High sensitivity C-reactive protein kit was obtained from MyBioSource (San Diego, CA, USA). Glucometer (EmpErOr) strips were from OKmeter (Hsinchu City, Taiwan).
Plant material
Fresh seeds of S. securidaca was purchased from the local market (Tehran, Iran) and identified by Herbarium of Pharmacy Faculty, Tehran University of Medical Sciences, Tehran, Iran. Voucher specimens were deposited with the herbarium code PMP-756 for further references. Almost 1000 g powdered seed was macerated in 4 L of 70% ethanol and placed in the dark at 40°C for 72 hours under gentle shaking. Finally, the resultant liquid was filtered and concentrated (115 g) under vacuum by a rotary evaporator (Stroglass, Italy) at 50°C.
Assessment of total phenol and flavonoid contents
The total phenolic and flavonoid contents of the crude extract were estimated using Folin-Ciocalteu and aluminum chloride (AlCl3) colorimetric methods, respectively as described by Bahadori and colleagues.19 Very briefly, after blending 20 µL of the sample with 100 µL Folin-Ciocalteu reagent (1:10) for 6 min, 80 µL NaHCO3 (7.5%) was added to the mixture and the absorbance was read at 740 nm, after 2 hours of incubation in the dark at 25°C. The total phenolic content was expressed as mg of gallic acid equivalents per g of samples (mg GAE/g) through the calibration curve with gallic acid. For assessment of total flavonoid content, 20 μL of HESS samples or a standard solution, rutin (1–200 μg/mL) was diluted using 60 μL of MeOH and 10 μL of AlCl3 (5%). Then, 10 μL of 0.5 M potassium acetate was added and the total volume was adjusted to 200 μL by distilled water. After 30 min, the absorbance was read at 415 nm and the results were expressed as rutin equivalents (REs/g sample).
Animals
Forty-eight male Wistar rats (4 weeks; 240 ± 7 g) were obtained from the animal center of Iran University of Medical Sciences, Faculty of Medicine, Tehran, Iran. The animals were housed in a standard specific pathogen-free condition at a temperature of 23 ± 2°C and humidity (60 ± 10%), under a 12:12-hour light-dark cycle. Free access to water and standard food laboratory was provided for rats. All rats were allowed to be adapted to their new surroundings for one week before the experimental procedures. An 18-gauge oral feeding needle was utilized for oral delivery of the treatments.
Study design and sampling
Six rats were randomly separated and considered as Group I, healthy control (NC). Citrate buffer dissolved STZ was injected intraperitoneally (IP) with a single dose of 55 mg/kg body weight to induce diabetes in the rest of the rats. Three days after the injection, blood sugar (BS) was measured by a digital glucometer (EmpEror, Korea) and animals with BS levels of 250 mg/kg or higher were considered to be diabetic. Diabetic rats were randomly categorized into seven groups (containing 6 animals in each) as follow: Group II, diabetes control (DC); Group III-V: Diabetic animals treated with 100, 200 and 400 mg/kg HESS/Body Weight (BW), respectively, (HESS-100, HESS-200, and HESS-400); Group VI: diabetic animals treated with GB 5 mg/kg BW (GB); Group VII and VIII: diabetic animals treated with both GB (5 mg/kg BW) and HESS 200 and 400 mg/kg BW, respectively, (GB+HESS-200 and GB+HESS-400). The selected doses of HESS were based on the previous study in which HESS was administered orally to diabetic rats for four weeks with the doses of 100 and 200 mg/kg.20 Another study has reported that HESS at higher doses (400 and 800 µg/mL) can significantly decrease lipolysis and adipogenesis without cytotoxicity in ex-vivo conditions.21 The dose of GB was adjusted based on the previous report.22
The oral dosing was administrated once a day from the fourth day after IP injection of STZ for 35 consecutive days. At the endpoint, the rats were anesthetized by chloroform. The blood was taken directly from the heart, and the specimens were immediately placed on ice (for 1 hour) and then centrifuged for 10 minutes at 1300×g. The serum was isolated and stored at -70°C for biochemical tests.
Assessment of lipid profile and PON1 activity
All of the biochemical analyses were performed using an automated biochemistry analyzer (BT2500, Italy). All of the lipid parameters including TG, cholesterol, HDL-C, and LDL-C were measured using standard assay kits based on the instructions and recommendations of the manufacturer in serum samples. VLDL-C was calculated as one-fifth of TG. The activity of PON1 was measured spectrophotometrically using Paraxone as a substrate based on the previously described procedure.23 The difference in absorbance of the enzymatic product (para-nitrophenol) to Paraxone was monitored at 412 nm for one minute.
Assessment of cardiovascular risk indices
AIP was calculated as base-10 logarithms of the ratio of the concentration of TG (mmol/L) to HDL-C (mmol/L) [log (TG/HDL-C)]. The values of nontraditional lipid profiles were calculated based on the formula described by Wu and coworkers, including non-HDL-C (TC minus HDL-C), TC/HDL-C, LDL-C/HDL-C (Cardiovascular risk, CVR)), non-HDL-C/HDL-C (atherogenic index, AI), TC∗TG∗LDL/HDL-C (lipoprotein combine index, LCI).24
Determination of lipid peroxidation
Lipid peroxidation assay was based on the conjugation ability of malondialdehyde (MDA) with 2-thiobarbituric acid (TBA) to form a pink product with a maximum absorbance at 532 nm.25
Inflammatory factors
Serum tumor necrosis factor-alpha (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) levels were measured by ELISA using Rat TNF-α ELISA kit and Rat High Sensitivity C-Reactive Protein kit respectively, according to the manufacturer’s protocol.
Statistical analysis
All of the data was expressed as mean ± standard deviation (SD). Differences between the means were compared by one-way analysis of variance (ANOVA-1) followed by the post hoc Tukey test. Statistical analysis was done using SPSS software, version 22 (IBM Corp., Chicago, IL, USA). Pearson’s correlation was also performed to test whether there is any correlation among PON1 activity with cardiovascular risk indices, inflammatory factors, and MDA. A P value of less than 0.05 was considered significant.
Results
Total phenol and flavonoid contents
The content of total flavonoids was measured 46 ± 1.7 mg quercetin equivalent (QE) per gram of dry weight (46 ± 1.7 mg QE/g D.W) and the content of total phenol was found to be 93.3 ± 1.5 gallic acid equivalent (GAE) per gram of dry weight (93.3 ± 1.5 mg QE/g D.W).
Lipid profile
At the end of the study (the final week), HESS-200 and HESS-400 groups exhibited significantly decreased ( P < 0.05) levels of BS compared to the DC group, but still, the levels were significantly higher ( P < 0.05) in comparison with the NC group. On the other hand, the lowest dose of HESS (100 mg/kg BW), had no significant effect on BS levels. It was found that GB alone was more effective than HESS in lowering BS, which could decrease BS levels very close to the normal range. The combination of herbal extract and standard drug also could reduce BS contents further, but no significant difference was detected between GB alone and in combination with HESS (of 200 mg/kg and 400 mg/kg doses) ( P > 0.05). The effects of treatment with HESS, GB, and GB+HESS on lipid profiles are depicted in Table 1. As can be seen, following the induction of diabetes, TC (~2.5 folds), TG (~1.4 folds), LDL-C (~1. 44 folds) and VLDL-C (~1.46 folds) levels were significantly increased and HDL-C (~1.5 folds) was significantly decreased as compared to the healthy rats ( P < 0.05). Administration of HESS at various doses could none significantly increase HDL-C and decrease the rest mentioned parameters dose-dependently, ( P > 0.05) as compared to the DC group, except for the heist dose of HESS (400 mg/kg), which could reduce TG and LDL levels significantly. GB alone could significantly restore lipid profile as compared to the DC group ( P < 0.05), however, it could not improve the profile to the point to be able to compare it with the healthy animals. Generally, the standard drug was also found to be more effective than the highest dose of herbal extract, except in the case of LDL and TG, in which no significant difference was detected between the HESS-400 and the GB groups. The co-administration of GB with HESS had also significant effects on the lipid profile, compared to the DC group and the herbal material could potentiate the effects of GB, but generally, there were no significant differences between the GB alone and the combinatorial treatment.
Table 1. Effects of various doses of HESS, GB and GB plus HESS on lipid profile in experimental groups .
| Groups | Parameters | |||||
| BS (mg/dL) | Cholesterol (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C(mg/dL) | VLDL-C (mg/dL) | |
| NCa | 90.6±5b-e | 86.92±2.9b-h | 64.01±4.5b-h | 42.02±1.83b-e | 35.93±2.07b-d | 12.80±0.90b-h |
| DCb | 324.6±14a,c-h | 231.70±8.8a,f-h | 93.35±5.7a,f-h | 28.86±0.54a,d-h | 51.80±1.39a,f-h | 18.67±1.15a,f-h |
| HESS-100c | 221.4±13a,b,d-h | 231.52±7.8a,f-h | 94.25±4.7a,f-h | 29.49±2.81a,e-h | 51.77±2.54a,f-h | 18.85±0.95a,f-h |
| HESS-200d | 170.3±27-a-c,f-h | 218.66±10.6a,f-h | 87.67±3.0a,g,h | 34.21±2.69a,b,f-h | 45.97±4.39a,g,h | 17.53±0.61a,g,h |
| HESS-400e | 159.4±33-a-c,f-h | 218.37±12.9a,f-h | 86.51±2.4a,h | 35.47±2.40-a-c,h | 45.05±4.25a,h | 17.30±0.48a,h |
| GBf | 105.4±8b-e | 111.78±7.4a-e | 80.60±2.9a-c | 40.45±3.38b-d | 42.10±4.29b,c | 16.1±0.59a-c |
| GB+HESS-200g | 99.3±5b-e | 105.30±7.5a-e | 78.97±0.9a-d | 40.95±3.20b-d | 38.49±3. 41b-d | 15.78±0.20a-d |
| GB+HESS-400h | 99.8±2b-e | 106.28±8.0a-e | 78.36±1.27a-e | 41.58±2.58b-e | 37.81±2.31b-e | 15.67±0.25a-e |
| ANOVA ( P value) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
The variables are presented as means ± SD. NC (normal control); DC (Diabetic control); HESS (hydroalcoholic extract of S. securidaca seeds); GB (glibenclamide).Groups at the left column have been defined by superscript letters (a–h). Superscript letters (a–f) within a column denote significant differences ( P < 0.05).
Cardiovascular risk indices
The nontraditional lipid profiles, including non-HDL-C, TC/HDL-C, CVR, AI, LCI, and AIP were calculated and are depicted in Table 2. All cardiac risk indices were much significantly higher in the diabetic control group than healthy control ( P < 0.05). Treatment with a lower dose of HESS (100 mg/kg BW) was not effective in normalizing the indices. However, the middle and higher doses (200 and 400 mg/kg BW) were relatively effective and could significantly decrease the indices as compared to the diabetic control animals ( P < 0.05), although the levels were still significantly higher than those in healthy rats ( P < 0.05). GB was more effective than the highest dose of HESS in reducing the levels of cardiovascular risk indices. The reductant effect of GB on indices was strengthened when it was administrated in combination with HESS, especially by HESS dose of 200 mg/kg BW.
Table 2. Effects of various doses of HESS, GB and GB plus HESS on cardiovascular risk indices in experimental groups .
| Groups | Parameters | |||||
| non-HDL-C | TC/HDL-C | CVR | AI | LCI | AIP | |
| NCa | 44.90±4.38b-h | 2.07±0.15b-e | 0.85±0.03b-f | 1.07±0.15b-f | 1.39±0.13b-f | 0.18±0.04b-h |
| DCb | 202.84±8.81a,d-h | 8.03±0.32a,d-h | 1.80±0.07a,d-h | 7.03±0.32a,d-h | 11.33±0.83a,d-h | 0.50±0.03a,d-h |
| HESS-100c | 202.02±8.87a,c-h | 7.91±084a,d-h | 1.77±0.23a,d-h | 6.91±0.84a,d-h | 11.23±1.57a,d-h | 0.49±0.02a,d-h |
| HESS-200d | 184.25±12.62a-c,f-h | 6.40±077a-c,f-h | 1.35±0.19a-c,f-h | 5.40±0.77a-c,f-h | 7.53±1.15a-c,f-h | 0.41±0.03a-c,f-h |
| HESS-400e | 183.15±15.57a-c,f-h | 6.25±0.83a-c,f-h | 1.28±0.13a-c,f-h | 5.25±0.83a-c,f-h | 7.11±1.26a-c,f-h | 0.39±0.04a-c,f-h |
| GBf | 71.13±9.29a-e | 2.77±0.38b-d | 1.04±0.12b-e | 1.77±0.38b-e | 2.75±0.45a-e | 0.29±0.04a-e |
| GB+HESS-200g | 63.94±10.07a-f | 2.57±0.37b-d | 0.94±0.15b-e | 1.57±0.37b-e | 2.29±0.47b-e | 0.28±0.04a-e |
| GB+HESS-400h | 66.31±11.24a-e | 2.69±0.44b-d | 0.95±0.13b-e | 1.69±0.44b-e | 2.34±0.53b-e | 0.29±0.04a-e |
| ANOVA ( P value) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
The variables are presented as means ± SD. NC (normal control); DC (diabetic control); HESS (hydroalcoholic extract of S. securidaca seeds); GB (glibenclamide).Groups at the left column have been defined by superscript letters (a–h). Superscript letters (a–f) within a column denote significant differences ( P < 0.05). CVR (cardiovascular risk): LDL-C/HDL-C; AI (atherogenic index): Non-HDL-C/HDL-C; LCI (Lipoprotein combine index): TC∗TG∗LDL/HDL-C; AIP (Atherogenic index of plasma): log (TG/HDL-C).
PON1 and inflammatory factors
Table 3 demonstrates the effects of diabetes induction and treatment with various doses of HESS and GB, in combination and alone on PON1 activity, MDA, and the inflammatory indicators. The activities of PON1, antioxidant, and antiatherogenic enzyme, were significantly decreased following the IP injection of STZ as compared to those of healthy animals ( P < 0.001). Also, MDA concentrations were significantly elevated in diabetic rats ( P < 0.05). Although the treatment with HESS restored PON1 activity dose-dependently, the combinatorial therapy with GB and HESS was found to be more effective than HESS alone. Furthermore, the standard drug, GB had more profound effects on PON1 activity as compared to various doses of HESS and could significantly increase the enzymatic activity (140.0±6 µU/L for GB vs. 126.0±6 µU/L for HESS-400). As can be seen from Table 3, the herbal treatment could decline MDA contents in diabetic animals dose-dependently. Although the lower (100 mg/kg) and middle (200 mg/kg) doses could not decrease MDA levels significantly ( P > 0.05) as compared to those of healthy control, the higher dose (400 mg/kg) dramatically reduced MDA concentrations close to the normal range. GB alone also had no significant effects on MDA concentrations, but the combination of GB and HESS (400 mg/kg) decreased MDA content significantly ( P < 0.05). Diabetes induction resulted in increased levels of TNF-α and hs-CRP as compared to the intact animals. Administration of HESS at various doses alone and in combination with GB had only minor effects on the aforementioned parameters. All the treatments could none-significantly decrease TNF-α and hs-CRP levels ( P > 0.05). The effects of GB alone on both parameters were comparable to that of HESS with a dose of 200 mg/kg BW.
Table 3. Effects of various doses of HESS, GB and GB plus HESS on PON1, lipid peroxidation and the inflammatory factors in experimental groups .
| Groups | Parameters | ||||
| PON1 (µU/L) | MDA (µM/mL) | TNF-α (PM/mL) | hs -CRP (ng/mL) | ||
| NCa | 143.6±6b | 0.021±0.002b-h | 19.8±1.1b,c | 1.8±0.3b,c | |
| DCb | 80.2±16a,d-h | 0.039±0.002a,d,e,g,h | 21.6±1.3a,d,f,g | 2.4±0.3a,e-d | |
| HESS-100c | 82.2±17a,d-h | 0.039±0.002a,d,e,g,h | 21.7±1.7a,d,g,h | 2.29±0.3a,d,e,g,h | |
| HESS-200d | 121.0±16b,c,h | 0.034±0.002a-c,e,h | 20.4±.9 | 1.81±0.4b,c | |
| HESS-400e | 126.0±6b,c | 0.029±0.002a-d,f,g | 19.6±1.2b,c | 1.78±0.2b,c | |
| GBf | 140.0±6 b,c | 0.035±0.002a-c,e,g,h | 20.2±1.0 | 1.91±0.4b | |
| GB+HESS-200g | 143.2±7b,c | 0.034±0.001a-c,e,h | 19.6±1.0b,c | 1.79±0.2b,c | |
| GB+HESS-400h | 146.60±10b-d | 0.029±0.002a-d,f,g | 18.8±1.1b,c | 1.73±0.1b,c | |
| ANOVA ( P value) | 0.001 | 0.001 | 0.013 | 0.016 | |
The variables are presented as means ± SD. NC (normal control); DC (diabetic control); HESS (hydroalcoholic extract of S. securidaca seeds); GB (glibenclamide).Groups at the left column have been defined by superscript letters (a–h). Superscript letters (a–f) within a column denote significant differences ( P < 0.05).
Correlations between PON1 activity and risk factors
Table 4 shows the correlations between PON1 activity with cardiovascular risk indices, MDA, and inflammatory biomarkers. PON1 activity was negatively correlated with the aforementioned parameters ( P < 0.05) and positively with HDL-C.
Table 4. Correlations between PON1 activity with cardiovascular risk indices, inflammatory factors, and MDA .
| Variables | All (n=48) | |
| r | P value | |
| HDL-C | 0.872 | 0.001 |
| non-HDL-C | -0.822 | 0.001 |
| TC/HDL-C | -0.897 | 0.001 |
| CVR | - 0.938 | 0.001 |
| AI | -0.897 | 0.001 |
| LCI | -0.921 | 0.001 |
| AIP | -0.868 | 0.001 |
| MDA | -0.641 | 0.001 |
| TNF-α | -0.591 | 0.001 |
| Hs-CRP | - 0.458 | 0.003 |
Discussion
Securigera securidaca is a well-known plant for its anti-diabetic properties and several researchers from Iran or other countries have confirmed its hypoglycemic and lipid-lowering effects using various models of diabetic or hyperlipidemic animals.3,6,7,21,26 Phytochemical analysis of the plant also has been done and several bioactive constitutes have been isolated.2,27 However, there are still gaps that should be covered. First of all, none of these studies have evaluated the effects of HESS on PON1 activity in diabetic animals. Secondly, the effects of herbal treatment on cardiovascular risk indices have not yet been calculated and finally, none of the previous studies have assessed the combinatorial effects of HESS and GB. Therefore, we designed the current study to cover these shortages.
Following the induction of diabetes, a dramatic increase in TG, cholesterol, LDL-C, and VLDL-C accompanied by a decrease in HDL-C was recorded. Rajaei and colleagues have reported similar findings following IP injection of STZ to Wistar rats. They observed that the treatment of the animals with 100 mg/kg BW HESS had no significant effects on the aforementioned parameters, which are consistent with our results. However, according to their study, HESS at the dose of 200 mg/kg BW could decrease cholesterol, LDL-C and increase HDL-C with no effects on TG.26 In contrast, our data shows HESS even at a higher dose (400 mg/kg) cannot significantly alter the parameters. The observed difference can probably be attributed to the gender of animals, as in this study, male Wistar rats were used, but in that study, the animals were female. The other difference was also the administration route of HESS. Rajaei et al supplemented the herbal extract in drinking water, but we used gastric gavage to deliver HESS extract.26
Treatment of the animals with GB could significantly regulate the lipid profile. Interestingly, we observed that co-administration of HESS with GB could potentiate the effects of the standard drug. GB is inferior to metformin in the drug treatment of gestational diabetes.28 Furthermore, it is associated with increased oxidative stress and negative chronotropic effects in diabetic patients.29,30 In a huge attempt to improve treatment quality with GB, it has been co-administered with Allium sativum, and Zingiber officinale Roscoe extracts, as well as honey. The results of all three studies are promising and could be important in reducing the dose of GB to achieve an enhanced therapeutic effect with minimal side effects.11,31,32 To the authors' knowledge, this is the first study that evaluates the effects of co-administration of GB with HESS.
Various cardiovascular risk indices were calculated in this study. As expected, all of the indices were remarkably increased following induction of diabetes, and the lower dose (100 mg/kg) of HESS was not effective in decreasing them. However, the higher doses (200 and 400 mg/kg) could relatively reduce the indices. GB was more effective than the highest dose of HESS in decreasing all of the risk indices, and its effect was potentiated when co-administrated with the higher doses of HESS. No data regarding the effects of HESS on cardiovascular risk indices are available to be able to be compared with our findings. Among the various indices, AIP is a novel and strong marker for predicting the risk of coronary heart disease (CHD) and can be used as a regular monitoring index of CHD in everyday practice, especially in persons with other cardiovascular risk factors.18,24 Dobiášová et al concluded from their studies that AIP inversely correlates with LDL-C particle size. They defined three ranges of -0.3 to 0.1, 0.1 to 0.24, >0.24 for AIP that could be associated with a low, medium, and high risk of CVD, respectively.33 Accordingly, an increase in AIP is proportional to decrease in LDL-C particle diameter or rise in small dense LDL (sdLDL) which is more susceptible to oxidation, and formation of foam cells and the creation of atherosclerotic plaques.34 Medium and high doses of HESS significantly increased reduced levels of HDL-C in diabetic rats. Again, GB alone was more effective than the highest dose of HESS in this regard, which was improved by the administration of GB in combination with HESS. As it is known, the antiatherogenic properties of HDL-C are applied by transporting peripheral cholesterol to the liver and having antioxidant enzyme of PON. These results were confirmed by the effects of HESS and GB on the serum MDA levels; because high levels of cardiovascular risk indices are associated with increased lipid peroxidation and MDA concentrations in diabetes. The MDA lowering effects of HESS was dose-dependent, but GB did not reduce MDA levels, which were comparable with DC rats. In agreement with our findings, Erejuwa et al observed no significant effects on MDA content following oral gavage of GB with a dose of 0.6 mg/kg for four weeks to STZ-induced diabetic rats.31 Parallel to our results, they also noticed that co-administration of honey with GB could improve its antioxidant properties. Our data clearly shows that HESS itself has cardio-protective effects and can potentiate GB's effects. Interestingly, Tofighi et al have determined a considerable amount of cardiac glycosides in the in-vitro culture of S. securidaca, suggesting, it's beneficial effects on the cardiovascular system.35
As depicted in Table 3, the activity of PON1 significantly dropped following the induction of diabetes. In agreement, several human studies have shown a decreased activity of PON1 in both types of diabetes.36-39 Furthermore, Garjani et al reported reduced activity of PON1 in hypercholesterolemic rats. However, they observed that oral administration of the animals with various doses of HESS, ranging from 50-200 mg/kg for 20 consecutive days had no significant effects in restoring PON1 activity.6 In contrast, our data clearly shows that the higher doses of HESS (200 and 400 mg/kg BW) after administration for 35 days are completely restorative and can significantly increase PON 1 activity close to the normal range. Interestingly, we observed that combinatorial therapy could increase PON1 activity even higher than a normal range, suggesting an enhanced antioxidant status of the diabetic animals and improved efficacy of GB, probably due to the presence of antioxidant ingredients in the herbal extract. With this aspect, phytochemical analysis of the aqueous and ethanolic extracts of the plant has proven the presence of flavonoids, phenolic acids, cardenolides, tannins, steroidal, and pentacyclic triterpenoid-type saponins.40,41 Polyphenols, especially flavonoids and phenolic acids, effectively reduce the oxidative stress, increase the antioxidant defense system, and regulate the balance between antioxidant/oxidant.42 In agreement with our results, Kıyıcı et al reported that supplementation of rats with grape seed extract which is rich in flavonoids could elevate the activity of PON1 in both healthy and STZ-induced diabetic animals.23 In this study, we measured the total phenolic content of HESS to be 93.3 ± 1.5 mg GAE/g extract, which is significantly lower than that of the study (147.14 ± 2.46 mg GAE/g extract) reported by Tofighi and colleagues.35 With this aspect, it has been claimed that several factors including climate and soil nutrients impact the chemical composition of plants.43
Studies have shown elevated levels of TNF-α and hs-CRP in diabetic cases due to severe oxidative stress, which are considered relevant to atherosclerotic progression.44 This study also showed an increase in serum levels of TNF-α and hs-CRP in diabetic rats compared to healthy control, but the increases were not statistically significant. HESS (dose-dependently) and GB, and their combination decreased both serum hs-CRP and TNF-α levels in diabetic rats, but still, the values were in the range between those in non-treated diabetic and healthy rats. The highest dose of HESS was slightly more effective than GB in reducing the pro-inflammatory factors. A significant negative correlation was detected between PON1 activity and both inflammatory biomarkers. Kumon et al reported that increased levels of proinflammatory cytokines such as IL-1 and TNF-α downregulated mRNA expression of PON1 in HepG2 cells.45 Ferretti et al found a lower PON1: hs-CRP ratio and high levels of lipid hydroperoxides in patients suffering from Prader-Willi syndrome, and in obese individuals.46 Consistent with our findings, Cheraghi et al and Jamor et al in separate studies detected a positive association among PON1 and HDL-C, and a negative correlation between PON1 and AI.47,48
Anti-inflammatory activity of polyphenols highlights again their valuable roles as therapeutic materials in the prevention and treatment of chronic diseases related to inflammation such as diabetes, obesity, cancers, and CHD. Phenolic compounds were shown to decrease blood hs-CRP levels, a probable agent in the development of atherosclerosis by inhibiting cytokine-induced CRP promoter activity. They modulate NF-kB activation and downregulate multiple key regulators involved in inflammatory responses such as TNF-α, IL-1, and IL-6.49 In vitro and in vivo studies have reported, these compounds eliminate atherogenic lesions by elevating PON1 activity, improving LDL-C/HDL-C ratio and inhibiting LDL-C oxidation. Combined data indicates that phenolic compounds present in different parts of plants have great potential in the prevention and treatment of chronic diseases such as diabetes, obesity, cancers, and CHD.
Conclusion
Taking together, our data clearly shows that HESS has antihyperlipidemic effects and can regulate the lipid profile. Furthermore, it can restore the activity of an important enzyme PON1 and improve cardiovascular risk indices suggesting its cardioprotective effects. The level of lipid peroxidation also was decreased following treatment with HESS. However, the herbal extract had only minor effects on inflammatory indicators. It was also observed that HESS could potentiate the efficacy of the standard drug, GB. In this study, we only measured serum PON1 activity. However, it is recommended that the activity should be measured in tissues, particularly the liver, to have better insight about the oxidative processes. Such determinations should be covered in future studies.
Acknowledgments
This work was supported by the Iran University of Medical Sciences. Thanks to the members of the Biochemistry Department of Iran University of Medical Sciences for their favors in improving this study.
Funding sources
This work was supported by Iran University of Medical Science (grant number: 4352).
Ethical statement
All laboratory animal management was approved by Iran University of Medical Sciences (Tehran, Iran) Committee, and all procedures of the current research were performed at the animal housing department, Faculty of Medicine.
Competing interests
There are no conflicts of interest to declare.
Authors’ contribution
EB proposed the original idea, designed the study, obtained the funding, and supervised the project. SAF handled, housed and sampled the animals. MB helped SAF in animal management. MK and NF performed the biochemical tests. SAF, AN and MB collected the required information for manuscript writing EB and AN performed data analysis/interpretation. AN and SAF drafted the manuscript. EB, AN, and SAF critically revised the manuscript. All the authors significantly contributed to the writing of the article.
Research Highlights
What is the current knowledge?
√ The high dose (400 mg/kg) of HESS could significantly improve lipid profile, restore PON1 activity, and decrease cardiovascular risk indices in diabetic animal.
√ Combination HESS with GB enhanced the aforementioned effects in diabetic animal, but had minor effects on inflammatory indicators.
What is new here?
√ Both HESS and GB increased PON1 activity; GB was more effective in this respect.
√ HESS was stronger than GB in antiatherogenic properties.
√ HESS properties are attributed to its phenolic and flavonoid contents.
References
- 1.Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 44 million participants. Lancet. 2016;387:1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim RM, El-Halawany AM, Saleh DO, El Naggar EMB, El-Shabrawy AE-RO, El-Hawary SS. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev Bras Farmacogn. 2015;25:134–41. doi: 10.1016/j.bjp.2015.02.008. [DOI] [Google Scholar]
- 3.Ali A, Mohamed M, Kamel M, Fouad M, Spring O. Studies on Securigera securidacea (L) Deg et Dörfl(Fabaceae) seeds, an antidiabetic Egyptian folk medicine. Die Pharmazie. 1998;53:710–5. [PubMed] [Google Scholar]
- 4.Hosseinzadeh H, Ramezani M, Danaei A. Antihyperglycaemic effect and acute toxicity of Securigera Securidaca L seed extracts in mice. Phytother Res. 2002;16:745–7. doi: 10.1002/ptr.1020. [DOI] [PubMed] [Google Scholar]
- 5.Rajaei Z, Hadjzadeh MA, Moradi R, Ghorbani A, Saghebi A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in streptozotocin-induced diabetic rats. Adv Biomed Res. 2015;4:33. doi: 10.4103/2277-9175.150427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garjani A, Fathiazad F, Zakheri A. et al. The effect of total extract of Securigera securidaca L seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol. 2009;126:525–32. doi: 10.1016/j.jep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Pouramir M, Shahaboddin ME, Moghadamnia A-A, Parastouei K. To study the effects of Securigera securidaca (L) seed against alloxan-induced hyperglycemia. J Med Plant Res. 2011;5:3188–91. doi: 10.1007/s11418-016-1060-7. [DOI] [Google Scholar]
- 8.Rambiritch V, Maharaj B, Naidoo P. Glibenclamide in patients with poorly controlled type 2 diabetes: a 12-week, prospective, single-center, open-label, dose-escalation study. Clin Pharmacol. 2014;6:63. doi: 10.2147/CPAA.S54809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamprianou S, Gysemans C, Bou Saab J, Pontes H, Mathieu C, Meda P. Glibenclamide Prevents Diabetes in NOD Mice. PLoS One. 2016;11:e0168839. doi: 10.1371/journal.pone.0168839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erejuwa OO, Sulaiman SA, Wahab MSA, Sirajudeen KNS, Salleh MSM, Gurtu S. Glibenclamide or metformi combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int J Biol Sci. 2011;7:244–52. doi: 10.7150/ijbs.7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poonam T, Prakash GP, Kumar LV. Influence of Allium sativum extract on the hypoglycemic activity of glibenclamide: an approach to possible herb-drug interaction. Drug Metabol Drug Interact. 2013;28:225–30. doi: 10.1515/dmdi-2013-0031. [DOI] [PubMed] [Google Scholar]
- 12.Bergmeier C, Siekmeier R, Gross W. Distribution spectrum of paraoxonase activity in HDL fractions. Clin Chem. 2004;50:2309–15. doi: 10.1373/clinchem.2004.034439. [DOI] [PubMed] [Google Scholar]
- 13.van Himbergen TM, van Tits LJ, Roest M, Stalenhoef AF. The story of PON1: how an organophosphate-hydrolysing enzyme is becoming a player in cardiovascular medicine. Neth J Med. 2006;64:34–8. [PubMed] [Google Scholar]
- 14.Dullaart RP, de Vries R, Sluiter WJ, Voorbij HA. High plasma C-reactive protein (CRP) is related to low paraoxonase-I (PON-I) activity independently of high leptin and low adiponectin in type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:221–6. doi: 10.1111/j.1365-2265.2008.03306.x. [DOI] [PubMed] [Google Scholar]
- 15.Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res. 2011;42:219–25. doi: 10.1016/j.arcmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Mackness MI, Durrington PN, Mackness B. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs. 2004;4:211–7. doi: 10.2165/00129784-200404040-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine (Baltimore) 2017;96:e8058–e. doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G. et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240. doi: 10.1186/1476-511X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahadori MB, Zengin G, Bahadori S, Dinparast L, Movahhedin N. Phenolic composition and functional properties of wild mint (Mentha longifolia var calliantha (Stapf) Briq) Int J Food Prop. 2018;21:183–93. doi: 10.1080/10942912.2018.1440238. [DOI] [Google Scholar]
- 20.Rajaei Z, Hadjzadeh MA, Moradi R, Ghorbani A, Saghebi A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in streptozotocin-induced diabetic rats. Adv Biomed Res. 2015:4. doi: 10.4103/2277-9175.150427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghorbani A, Moradi Marjaneh R, Rajaei Z, Hadjzadeh MA. Effects of Securigera securidaca Extract on Lipolysis and Adipogenesis in Diabetic Rats. Cholesterol. 2014;2014:582106. doi: 10.1155/2014/582106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Paramonova N, Isajeva D. et al. Comparison of the effects of glibenclamide on metabolic parameters, GLUT1 expression, and liver injury in rats with severe and mild streptozotocin-induced diabetes mellitus. Medicina (Kaunas) 2012;48:532–43. doi: 10.3390/medicina48100078. [DOI] [PubMed] [Google Scholar]
- 23.Kiyici A, Okudan N, Gökbel H, Belviranli M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J Med Food. 2010;13:725–8. doi: 10.1089/jmf.2009.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T-T, Gao Y, Zheng Y-Y, Ma Y-T, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197. doi: 10.1186/s12944-018-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 26.Alizadeh-Fanalou S, Babaei M, Hosseini A, Azadi N, Nazarizadeh A, Shojaii A. et al. Effects of Securigera Securidaca seed extract in combination with glibenclamide on antioxidant capacity, fibroblast growth factor 21 and insulin resistance in hyperglycemic rats. J Ethnopharmacol. 2020;248:112331. doi: 10.1016/j.jep.2019.112331. [DOI] [PubMed] [Google Scholar]
- 27.Tofighi Z, Moradi-Afrapoli F, Ebrahimi SN, Goodarzi S, Hadjiakhoondi A, Neuburger M. et al. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J Nat Med. 2017;71:272–80. doi: 10.1007/s11418-016-1060-7. [DOI] [PubMed] [Google Scholar]
- 28.alsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JS, Lin SD, Lee WJ, Su SL, Lee IT, Tu ST. et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2011;33:1932–42. doi: 10.1016/j.clinthera.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Sundaresan P, Lykos D, Daher A, Diamond T, Morris R, Howes LG. Comparative effects of glibenclamide and metformin on ambulatory blood pressure and cardiovascular reactivity in NIDDM. Diabetes Care. 1997;20:692. doi: 10.2337/diacare.20.5.692. [DOI] [PubMed] [Google Scholar]
- 31.Erejuwa OO, Sulaiman SA, Wahab MSA, Salam SKN, Salleh MSM, Gurtu S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci. 2010;11:2056–66. doi: 10.3390/ijms11052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Omaria IL, Afifib FU, Salhaba AS. Therapeutic effect and possible herb drug interactions of ginger (Zingiber officinale Roscoe, Zingiberaceae) crude extract with glibenclamide and insulin. Pharmacogn Commun. 2012;2(1):12–20. doi: 10.5530/pc.2012.1.4. [DOI] [Google Scholar]
- 33.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34:583–8. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197. doi: 10.1186/s12944-018-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tofighi Z, Ghazi SN, Hadjiakhoondi A, Yassa N. Determination of cardiac glycosides and total phenols in different generations of Securigera securidaca suspension culture. Research Journal of Pharmacognosy. 2016;3:23–31. [Google Scholar]
- 36.Namitha D, Nusrath A, Rajeshwari A, Rani NA. Serum paraoxonase levels in type 2 diabetes mellitus patients: a case control study. J Med Sci Health. 2015;1:14–8. doi: 10.46347/jmsh.2015.v01i02.003. [DOI] [Google Scholar]
- 37.Gupta N, Binukumar BK, Singh S, Sunkaria A, Kandimalla R, Bhansali A. et al. Serum paraoxonase-1 (PON1) activities (PONase/AREase) and polymorphisms in patients with type 2 diabetes mellitus in a North-West Indian population. Gene. 2011;487:88–95. doi: 10.1016/j.gene.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Javadzadeh A, Ghorbanihaghjo A, Bahreini E, Rashtchizadeh N, Argani H, Alizadeh S. Serum paraoxonase phenotype distribution in exudative age-related macular degeneration and its relationship to homocysteine and oxidized low-density lipoprotein. Retina. 2012;32:658–66. doi: 10.1097/IAE.0b013e31822529b1. [DOI] [PubMed] [Google Scholar]
- 39.Wegner M, Pioruńska-Stolzmann M, Araszkiewicz A, Zozulińska-Ziółkiewicz D, Wierusz-Wysocka B. Evaluation of paraoxonase 1 arylesterase activity and lipid peroxide levels in patients with type 1 diabetes. Pol Arch Med Wewn. 2011;121:448–54. doi: 10.20452/pamw.1113. [DOI] [PubMed] [Google Scholar]
- 40.Zatula V, Chernobrovaya N, Kolesnikov D. A chemical study of the structure of securigenin and its bioside securidaside. Chem Nat Compd. 1966;2:359–60. doi: 10.1007/BF00564226. [DOI] [Google Scholar]
- 41.Hosseinzadeh H, Ramezani M, Danaei AR. Antihyperglycaemic effect and acute toxicity of Securigera Securidaca L seed extracts in mice. Phytother Res. 2002;16:745–7. doi: 10.1002/ptr.1020. [DOI] [PubMed] [Google Scholar]
- 42.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zubair M, Malik A, Ahmad J. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J Endocrinol Metab. 2012;16:769–76. doi: 10.4103/2230-8210.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumon Y, Suehiro T, Ikeda Y, Hashimoto K. Human paraoxonase-1 gene expression by HepG2 cells is downregulated by interleukin-1beta and tumor necrosis factor-alpha, but is upregulated by interleukin-6. Life Sci. 2003;73:2807–15. doi: 10.1016/s0024-3205(03)00704-5. [DOI] [PubMed] [Google Scholar]
- 46.Ferretti G, Bacchetti T, Masciangelo S, Grugni G, Bicchiega V. Altered inflammation, paraoxonase-1 activity and HDL physicochemical properties in obese humans with and without Prader-Willi syndrome. Dis Model Mech. 2012;5:698‐705. doi: 10.1242/dmm.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheraghi M, Shahsavari G, Maleki A, Ahmadvand H. Paraoxonase 1 Activity, Lipid Profile, and Atherogenic Indexes Status in Coronary Heart Disease. Rep Biochem Mol Biol. 2017;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Jamor P, Ahmadvand H, Birjandi M, Ebadi Sharafabad B. Activity of serum paraoxonase 1, lipid profile and atherogenic indexes in diabetic induced rats treated with alpha lipoic acid. J Nephropathol. 2018;7:241–7. doi: 10.15171/jnp.2018.49. [DOI] [Google Scholar]
- 49.Aviram M, Fuhrman B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann N Y Acad Sci. 2002;957:146–61. doi: 10.1111/j.1749-6632.2002.tb02913.x. [DOI] [PubMed] [Google Scholar]