Abstract
Maintaining oxygen homeostasis is a most basic cellular process for adapting physiological oxygen variations, and its abnormality typically leads to various disorders in the human body. The key molecules of the oxygen-sensing system include the transcriptional regulator hypoxia-inducible factor (HIF), which controls a wide range of oxygen responsive target genes (eg, EPO and VEGF), certain members of the oxygen/2-oxoglutarate–dependent dioxygenase family, including the HIF proline hydroxylase (PHD, alias EGLN), and an E3 ubiquitin ligase component for HIF destruction called von Hippel–Lindau. In this review, we summarize the physiological role and highlight the pathologic function for each protein of the oxygen-sensing system. A better understanding of their molecular mechanisms of action will help uncover novel therapeutic targets and develop more effective treatment approaches for related human diseases, including cancer.
The History of Research on Oxygen-Sensing Pathway
Taking oxygen and transforming it into energy is the most fundamental biological step for every single vertebrate organism to live. It is well known that oxygen integration during cellular respiration, which commonly takes place in mitochondria, is the key metabolic step to convert food into biochemical energy (eg, ATP). The 1931 Nobel Prize in Physiology or Medicine was awarded to Otto Warburg for elucidating that this biological oxidation conversion is actually an enzymatic process. However, a more fundamental question remained unclear then, especially in mammals, on how oxygen could be sensed in cells, adapted by its physiological availability, and used through various cellular processes. Scientists then uncovered a particular oxygen-sensing system in mammal cells, which was a breakthrough discovery that opened a brand-new scientific field.1, 2, 3 The main scientists involved in this finding are Drs. Gregg Semenza, William Kaelin, and Peter Ratcliffe. Because of their extraordinary accomplishments in discovering the oxygen-sensing system, they shared the Lasker Basic Medical Research Award in 2016, and then they were awarded the 2019 Nobel Prize in Physiology or Medicine.
The exploration of oxygen-sensing discovery started from the finding of erythropoietin (EPO), a kidney-derived glycoprotein hormone first discovered in the early twentieth century, purified in 1977,4 and cloned years later in 1985.5 Although it was known long ago that EPO stimulates the bone marrow to produce red blood cells, thereby increasing the blood oxygen-carrying capacity, and EPO level increased under a specific physiological condition when cells were in shortage of oxygen, the molecular mechanism of how the body sensed a lack of oxygen to control the EPO expression remained unclear. The low oxygen level status in the body was termed hypoxia in contrary to the normoxia (physiological oxygen status). Semenza et al6 were the first to study how the EPO gene was regulated under varying oxygen status. In 1991, by using gene-modified mice, they identified the existence of hypoxia response element that allowed some specific nuclear factors to bind in the 3′ enhancer region of the EPO gene and regulate its transcription when liver and kidney cells underwent hypoxia.6 In 1993, both Semenza7 and Ratcliffe8 and colleagues found that hypoxia response element binding activity functioned in various types of cells, therefore broadening the knowledge that the oxygen-sensing system existed universally across cells and species. Identification of the factor bound to EPO gene was the next question they sought to address. Two years later, Semenza and colleagues9 purified the nuclear factor biochemically and named it as the hypoxia-inducible factor 1 (HIF-1). In addition, they uncovered that HIF-1 acted as a heterodimer form composed of the oxygen-labile subunit HIF-1α and the constitutively expressed subunit HIF-1β (alias aryl hydrocarbon receptor nuclear translocator).9 Meanwhile, Semenza and colleagues10 expanded the function of HIF-1 by finding that vascular endothelial growth factor (VEGF) is also one of its targets, confirming it further by observing an embryonic lethality phenotype due to angiogenesis and erythropoiesis deficiency in HIF-1α mutant mice.11 These findings were important enough to place HIF-1 at the central position of oxygen-sensing machinery by regulating vascular development and oxygen transport in the blood system.
Interestingly, comparing with the constitutively expressed pattern of HIF-1β, HIF-1α protein level is rigorously modulated, depending on oxygen content. HIF-1α is induced only under hypoxic conditions, whereas its mRNA level remains constitutively expressed, indicating mechanisms of post-translational regulation for HIF-1α protein stability. The answer to this question came from another angle contributed by William Kaelin. While Kaelin was an oncologist studying an inherited syndrome called von Hippel–Lindau (VHL) disease, he found that patients with VHL gene mutations were at increased risk of developing certain cancers, such as kidney cancer. Kaelin and his research group12 uncovered that VHL forms a complex with elongin B and C and Cullin 2 as an E3 ubiquitin ligase that labels protein with ubiquitin markers for subsequent proteasome degradation. The linkage between VHL and HIF-1 came from the clues that VHL deletion up-regulated VEGF and many other HIF-dependent targets.13
The last piece of the puzzle for the oxygen-sensing pathway was how oxygen participated in this VHL-HIF pathway. In 2001, Ratcliffe14 and Kaelin15 and colleagues published articles reporting that HIF-1α underwent proline hydroxylated under normal oxygen levels and VHL could specifically recognize this hydroxylation form of HIF-1α. Kaelin,16 McKnight,17 and Ratcliffe18 and colleagues further unveiled that a family of prolyl-4-hydroxylases (PHDs, alias EGLNs) was responsible for catalyzing these prolyl hydroxylation events on HIF. Besides the proline residue, an asparagine hydroxylated form of HIF-1α that blocks its affinity with other transcriptional coactivators instead of affecting its stability was later identified.19 It turned out this modification is catalyzed by the factor-inhibiting HIF-1 protein, whose activity also needs the presence of oxygen, suggesting a secondary pathway ensuring that the HIF-1 function could be inactivated under normoxic conditions.
Understanding the oxygen-sensing system, especially in humans, is of significant importance not only to peek at the body's natural secrets but also to examine the physiological relevance of the oxygen-sensing pathway in both normal and abnormal oxygen status. In fact, dysregulation of the oxygen-sensing pathway could lead to a large number of human diseases, such as stroke, renal/cardiovascular disease, inflammatory diseases, and cancer. Uncovering the mechanisms associated with this pathway is critical and deserves great efforts to develop therapeutic strategies accordingly. In this review, we will discuss the current understanding of the oxygen-sensing system in the field from the perspective of disease and summarize the ongoing and potential therapeutic implications.
Current Understanding of the Oxygen-Sensing System
HIF, a Double-Faced Master Regulator of Oxygen Homeostasis
The HIF proteins are a family of evolutionarily conserved transcription factors with basic helix–loop-helix-Per-Arnt-Sim (PAS) domain at the N terminal. Each of them is in a heterodimeric form constituted by an oxygen-sensitive α subunit and a constitutive β subunit through the helix-loop-helix-PAS domain. At the C terminal, there exists an oxygen-dependent degradation domain where hydroxylation modifications take place by the PHDs. There are three homologs of the α unit (HIF-1α, HIF-2α, and HIF-3α). Although HIF-1α is expressed ubiquitously, the expression of the other two is in a cell line/tissue-restricted manner. The function of HIF-1α and HIF-2α is relatively well studied in comparison with HIF-3α. The complexity of the HIF protein system suggests a fine defined adaption to diverse cellular processes during hypoxia.
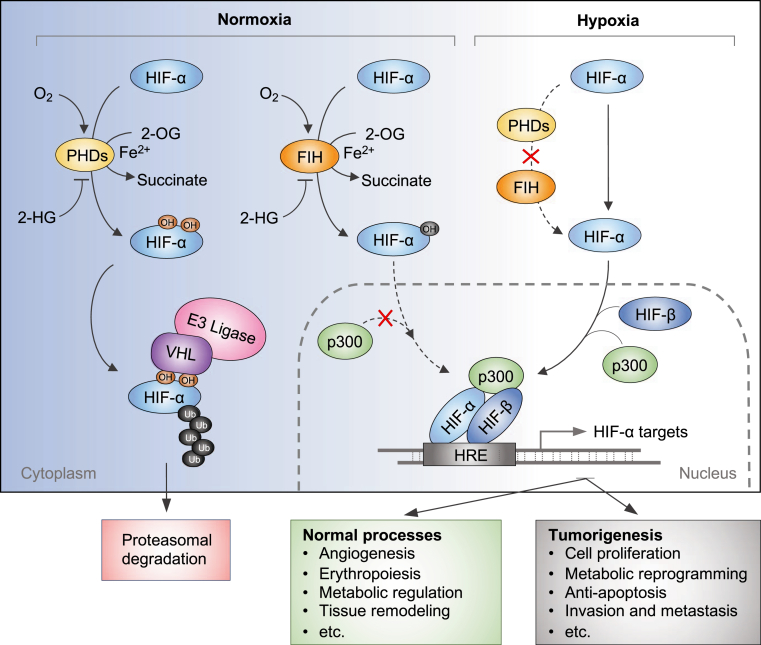
The HIFs transcriptionally control a large body of genes on the basis of their protein availability determined by oxygen level. When cells have normal oxygen levels, HIF-α is constantly degraded. Under hypoxic conditions, HIF-α is exempted from VHL degradation, followed by the stabilized HIF heterodimer bound to hypoxia response elements within promoters to switch on the transcription of its target genes (Figure 1). Interestingly, emerging evidence shows that HIF protein can be stabilized by increase in reactive oxygen species when cells undergo oxidative stress in some cancers.20, 21, 22 The accumulation of HIF consequently helps the cell to adaptively respond to oxidative stress.23
Figure 1.
Schematic review of the hypoxia-inducible factor (HIF) system controlled by oxygen content. Under normoxic conditions, prolyl hydroxylases (PHDs) catalyze the hydroxylation of two proline residues within the oxygen-dependent degradation domain of HIF-α. These hydroxylation modifications allow the von Hippel–Lindau (VHL) E3 ubiquitin ligase complex to bind and catalyzes ubiquitination on HIF-α, which eventually leads to its proteasomal degradation. On the other hand, the factor inhibiting HIF-1 (FIH) catalyzes an additional hydroxylation on the asparagine residue of HIF-α. The asparagine hydroxylation blocks the transcriptional co-activator p300 from binding with HIF-α, thereby inhibiting HIF transcriptional activity. Both PHDs and FIH belong to the 2-oxoglutarate (2-OG)–dependent dioxygenase family that uses oxygen and 2-OG as cosubstrates and Fe(II) as cofactor to catalyze the hydroxylation reaction. Their enzymatic activity can be repressed by some oncogenic metabolic intermediates, such as 2-hydroxyglutarate (2-HG). Under hypoxic conditions, the activity of PHDs and FIH is inhibited because of lack of oxygen. Unhydroxylated HIF-α translocates to the nucleus, forms a complex with HIF-β and p300, and activates transcription of HIF target genes. HRE, hypoxia response element; OH, hydroxide.
Using chromatin immunoprecipitation assays, gene expression assays, and pan-genomic studies, numerous genes that were directly controlled by HIFs have been identified.24, 25, 26 These target genes are involved in multiple fundamental cellular pathways, such as metabolic regulation,27 erythropoiesis,28 angiogenesis,29 tissue remodeling,30 and wound healing.31 The transcriptional adaptations, on one hand, ensure that normal cells or tissues survive in a severe oxygen environment; on the other hand, they drive tumor progression and many other disorders when cells undergo oxygen-sensing dysfunction and subsequent oncogenic gene expression. In the perspective of tumorigenesis, HIF plays a critical role to promote various oncogenic processes in cancers, including cell proliferation, metabolic reprogramming, anti-apoptosis, epithelial-mesenchymal transition, invasion, and metastasis.32 HIF is also involved in resistance to chemotherapy and radiotherapy.33 Notably, in solid tumors, certain regions usually undergo hypoxic status because of abnormal blood vessel development.33 Accordingly, this is accompanied by elevated HIF-α levels in many solid tumors.33 Moreover, both hypoxia and accumulation of HIF-α are associated with the worse prognosis in cancer patients.33
Proline Hydroxylation, the Fate Code of HIF and Others
There are multiple hydroxylation forms in terms of the amino acid residue modified in proteins, including proline, asparagine, aspartate, lysine, and histidine, while the predominant residue is proline. Prolyl hydroxylation was first identified and most abundant in secreted collagen proteins.34 Afterward, prolyl hydroxylation modifications had been identified in intercellular proteins. Among them, the best-characterized case is the hydroxylation on the HIF proteins by the PHDs (or EHLNs). In physiological oxygen conditions, PHDs hydroxylate HIF-1α within its oxygen-dependent degradation domain at two conserved prolyl residues at Pro402 and Pro564 and HIF-2α at Pro405 and Pro531. Consequently, the hydroxylated proline residues serve as unique codes that allow the VHL and thereby its functional complex E3 ubiquitin ligase to bind and polyubiquitylate the HIF-α, which is subsequently followed by proteasomal degradation (Figure 1). By far, three PHD family proteins have been identified in mammals [namely, PHD1, PHD2, and PHD3 (alias the egg-laying-defective nine proteins, EGLN2, EGLN1, and EGLN3, accordingly).35 PHD2/EGLN1 is the major HIF prolyl hydroxylation enzyme in vivo, which may largely depend on its ubiquitous expression pattern, whereas PHD1/EGLN2 and PHD3/EGLN3 are somehow tissue context dependent.36
A large body of recent studies revealed some novel PHD substrates beyond HIF, and it is important to emphasize that most of these modifications are significantly associated with human diseases. Discovering new HIF-independent prolyl hydroxylation pathways, therefore, is of great importance. Our previous study identified forkhead box O3 (FOXO3a) as a PHD1/EGLN2 prolyl hydroxylase substrate via in vitro hydroxylation screening.37 Notably, emerging evidence indicates that FOXO3a is a tumor suppressor in several cancer models, including leukemia, breast, and prostate cancer.38, 39, 40 Using mass spectrometry, two specific prolyl residues, Pro426 and Pro437, were identified to be hydroxylated by PHD1/EGLN2, leading to the dissociation of ubiquitin specific peptidase 9 X-linked (USP9x) deubiquitinase. Consequently, FOXO3a is destructed through proteasome, therefore activating the transcription of Cyclin D1 and breast tumorigenesis.40 In a recent report, Guo et al41 demonstrated that Akt is another important substrate of PHD2/EGLN1. Hydroxylation at Pro125 and Pro313 residues of Akt promoted its interaction with VHL, where VHL served as an adaptor protein by binding to the phosphatase 2A (PP2AC) that contributed to the Akt dephosphorylations. In pathologic states, when cells undergo hypoxia or VHL loss, Akt is aberrantly activated, promoting cell proliferation and tumor progression.41 Although it seems that hypoxia negatively regulates p53 in different systems,42 it is important to elucidate the connection of this critical tumor suppressor with the oxygen-sensing system. Two recent studies revealed the direct linkage of p53 with PHD1/EGLN2 and PHD3/EGLN3, respectively.43,44 Deschoemaeker et al43 found that p53 activity was attenuated by PHD1/EGLN2 in a hydroxylation-dependent manner in colorectal cancer, although no specific hydroxylation site was identified. Rodriguez et al44 then demonstrated that p53 can be hydroxylated by PHD3/EGLN3 at Pro359, which increases p53 stability by enhancing its ubiquitination by ubiquitin specific peptidase 7/10 (USP7/10). Interestingly, PHD3/EGLN3 also stabilizes p53 in a hydroxylase-independent manner,45 suggesting a more complicated regulation network that deserves further investigation. Other important proteins that were subjected to PHD-mediated prolyl hydroxylation include I-kappaB kinase β (IKKβ), mitogen-activated protein kinase 6 (MAPK6), dual-specificity tyrosine phosphorylation-regulated kinase 1A/1B (DYRK1A/B), N-myc downstream-regulated gene 3 (NDRG3), EPO receptor, and zinc fingers and homeoboxes 2 (ZHX2) and were therefore involved in the oxygen system and showed linkage with diseases. It is not possible to review all the details herein because of space limitations, as some of these targets were reviewed previously.46
VHL, the Proteolysis Executor beyond HIF
The physiological/pathologic function of VHL was constantly explored since the first description of VHL disease in the early 1990s.47 Hereditary VHL mutations consequently develop a series of cancer syndrome in the central nervous system, pancreas, and kidney.48 Among them, the clear cell renal cell carcinoma (ccRCC) leads the major death incidence, which constitutes approximately 70% of morbidity of all renal malignancies and harbors >90% of VHL loss-of-function mutations.49 The tumor-suppressive function of VHL largely relies on its proteolytical degradation to HIF-α with the assistance of oxygen and PHD hydroxylation.50 This was verified by the overproduction of both HIF-1α and HIF-2α or HIF-2α alone in the VHL mutant ccRCC patients.51 In addition, emerging evidence indicated the existence of HIF-independent pathways in the environment controlled by VHL, as some studies demonstrated that the simple inhibition of the HIF system might not be enough to prevent tumor progression.52 In fact, a few important VHL substrates other than HIF have been identified.
By developing an in vitro VHL capture-based binding assay combined with a genome-wide screening strategy, it was recently demonstrated the ZHX2 and the Scm-like with four malignant brain tumor domains 1 (SFMBT1) transcription factors served as novel VHL substrates in ccRCC.53,54 Mechanistically, ZHX2 protein, similar to the expression pattern of HIF, accumulated in VHL-deficient ccRCC patients, but not in VHL wild-type or normal tissues. Mass spectrometry data detected three potential prolyl hydroxylation sites at Pro427, Pro440, and Pro464 that might be important for VHL binding and proteasomal degradation, further suggesting that ZHX2 is also subjected to the supervision of the oxygen signaling pathway. Subsequent functional studies verified that ZHX2 promoted ccRCC carcinogenesis by controlling RELA/p65 nuclear localization and NF-κB activation.53
Another potential VHL substrate regulated by similar oxygen/PHD/VHL machinery is the NDRG3. By observing that NDRG3 was highly accumulated under hypoxic conditions in diverse cell types, Lee et al52 demonstrated that NDRG3 was potentially hydroxylated by PHD2/EGLN1 on the Pro294 followed by its binding with VHL for ubiquitination and degradation. Interestingly, hypoxia induced NDRG3 accumulation through binding with the glycolytic end product lactate, which blocked recognition by VHL and subsequent destruction. Stabilized NDRG3 then contributed to its downstream rapidly accelerated fibrosarcoma (Raf)–extracellular signal-regulated kinase (ERK) 1/2 kinase signaling during tumor development.52
Other potential VHL substrates also have been identified, including EPO receptor (EPOR),55 transcription factor B-Myb,56 actin cross-linker filamin A (FLNA),57 centrosomal protein 68 (CEP68),58 ceramide kinase-like protein (CERKL),59 human RNA polymerase II seventh subunit (hsRPB7),60 and euchromatic histone-lysine methyltransferase 2 (EHMT2).61 In all of these cases, VHL acts as an executor determining the abundance of multifunctional master regulatory proteins largely based on its E3 ubiquitin ligase-dependent activities. In addition, VHL also targets other proteins in an E3 ubiquitin-ligase–independent manner with or without the involvement of oxygen signal. Proteins subjected to this regulatory manner by VHL are, for example, TANK binding kinase 1 (TBK1),62 aldehyde dehydrogenase 2 (ALDH2),63 p53,64 Akt,41 and caspase recruitment domain family member 9 (CARD9).65 Nevertheless, these findings highlight the critical role of VHL in the oxygen-signaling pathway and multiple functions as a tumor suppressor controlling the abundance or activity of its substrates in various diseases (Figure 2).
Figure 2.
The multiple functions of von Hippel–Lindau protein (VHL). VHL suppresses tumor and relies on its E3 ligase-dependent function. Proline hydroxylation modification by the prolyl hydroxylases (PHDs) on the proteins is the prerequisite for VHL to recognize and ubiquitinate its substrates; the ubiquitinated protein is subsequently degraded by the proteasome. Hypoxia suppresses the activity of PHDs and leads to the accumulation of these VHL substrates. On the other hand, VHL can modulate kinase activity, protein stability, and transcriptional activity of some important proteins through an E3 ligase-independent manner. VHL loss or mutations lead to accumulation or dysregulation of its substrates, which causes diseases, such as clear cell renal cell carcinoma (ccRCC). AKT, protein kinase B; ALDH2, aldehyde dehydrogenase 2; CARD9, caspase recruitment domain family member 9; EPOR, erythropoietin receptor; HIF-α, hypoxia-inducible factor-α; NDRG3, N-myc downstream-regulated gene 3; OH, hydroxide; SFMBT1, Scm like with four mbt domains 1; TBK1, TANK binding kinase 1; ZHX2, zinc fingers and homeoboxes 2.
2-OG–Dependent Enzymes, Novel Oxygen Sensors Link Oxygen with Diseases
2-Oxoglutarate (2-OG)–dependent dioxygenases are a group of superfamily enzymes catalyzing a broad range of oxidative reactions using oxygen as cosubstrate.66 Beside oxygen, activities of the 2-OG–dependent enzymes also largely rely on Fe(II), the tricarboxylic acid cycle intermediate 2-OG, and ascorbate in some cases.66 Till date, there are approximately 70 2-OG–dependent oxygenase enzymes in mammals, including the HIF prolyl hydroxylase proteins (PHDs or EGLNs), as mentioned above. They play diverse roles in many cellular pathways, including protein modifications (eg, collagen hydroxylation, histone demethylation), nucleic acid modification (eg, DNA/RNA demethylation/hydroxylation), fatty acid metabolism (eg, carnitine biosynthesis), and oxygen sensing.67
Factor-inhibiting HIF is another HIF hydroxylase that can hydroxylate the asparagine residue of HIF-1 (Asn803) and HIF-2 (Asn851).68 The asparagine hydroxylation inhibits HIF transcriptional activity by blocking the binding with the transcriptional co-activator p300/cyclic AMP response element-binding protein.19 Factor-inhibiting HIF also mediates hydroxylation on other important substrates [eg, asparagine 22 (Asn22) of the ovarian tumor domain containing ubiquitin aldehyde binding protein 1 (OTUB1)],69 which drives cancer progression, metastasis, and chemotherapy resistance in colon, prostate, and breast cancers.70, 71, 72
The histone lysine demethylases (KDMs) are a group of important epigenetic regulatory proteins that shape the chromatin structure and gene transcription. They account for the largest subfamily of 2-OG dioxygenases with approximately 20 members.73 Some of them were intensively studied in diseases, such as the H3K27 demethylase KDM6A, lysine-specific demethylase 1 (LSD1), Jumonji domain-containing protein 3 (JMJD3), and JMJD6. Because of relatively high affinity with oxygen, in some cases, they serve as an oxygen sensor in the process of histone demethylation and hypoxic reprogramming. There are a couple of examples reported most recently, showing that KDM6A, KDM5A, or KDM3A can directly sense oxygen level, albeit control cell fate through diverse downstream cascades. The laboratory of Kaelin74 and Rocha75 and colleagues independently found a direct link between hypoxia and histone methylation. These processes were mediated by KDM6A/5A directly other than through the hypoxic induction of the HIF-dependent pathway. KDM6A, but not its paralog KDM6B, uses oxygen to promote H3K27 demethylation while its function is totally abolished under hypoxia.74 Qian et al76 also reported the KDM3A links the oxygen availability to mitochondrial biogenesis. Mechanistically, under normoxic conditions, KDM3A binds to proliferator-activated receptor-γ coactivator-1α and demethylates proliferator-activated receptor-γ coactivator-1α at lysine 224, thus increasing proliferator-activated receptor-γ coactivator-1α′s activity that is essential for nuclear respiratory factor 1/2 (NRF1/NRF2)-mediated transcriptional events for mitochondrial biogenesis.76
On the other hand, activities of 2-OG–dependent enzymes can be repressed by some oncogenic metabolic intermediates [eg, D-2-hydroxyglutarate (D-2-HG), succinate, and fumarate] derived from mutation of several key metabolic enzymes.77,78 For instance, D-2-HG can directly bind with and modulate the enzymatic activity of the ten-eleven translocation 2 (TET2), a 5-methylcytosine hydroxylase that modulates DNA demethylation by converting 5-methylcytosine to 5-hydroxymethylcytosine. D-2-HG is typically accumulated because of the mutation of isocitrate dehydrogenase 1 (IDH1) or IDH2, which leads to loss of its enzymatic activity, but gain of function by catalyzing the conversion from 2-OG to D-2-HG in IDH1 or IDH2 mutated gliomas and acute myelogenous leukemia.79,80 Functionally, D-2-HG dampens the catalytic activity of TET2 by competing 2-OG out from the TET2 enzymatic pocket because of their structural similarity.77 These studies highlight a pivotal role of TET2 connecting pernicious metabolic signals with the epigenetic dysregulation in cancers. However, there is no direct evidence showing how oxygen change leads to the accumulation of these metabolic intermediates. It is reasonable to speculate that oxygen might play role in the process because succinate and fumarate are important metabolic intermediates of the tricarboxylic acid cycle, which largely depends on the oxygen content of the cell. Interestingly, hypoxia can selectively induce L-2-hydroxyglutarate (L-2-HG) under the wild-type IDH1/2 gene background that suppresses the activity of histone lysine demethylase KDM4C and enhancement of H3K9me3 accumulation in glioblastoma.81
The critical roles of 2-OG–dependent enzymes in diverse biological processes and the complexity of their enzymatic regulation by various nutrient signals and metabolic intermediates suggest their central position between metabolic signaling (eg, oxygen) and downstream signaling cascades, and high vulnerability of disease due to oncometabolite accumulation and post-translational modifications of their substrates. This was confirmed by a most recent pan-cancer prognostic study suggesting that the expression of several family members of the 2-OG–dependent enzymes was associated with poor clinical outcomes across multiple cancer types.82 Nevertheless, the pathophysiological mechanisms of a considerable part of the 2-OG–dependent enzymes remain unclear, which deserve further investigations.
The Therapeutic Implications of the Oxygen-Sensing System
Given the significant role of the HIF signaling pathway in many human diseases, therapeutic applications targeting HIF have been developed for related diseases. There are several approaches to target HIF signaling, including targeting the HIF mRNA expression, protein synthesis, nuclear translocation, dimerization of HIF-α and HIF-β, HIF–hypoxia response element interaction, co-activator recruitment, and transcriptional activity. In addition to directly targeting the oxygen/PHD/HIF/VHL cascade, oxygen-independent oncogenic pathways that activate HIF may also be considered as druggable targets for inhibiting the HIF system. These oncogenic pathways are typically mediated by some key regulatory proteins [eg, phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR),83 mouse double minute 2 homolog (Mdm2),84 and heat shock protein 90 (Hsp90)].85 In fact, on the basis of these principles, several HIF inhibitors, as well as inhibitory candidates targeting the HIF pathway indirectly, have been developed and evaluated in the preclinical and clinical setting (Figure 3).
Figure 3.
Targeting the hypoxia-inducible factor (HIF) system with multiple therapeutic approaches. The schematic shows several examples for intervening in the HIF pathway in different layers, including modulating the mRNA translation, protein stability, dimerization, and transcriptional activity of HIF. Translation of HIF-α can be activated by the phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, which makes it possible to modulate HIF-α protein level by using inhibitors targeting either PI3K (wortmannin, LY294002, and GDC-0941) or mTOR (rapamycin and PP242). EZN-2698 is an antisense RNA antagonist that specifically binds and inhibits the translation of HIF-1α mRNA. Another translational inhibitory agent includes PX-478. The histone deacetylase inhibitor vorinostat can block the heat shock protein 90 (Hsp90)–mediated HIF-α protein stabilization and transcriptional activation. Other agents modulate the HIF-α transcriptional activity, including the factor-inhibiting HIF (FIH) inhibitor dimethyloxalylglycine and proteasome inhibitor bortezomib. MG132 can stabilize HIF-α protein by direct blocking proteasomal degradation. PT2385, PT2399, and PT2977 are selective compounds that allosterically disrupt the heterodimerization of HIF-2α and HIF-1β. HRE, hypoxia response element; mTORC, mTOR complex; OH, hydroxide; p300, a transcriptional co-activator; PHD, prolyl-4-hydroxylase; Ub, ubiquitin; VHL, von Hippel–Lindau.
Inhibitors of HIF-1α
The synthetic oligonucleotide EZN-2968 is an antisense 16-bp nucleotide that serves an RNA antagonist of human HIF-1α. Binding of EZN-2968 to HIF-1α mRNA specifically attenuates HIF-1α protein level in a dose-dependent manner but not HIF-2α at relative low concentration.86 EZN-2698 treatment leads to HIF-1α level decrease under hypoxia and growth deficiency in various cancer cells in vitro, and reduction of tumor in prostate cancer cell line xenograft model in vivo. Two phase 1 clinical trials have been conducted previously, testing EZN-2698 efficacy in small cohorts, and showed the proof of concept for this drug.87 Recently, a third phase 1 trial was finished in patients with hepatocellular carcinoma, further confirming its activity within one cycle of therapy.88 Further trials need to be investigated with more patients.
CRLX-101 is a β-cyclodextrin–based nanoparticle-drug conjugate containing pendant carboxylate groups, camptothecin, and polyethylene glycol.89 The active moiety of CRLX-101 is camptothecin, a potent inhibitor of HIF-1α that prevents HIF-1α protein accumulation. The design of this drug is to achieve the slow release of camptothecin specifically in tumors while sparing normal tissues. CRLX-101 has shown encouraging efficacy in patients with advanced solid malignancies in a combined phase 1/2a trial.90 Besides, CRLX-101 also improved efficacy in metastatic triple-negative breast cancer mouse models in single or combination preclinical studies.91 Currently, a phase 2 clinical trial is under evaluation for the potency of CRLX-101 in combination with the VEGF inhibitor bevacizumab for multiple tumor types (NCT01652079).
IDF-11774 is an (E)-phenoxyacrylic amide and a more potent derivative of the LW6, which was previously developed to inhibit accumulation of HIF-1α by inducing the expression of VHL.92 Several studies indicated that IDF-11774 decreases HIF-1α level through multiple mechanisms, not simply by increasing VHL-mediated HIF-1α degradation in colorectal cancer models both in vitro and in vivo, but also by inhibiting mitochondrial respiration and increasing intracellular oxygen level,93 or acting as Hsp70 (heat shock protein 70) inhibitor.94 IDF-11774 is currently under a phase 1 clinical investigation for cancer therapy by the Korea Food and Drug Administration.
Vorinostat, a synthetic suberoylanilide hydroxamic acid, is a US Food and Drug Administration–approved pan histone deacetylase inhibitor. It decreases HIF-1/2α activity by promoting the degradation of HIF-1/2α and shows efficacy in both solid tumors (hepatocellular carcinoma, osteosarcoma, and glioblastoma) and hematologic malignancies. Besides, the US Food and Drug Administration has approved it for the treatment of patients with cutaneous T-cell lymphoma on the basis of a phase 2 single-arm clinical trial.95 Inconsistent mechanisms have been reported for the suppression function of HIF-1/2α mediated by vorinostat. It was previously shown that vorinostat promotes acetylation of Hsp90 and promotes HIF-1/2α destruction via a ubiquitin-dependent pathway.96 In addition, vorinostat potently blocks HIF-1α nuclear translocation through direct acetylation of its associated chaperone Hsp90.96 Other studies suggested vorinostat increases interactions between HIF-1α and Hsp70 and results in subsequent HIF-1α degradation via the 20S proteasome-mediated nonubiquitin degradation pathway.97
Inhibitors of HIF-2α
A hallmark in VHL-null ccRCC tumor development is constitutive expression and accumulation of HIF-2α, a key oncogenic driver in ccRCC progression, whereas HIF-1α may behave as a tumor suppressor.98,99 This suggests the potential of HIF-2α as a therapeutic target in ccRCC. Two first-in-class, orally administered selective small-molecule inhibitors were developed by Peloton Therapeutics based on research from the University of Texas Southwestern Medical Center.100,101 They screened two closely related compounds, PT2385 and PT2399, using a structure-based drug design strategy based on the PAS-B domains of HIF-2α and HIF-1β. Therefore, PT2385 and PT2399 allosterically disrupt the heterodimerization of HIF-2α and HIF-1β and block the transcription of oncogenes during tumorigenesis. Both of them showed antitumor efficacy in a subset of kidney cancer cell lines and in mouse xenograft models of ccRCC. Significantly, two back-to-back studies investigated the mechanism of action for PT2399 and explored its antitumor mechanism and potency in ccRCC models.102,103 Specifically, HIF-2α target genes, such as VEGFA, SLC2A1 (alias GLUT1), SERPINE1 (PAI-1), and CCND1, were drastically reduced on PT2399 treatment. In addition, PT2399 was found to lead tumor regression in orthotopic xenografts and primary/metastatic mouse models. Notably, PT2399 showed better activity than the sunitinib, a currently US Food and Drug Administration–approved receptor tyrosine kinase inhibitor for the treatment of renal cell carcinoma. It is noteworthy that a phase 1 dose escalation/expansion and combination with nivolumab clinical trial has been conducted for PT2385, demonstrating its safety and promising clinical outcome (NCT02293980). Meanwhile, several single doses and combination studies with PT2385 and PT2399 are under investigation.
Inhibitors of Prolyl Hydroxylase
In addition to targeting HIF signaling in cancers, there has been increasing interest in using the PHD inhibitors to treat anemia in patients with chronic kidney disease,104 ischemic disease,105 heart failure,106 and inflammatory bowel diseases.107 EPO deficiency is one of the major causes of the anemia associated with kidney disease, which used to be treated with recombinant human EPO or other erythropoiesis-stimulating agents. However, the limitation of relatively high cost and related adverse events of these therapeutic approaches hampers the clinical outcome. On the other hand, the rationale of targeting the PHDs that directly stimulate HIF-mediated EPO expression paves the way for new drug development. To date, there are several pan-prolyl hydroxylase inhibitors, which are at the late stage of clinical trials for treatment of anemia, that have been developed, including roxadustat (FG-4592), daprodustat (GSK-1278863), vadadustat (AKB-6548), and molidustat (BAY 85-3934). Of note, roxadustat is the second-generation inhibitor codeveloped by FibroGen, AstraZeneca, and Astellas to replace FG-2216, which was the first promising compound; however, it was halted later because of a safety issue. Notably, roxadustat was recently approved in China for the treatment of kidney failure patients who develop anemia.108
Current Therapeutic Approaches
Significant efforts have been made to develop several inhibitors for various diseases by targeting the HIF pathway directly or indirectly. Although most of the inhibitors have been well documented in the research literature, only a few of them were successfully translated into the clinical setting. This is largely attributable to several aspects, including specificity, potency, and the relatively low response of the inhibitors. Therefore, further investigations are warranted to promote the clinical benefit from the bench-side to the bedside. In the meantime, some adverse effects caused by HIF/pan-PHD inhibition should be taken into consideration. In addition, one should be aware of the complexity of the HIF system because of highly interconnected molecular cascades and overlapped pathways, probably making it more challenging to develop highly selective inhibitors. For instance, the elevation of HIF levels is usually observed in human diseases, like cancers; therefore, it is feasible to inhibit HIF or some of its downstream molecules, such as VEGF, in some diseases. On the other hand, other studies provided evidence that normalization of blood vessels by PHD inhibition hampers tumor cell invasion and metastasis.109 Therefore, identification of hypoxia-associated biomarkers and more detailed understanding of molecular mechanisms affected by hypoxia in certain types of diseases are helpful for designing and choosing specific therapeutic strategy.
In addition, the development of specific enzymatic inhibitors for other 2-OG–dependent dioxygenase family members may provide novel therapeutic implications. By taking advantage of multiple approaches in structure-based drug design, high-throughput compound screening, and structure-activity relationship studies, promising inhibitors for several histone lysine demethylases (eg, LSD1 and jmjC domain-containing demethylases) are currently under investigation.110 Because of the high structural similarity of the group members, and the poor cellular permeability for some of the current inhibitors (eg, metal chelators and 2-OG analogs), some considerable challenges should be addressed before these inhibitors can be used in clinical applications.
Conclusions
This review summarizes the current knowledge of the oxygen-sensing pathway and the related potential therapeutic approaches. In summary, the oxygen-sensing system comprises a large body of molecular components and they can be divided into several functional modules on the basis of their fundamental role: i) the oxygen sensor and molecular modifier (PHDs and other 2-OG–dependent dioxygenases); ii) the master transcriptional regulatory proteins (eg, HIF); iii) the protein modulator (VHL or other modification readers); and iv) the functional genes in response to specific transcriptional regulation (eg, EPO and VEGF). The core components that drive the functionality of the machinery for each module are becoming increasingly clear while leaving the rest to be investigated. The PHD-HIF-VHL axis remains the best-characterized oxygen signaling pathway in the system; however, the existence of novel molecules involved in these pathways is largely unknown. Of note, for the 2-OG–dependent dioxygenases whose enzymatic activity is determined by oxygen content, the role of most of these enzymes in the oxygen sensing setting needs further investigation. Additionally, deciphering the role of 2-OG–dependent dioxygenases in different oxygenic conditions is meaningful and has raised increasing attention in recent years, given their broad substrate spectrum, diverse functions, and close relationship to various diseases. Several pioneering studies have suggested that some members of the 2-OG–dependent dioxygenase family could function as important oxygen sensors that directly mediate epigenetic modification on histones or post-translational modification on transcriptional factor coactivator, and hence, control cell fate independent of HIF-mediated responses.74, 75, 76 These findings suggest that cells might employ multiple oxygen-sensing machineries to govern a variety of cellular processes in different layers. Therefore, uncovering and understanding these general mechanisms both in physiological and pathologic states are useful and critical for developing novel pharmacologic therapeutics for the treatment of related diseases.
Footnotes
Supported by the National Cancer Institute grants R01CA211732 and R21CA223675, American Cancer Society Research Scholar Award TBE-132187, Department of Defense Kidney Cancer Research Program grant W81XWH1910813, and Cancer Prevention and Research Institute of Texas (CPRIT) grant RR190058 (Q.Z.).
Disclosures: None declared.
The American Society for Investigative Pathology (ASIP) Cotran Early Career Investigator Award recognizes early career investigators with demonstrated excellence as an investigator with recently established or emerging independence and with a research focus leading to an improved understanding of the conceptual basis of disease. Qing Zhang, recipient of the ASIP 2020 Cotran Early Career Investigator Award, is scheduled to deliver a lecture entitled “Studying Oxygen Sensing Pathways in Cancers” on November 7, 2020, at the 2020 Pathobiology for Investigators, Students, and Academicians meeting in Boston, MA.
References
- 1.Semenza G.L. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am J Physiol Cell Physiol. 2011;301:C550–C552. doi: 10.1152/ajpcell.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safran M., Kaelin W.G. Jr: HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratcliffe P.J. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyake T., Kung C.K.H., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 5.Lin F.K., Suggs S., Lin C.H., Browne J.K., Smalling R., Egrie J.C., Chen K.K., Fox G.M., Martin F., Stabinsky Z., Badrawi S.M., Lai P.H., Goldwasser E. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza G.L., Nejfelt M.K., Chi S.M., Antonarakis S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G.L., Semenza G.L. General involvement of hypoxia-inducible factor-I in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Inducible operation of the erythropoietin-3' enhancer in multiple cell-lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-Pas heterodimer regulated by cellular O-2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D., Semenza G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer N.V., Kotch L.E., Agani F., Leung S.W., Laughner E., Wenger R.H., Gassmann M., Gearhart J.D., Lawler A.M., Yu A.Y., Semenza G.L. Cellular and developmental control of O-2 homeostasis by hypoxia-inducible factor 1 alpha. Gene Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kibel A., Iliopoulos O., DeCaprio J.A., Kaelin W.G., Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 13.Iliopoulos O., Levy A.P., Jiang C., Kaelin W.G., Jr., Goldberg M.A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., Maxwell P.H., Pugh C.W., Ratcliffe P.J. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 15.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J.M., Lane W.S., Kaelin W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 16.Ivan M., Haberberger T., Gervasi D.C., Michelson K.S., Gunzler V., Kondo K., Yang H., Sorokina I., Conaway R.C., Conaway J.W., Kaelin W.G., Jr. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 18.Epstein A.C.R., Gleadle J.M., McNeill L.A., Hewitson K.S., O'Rourke J., Mole D.R., Mukherji M., Metzen E., Wilson M.I., Dhanda A., Tian Y.M., Masson N., Hamilton D.L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P.H., Pugh C.W., Schofield C.J., Ratcliffe P.J. C-elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 19.Lando D., Peet D.J., Gorman J.J., Whelan D.A., Whitelaw M.L., Bruick R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Gene Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S., Hallis S.P., Jung K.A., Ryu D., Kwak M.K. Impairment of HIF-1 alpha-mediated metabolic adaption by NRF2-silencing in breast cancer cells. Redox Biol. 2019;24:101210. doi: 10.1016/j.redox.2019.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shatrov V.A., Sumbayev V.V., Zhou J., Brune B. Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1alpha (HIF-1alpha) accumulation via redox-dependent mechanisms. Blood. 2003;101:4847–4849. doi: 10.1182/blood-2002-09-2711. [DOI] [PubMed] [Google Scholar]
- 22.Calvani M., Comito G., Giannoni E., Chiarugi P. Time-dependent stabilization of hypoxia inducible factor-1 alpha by different intracellular sources of reactive oxygen species. PLoS One. 2012;7:e38388. doi: 10.1371/journal.pone.0038388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H.S., Zhou Y.N., Li L., Li S.F., Long D., Chen X.L., Zhang J.B., Feng L., Li Y.P. HIF-1 alpha protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. doi: 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schodel J., Oikonomopoulos S., Ragoussis J., Pugh C.W., Ratcliffe P.J., Mole D.R. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:E207–E217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manalo D.J., Rowan A., Lavoie T., Natarajan L., Kelly B.D., Ye S.Q., Garcia J.G.N., Semenza G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 26.Schodel J., Mole D.R., Ratcliffe P.J. Pan-genomic binding of hypoxia-inducible transcription factors. Biol Chem. 2013;394:507–517. doi: 10.1515/hsz-2012-0351. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H.F., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K.I., Dang C.V., Semenza G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg M.A., Dunning S.P., Bunn H.F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 29.Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth-factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 30.Graham C.H., Forsdike J., Fitzgerald C.J., Macdonald-Goodfellow S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int J Cancer. 1999;80:617–623. doi: 10.1002/(sici)1097-0215(19990209)80:4<617::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Botusan I.R., Sunkari V.G., Savu O., Catrina A.I., Grunler J., Lindberg S., Pereira T., Yla-Herttuala S., Poellinger L., Brismar K., Catrina S.B. Stabilization of HIF-1 alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akanji M.A., Rotimi D., Adeyemi O.S. Hypoxia-inducible factors as an alternative source of treatment strategy for cancer. Oxid Med Cell Longev. 2019;2019:8547846. doi: 10.1155/2019/8547846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 34.Pihlajaniemi T., Myllyla R., Kivirikko K.I. Prolyl 4-hydroxylase and its role in collagen-synthesis. J Hepatol. 1991;13:S2–S7. doi: 10.1016/0168-8278(91)90002-s. [DOI] [PubMed] [Google Scholar]
- 35.Kaelin W.G., Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–345. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oehme F., Ellinghaus P., Kolkhof P., Smith T.J., Ramakrishnan S., Hutter J., Schramm M., Flamme I. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem Biophys Res Commun. 2002;296:343–349. doi: 10.1016/s0006-291x(02)00862-8. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X.N., Zhai B., Koivunen P., Shin S.J., Lu G., Liu J.Y., Geisen C., Chakraborty A.A., Moslehi J.J., Smalley D.M., Wei X., Chen X., Chen Z.M., Beres J.M., Zhang J., Tsao J.L., Brenner M.C., Zhang Y.Q., Fan C., DePinho R.A., Paik J., Gygi S.P., Kaelin W.G., Zhang Q. Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Gene Dev. 2014;28:1429–1444. doi: 10.1101/gad.242131.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J.Y., Zong C.S., Xia W.Y., Yamaguchi H., Ding Q.Q., Xie X.M., Lang J.Y., Lai C.C., Chang C.J., Huang W.C., Huang H., Kuo H.P., Lee D.F., Li L.Y., Lien H.C., Cheng X.Y., Chang K.J., Hsiao C.D., Tsai F.J., Tsai C.H., Sahin A.A., Muller W.J., Mills G.B., Yu D.H., Hortobagyi G.N., Hung M.C. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consolaro F., Ghaem-Maghami S., Bortolozzi R., Zona S., Khongkow M., Basso G., Viola G., Lam E.W.F. FOXO3a and posttranslational modifications mediate glucocorticoid sensitivity in B-ALL. Mol Cancer Res. 2015;13:1578–1590. doi: 10.1158/1541-7786.MCR-15-0127. [DOI] [PubMed] [Google Scholar]
- 40.Hillion J., LeConiat M., Jonveaux P., Berger R., Bernard O.A. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997;90:3714–3719. [PubMed] [Google Scholar]
- 41.Guo J.P., Chakraborty A.A., Liu P.D., Gan W.J., Zheng X.N., Inuzuka H., Wang B., Zhang J.F., Zhang L.L., Yuan M., Novak J., Cheng J.Q., Toker A., Signoretti S., Zhang Q., Asara J.M., Kaelin W.G., Wei W.Y. pVHL suppresses kinase activity of Akt in a proline-hydroxylation-dependent manner. Science. 2016;353:929–932. doi: 10.1126/science.aad5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sermeus A., Rebucci M., Fransolet M., Flamant L., Desmet D., Delaive E., Arnould T., Michiels C. Differential effect of hypoxia on etoposide-induced DNA damage response and p53 regulation in different cell types. J Cell Physiol. 2013;228:2365–2376. doi: 10.1002/jcp.24409. [DOI] [PubMed] [Google Scholar]
- 43.Deschoemaeker S., Di Conza G., Lilla S., Martin-Perez R., Mennerich D., Boon L., Hendrikx S., Maddocks O.D., Marx C., Radhakrishnan P., Prenen H., Schneider M., Myllyharju J., Kietzmann T., Vousden K.H., Zanivan S., Mazzone M. PHD1 regulates p53-mediated colorectal cancer chemoresistance. EMBO Mol Med. 2015;7:1350–1365. doi: 10.15252/emmm.201505492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez J., Herrero A., Li S., Rauch N., Quintanilla A., Wynne K., Krstic A., Acosta J.C., Taylor C., Schlisio S., von Kriegsheim A. PHD3 regulates p53 protein stability by hydroxylating proline 359. Cell Rep. 2018;24:1316–1329. doi: 10.1016/j.celrep.2018.06.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y., Gao Q., Xue Y., Li X., Xu L., Li C., Qin Y., Fang J. Prolyl hydroxylase 3 stabilizes the p53 tumor suppressor by inhibiting the p53-MDM2 interaction in a hydroxylase-independent manner. J Biol Chem. 2019;294:9949–9958. doi: 10.1074/jbc.RA118.007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zurlo G., Guo J., Takada M., Wei W., Zhang Q. New insights into protein hydroxylation and its important role in human diseases. Biochim Biophys Acta. 2016;1866:208–220. doi: 10.1016/j.bbcan.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaelin W.G., Jr. The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun. 2005;338:627–638. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 48.Kaelin W.G., Jr., Maher E.R. The VHL tumour-suppressor gene paradigm. Trends Genet. 1998;14:423–426. doi: 10.1016/s0168-9525(98)01558-3. [DOI] [PubMed] [Google Scholar]
- 49.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vriend J., Reiter R.J. Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: a review. Biochim Biophys Acta. 2016;1865:176–183. doi: 10.1016/j.bbcan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Kaelin W.G. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 52.Lee D.C., Sohn H.A., Park Z.Y., Oh S., Kang Y.K., Lee K.M., Kang M., Jang Y.J., Yang S.J., Hong Y.K., Noh H., Kim J.A., Kim D.J., Bae K.H., Kim D.M., Chung S.J., Yoo H.S., Yu D.Y., Park K.C., Yeom Y.I. A lactate-induced response to hypoxia. Cell. 2015;161:595–609. doi: 10.1016/j.cell.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Wu T., Simon J., Takada M., Saito R., Fan C., Liu X.D., Jonasch E., Xie L., Chen X., Yao X., Teh B.T., Tan P., Zheng X., Li M., Lawrence C., Fan J., Geng J., Liu X., Hu L., Wang J., Liao C., Hong K., Zurlo G., Parker J.S., Auman J.T., Perou C.M., Rathmell W.K., Kim W.Y., Kirschner M.W., Kaelin W.G., Jr., Baldwin A.S., Zhang Q. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science. 2018;361:290–295. doi: 10.1126/science.aap8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X.J., Simon J.M., Xie H.B., Hu L.X., Wang J., Zurlo G., Fan C., Ptacek T.S., Herring L., Tan X.M., Li M., Baldwin A.S., Kim W.Y., Wu T., Kirschner M.W., Gong K., Zhang Q. Genome-wide screening identifies SFMBT1 as an oncogenic driver in cancer with VHL loss. Mol Cell. 2020;77:1294–1306. doi: 10.1016/j.molcel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heir P., Srikumar T., Bikopoulos G., Bunda S., Poon B.P., Lee J.E., Raught B., Ohh M. Oxygen-dependent regulation of erythropoietin receptor turnover and signaling. J Biol Chem. 2016;291:7357–7372. doi: 10.1074/jbc.M115.694562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okumura F., Uematsu K., Byrne S.D., Hirano M., Joo-Okumura A., Nishikimi A., Shuin T., Fukui Y., Nakatsukasa K., Kamura T. Parallel regulation of von Hippel-Lindau disease by pVHL-mediated degradation of B-Myb and hypoxia-inducible factor alpha. Mol Cell Biol. 2016;36:1803–1817. doi: 10.1128/MCB.00067-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segura I., Lange C., Knevels E., Moskalyuk A., Pulizzi R., Eelen G., Chaze T., Tudor C., Boulegue C., Holt M., Daelemans D., Matondo M., Ghesquiere B., Giugliano M., Ruiz de Almodovar C., Dewerchin M., Carmeliet P. The oxygen sensor PHD2 controls dendritic spines and synapses via modification of filamin A. Cell Rep. 2016;14:2653–2667. doi: 10.1016/j.celrep.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin H., Zheng L., Liu W., Zhang D., Li W., Yuan L. Rootletin prevents Cep68 from VHL-mediated proteasomal degradation to maintain centrosome cohesion. Biochim Biophys Acta Mol Cell Res. 2017;1864:645–654. doi: 10.1016/j.bbamcr.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Chen J., Liu F., Li H., Archacki S., Gao M., Liu Y., Liao S., Huang M., Wang J., Yu S., Li C., Tang Z., Liu M. pVHL interacts with ceramide kinase like (CERKL) protein and ubiquitinates it for oxygen dependent proteasomal degradation. Cell Signal. 2015;27:2314–2323. doi: 10.1016/j.cellsig.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Na X., Duan H.O., Messing E.M., Schoen S.R., Ryan C.K., di Sant'Agnese P.A., Golemis E.A., Wu G. Identification of the RNA polymerase II subunit hsRPB7 as a novel target of the von Hippel-Lindau protein. EMBO J. 2003;22:4249–4259. doi: 10.1093/emboj/cdg410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casciello F., Al-Ejeh F., Kelly G., Brennan D.J., Ngiow S.F., Young A., Stoll T., Windloch K., Hill M.M., Smyth M.J., Gannon F., Lee J.S. G9a drives hypoxia-mediated gene repression for breast cancer cell survival and tumorigenesis. Proc Natl Acad Sci U S A. 2017;114:7077–7082. doi: 10.1073/pnas.1618706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu L.X., Xie H.B., Liu X.J., Potjewyd F., James L.I., Wilkerson E.M., Herring L.E., Xie L., Chen X., Cabrera J.C., Hong K., Liao C.H., Tan X.M., Baldwin A.S., Gong K., Zhang Q. TBK1 is a synthetic lethal target in cancer with VHL loss. Cancer Discov. 2020;10:460–475. doi: 10.1158/2159-8290.CD-19-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y.H., Wu Z.X., Xie L.Q., Li C.X., Mao Y.Q., Duan Y.T., Han B., Han S.F., Yu Y., Lu H.J., Yang P.Y., Xu T.R., Xia J.L., Chen G.Q., Wang L.S. VHL deficiency augments anthracycline sensitivity of clear cell renal cell carcinomas by down-regulating ALDH2. Nat Commun. 2017;8:15337. doi: 10.1038/ncomms15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roe J.S., Kim H., Lee S.M., Kim S.T., Cho E.J., Youn H.D. p53 Stabilization and transactivation by a von Hippel-Lindau protein. Mol Cell. 2006;22:395–405. doi: 10.1016/j.molcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Yang H., Minamishima Y.A., Yan Q., Schlisio S., Ebert B.L., Zhang X., Zhang L., Kim W.Y., Olumi A.F., Kaelin W.G., Jr. pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28:15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hausinger R.P. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 67.Islam M.S., Leissing T.M., Chowdhury R., Hopkinson R.J., Schofield C.J. 2-Oxoglutarate-dependent oxygenases. Annu Rev Biochem. 2018;87:585–620. doi: 10.1146/annurev-biochem-061516-044724. [DOI] [PubMed] [Google Scholar]
- 68.Schofield C.J., Ratcliffe P.J. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 69.Scholz C.C., Rodriguez J., Pickel C., Burr S., Fabrizio J.A., Nolan K.A., Spielmann P., Cavadas M.A.S., Crifo B., Halligan D.N., Nathan J.A., Peet D.J., Wenger R.H., Von Kriegsheim A., Cummins E.P., Taylor C.T. FIH regulates cellular metabolism through hydroxylation of the deubiquitinase OTUB1. PLoS Biol. 2016;14:e1002347. doi: 10.1371/journal.pbio.1002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karunarathna U., Kongsema M., Zona S., Gong C., Cabrera E., Gomes A.R., Man E.P., Khongkow P., Tsang J.W., Khoo U.S., Medema R.H., Freire R., Lam E.W. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene. 2016;35:1433–1444. doi: 10.1038/onc.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X., Jiang W.N., Wang J.G., Chen H. Colon cancer bears overexpression of OTUB1. Pathol Res Pract. 2014;210:770–773. doi: 10.1016/j.prp.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Iglesias-Gato D., Chuan Y.C., Jiang N., Svensson C., Bao J., Paul I., Egevad L., Kessler B.M., Wikstrom P., Niu Y., Flores-Morales A. OTUB1 de-ubiquitinating enzyme promotes prostate cancer cell invasion in vitro and tumorigenesis in vivo. Mol Cancer. 2015;14:8. doi: 10.1186/s12943-014-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson C., Tumber A., Che K., Cain P., Nowak R., Gileadi C., Oppermann U. The roles of Jumonji-type oxygenases in human disease. Epigenomics. 2014;6:89–120. doi: 10.2217/epi.13.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakraborty A.A., Laukka T., Myllykoski M., Ringel A.E., Booker M.A., Tolstorukov M.Y., Meng Y.J., Meier S.R., Jennings R.B., Creech A.L., Herbert Z.T., McBrayer S.K., Olenchock B.A., Jaffe J.D., Haigis M.C., Beroukhim R., Signoretti S., Koivunen P., Kaelin W.G., Jr. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363:1217–1222. doi: 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batie M., Frost J., Frost M., Wilson J.W., Schofield P., Rocha S. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science. 2019;363:1222–1226. doi: 10.1126/science.aau5870. [DOI] [PubMed] [Google Scholar]
- 76.Qian X., Li X., Shi Z., Bai X., Xia Y., Zheng Y., Xu D., Chen F., You Y., Fang J., Hu Z., Zhou Q., Lu Z. KDM3A senses oxygen availability to regulate PGC-1alpha-mediated mitochondrial biogenesis. Mol Cell. 2019;76:885–895. doi: 10.1016/j.molcel.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Losman J.A., Kaelin W.G. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Gene Dev. 2013;27:836–852. doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nowicki S., Gottlieb E. Oncometabolites: tailoring our genes. FEBS J. 2015;282:2796–2805. doi: 10.1111/febs.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gross S., Cairns R.A., Minden M.D., Driggers E.M., Bittinger M.A., Jang H.G., Sasaki M., Jin S., Schenkein D.P., Su S.M., Dang L., Fantin V.R., Mak T.W. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., Fantin V.R., Jang H.G., Jin S., Keenan M.C., Marks K.M., Prins R.M., Ward P.S., Yen K.E., Liau L.M., Rabinowitz J.D., Cantley L.C., Thompson C.B., Vander Heiden M.G., Su S.M. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Intlekofer A.M., Dematteo R.G., Venneti S., Finley L.W., Lu C., Judkins A.R., Rustenburg A.S., Grinaway P.B., Chodera J.D., Cross J.R., Thompson C.B. Hypoxia induces production of L-2-hydroxyglutarate. Cell Metab. 2015;22:304–311. doi: 10.1016/j.cmet.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang W.H., Forde D., Lai A.G. Dual prognostic role of 2-oxoglutarate-dependent oxygenases in ten cancer types: implications for cell cycle regulation and cell adhesion maintenance. Cancer Commun (Lond) 2019;39:23. doi: 10.1186/s40880-019-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang B.H., Jiang G., Zheng J.Z., Lu Z., Hunter T., Vogt P.K. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 84.Ravi R., Mookerjee B., Bhujwalla Z.M., Sutter C.H., Artemov D., Zeng Q., Dillehay L.E., Madan A., Semenza G.L., Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 85.Isaacs J.S., Jung Y.J., Mimnaugh E.G., Martinez A., Cuttitta F., Neckers L.M. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 86.Greenberger L.M., Horak I.D., Filpula D., Sapra P., Westergaard M., Frydenlund H.F., Albaek C., Schroder H., Orum H. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008;7:3598–3608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 87.Jeong W., Rapisarda A., Park S.R., Kinders R.J., Chen A., Melillo G., Turkbey B., Steinberg S.M., Choyke P., Doroshow J.H., Kummar S. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014;73:343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J., Contratto M., Shanbhogue K.P., Manji G.A., O'Neil B.H., Noonan A., Tudor R., Lee R. Evaluation of a locked nucleic acid form of antisense oligo targeting HIF-1 alpha in advanced hepatocellular carcinoma. World J Clin Oncol. 2019;10:149–160. doi: 10.5306/wjco.v10.i3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng J.J., Khin K.T., Jensen G.S., Liu A.J., Davis M.E. Synthesis of linear, beta-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjug Chem. 2003;14:1007–1017. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 90.Weiss G.J., Chao J., Neidhart J.D., Ramanathan R.K., Bassett D., Neidhart J.A., Choi C.H.J., Chow W., Chung V., Forman S.J., Garmey E., Hwang J., Kalinoski D.L., Koczywas M., Longmate J., Melton R.J., Morgan R., Oliver J., Peterkin J.J., Ryan J.L., Schluep T., Synold T.W., Twardowski P., Davis M.E., Yen Y. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest New Drugs. 2013;31:986–1000. doi: 10.1007/s10637-012-9921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pham E., Yin M., Peters C.G., Lee C.R., Brown D., Xu P., Man S., Jayaraman L., Rohde E., Chow A., Lazarus D., Eliasof S., Foster F.S., Kerbel R.S. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016;76:4493–4503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 92.Lee K., Kang J.E., Park S.K., Jin Y., Chung K.S., Kim H.M., Lee K., Kang M.R., Lee M.K., Song K.B., Yang E.G., Lee J.J., Won M. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010;80:982–989. doi: 10.1016/j.bcp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 93.Ban H.S., Kim B.K., Lee H., Kim H.M., Harmalkar D., Nam M., Park S.K., Lee K., Park J.T., Kim I., Lee K., Hwang G.S., Won M. The novel hypoxia-inducible factor-1alpha inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis. 2017;8:e2843. doi: 10.1038/cddis.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ban H.S., Naik R., Kim H.M., Kim B.K., Lee H., Kim I., Ahn H., Jang Y., Jang K., Eo Y., Bin Song K., Lee K., Won M. Identification of targets of the HIF-1 inhibitor IDF-11774 using alkyne-conjugated photoaffinity probes. Bioconjug Chem. 2016;27:1911–1920. doi: 10.1021/acs.bioconjchem.6b00305. [DOI] [PubMed] [Google Scholar]
- 95.Mann B.S., Johnson J.R., Cohen M.H., Justice R., Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 96.Zhang C., Yang C., Feldman M.J., Wang H., Pang Y., Maggio D.M., Zhu D., Nesvick C.L., Dmitriev P., Bullova P., Chittiboina P., Brady R.O., Pacak K., Zhuang Z. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8:56110–56125. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong X., Lin Z., Liang D., Fath D., Sang N., Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinez-Saez O., Gajate Borau P., Alonso-Gordoa T., Molina-Cerrillo J., Grande E. Targeting HIF-2 alpha in clear cell renal cell carcinoma: a promising therapeutic strategy. Crit Rev Oncol Hematol. 2017;111:117–123. doi: 10.1016/j.critrevonc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Shen C., Beroukhim R., Schumacher S.E., Zhou J., Chang M., Signoretti S., Kaelin W.G. Genetic and functional studies implicate HIF1 alpha as a 14q kidney cancer suppressor gene. Cancer Discov. 2011;1:222–235. doi: 10.1158/2159-8290.CD-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheuermann T.H., Li Q., Ma H.W., Key J., Zhang L., Chen R., Garcia J.A., Naidoo J., Longgood J., Frantz D.E., Tambar U.K., Gardner K.H., Bruick R.K. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9:271–276. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheuermann T.H., Tomchick D.R., Machius M., Guo Y., Bruick R.K., Gardner K.H. Artificial ligand binding within the HIF2 alpha PAS-B domain of the HIF2 transcription factor. Proc Natl Acad Sci U S A. 2009;106:450–455. doi: 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W., Hill H., Christie A., Kim M.S., Holloman E., Pavia-Jimenez A., Homayoun F., Ma Y., Patel N., Yell P., Hao G., Yousuf Q., Joyce A., Pedrosa I., Geiger H., Zhang H., Chang J., Gardner K.H., Bruick R.K., Reeves C., Hwang T.H., Courtney K., Frenkel E., Sun X., Zojwalla N., Wong T., Rizzi J.P., Wallace E.M., Josey J.A., Xie Y., Xie X.J., Kapur P., McKay R.M., Brugarolas J. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho H., Du X.L., Rizzi J.P., Liberzon E., Chakraborty A.A., Gao W.H., Carvo I., Signoretti S., Bruick R.K., Josey J.A., Wallace E.M., Kaelin W.G. On-target efficacy of a HIF-2 alpha antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barrett T.D., Palomino H.L., Brondstetter T.I., Kanelakis K.C., Wu X.D., Yan W., Merton K.P., Schoetens F., Ma J.Y., Skaptason J., Gao J.J., Tran D.T., Venkatesan H., Rosen M.D., Shankley N.P., Rabinowitz M.H. Prolyl hydroxylase inhibition corrects functional iron deficiency and inflammation-induced anaemia in rats. Br J Pharmacol. 2015;172:4078–4088. doi: 10.1111/bph.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bernhardt W.M., Campean V., Kany S., Jurgensen J.S., Weidemann A., Warnecke C., Arend M., Klaus S., Gunzler V., Amann K., Willam C., Wiesener M.S., Eckardt K.U. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 106.Philipp S., Jurgensen J.S., Fielitz J., Bernhardt W.M., Weidemann A., Schiche A., Pilz B., Dietz R., Regitz-Zagrosek V., Eckardt K.U., Willenbrock R. Stabilization of hypoxia inducible factor rather than modulation of collagen metabolism improves cardiac function after acute myocardial infarction in rats. Eur J Heart Fail. 2006;8:347–354. doi: 10.1016/j.ejheart.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 107.Marks E., Goggins B.J., Cardona J., Cole S., Minahan K., Mateer S., Walker M.M., Shalwitz R., Keely S. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm Bowel Dis. 2015;21:267–275. doi: 10.1097/MIB.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 108.Chen N., Hao C., Peng X., Lin H., Yin A., Hao L., Tao Y., Liang X., Liu Z., Xing C., Chen J., Luo L., Zuo L., Liao Y., Liu B.C., Leong R., Wang C., Liu C., Neff T., Szczech L., Yu K.P. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 109.Mazzone M., Dettori D., de Oliveira R.L., Loges S., Schmidt T., Jonckx B., Tian Y.M., Lanahan A.A., Pollard P., de Almodovar C.R., De Smet F., Vinckier S., Aragones J., Debackere K., Luttun A., Wyns S., Jordan B., Pisacane A., Gallez B., Lampugnani M.G., Dejana E., Simons M., Ratcliffe P., Maxwell P., Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jambhekar A., Anastas J.N., Shi Y. Histone lysine demethylase inhibitors. Cold Spring Harb Perspect Med. 2017;7:a026484. doi: 10.1101/cshperspect.a026484. [DOI] [PMC free article] [PubMed] [Google Scholar]