Abstract
Introduction
Infertility is mediated by several changes system‐wide. These changes are likely to cause other systems‐related pathologies, such as changes in systemic immune response, particularly inflammatory response can lead to cardiovascular diseases and breast cancer.
Methods
These morbidities can exist immediately or years after the diagnosis of infertility. Therefore, understanding the mechanism is important to move toward therapeutic interventions.
Results
Several extragonadal pathologies are reported due to infertility, as well as, how these might also contribute to reproductive disabilities. Detailed evidence are still not present that can give stronger result.
Conclusion
This review highlights some of the most frequent comorbidities that are seen in infertile women, hence requiring a need for complete clinical screening and care, as well as diagnosis and treatment in early stages.
Keywords: extragonadal comorbidities, infertile women, infertility
Several extragonadal pathologies are reported due to infertility, as well as how these might also contribute to reproductive disabilities. Women presenting subfertility or infertility need to be screened for abovementioned disorder. Adaptions in lifestyle might subsidize these effects.

Abbreviations
- ASA
anti‐sperm antibodies
- cGMP
cyclic guanosine monophosphate
- DHEAS
dehydroepiandrosterone sulfate
- GLP
glucagon‐like peptide
- IFN‐γ
interferon gamma
- MCP
monocyte chemoattractant protein‐1
- PCOS
polycystic ovary syndrome
- PDE
cyclic nucleotide phosphodiesterase
- SCF
stem cell factor
- SM
sphingomyelin
- TGF‐β
transforming growth factor‐β
- TNF‐α
tumor necrosis factor‐α
- VAT
visceral adipocyte tissues
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Up to 18% of general population is prone to infertility. 1 However, fewer studies target coexisting pathologies with infertility. Infertility is not a remote pathology and it can proffer effects on various systems in the body. Women infertility can have several causes, such as polycystic ovary syndrome (PCOS), endometriosis, tubal blockage, and hydrosalpinges. 2 , 3 , 4 Despite, availability of diversity of treatment options, prevalence extragonadal pathologies might influence the treatment outcomes for infertility. 5 , 6
Certain dietary intake can also elevate the risk to attain infertility. 7 A study reports genital infections like those in vagina, uterus, and ovaries that greatly contributes to infertility rather than cardiovascular factors. 8
PCOS is characterized by various phenotypes (Figure 1) and thereby existence of comorbidities varies according to each category. In general, these women present elevated body mass index (BMI), follicle count and duration of menstrual cycles, hyperlipidemia, hyperandrogenism, insulin resistance, inflammation, and alterations in the morphology of ovaries. 9
Figure 1.
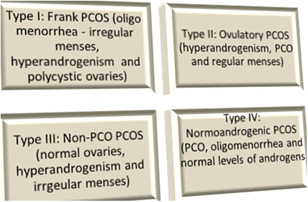
Various phenotypes of polycystic ovary syndrome
Similarly, endometriosis is an inflammatory disease, which is seen as the growth of endometrium tissues outside the uterus, where nearly half of such cases results in infertility. Several causes have been suggested, including housing of endometrial piece during menstruation near fallopian tube and immune dysfunction. 10 Common symptoms are seen in the form of pelvic pain, absence of menses, increase in the levels of estrogens, and abnormal growth of endometrium. As the core of the disease, immune system plays a chief role in infertility, particularly due to inhibition of the activity of lymphocytes, natural killer (in peritoneal cavity) and cytotoxic T cell and aggravated macrophage and inflammatory response. 11 Furthermore, the disturbance in production of cytokines, chemokines, and hormones has also found to worsen the disease. 12 Abnormal production of uterine natural killer cells results in decrease in stem cell factor (SCF) in endometriosis, leading to infertility. 13 Alteration in the Th‐1/Th‐2 skew, elevation of Th‐1 producing tumor necrosis factor‐alpha (TNF‐α), interleukin‐2 (IL‐2), and IL‐4 from Th‐2 promotes development of the pathology. This is mediated by the overexpression of T‐bet transcription factor leading to overproduction of Th‐1 and GATA3 for that of Th‐2, whereas decrease in the Foxp3‐caused Treg cells is inspected in endometriosis infertile women. 14 , 15 Nonetheless, in later phase of the development of endometriosis, endocrine and immune systems, together, are thought to play their role in the development of the disease. 16
In addition, immune system is also chiefly involved in unexplained infertility. Treg cells are thought to play critical immunosuppressive role for fetus. Decrease in the levels of CD4+, CD25+, Foxp3 cells, transforming growth factor‐β (TGF‐β), lymphocyte adhesion, and chemotaxis are associated with idiopathic infertility. Additionally, anti‐sperm antibodies (ASA) can elicit immunity in both, men and women. Blood barrier safeguards exposure of immune cells with ASA in men, whereas, immunoregulatory mechanism of vagina and cervix play protective role in females. Sperm entering women with ASA are prone to be phagocytized. Also, presence of antibodies against antigen in seminal fluid also can lead to infertility. 17
Other alterations are also reported in immune system due to chronic inflammation, as a result of infertility. This includes IL‐4 and ‐6, IFN‐γ (interferon gamma), and TNF‐α levels greater than control. 18
This review is designed to highlight the studies and evidence present in regard to infertility and its extragonadal manifestations. Severity of the disease might call for the need of medical screening and subsequent treatment. We have discussed the systemic changes that can lead to prevalence of the diseases mentioned below.
2. CARDIOVASCULAR DISEASES, METABOLIC SYNDROME, AND DIABETES MELLITUS
Infertility in women is strongly linked with the development of metabolic syndrome (dyslipidemia, hypertension, insulin resistance, and obesity) and various cardiovascular abnormalities 15 . Even in women with infertility to certain extent and frequent miscarriages, the risk of cardiomyopathy is substantial. 19
Numerous studies have shown incidence of cardiovascular diseases (CVDs) in infertile couples. They possess artherogenic characteristics such as increased serum cholesterol, triglycerides, C‐reactive protein (inflammation marker), reduction in high‐density lipoproteins, homocysteine, vascular endothelial growth factor (VEGF), and endothelial plasminogen activator inhibitor, 20 which can be plausible outcomes of infertility‐mediated oxidative stress, increased levels of antimullerian hormone, 21 or reduction in estrogen levels. 22 To it, anomalies in menstrual cycles and infertility due to ovarian disorders 23 also increase the risk of CVD, with or without hyperglycemia. 24 , 25 In a studies, it is also seen that cardiovascular disorders, mostly increased lipid prolife, 26 adds to the risk to gestational diabetes, increased birth weight (above 4 kg, macrosomia), pre‐eclampsia and impairment in the release of bile from the liver. High‐density lipoprotein, on the other hand, provides health benefits against these. 27 , 28 Abnormalities in childhood lipid profiles are also related to prospective pregnancy complications and sterility. 28 , 29 Endometriosis, as a consequence of chronic inflammation, can lead to angina, coronary artery disease, hypertension, hypercholesterolemia, and myocardial infraction. 30 , 31 Abnormalities in endometrium are noted due to diabetes, obesity, hyperglycemia, and hyperlipidemia. 32
PCOS patients also appear to have increased levels of palmitoyl sphingomyelin (SM), cyclic guanosine monophosphate (cGMP), and dehydroepiandrosterone sulfate (DHEAS). 33 Elevated levels of sphingolipids are also one of the significant findings in CVD. They induce inflammatory response, plaque formation, valvular impairment, and ischemic heart disease. 34 Nonetheless, contradictory outcomes are reported about association of DHEA with CVD. 35 Up to 40% of PCOS patients present metabolic syndrome. These patients may present insulin resistance along with obesity. 19 Cyclic nucleotide phosphodiesterase (PDE) is also involved in second messenger cGMP signaling. Deficiency of its isoforms, causing breakage of cell cycle, is reported to be linked with development of smooth muscles‐related CVDs such as atherosclerosis and development of metabolic syndrome. PDE3A (isoform) knockout mice, along with cardiovascular abnormalities also is deficit of matured oocytes and are barren. 36
Flow‐mediated dilation (FMD), referring to the widening in the diameter of artery due to increased flow rate, is one of the significant markers of atherosclerosis due to endothelial dysfunction. 37 Decrease in FMD in PCOS patients has been reported in several studies. Meta‐analysis concluded that more than 20% of these patients were at the risk of developing CVD due to a decrease in FMD. This might be due to visceral adiposity, oxidative stress, inflammatory response, and reduction of nitric oxide (NO) levels. 38 Several evidence are presented in regard to the role of NO is PCOS. Krishna et al 39 provided a detailed study concerning the role of NO and endothelial dysfunction in PCOS women. Owing to exacerbated inflammatory response and downregulated Treg immune action, studied revealed that decrease in NO levels is mediated by the downregulation of the enzyme involved in the suppression on the levels of l‐arginine (precursor of NO) by reducing the transcripts of the enzymes that arbitrate biochemical conversions and arginine transporter of arginine (cationic amino acid transporter). Additionally, increase in arginine degrader and asymmetric dimethylarginine (competitive inhibitor) also adds to this facilitates reduction in nitrite and nitrate (end products of NO). Conversely, treating PCOS women with clomiphene along with nitric oxide increases the chances of conceiving. 40 However, contradictory animal models have been presented. 41
Correspondingly, several studies have pointed out toward the concomitance of obesity, insulin resistance, and other metabolic disorders in all four phenotypes of PCOS. 26 Hyperinsulinemia is characterized by excessive release of luteinizing hormone and obesity in polycystic ovary (PCO) patients independent of other factors. 42 Insulin resistance is these patients is also depicted by the enhancement of inflammation (IL‐6 and TNF‐α), glycosylation end‐product leukocyte adhesion, and mitochondrial oxidative stress. 20 , 43 Additionally, mutations in the mitochondrial RNA is also associated severity of the symptoms. 44 Treatment of oxidative stress leads to reduction in apoptosis and has therapeutic potencies in this area. 45
Phenotype I is characterized by highest levels of fasting insulin, hypervolemic ovaries with greatest prevalence of cysts whereas, IV exhibits highest levels of luteinizing hormone (LH). 46 , 47 Bil et al 48 showed in a study that phenotype I and II are at the greater risk to develop metabolic syndrome as compared with the other phenotypes, owing to the visceral adiposity index. Obesity in PCOS women is evident due to hyperandrogensim. It is established that cytokines released from adipocytes, adipokines particularly adiponectin, play significant role. Its expression inversely regulates adiposity, inflammation, lipid profile, and levels of glucose and insulin. 49 Reduction in the levels of high molecular adiponectin is associated with cardiovascular disorder in type II diabetic patients. 50 Recent study has shown that this isoform is also involved in the comorbidities of polycystic ovaries due to elevated sympathetic activity. 51 Due to vast amount of evidence in relation with insulin resistance and hyperglycemia, studies have vividly shown the risk of diabetes embedded in infertile patients particularly due to tubal blockade and oligo or anovulation. 52 Early animal studies have shown that expression of Cdk4 in beta islet of pancreas and pituitary gland is involved in anterior pituitary development, affecting secretion of prolactin. Complete absence of transcripts can lead to diabetes and infertility, concurrently. 53
Additionally, raised levels of androgens in PCOS women are also seen by the reduction in the expression of adiponectin receptors. This is likely due to the impairment in the release of androgens by theca cells mediated by low levels of circulating adiponectin. 49 Visceral adiposity in affected women is seen perceived by the increase in expression of chemerin and lipocalin‐2 (types of adipokines) in visceral adipocyte tissues (VAT), which is normally seen in men and opposite in healthy women 54 (Figure 2).
Figure 2.
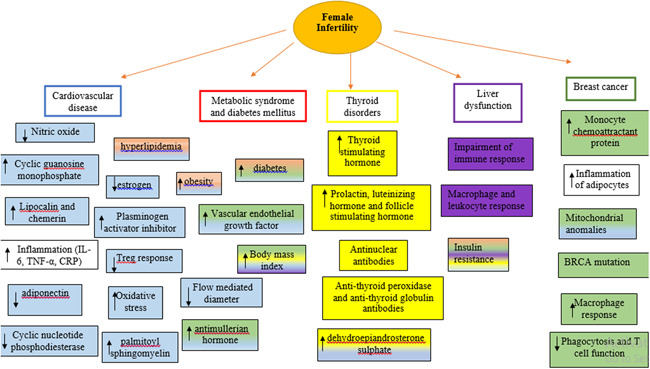
Effects of extragonadal comorbidities in relation to infertility. Figure shows changes that can lead that are seen in case of infertility and respective pathological condition (blue color: cardiovascular disease, red color: metabolic syndrome and diabetes, yellow color: thyroid dysfunction, purple color: liver disease, green color: breast cancer). BRCA, breast related cancer antigen; CRP, C‐reactive protein; IL‐6, interleukin 6; TNF‐α, tumor necrosis factor‐alpha
3. THYROID DYSFUNCTION
Alteration in the levels of thyroid hormone is strongly related to female infertility. Hypothyroidism (elevated thyroid stimulating hormone, TSH) is also characterized by hyperprolactemia (increase in serum prolactin levels). 55 Quintino‐Moroet al 56 provided a study on Graves' disease (GD) and Hashimoto's thyroiditis (HT) and onset of infertility. It was found that approximately 50% of women presenting GD and HT are sterile, especially those aged greater than 35 years. Presence of antithyroid peroxidase and antithyroid globulin antibodies leads to the destruction of the gland. These autoimmune antibodies are likely to cause abortion and can chiefly contribute to infertility. 57 Hypothyroidism is prevalent in infertile women and strongly affects the ability to conceive. Patients with hyperprolactemia and hypothyroidism responds significantly to thyroid hormone treatment and are able to conceive. 58 , 59 Increase in the production of prolactin and TSH can lead to excessive production of luteinizing and follicle‐stimulating hormone, DHEA, and accumulation of collagen in ovaries; hence, directing the formation of cysts in ovaries. Coexistence of chronic lymphocytic thyroiditis and PCOS and goiter and PCOS is also of greater prevalence than controls. 60 Correlation between these pathological conditions can be comprehended by various evidence. Primarily, obesity and insulin resistance strongly associate with them. Insulin resistance suppresses deiodinase‐2 activity in pituitary gland, henceforth, decreasing T3, elevating TSH, and eliciting inflammatory response. Besides, obesity causes a rise in thyrotropin‐releasing hormone, advancing hypothyroidism. 60 Presence of autoantibodies, antinuclear antibodies in PCOS patients, might as well cause autoimmune thyroid disease. 61 , 62
Women with adenomyosis are also at higher risk to develop thyroid cancer 63 while on the other hand, hypothyroidism can lead to the endometrial cancer 64 (Figure 2).
4. BREAST CANCER
Breast cancer is the second most recurrent type of cancer, dominant in women. Its association with infertility is studied widely with both, positive and negative, outcomes been strongly evident.
Breast cancer is associated with obesity and insulin resistance by immune‐system‐mediated hyperinsulinemia in adipocytes. Hyperproduction of macrophages instigates inflammatory response, as described above. M1 phenotype (inflammatory activity) is seen in the initial stage of tumorigenesis. Later macrophages switch to M2 phenotype—tumor‐associated macrophages (anti‐inflammatory and regulatory) and expresses VEGF, fibroblast growth factor, and monocyte chemoattractant protein‐1 (MCP). M1 also impedes action of insulin, leading to insulin resistance, diabetes mellitus, and obesity. 65 In breast cancer, it inhibits phagocytosis and T‐cell function and promotes angiogenesis. 66 To it, adipose tissue inflammation also corresponds to the inflammation in mammary glands. 67 Contribution of estrogen is also noteworthy in breast cancer due to the activation of proinflammatory cytokines and adipokines. 68 Adding to all these indications, it can be determined that infertility‐related pathogenesis such as PCOS, add significance to the incidence of breast cancer. However, studies have failed to find significant relationship. 69 , 70 , 71 Assistive reproductive technology is also chiefly associated with the development of breast cancer due to induction of hormones. 72 Women with mutations in BRAC (breast cancer) genes are seen to have decreased ovarian reserved and response rate as compared with controls, marked by the reduction in anti‐Müllerian hormone concentrations. 73 , 74 , 75 Likewise, women with endometriosis also possess risk of acquiring breast cancer although, in this case as well, convincing evidence are not present. 76 Studies have also depicted the interrelation between ovarian and breast cancer. BRCA1 mutation (ex9‐12del) is associated with 35% and 29% of ovarian and breast cancer, respectively. 77 Mutations in these genes are also seen to cause miscarriages and decrease the chances of having children, 78 due to its expression in germline cells and blastocysts. In addition, impairment of BRCA‐mediated DNA double‐stranded repair machine in oocytes can lead to the aging of eggs and alleviate follicular reserve 79 (Figure 2).
5. LIVER DISEASE
Nonalcoholic fatty acid liver disease (NAFLD) includes several diseases such as fibrosis, cirrhosis, hepatic steatosis, and liver cancer and mostly coexist with insulin resistance, oxidative stress, mitochondrial anomalies, obesity, diabetes, and metabolic syndrome 80 . It can also lead to cardiovascular morbidities. 81 Reduction in the levels of adipokines, instigation of local and systemic inflammatory response by adipocytes, macrophages, and leukocytes play critical role in the pathogenesis of NAFLD. 82 Al‐Jaroudi et al 83 studied the prevalence of NAFLD in PCO women and found that 60% of women presenting polycystic ovaries had liver disease with an increase in BMI and hyperlipidemia. Hyperandrogensim in PCOS is also correlated with NAFLD. 84 Ovariectomy in endometrial cancer patients increases the risk of developing NAFLD, proportional to the increase in period between the operation and the development of the disease (up to 40% in 5 years of the surgery) along with the occurrence of diabetes and insulin resistance. 85
Hepatitis B virus (HBV) leads to infertility directed by tubal and uterine causes. Impaired immune response is supposedly one of the noteworthy linkage between the two. 86 Hepatitis can also affect the success of in vitro fertilization (IVF). 87 , 88
Glucagon‐like peptide (GLP) regulates secretion of insulin and maintains the balance between the requirement of sugar in the body by causing excess to be converted into glycogen. Its deficiency can lead to diabetes mellitus (type II) and recent case report has stated that infertile PCOS women when treated with GLP showed improvement in insulin resistance and ovarian function, with successful pregnancy. 89 It also enhances hypothalamus‐pituitary‐mediated gonadal functions by increasing the secretion of luteinizing hormone. 90 Similarly, lipin 1, expressed in liver, regulates the levels of estrogen. Animal models have shown that reduction in the levels of lipin is seen in diabetic mice with impaired fertility and elevated serum estrogen levels 91 (Figure 2).
6. PSYCHOLOGICAL STRESS
Inability to conceive can impose psychological changes in sterile people.
Recent systematic review concluded that infertile women has greater prevalence of psychiatric disorders than controls. 1 These women show a decrease in the ability to cope with the stress (resilience). 92
Estrogen is widely studied for its role in temperamental changes. It is evident that it plays a significant role in regulation of mood and acts as antidepressants. 93 Women perceiving assisted reproductive technology (ART) as the source to conceive child also present pretreatment depression/major depressive disorder, which impacts the success of the procedure. 94 , 95 Up to 60% of sterile women present depression, obsession, paranoia, and anxiety, especially those at younger age and in women. 96 , 97 , 98 To it, miscarriages can also cause posttraumatic stress disorder. Psychological consultation is likely to improve chances of being pregnant and improve mental well‐being of infertile patients. 99 Mind and body program, writing, and mindfulness are also effective in reducing this stress, thereby increasing the rate of pregnancy through IVF. 100 , 101 , 102
Interestingly, stress can also induce reproductive dysfunction. Individual presented with long‐term stress are at greater incidence to face infertility‐related outcomes as compared with controls. In addition, people suffering from infertility, seeking ART for fertilization also undergo financial and emotional anxiety. It results in overall decline in the success rate of IVF. Stress can lead to reduction in fertility by mediating the release of gonadotrophin‐inhibiting hormone, increasing the activity of sympathetic, and non‐sympathetic system leading to the release of corticotrophin‐releasing hormone and glucocorticoids, which can affect follicular growth, suppression of gonadotrophin‐releasing hormone by GABA (gamma aminobutyric acid) along with decline in kisspeptin expression. Besides, ghrelin (increased in response to psychological stress) also plays a significant role in the release of LH, ovarian development and steroidogenesis, and pregnancy. 103 Figure 3 summarizes how stress poses effects on fertility.
Figure 3.
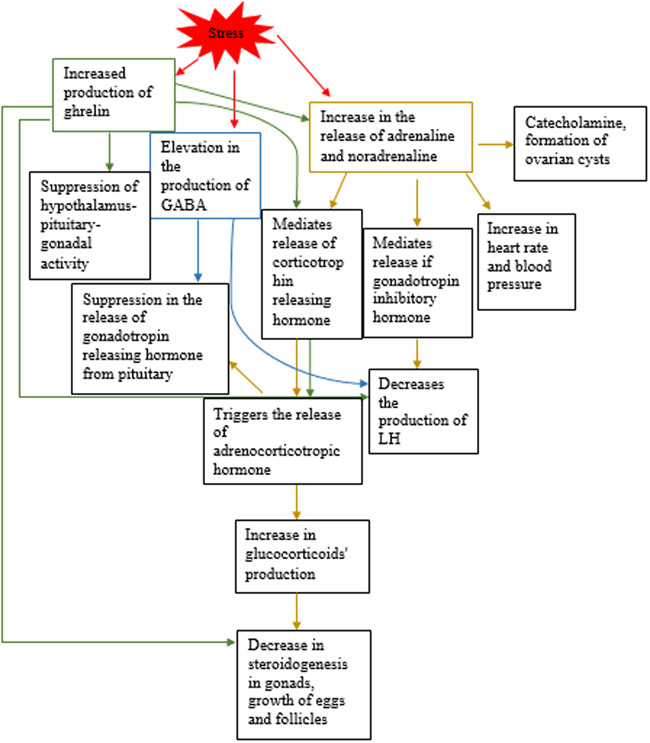
Stress induces the production of ghrelin (green), GABA (blue), and adrenaline and noradrenaline (yellow). The figure shows how they pose effects on reproductive system and have potencies to cause reproductive dysfunction. GABA, gamma aminobutyric acid; LH, luteinizing hormone
7. CONCLUSION
Several extragonadal pathologies are reported due to infertility, as well as, how these might also contribute to reproductive disabilities. Detailed evidence are still not present that can give stronger result. However, women presenting subfertility or infertility need to be screened for abovementioned disorder. Adaptions in lifestyle might minimize these effects. Additionally, treatment of these disease might aid betterment in ART results and cause pregnancy. Similarly, treating grounds of infertility, such as PCOS, might also diminish presentation of these morbidities.
CONFLICTS OF INTEREST
All the fees provided by research center fund and deployed accordingly.
AUTHOR CONTRIBUTIONS
NL: conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. NK: designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. YK: coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ETHICS STATEMENT
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Lorzadeh N, Kazemirad N, Kazemirad Y. Human immunodeficiency: Extragonadal comorbidities of infertility in women. Immun Inflamm Dis. 2020;8:447–457. 10.1002/iid3.327
REFERENCES
- 1. Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility‐associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34(2):167‐177. 10.1007/s10815-016-0836-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fadhlaoui A, Bouquet de la Jolinière J, Feki A. Endometriosis and infertility: how and when to treat? Front Surg. 2014;1:24 10.3389/fsurg.2014.00024 24‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics. 2015;70(11):765‐769. 10.6061/clinics/2015(11)09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharya S, Johnson N, Tijani HA, Hart R, Pandey S, Gibreel AF. Female infertility. BMJ Clin Evid. 2010;2010:0819 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21406133 https://www.ncbi.nlm.nih.gov/pmc/PMC3217752/ [PMC free article] [PubMed] [Google Scholar]
- 5. Fica S, Albu A, Constantin M, Dobri GA. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1(4):415‐422. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20108521 https://www.ncbi.nlm.nih.gov/pmc/PMC3018970/ [PMC free article] [PubMed] [Google Scholar]
- 6. Lorzadeh N, Kazemirad N. Infertility in Light of in vitro Fertilization and Intracytoplasmic Sperm Injection: Treatments and Associated Outcomes. Current Women's Health Reviews. 2020;16 10.2174/1573404816999200511102307. [Google Scholar]
- 7. Rossi BV, Abusief M, Missmer SA. Modifiable risk factors and infertility: what are the connections? Am J Lifestyle Med. 2016;10(4):220‐231. 10.1177/1559827614558020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tao X, Ge S‐Q, Chen L, Cai L‐S, Hwang M‐F, Wang C‐L. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population‐based nested controlled study. Clinics. 2018;73:e364 10.6061/clinics/2018/e364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark NM, Podolski AJ, Brooks ED, et al. Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology: an assessment of over 100 consecutive women self‐reporting features of polycystic ovary syndrome. Reprod Sci. 2014;21(8):1034‐1043. 10.1177/1933719114522525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lorzadeh N, Kazemirad N. The Role of Natural Killer Cells and Mast Cells in Female Infertility and Associated Treatment Outcomes. Current Women's Health Reviews. 20020;16(2):102–106. 10.2174/1573404816666200206111550. [DOI] [Google Scholar]
- 11. Lorzadeh N, Kazemirad N. Application of stem cells to infertility treatment with emphasis on mesenchymal stem cells and ovarian stem cells. American Journal of Perinatology. 2018;35(12):1142–1147. 10.1055/s-0038-1646948. [DOI] [PubMed] [Google Scholar]
- 12. Lin YH, Chen YH, Chang HY, Au HK, Tzeng CR, Huang YH. Chronic niche inflammation in endometriosis‐associated infertility: current understanding and future therapeutic strategies. Int J Mol Sci. 2018;19(8):2385 10.3390/ijms19082385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thiruchelvam U, Wingfield M, O'Farrelly C. Increased uNK progenitor cells in women with endometriosis and infertility are associated with low levels of endometrial stem cell factor. Am J Reprod Immunol. 2016;75(4):493‐502. 10.1111/aji.12486 [DOI] [PubMed] [Google Scholar]
- 14. Koval HD, Chopyak VV, Kamyshnyi OM, Kurpisz MK. Transcription regulatory factor expression in T‐helper cell differentiation pathway in eutopic endometrial tissue samples of women with endometriosis associated with infertility. Cent Eur J Immunol. 2018;43(1):90‐96. 10.5114/ceji.2018.74878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghafarzadeh M, Shakarami A, Yari F, Namdari P. The comparison of side effects of methyldopa, amlodipine, and metoprolol in pregnant women with chronic hypertension. Hypertension in Pregnancy. 2020;1–5. 10.1080/10641955.2020.1766489. [DOI] [PubMed] [Google Scholar]
- 16. Burns KA, Thomas SY, Hamilton KJ, Young SL, Cook DN, Korach KS. Early endometriosis in females is directed by immune‐mediated estrogen receptor alpha and IL‐6 cross‐talk. Endocrinology. 2018;159(1):103‐118. 10.1210/en.2017-00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brazdova A, Senechal H, Peltre G, Poncet P. Immune aspects of female infertility. Int J Fertil Steril. 2016;10(1):1‐10. 10.22074/ijfs.2016.4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trunov A, Obukhovа O, Gorbenko O, Shvayk A, Trunovа L. Cytokines and infertility influence of cytokines and local inflammation in women of reproductive age with infertility. J Cytokine Biol. 2016;1(1):102 10.4172/2576-3881.1000102 [DOI] [Google Scholar]
- 19. Park K, Wei J, Minissian M, Bairey Merz CN, Pepine CJ. Adverse pregnancy conditions, infertility, and future cardiovascular risk: implications for mother and child. Cardiovasc Drugs Ther. 2015;29(4):391‐401. 10.1007/s10557-015-6597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bajuk Studen K, Pfeifer M. Cardiometabolic risk in polycystic ovary syndrome. Endocr Connect. 2018;7(7):R238‐r251. 10.1530/ec-18-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verit FF, Akyol H, Sakar MN. Low antimullerian hormone levels may be associated with cardiovascular risk markers in women with diminished ovarian reserve. Gynecol Endocrinol. 2016;32(4):302‐305. 10.3109/09513590.2015.1116065 [DOI] [PubMed] [Google Scholar]
- 22. Verit FF, Keskin S, Omer B, Yalcinkaya S, Sakar N. Is there any relationship between cardiovascular risk markers and young women with diminished ovarian reserve? Gynecol Endocrinol. 2014;30(10):697‐700. 10.3109/09513590.2014.922948 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Xiong X, Bazzano L, Harville EW. Childhood cardiovascular health and subfertility: the Bogalusa Heart Study. Pediatr Res. 2018;84(5):625‐631. 10.1038/s41390-018-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verit FF. High sensitive serum C‐reactive protein and its relationship with other cardiovascular risk factors in normoinsulinemic polycystic ovary patients without metabolic syndrome. Arch Gynecol Obstet. 2010;281(6):1009‐1014. 10.1007/s00404-009-1226-6 [DOI] [PubMed] [Google Scholar]
- 25. Verit FF, Yildiz Zeyrek F, Zebitay AG, Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med. 2017;44(1):28‐32. 10.5653/cerm.2017.44.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shakarami A, Ghafarzadeh M, Yari F, Fathi L. Association between maternal serum uric acid and preeclampsia. Archives of Physiology and Biochemistry. 2020;1–4. 10.1080/13813455.2020.1773863. [DOI] [PubMed] [Google Scholar]
- 27. Jin W‐Y, Lin S‐L, Hou R‐L, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population‐based study from China. BMC Pregnancy Childbirth. 2016;16:60 10.1186/s12884-016-0852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Guan Q, Zhao J, et al. Association of maternal serum lipids at late gestation with the risk of neonatal macrosomia in women without diabetes mellitus. Lipids Health Dis. 2018;17(1):78 10.1186/s12944-018-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wallace M, Bazzano L, Chen W, Harville E. Maternal childhood cardiometabolic risk factors and pregnancy complications. Ann Epidemiol. 2017;27(7):429‐434. 10.1016/j.annepidem.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension. 2017;70(1):59‐65. 10.1161/hypertensionaha.117.09056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257‐264. 10.1161/CIRCOUTCOMES.115.002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozdemir S, Batmaz G, Ates S, Celik C, Incesu F, Peru C. Relation of metabolic syndrome with endometrial pathologies in patients with abnormal uterine bleeding. Gynecol Endocrinol. 2015;31(9):725‐729. 10.3109/09513590.2015.1058355 [DOI] [PubMed] [Google Scholar]
- 33. Fan X, Jiang J, Huang Z, et al. UPLC/Q‐TOF‐MS based plasma metabolomics and clinical characteristics of polycystic ovarian syndrome. Mol Med Rep. 2019;19(1):280‐292. 10.3892/mmr.2018.9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kikas P, Chalikias G, Tziakas D. Cardiovascular implications of sphingomyelin presence in biological membranes. Eur Cardiol. 2018;13(1):42‐45. 10.15420/ecr.2017:20:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu T‐T, Chen Y, Zhou Y, et al. Prognostic value of dehydroepiandrosterone sulfate for patients with cardiovascular disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6(5):e004896 10.1161/JAHA.116.004896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begum N, Shen W, Manganiello V. Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: implications in cardiovascular diseases and infertility. Curr Opin Pharmacol. 2011;11(6):725‐729. 10.1016/j.coph.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maruhashi T, Soga J, Fujimura N, et al. Relationship between flow‐mediated vasodilation and cardiovascular risk factors in a large community‐based study. Heart. 2013;99(24):1837‐1842. 10.1136/heartjnl-2013-304739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sprung VS, Atkinson G, Cuthbertson DJ, et al. Endothelial function measured using flow‐mediated dilation in polycystic ovary syndrome: a meta‐analysis of the observational studies. Clin Endocrinol. 2013;78(3):438‐446. 10.1111/j.1365-2265.2012.04490.x [DOI] [PubMed] [Google Scholar]
- 39. Krishna MB, Joseph A, Thomas PL, Dsilva B, Pillai SM, Laloraya M. Impaired arginine metabolism coupled to a defective redox conduit contributes to low plasma nitric oxide in polycystic ovary syndrome. Cell Physiol Biochem. 2017;43(5):1880‐1892. 10.1159/000484107 [DOI] [PubMed] [Google Scholar]
- 40. Mahran A, Abdelmeged A, Shawki H, Moheyelden A, Ahmed AM. Nitric oxide donors improve the ovulation and pregnancy rates in anovulatory women with polycystic ovary syndrome treated with clomiphene citrate: a RCT. Int J Reprod Biomed. 2016;14(1):9‐14. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27141543 https://www.ncbi.nlm.nih.gov/pmc/PMC4837925/ [PMC free article] [PubMed] [Google Scholar]
- 41. Hassani F, Karami M, D P, Jalali Nadoushan MR, Yazdi PE. Nitric oxide‐induced polycystic ovaries in the wistar rat. Int J Fertil Steril. 2012;6(2):111‐116. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25493168 https://www.ncbi.nlm.nih.gov/pmc/PMC4258239/ [PMC free article] [PubMed] [Google Scholar]
- 42. Wu S, Divall S, Nwaopara A, et al. Obesity‐induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63(4):1270‐1282. 10.2337/db13-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Victor VM, Rovira‐Llopis S, Banuls C, et al. Insulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: role of myeloperoxidase. PLOS One. 2016;11(3):e0151960 10.1371/journal.pone.0151960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mitochondrial tRNA(Leu(UUR)) C3275T, tRNA(Gln) T4363C and tRNA(Lys) A8343G mutations may be associated with PCOS and metabolic syndrome. Gene. 2018;642:299‐306. 10.1016/j.gene.2017.11.049 [DOI] [PubMed] [Google Scholar]
- 45. Ding Y, Jiang Z, Xia B, Zhang L, Zhang C, Leng J. Mitochondria‐targeted antioxidant therapy for an animal model of PCOS‐IR. Int J Mol Med. 2019;43(1):316‐324. 10.3892/ijmm.2018.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alizadeh R, Fard ZA. Renal impairment and analgesia: From effectiveness to adverse effects. Journal of cellular physiology. 2019;234(10):17205–17211. 10.1002/jcp.28506. [DOI] [PubMed] [Google Scholar]
- 47. Al‐Jefout M, Alnawaiseh N, Al‐Qtaitat A. Insulin resistance and obesity among infertile women with different polycystic ovary syndrome phenotypes. Sci Rep. 2017;7(1):5339 10.1038/s41598-017-05717-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bil E, Dilbaz B, Cirik DA, Ozelci R, Ozkaya E, Dilbaz S. Metabolic syndrome and metabolic risk profile according to polycystic ovary syndrome phenotype. J Obstet Gynaecol Res. 2016;42(7):837‐843. 10.1111/jog.12985 [DOI] [PubMed] [Google Scholar]
- 49. Comim FV, Hardy K, Franks S. Adiponectin and its receptors in the ovary: further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLOS One. 2013;8(11):e80416 10.1371/journal.pone.0080416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horáková D, Azeem K, Benešová R, et al. Total and high molecular weight adiponectin levels and prediction of cardiovascular risk in diabetic patients. Int J Endocrinol. 2015;2015:545068 10.1155/2015/545068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shorakae S, Abell SK, Hiam DS, et al. High‐molecular‐weight adiponectin is inversely associated with sympathetic activity in polycystic ovary syndrome. Fertil Steril. 2018;109(3):532‐539. 10.1016/j.fertnstert.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 52. Tobias DK, Gaskins AJ, Missmer SA, et al. History of infertility and risk of type 2 diabetes mellitus: a prospective cohort study. Diabetologia. 2015;58(4):707‐715. 10.1007/s00125-015-3493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin J, Hunt SL, Dubus P, et al. Genetic rescue of Cdk4 null mice restores pancreatic beta‐cell proliferation but not homeostatic cell number. Oncogene. 2003;22(34):5261‐5269. 10.1038/sj.onc.1206506 [DOI] [PubMed] [Google Scholar]
- 54. Martínez‐García MÁ, Montes‐Nieto R, Fernández‐Durán E, Insenser M, Luque‐Ramírez M, Escobar‐Morreale HF. Evidence for masculinization of adipokine gene expression in visceral and subcutaneous adipose tissue of obese women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2013;98(2):E388‐E396. 10.1210/jc.2012-3414 [DOI] [PubMed] [Google Scholar]
- 55. Sharma N, Baliarsingh S, Kaushik GG. Biochemical association of hyperprolactinemia with hypothyroidism in infertile women. Clin Lab. 2012;58(7‐8):805‐810. [PubMed] [Google Scholar]
- 56. Quintino‐Moro A, Zantut‐Wittmann DE, Tambascia M, Machado Hd C, Fernandes A. High prevalence of infertility among women with Graves' disease and Hashimoto's thyroiditis. Int J Endocrinol. 2014;2014:982705 10.1155/2014/982705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhong YP, Ying Y, Wu HT, et al. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9(2):121‐125. 10.7150/ijms.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Priya DM, Akhtar N, Ahmad J. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Indian J Endocrinol Metab. 2015;19(4):504‐506. 10.4103/2230-8210.159058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verma I, Sood R, Juneja S, Kaur S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int J Appl Basic Med Res. 2012;2(1):17‐19. 10.4103/2229-516x.96795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singla R, Gupta Y, Khemani M, Aggarwal S. Thyroid disorders and polycystic ovary syndrome: an emerging relationship. Indian J Endocrinol Metab. 2015;19(1):25‐29. 10.4103/2230-8210.146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Samsami Dehaghani A, Karimaghaei N, Parsanezhad ME, Malekzadeh M, Mehrazmay M, Erfani N. Anti‐nuclear antibodies in patients with polycystic ovary syndrome before and after laparoscopic electrocauterization. Iran J Med Sci. 2013;38(2 suppl):187‐190. [PMC free article] [PubMed] [Google Scholar]
- 62. Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res. 2014;2014:150239 10.1155/2014/150239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeh CC, Su FH, Tzeng CR, Muo CH, Wang WC. Women with adenomyosis are at higher risks of endometrial and thyroid cancers: A population‐based historical cohort study. PLOS One. 2018;13(3):e0194011 10.1371/journal.pone.0194011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Y, Zhou R, Wang J. Relationship between hypothyroidism and endometrial cancer. Aging Dis. 2019;10(1):190‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rose DP, Gracheck PJ, Vona‐Davis L. The interactions of obesity, inflammation and insulin resistance in breast cancer. Cancers. 2015;7(4):2147‐2168. 10.3390/cancers7040883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP. Tumor‐associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev. 2018;70:178‐189. 10.1016/j.ctrv.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 67. Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4(7):1021‐1029. 10.1158/1940-6207.Capr-11-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity‐associated cancer. Clin Cancer Res. 2013;19(22):6074‐6083. 10.1158/1078-0432.Ccr-12-2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ding DC, Chen W, Wang JH, Lin SZ. Association between polycystic ovarian syndrome and endometrial, ovarian, and breast cancer: a population‐based cohort study in Taiwan. Medicine. 2018;97(39):e12608 10.1097/md.0000000000012608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kok VC, Tsai HJ, Su CF, Lee CK. The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis: a population‐based study. Int J Gynecol Cancer. 2015;25(6):968‐976. 10.1097/igc.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 71. Shobeiri F, Jenabi E. The association between polycystic ovary syndrome and breast cancer: a meta‐analysis. Obstet Gynecol Sci. 2016;59(5):367‐372. Retrieved from http://synapse.koreamed.org/DOIx.php?id=10.5468%2Fogs.2016.59.5.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reigstad MM, Larsen IK, Myklebust TA, et al. Risk of breast cancer following fertility treatment—a registry based cohort study of parous women in Norway. Int J Cancer. 2015;136(5):1140‐1148. 10.1002/ijc.29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240‐244. 10.1200/jco.2009.24.2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Phillips KA, Collins IM, Milne RL, et al. Anti‐Mullerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31(5):1126‐1132. 10.1093/humrep/dew044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang ET, Pisarska MD, Bresee C, et al. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102(6):1723‐1728. 10.1016/j.fertnstert.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pontikaki A, Sifakis S, Spandidos DA. Endometriosis and breast cancer: a survey of the epidemiological studies. Oncol Lett. 2016;11(1):23‐30. 10.3892/ol.2015.3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Villarreal‐Garza C, Alvarez‐Gomez RM, Perez‐Plasencia C, et al. Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico. Cancer. 2015;121(3):372‐378. 10.1002/cncr.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith KR, Hanson HA, Hollingshaus MS. BRCA1 and BRCA2 mutations and female fertility. Curr Opin Obstet Gynecol. 2013;25(3):207‐213. 10.1097/GCO.0b013e32835f1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA‐mutated breast cancer patients. Cancer Treat Rev. 2017;59:61‐70. 10.1016/j.ctrv.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 80. Aghsaeifard Z, Alizadeh R, Bagheri N. Association between neutrophil gelatinase‐associated lipocalin (NGAL) and iron profile in chronic renal disease. Archives of physiology and biochemistry. 2020;1–5. 10.1080/13813455.2020.1720742. [DOI] [PubMed] [Google Scholar]
- 81. Okubo H, Kushiyama A, Nakatsu Y, et al. Roles of gut‐derived secretory factors in the pathogenesis of non‐alcoholic fatty liver disease and their possible clinical applications. Int J Mol Sci. 2018;19(10):3064 10.3390/ijms19103064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9(4):387 10.3390/nu9040387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Al‐Jaroudi D, Jamaat S, Kaddour O, Wani T, Al‐Badr A. Non‐alcoholic fatty liver disease in infertile women with polycystic ovarian syndrome: a prospective series. Int J Women's Health Wellness. 2017;3(1):047. [Google Scholar]
- 84. Rocha ALL, Faria LC, Guimaraes TCM, et al. Non‐alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta‐analysis. J Endocrinol Invest. 2017;40(12):1279‐1288. 10.1007/s40618-017-0708-9 [DOI] [PubMed] [Google Scholar]
- 85. Matsuo K, Gualtieri MR, Cahoon SS, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause. 2016;23(2):189‐196. 10.1097/gme.0000000000000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lao TT, Mak JSM, Li TC. Hepatitis B virus infection status and infertility causes in couples seeking fertility treatment‐indicator of impaired immune response? Am J Reprod Immunol. 2017;77(4):e12636 10.1111/aji.12636 [DOI] [PubMed] [Google Scholar]
- 87. Shaw‐Jackson C, Capraro M, Ameye L, et al. In vitro fertilization for women infected by hepatitis C virus: a matched case‐control study and a systematic literature review. J Assist Reprod Genet. 2017;34(5):587‐597. 10.1007/s10815-017-0892-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shi L, Liu S, Zhao W, Zhou H, Ren W, Shi J. Hepatitis B virus infection reduces fertilization ability during in vitro fertilization and embryo transfer. J Med Virol. 2014;86(7):1099‐1104. 10.1002/jmv.23908 [DOI] [PubMed] [Google Scholar]
- 89. Yang Q, Wang F. Successful pregnancy after improving insulin resistance with the glucagon‐like peptide‐1 analogue in a woman with polycystic ovary syndrome: a case report and review of the literature. Gynecol Obstet Invest. 2016;81(5):477‐480. 10.1159/000446951 [DOI] [PubMed] [Google Scholar]
- 90. Mallo F, González‐Matías LC, Romaní‐Pérez M, Outeiriño‐Iglesias V, Vigo E. GLP‐1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology. 2015;156(11):4226‐4237. 10.1210/en.2014-1978%JEndocrinology [DOI] [PubMed] [Google Scholar]
- 91. Gowri PM, Sengupta S, Katzenellenbogen BS, Bertera S. Lipin1 regulation by estrogen in uterus and liver: implications for diabetes and fertility. Endocrinology. 2007;148(8):3685‐3693. 10.1210/en.2006-1728 [DOI] [PubMed] [Google Scholar]
- 92. Sexton MB, Byrd MR, von Kluge S. Measuring resilience in women experiencing infertility using the CD‐RISC: examining infertility‐related stress, general distress, and coping styles. J Psychiatr Res. 2010;44(4):236‐241. 10.1016/j.jpsychires.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 93. Dumas JA, Albert KM, Naylor MR, Sites CK, Benkelfat C, Newhouse PA. The effects of age and estrogen on stress responsivity in older women. Am J Geriatr Psychiatry. 2012;20(9):734‐743. 10.1097/JGP.0b013e31825c0a14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Holley SR, Pasch LA, Bleil ME, Gregorich S, Katz PK, Adler NE. Prevalence and predictors of major depressive disorder for fertility treatment patients and their partners. Fertil Steril. 2015;103(5):1332‐1339. 10.1016/j.fertnstert.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sejbaek CS, Hageman I, Pinborg A, Hougaard CO, Schmidt L. Incidence of depression and influence of depression on the number of treatment cycles and births in a national cohort of 42,880 women treated with ART. Hum Reprod. 2013;28(4):1100‐1109. 10.1093/humrep/des442 [DOI] [PubMed] [Google Scholar]
- 96. Karimzadeh M, Salsabili N, Akbari Asbagh F, Teymouri R, Pourmand G, Soleimanieh Naeini T. Psychological disorders among Iranian infertile couples undergoing assisted reproductive technology (ART). Iran J Public Health. 2017;46(3):333‐341. [PMC free article] [PubMed] [Google Scholar]
- 97. Lakatos E, Szigeti JF, Ujma PP, Sexty R, Balog P. Anxiety and depression among infertile women: a cross‐sectional survey from Hungary. BMC Womens Health. 2017;17(1):48 10.1186/s12905-017-0410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pasch LA, Holley SR, Bleil ME, Shehab D, Katz PP, Adler NE. Addressing the needs of fertility treatment patients and their partners: are they informed of and do they receive mental health services? Fertil Steril. 2016;106(1):209‐215 e2 10.1016/j.fertnstert.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 99. Hammerli K, Znoj H, Barth J. The efficacy of psychological interventions for infertile patients: a meta‐analysis examining mental health and pregnancy rate. Hum Reprod Update. 2009;15(3):279‐295. 10.1093/humupd/dmp002 [DOI] [PubMed] [Google Scholar]
- 100. Domar AD, Rooney KL, Wiegand B, et al. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertil Steril. 2011;95(7):2269‐2273. 10.1016/j.fertnstert.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 101. Frederiksen Y, O'Toole MS, Mehlsen MY, et al. The effect of expressive writing intervention for infertile couples: a randomized controlled trial. Hum Reprod. 2017;32(2):391‐402. 10.1093/humrep/dew320 [DOI] [PubMed] [Google Scholar]
- 102. Li J, Long L, Liu Y, He W, Li M. Effects of a mindfulness‐based intervention on fertility quality of life and pregnancy rates among women subjected to first in vitro fertilization treatment. Behav Res Ther. 2016;77:96‐104. 10.1016/j.brat.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 103. Sominsky L, Hodgson DM, McLaughlin EA, Smith R, Wall HM, Spencer SJ. Linking stress and infertility: a novel role for ghrelin. Endocr Rev. 2017;38(5):432‐467. 10.1210/er.2016-1133 [DOI] [PubMed] [Google Scholar]


