Abstract
The feeling of being addressed is the first step in a complex processing stream enabling successful social communication. Social impairments are a relevant characteristic of patients with major depressive disorder (MDD). Here, we investigated a mechanism which—if impaired—might contribute to withdrawal or isolation in MDD, namely, the neural processing of social cues such as body orientation and gesture. During funtional magnetic resonance imaging (fMRI) data acquisition, 33 patients with MDD and 43 healthy control subjects watched video clips of a speaking actor: one version with a gesture accompanying the speech and one without gesture. Videos were filmed simultaneously from two different viewpoints: one with the actor facing the viewer head‐on (frontal) and one side‐view (lateral). After every clip, the participants were instructed to evaluate whether they felt addressed or not. Despite overall comparable addressment ratings and a large overlap in activation patterns in MDD and healthy subjects for gesture processing, the anterior cingulate cortex, bilateral superior/middle frontal cortex, and right angular gyrus were more strongly activated in patients than in healthy subjects for the frontal conditions. Our analyses revealed that patients showed specifically higher activation than healthy subjects for the frontal condition without gesture in regions including the posterior cingulate cortex, left prefrontal cortex, and the left hippocampus. We conclude that MDD patients can recognize and interpret social cues such as gesture or body orientation; however, they seem to require more neural resources. This additional effort might affect successful communication and contribute to social isolation in MDD.
Keywords: body orientation, depression, fMRI, gesture, language, social cues
1. INTRODUCTION
Social contact and participation in a community are essential for human well‐being. Daily conversations allow us to build relationships and exchange our thoughts and ideas. Actual and perceived loneliness is a major risk factor for poor health and early mortality (Holt‐Lunstad, Smith, Baker, Harris, & Stephenson, 2015). Social isolation and dysfunctional interpersonal relationships, however, are common problems among patients suffering from major depressive disorder (MDD) (Coyne, 1976; Hautzinger, Linden, & Hoffman, 1982; Youngren & Lewinsohn, 1980). Both excessive interpersonal feedback seeking and negative feedback seeking are relatively specific dysfunctional communication patterns in depression (Hames, Hagan, & Joiner, 2013). Besides the severity of the index episode, lack of social support is an important predictor of later depressive episodes (Saris, Aghajani, van der Werff, van der Wee, & Penninx, 2017; Spijker et al., 2004) and can also hinder recovery (Carpenter, 2017). Even after complete remission of depressive symptoms, residual impairments of social functioning can remain (Saris et al., 2017). Whether MDD causes or is caused by social isolation (or both) remains unclear so far. A dysfunctional processing of social cues such as gesture or body orientation might contribute to these social impairments.
Social communication relies on various cognitive processes such as affective learning of presented content, recognition of and response to socioaffective stimuli, embodied simulation of the perceived social cues, higher‐level representation of mental states and the ability to regulate one's judgment in a context sensitive manner (Ochsner, 2008). The feeling of involvement of the subject/patient himself/herself changes the emotional engagement and responsiveness to others; hence, it constitutes an important first step of social communication (Schilbach et al., 2013). On a neural level, regions related to theory of mind and intention understanding—such as the medial prefrontal cortex and premotor cortex—activate more strongly when the subject is being addressed directly, compared to when talking about others (Ciaramidaro, Becchio, Colle, Bara, & Walter, 2014). Parts of the default mode network and anterior cingulate cortex (ACC) seem to be generally involved in social cognition (Mars et al., 2012). This network includes areas such as the dorsomedial prefrontal cortex, temporal parietal junction, precuneus, posterior cingulate cortex (PCC), and amygdala (Saxe, 2006; Saxe & Powell, 2006), middle and ventral prefrontal cortex, middle temporal gyrus (Wheatley, Milleville, & Martin, 2007), and temporal pole (Koster‐Hale et al., 2017), which are also known as being involved in representing thoughts and feelings of other persons. Moreover, social functioning in natural contexts is based on the processing and integration of multiple (verbal and nonverbal) communication channels, including body orientation, gesture, and speech (Bucci, Startup, Wynn, Baker, & Lewin, 2008; Potthoff & Seitz, 2015; Straube, Green, Jansen, Chatterjee, & Kircher, 2010). The direction of gaze, for example, can influence speech processing and help understand a speaker's message (Holler et al., 2014; Holler et al., 2015). Similarly, body orientation influences the neural processing of speech and gesture (Straube et al., 2010) and the feeling of addressment (Nagels, Kircher, Steines, & Straube, 2015).
There is some first evidence of dysfunctional social communication in MDD patients: a negative misinterpretation of neutral faces in MDD patients (Rubinow & Post, 1992; Surguladze et al., 2004) as well as negative expectations and cognition concerning social rejection have been observed (Ehnvall et al., 2014; Kube et al., 2018). Depressive patients engage less in eye contact, use fewer gestures (Hames et al., 2013; Kazdin, Sherick, Esveldt‐Dawson, & Rancurello, 1985), and show less modulation in their voice (Youngren & Lewinsohn, 1980). Moreover, MDD patients show a lower activation in the neural reward system (e.g., nucleus accumbens) during social interaction (Germine, Garrido, Bruce, & Hooker, 2011; Hsu et al., 2015) and have difficulties attributing mental states to others (Bora & Berk, 2016). These deficits are correlated with symptom severity in depressive patients (Bora & Berk, 2016) and with a risk of depressive symptoms in healthy subjects (HS) (Bora & Berk, 2016; Inoue, Yamada, & Kanba, 2006). Furthermore, it has been suggested that MDD patients have a reduced motivation for social interaction in general (Kupferberg, Bicks, & Hasler, 2016). The direct behavioral reactions of MDD patients to social cues such as gesture or body orientation have not been examined so far.
In the past, we have identified regions involved in the processing of social cues such as gesture and body orientation, that is, the ACC, left fusiform gyrus, supplementary motor area (SMA), left inferior frontal gyrus (IFG), and right insula (Nagels, Kircher, Steines, & Straube, 2015). Most of these regions have been associated with attributing mental states to others (Spunt, Satpute, & Lieberman, 2011), which also seem to be impaired in MDD patients (Bora & Berk, 2016; Inoue et al., 2006; Wang, Wang, Chen, Zhu, & Wang, 2008). In a previous study with healthy students, an actor facing the participant head‐on (frontal view) while talking as well as using gestures was rated as more addressing than the same actor facing to the side (lateral view) or talking without gestures (Nagels, Kircher, Steines, & Straube, 2015). Furthermore, stronger activations of the ACC as well as left cerebellum/fusiform gyrus were revealed for the participant facing condition (frontal > lateral) in a gesture compared to a no‐gesture context. By contrast, increased activations of the SMA, left IFG, and right insula were found for the effect of gesture versus no‐gesture when the speaker was seen from a lateral instead of a frontal perspective. Results indicate that gesture use and body orientation contribute to the feeling of being addressed; they together influence neural processing in brain regions involved in motor simulation, empathy, and mentalizing. While data about these processes in MDD are missing, a decrease of activation in the left ACC, left IFG, and left insula during resting state in MDD patients has been described (Fitzgerald, Laird, Maller, & Daskalakis, 2008). A more recent functional network analysis also revealed alterations in networks including the left precentral gyrus, left angular gyrus, left IFG, and bilateral Rolandic operculum (Lai, Wu, & Hou, 2017). These studies are a first hint that brain regions relevant for the processing of social cues such as body orientation and gesture could be affected by depression.
The aim of the present study was to investigate a potential neural mechanism underlying the interpretation of social cues such as body orientation and gesture in patients with MDD. As was done in a previous study on HS (Nagels, Kircher, Steines, & Straube, 2015), we used video clips of an actor speaking simple sentences, once with an iconic gesture (IC) accompanying the speech and once without gesture (no gesture [NG]), filmed simultaneously from a frontal and lateral viewpoint. After every clip, the participants had to evaluate whether they felt addressed or not. We expected patients with MDD to be less sensitive to social cues, leading to generally reduced addressment ratings—compared to healthy subjects—for both frontal and both gesture conditions, with the biggest reduction in the combined condition (IC frontal).
For the neural processing of gesture (gesture > no gesture) we expected both groups to activate occipital, posterior temporal brain areas as well as prefrontal brain regions, as found in previous experiments of ours (Nagels, Kircher, Steines, & Straube, 2015; Straube et al., 2010; Straube, Green, Sass, Kirner‐Veselinovic, & Kircher, 2013). In contrast, we hypothesized that for the main effect of body orientation (frontal > lateral) HS would activate the right occipital/fusiform gyrus and left prefrontal regions (Nagels, Kircher, Steines, & Straube, 2015; Schilbach et al., 2006; Straube et al., 2010) while activation patterns of patients with MDD would deviate from those of the HS. Either increased activation to compensate for processing dysfunctions or decreased activation as a direct result of aberrant processing were expected. Moreover, we hypothesized different activations in the ACC, PCC, angular gyrus, left IFG, and insula among the groups as an interaction effect of gesture and body orientation (Straube et al., 2010; Straube, Green, Chatterjee, & Kircher, 2011).
2. METHODS
2.1. Participants
Thirty‐eight patients with the main diagnosis of MDD according to the Diagnostic and Statistical Manual of Mental Disorders, fith edition criteria (Falkai & Döpfner, 2015) and 48 healthy subjects (HS) were recruited. Inclusion criteria for both patients and healthy subjects were native fluency in German and no impairment of seeing and hearing. We did not exclude patients with substance dependence because of the high comorbidity in MDD patients (Davis, Uezato, Newell, & Frazier, 2008) to maintain the representativity of a clinical sample. Explanatory analyses showed no significant difference between patients with and without a comorbid substance dependency. There were five retrospective exclusions in the MDD group and five in the control group: exclusion of data sets from four patients and the HS due to poor image quality (see below) and one patient developed a manic episode after participation.
Clinical and demographic data can be found in Table 1.
TABLE 1.
Demographic and clinical characteristics of patients with major depressive disorder (MDD) and healthy subjects (HS)
| MDD | HS | p | |
|---|---|---|---|
| (n = 33) | (n = 43) | ||
| Mean (SD) | Mean (SD) | ||
| Age (years) | 37.24 (12.33) | 36.47 (11.21) | .775 |
| Male/female | 18/15 | 31/12 | .113 |
| University entrance diploma/no university entrance diploma | 16/17 | 25/18 | .403 |
| Neurocognition | |||
| Verbal fluency test—animals (correct answers) | 21.85 (6.25) | 24.32 (6.16) | .098 |
| Verbal fluency test—p (correct answers) | 12.18 (4.04) | 12.22 (4.10) | .972 |
| Verbal fluency test—alternating words (correct answers) | 13.27 (4.23) | 16.08 (2.76) | .001 |
| Digit span (total) | 13.97 (4.21) | 14.93 (3.60) | .291 |
| Trail making test (TMT‐B–TMT‐A) | 45.18 (43.48) | 55.34 (50.52) | .709 |
| d2—total number of correct responses | 414.21 (95.68) | 464.31 (85.99) | .032 |
| Psychopathology | |||
| HAMD a | 13.17 (6.13) | – | – |
| Empathy (E scale) | 77.64 (12.91) | 76.10 (13.88) | .787 |
| BAG total | 3.07 (0.46) | 3.31 (0.58) | .057 |
| BAG I (perception) | 3.49 (0.72) | 3.66 (0.70) | .289 |
| BAG II (production) | 2.77 (0.77) | 2.96 (0.94) | .340 |
| BAG III (social production) | 3.65 (0.90) | 4.21 (0.62) | .002 |
| BAG IV (social perception) | 2.26 (0.87) | 2.40 (0.93) | .505 |
Note: d2: d2 test of sustained attention (Schmidt‐Atzert & Brickenkamp, 2017); BAG: brief self‐rating scale for the assessment of individual differences in gesture perception and production (Nagels, Kircher, Steines, Grosvald, & Straube, 2015); HAMD: Hamilton Depression Scale (Hamilton, 1960); Empathy: “questionnaire for the assessment of empathy” (E scale) (Leibetseder, Laireiter, Riepler, & Köller, 2001).
HAMD scores were only acquired in patients with MDD.
All participants gave written informed consent prior to the beginning of the study and received 50 Euro for their participation. The study was approved by the local ethics committee.
2.2. Stimuli
For a complete description of the stimulus set as well as the evaluation and selection procedure, see Nagels, Kircher, Steines, and Straube (2015). Twenty German sentences were presented to the participants as short videos. The grammatical structure was consistent across all sentences: subject–predicate–object. Each sentence was presented once with an iconic co‐speech gesture (IC) and once without any gesture (NG). The co‐speech gesture was performed in a natural way, conform with the content of the sentences, for example, “The man caught a big fish,” while the actor is indicating the size of the fish with his hands. For 0.5 s at the beginning and at the end of each clip the actor neither spoke nor moved. Two cameras simultaneously filmed the actor while speaking, so that only the viewpoint differed between the frontal and the lateral condition (see Figure 1). Four different experimental sets consisting of the same stimuli but in counterbalanced sequential arrangements regarding body orientation and gesture presence were created to reduce sequence effects. Each stimulus set consisted of 80 video clips in total (40 frontal, 40 lateral conditions).
FIGURE 1.
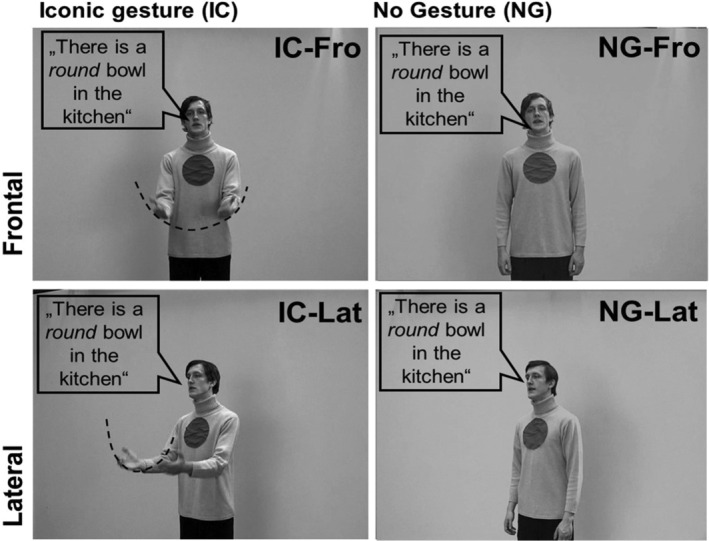
Experimental design and stimuli. Illustration of the four different conditions: (1) a frontal facing body orientation of the actor with an iconic co‐speech gesture (IC‐Fro), (2) a frontal facing body orientation of the actor with no gesture (NG‐Fro), (3) a lateral view of the actor with an iconic co‐speech gesture (IC‐Lat), (4) a lateral view of the actor with no gesture (NG‐Lat; see Nagels, Kircher, Steines, & Straube, 2015). Fro, frontal view; IC, iconic gesture; Lat: lateral view; NG, no gesture
2.3. Experimental design and procedures
Prior to the fMRI scanning procedure, participants saw and evaluated four practice trials (videos were not part of the experiment) to make sure that they understood the task. For the fMRI experiment, MRI‐compatible headphones together with earplugs were used to optimize scanner noise reduction. Stimuli were presented in the middle of the video screen using presentation software (Version 18.3, Neurobehavioral Systems). The 20 videos for each of the four conditions were presented in a pseudorandomized and counterbalanced order across subjects. Subsequent to the presentation of each video, a low‐level baseline with a variable duration of 3,750—6,750 ms (mean = 5,000 ms) followed. This baseline consisted of a blank gray screen. A similar experimental procedure was used in a previous study of ours (for details, see, for example, Straube et al., 2010). For each stimulus, participants were asked to evaluate whether they felt addressed or not, taking into account the whole video. To give their answer, participants were instructed to press a button for “yes” or “no” on an magnetic resonsance (MR)‐compatible response device fixed to their left leg. Thus, feeling addressed resulted in a button press with the left middle finger, not feeling addressed resulted in a left index finger button press. Participants were further instructed to respond directly after the video had disappeared from the screen. For the statistical analysis, the ratings (number of yes responses for the 20 videos per condition) were transformed into percentage of “yes” responses related to all responses of one condition for each subject and condition.
2.4. Behavioral data analyses
A mixed‐design analysis of variance (ANOVA) was performed using IBM SPSS Statistics 22 with the within‐subject factors gesture (IC, NG) and body orientation (Fro, Lat) and the between‐subject factor group (HS vs. MDD) to analyze the addressment ratings. The alpha level was set at 5%, and a Greenhouse–Geisser correction was used whenever necessary.
2.5. fMRI data acquisition
MRI data were collected on a Siemens 3 Tesla MR Magnetom Trio Trim scanner. To reduce motion artifacts, subjects' heads were fixated using foam pads. MR‐compatible headphones were used.
Subsequent to the acquisition of functional data, T1‐weighted high‐resolution images were acquired for each subject. Functional data were acquired using a T2‐weighted echo planar image (EPI) sequence (repetition time = 2,000 ms; echo time = 30 ms; flip angle = 90°). The volume included 33 transversal slides (slice thickness = 3.6 mm; interslice gap = .36 mm; field of view = 230 mm, voxel resolution = 3.6 mm2]. In total, 420 functional volumes were acquired in each subject.
2.6. fMRI data analysis
fMRI data were analyzed with the statistical parametric mapping software (SPM12, v6685, Wellcome Trust Centre for Neuroimaging, London, UK) implemented in MATLAB Version 7.9.0 (release R2009b, The MathWorks, Inc., Natick, MA). The first five images were discarded from the analysis.
All images were scanned for artifacts and movement patterns were displayed before the analysis. Subjects who showed significant artifacts or moved further than 3 mm were excluded from the analysis.
During preprocessing, the images were first realigned to the mean image and then normalized into Montreal Neurological Institute (MNI) space using the unified segmentation routine with the mean EPI image of each subject as “image to align.” The normalized data (resliced voxel size: 2 mm3) were then smoothed with an 8 mm3 Gaussian kernel.
As in previous studies (e.g., Nagels, Kircher, Steines, & Straube, 2015), an event‐related design was used to measure the blood‐oxygen‐level dependent (BOLD) responses for each video belonging to one of the four different experimental conditions (NG‐Fro, NG‐Lat, IC‐Fro, IC‐Lat). In the single subject (first‐level) analysis, the anticipated hemodynamic response at the defined points of speech and gesture co‐occurrence (defined as the onset of the keyword within each sentence) for each event type was modeled by a canonical hemodynamic response function (Friston et al., 1998). The function was convolved with the event sequence, with a fixed event duration of 1 s, and the onsets corresponding to the speech–gesture integration points to fit the stimulus conditions in a general linear model. The fixed event duration of 1 s was selected to get a broader range of data around the assumed time point of speech and gesture processing and has also been applied successfully in previous studies of co‐verbal gesture processing (Green et al., 2009; Kircher et al., 2009; Nagels, Kircher, Steines, & Straube, 2015; Straube et al., 2010; Straube, Green, Weis, Chatterjee, & Kircher, 2009). Movement parameters of each subject were implemented as multiple regressors into the data analyses to correct for head movement during data acquisition.
A flexible‐factorial analysis implemented in SPM 12 was conducted with the four baseline‐contrast images (one for each condition) for each participant within each group (= eight conditions in total). In this group analysis, specific contrasts of interest were defined (see below). Subsequently, a three‐level 2 × 2 × 2 mixed design ANOVA with two within subject factors (gesture, body orientation) and one between‐subject factor (group) was performed in SPSS for analyses with extracted data of cluster activation (eigenvariate).
We performed a Monte–Carlo simulation of the brain volume to establish an appropriate voxel contiguity threshold (Slotnick & Schacter, 2004; see also Green et al., 2009; Kircher et al., 2009; Straube, Green, Chatterjee, et al., 2011). This correction has the advantage of higher sensitivity to smaller effect sizes, while still correcting for multiple comparisons across the whole brain volume (Slotnick, 2017). This procedure was chosen to increase comparability to previous investigations in HS (Nagels, Kircher, Steines, & Straube, 2015).
Assuming an individual voxel Type I error of uncorrected p < .005, a cluster extent of 77 contiguous resampled voxels was indicated as necessary to correct for multiple voxel comparisons at p < .05 (for further information, see Data S1). The reported voxel coordinates of activation peaks are located in MNI space.
For the anatomical localization, functional data were referenced to automatic anatomical labeling (AAL) (Tzourio‐Mazoyer et al., 2002). Complete lists of extended AAL cluster labels for each contrast are provided in Data S1.
2.7. Contrasts of interest
First, main effects for gesture (IC > NG) and body orientation (Fro > Lat) were calculated to detect involved brain regions for the different social cues. T tests for both factors separately were performed with MDD > HS to investigate potential compensation processes and HS > MDD to reveal areas related to dysfunctional processing. Secondly, we performed a conjunction analysis to identify communalities between the groups. Finally, we performed an analysis for a potential interaction effect group × gesture × body orientation (in the flexible‐factorial analyses implemented in SPM 12), to reveal group differences in BOLD signal changes depending on both factors: gesture and body orientation.
3. RESULTS
3.1. Behavioral results
The results of the addressment ratings are presented in Table 2 and Figure 2.
TABLE 2.
Addressment ratings in percentage
| Group | Gesture | Body orientation | M | SE | 95% CI | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| MDD | NG | Fro | 35.30 | 6.58 | 22.1 | 48.4 |
| Lat | 17.42 | 3.69 | 10.1 | 24.8 | ||
| IC | Fro | 75.15 | 4.73 | 65.7 | 84.6 | |
| Lat | 54.85 | 6.42 | 42.1 | 67.6 | ||
| HS | NG | Fro | 44.41 | 5.83 | 32.8 | 56.0 |
| Lat | 10.95 | 3.27 | 4.4 | 17.5 | ||
| IC | Fro | 81.55 | 4.19 | 73.2 | 89.9 | |
| Lat | 43.69 | 5.69 | 32.4 | 55.0 | ||
| Group | Gesture | M | SE | 95% CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| MDD | NG | 26.36 | 3.87 | 18.6 | 34.1 |
| IC | 65.00 | 4.26 | 56.5 | 73.5 | |
| HS | NG | 27.68 | 3.43 | 20.8 | 34.5 |
| IC | 62.62 | 3.77 | 55.1 | 70.1 | |
| Group | Body orientation | M | SE | 95% CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| MDD | Fro | 55.23 | 4.56 | 46.1 | 64.3 |
| Lat | 36.14 | 3.82 | 28.5 | 43.8 | |
| HS | Fro | 62.98 | 4.05 | 54.9 | 71.0 |
| Lat | 27.32 | 3.39 | 20.6 | 34.1 | |
Note: The first part is displaying the descriptive statistics for all conditions separately. In the second part, the gesture conditions were averaged over both body orientation conditions each. The third part was performed equivalently for body orientation.
Abbreviations: CI, confidence interval; Fro, frontal view; HS, healthy subjects; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder.
We found a significant main effect of the factor gesture (F[1, 74] = 66.00; p < .001; η 2 = .471) with a higher frequency of positive addressment ratings for the IC condition (M IC = 65.00%; SE IC = 4.26) than for the condition without gesture (M NG = 26.36%; SE NG = 3.87). There was also a significant main effect of the body orientation (F[1, 74] = 35.03; p < .001; η 2 = .321) that consisted of a higher proportion of addressment ratings for the frontal orientation of the actor (M FRO = 55.23%; SE FRO = 4.56) than for the lateral orientation (M LAT = 36.14%; SE LA T = 3.82).
Furthermore, we found a trend for interaction between group and body orientation (F[1, 74] = 3.631; p = .061, η 2 = .047). There was no significant interaction of group × body orientation × gesture (F[1, 74] = .085; p = .772), nor were there significant interaction effects of gesture × body orientation (F[1, 74] = 1.590; p = .211) or group × gesture (F[1, 74] = .252; p = .617) and the inter subject factor group only (F[1,74] = .021, p = .886).
3.2. fMRI results
3.2.1. Main effect of gesture and group interactions
The main effect of gesture (across groups and body orientations) revealed more BOLD response (further on, the term activation is used to describe the BOLD response) in bilateral occipital, posterior temporal, hippocampal as well as frontal areas including the left IFG for the condition with gesture in comparison to the condition without gesture. Details for the separate groups can be seen in Tables S1 and S2. For an extended list of AAL toolbox cluster labels, see also Data S1.
A conjunction analysis between MDD patients and HS revealed a similar increase of activation in occipital and temporal regions, including the hippocampus, Rolandic operculum, and fusiform gyrus, as well as in frontal regions, for example, the superior, inferior, and middle frontal gyrus and the precentral gyrus in both hemispheres (see Figure 3 and Table 3) for the contrast of gesture (IC > NG).
FIGURE 2.
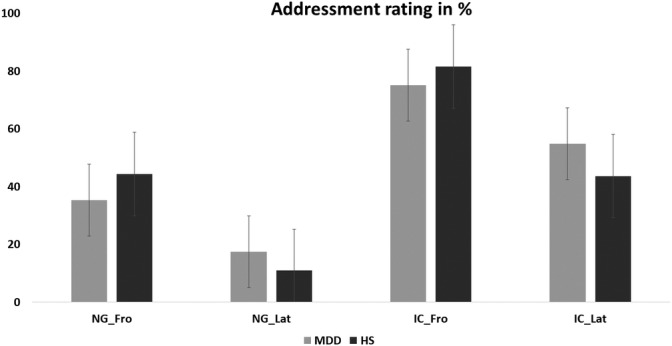
Addressment rating of major depressive disorder (MDD) patients and control group in percentage
TABLE 3.
Group communalities and differences in activation for the main effect of gesture
| Contrast | Anatomical region | Cluster extent | Hem. | No. voxels | t Value | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Group communalities: (HS‐IC > HS‐NG) ∩ (MDD‐IC > MDD‐NG) | Fusiform gyrus | Middle, superior, and inferior temporal gyrus, middle and inferior occipital lobe, Rolandic operculum | R | 12,227 | 22.06 | 50 | −62 | −2 |
| Middle, superior, and inferior occipital lobe, middle and inferior temporal lobe, fusiform gyrus and Cuneus | L | 11,450 | 18.74 | −46 | −72 | 2 | ||
| Hippocampus | Thalamus and lingual gyrus | R | 408 | 6.60 | 16 | −28 | 0 | |
| Precentral gyrus | Middle, superior, and inferior frontal gyrus | R | 982 | 5.32 | 54 | 10 | 38 | |
| Hippocampus | Thalamus and lingual gyrus | L | 304 | 5.02 | −16 | −28 | 0 | |
| Precentral gyrus | Postcentral gyrus, middle, superior, and inferior frontal gyrus | L | 731 | 4.52 | −44 | −2 | 56 | |
| Group differences: (MDD‐IC > MDD‐NG) > (HS‐IC > HS‐NG) | Fusiform Gyrus | Cerebellum | L | 92 | 4.45 | −36 | −46 | −28 |
| Group differences: (HS‐IC > HS‐NG) > (MDD‐IC > MDD‐NG) | Putamen | R | 97 | 3.19 | 26 | −12 | 16 |
Note: Coordinates in Montreal Neurological Institute (MNI) space, cluster extent, and t values of the conjunction and interactions of group by condition. Cluster level corrected at p < .05.
Abbreviations: Fro, frontal view; HS, healthy subjects; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder.
Beside these commonalities, group differences revealed that MDD patients showed stronger activations in the left cerebellum and fusiform gyrus for ICs in comparison to the NG condition, whereas HS activated more in the right putamen (group × gesture interactions; see Table 3). Effect sizes estimations based on the extracted eigenvariate of the respective clusters revealed for the interaction of group × gesture in the cerebellum an effect size of η 2 = .134 and in the right putamen of η 2 = .174.
Correlation analyses for the patient group did not show any significant correlation between BOLD activation and depressive symptoms using the Hamilton Depression Scale (HAMD). There was no correlation between addressment rating and BOLD activation for both groups.
3.2.2. Main effect of body orientation and group interactions
The main effect of body orientation (Fro > Lat; across groups and gesture conditions) revealed more activation for the frontal condition in comparison to the lateral condition in the middle occipital lobe and inferior frontal areas. Among the HS, we found an increased activation for this contrast predominantly in the right fusiform gyrus, left IFG, precentral gyrus, and middle occipital lobe. In addition, MDD patients revealed more activation in bilateral occipital lobe, insula, left hippocampus, and cerebellum for the same contrast. More details can be found in Table S3 and S4.
A conjunction analysis revealed for both groups shared increased activation for a frontal body orientation in comparison to the lateral orientation in the left precentral gyrus, IFG, and right superior and middle occipital gyrus, cuneus, and fusiform gyrus (see Table 4; Figure 4).
TABLE 4.
Group communalities and differences in activation for the main effect of body orientation
| Contrast | Anatomical region | Cluster extent | Hem. | No. voxels | t Value | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Group communalities: (HS‐Fro > HS‐Lat) ∩ (MDD‐Fro > MDD‐Lat) | Fusiform gyrus | Lingual gyrus, Calcarine gyrus, middle and superior occipital gyrus, Cuneus | R | 823 | 9.72 | 22 | −82 | 10 |
| IFG | Precentral gyrus | L | 153 | 3.60 | −42 | 2 | 32 | |
| Group differences: (MDD‐Fro > MDD‐Lat) > (HS‐Fro > HS‐Lat) | SMA | Middle and superior frontal gyrus, ACC, MCC, and SMA | L | 571 | 4.37 | −14 | 30 | 36 |
| Angular gyrus | Middle occipital gyrus, inferior parietal gyrus | R | 324 | 4.32 | 42 | −74 | 32 | |
| ACC | Middle and superior frontal gyrus and MCC | R | 522 | 3.78 | 30 | 22 | 40 | |
| Group differences: (HS‐Fro > HS‐Lat) > (MDD‐Fro > MDD‐Lat) | No significant clusters |
Note: Coordinates in MNI space, cluster extent and t values of the interaction of group by condition. Cluster level corrected at p < .05.
Abbreviations: ACC, anterior cingulate cortex; Fro, frontal view; HS, healthy subjects; IC, iconic gesture; Lat: lateral view; MCC, middle cingulate cortex; MDD, major depressive disorder; MNI, Montreal Neurological Institute; NG, no gesture; SMA, supplementary motor area.
FIGURE 3.

Main effect of gesture (IC > NG) in MDD patients (red), healthy subjects (green) and common activation (yellow) for gesture in patients and healthy subjects (yellow). The bar graph at the bottom of Figure 3 shows the extracted eigenvariate of the right precentral cluster of the conjunction analyses (54, 10, 38). It is representative of all yellow regions demonstrating more activation for the gesture conditions in both groups (HS and MDD). The error bars of the bar graphs are indicating the SE of the mean. Fro, frontal view; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder
Our analyses for a potential interaction (group × body orientation) revealed that MDD patients show higher activations than HS for the contrast of body orientation (Fro > Lat) in bilateral superior and middle frontal gyrus, anterior and middle cingulate cortex in both hemispheres, left SMA, right angular gyrus, right inferior parietal gyrus, and middle occipital gyrus (see Figure 4 and Table 4). Effect sizes estimations based on the extracted eigenvariates of the SFG/MFG/ACC cluster revealed for the interaction of group × body orientation an effect size of η 2 = .174.
There was no significantly increased activation for HS compared to MDD patients for the same contrast (Fro > Lat).
Correlation analyses for the patient groups revealed that BOLD activation in the left ACC/MFG/SMA cluster during the lateral NG condition correlated with depressive mood, measured with the subscale of the HAMD scale (r = −.433, p = .012), suggesting that depressive mood is related to reduced activation during the condition where no addressment cues are provided. There was no correlation between addressment rating and BOLD activation for both groups.
3.2.3. Interaction between group, gesture, and body orientation
Finally, we performed an analysis for an interaction effect between group, gesture, and body orientation (group × gesture × body orientation; for gesture × body orientation across groups, see Data S1).
In the conditions without gesture, MDD patients showed lower activations in comparison to the HS in the left vermis and cerebellum for the frontal condition than in the lateral conditions. The HS revealed lower activation in comparison to MDD patients in left hippocampus, lingual gyrus, precuneus, PCC, SFG, MFG, and bilateral basal ganglia in the frontal body orientation in comparison to the lateral body orientation not using any gesture (see Figure 5 and Table5). Effect sizes estimations based on the extracted eigenvariates of the PCC cluster revealed for the interaction of group × gesture × body orientation an effect size of η 2 = .101.
FIGURE 4.
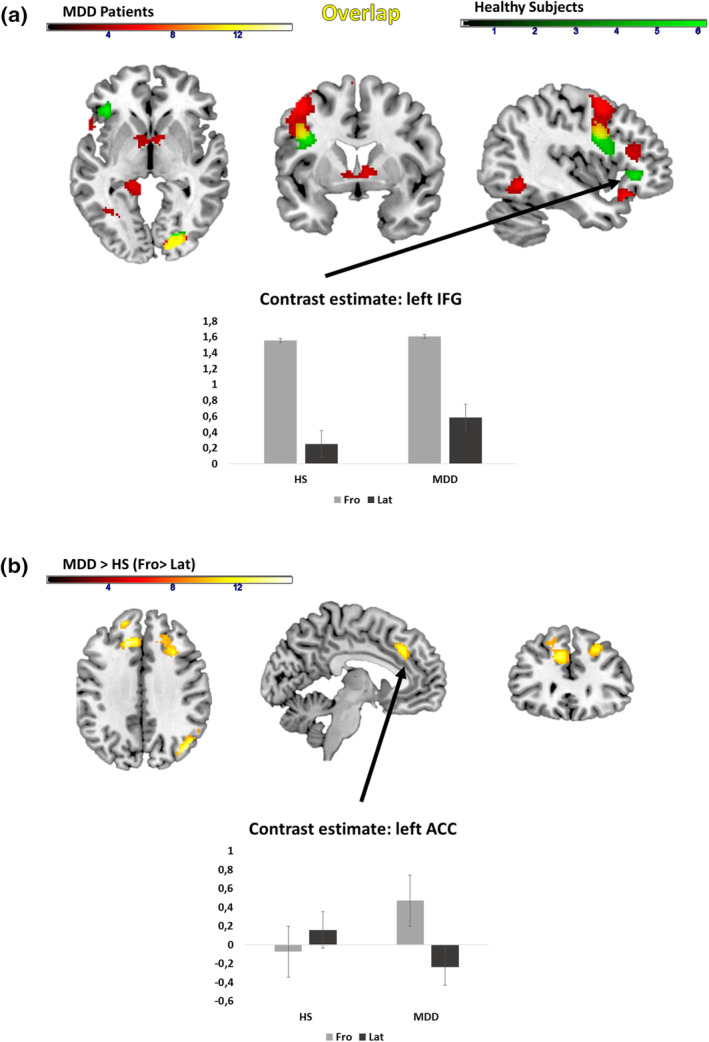
Main effect of orientation in MDD patients (red), healthy subjects (green) and shared activations in MDD patients and healthy subjects (yellow). (a) Conjunction analysis for shared activations between MDD patients and HS. (b) Interaction between group and body orientation. Bar graphs show the extracted eigenvariates of the respective clusters of the conjunction (a) or interaction effect (b). The error bars of the bar graphs are indicating the SE of the mean. Fro, frontal view; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder
TABLE 5.
Interaction between group, body orientation and use of gesture
| Contrast | Anatomical region | Cluster extent | Hem. | No. voxels | t Value | x | y | z |
|---|---|---|---|---|---|---|---|---|
| MDD (IC_Fro > IC_Lat) > (NG_Fro > NG_Lat) > HS (IC_Fro > IC_Lat) > (NG_Fro > NG_Lat) | Cerebellum | Vermis | L | 188 | 3.28 | −12 | −36 | −32 |
| HS (IC_Fro > IC_Lat) > (NG_Fro > NG_Lat) > MDD (IC_Fro > IC_Lat) > (NG_Fro > NG_Lat) | Superior frontal gyrus | Middle frontal gyrus | L | 84 | 3.47 | −26 | 46 | 30 |
| Hippocampus | Lingual gyrus, precuneus, thalamus, PCC, nucleus caudatus | L | 192 | 3.32 | −26 | −42 | 4 | |
| PCC | PCC, thalamus, nucleus caudatus | L | 85 | 3.19 | −12 | −46 | 30 | |
| Thalamus | Putamen, globus pallidus | R | 100 | 3.07 | 4 | −20 | 12 |
Note: Coordinates (in MNI space), cluster extent and t values of the interaction of group by condition. Cluster level corrected at p < .05.
Abbreviations: Fro, frontal view; HS, healthy subjects; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder; MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex.
FIGURE 5.
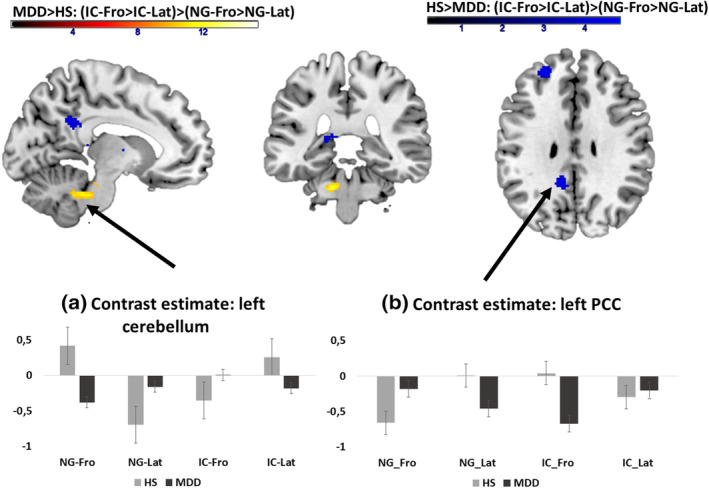
The interaction effect for the factors group × gesture × body orientation in (a) left cerebellum and (b) left posterior cingulate cortex, hippocampus, superior frontal gyrus (SFG), middle frontal gyrus (MFG), and basal ganglia. In the cerebellum, patients showed a higher activation for the condition IC‐Fro in comparison to healthy subjects, whereas they show an increased activation in the PCC for the same condition. Bar graphs show the extracted eigenvariates of the respective clusters, illustrating the activation pattern across conditions for HS (blue) and patients (MDD, red). The error bars of the bar graphs are indicating the SE of the mean. Fro, frontal view; HS, healthy subjects; IC, iconic gesture; Lat: lateral view; NG, no gesture; MDD, major depressive disorder; PCC, posterior cingulate cortex
Using gesture, the pattern was contrariwise: MDD showed higher activation for frontal conditions than lateral conditions and the HS group higher activation for frontal conditions than lateral conditions in the regions described above. Interestingly, the group difference in activation was especially pronounced in the IC‐Fro condition (see Figure 5).
Correlation analyses revealed that BOLD activation of left SFG for the lateral gesture condition (IC‐Lat) correlated with the empathy rating across both groups (r = −.316, p = .005), but not in the patient group (r = −.259, p = .145).
The contrast estimates (extracted eigenvariate) of the PCC cluster for the frontal gesture condition (IC‐Fro) correlated negatively with the HAMD score for depressive mood (r = −.383, p = .028). During the lateral gesture condition contrast estimates of the PCC cluster were related to the post experimental rating (how easy was the task for the participant), indicating that more activity was related to higher ratings of easiness across both groups (r = .290, p = .011).
These data suggest that different aspects of psychopathology are related to the neural processing of body orientation (with or without gesture) in patients with MDD.
There was no correlation between addressment rating and BOLD activation in both groups.
4. DISCUSSION
During social interpersonal interaction multiple cues such as body orientation and gesture can generate a feeling of addressment. Here, we showed for the first time the contribution of body orientation and gesture for the feeling of being addressed in MDD patients. Despite a similar rating and large overlap in activation patterns in MDD and HS for the effect of gesture as well as for body orientation, we found patterns of increased activation in patients (vs. HS) for the frontal body orientation in the right angular gyrus and the bilateral prefrontal cortex including ACC and for gesture in the left fusiform gyurs. Furthermore, our analyses revealed interaction effects with a first hint for a distinguished processing for the integration of social cues. Especially for the most addressing condition IC‐Fro, we found lower activation in PCC and SFG in patients with MDD compared to HS. Hence, we conclude that MDD patients are well capable of recognizing and interpreting social cues though they seem to need more neural resources to evaluate social cues separately, whereas the integration of both cues leads to a lower activation in regions of self‐reference.
Furthermore, our results support the conclusion of previous studies (Holler et al., 2015; Nagels, Kircher, Steines, & Straube, 2015; Saggar, Shelly, Lepage, Hoeft, & Reiss, 2014; Straube et al., 2010), indicating the important role of body orientation and co‐speech gesture for social interaction.
4.1. The neural processing of gesture
In line with studies of gesture processing in HS (Green et al., 2009; Saggar et al., 2014; Straube, Green, Bromberger, & Kircher, 2011), the control group and patient group showed similar activations for the effect of gesture (IC > NG) in regions involved in visual/motion processing (i.e., occipital brain regions) and mentalizing (i.e., frontoparietal areas, precentral cortex and hippocampus). Thus, the general processing of gestures seems to be intact. However, MDD patients showed higher activations in the fusiform gyrus and cerebellum, whereas HS revealed more activation for gesture in the putamen, giving a first hint regarding differential processing of gestures in patients with MDD. Thus, patients recruited more visuomotor‐related areas to process gesture information, suggesting an increased effort to use gesture related information to solve the addressment rating task.
4.2. The neural processing of body orientation
Regarding the neural processing of body orientation, we found higher activations for the frontal conditions in both groups in precentral areas: the left occipital cortex and fusiform gyrus. This supports previous findings (Nagels, Kircher, Steines, & Straube, 2015) that the left fusiform gyrus plays an important role for processing body orientation as a social cue and is consistent with our hypothesis.
A greater activity in precentral areas and SMA for the frontal conditions in comparison to lateral condition may reflect a mentalizing or motor simulation process activated by the evaluation of an addressing frontal body orientation. Interestingly, there was a higher activation in the superior and middle frontal gyrus, cingulate cortex, SMA, and parietooccipital regions for the frontal body orientation in MDD patients compared to HS (see Figure 4), indicating a possible compensation process for mentalizing dysfunctions. The cingulate cortex and the anterior part (ACC) are involved in various processes, such as stimuli assessment (Zysset, Huber, Ferstl, & von Cramon, 2002) and empathy (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Olsson & Ochsner, 2008) and are associated with the processing of social cues (Nagels, Kircher, Steines, & Straube, 2015; Straube et al., 2010). Middle and inferior frontal areas were also found in HS being more activated for frontal body orientation (Straube et al., 2010). In our study, these areas—in addition the right angular gyrus—also showed an increased activation for frontal body orientation in MDD patients in comparison to HS. Thus, MDD patients seem to need higher activation levels in the cingulate cortex, frontal areas, and the right angular gyrus for the interpretation of body orientation to achieve the same behavioral output as HS.
4.3. The interaction of body orientation and gesture
The interaction of body orientation and gesture for patients compared to healthy control subjects revealed more activation in the cerebellum for the IC‐Fro condition but less activation in PCC and prefrontal cortex.
The combination of body orientation and gesture is certainly relevant. However, what is not clear is their meaning regarding the addressment in the conditions NG‐Fro and IC‐Lat. In these two ambiguous conditions, MDD patients showed higher activations in PCC, hippocampus, MFG, SFG, and basal ganglia than HS. As these regions had been considered for attributing mental states to others (Saxe, 2006), increased activity during this task could be necessary for patients to evaluate these ambiguous video conditions and interpret them comparable to HS.
These findings could also be interpreted as a potential neural correlate of the interpretation bias in depression (Everaert, Bronstein, Cannon, & Joormann, 2018; Everaert, Podina, & Koster, 2017). Thus, their difficulties to interpret and dissolve ambiguous social cues may be reflected in their higher activations of the concerned brain regions. Furthermore, the lower activation in PCC and prefrontal cortex for the conditions IC‐Fro and NG‐Lat may reflect a deviation of self‐referential processes (Brewer, Garrison, & Whitfield‐Gabrieli, 2013, 2013; Whitfield‐Gabrieli et al., 2011; Yaoi, Osaka, & Osaka, 2015). Intriguingly, bar graphs indicate that activation seems to be lowest for patients and group differences highest in the condition were both addressment cues are present (IC‐Fro). Thus, compensatory processes for separate processing of gesture and body cues might be necessary as integration of both cues is impaired, as reflected by reduced BOLD response for the facing gesture condition.
Especially in situations with more than one counterpart, it is important to recognize and interpret social cues to feel involved and to address another person correctly. Therefore, we think that being addressed is the first step in a complex social–emotional processing stream leading to functioning communication (Ochsner, 2008). Our data suggest that this first step is already impaired in patients with MDD or, at least, that patients might need more neural resources in order to interpret social communicative cues. Patients with MDD in particular suffer from social isolations and dysfunctional relationships (Coyne, 1976; Hautzinger et al., 1982; Youngren & Lewinsohn, 1980). Even though it remains unclear whether MDD causes or is caused by social isolation (Brakemeier, Normann, & Berger, 2008), there is evidence for the theory that MDD is a learnt reaction to social rejection (Kube et al., 2018) and missing reinforcement in social situations (Airaksinen, Wahlin, Larsson, & Forsell, 2006; Brown, Harris, Hepworth, & Robinson, 1994; Saris et al., 2017; Spijker et al., 2004). Our data cannot disentangle related assumptions; however, we can provide first evidence for differences in neural processing of social cues such as gesture and body orientation, which might contribute to social impairments. Especially the fact that more neural resources seem to be necessary for patients with MDD to evaluate social cues could hint to a higher barrier for successful social interaction. Together, with reduced motivation/drive this effort might often be avoided in natural social context.
5. CONCLUSIONS
Despite a similar rating in MDD and the healthy subject group, several regions including the ACC (for the frontal body orientation condition) appeared more activated in patients than in healthy subjects. Hence, we conclude that MDD patients are well capable of recognizing and interpreting social cues, such as gesture or body orientation, though they seem to need more neural capacity in mentalizing regions to achieve the same cue depended evaluations of their own addressment. These findings are a first indication that MDD patients do not feel less addressed in general but may be more strained while identifying and processing the relevant social cues. This might be especially true for situations with multiple addressment cues, which have to be integrated to induce self‐referential processes along with a feeling of being addressed. Though it must be considered that feeling addressed is only one component of our complex interpersonal communication, it is particularly relevant for being accepted as an interaction partner.
Supporting information
Data S1. Cluster correction
Table S1. Activations for the main effect of gesture in MDD patients
Table S2. Activations for the main effect of gesture in healthy subjects
Table S3. Main effect of body orientation in MDD patients
Table S4. Main effect of body orientation in healthy subjects
Table S5. Main effect of gesture (IC > NG); extended cluster labels
Table S6. Main effect of gesture (NG > IC), extended cluster labels
Table S7. Main effect of body orientation (Fro > Lat); extended cluster labels
Table S8. Main effect of body orientation (Lat > Fro); extended cluster labels
Table S9. Conjunction analysis for the main effect of gesture (IC > NG); extended cluster labels
Table S10. Conjunction analysis of the main effect of body orientation (Fro > Lat); extended cluster labels
Table S11. Interaction between group (MDD > HS) and body orientation(Fro > Lat); extended cluster labels
Table S12. Interaction between group (HS > MDD) and gesture (IC > NG); extended cluster labels
Table S13. Interaction between group (MDD > HS) and gesture (IC > NG); extended cluster labels
Table S14. Interaction between body orientation (Fro > Lat) and gesture (IC > NG); extended cluster labels
Table S15. Main effect of body orientation (Lat > Fro) (extended cluster labels)
Table S16. Interaction between group (HS > MDD), gesture (IC > NG) and body orientation (Fro>Lat); extended cluster labels
Table S17. Interaction between group (MDD > HS), gesture (IC > NG) and body orientation (Fro > Lat); extended cluster labels
ACKNOWLEDGMENT
This research project is supported by a grant from the “Deutsche Forschungsgemeinschaft” (project no. DFG: Ki 588/6‐2; STR 1146/11‐2; B. S. is funded by STR 1146/15‐1) and the Rhön clinic (project no. FI_3).
Suffel A, Nagels A, Steines M, Kircher T, Straube B. Feeling addressed! The neural processing of social communicative cues in patients with major depression. Hum Brain Mapp. 2020;41:3541–3554. 10.1002/hbm.25027
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Number: DFG STR 1146/11‐2; KI 588/6‐2
DATA AVAILABILITY STATEMENT
The data that support the findings on the level of anonymised preprocessed data and group analyses of this study are available on request from the corresponding author, B. S. The raw data are not publicly available due to ethical restrictions (participants' privacy).
REFERENCES
- Airaksinen, E. , Wahlin, A. , Larsson, M. , & Forsell, Y. (2006). Cognitive and social functioning in recovery from depression: Results from a population‐based three‐year follow‐up. Journal of Affective Disorders, 96(1–2), 107–110. 10.1016/j.jad.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Bora, E. , & Berk, M. (2016). Theory of mind in major depressive disorder: A meta‐analysis. Journal of Affective Disorders, 191, 49–55. 10.1016/j.jad.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Brakemeier, E.‐L. , Normann, C. , & Berger, M. (2008). Atiopathogenese der unipolaren depression. Neurobiologische und psychosoziale Faktoren [The etiopathogenesis of unipolar depression. Neurobiological and psychosocial factors]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, 51(4), 379–391. 10.1007/s00103-008-0505-x [DOI] [PubMed] [Google Scholar]
- Brewer, J. A. , Garrison, K. A. , & Whitfield‐Gabrieli, S. (2013). What about the "self" is processed in the posterior cingulate cortex? Frontiers in Human Neuroscience, 7, 647 10.3389/fnhum.2013.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G. W. , Harris, T. O. , Hepworth, C. , & Robinson, R. (1994). Clinical and psychosocial origins of chronic depressive episodes. II. A patient enquiry. The British Journal of Psychiatry: The Journal of Mental Science, 165(4), 457–465. [DOI] [PubMed] [Google Scholar]
- Bucci, S. , Startup, M. , Wynn, P. , Baker, A. , & Lewin, T. J. (2008). Referential delusions of communication and interpretations of gestures. Psychiatry Research, 158(1), 27–34. 10.1016/j.psychres.2007.07.004 [DOI] [PubMed] [Google Scholar]
- Carpenter, W. T. (2017). Social withdrawal as psychopathology of mental disorders. Neuroscience and Biobehavioral Reviews, 97, 85–86. 10.1016/j.neubiorev.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Carr, L. , Iacoboni, M. , Dubeau, M.‐C. , Mazziotta, J. C. , & Lenzi, G. L. (2003). Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5497–5502. 10.1073/pnas.0935845100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro, A. , Becchio, C. , Colle, L. , Bara, B. G. , & Walter, H. (2014). Do you mean me? Communicative intentions recruit the mirror and the mentalizing system. Social Cognitive and Affective Neuroscience, 9(7), 909–916. 10.1093/scan/nst062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. C. (1976). Toward an interactional description of depression. Psychiatry, 39(1), 28–40. [DOI] [PubMed] [Google Scholar]
- Davis, L. , Uezato, A. , Newell, J. M. , & Frazier, E. (2008). Major depression and comorbid substance use disorders. Current Opinion in Psychiatry, 21(1), 14–18. 10.1097/YCO.0b013e3282f32408 [DOI] [PubMed] [Google Scholar]
- Ehnvall, A. , Mitchell, P. B. , Hadzi‐Pavlovic, D. , Parker, G. , Frankland, A. , Loo, C. , … Perich, T. (2014). Rejection sensitivity and pain in bipolar versus unipolar depression. Bipolar Disorders, 16(2), 190–198. 10.1111/bdi.12147 [DOI] [PubMed] [Google Scholar]
- Everaert, J. , Bronstein, M. V. , Cannon, T. D. , & Joormann, J. (2018). Looking through tinted glasses: Depression and social anxiety are related to both interpretation biases and inflexible negative interpretations. Clinical Psychological Science, 6(4), 517–528. 10.1177/2167702617747968 [DOI] [Google Scholar]
- Everaert, J. , Podina, I. R. , & Koster, E. H. W. (2017). A comprehensive meta‐analysis of interpretation biases in depression. Clinical Psychology Review, 58, 33–48. 10.1016/j.cpr.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Falkai P., & Döpfner M. (Eds.). (2015). Diagnostisches und statistisches Manual psychischer Störungen—DSM‐5®. Göttingen: Hogrefe; Retrieved from. http://sub-hh.ciando.com/book/?bok_id=1792418 [Google Scholar]
- Fitzgerald, P. B. , Laird, A. R. , Maller, J. , & Daskalakis, Z. J. (2008). A meta‐analytic study of changes in brain activation in depression. Human Brain Mapping, 29(6), 683–695. 10.1002/hbm.20426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Fletcher, P. , Josephs, O. , Holmes, A. , Rugg, M. D. , & Turner, R. (1998). Event‐related fMRI: Characterizing differential responses. NeuroImage, 7(1), 30–40. 10.1006/nimg.1997.0306 [DOI] [PubMed] [Google Scholar]
- Germine, L. T. , Garrido, L. , Bruce, L. , & Hooker, C. (2011). Social anhedonia is associated with neural abnormalities during face emotion processing. NeuroImage, 58(3), 935–945. 10.1016/j.neuroimage.2011.06.059 [DOI] [PubMed] [Google Scholar]
- Green, A. , Straube, B. , Weis, S. , Jansen, A. , Willmes, K. , Konrad, K. , & Kircher, T. (2009). Neural integration of iconic and unrelated coverbal gestures: A functional MRI study. Human Brain Mapping, 30(10), 3309–3324. 10.1002/hbm.20753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames, J. L. , Hagan, C. R. , & Joiner, T. E. (2013). Interpersonal processes in depression. Annual Review of Clinical Psychology, 9, 355–377. 10.1146/annurev-clinpsy-050212-185553 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger, M. , Linden, M. , & Hoffman, N. (1982). Distressed couples with and without a depressed partner: An analysis of their verbal interaction. Journal of Behavior Therapy and Experimental Psychiatry, 13(4), 307–314. [DOI] [PubMed] [Google Scholar]
- Holler, J. , Kokal, I. , Toni, I. , Hagoort, P. , Kelly, S. D. , & Özyürek, A. (2015). Eye'm talking to you: Speakers' gaze direction modulates co‐speech gesture processing in the right MTG. Social Cognitive and Affective Neuroscience, 10(2), 255–261. 10.1093/scan/nsu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler, J. , Schubotz, L. , Kelly, S. , Hagoort, P. , Schuetze, M. , & Özyürek, A. (2014). Social eye gaze modulates processing of speech and co‐speech gesture. Cognition, 133(3), 692–697. 10.1016/j.cognition.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Holt‐Lunstad, J. , Smith, T. B. , Baker, M. , Harris, T. , & Stephenson, D. (2015). Loneliness and social isolation as risk factors for mortality: A meta‐analytic review. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 10(2), 227–237. 10.1177/1745691614568352 [DOI] [PubMed] [Google Scholar]
- Hsu, D. T. , Sanford, B. J. , Meyers, K. K. , Love, T. M. , Hazlett, K. E. , Walker, S. J. , … Zubieta, J.‐K. (2015). It still hurts: Altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Molecular Psychiatry, 20(2), 193–200. 10.1038/mp.2014.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, Y. , Yamada, K. , & Kanba, S. (2006). Deficit in theory of mind is a risk for relapse of major depression. Journal of Affective Disorders, 95(1–3), 125–127. 10.1016/j.jad.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Kazdin, A. E. , Sherick, R. B. , Esveldt‐Dawson, K. , & Rancurello, M. D. (1985). Nonverbal behavior and childhood depression. Journal of the American Academy of Child Psychiatry, 24(3), 303–309. 10.1016/s0002-7138(09)61091-8 [DOI] [PubMed] [Google Scholar]
- Kircher, T. , Straube, B. , Leube, D. , Weis, S. , Sachs, O. , Willmes, K. , … Green, A. (2009). Neural interaction of speech and gesture: Differential activations of metaphoric co‐verbal gestures. Neuropsychologia, 47(1), 169–179. 10.1016/j.neuropsychologia.2008.08.009 [DOI] [PubMed] [Google Scholar]
- Koster‐Hale, J. , Richardson, H. , Velez, N. , Asaba, M. , Young, L. , & Saxe, R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others' beliefs. NeuroImage, 161, 9–18. 10.1016/j.neuroimage.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube, T. , Siebers, V. H. A. , Herzog, P. , Glombiewski, J. A. , Doering, B. K. , & Rief, W. (2018). Integrating situation‐specific dysfunctional expectations and dispositional optimism into the cognitive model of depression—A path‐analytic approach. Journal of Affective Disorders, 229, 199–205. 10.1016/j.jad.2017.12.082 [DOI] [PubMed] [Google Scholar]
- Kupferberg, A. , Bicks, L. , & Hasler, G. (2016). Social functioning in major depressive disorder. Neuroscience and Biobehavioral Reviews, 69, 313–332. 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Lai, C.‐H. , Wu, Y.‐T. , & Hou, Y.‐M. (2017). Functional network‐based statistics in depression: Theory of mind subnetwork and importance of parietal region. Journal of Affective Disorders, 217, 132–137. 10.1016/j.jad.2017.03.073 [DOI] [PubMed] [Google Scholar]
- Leibetseder, M. , Laireiter, A.‐R. , Riepler, A. , & Köller, T. (2001). E‐Skala: Fragebogen zur Erfassung von Empathie—Beschreibung und psychometrische Eigenschaften. Zeitschrift für Differentielle und Diagnostische Psychologie, 22(1), 70–85. 10.1024//0170-1789.22.1.70 [DOI] [Google Scholar]
- Mars, R. B. , Neubert, F.‐X. , Noonan, M. P. , Sallet, J. , Toni, I. , & Rushworth, M. F. S. (2012). On the relationship between the "default mode network" and the "social brain". Frontiers in Human Neuroscience, 6, 189 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels, A. , Kircher, T. , Steines, M. , Grosvald, M. , & Straube, B. (2015). A brief self‐rating scale for the assessment of individual differences in gesture perception and production. Learning and Individual Differences, 39, 73–80. 10.1016/j.lindif.2015.03.008 [DOI] [Google Scholar]
- Nagels, A. , Kircher, T. , Steines, M. , & Straube, B. (2015). Feeling addressed! The role of body orientation and co‐speech gesture in social communication. Human Brain Mapping, 36(5), 1925–1936. 10.1002/hbm.22746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. (2008). The social‐emotional processing stream: Five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry, 64(1), 48–61. 10.1016/j.biopsych.2008.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson, A. , & Ochsner, K. N. (2008). The role of social cognition in emotion. Trends in Cognitive Sciences, 12(2), 65–71. 10.1016/j.tics.2007.11.010 [DOI] [PubMed] [Google Scholar]
- Potthoff, D. , & Seitz, R. J. (2015). Role of the first and second person perspective for control of behaviour: Understanding other people's facial expressions. Journal of Physiology, Paris, 109(4–6), 191–200. 10.1016/j.jphysparis.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Rubinow, D. R. , & Post, R. M. (1992). Impaired recognition of affect in facial expression in depressed patients. Biological Psychiatry, 31(9), 947–953. [DOI] [PubMed] [Google Scholar]
- Saggar, M. , Shelly, E. W. , Lepage, J.‐F. , Hoeft, F. , & Reiss, A. L. (2014). Revealing the neural networks associated with processing of natural social interaction and the related effects of actor‐orientation and face‐visibility. NeuroImage, 84, 648–656. 10.1016/j.neuroimage.2013.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris, I. M. J. , Aghajani, M. , van der Werff, S. J. A. , van der Wee, N. J. A. , & Penninx, B. W. J. H. (2017). Social functioning in patients with depressive and anxiety disorders. Acta Psychiatrica Scandinavica, 136(4), 352–361. 10.1111/acps.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe, R. (2006). Uniquely human social cognition. Current Opinion in Neurobiology, 16(2), 235–239. 10.1016/j.conb.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Saxe, R. , & Powell, L. J. (2006). It's the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science, 17(8), 692–699. 10.1111/j.1467-9280.2006.01768.x [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Timmermans, B. , Reddy, V. , Costall, A. , Bente, G. , Schlicht, T. , & Vogeley, K. (2013). Toward a second‐person neuroscience. The Behavioral and Brain Sciences, 36(4), 393–414. 10.1017/S0140525X12000660 [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Wohlschlaeger, A. M. , Kraemer, N. C. , Newen, A. , Shah, N. J. , Fink, G. R. , & Vogeley, K. (2006). Being with virtual others: Neural correlates of social interaction. Neuropsychologia, 44(5), 718–730. 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Schmidt‐Atzert, L. , & Brickenkamp, R. (2017). Test d2‐R ‐ Elektronische Fassung des Aufmerksamkeits‐ und Konzentrationstests d2‐R Göttingen: Hogrefe; https://www.testzentrale.de/shop/elektronische-fassung-des-aufmerksamkeits-und-konzentrationstests.html. [Google Scholar]
- Slotnick, S. D. (2017). Cluster success: fMRI inferences for spatial extent have acceptable false‐positive rates. Cognitive Neuroscience, 8(3), 150–155. 10.1080/17588928.2017.1319350 [DOI] [PubMed] [Google Scholar]
- Slotnick, S. D. , & Schacter, D. L. (2004). A sensory signature that distinguishes true from false memories. Nature Neuroscience, 7(6), 664–672. 10.1038/nn1252 [DOI] [PubMed] [Google Scholar]
- Spijker, J. , de Graaf, R. , Bijl, R. V. , Beekman, A. T. F. , Ormel, J. , & Nolen, W. A. (2004). Determinants of persistence of major depressive episodes in the general population. Results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Journal of Affective Disorders, 81(3), 231–240. 10.1016/j.jad.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Spunt, R. P. , Satpute, A. B. , & Lieberman, M. D. (2011). Identifying the what, why, and how of an observed action: An fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience, 23(1), 63–74. 10.1162/jocn.2010.21446 [DOI] [PubMed] [Google Scholar]
- Straube, B. , Green, A. , Bromberger, B. , & Kircher, T. (2011). The differentiation of iconic and metaphoric gestures: Common and unique integration processes. Human Brain Mapping, 32(4), 520–533. 10.1002/hbm.21041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube, B. , Green, A. , Chatterjee, A. , & Kircher, T. (2011). Encoding social interactions: The neural correlates of true and false memories. Journal of Cognitive Neuroscience, 23(2), 306–324. 10.1162/jocn.2010.21505 [DOI] [PubMed] [Google Scholar]
- Straube, B. , Green, A. , Jansen, A. , Chatterjee, A. , & Kircher, T. (2010). Social cues, mentalizing and the neural processing of speech accompanied by gestures. Neuropsychologia, 48(2), 382–393. 10.1016/j.neuropsychologia.2009.09.025 [DOI] [PubMed] [Google Scholar]
- Straube, B. , Green, A. , Sass, K. , Kirner‐Veselinovic, A. , & Kircher, T. (2013). Neural integration of speech and gesture in schizophrenia: Evidence for differential processing of metaphoric gestures. Human Brain Mapping, 34(7), 1696–1712. 10.1002/hbm.22015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube, B. , Green, A. , Weis, S. , Chatterjee, A. , & Kircher, T. (2009). Memory effects of speech and gesture binding: Cortical and hippocampal activation in relation to subsequent memory performance. Journal of Cognitive Neuroscience, 21(4), 821–836. 10.1162/jocn.2009.21053 [DOI] [PubMed] [Google Scholar]
- Surguladze, S. A. , Young, A. W. , Senior, C. , Brébion, G. , Travis, M. J. , & Phillips, M. L. (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology, 18(2), 212–218. 10.1037/0894-4105.18.2.212 [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wang, Y.‐G. , Wang, Y.‐Q. , Chen, S.‐L. , Zhu, C.‐Y. , & Wang, K. (2008). Theory of mind disability in major depression with or without psychotic symptoms: A componential view. Psychiatry Research, 161(2), 153–161. 10.1016/j.psychres.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Wheatley, T. , Milleville, S. C. , & Martin, A. (2007). Understanding animate agents: Distinct roles for the social network and mirror system. Psychological Science, 18(6), 469–474. 10.1111/j.1467-9280.2007.01923.x [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , Moran, J. M. , Nieto‐Castañón, A. , Triantafyllou, C. , Saxe, R. , & Gabrieli, J. D. E. (2011). Associations and dissociations between default and self‐reference networks in the human brain. NeuroImage, 55(1), 225–232. 10.1016/j.neuroimage.2010.11.048 [DOI] [PubMed] [Google Scholar]
- Yaoi, K. , Osaka, M. , & Osaka, N. (2015). Neural correlates of the self‐reference effect: Evidence from evaluation and recognition processes. Frontiers in Human Neuroscience, 9, 383 10.3389/fnhum.2015.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren, M. A. , & Lewinsohn, P. M. (1980). The functional relation between depression and problematic interpersonal behavior. Journal of Abnormal Psychology, 89(3), 333–341. [DOI] [PubMed] [Google Scholar]
- Zysset, S. , Huber, O. , Ferstl, E. , & von Cramon, D. Y. (2002). The anterior frontomedian cortex and evaluative judgment: An fMRI study. NeuroImage, 15(4), 983–991. 10.1006/nimg.2001.1008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Cluster correction
Table S1. Activations for the main effect of gesture in MDD patients
Table S2. Activations for the main effect of gesture in healthy subjects
Table S3. Main effect of body orientation in MDD patients
Table S4. Main effect of body orientation in healthy subjects
Table S5. Main effect of gesture (IC > NG); extended cluster labels
Table S6. Main effect of gesture (NG > IC), extended cluster labels
Table S7. Main effect of body orientation (Fro > Lat); extended cluster labels
Table S8. Main effect of body orientation (Lat > Fro); extended cluster labels
Table S9. Conjunction analysis for the main effect of gesture (IC > NG); extended cluster labels
Table S10. Conjunction analysis of the main effect of body orientation (Fro > Lat); extended cluster labels
Table S11. Interaction between group (MDD > HS) and body orientation(Fro > Lat); extended cluster labels
Table S12. Interaction between group (HS > MDD) and gesture (IC > NG); extended cluster labels
Table S13. Interaction between group (MDD > HS) and gesture (IC > NG); extended cluster labels
Table S14. Interaction between body orientation (Fro > Lat) and gesture (IC > NG); extended cluster labels
Table S15. Main effect of body orientation (Lat > Fro) (extended cluster labels)
Table S16. Interaction between group (HS > MDD), gesture (IC > NG) and body orientation (Fro>Lat); extended cluster labels
Table S17. Interaction between group (MDD > HS), gesture (IC > NG) and body orientation (Fro > Lat); extended cluster labels
Data Availability Statement
The data that support the findings on the level of anonymised preprocessed data and group analyses of this study are available on request from the corresponding author, B. S. The raw data are not publicly available due to ethical restrictions (participants' privacy).


