Abstract
Previous research in young adults has demonstrated that both motor learning and transcranial direct current stimulation (tDCS) trigger decreases in the levels of gamma‐aminobutyric acid (GABA) in the sensorimotor cortex, and these decreases are linked to greater learning. Less is known about the role of GABA in motor learning in healthy older adults, a knowledge gap that is surprising given the established aging‐related reductions in sensorimotor GABA. Here, we examined the effects of motor learning and subsequent tDCS on sensorimotor GABA levels and resting‐state functional connectivity in the brains of healthy older participants. Thirty‐six older men and women completed a motor sequence learning task before receiving anodal or sham tDCS to the sensorimotor cortex. GABA‐edited magnetic resonance spectroscopy of the sensorimotor cortex and resting‐state (RS) functional magnetic resonance imaging data were acquired before and after learning/stimulation. At the group level, neither learning nor anodal tDCS significantly modulated GABA levels or RS connectivity among task‐relevant regions. However, changes in GABA levels from the baseline to post‐learning session were significantly related to motor learning magnitude, age, and baseline GABA. Moreover, the change in functional connectivity between task‐relevant regions, including bilateral motor cortices, was correlated with baseline GABA levels. These data collectively indicate that motor learning‐related decreases in sensorimotor GABA levels and increases in functional connectivity are limited to those older adults with higher baseline GABA levels and who learn the most. Post‐learning tDCS exerted no influence on GABA levels, functional connectivity or the relationships among these variables in older adults.
Keywords: aging, functional connectivity, functional neuroimaging, gamma‐aminobutyric acid, motor learning
Gamma‐aminobutyric acid (GABA)‐edited magnetic resonance spectroscopy of the sensorimotor cortex and resting‐state (RS) functional magnetic resonance imaging data were acquired before and after a motor learning task and either anodal or sham transcranial direct current stimulation (tDCS). Results demonstrated that neither motor sequence learning nor subsequent tDCS significantly modulated GABA+ levels or RS functional connectivity at the group level in older adults. However, individual differences in the learning‐related modulations in GABA+ levels were associated with the participants' age, baseline GABA+ levels, and the magnitude of learning. Higher baseline GABA was also linked to a learning‐related increase in RS functional connectivity between motor task‐relevant regions, including bilateral motor cortices.
1. INTRODUCTION
Gamma‐aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system, plays a vital role in maintaining the excitation/inhibition balance that is essential for optimal brain functioning, including the neural processes underlying learning and memory (e.g., Le Roux, Amar, Moreau, Baux, & Fossier, 2008; Trepel & Racine, 2000). Previous research in young adults has demonstrated that learning a novel motor skill triggers a decrease in sensorimotor cortical GABA levels, as assessed by magnetic resonance spectroscopy (MRS; Floyer‐Lea, Wylezinska, Kincses, & Matthews, 2006; Kolasinski et al., 2019; Sampaio‐Baptista et al., 2015). These learning‐related decreases in the level of inhibitory GABA are thought to favor synaptic plasticity (Stagg, 2014; Stagg, Bachtiar, & Johansen‐Berg, 2011b) and are paralleled by increased functional connectivity among task‐relevant brain regions in the resting state (RS) (Bachtiar, Near, Johansen‐Berg, & Stagg, 2015; Stagg et al., 2014).
While research on the relationship between motor learning and GABA levels is abundant in young adults, considerably less is known in the context of healthy aging. This gap in the available literature is surprising given that older age is associated with deficits in motor learning and memory processes (e.g., King, Fogel, Albouy, & Doyon, 2013) as well as reduced GABA levels in multiple brain regions, including the sensorimotor cortex (Cassady et al., 2019; Chalavi et al., 2018; Cuypers et al., 2020; Gao et al., 2013; Grachev & Apkarian, 2001; Hermans, Leunissen, et al., 2018). It could be speculated that the lower GABA levels observed in older adults may compromise the reduction in GABA that is known to be critical for successful motor learning. This potential explanation, however, has yet to be adequately examined and is indeed a focus of the current investigation.
Interestingly, one avenue that has shown potential to facilitate motor learning and memory processes in both young and older adults is transcranial direct current stimulation (tDCS; e.g., Nitsche et al., 2003; Rumpf et al., 2017; Stagg, Jayaram, et al., 2011, but see Chen et al., 2019; Lopez‐Alonso et al., 2018; Vancleef, Meesen, Swinnen, & Fujiyama, 2016, for examples of no effect of stimulation). Anodal tDCS applied over the primary motor cortex has been shown to reduce sensorimotor GABA levels and increase connectivity within motor RS networks in young individuals (Antonenko et al., 2019; Bachtiar et al., 2015, 2018; Kim, Stephenson, Morris, & Jackson, 2014; Patel et al., 2019; Stagg et al., 2009, 2014; Stagg, Bachtiar, & Johansen‐Berg, 2011a). More importantly, this tDCS‐induced decrease in GABA has been shown to be positively correlated with the magnitude of motor learning (Kim et al., 2014; Stagg et al., 2011a). These data collectively suggest that tDCS may enhance motor learning processes by modulating GABA levels and RS functional connectivity in motor‐related regions. Antonenko et al. (2017) extended this line of research to an aging population and demonstrated that anodal tDCS to the motor cortex—relative to a sham stimulation condition—decreased sensorimotor GABA and functional connectivity in older adults. However, learning was not investigated in this study and thus the link among motor learning and modulations in GABA and RS connectivity in an aging population remains unknown.
Here, we examined the effects of motor sequence learning (MSL) and subsequent tDCS to the primary motor cortex on sensorimotor cortical GABA levels and functional communication between motor task‐relevant regions in healthy older adults. MRS from the sensorimotor cortex and whole brain RS functional magnetic resonance imaging (fMRI) data were obtained prior to and following the completion of a motor learning task and either anodal or sham tDCS. On the one hand, the well‐documented aging‐associated reductions in GABA levels (Cassady et al., 2019; Chalavi et al., 2018; Gao et al., 2013; Grachev & Apkarian, 2001; Hermans, Leunissen, et al., 2018) may mitigate the learning‐ and tDCS‐induced modulations in GABA and RS functional connectivity that have been previously observed in healthy young individuals. On the other hand, and based on the aforementioned tDCS study (Antonenko et al., 2017), learning‐ and stimulation‐related modulations may be predominantly preserved in healthy aging despite reductions in baseline GABA levels. The current study aims to differentiate these two alternatives.
2. METHODOLOGY
2.1. Participants
Healthy, right‐handed (Oldfield, 1971) older adults (>60 years of age) were recruited from Leuven and the surrounding area to serve as participants. Eligibility criteria included nonsmokers, free of psychoactive and sleep‐aid medications, no known history of neurological, psychological, psychiatric or sleep disorders (based on self‐report), no signs of cognitive impairment, as indicated by the Mini‐Mental State Examination (Cockrell & Folstein, 1988), and free of MRI and tDCS contra‐indications. To ensure minimal experience in tasks requiring dexterous finger movements similar to that employed in the current study, professional musicians and typists were excluded. Similarly, individuals that previously participated in research involving a MSL task were excluded. Those who worked night shifts or took trans‐meridian trips in the month prior to the experiment were also not permitted to participate. Of the 44 participants who met these criteria and were assigned to an experimental group (anodal or sham tDCS; see below for details), 8 were excluded from all analyses: 4 failed to appropriately perform the MSL task (i.e., >2.5 SD below the mean for sequence accuracy) and 4 had unuseable/missing data from one of the MRS acquisition time points. Participant characteristics of the 36 individuals included in the MRS and behavioral data analyses are provided in Table 1. Note that an additional three participants (one anodal, two sham) were excluded from RS connectivity analyses due to excessive head motion during acquisition. Specifically, frame‐wise displacement exceeded 0.5 mm in more than 33% of the acquired volumes in at least one RS time point. Accordingly, less than 100 volumes were remaining for analyses after scrubbing (i.e., see details in RS fMRI preprocessing section below) of these high‐motion volumes and thus these individuals were removed from further RS analyses only.
TABLE 1.
Participant characteristics
| Anodal | Sham | Group differences | |
|---|---|---|---|
| n | 17 | 19 | – |
| Females | 8 | 8 | – |
| Mean age (years) | 66.6 ± 4.1 | 67.6 ± 4.4 | p = .47 |
| Handedness | 86.4 ± 20.8 | 89.9 ± 14.7 | p = .55 |
| BAI | 3.8 ± 4.1 | 3.2 ± 3.8 | p = .62 |
| BDI | 2.8 ± 4.4 | 3.8 ± 2.5 | p = .39 |
| PSQI | 2.4 ± 1.4 | 3.3 ± 2.0 | p = .12 |
| MMSE | 29.4 ± 0.9 | 29.4 ± 1.1 | p = .96 |
| SSS | 1.9 ± 0.8 | 1.9 ± 0.6 | p = .95 |
| PVT (ms) | 396 ± 29 | 399 ± 31 | p = .78 |
Note: Group means ± SD for participant characteristics, standardized questionnaires as well as the vigilance assessments administered at time of testing. Handedness scores are from (Oldfield, 1971). Statistical information in the column “Group Differences” reflect results from independent samples t tests. The two groups did not differ on any of the measures.
Abbreviations: BAI, Beck's anxiety inventory (Beck, Epstein, Brown, & Steer, 1988); BDI, Beck's Depression Inventory (Beck, Steer, Ball, & Ranieri, 1996); MMSE, Mini‐Mental State Examination (Cockrell & Folstein, 1988); PSQI, Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989); PVT, psychomotor vigilance task (Dinges & Powell, 1985); SSS, Stanford Sleepiness Scale (MacLean, Fekken, Saskin, & Knowles, 1992).
Written informed consent was obtained before testing. The local ethics committee for biomedical research approved all experimental procedures. Participants received monetary compensation for their time, effort, and incurred travel costs.
2.2. Experimental design
Following a screening session in which questionnaires were completed to assess eligibility, participants returned to the University Hospital of KU Leuven. Note that the full protocol consisted of a second experimental session later on the same testing day (see Figure S1); however, the focus of the current manuscript is limited to the motor learning‐ and stimulation‐induced changes in GABA levels and RS functional connectivity assessed within a single experimental session (Figure 1).
FIGURE 1.

Following completion of a MSL task with the nondominant left hand inside the MRI scanner, participants were randomly assigned to either an anodal or sham tDCS experimental condition (anodal electrode centered above right primary motor cortex). MRS from the right sensorimotor cortex (contralateral to the hand used to perform the motor task) and whole brain RS fMRI data were obtained prior to and following the MSL task/tDCS intervention. The experiment was completed between approximately 11 a.m. and 1 p.m. for all participants. The approximate durations (in minutes) for each of the various measurements are provided in the figure. As the number of sequence repetitions was held constant in the MSL task, the duration of the training session varied depending on the participants movement speed (mean = 16 min; range = 12–24 min). Note that participants were removed from the MRI scanner and taken to an adjacent testing room to complete the stimulation session. The tDCS electrode positions were marked, but not attached, prior to entering the scanner in order to minimize the time between end of the learning episode and the onset of stimulation (mean duration = 8 min; range = 5–16 min). As the participants were removed from the scanner for the tDCS, the delay between post‐learning/stimulation MRS time point and the end of MSL training and the end of stimulation varied. The post‐MRS measurements started approximately 32 min (range = 27–40) and 8 min (range = 5–15) after the completion of the MSL and stimulation, respectively. Previous research in young healthy adults has indicated that modulations in GABA are detectable up to 20–50 min after a learning or stimulation intervention (Bachtiar et al., 2018; Floyer‐Lea et al., 2006; Patel et al., 2019). Changes in RS connectivity have been observed hours following motor learning (e.g., Sami, Robertson, & Miall, 2014). fMRI, functional magnetic resonance imaging; GABA, gamma‐aminobutyric acid; MRS, magnetic resonance spectroscopy; MSL, motor sequence learning; tDCS, transcranial direct current stimulation; RS, resting state
Participants first completed the psychomotor vigilance test (PVT; Dinges & Powell, 1985) and the Stanford sleepiness scale (SSS; MacLean et al., 1992) to provide objective and subjective, respectively, measures of vigilance at the time of testing (results presented in Table 1). Participants were subsequently positioned supine in the MRI scanner at approximately 11 a.m. Following localizer and structural brain scans, baseline (i.e., prior to the motor task and tDCS) RS fMRI and MRS data were acquired (see below for scan details). All participants then completed a MSL task while functional blood oxygen level‐dependent (BOLD) images were obtained. Note that analyses of task‐related imaging data for this manuscript are restricted to identifying regions of interest (ROIs) for the analysis of RS fMRI data. Following the completion of the MSL task with their nondominant left hand, participants were removed from the MR scanner and taken to an adjacent testing room to complete either anodal or sham tDCS (randomly assigned; see below for details) applied to the right motor cortex (i.e., contralateral to the hand used to practice the task). We elected to remove participants from the scanner for the tDCS; otherwise, participants would have remained in the scanner for two consecutive hours in order to complete the full imaging protocol. tDCS was administered immediately after (i.e., in the offline state), as opposed to during (i.e., online), the learning session in order to avoid confounds related to the known beneficial effects of online stimulation on motor performance (e.g., Hamoudi et al., 2018; Hummel et al., 2010; Nitsche et al., 2003; Zimerman et al., 2013). Specifically, with an online stimulation protocol, any observed effects on GABA or RS connectivity could be attributed to tDCS directly influencing GABA/connectivity; or, alternatively, tDCS enhancing motor performance which in turn influences GABA/connectivity (i.e., an indirect effect of tDCS on GABA/connectivity). Our design then allows us to differentiate the effect of learning only (i.e., sham group) versus learning potentiated by stimulation (i.e., anodal group) on GABA levels and connectivity patterns. Thus, the differences between the anodal and sham groups reflect the effect of anodal tDCS beyond any motor learning‐related effects. After completing the tDCS session, participants immediately returned to the scanner and post‐learning/stimulation RS fMRI and MRS sequences were completed. It is worth explicitly mentioning that, as stimulation was administered immediately after motor task performance in our design, we aimed to examine the effects of motor learning and subsequent tDCS on GABA and functional connectivity and not the influence of tDCS on motor behavior as investigated in previous research (e.g., Reis & Fritsch, 2011).
2.3. Motor sequence learning task
MSL was tested with an adapted version of the sequential finger tapping task (Karni et al., 1995) that has been employed extensively by members of our team (Dan, King, Doyon, & Chan, 2015; Fogel et al., 2014; King, Saucier, et al., 2017). Prior to the initial training session, participants were shown a PowerPoint (Microsoft Corporation, Redmond, WA) presentation providing detailed instructions on the series of tasks described below. While positioned supine in the scanner, participants were instructed to use fingers of their (nondominant) left hand and the corresponding buttons of a custom‐made MR‐compatible response box with four buttons in order to perform an explicitly known sequence of finger presses: 4–1–3–2–4, where 1–4 correspond to the index, middle, ring, and little fingers, respectively. To ensure that participants knew the appropriate sequence of finger movements prior to beginning the MSL task, they completed three consecutive sequences slowly and without errors. Subsequently, for the training phase, participants were then asked to execute the sequence of finger movements as fast as possible while making as few errors as possible. Participants were required to continuously repeat the motor sequence when a fixation cross, displayed on the rear‐projected screen, became green and to rest when the cross was red (20‐s rest breaks). Unbeknownst to the participants, the cross remained green until the completion of 60 presses (ideally corresponding to 12 correct sequences). Participants were instructed to return to the beginning of the sequence if an error was made. The initial training session consisted of 12 blocks of practice followed by an immediate (~1–2‐min pause) posttest of four blocks, affording an end‐of‐training performance assessment following the dissipation of mental and physical fatigue.
During the MSL session, the timing of all key presses was recorded for subsequent data processing. The primarydependent measure was sequence duration, which reflects movement speed and was computed as the average time in seconds to complete a correct sequence within a block (i.e., smaller values are indicative of increased speed). Although sequence accuracy can also be a viable measure and has been used extensively in previous research (e.g., Albouy et al., 2016; Dan et al., 2015; Dolfen, King, Schwabe, Swinnen, & Albouy, 2019), the percentage of correct transitions was high in the current study (92% on average) and participants, on average, did not exhibit practice‐dependent modulations in accuracy. As such, we elected to only report results related to the speed measure in the main text. Additional information on movement accuracy is provided in Figure S2.
2.4. Transcranial direct current stimulation
Parameters for the post‐learning tDCS were selected based on previous research by members of our team (Rumpf et al., 2017, 2018). Specifically, stimulation was administered with a DC‐Stimulator Plus from Neuroconn (Ilmenau, Germany). Two 5 × 5‐cm2 rubber electrodes were placed inside sponges soaked in a saline solution. The anode was placed at the C4 position defined in the international 10–20 system, which is located approximately over the hand area of the right primary motor cortex (i.e., contralateral to the hand used to perform the motor task). The cathode was placed on the left supraorbital region contralateral to the anode. For the anodal stimulation group, current was increased in a ramp‐like manner over a duration of 8 s until the desired current of 1 mA was reached (surface current density = 0.04 mA/cm2). This stimulation intensity is identical to earlier work demonstrating tDCS‐induced modulations of GABA and functional connectivity in younger and older adults (Antonenko et al., 2017; Bachtiar et al., 2015) as well as improvements in motor performance in healthy older individuals (Rumpf et al., 2017). Although stimulation intensity was matched to these previous studies, note that the size of the electrodes in the current research (5 × 5 cm2) was smaller. The duration of stimulation at 1 mA was 15 min. For sham stimulation, current ramped to and stayed at 1 mA for only 30 s, and then faded out over a duration of 8 s. This procedure is successful in blinding the subject to experimental condition (Gandiga, Hummel, & Cohen, 2006; Nitsche et al., 2008). The researcher was also blind to stimulation condition using the “study mode” available in the DC‐Stimulator Plus. Consistent with earlier research (Rumpf et al., 2017), participants viewed nature images without sound during the stimulation session.
2.5. Brain imaging data acquisition and processing
A Philips (Philips Healthcare, Amsterdam, the Netherlands) Achieva 3.0T MRI system with 32‐channel head coil was used for image acquisition.
2.5.1. Structural images
A three‐dimensional (low‐resolution) T1‐weighted structural image was acquired with a magnetization‐prepared rapid‐acquisition gradient‐echo (MPRAGE) sequence prior to each of the MRS time points (repetition time (TR) /echo time (TE) = 9.6/4.6 ms; voxel size = 1.2 × 1.2 × 2.0 mm3; field of view = 250 × 250 × 222 mm3; 111 coronal slices). These lower‐resolution images were acquired due to time constraints during the experimental protocol. They were of sufficient quality to position the MRS voxel, but not for subsequent data processing. Therefore, a high‐resolution T1‐weighted structural image was acquired with a MPRAGE sequence during a separate scanning session later in the same day (TR/TE = 9.6/4.6 ms; voxel size = 0.98 × 0.98 × 1.2 mm3; field of view = 250 × 250 × 192 mm3; 160 coronal slices). This high‐resolution T1‐weighted image was independently coregistered to the two low‐resolution images with SPM12, thus resulting in a high‐resolution structural image for each MRS time point that was used in data processing.
2.5.2. Magnetic resonance spectroscopy
The quantification of GABA in a predefined ROI was assessed via in‐vivo proton (1H) MRS (Mullins et al., 2014; Puts & Edden, 2012). For each of the two MRS time points in the current study, spectra were acquired from the right sensorimotor cortex (contralateral to the hand used to perform the motor task, but directly below the anodal tDCS electrode positioned at C4). The exact positioning of the 3 × 3 × 3 cm3 MRS voxel was based on the acquired time point‐specific T1 image, centered above the right hand knob area (Yousry et al., 1997) and rotated in the coronal and sagittal planes to align with the cortical surface of the brain (Figure 2(a)). The average within‐subject overlap in voxel position for the two time points was equal to 83.88 ± 7.1% (mean ± SD), suggesting high consistency in the placement of the MRS voxels (see Figure S3 for heat maps depicting the degree of spatial overlap across all participants and time points). Data were acquired using the Mescher–Garwood point resolved spectroscopy sequence (Mescher, Merkle, Kirsch, Garwood, & Gruetter, 1998), with parameters similar to previous research (Hermans, Levin, et al., 2018; Maes et al., 2018; Mikkelsen et al., 2017; 14‐ms editing pulses applied at an offset of 1.9 ppm in the on experiment and 7.46 ppm in the off experiment, TR = 2 s, TE = 68 ms, 2‐kHz spectral width, multiple optimizations insensitive suppression train water suppression, 320 averages, scan duration of 11 min, 12 s). Sixteen water‐unsuppressed averages were acquired from the same voxel and interleaved to allow for real‐time frequency correction (Edden et al., 2016), which is of particular importance after fMRI scanning (Harris et al., 2014). Scan parameters were identical for the two MRS time points. During data acquisition, a dark screen (i.e., no visual stimulus) was presented; participants were simply instructed to remain still for the duration of the scan.
FIGURE 2.
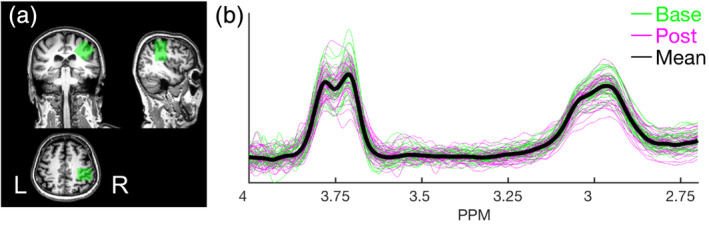
(a) Depiction of MRS voxel positioning in the right sensorimotor cortex of a randomly selected participant and time point. The MRS voxel is overlaid on the participant and time point‐specific T1 structural image. Image created in the software MRIcro available at https://www.mccauslandcenter.sc.edu/crnl/mricro. (b) Spectra from all participants and time points included in the analyses (n = 72; 36 participants × two time points). GABA+ peak is visible at 3 ppm. Green and magenta represent the baseline and post‐learning/stimulation time points, respectively (thick black line = mean spectrum collapsed across time points and participants). GABA, gamma‐aminobutyric acid; MRS, magnetic resonance spectroscopy
MRS data were analyzed with the Gannet software 3.0 toolkit (Edden, Puts, Harris, Barker, & Evans, 2014). Individual frequency domain spectra were frequency‐ and phase‐corrected using spectral registration (Near et al., 2015). Data were subsequently filtered with a 3‐Hz exponential line broadening. Individual ON and OFF spectra were averaged and subtracted, resulting in an edited difference spectrum. The GABA signal from this difference spectrum was modeled at 3 ppm with a single Gaussian peak and a 5‐parameter Gaussian model whereas the unsuppressed water signal, to be used as the reference compound (Mikkelsen et al., 2019), was fit with a Gaussian–Lorentzian model. The integrals of the modeled data were then used to quantify uncorrected GABA levels. It should be emphasized that this scheme edits GABA as well as macromolecules at 3 ppm (Edden, Puts, & Barker, 2012; Rothman, Petroff, Behar, & Mattson, 1993) and thus GABA levels reported herein are referred to as GABA+ (i.e., GABA+ macromolecules). To adjust GABA+ levels for heterogeneity in voxel tissue composition, MRS voxels coregistered to the high‐resolution T1 were segmented into different tissue fractions (gray matter [GM], white matter [WM], and cerebrospinal fluid [CSF]) with SPM12. These voxel compositions were used to compute tissue‐corrected GABA+; specifically, it was assumed that GABA+ levels are negligible in CSF and twice as high in GM relative to WM (Harris, Puts, & Edden, 2015). Tissue‐specific relaxation and water visibility values were also considered as in Harris et al. (2015). Last, GABA+ levels were normalized to the average voxel composition in our sample of older adults (Harris et al., 2015). As such, the reported GABA+ values reported in this manuscript, specified in institutional units (i.u.), correspond to the “QuantNormTissCorrGABAiu” variable in Gannet 3.0.
Quality of the MRS data was assessed with the following measures: GABA signal‐to‐noise ratio, fit error of the GABA peak, SD of the water frequency offset and linewidth (LW), quantified as the full‐width half‐maximum (FWHM) of the modeled N‐acetylaspartate (NAA) signal. These data quality metrics and MRS voxel tissue fractions, as well as corresponding statistical analyses to assess potential effects of experimental group and MRS time point, are detailed in Table S1. In brief, the averaged quality and tissue fraction values are comparable to a recent, large multicenter study (Mikkelsen et al., 2017) as well as in previous research in our laboratory (Hermans, Levin, et al., 2018; Maes et al., 2018). There were no group differences (i.e., anodal vs. sham) or group × time point effects in any of the data quality metrics or tissue fractions. There were, however, significant time point effects for NAA LW and the SD of the water frequency offset, with the post‐learning/stimulation time point having significantly worse data quality. Although these time point effects were unexpected, the differences in data quality did not exert a significant influence on GABA+ levels (Figure S4). Specifically, the GABA+ levels were uncorrelated with both NAA LW and SD of the water frequency offset (both p > .68).
2.5.3. Resting‐state fMRI
Functional RS data were acquired with an ascending gradient echoplanar imaging (EPI) pulse sequence for T2*‐weighted images (TR = 2,500 ms; TE = 30 ms; flip angle = 90°; 45 transverse slices; interslice gap = 0.25 mm; voxel size = 2.5 × 2.5 × 3 mm3; field of view = 200 × 200 × 146 mm3; matrix = 80 × 78; 162 dynamic scans plus four dummy scans discarded at the beginning of the sequence). During data acquisition, a dark screen (i.e., no visual stimuli) was presented; participants were instructed to remain still, close their eyes and to not think of anything in particular for the duration of the scan.
Preprocessing
Functional volumes were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; Wellcome Department of Neuroimaging Neuroscience, London, UK) implemented in Matlab. Specifically, each participant's functional volumes were realigned to the first volume of the session using rigid body transformations and then slice time corrected to the middle slice (reference slice = 22). Functional images were coregistered to the high resolution T1‐weighted anatomical image using a rigid body transformation optimized to maximize the normalized mutual information between the two images. The structural image was segmented into GM, WM, CSF, bone, soft tissue and background. An average subject‐based template was created using DARTEL in SPM 12 and registered to the Montreal Neurological Institute (MNI) space. All functional and anatomical images were then normalized to the resulting template.
Head motion in six dimensions (i.e., rotations and linear translations in three planes of movement) was quantified for each subject during the RS scan as a result of the functional realignment preprocessing step. Across all subjects included in the RS analyses (n = 33) and the two time points, the average ± SD of the maximum displacements across all 162 volumes and the three planes of movement were 0.51 ± 0.33 mm and 0.57 ± 0.40° for translations and rotations, respectively, suggesting minimal participant head movement. Critically, there were no significant group, time point, or group by time point effects on these movement parameters (all p > .10; see Table S2).
Defining motor task‐relevant ROIs
We were interested in the effect of learning/stimulation on RS functional connectivity between the right motor cortex (i.e., the same region from which GABA data were acquired) and other task‐relevant areas. As such, task‐based functional connectivity analyses were used to identify which areas of the brain exhibited a significant increase in connectivity with right M1 as a function of performance improvements. To do this, we performed PsychoPhysiological Interaction (PPI) analyses on fMRI data collected during task practice with an ascending gradient EPI pulse sequence for T2*‐weighted images (TR = 3,000 ms; TE = 30 ms; flip angle = 90°; 54 transverse slices; interslice gap = 0.20 mm; voxel size = 2.5 × 2.5 × 2.5 mm3; field of view = 210 × 210 × 146 mm3; matrix = 84 × 82; 2 dummy scans discarded at the beginning of the run). PPI analyses were seeded in the right primary motor cortex that exhibited the local maximum of brain activity during MSL task practice. Specifically, and similar to our previous work (King, Saucier, et al., 2017), fMRI data were preprocessed similarly as described above and then were analyzed in two serial steps to account for intraindividual (fixed effects) and interindividual (random effects) variance. Changes in brain responses were estimated, for each subject, by a model including the responses to the learned sequence and their linear modulation by performance speed (mean time to perform a correct sequence per block). These regressors consisted of box cars convolved with the canonical hemodynamic response function. High‐pass filtering was implemented in the design matrix using a cutoff period of 128 s to remove slow drifts from the time series. Serial correlations in fMRI signal were estimated using an autoregressive (Order 1) plus white noise model and a restricted maximum likelihood algorithm. Linear contrasts tested the main effect of task practice at the individual level were then further spatially smoothed (Gaussian kernel 6‐mm FWHM) and entered in a second‐level analysis, accounting for intersubject variance and allowing inferences to be made at the population level. A one‐sample t test was run on the entire sample, resulting in an activation map of the t‐statistic (SPM[T]) thresholded a p < .001 uncorrected. The main effect of practice at the group level revealed a peak of activation within right M1 (MNI coordinate: x = 38, y = −20, z = 54) that was subsequently used as a seed region for PPI analyses.
PPI analyses were conducted to test the changes in functional connectivity of this right M1 seed region with the rest of the brain as a function of task practice. To do so, time series were extracted from a 6‐mm radius sphere centered on the right M1 seed region identified as described above. Importantly, this sphere was located within the group‐averaged MRS voxel (see Figure S5). Linear models were generated, at the individual level, with a first regressor representing the practice of the motor sequence modulated by performance speed, a second regressor corresponding to the BOLD signal in the M1 seed and a third regressor representing the interaction between the first (psychological) and second (physiological) regressors. To build this regressor, the underlying neuronal activity was first estimated by a parametric empirical Bayes formulation, combined with the psychological factor, and subsequently convolved with the hemodynamic response function (Gitelman, Penny, Ashburner, & Friston, 2003). The linear contrast testing for the interaction between the psychological and physiological regressors at the individual level was then further spatially smoothed (Gaussian kernel 6‐mm FWHM) and entered in a second‐level analysis, accounting for intersubject variance and allowing inferences to be made at the population level. A one‐sample t test including all participants was performed to assess which brain regions exhibited increased functional connectivity with the right M1 seed as a function of performance improvement (p < .001 uncorrected). A significant PPI therefore indicated a change in the regression coefficient (i.e., a change in the strength of the functional interaction) between any reported brain area and the M1 seed, related to improvement in performance speed during training. From this connectivity map, we extracted the coordinates of the local maxima within clusters that were at least 20 voxels in size and greater than 20 mm apart from one another. This resulted in 14 regions, including left M1, left supplementary motor area, left superior parietal cortex (two regions), left precuneus, bilateral rolandic operculum, bilateral frontal cortices, bilateral cerebellar lobule VIII, left cerebellar lobule VI, and cerebellar vermis. A ROI with a 6‐mm radius sphere centered on each peak voxel was then built with the MarsBAR toolbox in Matlab and used in subsequent RS functional connectivity analyses. Figure S5 and Table S3depict and list, respectively, these 15 ROIs constituting the task‐relevant motor network (i.e., the initial right M1 seed identified based on the activation analysis plus the 14 regions that exhibited increased connectivity with right M1 as a function of performance improvements as revealed by the PPI analysis).
Functional connectivity of the task‐relevant motor network during RS
The analysis pipeline was conducted in Matlab and was similar to that employed in our previous research (King et al., 2018). Prior to running the connectivity analyses, additional preprocessing steps were completed to remove variance from spurious sources. First, to minimize the impact of motion on the correlations between ROIs, data were “scrubbed” to remove volumes in which the scan‐to‐scan displacement exceeded 0.5 mm (e.g., Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Data were then high‐pass filtered with a cutoff of 0.01 Hz. Regression analyses were performed on the fMRI time series, including the six‐dimensional head motion realignment parameters, the realignment parameters squared, their derivatives, the square of the derivatives, as well as the first three principal component time series extracted from WM and CSF masks as regressors. The resulting residuals were then low‐pass filtered with a cutoff of 0.08 Hz. Data filtering served to minimize high‐frequency noise that may be the result of cardiac and respiratory factors (Fox et al., 2005; Fox & Raichle, 2007).
At the individual level, the time‐series across all voxels within each region were averaged and Pearson correlation coefficients between the right M1 seed and the other 14 ROIs were computed. We restricted our analyses to right M1 connectivity to match the GABA data acquired from the right sensorimotor cortex. Each correlation coefficient r was converted to z‐values with the formula:
where nvols = number of BOLD volumes recorded (Fox et al., 2005). Statistical analyses of the correlation data were performed on these z‐values.
2.6. Statistical analyses
Behavioral data were analyzed using repeated measures analysis of variances (ANOVAs) on the dependent measure sequence duration for the training and test runs separately with block and group (anodal/sham) as factors of interest. In case of violation of the sphericity assumption, Greenhouse–Geisser corrections were applied. In order to assess the relationship between motor learning and measures of GABA+ levels, a learning magnitude measure was computed as the percent decrease in sequence duration from the first block of the initial training run to the last three blocks of the immediate posttest run. This measure was computed based on the last three—as opposed to four—blocks of the test run, as performance was stable across this subset of blocks (i.e., no significant block effect; see Section 3). An independent samples t test was used to assess group differences in learning magnitude. It is worth explicitly stating that no group (i.e., anodal/sham) differences were expected on the motor task, as the stimulation was administered after the learning episode. Threshold for significance was set at α = .05 for all behavioral contrasts.
GABA+ levels were analyzed using a repeated measures ANOVA with time point (baseline, post‐learning/stimulation) and experimental group (anodal/sham) as factors of interest (α = .05). We then adopted an individual differences approach in order to assess the pairwise relationships—and the modulatory influence of experimental group—among the following four variables of interest: age, MSL magnitude, baseline GABA+, and percent change in GABA+ across the two time points (i.e., reflecting learning/stimulation‐induced modulations). For each of the six pairs of variables, within‐group (i.e., anodal/sham) Pearson's correlation coefficients between the two variables of interest were transformed to z‐scores using Fishers' r‐to‐z transformation and these z‐scores were statistically compared to assess if the stimulation modulated the relationship between the two continuous variables. A single and z‐transformed Pearson's correlation coefficient was subsequently computed across groups when the within‐group correlation coefficients were not statistically different from one another (i.e., effectively collapsing across the nonsignificant factor of group). Within each family of hypothesis tests, corrections for multiple correlations were conducted using the false discovery rate (FDR) method with the desired FDR value for significance testing set to 0.05.
With respect to the RS connectivity data, we first assessed connectivity during the baseline time point (i.e., prior to the motor learning session) to determine if the ROIs extracted based on the task‐related functional connectivity analyses (i.e., PPI) were connected to the right M1 seed at rest. To this end, one‐sample t‐tests were conducted on the z‐transformed correlation coefficients to assess baseline connectivity between the right M1 seed and each of the 14 ROIs. Next, to assess group and time point effects, RS connectivity scores were analyzed with a repeated measures ANOVA with time point (baseline, post‐learning/stimulation) and experimental group (anodal/sham) as factors of interest (α = .05). We then adopted an individual differences approach to assess the relationships between baseline connectivity and the following set of variables: age, baseline GABA, percent change in GABA across the two time points and MSL magnitude. Similarly, we assessed the relationship between the intersession change in connectivity, computed as baseline connectivity subtracted from post‐learning/stimulation connectivity, and the above‐mentioned set of variables. We followed the identical correlational approach as outlined in the preceding paragraph. Specifically, within‐group (i.e., anodal/sham) Pearson's correlation coefficients were computed and transformed with Fishers' r‐to‐z to quantify the relationship between change in connectivity and the specific variable of interest (i.e., age, learning magnitude, baseline GABA, and change in GABA). An across‐group single correlation coefficient was computed if the within‐group correlations did not statistically differ. For all connectivity analyses, the statistical probabilities associated with each family of hypothesis tests were considered significant if surviving the FDR method for multiple comparisons (i.e., correcting among the 14 seed/ROI connectivity pairs). The desired FDR value for significance testing was set to 0.05. For completeness, figures indicate significance at thresholds of both p(FDR) < .05 as well as p(uncorrected) < .05.
3. RESULTS
3.1. Motor sequence learning
To verify that participants learned the motor sequence and to a similar extent in the two experimental groups, a 2 (anodal/sham) × 12 (practice blocks) ANOVA on the dependent measure sequence duration was conducted for the initial training run. Sequence duration decreased across the 12 blocks of training, as indicated by a significant main effect of block (Figure 3a; F 3.58,121.86 = 61.52, p < .001). Both the group effect (F 1,34 = .25, p = .62) and the block × group interaction were not significant (F 3.58,121.86 = 0.94, p = .44), as expected. A similar pattern of results was obtained for the analysis of performance during the immediate post‐training test (block: F 2.20,74.63 = 8.75, p < .001; group: F 1,34 = 1.11, p = .30; block × group: F 2.20,74.63 = 2.02, p = .14) and a performance plateau was achieved across the last three blocks, as indicated by a non‐significant effect of block (F 22,68 = 0.63, p = .54). These results collectively indicate that, as expected, the two groups learned the explicit sequence of finger movements to a similar extent. This was further supported by the lack of a statistically significant group effect on the learning magnitude measure, computed as the percent improvement from the beginning of the training session to the end (last three blocks) of the test session (Figure 3b; t 34 = 1.72, p = .095).
FIGURE 3.
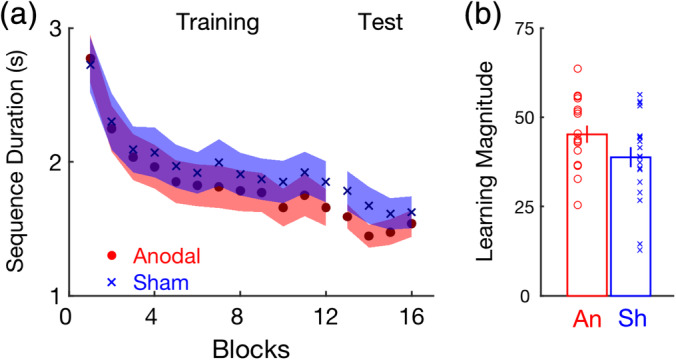
(a) Sequence Duration plotted for the anodal and sham tDCS experimental groups across the 12 blocks of training and the four blocks of the posttest (Test) administered immediately following training. Shaded regions represent SEM. (b) Learning magnitude, computed as the percent change from the first block of the training run to the last three blocks of the test run, plotted for the two experimental groups. Higher scores are indicative of greater within‐session learning. Error bars = SEM. Small circles and crosses depict individual data for the anodal and sham groups, respectively. An, anodal; SEM, standard error of mean; Sh, sham; tDCS, transcranial direct current stimulation
3.2. Sensorimotor cortex GABA+ levels
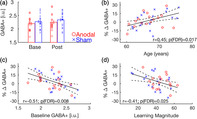
GABA+ levels did not significantly differ between the two experimental groups or across the two MRS time points (Figure 4a; Session main effect: F 1,34 = 1.42, p = .24; group: F 1,34 = 1.30, p = .26; session × group: F 1,34 = 0.02, p = .90). This indicates that, at the group level, neither motor learning nor subsequent anodal tDCS significantly modulated GABA+ levels in healthy older adults. We assessed the effect of group (anodal/sham) on the pairwise relationships among the following four variables of interest: age, MSL magnitude, baseline GABA+, and percent change in GABA+ levels across the two time points. As the within‐group Pearson's correlation coefficients did not statistically differ between the two experimental groups for each of the six pairs of variables (all uncorrected p > .14; see Tables S4 and S5), correlations were conducted across the anodal and sham groups. Results revealed that changes in GABA+ levels from the baseline to the post‐learning/stimulation session were significantly correlated with age, baseline GABA+ levels and the magnitude of motor learning (Figure 4b–d; see Table 2 for full correlational matrix specifying all six pairwise relationships). Participants that were younger, had higher baseline GABA+ levels, and exhibited the greatest amount of motor learning were more likely to exhibit a decrease in GABA+ across the learning/stimulation interval.
FIGURE 4.
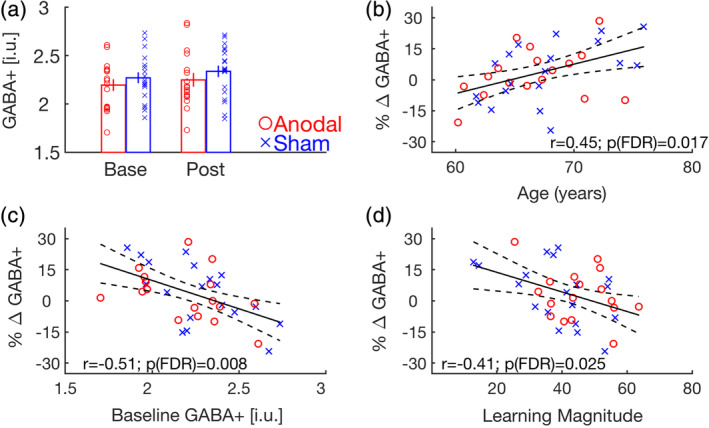
(a) GABA+ levels plotted for the anodal (red) and sham (blue) stimulation groups for the baseline and post‐learning/stimulation sessions. Error bars = SEM. Small circles and crosses depict individual data for the anodal and sham groups, respectively. See Figure S6 for a depiction of the intraindividual change in GABA+ levels across the two time‐points. (b–d) Relationships between % change in GABA+ levels between the two MRS sessions and age (b), Baseline GABA+ levels (c) and motor sequence learning magnitude (d). Solid dark lines represent linear regression fits; dashed lines depict 95% prediction intervals of the linear function. The relationships between any pair of variables were significant across groups (n = 36). For completeness, however, the anodal and sham participants are shown in red circles and blue crosses, respectively. All three correlations survive FDR correction for multiple comparisons (FDR‐adjusted p‐values provided in the plots). Collectively, these three variables account for 41.24% of the variance in % change in GABA+. FDR, false discovery rate; GABA, gamma‐aminobutyric acid; i.u., institutional units; MRS, magnetic resonance spectroscopy; SEM, standard error of mean
TABLE 2.
Correlations among age, GABA+ measures and motor sequence learning magnitude
| Age | Base GABA+ | % ΔGABA+ | Learn Mag | |
|---|---|---|---|---|
| Age | – | – | – | – |
| Base GABA+ | −.32 (.056) | – | – | – |
| % ΔGABA+ | .45 (.006) | −.51 (.001) | – | – |
| Learn Mag | −.29 (.083) | .22 (.196) | −.41 (.013) | – |
Note: Pearson's correlation coefficients and corresponding uncorrected p values (parentheses) are shown for each pair of variables. Those in bold remained significant after correction for multiple comparisons using the FDR approach (adjusted critical p = .013). Correlations were conducted across the two experimental groups (n = 36), but see Tables S4 and S5 for within‐group correlations and group comparisons of the within‐group correlation coefficients, respectively.
Abbreviations: Base, baseline time point; FDR, false discovery rate; GABA, gamma‐aminobutyric acid; % ΔGABA+, percent change in GABA+ from the baseline to post‐learning/stimulation magnetic resonance spectroscopy time points; Learn Mag, magnitude of motor sequence learning in the initial training session.
To ensure that the significant relationships among GABA+ measures, age and motor learning (Figure 4b–d) were not attributed to the decrease of some data quality markers during the post‐learning/stimulation MRS time point (Table S1), we computed partial correlations among our variables of interest (i.e., age, baseline GABA, change in GABA, and learning magnitude) after controlling for the NAA LW and SD of the water frequency offset in the post‐learning/stimulation time point (Tables S6 and S7). Critically, the significant relationships reported in the main text remained after removing the variance explained by these MRS data quality metrics.
As a final point of emphasis, the relationship between change in GABA+ and learning magnitude (Figure 4d) appears to be specific to learning and not motor execution per se. There was no significant relationship between change in GABA+ and individuals' average performance speed during the training session (averaged time to complete a sequence; r = .18; p = .28). Moreover, the partial correlation between change in GABA+ and MSL magnitude remained significant after accounting for the averaged time to complete a sequence (r = −.43; p = .011).
3.3. RS functional connectivity
RS connectivity between right M1 and the identified motor task‐relevant network is shown in Figure 5. One‐sample t tests performed across all participants on the baseline z‐transformed correlation coefficients, representing connectivity prior to MSL, are shown in the top row. As expected, the vast majority of these ROIs, including left M1, SMA, left superior parietal cortex, left precuneus, bilateral rolandic operculum, and three cerebellar ROIs (bilateral lobule VIII and left lobule VI), were significantly connected with the right M1 seed at rest prior to the task. Thus, and unsurprisingly, the regions that exhibited a significant increase in connectivity with the right M1 seed as a function of performance improvements during the MSL task were also significantly connected with the right motor cortex at rest prior to the task.
FIGURE 5.
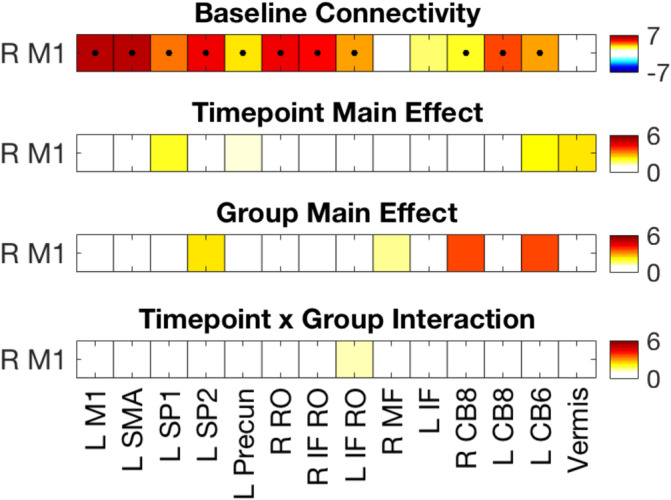
RS functional connectivity between the right M1 seed and the 14 motor‐task relevant ROIs. Top row depicts connectivity during the baseline RS time point collapsed across the two groups (n = 33). Values represent t statistics based on the z‐transformed correlations. Tests of statistical significance were based on a one‐sample t test corrected for multiple comparisons with a FDR threshold set to 0.05. Corrected p value threshold is .0297. • = p(FDR) < .05; o = p(uncorrected) < .05. The bottom three rows depict main effect of time point, group, and the time point by group interaction. Values represent F statistics based on the repeated measures ANOVA conducted on the z‐transformed correlations. There were no significant time point, group, or time point × group effects. The numbers 6 and 8 represent the cerebellar lobules. ANOVA, analysis of variance; CB, cerebellar; FDR, false discovery rate; IF, inferior frontal; L, left; M1, primary motor cortex; MF, medial frontal; Precun, precuneus; R, right; RO, rolandic operculum; ROIs, regions of interest; RS, resting state; SMA, supplementary motor area; SP, superior parietal
Functional connectivity between the right M1 seed and the motor task‐relevant network did not significantly differ between the two experimental groups or across the two RS time points (bottom three rows of Figure 5). These data indicate that, at the group level and similar to the results on GABA+ levels, neither motor learning nor subsequent anodal tDCS significantly modulated functional connectivity between the right motor cortex and other task‐relevant regions in healthy older adults.
We first assessed the relationships between baseline functional connectivity and age, MSL magnitude, baseline GABA+ and percent change in GABA+ across the two MRS time points. The anodal and sham experimental groups did not differ in the relationships between baseline RS connectivity and any of the aforementioned variables after correction for multiple comparisons. Subsequent correlation analyses collapsed across the two groups also did not reveal any significant relationships after correction for multiple comparisons between baseline connectivity and age, baseline GABA+, learning magnitude, and change in GABA+ (see Figure S7). Of note, and consistent with previous research in young (Bachtiar et al., 2015; Stagg et al., 2014) and older adults (Antonenko et al., 2017), baseline connectivity between bilateral M1s did exhibit a negative relationship with baseline GABA+ (r = −.35; p = .043). That is, higher baseline GABA+ levels were related to lower M1‐M1 functional connectivity. This result, however, did not survive FDR correction for multiple comparisons.
Last, the relationships between the change in connectivity across the two RS time points and age, MSL magnitude, baseline GABA+, and percent change in GABA+ were assessed via the computation of Pearson's correlation coefficients. Critically, the anodal and sham stimulation groups did not differ in the relationships between change in connectivity and any of the variables of interest after correction for multiple comparisons (see Figures S8 and S9). Correlation analyses across the two groups revealed that the changes in connectivity between right M1 and two task‐relevant regions (i.e., left M1 and left superior parietal cortex) were significantly and positively correlated with baseline GABA+ levels (Figure 6). That is, individuals with higher baseline GABA+ levels in the right sensorimotor cortex were more likely to exhibit a motor learning‐related increase in functional connectivity between the right M1 seed and two nodes of a task‐relevant network. As these effects did not differ between the two groups, it can be inferred that learning, rather than stimulation‐mediated changes in functional connectivity are related to the level of GABA during baseline. Age, change in GABA+ levels and learning magnitude were not related, after FDR correction, to the change in connectivity across the learning/stimulation interval in any of the seed/ROI pairs (Figure 6).
FIGURE 6.
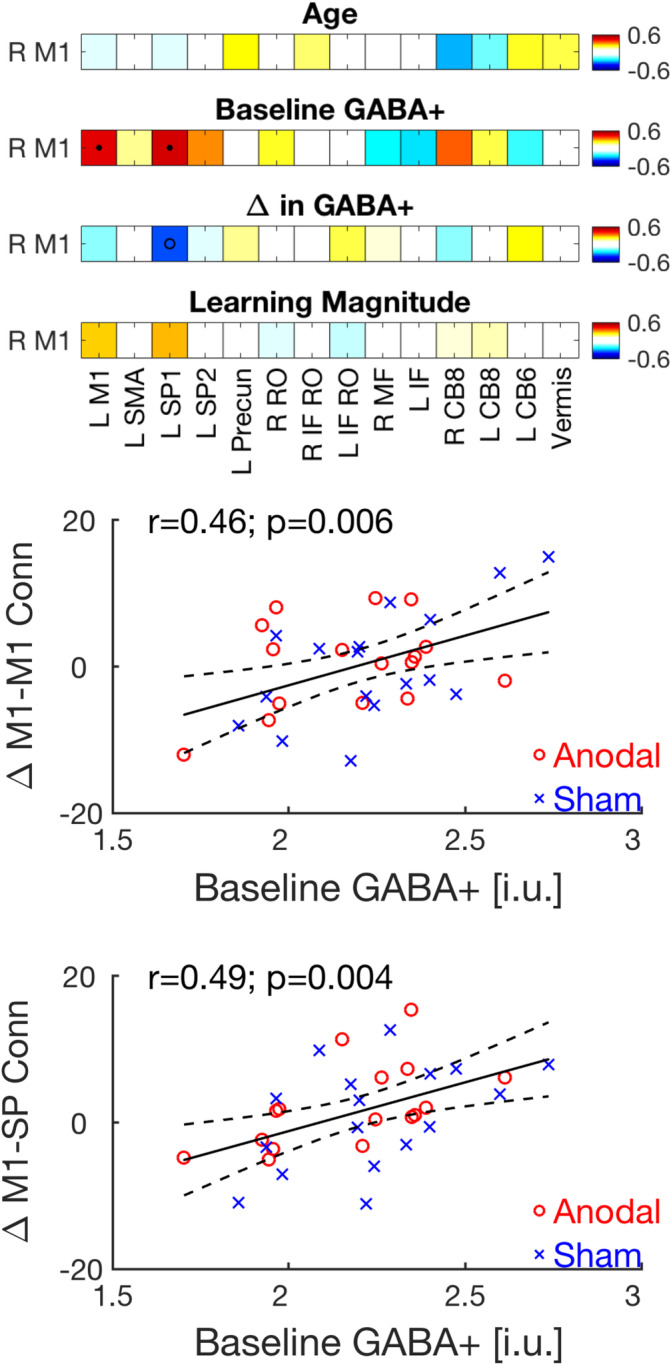
(a) Relationships between the change in RS functional connectivity between the right M1 seed and the 14 motor task‐relevant ROIs across the two RS time points and age (top row), baseline GABA+ levels (second from top), change in GABA+ (second from bottom) and motor sequence learning magnitude (bottom row). Values represent Pearson's correlation coefficients conducted across the anodal and sham groups (n = 33). Tests of statistical significance were based on comparisons of the coefficients to a correlation of 0 and corrected for multiple comparisons with a FDR threshold set to 0.05. Corrected p value threshold for the relationships between change in connectivity and baseline GABA+ was .0064. • = p(FDR) < .05; o = p(uncorrected) < .05. (b,c) Significant relationships between baseline GABA+ and the change in connectivity across the two RS time points between right M1 and left M1 (b) and left SP cortex (c) are depicted. Solid dark lines represent linear regression fits; dashed lines depict 95% prediction intervals of the linear function. Correlations were conducted across the two experimental groups (n = 33), but the anodal and sham participants are shown in red circles and blue crosses, respectively. Note that these significant correlations survive FDR correction for multiple comparisons. The numbers 6 and 8 represent the cerebellar lobules. CB, cerebellar; FDR, false discovery rate; GABA, gamma‐aminobutyric acid; IF, inferior frontal; i.u., institutional units; L, left; M1, primary motor cortex; MF, medial frontal; Precun, precuneus; R, right; RO, rolandic operculum; ROIs, regions of interest; RS, resting state; SMA, supplementary motor area; SP, superior parietal
4. DISCUSSION
Our results demonstrate that although MSL did not significantly modulate GABA+ levels or RS functional connectivity at the group level, individual differences in the learning‐related modulations in GABA+ levels were associated to the participants' age, baseline GABA+ levels and the magnitude of learning. That is, the younger old participants with higher baseline GABA+ levels and who exhibited the greatest amount of learning were more likely to show the learning‐related decrease in GABA that has been previously observed in young adults. Higher baseline GABA was also linked to a learning‐related increase in RS functional connectivity between motor task‐relevant regions, including bilateral motor cortices. Post‐learning anodal tDCS exerted no influence on GABA+ levels, functional connectivity or the relationships among GABA, connectivity and behavioral measures in healthy older adults.
4.1. No effects of post‐learning tDCS on GABA levels or RS functional connectivity
Previous research has demonstrated that anodal tDCS triggered a decrease in sensorimotor GABA relative to a sham control in both young and older individuals (Antonenko et al., 2017; Bachtiar et al., 2015; Kim et al., 2014; Patel et al., 2019; Stagg et al., 2009, 2011a). The lack of an effect of post‐learning tDCS reported in the current manuscript is thus not entirely in line with this earlier research. There are a few key methodological differences that deserve mentioning. First, in the current experiment, tDCS followed the completion of the MSL protocol. It could be suggested that the learning task masked any stimulation‐induced changes in GABA. Although we cannot completely discount this possibility, it is unlikely given our results. Specifically, the most likely scenario of a learning task preventing subsequent tDCS‐induced modulations of GABA levels would be the presence of a learning‐induced floor effect on GABA levels; that is, tDCS would fail to decrease GABA beyond what was already observed with learning. However, there was no learning‐related modulation in GABA within our sham group and thus learning by itself did not cause GABA levels to reach a “floor” that then masked further stimulation‐induced decreases. Nonetheless, without additional experimental groups that did not complete the learning task, we are limited in making direct comparisons to Antonenko et al. (2017). Second, and consistent with previous research (e.g., Albouy et al., 2016; Karni et al., 1995; King, Saucier, et al., 2017), participants in the current experiment completed the MSL task with the nondominant (left) hand and thus tDCS was administered to the contralateral (right) hemisphere. Conversely, earlier research has predominantly stimulated the dominant, left hemisphere (e.g., Antonenko et al., 2017; Stagg et al., 2009, 2011b). There is evidence suggesting that both GABA levels (Cuypers et al., 2020) and the behavioral effects of tDCS differ between the two hemispheres (Schambra et al., 2011); however, any hemispheric differences of tDCS on GABA levels or functional connectivity have yet to be examined.
It is worth noting that anodal stimulation in the work of Antonenko et al. (2017) exhibited decreased GABA in healthy older adults relative to the sham condition, but not a decrease in absolute terms. Anodal tDCS actually resulted in an averaged 8% increase in GABA, a change that was significantly less than the 18% increase following sham stimulation. The authors suggested such increases in GABA levels may be attributed to technical, scanner‐related issues, such as MR gradient‐induced frequency drifts (Harris et al., 2014). Nonetheless, this result is different from the available literature in healthy young individuals, in which anodal stimulation resulted in an absolute decrease in GABA (Bachtiar et al., 2015; Kim et al., 2014; Patel et al., 2019; Stagg et al., 2009, 2011b).
In addition to modulations of GABA, previous research has demonstrated that anodal tDCS decreased connectivity within a motor network as well as between motor and posterior (visual) areas in older adults (Antonenko et al., 2017, 2018). It was suggested that the stimulation may serve to mitigate known aging‐associated increases in functional connectivity (e.g., Damoiseaux, 2017). In contrast, post‐learning tDCS in our study did not modulate RS connectivity among motor task‐relevant regions. Similar to above, we cannot definitively rule out the possibility that post‐learning anodal tDCS differentially influences connectivity relative to stimulation in the absence of a learning task. Alternatively, perhaps the inconsistency in the results across studies can be best conceptualized within the growing body of literature highlighting the heterogeneous nature of transcranial electrical stimulation (see Wiethoff, Hamada, & Rothwell, 2014). The neurophysiological effects of tDCS are dependent on multiple interconnected factors, including the strength and flow of the stimulation‐induced electrical field, individual variability in the anatomical features of the brain and skull, baseline neurochemical levels, as well as baseline resting motor thresholds (Antonenko et al., 2019; Datta, Truong, Minhas, Parra, & Bikson, 2012; Filmer, Ehrhardt, Bollmann, Mattingley, & Dux, 2019; Laakso, Tanaka, Koyama, De Santis, & Hirata, 2015; Labruna et al., 2019; Opitz, Paulus, Will, Antunes, & Thielscher, 2015). It is vital for the field to better understand the effect of these interindividual factors in order for approaches such as tDCS to be considered a reliable option to modulate underlying neurophysiology and ultimately behavior.
4.2. Influence of motor learning on GABA levels and functional connectivity
The lack of a significant effect of motor learning on GABA levels and functional connectivity at the group level in healthy older adults is in contrast to previous research in young individuals (e.g., Albert, Robertson, & Miall, 2009; Floyer‐Lea et al., 2006; Kolasinski et al., 2019; Sami et al., 2014; Solesio‐Jofre et al., 2018). As healthy aging is associated with significant reductions in GABA (e.g., Chalavi et al., 2018; Gao et al., 2013; Hermans, Leunissen, et al., 2018), it is likely that lower baseline GABA levels observed in older adults effectively prevented substantial group‐level decreases across the two MRS time points (i.e., less room to exhibit a significant modulation). This interpretation is consistent with our correlation analyses. The younger old participants with higher baseline GABA levels (i.e., similar to previously reported estimates for young adults collected on the same MR scanner and with similar acquisition parameters; see Hermans, Leunissen, et al., 2018) were more likely to exhibit the learning‐related decreases in GABA and increases in RS connectivity that have been previously reported in young adults (e.g., Albert et al., 2009; Floyer‐Lea et al., 2006; Kolasinski et al., 2019; Sami et al., 2014). Perhaps most importantly, those that exhibited greater learning during the training session also exhibited greater decreases in GABA, effectively linking behavior to changes at the brain level. These data collectively suggest that higher (i.e., “young‐like”) sensorimotor GABA levels in older adults are favorable for the neuroplastic processes that underlie successful motor learning.
These results are in line with previous research that assessed motor cortical inhibition across the adult lifespan with a short‐interval intracortical inhibition protocol (Heise et al., 2013). This study demonstrated that aging‐related reductions in motor cortical RS inhibition were associated with worse motor performance as well as an impaired modulation of inhibition during movement preparation. Thus, and analogous to the current research, older individuals with young‐like levels of cortical inhibition showed an enhanced modulatory capacity that was linked to better motor functioning (Heise et al., 2013).
It is possible that the significant relationship between baseline GABA levels and the change in GABA across the learning/stimulation interval reported in the current study reflects, in part, a homeostatic process that maintains GABA levels within bounds. In other words, individuals with low or high baseline GABA levels tended to regress toward the mean after learning/stimulation. Although such a homeostatic process is certainly plausible, a critical finding of this research is that such a regulatory mechanism was found to be significantly associated with the plasticity observed at the behavioral level (i.e., the magnitude of motor learning). Interestingly, an analogous relationship has been previously and extensively observed with respect to the homeostatic regulation of synaptic strength during sleep and learning processes (Tononi & Cirelli, 2006, 2014).
To date, investigations into GABA and motor learning in older adults are relatively limited, making it difficult to situate the current results within the available aging literature. Nonetheless, a recent study from our own group indicated that practice on a bimanual, visuomotor tracking task failed to modulate sensorimotor cortical GABA levels in older adults (Chalavi et al., 2018). It is worth noting, however, that the same paradigm induced no changes in sensorimotor GABA in young individuals. Thus, the lack of an effect in older adults in this earlier study appears to not be the result of an aging‐associated impairment, but perhaps can be attributed to the nature of the specific task employed. Consistent with such an interpretation, occipital—but not sensorimotor—GABA levels decreased in both young and older adults as a result of a random practice regime for this visuomotor task (Chalavi et al., 2018).
4.3. Limitations
There are several limitations that warrant further discussion. First, and applicable to all MRS of GABA experiments, the estimates derived from such an approach reflect the total amount of GABA within the voxel and thus it is not possible to assess the relative contributions of the various pools of GABA found in the brain (see Stagg, 2014; Stagg et al., 2011a; Stagg, Bestmann, et al., 2011, for expanded discussion). There is evidence suggesting that MRS‐quantified GABA reflects extracellular pools of GABA, rather than GABAergic synaptic transmission (Dyke et al., 2017; Stagg, Bestmann, et al., 2011). As such, it has been proposed that MRS‐measured GABA levels can best be conceptualized as markers of GABAergic tone (Rae, 2014). This issue, however, is an ongoing debate and thus all results must be interpreted in the context of the limitation outlined above. Second, it is worth explicitly stating that the primary, statistically significant results reported in the current research are based on correlations and thus we are restricted to discussing associations among variables of interest as opposed to inferring causality of the effects. Moreover, many of the variables of interest (i.e., age, MSL magnitude, baseline GABA, and modulation in GABA) are interrelated (see Table 2), making it difficult to decipher the precise relationship between any two variables independent of the influence of the remaining factors of interest. Third, the current research did not include groups of young participants. Even though we are comfortable making comparisons with the literature based on the plethora of previous studies highlighted above that demonstrated learning‐ and tDCS‐dependent modulations in GABA and functional connectivity, the inclusion of young adults would have facilitated interpretation of the results in the context of aging‐associated changes. Fourth, and as discussed earlier, additional groups of participants that completed the anodal or sham stimulation in the absence of learning would have provided a more direct assessment of the effects of tDCS on GABA levels and RS functional connectivity in older adults (see Antonenko et al., 2017). Similarly, the inclusion of additional groups that completed an analogous motor task without a learning component would allow us to more clearly differentiate the effects of motor execution versus motor learning on both GABA levels and functional connectivity in older individuals (see Floyer‐Lea et al., 2006). Last, the assessment of relevant neurometabolites in our experiment was limited to GABA in the sensorimotor cortex. Examining additional neurometabolites (see Levin et al., 2019) and acquiring data from other ROIs, including motor learning‐relevant deep structures such as the hippocampus and striatum (see Albouy, King, Maquet, & Doyon, 2013; King, Hoedlmoser, Hirschauer, Dolfen, & Albouy, 2017, for reviews), would be of great interest for future research.
5. CONCLUSIONS
Although MSL did not significantly modulate sensorimotor GABA or RS functional connectivity at the group level in older adults, those participants with “young‐like” GABA levels exhibited similar learning‐dependent changes in both GABA and connectivity as previously observed in healthy young individuals. This result suggests that the well‐documented age‐related reductions in cortical GABA levels may compromise the neuroplastic processes known to underlie successful motor learning in young adults. We found no evidence that post‐learning anodal tDCS influenced GABA, functional connectivity or the relationships among GABA, connectivity, and behavioral measures in older individuals.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1. Supporting Information
Figure S1. The full experimental protocol consisted of initial training and 6hr retest sessions on the same day. The main text presents data from the initial training session only. RS fMRI = resting state functional magnetic resonance imaging. MRS = magnetic resonance spectroscopy of right sensorimotor cortex (contralateral to hand used to perform motor task). MSL = motor sequence learning. tDCS = transcranial direct current stimulation. DWI = diffusion weighted imaging
Figure S2. Percentage of correct transitions, reflecting sequence accuracy, plotted for the two experimental groups across the 12 blocks of training and the 4 blocks of the immediate posttest. Red and blue depict anodal and sham tDCS groups, respectively. Shaded regions represent SEM. A 12 (Block) × 2 (Group) ANOVA on the training run revealed no significant Group main effect (F 1,34 = 0.046, p = .83), Block main effect (F 4.61,156.81 = 1.86, p = .11) or Group by Block interaction (F 4.61,156.81 = 1.32, p = .26). Similarly, there were no significant Group (F 1,34 = 0.015, p = 0.90), Block (F 1.37,46.57 = 1.22, p = .29), or Block by Group effects during the immediate posttraining test (F 1.37,46.57 = 0.065, p = .87)
Figure S3. The T1‐weighted structural images and MRS voxel masks were normalized to MNI space in SPM12. The normalized and binarized voxel masks (n = 72; 36 participants × 2 sessions) were then summed and overlaid on an MNI template to depict the spatial overlap of the MRS voxels. The high spatial overlap indicates high consistency in voxel placement across participants and time points
Table S1. MRS data quality and voxel composition metrics for the 2 experimental groups and 2 time points
Figure S4. GABA+ levels (institutional units; i.u.) plotted as a function of NAA Line Width (Hz; top) and the SD of the Water Frequency Offset (bottom). Correlations were conducted across the two experimental groups and MRS time points (n = 72), but the groups and time points are depicted in the scatterplot (red = Anodal; blue = Sham; circles = baseline session; crosses = postlearning/stimulation session). Solid dark lines represent linear regression fits; dashed lines depict 95% prediction intervals of the linear function. Results indicate that there were no significant relationships between data quality metrics and GABA+ levels
Table S2. Head motion during the resting state acquisition sequences for the 2 experimental groups and 2 time points
Figure S5. A. Right M1 seed (white sphere) identified based on task‐related brain activity and used in subsequent functional connectivity analyses is located within the MRS voxel (red). B. Seed regions that demonstrated a significant increase in connectivity with the right M1 seed as a function of performance improvements are overlaid on an MNI template. See Table S2 for MNI coordinates of the 15 seeds identified and used in subsequent resting state functional connectivity analyses. Note that slices were selected in order to offer a representative depiction and thus not every single seed is visible in the images shown. C. All seeds are depicted in a glass brain from posterior (left) and sagittal views (right). Images were created using the software MRIcro and MRIcroGL (https://www.mccauslandcenter.sc.edu/crnl/tools)
Table S3. Regions of interest used in the resting state functional connectivity analyses
Figure S6. Individual GABA+ levels (institutional units; i.u.) for the baseline and postlearning/stimulation sessions are plotted pairwise for the anodal (red) and sham (blue) stimulation groups (small open circles)
Table S4. Correlations among age, GABA+ measures and motor sequence learning magnitude within the 2 experimental groups
Table S5. Group (anodal / sham) differences in the correlations among age, GABA+ measures and motor sequence learning magnitude
Table S6. Partial correlations among age, GABA+ measures and motor sequence learning magnitude controlling for the effects of NAA LW
Table S7. Partial correlations among age, GABA+ measures and motor sequence learning magnitude controlling for the effects of the SD of the Frequency offset
Figure S7. Relationships between baseline functional connectivity between the right M1 seed and the 14 motor task‐relevant ROIs and age (top row), baseline GABA+ levels (2nd from top), change in GABA+ (2nd from bottom) and motor sequence learning magnitude (bottom row). Values represent Pearson's correlation coefficients conducted across the anodal and sham groups (n = 33). Tests of statistical significance were based on comparisons of the coefficients to a correlation of 0 and corrected for multiple comparisons with a False Discovery Rate (FDR) threshold set to 0.05. None of the seed/ROI pairs were significantly correlated with a variable of interest after correction for multiple comparisons. It is worth emphasizing that baseline connectivity between bilateral M1s did exhibit a negative relationship with baseline GABA+ levels before correction for multiple comparisons (r = −0.35; p = .043). That is, higher baseline GABA+ levels were related to lower M1‐M1 functional connectivity. Although this result is analogous to previous research in both young (Bachtiar, Near, Johansen‐Berg, & Stagg, 2015; Stagg et al., 2014) and older adults (Antonenko et al., 2017), it should be interpreted with caution as it did not survive FDR correction. o = p(uncorrected)<.05. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
Figure S8. A. Relationships between the change in RS functional connectivity between the right M1 seed and the 14 motor task‐relevant ROIs across the two RS time points and age, baseline GABA+ levels, change in GABA+ and motor sequence learning magnitude separately for the anodal (Panel A; n = 16) and sham (B; n = 17) stimulation groups. Values represent Pearson's correlation coefficients. Within‐group tests of statistical significance were based on comparisons of the coefficients to a correlation of 0 and corrected for multiple comparisons with a False Discovery Rate (FDR) threshold set to 0.05. o = p(uncorrected) < .05. No seed/ROI pair survived correction for multiple comparison. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
Figure S9. Uncorrected p‐values (thresholded at p = 0.1) corresponding to a test of group differences in the relationships between the change in RS functional connectivity and age, baseline GABA+ levels, change in GABA+ and motor sequence learning magnitude (n = 16 and 17 in the anodal and sham groups, respectively). There were no significant differences between anodal and sham groups after correction for multiple comparison (all p(FDR) > .4) and thus the correlation coefficients presented in the main text are collapsed across the two groups. o = p(uncorrected) < .05. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
ACKNOWLEDGMENTS
This work was supported by the EU's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement (703490), the FWO Research Foundation Flanders (grants 1509616N, G099516N, G0D7918N, G089818N, and postdoctoral fellowship 132635), the Excellence of Science funding competition (EOS; 30446199), and the KU Leuven Special Research Fund (grants C16/15/070; C12/18/007). This study applies tools developed under National Institutes of Health (NIH) grants R01‐EB‐016089, R01‐023963, and P41‐EB015909; R. A. E. E. also receives salary support from these grants. N. A. J. P. receives salary support from NIH grant R00‐MH‐107719.
King BR, Rumpf J‐J, Verbaanderd E, et al. Baseline sensorimotor GABA levels shape neuroplastic processes induced by motor learning in older adults. Hum Brain Mapp. 2020;41:3680–3695. 10.1002/hbm.25041
Funding information Excellence of Science, Grant/Award Number: 30446199; Fonds Wetenschappelijk Onderzoek, Grant/Award Numbers: 132635, 1509616N, G089818N, G099516N, G0D7918N; H2020 Marie Skłodowska‐Curie Actions, Grant/Award Number: 703490; KU Leuven Special Research Fund, Grant/Award Numbers: C12/18/007, C16/15/070; National Institutes of Health, Grant/Award Numbers: P41‐EB015909, R00‐MH‐107719, R01‐023963, R01‐EB‐016089
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
REFERENCES
- Albert, N. B. , Robertson, E. M. , & Miall, R. C. (2009). The resting human brain and motor learning. Current Biology, 19(12), 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy, G. , King, B. R. , Maquet, P. , & Doyon, J. (2013). Hippocampus and striatum: Dynamics and interaction during acquisition and sleep‐related motor sequence memory consolidation. Hippocampus, 23(11), 985–1004. [DOI] [PubMed] [Google Scholar]
- Albouy, G. , King, B. R. , Schmidt, C. , Desseilles, M. , Dang‐Vu, T. , Balteau, E. , … Korman, M. (2016). Cerebral activity associated with transient sleep‐facilitated reduction in motor memory vulnerability to interference. Scientific Reports, 6, 34948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko, D. , Nierhaus, T. , Meinzer, M. , Prehn, K. , Thielscher, A. , Ittermann, B. , & Flöel, A. (2018). Age‐dependent effects of brain stimulation on network centrality. NeuroImage, 176, 71–82. [DOI] [PubMed] [Google Scholar]
- Antonenko, D. , Schubert, F. , Bohm, F. , Ittermann, B. , Aydin, S. , Hayek, D. , … Floel, A. (2017). tDCS‐induced modulation of GABA levels and resting‐state functional connectivity in older adults. The Journal of Neuroscience, 37(15), 4065–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko, D. , Thielscher, A. , Saturnino, G. B. , Aydin, S. , Ittermann, B. , Grittner, U. , & Flöel, A. (2019). Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stimulation, 12(5), 1159–1168. [DOI] [PubMed] [Google Scholar]
- Bachtiar, V. , Johnstone, A. , Berrington, A. , Lemke, C. , Johansen‐Berg, H. , Emir, U. , & Stagg, C. J. (2018). Modulating regional motor cortical excitability with noninvasive brain stimulation results in neurochemical changes in bilateral motor cortices. The Journal of Neuroscience, 38(33), 7327–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar V., Near J., Johansen‐Berg H., Stagg C. J. (2015). Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife, 4, 08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , Ball, R. , & Ranieri, W. (1996). Comparison of Beck Depression Inventories ‐IA and ‐II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Epstein, N. , Brown, G. , & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Buysse, D. J. , Reynolds, C. F. , Monk, T. H. , Berman, S. R. , & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Cassady, K. , Gagnon, H. , Lalwani, P. , Simmonite, M. , Foerster, B. , Park, D. , … Polk, T. A. (2019). Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage, 186, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalavi, S. , Pauwels, L. , Heise, K.‐F. , Zivari Adab, H. , Maes, C. , Puts, N. A. J. , … Swinnen, S. P. (2018). The neurochemical basis of the contextual interference effect. Neurobiology of Aging, 66, 85–96. [DOI] [PubMed] [Google Scholar]
- Chen, J. , McCulloch, A. , Kim, H. , Kim, T. , Rhee, J. , Verwey, W. B. , … Wright, D. L. (2019). Application of anodal tDCS at primary motor cortex immediately after practice of a motor sequence does not improve offline gain. Experimental Brain Research, 238, 1–9. 10.1007/s00221-019-05697-7 [DOI] [PubMed] [Google Scholar]
- Cockrell, J. R. , & Folstein, M. F. (1988). Mini‐Mental State Examination (MMSE). Psychopharmacology Bulletin, 24(4), 689–692. [PubMed] [Google Scholar]
- Cuypers, K. , Verstraelen, S. , Maes, C. , Hermans, L. , Hehl, M. , Heise, K. F. , … Swinnen, S. P. (2020). Task‐related measures of short‐interval intracortical inhibition and GABA levels in healthy young and older adults: A multimodal TMS‐MRS study. NeuroImage, 208, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux, J. S. (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. [DOI] [PubMed] [Google Scholar]
- Dan, E. , King, B. R. , Doyon, J. , & Chan, P. (2015). Motor sequence learning and consolidation in unilateral de novo patients with Parkinson's disease. PLoS One, 10(7), e0134291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, A. , Truong, D. , Minhas, P. , Parra, L. C. , & Bikson, M. (2012). Inter‐individual variation during transcranial direct current stimulation and normalization of dose using MRI‐derived computational models. Frontiers in Psychiatry, 3, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges, D. F. , & Powell, J. W. (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research Methods, Instruments, & Computers, 17(6), 652–655. [Google Scholar]
- Dolfen, N. , King, B. R. , Schwabe, L. , Swinnen, S. , & Albouy, G. (2019). Glucocorticoid response to stress induction prior to learning is negatively related to subsequent motor memory consolidation. Neurobiology of Learning and Memory, 158, 32–41. [DOI] [PubMed] [Google Scholar]
- Dyke, K. , Pépés, S. E. , Chen, C. , Kim, S. , Sigurdsson, H. P. , Draper, A. , … Jackson, S. R. (2017). Comparing GABA‐dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra‐high‐field MRI. NeuroImage, 152, 360–370. 10.1016/j.neuroimage.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Oeltzschner, G. , Harris, A. D. , Puts, N. A. J. , Chan, K. L. , Boer, V. O. , … Barker, P. B. (2016). Prospective frequency correction for macromolecule‐suppressed GABA editing at 3T. Journal of Magnetic Resonance Imaging, 44(6), 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Puts, N. A. J. , & Barker, P. B. (2012). Macromolecule‐suppressed GABA‐edited magnetic resonance spectroscopy at 3T. Magnetic Resonance in Medicine, 68(3), 657–661. 10.1002/mrm.24391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Puts, N. A. J. , Harris, A. D. , Barker, P. B. , & Evans, C. J. (2014). Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid‐edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging, 40(6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer, H. L. , Ehrhardt, S. E. , Bollmann, S. , Mattingley, J. B. , & Dux, P. E. (2019). Accounting for individual differences in the response to tDCS with baseline levels of neurochemical excitability. Cortex, 115, 324–334. [DOI] [PubMed] [Google Scholar]
- Floyer‐Lea, A. , Wylezinska, M. , Kincses, T. , & Matthews, P. M. (2006). Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. Journal of Neurophysiology, 95(3), 1639–1644. [DOI] [PubMed] [Google Scholar]
- Fogel, S. M. , Albouy, G. , Vien, C. , Popovicci, R. , King, B. R. , Hoge, R. , … Doyon, J. (2014). fMRI and sleep correlates of the age‐related impairment in motor memory consolidation. Human Brain Mapping, 35, 3625–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga, P. C. , Hummel, F. C. , & Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double‐blind sham‐controlled clinical studies in brain stimulation. Clinical Neurophysiology, 117(4), 845–850. [DOI] [PubMed] [Google Scholar]
- Gao, F. , Edden, R. A. E. , Li, M. , Puts, N. A. J. , Wang, G. , Liu, C. , … Barker, P. B. (2013). Edited magnetic resonance spectroscopy detects an age‐related decline in brain GABA levels. NeuroImage, 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman, D. R. , Penny, W. D. , Ashburner, J. , & Friston, K. J. (2003). Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage, 19(1), 200–207. [DOI] [PubMed] [Google Scholar]
- Grachev, I. D. , & Apkarian, A. V. (2001). Aging alters regional multichemical profile of the human brain: An in vivo 1 H‐MRS study of young versus middle‐aged subjects. Journal of Neurochemistry, 76(2), 582–593. [DOI] [PubMed] [Google Scholar]
- Hamoudi, M. , Schambra, H. M. , Fritsch, B. , Schoechlin‐Marx, A. , Weiller, C. , Cohen, L. G. , & Reis, J. (2018). Transcranial direct current stimulation enhances motor skill learning but not generalization in chronic stroke. Neurorehabilitation and Neural Repair, 32(4–5), 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. D. , Glaubitz, B. , Near, J. , John Evans, C. , Puts, N. A. J. , Schmidt‐Wilcke, T. , … Edden, R. A. E. (2014). Impact of frequency drift on gamma‐aminobutyric acid‐edited MR spectroscopy. Magnetic Resonance in Medicine, 72(4), 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. D. , Puts, N. A. J. , & Edden, R. A. E. (2015). Tissue correction for GABA‐edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging, 42(5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise, K.‐F. , Zimerman, M. , Hoppe, J. , Gerloff, C. , Wegscheider, K. , & Hummel, F. C. (2013). The aging motor system as a model for plastic changes of GABA‐mediated intracortical inhibition and their behavioral relevance. The Journal of Neuroscience, 33(21), 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, L. , Leunissen, I. , Pauwels, L. , Cuypers, K. , Peeters, R. , Puts, N. A. J. , … Swinnen, S. P. (2018). Brain GABA levels are associated with inhibitory control deficits in older adults. The Journal of Neuroscience, 38(36), 7844–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, L. , Levin, O. , Maes, C. , van Ruitenbeek, P. , Heise, K.‐F. , Edden, R. A. E. , … Cuypers, K. (2018). GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: An MRS‐TMS study. Neurobiology of Aging, 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Hummel, F. C. , Heise, K. , Celnik, P. , Floel, A. , Gerloff, C. , & Cohen, L. G. (2010). Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiology of Aging, 31(12), 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A. , Meyer, G. , Jezzard, P. , Adams, M. M. , Turner, R. , & Ungerleider, L. G. (1995). Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature, 377(6545), 155–158. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Stephenson, M. C. , Morris, P. G. , & Jackson, S. R. (2014). tDCS‐induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7 T magnetic resonance spectroscopy study. NeuroImage, 99, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, B. R. , Fogel, S. M. , Albouy, G. , & Doyon, J. (2013). Neural correlates of the age‐related changes in motor sequence learning and motor adaptation in older adults. Frontiers in Human Neuroscience, 7, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, B. R. , Hoedlmoser, K. , Hirschauer, F. , Dolfen, N. , & Albouy, G. (2017). Sleeping on the motor engram: The multifaceted nature of sleep‐related motor memory consolidation. Neuroscience & Biobehavioral Reviews, 80, 1–22. [DOI] [PubMed] [Google Scholar]
- King, B. R. , Saucier, P. , Albouy, G. , Fogel, S. , Rumpf, J. , Klann, J. , … Doyon, J. (2017). Cerebral activation during initial motor learning forecasts subsequent sleep‐facilitated memory consolidation in older adults. Cerebral Cortex, 27(2), 1588–1601. [DOI] [PubMed] [Google Scholar]
- King, B. R. , van Ruitenbeek, P. , Leunissen, I. , Cuypers, K. , Heise, K.‐F. , Santos Monteiro, T. , … Swinnen, S. P. (2018). Age‐related declines in motor performance are associated with decreased segregation of large‐scale resting state brain networks. Cerebral Cortex, 28(12), 4390–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski, J. , Hinson, E. L. , Divanbeighi Zand, A. P. , Rizov, A. , Emir, U. E. , & Stagg, C. J. (2019). The dynamics of cortical GABA in human motor learning. The Journal of Physiology, 597(1), 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso, I. , Tanaka, S. , Koyama, S. , De Santis, V. , & Hirata, A. (2015). Inter‐subject variability in electric fields of motor cortical tDCS. Brain Stimulation, 8(5), 906–913. [DOI] [PubMed] [Google Scholar]
- Labruna, L. , Stark‐Inbar, A. , Breska, A. , Dabit, M. , Vanderschelden, B. , Nitsche, M. A. , & Ivry, R. B. (2019). Individual differences in TMS sensitivity influence the efficacy of tDCS in facilitating sensorimotor adaptation. Brain Stimulation, 12, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux, N. , Amar, M. , Moreau, A. , Baux, G. , & Fossier, P. (2008). Impaired GABAergic transmission disrupts normal homeostatic plasticity in rat cortical networks. European Journal of Neuroscience, 27(12), 3244–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, O. , Weerasekera, A. , King, B. R. , Heise, K. F. , Sima, D. M. , Chalavi, S. , … Swinnen, S. P. (2019). Sensorimotor cortex neurometabolite levels as correlate of motor performance in normal aging: Evidence from a 1H‐MRS study. NeuroImage, 202, 116050. [DOI] [PubMed] [Google Scholar]
- Lopez‐Alonso, V. , Liew, S.‐L. , Fernández del Olmo, M. , Cheeran, B. , Sandrini, M. , Abe, M. , & Cohen, L. G. (2018). A preliminary comparison of motor learning across different non‐invasive brain stimulation paradigms shows no consistent modulations. Frontiers in Neuroscience, 12, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, A. , Fekken, G. , Saskin, P. , & Knowles, J. (1992). Psychometric evaluation of the Stanford Sleepiness Scale. Journal of Sleep Research, 1(1), 35–39. [DOI] [PubMed] [Google Scholar]
- Maes, C. , Hermans, L. , Pauwels, L. , Chalavi, S. , Leunissen, I. , Levin, O. , … Swinnen, S. P. (2018). Age‐related differences in GABA levels are driven by bulk tissue changes. Human Brain Mapping, 39(9), 3652–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher, M. , Merkle, H. , Kirsch, J. , Garwood, M. , & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11(6), 266–272. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, M. , Barker, P. B. , Bhattacharyya, P. K. , Brix, M. K. , Buur, P. F. , Cecil, K. M. , … Edden, R. A. E. (2017). Big GABA: Edited MR spectroscopy at 24 research sites. NeuroImage, 159, 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen, M. , Rimbault, D. L. , Barker, P. B. , Bhattacharyya, P. K. , Brix, M. K. , Buur, P. F. , … Edden, R. A. E. (2019). Big GABA II: Water‐referenced edited MR spectroscopy at 25 research sites. NeuroImage, 191, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, P. G. , McGonigle, D. J. , O'Gorman, R. L. , Puts, N. A. J. , Vidyasagar, R. , Evans, C. J. , … Edden, R. A. E. (2014). Current practice in the use of MEGA‐PRESS spectroscopy for the detection of GABA. NeuroImage, 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near, J. , Edden, R. , Evans, C. J. , Paquin, R. , Harris, A. , & Jezzard, P. (2015). Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magnetic Resonance in Medicine, 73(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M. A. , Cohen, L. G. , Wassermann, E. M. , Priori, A. , Lang, N. , Antal, A. , … Pascual‐Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–223. [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Schauenburg, A. , Lang, N. , Liebetanz, D. , Exner, C. , Paulus, W. , & Tergau, F. (2003). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience, 15(4), 619–626. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Opitz, A. , Paulus, W. , Will, S. , Antunes, A. , & Thielscher, A. (2015). Determinants of the electric field during transcranial direct current stimulation. NeuroImage, 109, 140–150. [DOI] [PubMed] [Google Scholar]
- Patel, H. J. , Romanzetti, S. , Pellicano, A. , Nitsche, M. A. , Reetz, K. , & Binkofski, F. (2019). Proton magnetic resonance spectroscopy of the motor cortex reveals long term GABA change following anodal transcranial direct current stimulation. Scientific Reports, 9(1), 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts, N. A. J. , & Edden, R. A. E. (2012). In vivo magnetic resonance spectroscopy of GABA: A methodological review. Progress in Nuclear Magnetic Resonance Spectroscopy, 60, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae, C. D. (2014). A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochemical Research, 39(1), 1–36. 10.1007/s11064-013-1199-5 [DOI] [PubMed] [Google Scholar]
- Reis, J. , & Fritsch, B. (2011). Modulation of motor performance and motor learning by transcranial direct current stimulation. Current Opinion in Neurology, 24(6), 590–596. [DOI] [PubMed] [Google Scholar]
- Rothman, D. L. , Petroff, O. A. , Behar, K. L. , & Mattson, R. H. (1993). Localized 1H NMR measurements of gamma‐aminobutyric acid in human brain in vivo. Proceedings of the National Academy of Sciences, 90(12), 5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf, J.‐J. , Dietrich, S. , Stoppe, M. , Fricke, C. , Weise, D. , Then Bergh, F. , & Classen, J. (2018). Compromised tDCS‐induced facilitation of motor consolidation in patients with multiple sclerosis. Journal of Neurology, 265(10), 2302–2311. [DOI] [PubMed] [Google Scholar]
- Rumpf, J.‐J. , Wegscheider, M. , Hinselmann, K. , Fricke, C. , King, B. R. , Weise, D. , … Classen, J. (2017). Enhancement of motor consolidation by post‐training transcranial direct current stimulation in older people. Neurobiology of Aging, 49, 1–8. [DOI] [PubMed] [Google Scholar]
- Sami, S. , Robertson, E. M. , & Miall, R. C. (2014). The time course of task‐specific memory consolidation effects in resting state networks. The Journal of Neuroscience, 34(11), 3982–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio‐Baptista, C. , Filippini, N. , Stagg, C. J. , Near, J. , Scholz, J. , & Johansen‐Berg, H. (2015). Changes in functional connectivity and GABA levels with long‐term motor learning. NeuroImage, 106, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra, H. M. , Abe, M. , Luckenbaugh, D. A. , Reis, J. , Krakauer, J. W. , & Cohen, L. G. (2011). Probing for hemispheric specialization for motor skill learning: A transcranial direct current stimulation study. Journal of Neurophysiology, 106(2), 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesio‐Jofre, E. , Beets, I. A. M. , Woolley, D. G. , Pauwels, L. , Chalavi, S. , Mantini, D. , & Swinnen, S. P. (2018). Age‐dependent modulations of resting state connectivity following motor practice. Frontiers in Aging Neuroscience, 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. (2014). Magnetic resonance spectroscopy as a tool to study the role of GABA in motor‐cortical plasticity. NeuroImage, 86, 19–27. [DOI] [PubMed] [Google Scholar]
- Stagg, C. J. , Bachtiar, V. , Amadi, U. , Gudberg, C. A. , Ilie, A. S. , Sampaio‐Baptista, C. , … Johansen‐Berg, H. (2014). Local GABA concentration is related to network‐level resting functional connectivity. eLife, 3, e01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Bachtiar, V. , & Johansen‐Berg, H. (2011a). The role of GABA in human motor learning. Current Biology, 21(6), 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Bachtiar, V. , & Johansen‐Berg, H. (2011b). What are we measuring with GABA magnetic resonance spectroscopy? Communicative & Integrative Biology, 4(5), 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Best, J. G. , Stephenson, M. C. , O'Shea, J. , Wylezinska, M. , Kincses, Z. T. , … Johansen‐Berg, H. (2009). Polarity‐sensitive modulation of cortical neurotransmitters by transcranial stimulation. The Journal of Neuroscience, 29(16), 5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Bestmann, S. , Constantinescu, A. O. , Moreno, L. M. , Allman, C. , Mekle, R. , … Rothwell, J. C. (2011). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. The Journal of Physiology, 589(Pt 23), 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Jayaram, G. , Pastor, D. , Kincses, Z. T. , Matthews, P. M. , & Johansen‐Berg, H. (2011). Polarity and timing‐dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia, 49(5), 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi, G. , & Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Medicine Reviews, 10(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Tononi, G. , & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel, C. , & Racine, R. J. (2000). GABAergic modulation of neocortical long‐term potentiation in the freely moving rat. Synapse, 35(2), 120–128. [DOI] [PubMed] [Google Scholar]
- Vancleef, K. , Meesen, R. , Swinnen, S. P. , & Fujiyama, H. (2016). tDCS over left M1 or DLPFC does not improve learning of a bimanual coordination task. Scientific Reports, 6(1), 35739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff, S. , Hamada, M. , & Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7(3), 468–475. [DOI] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Buettner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain: A Journal of Neurology, 120, 141–157. [DOI] [PubMed] [Google Scholar]
- Zimerman, M. , Nitsch, M. , Giraux, P. , Gerloff, C. , Cohen, L. G. , & Hummel, F. C. (2013). Neuroenhancement of the aging brain: Restoring skill acquisition in old subjects. Annals of Neurology, 73(1), 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Figure S1. The full experimental protocol consisted of initial training and 6hr retest sessions on the same day. The main text presents data from the initial training session only. RS fMRI = resting state functional magnetic resonance imaging. MRS = magnetic resonance spectroscopy of right sensorimotor cortex (contralateral to hand used to perform motor task). MSL = motor sequence learning. tDCS = transcranial direct current stimulation. DWI = diffusion weighted imaging
Figure S2. Percentage of correct transitions, reflecting sequence accuracy, plotted for the two experimental groups across the 12 blocks of training and the 4 blocks of the immediate posttest. Red and blue depict anodal and sham tDCS groups, respectively. Shaded regions represent SEM. A 12 (Block) × 2 (Group) ANOVA on the training run revealed no significant Group main effect (F 1,34 = 0.046, p = .83), Block main effect (F 4.61,156.81 = 1.86, p = .11) or Group by Block interaction (F 4.61,156.81 = 1.32, p = .26). Similarly, there were no significant Group (F 1,34 = 0.015, p = 0.90), Block (F 1.37,46.57 = 1.22, p = .29), or Block by Group effects during the immediate posttraining test (F 1.37,46.57 = 0.065, p = .87)
Figure S3. The T1‐weighted structural images and MRS voxel masks were normalized to MNI space in SPM12. The normalized and binarized voxel masks (n = 72; 36 participants × 2 sessions) were then summed and overlaid on an MNI template to depict the spatial overlap of the MRS voxels. The high spatial overlap indicates high consistency in voxel placement across participants and time points
Table S1. MRS data quality and voxel composition metrics for the 2 experimental groups and 2 time points
Figure S4. GABA+ levels (institutional units; i.u.) plotted as a function of NAA Line Width (Hz; top) and the SD of the Water Frequency Offset (bottom). Correlations were conducted across the two experimental groups and MRS time points (n = 72), but the groups and time points are depicted in the scatterplot (red = Anodal; blue = Sham; circles = baseline session; crosses = postlearning/stimulation session). Solid dark lines represent linear regression fits; dashed lines depict 95% prediction intervals of the linear function. Results indicate that there were no significant relationships between data quality metrics and GABA+ levels
Table S2. Head motion during the resting state acquisition sequences for the 2 experimental groups and 2 time points
Figure S5. A. Right M1 seed (white sphere) identified based on task‐related brain activity and used in subsequent functional connectivity analyses is located within the MRS voxel (red). B. Seed regions that demonstrated a significant increase in connectivity with the right M1 seed as a function of performance improvements are overlaid on an MNI template. See Table S2 for MNI coordinates of the 15 seeds identified and used in subsequent resting state functional connectivity analyses. Note that slices were selected in order to offer a representative depiction and thus not every single seed is visible in the images shown. C. All seeds are depicted in a glass brain from posterior (left) and sagittal views (right). Images were created using the software MRIcro and MRIcroGL (https://www.mccauslandcenter.sc.edu/crnl/tools)
Table S3. Regions of interest used in the resting state functional connectivity analyses
Figure S6. Individual GABA+ levels (institutional units; i.u.) for the baseline and postlearning/stimulation sessions are plotted pairwise for the anodal (red) and sham (blue) stimulation groups (small open circles)
Table S4. Correlations among age, GABA+ measures and motor sequence learning magnitude within the 2 experimental groups
Table S5. Group (anodal / sham) differences in the correlations among age, GABA+ measures and motor sequence learning magnitude
Table S6. Partial correlations among age, GABA+ measures and motor sequence learning magnitude controlling for the effects of NAA LW
Table S7. Partial correlations among age, GABA+ measures and motor sequence learning magnitude controlling for the effects of the SD of the Frequency offset
Figure S7. Relationships between baseline functional connectivity between the right M1 seed and the 14 motor task‐relevant ROIs and age (top row), baseline GABA+ levels (2nd from top), change in GABA+ (2nd from bottom) and motor sequence learning magnitude (bottom row). Values represent Pearson's correlation coefficients conducted across the anodal and sham groups (n = 33). Tests of statistical significance were based on comparisons of the coefficients to a correlation of 0 and corrected for multiple comparisons with a False Discovery Rate (FDR) threshold set to 0.05. None of the seed/ROI pairs were significantly correlated with a variable of interest after correction for multiple comparisons. It is worth emphasizing that baseline connectivity between bilateral M1s did exhibit a negative relationship with baseline GABA+ levels before correction for multiple comparisons (r = −0.35; p = .043). That is, higher baseline GABA+ levels were related to lower M1‐M1 functional connectivity. Although this result is analogous to previous research in both young (Bachtiar, Near, Johansen‐Berg, & Stagg, 2015; Stagg et al., 2014) and older adults (Antonenko et al., 2017), it should be interpreted with caution as it did not survive FDR correction. o = p(uncorrected)<.05. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
Figure S8. A. Relationships between the change in RS functional connectivity between the right M1 seed and the 14 motor task‐relevant ROIs across the two RS time points and age, baseline GABA+ levels, change in GABA+ and motor sequence learning magnitude separately for the anodal (Panel A; n = 16) and sham (B; n = 17) stimulation groups. Values represent Pearson's correlation coefficients. Within‐group tests of statistical significance were based on comparisons of the coefficients to a correlation of 0 and corrected for multiple comparisons with a False Discovery Rate (FDR) threshold set to 0.05. o = p(uncorrected) < .05. No seed/ROI pair survived correction for multiple comparison. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
Figure S9. Uncorrected p‐values (thresholded at p = 0.1) corresponding to a test of group differences in the relationships between the change in RS functional connectivity and age, baseline GABA+ levels, change in GABA+ and motor sequence learning magnitude (n = 16 and 17 in the anodal and sham groups, respectively). There were no significant differences between anodal and sham groups after correction for multiple comparison (all p(FDR) > .4) and thus the correlation coefficients presented in the main text are collapsed across the two groups. o = p(uncorrected) < .05. L = left; R = right; M1 = primary motor cortex; SMA = supplementary motor area; SP = superior parietal; Precun = precuneus; RO = rolandic operculum; IF = inferior frontal; MF = medial frontal; CB = cerebellar; the numbers 6 and 8 represent the cerebellar lobules
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.


