Abstract
Although the neural bases of numerical processing and memory have been extensively studied, much remains to be elucidated concerning the spectral and temporal dynamics surrounding these important cognitive processes. To further this understanding, we employed a novel numerical working memory paradigm in 28 young, healthy adults who underwent magnetoencephalography (MEG). The resulting data were examined in the time‐frequency domain prior to image reconstruction using a beamformer. Whole‐brain, spectrally‐constrained coherence was also employed to determine network connectivity. In response to the numerical task, participants exhibited robust alpha/beta oscillations in the bilateral parietal cortices. Whole‐brain statistical comparisons examining the effect of numerical manipulation during memory‐item maintenance revealed a difference centered in the right superior parietal cortex, such that oscillatory responses during numerical manipulation were significantly stronger than when no manipulation was necessary. Additionally, there was significantly reduced cortico‐cortical coherence between the right and left superior parietal regions during the manipulation compared to the maintenance trials, indicating that these regions were functioning more independently when the numerical information had to be actively processed. In sum, these results support previous studies that have implicated the importance of parietal regions in numerical processing, but also provide new knowledge on the spectral, temporal, and network dynamics that serve this critical cognitive function during active working memory maintenance.
Keywords: neural oscillations, numerical processing, superior parietal cortex, working memory
Using magnetoencephalography and a novel numerical working memory task, we examined the oscillatory neural dynamics supporting numerical working memory. Active mental manipulation of a number set during working memory maintenance preferentially desynchronized alpha oscillations in the right superior parietal cortex (SPC), and whole‐brain coherence imaging showed that this region decoupled from its left homologue during the same time window. These results suggest that numerical working memory requires specialized processing by the right SPC, which is distinct from the left SPC.
1. INTRODUCTION
Numerical processing is key to everyday cognitive function, and includes the processing of information such as times, dates, and financial transactions, to successfully navigate everyday life (Knops, 2017). Although numerical processing contributes heavily to mathematical computation, more basic ordering, memory, and organization of numbers is also a key foundation for higher‐level cognitive processes, such as those listed above. A specific area of numerical processing, numerical comparison, involves the ability to correctly identify the value and therefore the magnitude of a number compared to other numbers, and correctly use that knowledge to perform a task at hand. This basic ability to compare numbers plays a key role in arithmetic, and is the foundation of more complicated numerical operations within working memory (WM) that are needed in day to day functioning. Although numerical processing and its neural correlates have been studied extensively (Chochon, Cohen, van de Moortele, & Dehaene, 1999; Dehaene, Piazza, Pinel, & Cohen, 2003; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999; Eger et al., 2009; Feigenson, Dehaene, & Spelke, 2004; Knops, 2017; Knops & Willmes, 2014; Maruyama, Pallier, Jobert, Sigman, & Dehaene, 2012; Piazza, Izard, Pinel, Le Bihan, & Dehaene, 2004; Piazza, Pinel, Le Bihan, & Dehaene, 2007; Pinel, Dehaene, Rivière, & LeBihan, 2001), and many theoretical models of the underlying mechanisms have been developed (Dehaene et al., 2003; Dehaene & Changeux, 1993; Knops, 2017; Skagenholt, Träff, Västfjäll, & Skagerlund, 2018; Stoianov & Zorzi, 2012), a great deal remains to be understood about the spectral and temporal neural dynamics that serve number processing and numerical comparison, particularly during the active maintenance of numerical stimuli in WM.
In terms of its neural bases, numerical computation has been found to heavily involve bilateral parietal cortices (Dehaene et al., 1999, 2003; Maruyama et al., 2012), which also play a key role in visuospatial representation and mapping of saliency (Chambers, Payne, Stokes, & Mattingley, 2004; Kelley, Serences, Giesbrecht, & Yantis, 2008; Thiebaut de Schotten et al., 2011; Wiesman, Heinrichs‐Graham, Proskovec, McDermott, & Wilson, 2017; Wiesman & Wilson, 2018). Interestingly, the laterality of these responses seems to be important, with the right parietal cortex being active during both number comparison and arithmetic manipulation, while left parietal circuits seem to be much more active during arithmetic manipulation (Chochon et al., 1999). Besides the parietal cortices' general involvement in the symbolic representations of numbers (e.g., Arabic numerals), the intraparietal cortex also appears to be involved in one's understanding of numerosity or the basic sense of amounts without symbolic representation (Eger et al., 2009; Piazza et al., 2004; Piazza et al., 2007). In addition to their well‐defined role in numerical manipulation, the bilateral parietal cortices are also well‐established as components of WM networks (Majerus et al., 2006; Majerus et al., 2007; Sneve, Magnussen, Alnæs, Endestad, & D'Esposito, 2013), and are thought to be essential for the maintenance and active manipulation of short‐term memories. Thus, numerical WM might be expected to tax these regions particularly strongly.
Although previous research has identified the neural underpinnings of numerical processing and consistently found relevant neural activity in the parietal cortices (Chochon et al., 1999; Dehaene et al., 1999; Dehaene et al., 2003; Eger et al., 2009; Feigenson et al., 2004; Knops, 2017; Knops & Willmes, 2014; Maruyama et al., 2012; Piazza et al., 2007; Pinel et al., 2001), these studies have almost exclusively relied on fMRI, and thus have largely not examined the spectral and temporal dynamics of numerical manipulation and/or numerical WM. In fact, numerical processing studies using magnetoencephalography (MEG), which combines relatively high spatial resolution with extremely precise temporal resolution, are extremely rare. In addition, the cognitive paradigms that have been used to study numerical processing have primarily focused on simple arithmetical operations, and have not addressed the manipulation of previously‐encoded numerical stimuli within WM during a state of equivalent visual stimulation.
To specifically address the lack of research surrounding the spectral and temporal neural dynamics serving numerical manipulation, we have developed a novel numerical WM task, wherein encoded numerical representations are either manipulated or maintained in the context of a visually‐balanced maintenance period. Herein, we report on 28 healthy adult controls who completed this paradigm during MEG recording. Due to its high temporal and spatial precision, MEG allowed us to study the spatial, spectral, and temporal dynamics of the neural oscillations associated with numerical processing and manipulation in the human brain. In line with previous research, we hypothesized that numerical manipulation of items in WM would involve more widespread and stronger neural responses in parietal areas that have previously been identified as essential to numeric computation, compared to a control condition where no manipulation of such items was required. Specifically, due to literature suggesting the importance of bilateral parietal activity in numerical computation, we expected robust neural responses in the bilateral parietal cortices, with the most robust manipulation‐related activity in the right hemisphere. Further, we expected that these neural responses would be within oscillatory rhythms commonly associated with active processing in these regions (i.e., alpha oscillations in occipito‐parietal cortices). Finally, we also hypothesized that coherence between bilateral parietal cortices would be altered by numerical manipulation of items in WM, providing further evidence for the functional role of these regions during such numerical processing. Importantly, we did not have an a priori hypothesis regarding the direction of this coherence effect. It could be expected that the coherence between these regions would decrease, signaling a reduced communication between them during numerical manipulation and thus functional specialization of right versus left parietal cortex in such processing. Alternatively, enhanced coherence between these regions would also be interpretable as shared processing of the manipulated numerical stimuli. To investigate these hypotheses, we used a data‐driven approach, as this allows for a more unbiased approximation of the spectral, spatial, and temporal extents of the neural responses of interest in our specific sample, and enables discovery of other brain regions and oscillatory dynamics may contribute to numerical processing.
2. METHODS
2.1. Participants
We collected data from 28 healthy young adults for this study. All participants were between the ages of 19 and 31 years (15 females; Mean age = 24.00 years; SD = 3.16 years; 26 right‐handed). Exclusionary criteria included any medical illness affecting CNS function, any neurological disorder, history of head trauma, current substance abuse, and any nonremovable metal implants that would interfere with MEG data acquisition. The Institutional Review Board of the University of Nebraska Medical Center reviewed and approved this investigation. Written informed consent was obtained from each participant following a detailed description of the study. With the exception of the counter‐balancing of the task instructions (see Task Paradigm, below), all participants completed the same experimental protocol during the visit.
2.2. Task paradigm
This study utilized a novel numerical manipulation WM paradigm, designed to tap the comparison of numerical digits in the context of equivalent visual input (Figure 1). Briefly, participants were shown a centrally‐presented fixation surrounded by an empty horizontal grid for 1.0 s. A horizontal array of four integers between 1 and 9 then appeared at fixed locations within this grid for 1.2 s. Following this, the numbers disappeared and the screen was empty for 2.5 s. During this time, the value (i.e., the relative darkness/lightness of a color) of the grid changed to either a darker or lighter color than what was present during the encoding period, and this change denoted whether the subject was to mentally rearrange the numbers into numerically ascending order, or maintain the order of digits as originally seen. Importantly, the meaning of the light versus dark value change was counter‐balanced across participants, and the instructions given was the only difference between these counter‐balanced administrations. After the maintenance period, a single target integer, which was always a member of the previous set, appeared at a random position within the grid for 1.6 s, and the participant was instructed to respond whether this number was in the correct position based on the instructions to maintain/manipulate the sequence. Responses were recorded by right‐handed button presses, where the index finger indicated that the location of the number matched the maintained/manipulated sequence and the middle finger indicated that this location did not match. A total of 200 trials were completed per participant, and these trials were equally split and pseudorandomized between manipulate and maintain trial conditions. Total MEG recording time was about 21 min.
FIGURE 1.
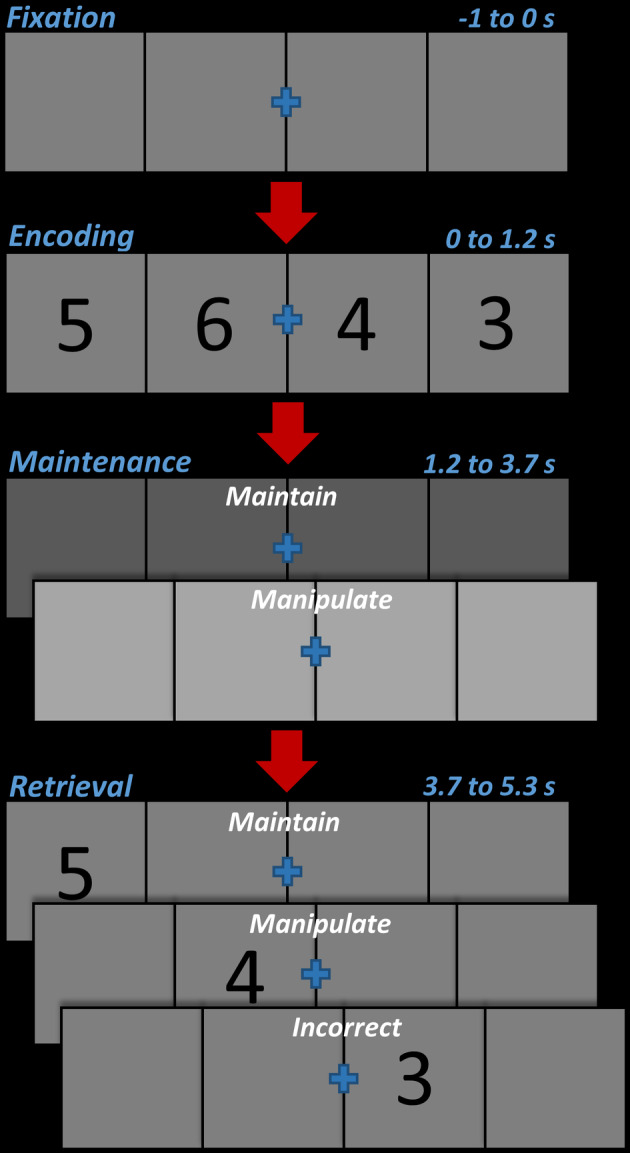
Numerical working memory manipulation task. Each trial began with an empty 4 × 1 grid with a blue fixation cross in the center presented for 1 s, followed by a 4 × 1 grid of single numerical digits (1–9) presented for 1.2 s (encoding). The digits then disappeared for 2.5 s (maintenance), and the underlying color of the grid became darker or lighter. The color indicated whether the participant was to maintain the original sequence, or rearrange the sequence into a numerically ascending order. After the 2.5 s, a 4 × 1 grid with a single probe digit was presented in any of the four boxes for 1.6 s (retrieval), and participants were tasked with responding (by button press) if the digit was in the correct box in the grid (right index finger) or not (right middle finger). It is important to note that the meaning of the light or dark color change was pseudorandomized between participants
2.3. MEG acquisition and coregistration
All recordings were conducted in a one‐layer magnetically‐shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta/MEGIN MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), the MEG data from each participant were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu & Simola, 2006; tSSS; Suma Taulu, Simola, & Kajola, 2005). Of note, the application of tSSS reduces the effects of interfering signals originating from outside of the persons head space, including signals related to the active shielding component of our environmental noise compensation.
Prior to starting the MEG experiment, four coils were attached to the subject's head and localized, together with the three fiducial points and scalp surface, with a 3‐D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT). Once the subject was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed for each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant's MEG data were coregistered with structural MRI data prior to source space analyses using BESA MRI (Version 2.0). These data were aligned in parallel to the anterior and posterior commissures and transformed into standardized space. Following source analysis (i.e., beamforming), each participant's 4.0 × 4.0 × 4.0 mm MEG functional images were transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.4. MEG time frequency transformation and statistics
Cardiac artifacts were removed from the data using signal‐space projection (SSP), which was accounted for during source reconstruction (Uusitalo & Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 6.3 s duration, with the onset of the encoding grid defined as time 0.0 s and the baseline being defined as −0.9 to −0.3 s prior to the onset of this grid. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, etc.) were rejected based on individual amplitude and gradient thresholds, supplemented with visual inspection. An average amplitude threshold of 1,210.71 (SD = 330.08) fT and an average gradient threshold of 83.62 (SD = 36.24) fT/s was used to reject artifacts. Further, only trials where participants responded correctly were used for analysis. This resulted in an average of 182.14 (SD = 11.57) trials per participant. Of note, there was no significant difference between the number of trials used between the manipulate and maintain conditions (t[28] = −.15; p = .88).
Artifact‐free epochs were transformed into the time‐frequency domain using complex demodulation (Hoechstetter et al., 2004; Kovach & Gander, 2016; Papp & Ktonas, 1977), and the resulting spectral power estimations per sensor were averaged over trials to generate time‐frequency plots of mean spectral density. These sensor‐level data were normalized using the respective bin's baseline power, which was calculated as the mean power during the −0.9 to −0.3 s time period. The specific time‐frequency windows used for imaging were determined by statistical analysis of the sensor‐level spectrograms across the entire array of gradiometers. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two‐stage procedure was followed to control for Type 1 error. In the first stage, paired‐samples t‐tests against baseline were conducted on each data point and the output spectrogram of t‐values was thresholded at p < .05 to define time‐frequency bins containing potentially significant oscillatory deviations relative to baseline across all participants. In Stage 2, time‐frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < .05) threshold, and a cluster value was derived by summing all of the t‐values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster‐values, and the significance level of the observed clusters (from Stage 1) were tested directly using this distribution (Ernst, 2004; Maris & Oostenveld, 2007). For each comparison, 1,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time‐frequency windows during the interval of interest (i.e., the maintenance period) that contained significant oscillatory events across all participants (final threshold of p < .05; two‐tailed) were subjected to a beamforming analysis, and these source images were then used to test our hypothesized effects. Further details regarding our data analysis pipeline can be found in (Alex I Wiesman & Wilson, 2020).
2.5. MEG source imaging and statistics
Cortical networks were imaged using dynamic imaging of coherent sources (DICS; Gross et al., 2001), which employs spatial filters in the frequency domain to calculate source power and/or coherence for the entire brain volume. The single images were derived from the cross‐spectral densities of all combinations of MEG gradiometers averaged over the time‐frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, we computed noise‐normalized, source power and/or coherence per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth (Hillebrand, Singh, Holliday, Furlong, & Barnes, 2005; Van Veen, van Drongelen, Yuchtman, & Suzuki, 1997). For neural response power, such images are typically referred to as pseudo‐t maps, with units (pseudo‐t) that reflect noise‐normalized power differences (i.e., active vs. passive) per voxel. In contrast, coherence images reflect noise‐normalized changes in connectivity from baseline levels between a prespecified seed voxel of interest and every other voxel in the brain. MEG preprocessing and imaging used the Brain Electrical Source Analysis (BESA V6.1) software.
Normalized differential source power was computed for the statistically‐determined time‐frequency bands (see below) over the entire brain volume per participant at 4.0 mm isotropic resolution. The resulting 3D maps of brain activity were first group‐averaged per time bin and condition to provide a visualization of the spatio‐temporal evolution of the response, before being averaged across all maintenance time bins (i.e., 1.2–3.7 s after onset of the encoding grid) per participant and condition. To assess the neuroanatomical basis of significant oscillatory neural response differences as a function of manipulation condition in a data‐driven manner, these averaged maps were subjected to whole‐brain paired samples t‐tests (i.e., manipulate vs maintain). To control for Type‐I error, we conducted nonparametric permutation testing using a cluster‐based permutation method similar to that performed on the sensor‐level spectrograms, with 10,000 permutations per comparison (final threshold of p < .05; two‐tailed). To further elucidate the temporal dynamics of numerical processing, we extracted peak voxel time series using the statistical comparison power images, and plotted the resulting time series per condition for visualization purposes. To compute the virtual sensors/voxel time series, we applied the sensor weighting matrix derived from the forward solution to the preprocessed signal vector, which yielded a time series for the specific voxel coordinates of interest.
To examine network‐level effects of numerical WM processing, a similar statistical approach was used on the coherence maps. Briefly, this consisted of averaging these whole‐brain coherence maps per participant and condition across the maintenance period, before performing voxel‐wise paired‐samples t‐tests across the whole brain volume, with follow‐on correction using cluster‐based permutation testing (10,000 permutations; final threshold of p < .05; two‐tailed). To limit the confound of source power, any region exhibiting significant conditional differences in coherence was entered into a posthoc analysis, where the source amplitudes of the seed and target regions were co‐varied out of this relationship to ensure that any significant differences found were not the result of biases resulting from systematic power differences.
3. RESULTS
3.1. Behavioral effects
Participants performed generally well on the WM task (mean RT = 992.95 ms, SD = 213.24 ms; accuracy = 91.07%; SD = 5.79%). There were no significant differences in reaction time (t[28] = −.77; p = .45) or accuracy (t[28] = −1.89; p = .07) as a function of condition (i.e., between manipulation and maintain trials).
3.2. Sensor‐ and source‐level responses to the WM task
Cluster‐based permutation testing (final threshold: p < .05; 10,000 permutations) of the sensor‐level spectrograms across both conditions revealed significant oscillatory responses in the alpha and beta bands during the entirety of the maintenance phase (Figure 2; 8–18 Hz, 1.2–3.7 s). Beamformer source imaging revealed that this response corresponded to robust decreases in 8–18 Hz power in bilateral parietal, occipital, and supramarginal regions. Qualitatively, these decreases were more robust and prolonged during manipulate trials compared to maintain trials (Figure 2).
FIGURE 2.
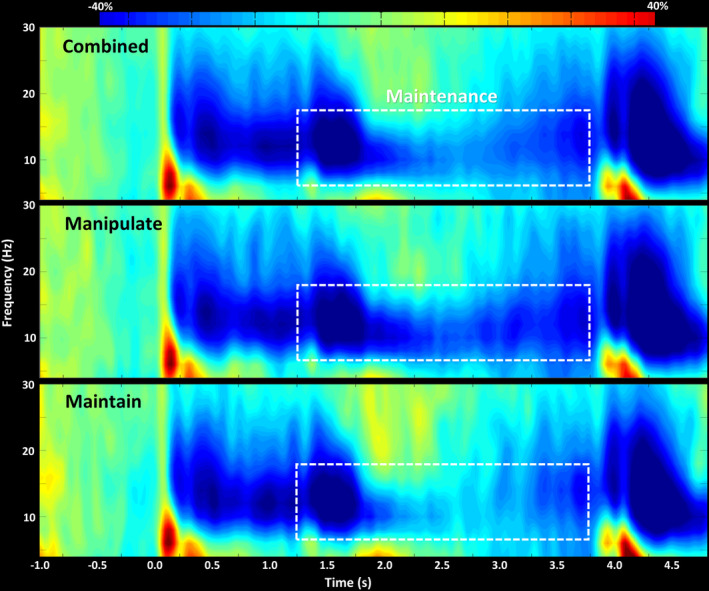
MEG sensor‐level neural responses. Time‐frequency spectrograms of a peak parietal‐occipital sensor (sensor label: MEG1943) that has been grand averaged across both conditions (top), manipulate trials only (middle), and maintain trials only (bottom). Time is shown on the x‐axis in seconds, and frequency is shown on the y‐axis in Hz. Changes in power are shown as percent change from baseline, with the scale color bar at the top. We found a significant decrease in alpha/beta response power across both encoding and maintenance periods. The white box demarcates the maintenance period that was of primary interest for this study, as this is when participants were performing differently (i.e., manipulating or maintaining) based on the task instructions
3.3. Conditional differences on alpha/beta oscillatory responses
Alpha/beta oscillatory responses during the maintenance period were much stronger, widespread, and prolonged in the manipulate compared to the maintain condition (Figure 3). This activity was strongest in the bilateral parietal and occipital areas, and clearly progressed anterior toward superior parietal and frontal areas in the manipulate condition. In contrast, alpha/beta activity was much weaker in the maintain condition and had largely dissipated after the first second of maintenance processing.
FIGURE 3.
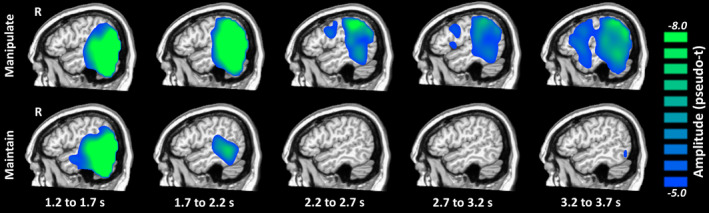
Right hemispheric neural responses in each condition. The beamformer maps for each condition have been averaged across all participants separately for each .5 s time bin and are shown above. As can be seen, there were strong alpha/beta decreases throughout the maintenance period in the manipulate condition, but these largely dissipated after 1 s in the maintain condition. In the initial time bin, this response was localized to the parietal and occipital regions for both conditions, but then weakened in the maintain condition and spread more anterior into the parietal and frontal regions in the manipulate condition. There was also a notable frontal shift in activity during the later maintenance periods in the manipulate condition
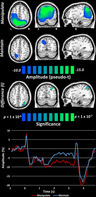
To statistically examine the effects of numerical manipulation on these neural oscillations, we used the participant level‐whole brain maps and averaged across all maintenance time bins per participant and condition, and then performed voxel‐wise paired‐samples t‐tests (Figure 4, top). Multiple comparisons were controlled using cluster‐based permutation testing, with a final significance cutoff of p < .05 and 10,000 permutations (see Section 2.5). We found that numerical manipulation was associated with significantly stronger alpha/beta responses in the right parietal region relative to the numerical maintain condition. To aid in visualization of the time course of the observed neural responses in each condition, virtual sensors were extracted from the peak voxel of this right superior parietal cluster and are plotted in Figure 4 (bottom).
FIGURE 4.
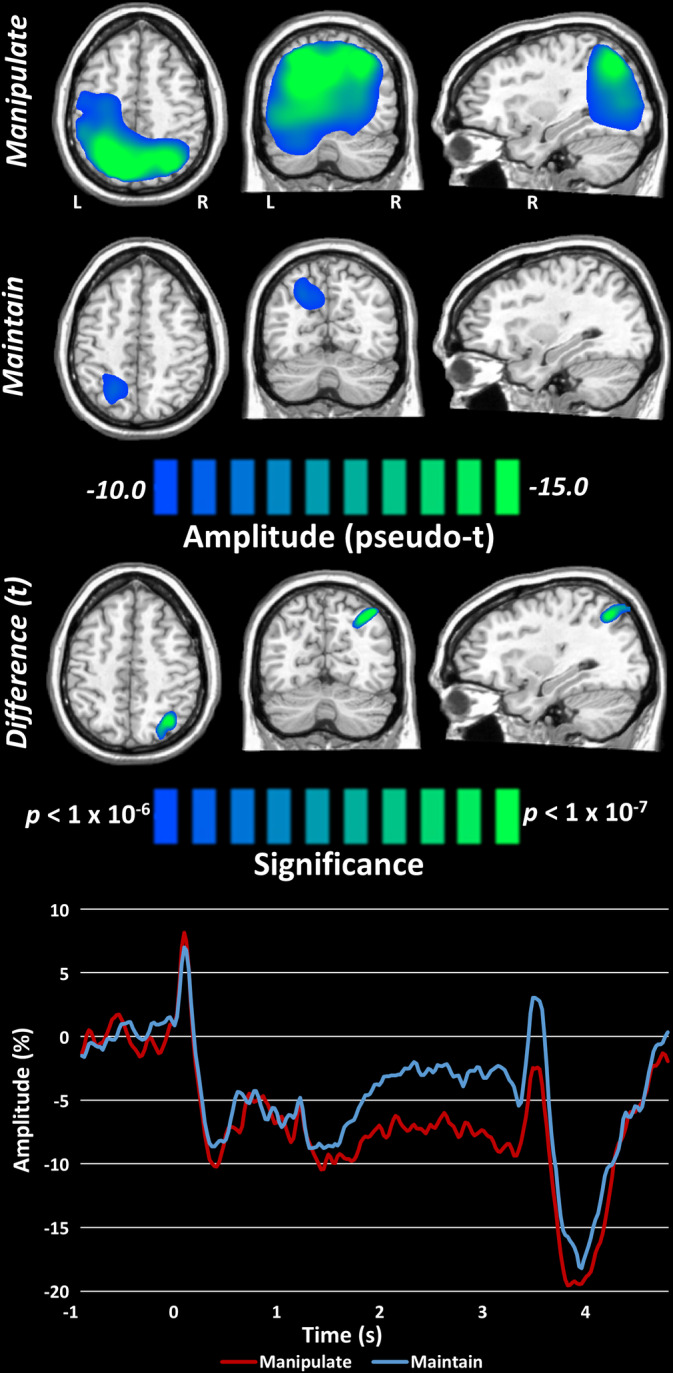
Effects of numerical manipulation on alpha/beta oscillations. (Top) Grand averages over the maintenance period time bins (i.e., the five bins represented in Figure 3) per condition revealed much stronger activity in the manipulate condition across widespread regions. Rigorous paired‐samples t‐tests with permutation testing for multiple comparisons revealed significantly stronger alpha/beta oscillatory activity in the right superior parietal region. Virtual sensor extraction from this region showed that the manipulate condition sustained a much stronger alpha/beta decrease relative to the maintain condition for about 2.0 s of the total 2.5 s maintenance period
Finally, to examine whether network connectivity with this right parietal peak differed as a function of numerical WM processing condition, we computed whole‐brain alpha/beta coherence maps during the maintenance period using the peak voxel from the significant amplitude difference as the seed, and then compared the output using voxel‐wise paired‐samples t‐tests and cluster‐based permutation tests (final threshold: p < .05; 10,000 permutations). Interestingly, we found a pattern of decreased coherence between the right and left superior parietal regions when participants were required to perform numerical manipulation, compared to when they just had to maintain the stimuli (Figure 5). Importantly, to limit the impact of potential confounds of power on coherence, we covaried out the power values of the seed (right superior parietal) and peak difference (left superior parietal) voxels and the significant difference in coherence remained.
FIGURE 5.
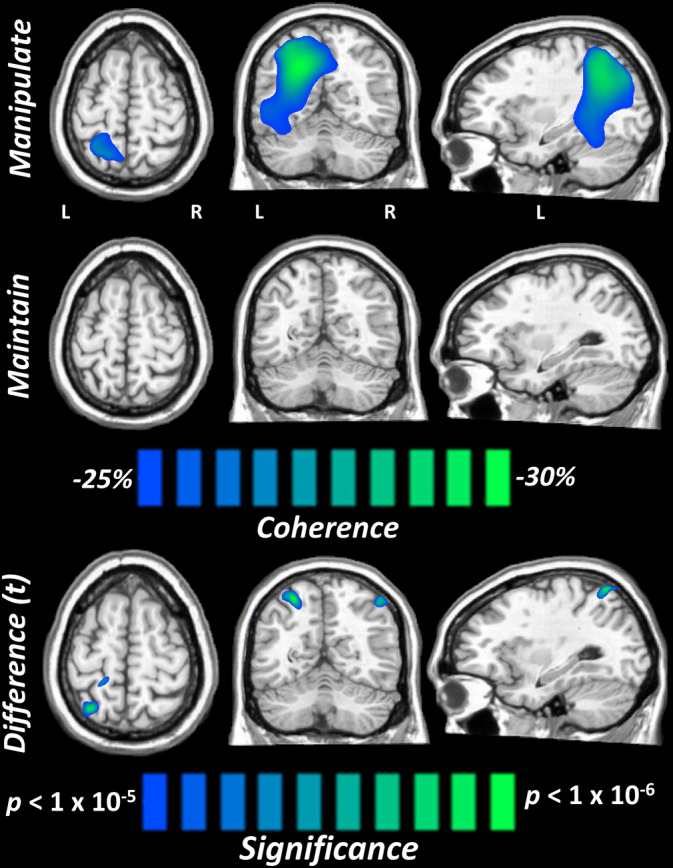
Coherence analysis of right superior parietal seed. (Top) Grand average coherence maps in each condition revealed decreased coherence between the right superior parietal seed and left parieto‐occipital cortices during the maintenance period relative to the baseline. This decrease was especially strong in the manipulate condition. (Bottom) Paired‐samples t‐tests showed significantly weaker coherence in the manipulate condition, which survived stringent multiple comparisons correction and covarying out the power of each response peak
4. DISCUSSION
Despite a voluminous literature examining numerical processing, very few studies have explored the underlying neural dynamics and network‐level processing of numerical manipulation during WM maintenance. In addition, the few studies that have examined such processing have relied on fMRI, which although exceedingly useful, lacks the temporal precision needed to understand the dynamics. Here, we applied the high temporal and spatial precision of MEG to explore these neural dynamics during performance of a novel digit reordering WM paradigm. Using advanced source imaging and whole‐brain statistical analyses, we found stronger alpha/beta oscillatory activity during the maintenance period when manipulation of the numbers was necessary, compared to when it was not. This difference was strongest in the bilateral parietal and occipital areas, and qualitatively displayed an anterior progression toward more superior parietal and frontal cortical areas later in the maintenance period. Moreover, the bilateral parietal regions displayed decreased coherence during the manipulation condition compared to the maintain condition, indicating that these regions were likely performing different functions toward the goal of numerical processing. These results, as well as their implications for future research in this field, are discussed below.
We found a robust decrease in the alpha/beta range (8–18 Hz) throughout the maintenance period during the numerical manipulation condition in bilateral brain regions, extending anteriorly from occipito‐parietal to frontal cortices. Such alpha decreases in posterior parieto‐occipital regions are known to represent the active processing of stimuli, and have been reported in a number of different tasks requiring the maintenance of encoded verbal stimuli (Embury, Wiesman, Proskovec, Heinrichs‐Graham, et al., 2018; Embury, Wiesman, Proskovec, Mills, et al., 2018; Heinrichs‐Graham & Wilson, 2015; Jensen & Mazaheri, 2010; McDermott, Badura‐Brack, Becker, Ryan, Bar‐Haim, et al., 2016; McDermott, Badura‐Brack, Becker, Ryan, Khanna, et al., 2016; Proskovec, Heinrichs‐Graham, & Wilson, 2016, 2019; Proskovec, Wiesman, Heinrichs‐Graham, & Wilson, 2018; van Dijk, Schoffelen, Oostenveld, & Jensen, 2008; Wiesman et al., 2016; Wilson et al., 2017). These patterns of alpha/beta activity also spread noticeably into cerebellar and posterior temporal regions in both conditions. The cerebellum is essential for a range of cognitive functions, and has been reported numerous times as being active during WM (Heinrichs‐Graham & Wilson, 2015; Proskovec et al., 2016) and semantic comprehension (Kujala et al., 2007), while the posterior temporal cortices are essential for the processing of visual stimuli, feature binding, and identification (i.e., the “what” pathway; Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999). Taken together, the recruitment of these distributed cortical regions for the maintenance of items in WM is well supported by previous literature.
Further, our data showed that the manipulate condition was associated with a qualitatively stronger and more widespread alpha/beta decrease throughout the majority of the maintenance period. In contrast, the maintain condition was associated with a weaker alpha/beta decrease initially, and this activity further decreased in strength rapidly as the maintenance period progressed. This is intuitive, as the manipulate condition requires greater processing, as participants were not only required to commit the digit sequence to WM, but also to actively rearrange that sequence in ascending numerical order for correct task completion. This greater processing requirement was reflected by a larger decrease in parietal and other regions required to complete the task. It is important to note that despite being a decrease in amplitude from basal levels of neural activity, the patterns observed throughout the maintenance phase in the alpha/beta frequency band actually represent the activation of these areas (Jensen & Mazaheri, 2010; Klimesch, 2012; Klimesch, Sauseng, & Hanslmayr, 2007), leading to a dis‐inhibition of these cortical regions during stimulus manipulation. Subsequent statistical testing of these data confirmed that the significant differences in alpha/beta activity were strongest over right superior parietal cortices, which is in agreement with previous studies of numerical manipulation (Chochon et al., 1999; Dehaene et al., 1999, 2003; Eger et al., 2009; Feigenson et al., 2004; Knops, 2017; Knops & Willmes, 2014; Maruyama et al., 2012; Piazza et al., 2007; Pinel et al., 2001), however until now the spectral and temporal parameters of this effect have remained unknown. Identifying these parameters is essential, as differing oscillatory frequencies have been found to index distinct cognitive functions in the human brain (Başar, Basar‐Eroglu, Karakas, & Schurmann, 2001), even when overlapping temporally in the same regions. Further, a number of neurological and psychiatric patient populations have been found to exhibit aberrant neural responses at specific frequencies of oscillatory activity (Badura‐Brack et al., 2017; Başar & Güntekin, 2013; Groff et al., 2020; Heinrichs‐Graham et al., 2014; Kurz, Wiesman, Coolidge, & Wilson, 2017; Lew et al., 2018; Spooner et al., 2018; Wiesman et al., 2016; Wiesman et al., 2018; Wilson, Heinrichs‐Graham, Proskovec, & McDermott, 2016; Wilson et al., 2013; Wilson, Lew, Spooner, Rezich, & Wiesman, 2019; Wilson, Rojas, Reite, Teale, & Rogers, 2007; Wilson et al., 2011), signaling the importance of a greater understanding of the spectral organization of human brain activity for potential neuromodulatory interventions.
Next, to identify the network‐level relationships between this right parietal region and the rest of the brain, we investigated differences in whole‐brain connectivity using a frequency‐resolved coherence beamformer. We found a relative decrease in coherence between the right and left superior parietal regions during the manipulate compared to the maintain condition, signifying reduced network connectivity when numerical manipulation in WM was necessary. Importantly, due to the potential confound of amplitude on coherence measurements, we also covaried out the effects of the seed and target region amplitude on this conditional difference, and our results remained significant. These results indicate that the right superior parietal cortex, which has been traditionally associated with numerical processing, actively disconnects from the left superior parietal to perform numerical manipulations. Given previous literature suggesting differing roles of the bilateral parietal regions in numerical processing (Chochon et al., 1999), this is additional evidence that these regions are performing discrete computations from each other during this kind of task. In other words, this decrease in coherence suggests that information sharing between the bilateral parietal cortices is reduced during numerical manipulation, and that the two regions appear to be functioning more independently during manipulation trials. However, future studies using methods with higher spatial precision will need to investigate whether this directly relates to enhanced processing in discrete local networks. Regardless, our new data are critical, as although left parietal recruitment in numerical processing has been widely established (Chochon et al., 1999; Dehaene et al., 1999, 2003; Dehaene & Changeux, 1993; Piazza et al., 2004, 2007; Pinel et al., 2001), this is the first report of significant disconnection between these regions during numerical manipulations.
Despite the robust effects observed on neural activity, behaviorally, there were no significant differences between manipulate and maintain conditions in terms of reaction time or accuracy. However, this null finding is not entirely surprising, as participants were given ample time to perform the required numerical reordering during the maintenance period. Further, it is likely that the increased recruitment of bilateral parietal networks to complete the numerical manipulation was sufficient to compensate for any differences in difficulty between the conditions, as all of our participants were healthy young adults with good cognitive abilities. In fact, the relatively long amount of time provided to participants for the manipulation was by design, and it was fortuitous that no behavioral differences emerged, as this allowed us to examine the underlying neurophysiology without any worry of confounding performance differences. Note that although condition effects on accuracy were trending, this was not a biasing factor here, as we only examined neural data from correct trials.
Before closing, it is important to note the limitations of this study. For one, our numerical WM manipulation task was relatively simple, and there is certainly motivation for future studies to incorporate more complex manipulation paradigms (e.g., longer sequences, more than two outcomes, etc.) that may have greater sensitivity to behavioral differences. Although concerns over performance differences would have to be considered in this circumstance, a more difficult task might also allow researchers to more effectively parse out which of the responses found here are contributing directly to certain aspects of numerical processing. Additionally, this task only considered the reordering of numerical stimuli, whereas the reordering of other stimulus types (e.g., letter stimuli) needs further study. Finally, although cluster‐based permutation testing is likely the best method for controlling Type‐I error in MEG data, it provides only weak evidence regarding the spatial/spectral/temporal extents of significant clusters. Thus, it is possible, and even likely, that additional brain regions are necessary for numerical WM processing in the healthy adult brain, and that our methods were simply too conservative to detect these other cortical areas. Despite these limitations, this study confirms the importance of parietal regions during numerical WM processing, while also adding essential new knowledge concerning the underlying temporal and spatial dynamics.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author, Dr. Tony W. Wilson, upon reasonable request.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This research was supported by grants R01‐MH103220 (Tony W. Wilson), R01‐MH116782 (Tony W. Wilson), R01‐MH118013 (Tony W. Wilson), R01‐DA047828 (Tony W. Wilson), R01‐MH121101 (Tony W. Wilson), RF1‐MH117032 (Tony W. Wilson), P20‐GM130447 (Tony W. Wilson), F31‐AG055332 (Alex I. Wiesman), and U54‐GM115458 (Elizabeth Heinrichs‐Graham) from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Koshy SM, Wiesman AI, Proskovec AL, et al. Numerical working memory alters alpha‐beta oscillations and connectivity in the parietal cortices. Hum Brain Mapp. 2020;41:3709–3719. 10.1002/hbm.25043
Funding information National Institutes of Health, Grant/Award Numbers: U54‐GM115458, F31‐AG055332, P20‐GM130447, R01‐MH121101, R01‐DA047828, R01‐MH118013, R01‐MH116782, R01‐MH103220, RF1‐MH117032
REFERENCES
- Badura‐Brack, A. S. , Heinrichs‐Graham, E. , McDermott, T. J. , Becker, K. M. , Ryan, T. J. , Khanna, M. M. , & Wilson, T. W. (2017). Resting‐state neurophysiological abnormalities in posttraumatic stress disorder: A magnetoencephalography study. Frontiers in Human Neuroscience, 11, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar, E. , Basar‐Eroglu, C. , Karakas, S. , & Schurmann, M. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39(2–3), 241–248. [DOI] [PubMed] [Google Scholar]
- Başar, E. , & Güntekin, B. (2013). Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. Supplements to Clinical Neurophysiology, 62, 303–341. [DOI] [PubMed] [Google Scholar]
- Chambers, C. D. , Payne, J. M. , Stokes, M. G. , & Mattingley, J. B. (2004). Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience, 7(3), 217–218. 10.1038/nn1203 [DOI] [PubMed] [Google Scholar]
- Chochon, F. , Cohen, L. , van de Moortele, P. F. , & Dehaene, S. (1999). Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience, 11(6), 617–630. [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , & Changeux, J. P. (1993). Development of elementary numerical abilities: A neuronal model. Journal of Cognitive Neuroscience, 5(4), 390–407. 10.1162/jocn.1993.5.4.390 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Piazza, M. , Pinel, P. , & Cohen, L. (2003). Three parietal circuits for number processing. Cognitive Neuropsychology, 20(3), 487–506. 10.1080/02643290244000239 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Spelke, E. , Pinel, P. , Stanescu, R. , & Tsivkin, S. (1999). Sources of mathematical thinking: Behavioral and brain‐imaging evidence. Science, 284(5416), 970–974. [DOI] [PubMed] [Google Scholar]
- Eger, E. , Michel, V. , Thirion, B. , Amadon, A. , Dehaene, S. , & Kleinschmidt, A. (2009). Deciphering cortical number coding from human brain activity patterns. Current Biology, 19(19), 1608–1615. 10.1016/j.cub.2009.08.047 [DOI] [PubMed] [Google Scholar]
- Embury, C. M. , Wiesman, A. I. , Proskovec, A. L. , Heinrichs‐Graham, E. , McDermott, T. J. , Lord, G. H. , … Wilson, T. W. (2018). Altered brain dynamics in patients with type 1 diabetes during working memory processing. Diabetes, 67(6), 1140–1148. 10.2337/db17-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury, C. M. , Wiesman, A. I. , Proskovec, A. L. , Mills, M. S. , Heinrichs‐Graham, E. , Wang, Y. P. , … Wilson, T. W. (2018). Neural dynamics of verbal working memory processing in children and adolescents. NeuroImage, 185, 191–197. 10.1016/j.neuroimage.2018.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M. (2004). Permutation methods: A basis for exact inference. Statistical Science, 19, 676–685 Institute of Mathematical Statistics. [Google Scholar]
- Feigenson, L. , Dehaene, S. , & Spelke, E. (2004). Core systems of number. Trends in Cognitive Sciences, 8(7), 307–314. 10.1016/j.tics.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Groff, B. R. , Wiesman, A. I. , Rezich, M. T. , O'Neill, J. , Robertson, K. R. , Fox, H. S. , … Wilson, T. W. (2020). Age‐related visual dynamics in HIV‐infected adults with cognitive impairment. Neurology‐Neuroimmunology Neuroinflammation, 7(3), e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Kujala, J. , Hamalainen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2015). Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex, 69, 121–130. 10.1016/j.cortex.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Wilson, T. W. , Santamaria, P. M. , Heithoff, S. K. , Torres‐Russotto, D. , Hutter‐Saunders, J. A. , … Gendelman, H. E. (2014). Neuromagnetic evidence of abnormal movement‐related beta desynchronization in Parkinson's disease. Cerebral Cortex, 24(10), 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand, A. , Singh, K. D. , Holliday, I. E. , Furlong, P. L. , & Barnes, G. R. (2005). A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping, 25(2), 199–211. 10.1002/hbm.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter, K. , Bornfleth, H. , Weckesser, D. , Ille, N. , Berg, P. , & Scherg, M. (2004). BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography, 16(4), 233–238. [DOI] [PubMed] [Google Scholar]
- Ishai, A. , Ungerleider, L. G. , Martin, A. , Schouten, J. L. , & Haxby, J. V. (1999). Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences of the United States of America, 96(16), 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, O. , & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, T. A. , Serences, J. T. , Giesbrecht, B. , & Yantis, S. (2008). Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex, 18(1), 114–125. 10.1093/cercor/bhm036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. (2012). α‐Band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. , Sauseng, P. , & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Research Reviews, 53(1), 63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Knops, A. (2017). Probing the neural correlates of number processing. The Neuroscientist, 23(3), 264–274. 10.1177/1073858416650153 [DOI] [PubMed] [Google Scholar]
- Knops, A. , & Willmes, K. (2014). Numerical ordering and symbolic arithmetic share frontal and parietal circuits in the right hemisphere. NeuroImage, 84, 786–795. 10.1016/j.neuroimage.2013.09.037 [DOI] [PubMed] [Google Scholar]
- Kovach, C. K. , & Gander, P. E. (2016). The demodulated band transform. Journal of Neuroscience Methods, 261, 135–154. 10.1016/j.jneumeth.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala, J. , Pammer, K. , Cornelissen, P. , Roebroeck, A. , Formisano, E. , & Salmelin, R. (2007). Phase coupling in a cerebro‐cerebellar network at 8–13 Hz during reading. Cerebral Cortex, 17(6), 1476–1485. [DOI] [PubMed] [Google Scholar]
- Kurz, M. J. , Wiesman, A. I. , Coolidge, N. M. , & Wilson, T. W. (2017). Children with cerebral palsy hyper‐gate somatosensory stimulations of the foot. Cerebral Cortex, 28(7), 2431–2438. 10.1093/cercor/bhx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, B. J. , McDermott, T. J. , Wiesman, A. I. , O'Neill, J. , Mills, M. S. , Robertson, K. R. , … Wilson, T. W. (2018). Neural dynamics of selective attention deficits in HIV‐associated neurocognitive disorder. Neurology, 91(20), e1860–e1869. 10.1212/WNL.0000000000006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus, S. , Bastin, C. , Poncelet, M. , van der Linden, M. , Salmon, E. , Collette, F. , & Maquet, P. (2007). Short‐term memory and the left intraparietal sulcus: Focus of attention? Further evidence from a face short‐term memory paradigm. NeuroImage, 35(1), 353–367. [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Poncelet, M. , van der Linden, M. , Albouy, G. , Salmon, E. , Sterpenich, V. , … Maquet, P. (2006). The left intraparietal sulcus and verbal short‐term memory: Focus of attention or serial order? NeuroImage, 32(2), 880–891. [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Maruyama, M. , Pallier, C. , Jobert, A. , Sigman, M. , & Dehaene, S. (2012). The cortical representation of simple mathematical expressions. NeuroImage, 61(4), 1444–1460. 10.1016/j.neuroimage.2012.04.020 [DOI] [PubMed] [Google Scholar]
- McDermott, T. J. , Badura‐Brack, A. S. , Becker, K. M. , Ryan, T. J. , Bar‐Haim, Y. , Pine, D. S. , … Wilson, T. W. (2016). Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cognitive, Affective, & Behavioral Neuroscience, 16(6), 1140–1149. 10.3758/s13415-016-0459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, T. J. , Badura‐Brack, A. S. , Becker, K. M. , Ryan, T. J. , Khanna, M. M. , Heinrichs‐Graham, E. , & Wilson, T. W. (2016). Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: An MEG study. Journal of Psychiatry & Neuroscience, 41(4), 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp, N. , & Ktonas, P. (1977). Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation, 13, 135–145. [PubMed] [Google Scholar]
- Piazza, M. , Izard, V. , Pinel, P. , Le Bihan, D. , & Dehaene, S. (2004). Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron, 44(3), 547–555. 10.1016/j.neuron.2004.10.014 [DOI] [PubMed] [Google Scholar]
- Piazza, M. , Pinel, P. , Le Bihan, D. , & Dehaene, S. (2007). A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron, 53(2), 293–305. 10.1016/j.neuron.2006.11.022 [DOI] [PubMed] [Google Scholar]
- Pinel, P. , Dehaene, S. , Rivière, D. , & LeBihan, D. (2001). Modulation of parietal activation by semantic distance in a number comparison task. NeuroImage, 14(5), 1013–1026. 10.1006/nimg.2001.0913 [DOI] [PubMed] [Google Scholar]
- Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2016). Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Human Brain Mapping, 37(6), 2348–2361. 10.1002/hbm.23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2019). Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. NeuroImage, 184, 256–265. 10.1016/j.neuroimage.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Wiesman, A. I. , Heinrichs‐Graham, E. , & Wilson, T. W. (2018). Beta oscillatory dynamics in the prefrontal and superior temporal cortices predict spatial working memory performance. Scientific Reports, 8(1), 8488 10.1038/s41598-018-26863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagenholt, M. , Träff, U. , Västfjäll, D. , & Skagerlund, K. (2018). Examining the triple code model in numerical cognition: An fMRI study. PLoS One, 13(6), e0199247 10.1371/journal.pone.0199247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneve, M. H. , Magnussen, S. , Alnæs, D. , Endestad, T. , & D'Esposito, M. (2013). Top–down modulation from inferior frontal junction to FEFs and intraparietal sulcus during short‐term memory for visual features. Journal of Cognitive Neuroscience, 25(11), 1944–1956. [DOI] [PubMed] [Google Scholar]
- Spooner, R. K. , Wiesman, A. I. , Mills, M. S. , O'Neill, J. , Robertson, K. R. , Fox, H. S. , … Wilson, T. W. (2018). Aberrant oscillatory dynamics during somatosensory processing in HIV‐infected adults. Neuroimage Clinics, 20, 85–91. 10.1016/j.nicl.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoianov, I. , & Zorzi, M. (2012). Emergence of a ‘visual number sense’ in hierarchical generative models. Nature Neuroscience, 15(2), 194–196. 10.1038/nn.2996 [DOI] [PubMed] [Google Scholar]
- Taulu, S. , & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51(7), 1759–1768. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Taulu, S. , Simola, J. , & Kajola, M. (2005). Applications of the signal space separation method. IEEE Transactions on Signal Processing, 53(9), 3359–3372. 10.1109/TSP.2005.853302 [DOI] [Google Scholar]
- Thiebaut de Schotten, M. , Dell'Acqua, F. , Forkel, S. J. , Simmons, A. , Vergani, F. , Murphy, D. G. , & Catani, M. (2011). A lateralized brain network for visuospatial attention. Nature Neuroscience, 14(10), 1245–1246. 10.1038/nn.2905 [DOI] [PubMed] [Google Scholar]
- Uusitalo, M. A. , & Ilmoniemi, R. J. (1997). Signal‐space projection method for separating MEG or EEG into components. Medical & Biological Engineering & Computing, 35(2), 135–140. [DOI] [PubMed] [Google Scholar]
- van Dijk, H. , Schoffelen, J. M. , Oostenveld, R. , & Jensen, O. (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. The Journal of Neuroscience, 28(8), 1816–1823. 10.1523/JNEUROSCI.1853-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen, B. D. , van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering, 44(9), 867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Wiesman, A. I. , Heinrichs‐Graham, E. , McDermott, T. J. , Santamaria, P. M. , Gendelman, H. E. , & Wilson, T. W. (2016). Quiet connections: Reduced fronto‐temporal connectivity in nondemented Parkinson's disease during working memory encoding. Human Brain Mapping, 37, 3224–3235. 10.1002/hbm.23237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , Heinrichs‐Graham, E. , Proskovec, A. L. , McDermott, T. J. , & Wilson, T. W. (2017). Oscillations during observations: Dynamic oscillatory networks serving visuospatial attention. Human Brain Mapping, 38(10), 5128–5140. 10.1002/hbm.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , O'Neill, J. , Mills, M. S. , Robertson, K. R. , Fox, H. S. , Swindells, S. , & Wilson, T. W. (2018). Aberrant occipital dynamics differentiate HIV‐infected patients with and without cognitive impairment. Brain, 141, 1678–1690. 10.1093/brain/awy097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , & Wilson, T. W. (2018). The impact of age and sex on the oscillatory dynamics of visuospatial processing. NeuroImage, 185, 513–520. 10.1016/j.neuroimage.2018.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , & Wilson, T. W. (2020). Attention modulates the gating of primary somatosensory oscillations. NeuroImage, 211, 116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , Proskovec, A. L. , & McDermott, T. J. (2016). Neuroimaging with magnetoencephalography: A dynamic view of brain pathophysiology. Translational Research, 175, 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , Robertson, K. R. , Sandkovsky, U. , O'Neill, J. , Knott, N. L. , … Swindells, S. (2013). Functional brain abnormalities during finger‐tapping in HIV‐infected older adults: A magnetoencephalography study. Journal of Neuroimmune Pharmacology, 8(4), 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Lew, B. J. , Spooner, R. K. , Rezich, M. T. , & Wiesman, A. I. (2019). Aberrant brain dynamics in neuroHIV: Evidence from magnetoencephalographic (MEG) imaging. Progress in Molecular Biology and Translational Science, 165, 285–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Proskovec, A. L. , Heinrichs‐Graham, E. , O'Neill, J. , Robertson, K. R. , Fox, H. S. , & Swindells, S. (2017). Aberrant neuronal dynamics during working memory operations in the aging HIV‐infected brain. Scientific Reports, 7, 41568 10.1038/srep41568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Rojas, D. C. , Reite, M. L. , Teale, P. D. , & Rogers, S. J. (2007). Children and adolescents with autism exhibit reduced MEG steady‐state gamma responses. Biological Psychiatry, 62(3), 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Slason, E. , Asherin, R. , Kronberg, E. , Teale, P. D. , Reite, M. L. , & Rojas, D. C. (2011). Abnormal gamma and beta MEG activity during finger movements in early‐onset psychosis. Developmental Neuropsychology, 36(5), 596–613. 10.1080/87565641.2011.555573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Dr. Tony W. Wilson, upon reasonable request.


