Abstract
Introduction: Chronic liver disease frequently accompanied by hepatic encephalopathy (HE). Changes in the permeability of the blood-brain barrier in HE, make an easier entrance of ammonia among other substances to the brain, which leads to neurotransmitter disturbances. Lactulose (LAC), causes better defecation and makes ammonia outreach of blood. Silymarin (SM) is a known standard drug for liver illnesses. The purpose of this research was to determine the results of LAC and SM combined treatment, on the changes in memory of cirrhotic male rats. Methods: The cirrhotic model established by treatment with thioacetamide (TAA) for 18 weeks. Cirrhotic rats randomized to four groups (n = 7): TAA group (received drinking water), LAC group (2 g/kg/d LAC in drinking water), SM group (50 mg/kg/d SM by food), SM+ LAC group (similar combined doses of both compounds) for 8 weeks. The control group received drinking water. The behavior examined by wire hanging (WH), passive avoidance (PA), and open field (OF) tests.
Results: Our findings showed that treatment with SM+LAC effectively increased PA latency, compared with the control group. The results showed that the administration of LAC and SM+LAC affected the number of lines crossed, the total distance moved and velocity in the OF tests.
Conclusion: SM and LAC have anti-inflammatory effects that are memory changing. It may be due to their useful effects. These results indicated that SM+LAC restored memory disturbance and irritated mood in the cirrhotic rats. Comparable neuroprotection was never previously informed. Such outcomes are extremely promising and indicate the further study of SM+LAC.
Keywords: Behavior, Lactulose, Liver cirrhosis, Memory, Silymarin, Wistar rat
Introduction
Fibrosis and cirrhosis have turned out to be significant endpoints of the liver in clinical trials of patients with chronic liver illnesses.1 Cirrhosis of the liver is the final morphological stage2 and pathological result3 of most liver diseases. At present, it is apparent that a dynamic two-way process is involved with a possibility of further progression, or regression of fibrosis/cirrhosis provided that causal treatment of the basic hepatologic disease is possible.2
Hepatic encephalopathy (HE) could be a serious neuropsychiatric syndrome that mostly happens in decompensated cirrhosis of the liver and includes a range of signs that extends from gentle cognitive defect to come.4 Low-grade cirrhosis is of common occurrence, however, it affects brain function, and its extension is not clearly understood.5 Minimal hepatic encephalopathy (MHE), HE subtype, is extremely common in patients with liver pathology (22%-74%). It is characterized as HE with cognitive shortfalls, that can be uncovered by psychometric testing, however, without grossly evident neurologic abnormalities.6 Though HE etiologies are not entirely understood, it is believed that in the pathogenesis of HE more than one underlying mechanism involved.4 Indeed, even a low-grade chronic liver failure (CLF) will disorder the higher-order brain capacities like cognition, motor coordination, and learning-memory among the rats.5
Thioacetamide (TAA) is a hepatotoxin that is activated by two consecutive S-oxidations.7 The hepatotoxicity of TAA has been known since 1948.8 It used for suitable animal models to develop acute and chronic liver damage. Utilizing different times, doses and routes of its administration, especially in water have done because of its similarity to human fibrosis and cirrhosis of the liver.9
Different agents such as lactulose (LAC),10 fish oil,11 and guanosine12 are effective against cognitive impairments observed in cirrhotic rats.
LAC is one of the clinically utilized HE drugs that has recently pulled in considerable consideration lately thanks to its neuroprotective properties.4,6,10 Pharmacodynamics of LAC make it a safe and efficacious drug in these indications.13 In the Morris behavioral experiment, the LAC administrated group of rats exhibited shorter escape latency time and received higher neurological scores point out that the cognitive impairments caused by HE enhanced by LAC.14 In- patients with chronic liver disease, LAC significantly improved health-related quality of life and MHE.6,15
The Silybum marianum (milk thistle) plant extract, silymarin (SM), contains different flavonolignans among which silybin is the major one.16 By considering the crucial functions of SM pathways in migration and mediating adhesion of cancer cells, SM represents a conceivable chemotherapeutic agent.17 SM improved memory impairments of HE.18
The point of the study was to address whether inducing an animal model of type C HE through TAA-induced cirrhosis would adversely affect associative learning, with the implication of the emotional memory function of rats using behavioral studies. The second objective was to determine the impact of treatments on the changes actuated in a rat model of neurotoxicity caused by TAA.
Materials and Methods
Chemicals and reagents
Formaldehyde solution (Dr, Mojallali Industrial chemical complex Co., Tehran, Iran) has been purchased. LAC was obtained from (Alborz Daru, Tehran, Iran), ketamine % 10 and xylazine % 2 were purchased from (Alfasa, Woerden, Netherlands), SM from the fruit of Silybum marianum extract was from (Sigma-Aldrich, St. Louis, USA), and TAA, Hydrochloric acid fuming and 2 - thiobarbituric acid were purchased from (Merck Co., Darmstadt, Germany) and Trichloroacetic acid was obtained from (Central Drug House (p) Ltd., Delhi, India).
Animals
Forty adult male Wistar rats (Pasture Institute, Karaj, Iran) with initial weight (210-275 g) were housed 3-4 animals per cage in an agreeable environment in Plexiglas-walled cages. Rats were fed commercial pellets (Behparvar Co., Tehran, Iran) and water ad libitum and were acclimatized for 2 weeks before the beginning of the study. The room that housed the rats was well kept at 22 ± 2°C, 55-65% dampness, in a light cycle inverted 12 h: 12 h dark/light: 7 am-7 pm). Animal welfare was carried out following the guidelines for the use and care of laboratory animals (1996, distributed by National Academy Press, 2101 Constitution Avenue. NW, Washington, DC 20055, USA) and in agreement with the opinion of the Bu-Ali Sina University ethics committee health guide for use and care of research facility animals. This investigation is a piece of doctorate thesis, which has been affirmed by the research committee of Bu-Ali Sina University as No. 2381770. To minimize the discomfort and pain of the animals, the greatest effort was made. Food and fluid consumption was measured daily, and body weights were measured weekly throughout the experiment.
Cirrhotic model induction
The week by week administration of TAA (Merck Co., Germany) in drinking water was the procedure used by Li et al19 and Laleman et al20 and to achieve cirrhosis. TAA received an initial concentration of 0.03% as depicted by male Wistar rats, in the water for 18 weeks successively. This was the only drinking water accessible for rats. As TAA gradual concentration in the rat body arises slowly. Weight loss was very slow, and only week after week rat weighing was done. Its concentration was adjusted week by week relying on the weight gain or weight loss of the animals.
Experimental procedures
After 18 weeks, all of the rats were haphazardly distributed between five groups: (1: Control, 2: TAA, 3: SM, 4: LAC and 5: SM+LAC, n = 7 per group). The following experiments were performed in all groups of animals, as follows.
Control group: rats were isolated and received normal tap water.
TAA group: The TAA-induced cirrhotic group received tap water ad libitum only.
SM group: The TAA-induced cirrhotic group treated with SM (50 mg/kg/d by food).21-23
LAC group: The TAA-induced cirrhotic group handled with LAC (2 g/kg/d by ingesting water).10,22,23
SM &LAC group: The TAA-induced cirrhotic group handled with SM (50 mg/kg/d by food), and LAC (2 g/kg/d by ingesting water).
Pharmacological and behavioral test
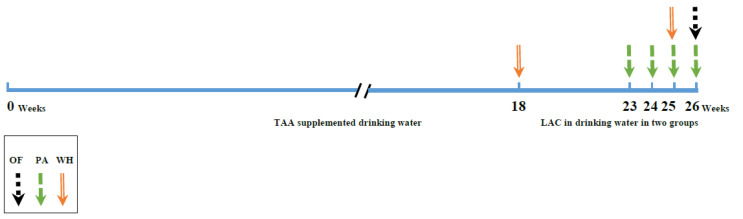
All behavioral assays performed in the following order: wire grip test, passive avoidance, and OF test (Fig. 1). As the liver has an important role in protein and energy metabolism. During cirrhosis muscle proteins metabolized. So the last day of TAA administration, the WH test has done. In the last month of the experiment, PA has done weekly and all tests have performed in the last week.
Fig. 1.
Experimental timeline (not to scale). Abbreviations: WH: Wire hanging test; PA: Passive avoidance test; OF: open field test; TAA: Thioacetamide; LAC: Lactulose; SM: Silymarin.
Behavioral analysis
Wire hanging (WH) test
The wire grasp test supports the strength and balance of the animals' muscles. Each rat was suspended on a horizontal steel wire (80 cm long, 5 mm in diameter) hanging on both forepaws. The rat was placed in a vertical position while in contact with the steel wire its forepaws were mounted. The rat was released every time the wire was grasped. For each animal, a stopwatch was used to measure the latency to fall. At an inter-trial interval of 30 minutes, every rat was tested three times.24
Passive avoidance test (PA)
The study designed to test the impacts of HE regression and its effects on acquisition and retention times of memory 7, 14, and 21 days in HE model rats.25 This experiment utilized to verify fear learning in cirrhotic rats and the feasible impact of treatments. The PA test consisted of habituation, acquisition, and retention phases. The passive avoidance apparatus (97 × 23 × 27cm) comprised of two compartments, one dark (64 × 23 × 27 cm), and one light compartment (33 × 23 × 27 cm), separated by a guillotine door (8×8.5 cm). the equipment’s ground network of comprised of 2.5 mm diameter stainless-steel bars at intervals of 1 cm, linked to a shock scrambler (Noortab Medical Engineering, Iran). During the habituation phase, animals have been put with their face confronting the wall within the lit compartment. The guillotine entryway was opened after 10 seconds, and the rat went into the dark compartment. Afterward a day, the acquisition phase started, the rat was set within the lit up compartment and after 10 seconds, the door was raised and the latency was measured to cross the compartment. Promptly afterward, the entryway was closed, a gentle electrical stun was quickly administered (1.5 mA, 3 seconds) to the rat the first time that the animal entered the dark compartment.26 The animal was kept for 20 seconds in the dark compartment and was brought back to the vivarium. Twenty-four hours later, it was checked for retrieval., The process was exactly similar to the acquisition phase except that there was no shock. Crossing latency into the dark compartment (Step-through latency: STL), and time in the dark compartment (TDC), as well as several entrances into the dark sector, was reported as contextual memory indicators were calculated in a 300-second interval.27 Around 10.00 and 13.00 hours, all behavioral activities are conducted.
Open field (OF) test
An open-field experiment used to test the conceivable impact of TAA and treatments on movement and anxiety-like behaviors.28 It conducted in a quiet room. Open-field has habituation phases comprised of presenting an animal to an open arena, a new surrounding condition with no aversive or appetizing stimuli, and allowing for a set measure of time to investigate it uninhibitedly. The animals were individually placed the apparatus consisted of a field made of Plexiglass box in a (60 × 60 × 50 cm), divided into 16 squares by lines painted on its floor. The open-field box remained on the floor in the room close to the walls and isolated from the remainder of the room with a white curtain.
Rats took to the test room in their domestic enclosures and put separately in the middle of the arena. At that point, for the following measurements of the overall number of lines crossed, the number of grooming and rearing (characterized as upstanding on their rear legs), the total distance moved, the speed, the entire time spent in the center or periphery in an OF were recorded for each rat, they were permitted to openly investigate the field for 3 minutes. Each animal was tested in the apparatus once.29 A vertically mounted video camera connected to a screen captured the test sessions, in a room for 180 seconds. Afterward, an expert analyzed the recorded experimental sessions. After each test, the rats were evacuated from the field and a cotton cloth.30 and 10% ethanol were used to clean the OF chamber to remove odorant cues.
Histopathological examination
The liver was removed and weighed immediately to verify the cirrhotic condition. Liver tissue fragments from all groups were collected for histopathological examinations. The left hepatic lobe tissue samples were stored separately in 10% buffered formalin for histomorphological examination. Routinely handled and implanted fixed tissues in paraffin. Masson's trichrome staining protocol performed (3-5 μm thickness) the paraffin-embedded liver tissue slices. The stained tissue slices were microscopically examined at 100 × magnifications after sealing the slides containing the tissue slices and watched and shot utilizing a light optical microscope (Zeiss Axio Star Plus, Jena, Germany). The validity of the TAA induces a cirrhotic rat model that has been well documented for studying biochemical and behavioral changes of liver cirrhosis.5,10 The model causes fibrosis, infiltration of inflammatory cells into periportal areas with bile duct proliferation,31 macronodular cirrhosis, portal hypertension and splenomegaly.19
Collagen proportionate zone utilizing digital image analysis
This technique is portrayed in detail somewhere else.32 In rundown, the Masson’s trichrome stained liver sections were utilized for digital image analysis. A digital camera (Canon PowerShot G 11) connected to a good personal computer (PC) archived the digital images. After the entire section computerized picture catch, collagen proportionate area (CPA), was measured with ImageJ 1.51 k examination software. At that time an RGB (Red, Green, and Blue) threshold was utilized to identify stained collagen territories and the collagen cover was determined as a pixel zone. A proportion of the CPA is communicated as a percentage. Huge blood vessels have been dodged. The CPA calculation incorporates a manual altering step to remove picture artifacts and operator-dependent thresholding to decide the stained territory of the section. The extent of collagen stain was characterized as a positive area/total area.
Determination of malondialdehyde (MDA) in brain tissues
Malondialdehyde (MDA) is one of the most as often as a possible utilized marker of lipid peroxidation.33 MDA precursor is being determined in tissues by the thiobarbituric acid (TBA) test.34 Based on the red condensation product which is produced from the reaction between TBA and MDA. The level of MDA was calculated as follows: C (M) = A/1.65 × 105. The absorption by a spectrophotometer of the upper organic layer was measured at 535 nm.35 MDA was expressed by gram tissue (nMol/g tissue) as nanomoles.
Analysis of statistics
All calculations were made utilizing the statistical package for the social sciences (SPSS) software package (version 23, IBM) for the analysis of data expressed. Results to describe the level of biochemical parameters are expressed as a mean ± standard mean error (SEM). Distribution normality has been tested using tests from Kolmogorov-Smirnov and Shapiro-Wilk. The statistical significance of differences between groups was assessed by one-way analysis of variance joined by post hoc analysis of the least significant differences. The paired t -test was used to assess muscle strength behavioral data. Statistically significant differences are considered at P < 0.05.
Results
Treatments effect on muscle strength
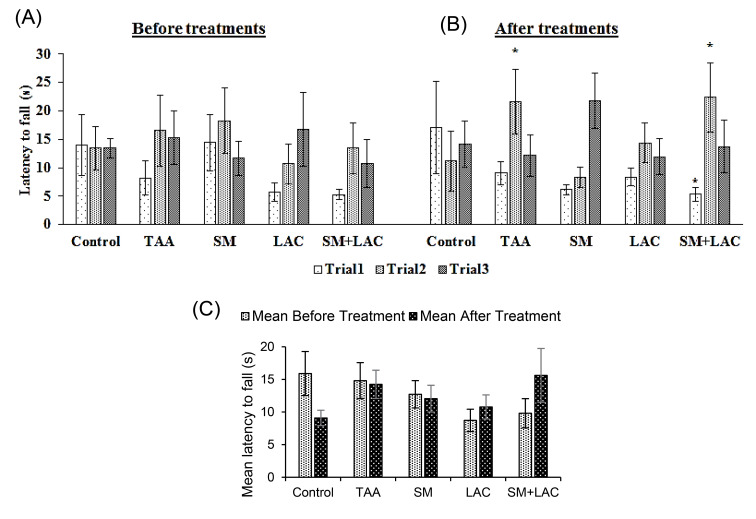
No significant within-trial effect found in all study groups before treatments ( P > 0.05) (Fig. 2A). In the first trial between SM+LAC and control groups, time on the rod was significantly decreased and increased in the second trial between TAA and SM+LAC groups in comparison with the control group of the study after treatments ( P < 0.05, Fig. 2B). There was no significant distinction between the five groups' muscle strength as measured in the WH test after treatments in contrast to before treatments ( P > 0.05, Fig. 2C).
Fig. 2.
The impact of SM and LAC chronic administration on muscle strength. (A) Evaluation of muscle strength by measurement of latency to fall in 3 trials before treatment administration. (B) Evaluation of muscle strength in 3 trials after treatment administration for eight weeks. (C) Comparing Mean of 3 trials of latency to fall before and after treatment administration. Abbreviations: TAA, Thioacetamide; LAC, Lactulose; SM, Silymarin; SM+LAC, Silymarin + Lactulose. *Compared with the control group P <0.05.
Impact of administration of SM and LAC on PA
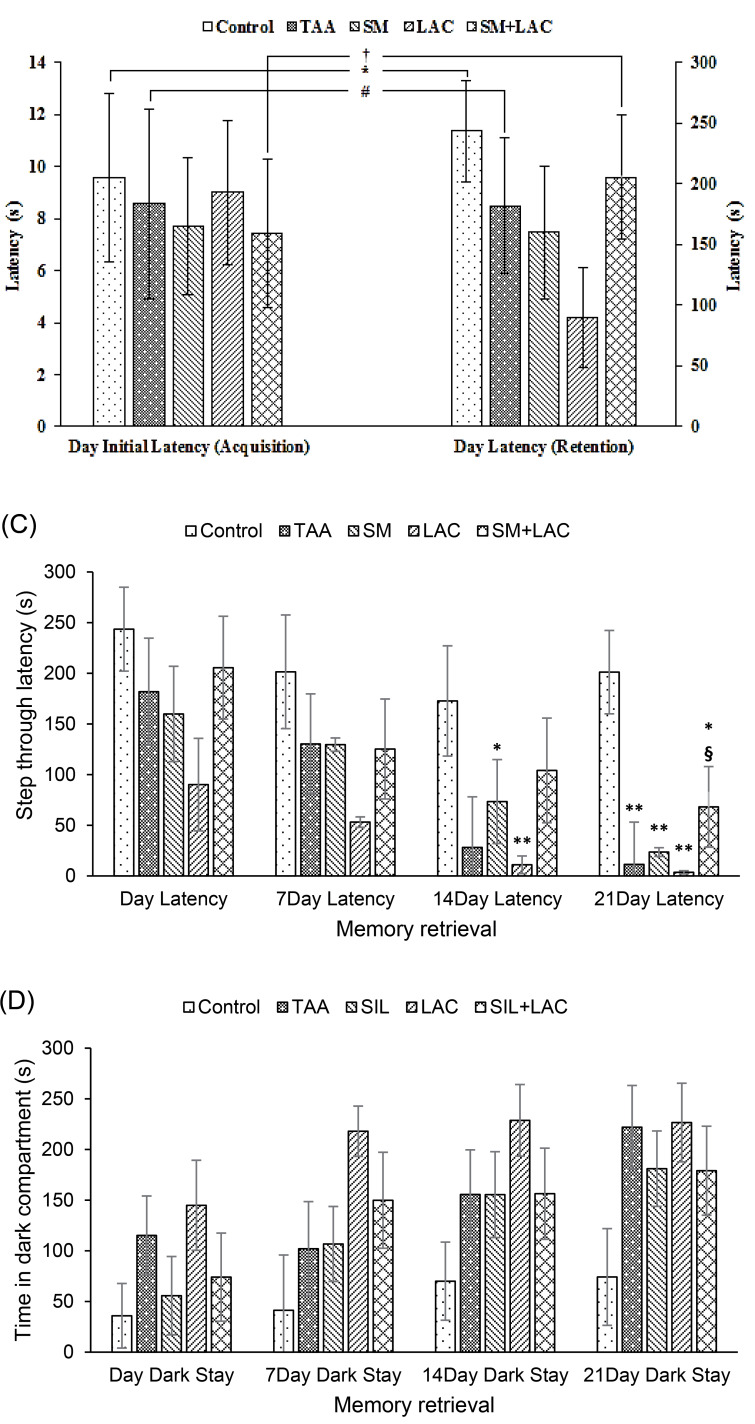
Data analysis showed that there was no difference in the latency between groups to enter the dark chamber ( P > 0.05, Fig. 3A) during the acquisition phase. Acquisition and retrieval phases of Wilcoxon’s two associated sample tests showed that there were discrepancies between Control, TAA, and SM+LAC groups ( P < 0.05, Fig. 3A-B).
Fig. 3.
The impact of cirrhosis and chronic treatment with SM and LAC on the learning in a PA learning paradigm. (A) The bar graph represents initial latencies of crossing to the dark chamber throughout acquisition within the step-through PA task of Control, TAA, SM, LAC, SM+LAC groups. (B) The bar graph represents latencies of crossing into the dark chamber throughout memory retrieval in the step-through PA task of Control, TAA, SM, LAC, SM+LAC groups. (A) + (B) Bar graph represents comparison of initial latencies and latencies of crossing to the dark chamber during memory acquisition and retrieval in the step-through PA task of Control, TAA, SM, LAC, SM+LAC groups. (C) Bar graph represent step through latencies of crossing to the dark chamber during memory retrieval in the step-through PA task of Control, TAA, SM, LAC, SM+LAC groups. (D) Bar graph represent time spend in dark compartment during memory retrieval in the step-through PA task of Control, TAA, SM, LAC, SM+LAC groups. (mean ± SEM). * P < 0.05, ** P < 0.01 in comparison with the control group. # P < 0.05 in comparison with the TAA group. § P < 0.05 in comparison with the LAC group. † P < 0.05 in comparison with the SM+LAC. Abbreviations TAA, Thioacetamide; LAC, Lactulose; SM, Silymarin; SM+LAC, Silymarin + Lactulose.
Cirrhotic rats demonstrated a decreased STL and had impaired memory retrieval compared to the control group. The administration of SM with LAC reversed the effect of TAA and showed an increased STL compared to the control group (Fig. 3C).
The time within the dark compartment was also modified in the cirrhotic groups compared to the control group. LAC group showed an increased TDC compared to the control group. SM and SM+LAC and counteracted this effect of TAA on PA learning and had a decreased TDC, whereas there was no significant contrast between the SM and SM+LAC groups and the control group, involving a defensive part for SM (Fig. 3D).
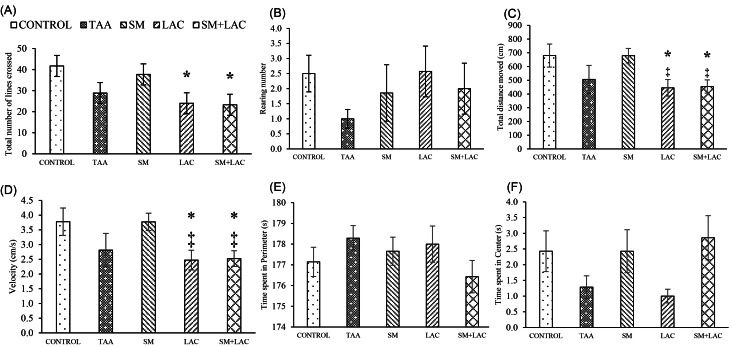
The effect of cirrhosis by OF on exploratory and anxiety-like behaviors
Fig. 4 shows, the total number of crossed lines (A) and the frequency of rearings (B) in the OF. Nevertheless, the locomotion and anxiety-like behaviors were changed in LAC and SM+LAC rats, compared to control regarding the number of lines crossed, the total distance moved and the velocity the statistical result obtained, was of statistical significance ( P < 0.05) (Fig. 4A, C-D). There were no significant differences in the time spent in the perimeter (E), time spent in center (F), and rearing frequency (B) parameters between the five study groups.
Fig. 4.
Impact of treatments on locomotion and anxiety-like behaviors in OF test. (A) The total number of lines crossed, (B) Rearing number, (C) Total distance moved, (D) speed, (E) the time spent within the perimeter and (F) time spent within the center in OF test in the control, TAA, LAC, SM and SM+LAC groups in all diagrams. * P < 0.05 vs. control; ‡ P < 0.05 vs. SM. Abbreviations: TAA, Thioacetamide; LAC, Lactulose; SM, Silymarin; SM+LAC, Silymarin + Lactulose.
Effects of SM and LAC and their combination administration on biochemical parameters
These effects have been studied through our previous work.23
Histopathological analysis
Analysis of histopathological characters
Fig. 5 appears all experimental groups' liver photomicrographs. Histopathological examination appeared that the livers of cirrhotic animals were marked by disorganization of the lobular cytoarchitecture, completely developed cirrhotic nodules encompassed by thick fibrous septa, notable bridging necrosis, pseudolobule, hepatocellular balloon-like changes, focal necrosis, inflammation and wide infiltration of inflammatory cells around the central veins, with the multiplication of the portal and periportal biliary ductules, widened portal spaces and areas of polymorphonuclear leukocyte penetration, and confluent necrosis, fibrosis, and hepatocytes within the cell death process were moreover apparent in the most part of the liver (Fig. 5B-D). Fibrotic changes were most considerable in the TAA group (Fig. 5B), but mildest in the SM+LAC group (Fig. 5E). None of these morphological modifications were watched in the control group it had normal histology in the liver parenchyma (Fig. 5A).
Fig. 5.
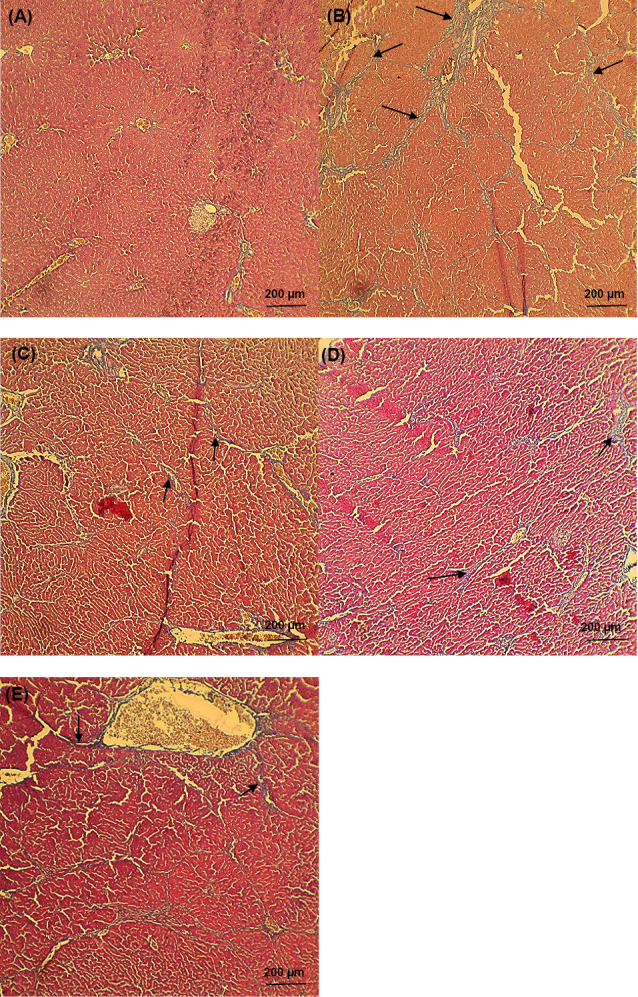
Histopathological examination of liver microscopy. Photomicrographs of (A) normal liver sections of control staining with Masson’s trichrome and (B-E) cirrhotic animals (black arrows presented the collagen fibers). Livers of cirrhotic animals exhibited disorganization of the lobular cytoarchitecture (B), with mild cirrhosis in the SM-treated rat on day 56 after TAA withdrawal (C) and areas of fibrosis with Minimal focal necrosis in LAC- treated rat (D), among strands of degenerating hepatocytes in SM+LAC- treated rat (E). None of these pathological modifications were seen in control animals (A). All of the pathophysiological examinations were performed under light microscopy at 100× magnification. Scale Bar: 200 µm. Abbreviations TAA: Thioacetamide; LAC: Lactulose; SM: Silymarin; SM+LAC: Silymarin + Lactulose.
Spontaneous resolution of cirrhosis was watched both microscopically and grossly after TAA withdrawal and treatment usage. Fibrous septa in the TAA group were dense and spreading over most parts of the liver, but slowly attenuated in the LAC group, and became thin in the SM group, interrupting but still visible SM+LAC group over the 56-day duration (Fig. 5). Irregular nodules slowly shrank on the surface of the liver, decreasing the number of nodules.
Collagen content
They were %17.42 ± 0.87 in the cirrhotic liver at week 8 (56 d), significantly higher than %11.56 ± 0.69 ( P < 0.01) in control liver (Table 1 and Fig. 5).
Table 1. Changes after TAA withdrawal in cirrhotic rats and different treatments administration .
| Rat group | Collagen content (%) | Brian MDA ( nMol /g). |
| Control rats | 11.56 ± 0.69 | 25.10 ± 0.86 |
| TAA-treated rats | ||
| TAA | 17.42 ± 0.87** | 27.11 ± 1.28 |
| SM | 16.12 ± 1.69* | 27.51 ± 2.57 |
| LAC | 17.32 ± 1.29** | 27.58 ± 1.16 |
| SM+LAC | 15.60 ± 1.65* | 28.90 ± 1.73 |
Quantification of contents of Collagen and oxidative stress marker (MDA) was determined in the liver and Brain tissue respectively.
MDA contents in brain tissue
The level of MDA content, a marker of lipid peroxidation, was higher in TAA, SM, LAC and SM+LAC groups than the control group without statistical differences among four groups (Table 1).
The level of serum MDA content and liver MDA content have been studied through our previous work.23
Discussion
The TAA model has been utilized to portray changes in neuromodulatory capacities of astrocytes in connection to the progression of HE36 oral feeding of TAA mirrors gentle hepatitis reflecting e.g. alcoholic liver disease37; It was administrated to rats that reflected cirrhotic situations, namely a chronic form of liver injury (groups II-V). Previous studies have shown spatial working memory deficits following TAA administration.38-40 Although the precise mechanisms of impairments do not seem to be yet clear, which are mainly caused by alteration of the metabolic activity of neural regions,38 an increase in cerebral histamine and pathology of the glutamatergic system,39 and alterations of acetylcholinesterase activity in brain limbic system regions40 have been proposed to underlie these impairments. Oxidative stress prevails in pons medulla and cerebral cortex during the TAA-induced acute liver failure in rats’ brains.41 Consequently, discovering ways to diminish these oxidative changes might facilitate to lessen the impairments saw in HC.
SM isolated from Silybum marianum (L.) Gaertn. is a mixture of bioactive flavonolignans, employed in the treatment of oxidative damage of the central nervous system by its capacity to avoid lipid peroxidation and replenishing the decreased glutathione levels.42 Therefore, it shows up that SM might apply its impact through these pathways on TAA-induced disabilities. More research is needed to explain the precise neural security mechanisms of SM in a cognitive disabilities model of TAA.
LAC, non-absorbable disaccharide, possessed neuroprotective effects on the cerebral injury,14 prohibit aggregation-associated neurodegenerative diseases43 have beneficial effects on HE manifestations and the avoidance of HE episodes,44 effectively improves cognitive function,45 increases blood ammonia and psychometric screening in MHE and reduces the danger of developing overt encephalopathy.46 The mechanism of action of LAC is not yet clearly characterized, however up to date investigations indicate that it works primarily through H2 generation by LAC fermentation of intestinal bacteria and Nrf2 activation,14 autophagy up-regulation,43 and early HE enhancement of neuroplasticity.45 While further studies are needed to explain the impact of LAC in combination with other medications.
The consequences of the present study demonstrated that impairments in learning, memory and motor function were ascertained in rats following utilization of TAA as an HC animal model. SM+LAC had a promising effect of neurocurative capacity against learning, memory, and motor function impairments prompted by TAA (Figs. 2-4).
Step-through PA learning is believed to be based on a neural circuit together with the hippocampus, amygdala, thalamus, parahippocampal cortices, and mammillary nuclei that are included within the learning and memory forms that empower context-dependent behavior.47 In the current study, the cognitive shortages were surveyed via step-through PA, consistent with previous studies,39,48 we have shown that chronic use of TAA appeared to be poorer recall latencies than the control group (Fig. 3C), proposing a shortfall in memory processes associated with this assignment but does not impairs spatial memory like acute condition.48 This performance of the TAA group in PA matches the detailed data of Méndez et al.39 who didn't watch any changes within the PA latency of rats treated with TAA but in our work significant alter of memory have been seen after 14 days (Fig. 3C). Such inconsistencies between experimental groups within the capacities of memory and learning might be because of the pathophysiological contrasts produced in the cirrhotic models. Cirrhotic rats endure from secondary liver failure through oxygen reactive species associated with inflammation, fibrosis, cirrhosis, and advancement in new nodule making. Portal-systemic shunts are becoming more and more shaped through the destructured liver parenchyma, i.e. intrahepatic portal-systemic shunts. The portal-systemic collateral circulation develops through splanchnic involvement and hyperdynamic circulation is made.49 So blood containing TAA or treatments depending on shunt degree cannot go under "first-pass metabolism" of the liver and by collateral circulation blood without change directly reach the brain. The different treatment makes a different level of changes in the pathophysiology of tissues and results in different memory effects. Surai has shown that treatment with SM alleviates TAA-induced memory impairments through several mechanisms that mediate this beneficial effect of SM.16 As we have discussed before LAC therapy could increase blood ammonia, induce psychometric hydrogen testing in MHE and lessen the danger of creating overt encephalopathy.46,50 We have demonstrated for the first time that TAA impairs spatial memory after three weeks, which could be protected through SM+LAC administration. We considered that the TAA rats and LAC rats indicated similar behavior not only in 14 and 21 days’ latency at PA (Fig. 3C) but also in the perimeter and central time on OF test (Fig. 4E-F). Further investigations are necessary to decide if LAC features a deteriorative impact on cirrhotic rat behavior. These outcomes are as per the results of Kawai et al in the new object recognition test.10 We propose that TAA-induced disabilities intercede through the oxidative stress pathway, which may be the situation for SM and LAC combination.42,50 Though these discoveries in addition to findings of this study indicate the need for further studies to clarify the exact fundamental mechanisms.
Liver histopathology reflected hepatocyte damage. When liver parenchyma was injured, the collagen levels were higher in TAA-induced chronic liver damage compared with control. Besides, liver histopathology was concordant with these data as diffuse necrosis and collagen septa were observed in groups II-V (Fig. 5).
As mentioned before, HE is a syndrome described by a depressed level of consciousness, and cognitive impairment, changes in personality have been seen in patients with liver dysfunction, for example, hepatitis and cirrhosis. The point-by-point mechanism underlying the pathogenesis of HE stays unclear. The TAA rats showed elevated blood levels of ammonia in biochemical analyses.10 Ammonia accumulates in blood as a result of the fact that harmed hepatocytes lack it's removing, so an excess of ammonia goes to the brain tissue. Ammonia reacts with glutamate in brain astrocytes and produces, glutamine, which in turn induce osmotic imbalance, which leads to a change of functions and form of astrocytes.51 In any case, Post-oral treatment of LAC, a clinically used HE medication, effectively diminished blood ammonia levels and reestablished the decreased immobility and reduced cognitive scores, with no consequences compared with the control on neurotransmitter contents within the brain.10 In our study LAC treatment was able to improve muscle strength levels (Fig. 2C), despite not affecting memory retrieval on PA (Fig. 3C). However, the result of the OF test showed that the LAC did not significantly improve rat locomotor activity and increased anxiety-like behaviors by reducing the time spent in the center since the time spent by the LAC group in the center of an OF was closer to that of the TAA group (Fig. 4F). LAC was also unable to reduce the impairments of cirrhotic hepatic structure damage (Fig. 5D) and to mitigate or reverse the content of collagen in the liver. LAC did not affect the reduced content of brain MDA (Table 1). Despite previous studies, our results do not confirm LAC’s effectiveness in changing motor behavior, reinforcing the value of further analysis in the treatment of cirrhosis-associated encephalopathy symptoms for its clinical usage. So as mentioned in previous studies,10 to explain the connections among LAC and neuronal systems further examinations are necessary. The results of this investigation likewise exhibited that utilization of SM+LAC could delay the improvement of behavioral changes and encephalopathy caused by liver malfunction to the related toxins in circulation according to behavioral assessments. Treatment with SM+LAC likewise improved motor activity, which is influenced by encephalopathy, proposed that the increased locomotor activity based on OF test results saw in SM+LAC treated rats might be the consequence of psychomotor incitement or diminished anxiety following SM+LAC treatment (Fig. 4E-F).
It is well known that histamine and serotonin (5-HT) were mainly elevated in the blood in TAA-induced cirrhosis.52 During the development of cirrhosis, therefore, the "down-regulation" of the histamine H1 and H2 receptors may be an important factor for the reduction of maximal binding capacity and binding ability of the two histamine receptors, and these changes of the receptors may lead to metabolic disorders of carbohydrates and phospholipids in the liver and lower the liver's ability to inactivate histamine.53 The osmotic laxative, LAC had little or no effect on the production of prostaglandin-like material, histamine, and 5-HT.54 The unabsorbed substance, such as LAC, also induced a decrease in rat ileum to short-circuit currents by bringing out histamine discharge from ileum mucosa.55 SM inhibits the dose-dependent manner of neutrophil-mediated histamine release.56 The degree of nitrate and inducible nitric oxide synthase (iNOS) significantly increases in cirrhotic patients. Increased nitric oxide synthase expression is therefore vital within the development of systemic hyperdynamic circulatory variations in cirrhosis.57 Stimulation of nitric oxide (NO) synthesis like the use of NO contributors may be beneficial in liver fibrosis. Numerous authors accept that activation of NO synthesis by pharmacological agents is promising in the treatment of liver fibrosis. For iNOS, rather than for expressed NOS (eNOS), there is a significant anti-fibrotic function.58 Chronic SM treatment, however, can improve liver enzyme activities and fibrosis caused by cirrhosis, yet could worsen the hemodynamic eNOS activity, especially by diminishing eNOS expression and expanding expression of caveolin-1, an eNOS inhibitor.59 LAC also exercised a colonic iNOS expression inhibition, which is unregulated as a result of the inflammatory status.60 We propose that the neurocurative effect of SM and LAC due to alterations in the NO, ammonia, and histamine pathways and their other effects combine so their synergic effect shows its best in the SM+LAC group and could be a good treatment for cirrhosis and HC. Though, in further studies, this hypothesis ought to be checked more. Nonetheless, it is an appropriate treatment for the study on various HC complications.
Conclusion
Taking everything into account, the conducted study has shown that chronic treatment with LAC and SM in combination have potential pharmaceutical use in cirrhotic livers. Our examinations have shown that SM+LAC alleviates the spatial and fear learning and memory impairment in rats with cirrhotic liver disease. SM+LAC combination could also be utilized as a potent neuroprotective agent that protecting the brain. Similar neuroprotection has never been informed before. To illuminate the precise mechanisms and possible adverse effects of chronic SM+LAC administration, more studies are needed. These outcomes are extremely promising and propose that SM+LAC ought to be tested additionally.
Acknowledgments
Authors like to acknowledge Isfahan University of Medical Sciences for their kindness in letting us use their laboratory and animal nest and for their technical support.
Funding sources
This work was done without any external funding.
Ethical statement
All appropriate global, regional, and/or institutional animals care and utilization rules have been pursued. All procedures conducted in animal-related examinations were as per the institution’s moral standards and policy in which the research is done. This article does not contain any examinations conducted by any of the researchers with human members.
Competing interests
None declared.
Authors’ contribution
Conceiving and planning the project, the main conceptual ideas, and the design of the experiments were performed by the MG and NM. The data collection and data analysis, the finalized draft and the latest version of the manuscript carried out by MG. The project was supervised by NM. The manuscript was revised by NM, HA, and MR. The final version of the manuscript has the approval of all authors.
Research Highlights
What is the current knowledge?
√ Passive avoidance learning impairments were not observed in similar cirrhotic rat model made in fewer days.
√ Cirrhotic rats had an increased level of malondialdehyde in other models.
√ Lactulose and silymarin combination effect against the motor and cognitive impairments have not worked before.
What is new here?
√ Spatial and passive avoidance learning impairments were observed in a current cirrhotic rat model.
√ Cirrhotic rats had an increased level of malondialdehyde.
√ Lactulose and silymarin combination has a curative effect against the motor and cognitive impairments induced by thioacetamide.
References
- 1.Schuppan D. Liver fibrosis: Common mechanisms and antifibrotic therapies. Clin Res Hepatol Gastroenterol. 2015;39:S51–S9. doi: 10.1016/j.clinre.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Hejda V. Cirrhosis of the liver and HCV. Vnitr Lek. 2015;61:4s13–23. [PubMed] [Google Scholar]
- 3.Zhou W-C, Zhang Q-B, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo M, Guo J-Y, Cao W-K. Inflammation: A novel target of current therapies for hepatic encephalopathy in liver cirrhosis. World J Gastroenterol. 2015;21:11815. doi: 10.3748/wjg.v21.i41.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Trigun S. Low grade cirrhosis induces cognitive impairment and motor dysfunction in rats: Could be a model for minimal hepatic encephalopathy. Neurosc Lett. 2014;559:136–40. doi: 10.1016/j.neulet.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 6.Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109:180–7. doi: 10.3238/arztebl.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarma D, Hanzlik RP. Synthesis of carbon‐14, carbon‐13 and deuterium labeled forms of thioacetamide and thioacetamide S‐oxide. J Labelled Comp Radiopharm. 2011;54:795–8. doi: 10.1002/jlcr.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajovsky H, Hu G, Koen Y, Sarma D, Cui W, Moore DS. et al. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem Res Toxicol. 2012;25:1955–63. doi: 10.1021/tx3002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbasi MH, Akhtar T, Malik IA, Fatima S, Khawar B, Mujeeb KA. et al. Acute and chronic toxicity of thioacteamide and alterations in blood cell indices in rats. J Cancer Ther. 2013;4:251. doi: 10.4236/jct.2013.41032. [DOI] [Google Scholar]
- 10.Kawai H, Ishibashi T, Kudo N, Kawashima Y, Mitsumoto A. Behavioral and biochemical characterization of rats treated chronically with thioacetamide: proposal of an animal model for hepatic encephalopathy associated with cirrhosis. J Toxicol Sci. 2012;37:1165–75. doi: 10.2131/jts.37.1165. [DOI] [PubMed] [Google Scholar]
- 11.Staziaki P, M Marques C, M Delattre A, De Paula Cioni B, Rufino M, Vila dos Santos F. et al. Fish oil has beneficial effects on behavior impairment and oxidative stress in rats subjected to a hepatic encephalopathy model. CNS Neurol Disord Drug Targets. 2013;12:84–93. doi: 10.2174/1871527311312010014. [DOI] [PubMed] [Google Scholar]
- 12.Paniz L, Calcagnotto M, Pandolfo P, Machado D, Santos G, Hansel G. et al. Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab Brain Dis. 2014;29:645–54. doi: 10.1007/s11011-014-9548-x. [DOI] [PubMed] [Google Scholar]
- 13.Schumann C. Medical, nutritional and technological properties of lactulose An update. Eur J Nutr. 2002:41. doi: 10.1007/s00394-002-1103-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhai X, Chen X, Shi J, Shi D, Ye Z, Liu W. et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic Biol Med. 2013;65:731–41. doi: 10.1016/j.freeradbiomed.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011;23:725–32. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 16.Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4:204–47. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EJ, Lee MY, Jeon YJ. Silymarin inhibits morphological changes in LPS-stimulated macrophages by blocking NF-κB pathway. Korean J Physiol Pharmacol. 2015;19:211–8. doi: 10.4196/kjpp.2015.19.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad MA, Rastanawi AA, El-Yamany MF. Alogliptin abates memory injuries of hepatic encephalopathy induced by acute paracetamol intoxication via switching-off autophagy-related apoptosis. Life Sci. 2018;215:11–21. doi: 10.1016/j.lfs.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol. 2002;36:488–93. doi: 10.1016/S0168-8278(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 20.Laleman W, Elst IV, Zeegers M, Servaes R, Libbrecht L, Roskams T. et al. A stable model of cirrhotic portal hypertension in the rat: thioacetamide revisited. Eur J Clin Invest. 2006;36:242–9. doi: 10.1111/j.1365-2362.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 21.Alshawsh MA, Abdulla MA, Ismail S, Amin ZA. Hepatoprotective Effects of Orthosiphon stamineus Extract on Thioacetamide-Induced Liver Cirrhosis in Rats. Evid Based Complement Alternat Med. 2011;2011:103039. doi: 10.1155/2011/103039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyoda-Hokaiwado N, Yasui Y, Muramatsu M, Masumura K, Takamune M, Yamada M. et al. Chemopreventive effects of silymarin against 1,2-dimethylhydrazine plus dextran sodium sulfate-induced inflammation-associated carcinogenicity and genotoxicity in the colon of gpt delta rats. Carcinogenesis. 2011;32:1512–7. doi: 10.1093/carcin/bgr130. [DOI] [PubMed] [Google Scholar]
- 23.Ghobadi Pour M, Mirazi N, Alaei H, Moradkhani S, Rajaei Z, Monsef Esfahani A. Effects of lactulose and silymarin on liver enzymes in cirrhotic rats. Can J Physiol Pharmacol. 2017;95:522–9. doi: 10.1139/cjpp-2016-0454. [DOI] [PubMed] [Google Scholar]
- 24.Van Wijk N, Rijntjes E, Van De Heijning B. Perinatal and chronic hypothyroidism impair behavioural development in male and female rats. Exp Physiol. 2008;93:1199–209. doi: 10.1113/expphysiol.2008.042416. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini N, Alaei H, Zarrindast MR, Nasehi M, Radahmadi M. Cholestasis progression effects on long-term memory in bile duct ligation rats. Adv Biomed Res. 2014;3:215. doi: 10.4103/2277-9175.143263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khajehpour L, Alizadeh-Makvandi A, Kesmati M, Eshagh-Harooni H. Involvement of basolateral amygdala GABAA receptors in the effect of dexamethasone on memory in rats. J Zhejiang Univ Sci B. 2011;12:900–8. doi: 10.1631/jzus.B1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khayam Haghighi S, vaez Mahdavi MR, Reza M, Reisi P, Alaei H. The Effects of Mid-Term Running Activity on Passive Avoidance Learning and Memory in Opioid Addicted Rats. Journal Of Isfahan Medical School (IUMS) 2009;27:507–18. [Google Scholar]
- 28. Hines TJ, Minton BR. Effects of environmental enrichment on rat behavior in the open field test. Proc Natl Conf Undergrad Res; March 29–31, 2012; Weber State University, Ogden, Utah.
- 29.Yu L, Jiang X, Zhang Y, Liao M, Ma R, Yu T. Antidepressant-like activity of Tetramethylpyrazine measured by chronic experimental method in rat model of depression. Pharmacol Pharm. 2012;3:52. doi: 10.4236/pp.2012.31008. [DOI] [Google Scholar]
- 30. Quillfeldt JA. Behavioral Methods to Study Learning and Memory in Rats. In: Andersen ML, S Tufik, editors. Rodent Model as Tools in Ethical Biomedical Research. Cham: Springer International Publishing; 2016. p. 271-311.
- 31.Jang J, Kang K, Kim Y, Kang Y, Lee I. Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transplant Proc. 2008;40:2700–3. doi: 10.1016/j.transproceed.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Gu K, Zhao JD, Ren ZG, Ma NY, Lai ST, Wang J. et al. A natural process of cirrhosis resolution and deceleration of liver regeneration after thioacetamide withdrawal in a rat model. Mol Biol Rep. 2011;38:1687–96. doi: 10.1007/s11033-010-0281-1. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. doi: 10.1093/clinchem/43.7.1209. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 35.Rajaei Z, Hadjzadeh M-A-R, Nemati H, Hosseini M, Ahmadi M, Shafiee S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J Med Food. 2013;16:206–10. doi: 10.1089/jmf.2012.2407. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783–8. doi: 10.1111/j.1478-3231.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- 37.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F. et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendez M, Mendez-Lopez M, Lopez L, Aller MA, Arias J, Arias JL. Basal and learning task-related brain oxidative metabolism in cirrhotic rats. Brain Res Bull. 2009;78:195–201. doi: 10.1016/j.brainresbull.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Mendez M, Mendez-Lopez M, Lopez L, Aller MA, Arias J, Arias JL. Associative learning deficit in two experimental models of hepatic encephalopathy. Behav Brain Res. 2009;198:346–51. doi: 10.1016/j.bbr.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Mendez M, Mendez-Lopez M, Lopez L, Aller MA, Arias J, Arias JL. Acetylcholinesterase activity in an experimental rat model of Type C hepatic encephalopathy. Acta Histochem. 2011;113:358–62. doi: 10.1016/j.acthis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Sathyasaikumar KV, Swapna I, Reddy PV, Murthy Ch R, Dutta Gupta A, Senthilkumaran B. et al. Fulminant hepatic failure in rats induces oxidative stress differentially in cerebral cortex, cerebellum and pons medulla. Neurochem Res. 2007;32:517–24. doi: 10.1007/s11064-006-9265-x. [DOI] [PubMed] [Google Scholar]
- 42.Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine. 2007;14:129–35. doi: 10.1016/j.phymed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Lee GC, Lin CH, Tao YC, Yang JM, Hsu KC, Huang YJ. et al. The potential of lactulose and melibiose, two novel trehalase-indigestible and autophagy-inducing disaccharides, for polyQ-mediated neurodegenerative disease treatment. Neurotoxicology. 2015;48:120–30. doi: 10.1016/j.neuro.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G. et al. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence? Metab Brain Dis. 2013;28:221–5. doi: 10.1007/s11011-012-9372-0. [DOI] [PubMed] [Google Scholar]
- 45.Yang N, Liu H, Jiang Y, Zheng J, Li DM, Ji C. et al. Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy. Neural Regen Res. 2015;10:1457–62. doi: 10.4103/1673-5374.165516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziada DH, Soliman HH, El Yamany SA, Hamisa MF, Hasan AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol. 2013;14:116–22. doi: 10.1016/j.ajg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Arias N, Mendez M, Arias JL. The importance of the context in the hippocampus and brain related areas throughout the performance of a fear conditioning task. Hippocampus. 2015;25:1242–9. doi: 10.1002/hipo.22430. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Rafei M, Amin MM, Hasan HF. Novel effect of Daflon and low-dose gamma-radiation in modulation of thioacetamide-induced hepatic encephalopathy in male albino rats. Hum Exp Toxicol. 2016 doi: 10.1177/0960327116637657. [DOI] [PubMed] [Google Scholar]
- 49.Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912–24. doi: 10.1016/j.jhep.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, Zhang W, Zhang R, Ruan X, Ren P, Lu B. Lactulose accelerates liver regeneration in rats by inducing hydrogen. J Surg Res. 2015;195:128–35. doi: 10.1016/j.jss.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 51.Strekalova OS, Uchaikin VF, Ipatova OM, Torkhovskaia TI, Medvedeva NV, Storozhakov GI. et al. [Comatose states: etiopathogenesis, experimental studies, treatment of hepatic coma] Biomed Khim. 2009;55:380–96. [PubMed] [Google Scholar]
- 52.Li XN, Huang CT, Wang XH, Leng XS, Du RY, Chen YF. et al. Changes of blood humoral substances in experimental cirrhosis and their effects on portal hemodynamics. Chin Med J (Engl) 1990;103:970–7. [PubMed] [Google Scholar]
- 53.Peng J, Leng X, Wei Y. [Changes of histamine receptors in the liver of the rat during the development of experimental cirrhosis] Zhonghua Wai Ke Za Zhi. 1996;34:113–6. [PubMed] [Google Scholar]
- 54.Capasso F, Mascolo N, Autore G, Romano V. Laxatives and the production of autacoids by rat colon. J Pharm Pharmacol. 1986;38:627–9. doi: 10.1111/j.2042-7158.1986.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, An N, Shaikh AS, Wang H, Xiao L, Liu H. et al. Hyperosmolarity evokes histamine release from ileum mucosa by stimulating a cholinergic pathway. Biochem Biophys Res Commun. 2017;493:1037–42. doi: 10.1016/j.bbrc.2017.09.093. [DOI] [PubMed] [Google Scholar]
- 56.Fantozzi R, Brunelleschi S, Rubino A, Tarli S, Masini E, Mannaioni PF. FMLP-activated neutrophils evoke histamine release from mast cells. Agents Actions. 1986;18:155–8. doi: 10.1007/BF01988009. [DOI] [PubMed] [Google Scholar]
- 57.Showpittapornchai U, Wattanasirichaigoon S, Pradidarcheep W. Predominant vascular dilatation with NOS expression in lung lower lobe of thioacetamide induced-cirrhotic rat. J Med Assoc Thai. 2012;95 Suppl 12:S99–104. [PubMed] [Google Scholar]
- 58.Lukivskaya O, Patsenker E, Lis R, Buko VU. Inhibition of inducible nitric oxide synthase activity prevents liver recovery in rat thioacetamide-induced fibrosis reversal. Eur J Clin Invest. 2008;38:317–25. doi: 10.1111/j.1365-2362.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 59.Cho YK, Yun JW, Park JH, Kim HJ, Park DI, Sohn CI. et al. Deleterious effects of silymarin on the expression of genes controlling endothelial nitric oxide synthase activity in carbon tetrachloride-treated rat livers. Life Sci. 2009;85:281–90. doi: 10.1016/j.lfs.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi LC, Rodriguez-Cabezas ME. et al. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–71. doi: 10.1097/01.mib.0000160808.30988.d9. [DOI] [PubMed] [Google Scholar]