SUMMARY
Research background
Kefir is a natural probiotic drink traditionally produced by milk fermentation using kefir grains. Kefir grains are composed of a complex population of bacteria and yeasts embedded in a polysaccharide-protein matrix. The geographic origin of kefir grains may largely influence their microbial composition and the associated kefir drink properties. Although the detailed bacterial composition of kefir grains from several geographic regions has been reported, to date, analogous data about the microbiome of Greek kefir are lacking. Hence, the aim of this study is to investigate the structure and the diversity of the bacterial community of Greek kefir grains.
Experimental approach
The bacterial community structure and diversity of two different kefir grains from distant geographic regions in Greece were examined via high-throughput sequencing analysis, a culture-independent metagenomic approach, targeting the 16S rRNA V4 variable region, in order to gain a deeper understanding of their bacterial population diversities.
Results and conclusions
Firmicutes (a phylum that includes lactic acid bacteria) was strikingly dominant amongst the identified bacterial phyla, with over 99% of the sequences from both kefir grains classified to this phylum. At the family level, Lactobacillaceae sequences accounted for more than 98% of the operational taxonomic units (OTUs), followed by Ruminococcaceae, Lahnospiraceae, Bacteroidaceae and other bacterial families of lesser abundance. Α relatively small number of bacterial genera dominated, with Lactobacillus kefiranofaciens being the most abundant in both kefir grains (95.0% of OTUs in kefir A and 96.3% of OTUs in kefir B). However, a quite variable subdominant population was also present in both grains, including bacterial genera that have been previously associated with the gastrointestinal tract of humans and animals, some of which are believed to possess probiotic properties (Faecalibacterium spp., Bacteroides spp., Blautia spp.). Differences among the bacterial profiles of the two grains were very small indicating a high homogeneity despite the distant geographic origin.
Novelty and scientific contribution;
This is the first study to deeply explore and report on the bacterial diversity and species richness of Greek kefir.
Key words: kefir, bacterial diversity, species richness, high-throughput sequencing, probiotic drink
INTRODUCTION
Kefir is a fermented dairy product with probiotic properties. It is mildly acidic, self-carbonated, with a creamy consistency and a unique flavour attributed to the bacterial and yeast fermentation products. The reported health benefits to the consumer are proposed to be associated with biochemical alterations in milk constituents during milk fermentation, such as the production of bioactive peptides and organic acids, and with the presence of probiotic microorganisms (1, 2). It is traditionally produced by inoculation of milk (primarily bovine milk, although other milk types can be used) with kefir grains, which comprise symbiotic communities of bacteria and yeast that are embedded in a polysaccharide-protein matrix. Kefir grains contain a variety of microbiota including lactic acid bacteria (LAB) and yeast, and occasionally acetic acid bacteria (3-7). Kefir grains of different origin contain distinct consortia of microorganisms (3, 7) and the microbial composition of kefir of different geographic origins has been investigated using culture-dependent and/or culture-independent methods (4, 8-10).
Metagenomics provides a powerful tool for the analysis of microbial communities that does not depend on culturing and has been increasingly used in studies involving food microbial communities (11). Hence, in the last decade, metagenomic analyses have been employed for the culture-independent, in-depth description of the microbial communities of kefir grains from Ireland (7, 12), Brazil (13), Tibet (14, 15), Turkey (16, 17), USA, Spain, Canada and Germany (7), Italy (7, 18), Belgium (19), Malaysia (20), France and the UK (7, 21).
In Greece, with the exception of one published study focusing on the fungal composition of kefir grains and kefir drinks (22), to date there is no literature on the composition of the Greek kefir microbial community. In particular, the bacterial diversity in Greek kefir grains has not been studied. The aim of this study is to explore the bacterial diversity and species richness of two kefir grains originating from distant geographic regions in Greece using a metagenomic approach. This is the first report on using metagenomic analysis to elucidate the microbiological composition of Greek kefir grains.
MATERIALS AND METHODS
Kefir grain samples
Kefir grains were obtained from two geographically distant artisanal kefir producers in Greece, located in Athens (kefir A) and Crete (kefir B). The sampling of grains was done aseptically; grains were transported to the laboratory in low-fat (1.5%) ultra-high temperature (UHT) milk under refrigeration (approx. 5 °C). Upon arrival to the laboratory, the grains were propagated in low-fat UHT milk at the 5% inoculation level (m/V) and incubated at 25 °C for 24 h. At the end of fermentation, the grains were filtered through a sterile sieve and washed with sterile normal saline. This grain propagation procedure was repeated five times and then the kefir grains were used for DNA extraction.
DNA isolation and high-throughput sequencing
Total DNA isolation was performed using 10 g of kefir grains that were placed in a stomacher filter bag with 90 mL of ¼ strength Ringer’s solution (Lab M Limited, Lancashire, UK). The sample was mixed at maximum speed in a Stomacher 400 Lab blender (Seward Medical, London, UK) for 15 min. The liquid was centrifuged at 17 590×g and 4 °C (model 7780; Kubota Corp., Tokyo, Japan) for 7 min. The pellet was resuspended in 20 mM Tris, 2 mM EDTA, 1% Triton X-100 and 30 mg/mL lysozyme (Merck, Darmstadt, Germany) and incubated at 37 °C for 1 h. After the incubation period, 200 μL were removed to a sterile microcentrifuge tube and 20 μL of proteinase K (Thermo Fisher Scientific Inc., Rochester, NY, USA) were added. The solution was incubated overnight at 55 °C. After this step, DNA was extracted using the GeneJET Whole Blood Genomic DNA Purification mini kit (Thermo Fisher Scientific Inc.) according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were determined in a SmartSpec™ Plus spectrophotometer (BioRad Inc., Hercules, CA, USA).
One hundred ng of each DNA sample were used for a polymerase chain reaction (PCR) using the HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA, USA). Primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACVSGGGTATCTAAT-3′) that target the 16S rRNA V4 variable region were used with a barcode on the forward primer (23). PCR conditions were as follows: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 60 °C for 40 s and 72 °C for 1 min, and a final elongation step at 72 °C for 5 min. Successful amplification was determined on a 2% agarose gel. Samples were purified using calibrated Ampure XP beads (Agencourt Bioscience Corporation, Danvers, MA, USA). Purified PCR products were used to prepare a DNA library by following the Illumina TruSeq DNA library preparation protocol (Illumina Inc., San Diego, CA, USA). Sequencing was performed at the Molecular Research Laboratory, Shallowater, TX, USA on an Illumina MiSeq instrument following the manufacturer’s guidelines.
Processing of sequencing data
Sequence data were processed using a commercial sequencing facility analysis pipeline (Molecular Research Laboratory) (23). In brief, sequences were joined and edited to remove the barcode and primer sequences. Sequences less than 150 bp and sequences with ambiguous base calls were removed. Next, sequences were denoised and clustered at 3% divergence to generate operational taxonomic units (OTUs), followed by the removal of chimeric sequences. Final OTUs were taxonomically classified using BLASTn (24) against a curated database derived from GreenGenes (25), Ribosomal Database Project (RDPII) (26) and the National Center for Biotechnology Information (NCBI) (27). All the sample raw reads have been deposited at NCBI and are available under the BioProject ID PRJNA635224.
Diversity assessment
Diversity indices were calculated at the species level (28). The Shannon entropy (H') was calculated as follows:
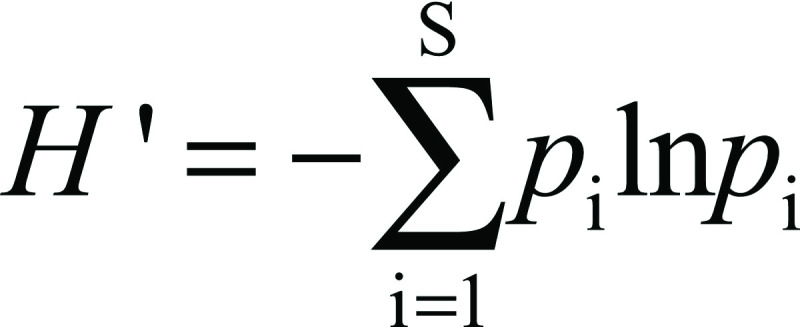 |
where pi is the proportion of species i relative to the total number of species, and S is the total number of species. Shannon's equitability (EH) was calculated by dividing H' by H'max (were H'max=lnS).
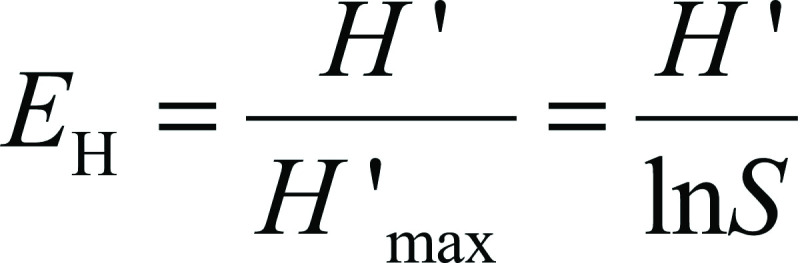 |
The Simpson (D) and Gini-Simpson's (DGS) diversity indices were calculated using the following equations, respectively:
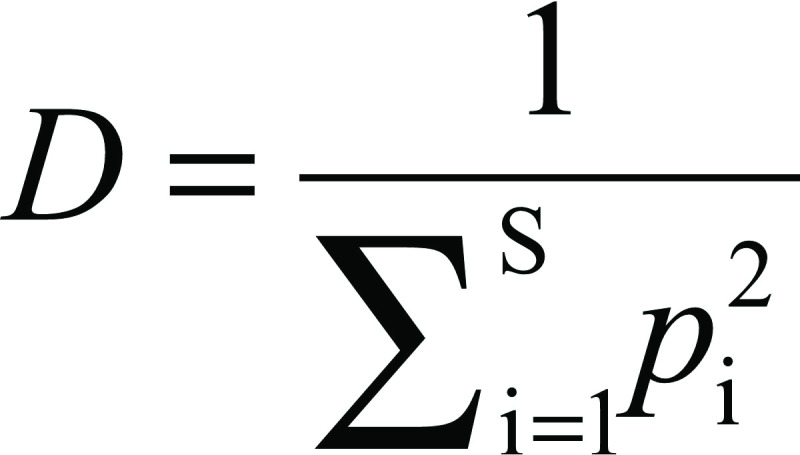 |
And
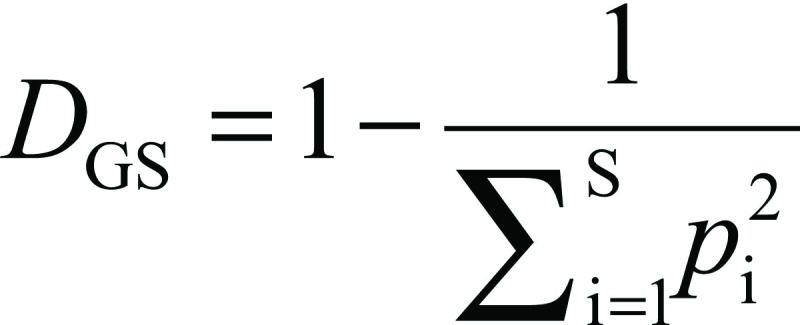 |
Hill numbers were calculated using the equation:
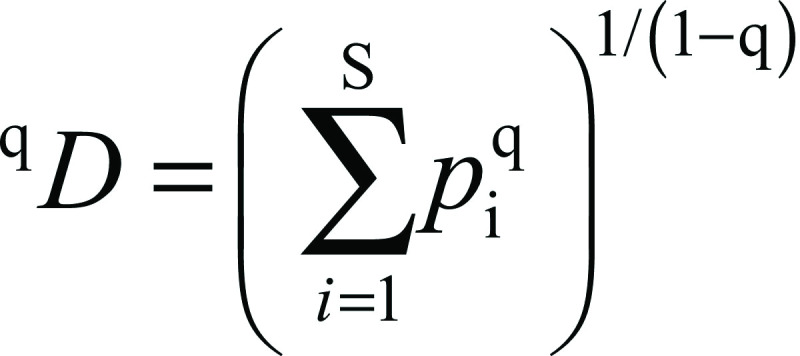 |
where q is the order of the diversity, a parameter that controls the sensitivity of the measure to the relative abundance of the species (29, 30). For q=0 the Hill number indicates species richness; in the limit as q approaches 1, the Hill number represents the exponential of Shannon's entropy index, whereas for q=2 it signifies the inverse of Simpson's concentration index. Alpha and beta diversity data were generated using QIIME (31). A principal coordinate analysis (PCoA) plot based on the unweighted UniFrac distance matrix was generated using EMPeror in QIIME (32).
RESULTS AND DISCUSSION
Sequencing results
The analysis of the microbiota of the two kefir grains by 16S amplicon sequencing generated a total of 305 072 raw sequences. Of these, 156 370 were from kefir A and 148 702 belonged to kefir B.
Bacterial diversity of Greek kefir grains
Diversity metrics in microbiome studies is used to infer the structure of a community with respect to species richness and evenness. Rarefaction curves (Fig. 1) of both samples showed a plateau as the number of sequences increased, indicating that the bacterial community of the two grains was adequately sampled. Diversity indices provide information about the composition of a community by considering the relative abundances of different species. Species richness is a measure of the total number of different species in a sample. At the species level, the microbiota of kefir A was composed of 61 distinct species versus 55 in kefir B. Since species richness does not incorporate any information about the relative abundance of a species, values of the Shannon entropy, Shannon's equitability, Simpson dominance and Gini-Simpson index were calculated and are reported in Table 1. A Shannon entropy index of 1 indicates that all species are equally represented in the bacterial community of a sample, whereas a high value of the Simpson’s index indicates low diversity. Based on the value for Shannon entropy (0.24 for kefir A and 0.18 for kefir B) and Simpson dominance (0.92 for kefir A and 0.94 for kefir B), we could describe the equitability, or evenness of individual distributions among species in the Greek kefir grain community, as relatively low. This is in line with other studies reporting on the presence of one or a few dominant species in kefir grain communities from different geographic locations (7, 13, 18, 33). Since both indices have limitations, the effective number of species, expressed by Hill numbers, was proposed as more informative metrics to quantify diversity (29, 30). Hill numbers were calculated for the two kefir grain samples and are presented in Table 2. As the order of q increases, low diversity values indicate a high degree of dominance in the community (29). As shown, the effective number of species, a metric that is better associated with dominance, was 1.27 for kefir A and 1.20 for kefir B. Based on the calculated diversity indices, the bacterial community in the two Greek kefir grains shows high dominance by a few bacterial species. Beta diversity (a measure of the difference between the entire microbial community in kefirs A and B) provides complementary information on community variation. Beta diversity was assessed by calculating the UniFrac distance metric, which is based on the fraction of branch length within a phylogenetic tree that is shared between two bacterial communities. PCoA of the microbial community of each sample based upon the unweighted (abundance independent) UniFrac distance matrix was performed in order to compare the diversity in the microbial composition between the two samples. Unweighted UniFrac considers only the presence or absence of lineages and provides information on community membership. The PCoA plot illustrated in Fig. 2. shows that the microbiota in the two kefir grains cluster together, indicating that the two microbial communities are evolutionarily similar.
Fig. 1.
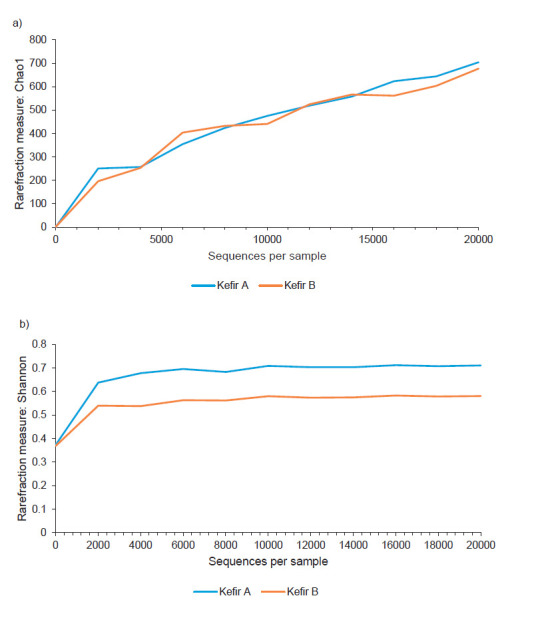
Rarefaction curves of both samples showed a plateau as the number of sequences increased indicating that the kefir community was sufficiently sampled: a) Chao1 is a non-parametric richness estimator, which estimates the number of species present as singletons or doubletons in a sample based on abundances and has units of number of species, b) Shannon index is an entropy measure, which provides the uncertainty in the species diversity of a randomly chosen individual in the community and has units of bits of information. Blue line represents kefir A (Athens) and red line represents kefir B (Crete)
Table 1. Alpha diversity indices for kefir A and B.
| Kefir A | Kefir B | ||
|---|---|---|---|
| Richness | 61 | 55 | |
| Shannon entropy | 0.237 | 0.181 | |
| Shannon's equitability | 0.058 | 0.045 | |
| Simpson dominance | 0.920 | 0.944 | |
| Gini-Simpson index | 0.080 | 0.057 |
Kefir A grain originated from Athens and kefir B from Crete
Table 2. Hill numbers for the order of q for kefir A and B.
| Hill number (qD) | |||
|---|---|---|---|
| Order q | Kefir A | Kefir B | |
| 0 | 61 | 55 | |
| 1 | 1.27 | 1.20 | |
| 2 | 1.09 | 1.06 | |
| 3 | 1.07 | 1.04 | |
| 4 | 1.06 | 1.04 | |
| 5 | 1.04 | 1.03 | |
Hill numbers (qD) measure diversity as the effective number of species. Hill number for q=0 indicates species richness, q=1 represents the exponential of Shannon's entropy index and q=2 the inverse of Simpson's concentration index. Kefir A grain originated from Athens and kefir B from Crete
Fig. 2.
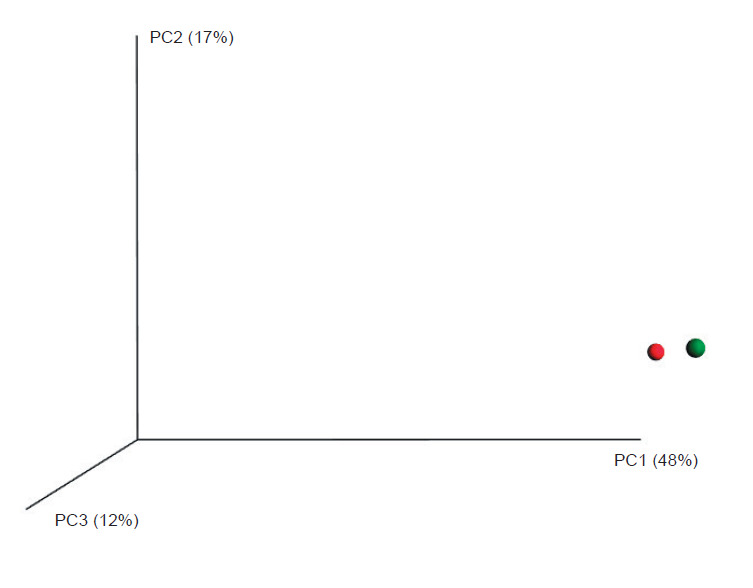
Principal coordinate analysis plot based on unweighted UniFrac distance matrices for kefir A (Athens, red circle) and kefir B (Crete, green circle). UniFrac measures the phylogenetic distance between sets of taxa in a phylogenetic tree as a fraction of branch length. This metric captures the total amount of evolution that is unique to each sample, reflecting adaptation to one environment that could be deleterious to the other. The percentages in the axis labels represent the percentages of variation explained by the principal coordinates
Bacterial profile of Greek kefir grains
The percentages of bacterial OTUs assigned to the phylum, family, genus and species levels of taxonomy are given in Fig. 3. The bacterial phylum Firmicutes, which includes LAB, dominated the bacterial OTUs, with 99.4 and 99.5% of the sequences of kefir A and kefir B, respectively, classified to this phylum (Fig. 3a). Lactobacillaceae was the dominant bacterial family in both kefir grains (approx. 98%) followed by Ruminococcaceae (0.6%), Lachnospiraceae (0.4%), Bacteroidaceae (0.2%), Syntrophomonadaceae (0.2%) and other families of minor representation (Fig. 3b).
Fig. 3.
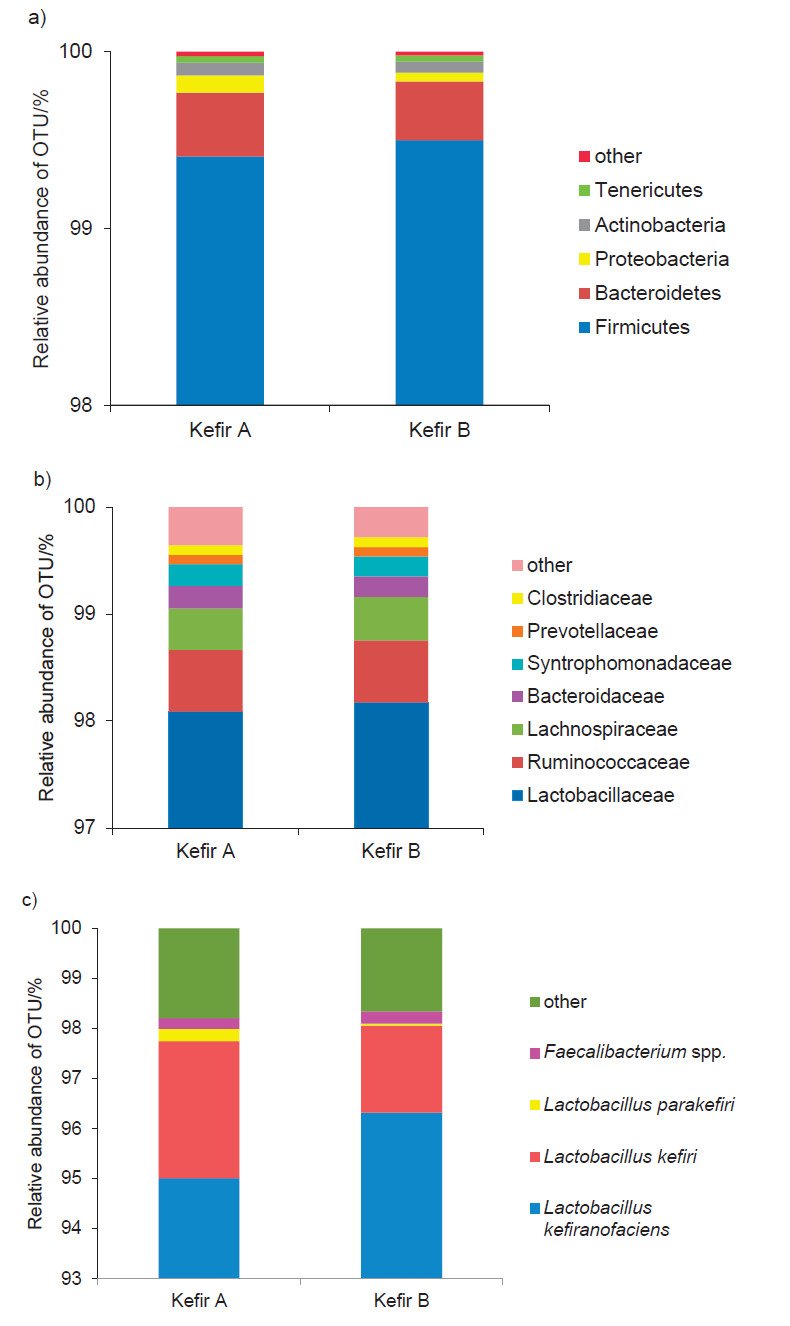
Relative abundance of bacterial OTUs (operational taxonomic units) at the a) phylum, b) family and c) genus/species levels detected by 16S metagenomic analysis of two kefir grains of distant geographic origin in Greece: kefir A (Athens) and kefir B (Crete)
The predominant species identified in both kefir grains was Lactobacillus kefiranofaciens, followed by Lb. kefiri and Lb. parakefiri. The percentages of OTUs assigned to these species were different in the two kefir grains, as shown in Fig. 3c. Lb. kefiranofaciens was more prevalent in kefir B (96.3 vs. 95.0% in kefir A), whereas Lb. kefiri and Lb. parakefiri OTUs were identified at higher percentages in kefir A (2.75 and 0.25% in kefir A, vs. 1.74 and 0.04% in kefir B). All three species are described as normal and frequent components of kefir drinks and kefir grains (12, 18, 19). Specifically, Lb. kefiranofaciens is classified as a homofermentative LAB (34), producing principally d-(–)-lactic acid (without gas) from glucose fermentation, and has been identified as the most abundant bacterial species in kefir grains originating from different parts of the world (13, 16, 18-20, 35). Lb. kefiranofaciens produces kefiran, a biopolymer (polysaccharide) possessing a variety of health benefits (anti-inflammatory, antibacterial, antitumour, antioxidant) and technological functionalities due to its favourable characteristics, such as its rheological behaviour, biocompatibility and emulsifying properties (36). Lb. kefiri and Lb. parakefiri (heterofermentative LAB) are also common kefir isolates around the world (13, 19). Lb. kefiranofaciens and Lb. kefiri are considered as two key LAB in the mechanism of kefir grain formation (37). OTUs of other species of lactobacilli that were recovered in very low or rare frequencies included Lb. crispatus, Lb. aviarius, Lb. agilis and Lb. salivarius (Table 3).
Table 3. Numbers and percentages of bacterial operational taxonomic units (OTUs) identified in two kefir grains from distant geographic origin in Greece (kefir A grain originating from Athens and kefir B from Crete).
| Bacterial genus or species | N(bacterial OTU) | N(bacterial OTU)/% | |||
|---|---|---|---|---|---|
| Kefir A | Kefir B | Kefir A | Kefir B | ||
| Lactobacillus kefiranofaciens | 142970 | 138125 | 95.005 | 96.318 | |
| Lactobacillus kefiri | 4133 | 2494 | 2.746 | 1.739 | |
| Lactobacillus parakefiri | 367 | 59 | 0.246 | 0.041 | |
| Faecalibacterium spp. | 321 | 351 | 0.215 | 0.247 | |
| Bacteroides spp. | 191 | 159 | 0.128 | 0.112 | |
| Pseudobutyrivibrio spp. | 119 | 120 | 0.080 | 0.084 | |
| Clostridium spp. | 118 | 99 | 0.079 | 0.070 | |
| Coprococcus spp. | 96 | 104 | 0.064 | 0.073 | |
| Blautia spp. | 85 | 100 | 0.057 | 0.070 | |
| Subdoligranulum spp. | 81 | 73 | 0.054 | 0.051 | |
| Acinetobacter johnsonii | 96 | 23 | 0.064 | 0.016 | |
| Olsenella spp. | 68 | 54 | 0.046 | 0.038 | |
| Lactobacillus crispatus | 46 | 28 | 0.031 | 0.020 | |
| Allobaculum spp. | 37 | 22 | 0.025 | 0.015 | |
| Ruminococcus spp. | 34 | 39 | 0.023 | 0.027 | |
| Bacteroides barnesiae | 32 | 31 | 0.021 | 0.022 | |
| Anaeroplasma spp. | 28 | 25 | 0.019 | 0.018 | |
| Shuttleworthia spp. | 25 | 26 | 0.017 | 0.018 | |
| Phascolarctobacterium spp. | 24 | 22 | 0.016 | 0.015 | |
| Paraprevotella | 21 | 23 | 0.014 | 0.016 | |
| Lactobacillus aviarius | 20 | 21 | 0.013 | 0.015 | |
| Parabacteroides spp. | 18 | 12 | 0.012 | 0.008 | |
| Oscillospira spp. | 15 | 9 | 0.010 | 0.006 | |
| Alistipes spp. | 13 | 15 | 0.009 | 0.011 | |
| Flavonifractor spp. | 13 | 8 | 0.009 | 0.006 | |
| Moryella | 13 | 15 | 0.009 | 0.011 | |
| Bacteroides salanitronis | 12 | 15 | 0.008 | 0.011 | |
| Lactobacillus spp. | 10 | 7 | 0.007 | 0.005 | |
| Anaerotruncus spp. | 9 | 8 | 0.006 | 0.006 | |
| Sutterella | 9 | 8 | 0.006 | 0.006 | |
| Thalassospira spp. | 8 | 9 | 0.005 | 0.006 | |
| Bacteroides plebeius | 6 | 1 | 0.004 | 0.001 | |
| Slackia spp. | 6 | 7 | 0.004 | 0.005 | |
| Clostridium orbiscindens | 5 | 7 | 0.003 | 0.005 | |
| Papillibacter spp. | 4 | 3 | 0.003 | 0.002 | |
| Roseburia | 4 | 9 | 0.003 | 0.006 | |
| Turicibacter spp. | 4 | 8 | 0.003 | 0.006 | |
| Anaerofilum spp. | 3 | 3 | 0.002 | 0.002 | |
| Bacillus spp. | 3 | 2 | 0.002 | 0.001 | |
| Bacteroides coprocola | 3 | 3 | 0.002 | 0.002 | |
| Clostridium lactatifermentans | 3 | 1 | 0.002 | 0.001 | |
| Leuconostoc mesenteroides dextranicum | 3 | 0 | 0.002 | 0.000 | |
| Mucispirillum spp. | 3 | 4 | 0.002 | 0.003 | |
| Pseudomonas fluorescens | 3 | 1 | 0.002 | 0.001 | |
| Bacteroides coprophilus | 2 | 7 | 0.001 | 0.005 | |
| Collinsella | 2 | 2 | 0.001 | 0.001 | |
| Enterococcus cecorum | 2 | 4 | 0.001 | 0.003 | |
| Lactobacillus agilis | 2 | 0 | 0.001 | 0.000 | |
| Lactobacillus salivarius | 2 | 1 | 0.001 | 0.001 | |
| Marvinbryantia | 2 | 4 | 0.001 | 0.003 | |
| Oscillibacter spp. | 2 | 3 | 0.001 | 0.002 | |
| Veillonella magna | 2 | 1 | 0.001 | 0.001 | |
| Aeriscardovia aeriphila | 1 | 0 | 0.001 | 0.000 | |
| Akkermansia | 1 | 0 | 0.001 | 0.000 | |
| Anaerostipes | 1 | 0 | 0.001 | 0.000 | |
| Bacteroides capillosus | 1 | 0 | 0.001 | 0.000 | |
| Barnesiella spp. | 1 | 0 | 0.001 | 0.000 | |
| Dorea | 1 | 0 | 0.001 | 0.000 | |
| Peptococcus spp. | 1 | 2 | 0.001 | 0.001 | |
| Prevotella spp. | 1 | 0 | 0.001 | 0.000 | |
| Pseudoflavonifractor spp. | 1 | 0 | 0.001 | 0.000 | |
| Fusobacterium mortiferum | 0 | 1 | 0.000 | 0.001 | |
| Megamonas spp. | 0 | 2 | 0.000 | 0.001 | |
| Megasphaera | 0 | 1 | 0.000 | 0.001 | |
| Oxalobacter spp. | 0 | 1 | 0.000 | 0.001 | |
Interestingly, OTUs from a variety of microorganisms which have been previously associated with the human gut microbiome or dairy animals’ rumen were noted at generally small but variable frequencies in our analyses. Hence, a noticeable percentage of OTUs in both kefir grains (approx. 0.22 and 0.25% in kefir A and B, respectively) corresponded to Faecalibacterium spp. To date, there is only one recognized species within this genus, F. prausnitzii, a butyrate-producing bacterium of the colon, which is considered as beneficial to humans and animals and is found in the mammalian and avian gut, but also occasionally isolated from bovine milk (38). To our knowledge, there is only one published study reporting on the presence of Faecalibacterium spp. in kefir grains (7) at very low frequencies (<1%) in the overall population.
Bacteroides spp. ranked third in terms of relative abundance, with approx. 0.13% in kefir A and 0.11% in kefir B. OTUs of six species within the genus Bacteroides were noted (albeit in minor to rare frequencies) in the kefir grains analyzed: B. barnesiae, B. coprocola, B. coprophilus, B. plebeius, B. salanitronis (detected in both kefir grains) and B. capillosus (in grain A only). Previously published studies on kefir have revealed sequences belonging to the same family (Bacteroidaceae) level of taxonomy and only one study (20) reported sequences to the species level (B. chinchillae, B. stercorirosoris and B. vulgatus). To our knowledge, these six bacterial species have never been previously associated with kefir. Bacteria of the genus Bacteroides are strictly anaerobic and reside in the gastrointestinal tract of humans and animals. Owing to their beneficial modulatory mechanisms (interactions with the host), members of Bacteroides spp. have received attention as promising candidates for next-generation probiotics (39).
A similarly minor frequency of OTUs (approx. 0.08%) across both grains were identified as Pseudobutyrivibrio spp., which are members of the Lachnospiraceae family. These organisms are Gram-negative anaerobic rods, which ferment a variety of carbohydrates, with major fermentation end-products being formate, butyrate and lactate. Pseudobutyrivibrio spp. are considered commensal bacteria in the rumen of dairy ruminants (40).
Similar and very low percentages of Clostridium spp. OTUs (0.07-0.08%) were noted in the two grains. At the species level, only rare sequences of C. orbiscindens (0.003 and 0.005%) and C. lactatifermentans (0.002 and 0.001%) were noted. C. orbiscindens is an abundant member of the human gut microbiome (41), whereas C. lactatifermentans is a member of the clostridial 16S rRNA cluster XIVb, originally described as a chicken caecum isolate (42) and was recently proposed to be re-classified in the novel genus Anaerotignum (43). Sequences belonging to the Clostridiaceae family have previously been reported from only one study in kefir (7).
Coprococcus spp. OTUs were noted in very small but similar frequencies in both grain samples (approx. 0.06-0.07%). To the best of our knowledge, bacteria belonging to this genus of strictly anaerobic cocci have never been previously associated with kefir grains. This genus includes four recognized species to date (44) and belongs to the order of Clostridiales (within the phylum Firmicutes). Coprococcus spp. are naturally present in human faeces and only rarely associated with human clinical specimens. Similarly, Blautia spp. OTUs were noted in very small frequencies in both grains (approx. 0.06-0.07%) and have not been previously associated with kefir. Blautia spp. are members of the gut microbiota and, according to recent findings, their relative abundance in the human gut is inversely associated with visceral fat accumulation in adults (45). Subdoligranulum spp. OTUs were also noted in very low frequencies in both grains (approx. 0.05%); to date, there is only one recognized species (S. variabile) within this genus of strictly anaerobic, Gram-negative, gut bacteria (46), with no previous association with kefir grains.
To our knowledge, Acinetobacter johnsonii (0.064% OTUs in kefir A and 0.016% in kefir B) has only been found in Malaysian kefir (20), although other members of the genus have been reported in Turkish (17) and Tibetan (14) kefir. Unlike other well-known pathogenic species of the genus (A. baumannii, a severe hospital-acquired pathogen), A. johnsonii is considered part of the normal human skin flora and has only rarely been associated with human disease (47), whereas recently, it has been shown to have the capacity to degrade polycyclic aromatic hydrocarbons (48).
Additional OTUs belonging to bacterial genera and species mostly associated with the gastrointestinal tract of humans and animals (e.g. Olsenella spp., Allobaculum spp., Ruminococcus spp., Shuttleworthia spp., Phascolarctobacterium spp., Paraprevotella spp. Parabacteroides spp., Oscillospira spp., Alistipes spp., Flavonifactor spp., Roseburia spp. Collinsella spp.) and never been previously associated with kefir, were also noted at rare (<0.05%) frequencies (Table 3). Whether some or all of these microorganisms are naturally present in kefir grains or came in as contaminants from human handlers during sequential kefir grain propagation (i.e. over the years during artisanal kefir making) remains unknown. Recent findings, however, imply that specific strains or species within some of these bacterial genera may be positively (49) or negatively (50) associated with human health. The microbiota of kefir drinks and their corresponding kefir grains can be quite different. The bacterial population of kefir milk is more consistent and less diverse than that of the corresponding kefir grains (7). Since we did not test kefir drinks made by the two grains, it is not known whether these minor species or genera will be present in the corresponding kefir drinks.
CONCLUSIONS
In this study, the bacterial composition of two Greek kefir grains originating from geographically distant areas (Athens and Crete) were evaluated using a high-throughput, sequencing-based approach. The study provided for the first time an in-depth analysis of the bacterial diversity and species richness of kefir grains in Greece. In terms of bacterial populations, both kefir grains were dominated by three species of lactobacilli, with Lb. kefiranofaciens being the principally dominant species. However, in contrast to the small variety of dominant species, a great variety of sub-dominant genera and species were identified. Based on published scientific literature, most of this sub-dominant bacterial flora has been associated with the gastrointestinal tract of humans and animals and has never been identified as part of the kefir community worldwide. Differences between the bacterial profiles of the two grains were very small, indicating a high homogeneity despite the distant geographic origin. This study provides novel data on the bacterial ecology of Greek kefir. The detailed composition of its microbiota will be valuable in order to screen for beneficial strains from this traditional probiotic dairy product.
ACKNOWLEDGEMENTS
The authors would like to thank the two Greek artisanal kefir producers for providing the kefir grains for analysis.
Footnotes
FUNDING: Part of this study was funded by a grant awarded to MSK by the Internal Research Fund of the American College of Thessaloniki.
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Bourrie BCT, Willing BP, Cotter PD. The microbiota and health promoting characteristics of the fermented beverage kefir. Front Microbiol. 2016;7:647. 10.3389/fmicb.2016.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DH, Jeong D, Kim H, Seo KH. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit Rev Food Sci Nutr. 2019;59(11):1782–93. 10.1080/10408398.2018.1428168 [DOI] [PubMed] [Google Scholar]
- 3.Plessas S, Nouska C, Mantzourani I, Kourkoutas Y, Alexopoulos A, Bezirtzoglou E. Microbiological exploration of different types of kefir grains. Fermentation (Basel). 2017;3(1):1 10.3390/fermentation3010001 [DOI] [Google Scholar]
- 4.Miguel MGCP, Cardoso PG, Lago LA, Schwan RF. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res Int. 2010;43(5):1523–8. 10.1016/j.foodres.2010.04.031 [DOI] [Google Scholar]
- 5.Rea MC, Lennartsson T, Dillon P, Drinan FD, Reville WJ, Heapes M, et al. Irish kefir-like grains: Their structure, microbial composition and fermentation kinetics. J Appl Bacteriol. 1996;81(1):83–94. 10.1111/j.1365-2672.1996.tb03286.x [DOI] [Google Scholar]
- 6.Taş TK, Ekinci FY, Guzel-Seydim ZB. Identification of microbial flora in kefir grains produced in Turkey using PCR. Int J Dairy Technol. 2012;65(1):126–31. 10.1111/j.1471-0307.2011.00733.x [DOI] [Google Scholar]
- 7.Marsh AJ, O’Sullivan O, Hill C, Ross RP, Cotter PD. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS One. 2013;8(7):e69371. 10.1371/journal.pone.0069371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzel-Seydim Z, Wyffels JT, Seydim AC, Greene AK. Turkish kefir and kefir grains: Microbial enumeration and electron microscopic observation. Int J Dairy Technol. 2005;58(1):25–9. 10.1111/j.1471-0307.2005.00177.x [DOI] [Google Scholar]
- 9.Loretan T, Mostert JF, Viljoen BC. Microbial flora associated with South African household kefir. S Afr J Sci. 2003;99(1-2):92–4. [Google Scholar]
- 10.Witthuhn RC, Schoeman T, Britz TJ. Isolation and characterization of the microbial population of different South African kefir grains. Int J Dairy Technol. 2004;57(1):33–7. 10.1111/j.1471-0307.2004.00126.x [DOI] [Google Scholar]
- 11.Ercolini D. High-throughput sequencing and metagenomics: Moving forward in the culture-independent analysis of food microbial ecology. Appl Environ Microbiol. 2013;79(10):3148–55. 10.1128/AEM.00256-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobson A, O’Sullivan O, Cotter PD, Ross P, Hill C. High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol Lett. 2011;320(1):56–62. 10.1111/j.1574-6968.2011.02290.x [DOI] [PubMed] [Google Scholar]
- 13.Leite AMO, Mayo B, Rachid CTCC, Peixoto RS, Silva JT, Paschoalin VMF, et al. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol. 2012;31(2):215–21. 10.1016/j.fm.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Gu F, He J, Xiao J, Chen Q, Ruan H, et al. Metagenome analysis of bacterial diversity in Tibetan kefir grains. Eur Food Res Technol. 2013;236:549–56. 10.1007/s00217-013-1912-2 [DOI] [Google Scholar]
- 15.Liu W, Zhang M, Xie J, Wang H, Zhao X, Chen B, et al. Comparative analyses of microbial community diversities of Tibetan kefir grains from three geographic regions. Int J Dairy Technol. 2019;72(4):536–44. 10.1111/1471-0307.12616 [DOI] [Google Scholar]
- 16.Nalbantoglu U, Cakar A, Dogan H, Abaci N, Ustek D, Sayood K, et al. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014;41:42–51. 10.1016/j.fm.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 17.Dertli E, Çon AH. Microbial diversity of traditional kefir grains and their role on kefir aroma. LWT – Food Sci Technol. 2017;85(Part A):151–7. 10.1016/j.lwt.2017.07.017 [DOI] [Google Scholar]
- 18.Garofalo C, Osimani A, Milanović V, Aquilanti L, De Filippis F, Stellato G, et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015;49:123–33. 10.1016/j.fm.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 19.Korsak N, Taminiau B, Leclercq M, Nezer C, Crevecoeur S, Ferauche C, et al. Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J Dairy Sci. 2015;98(6):3684–9. 10.3168/jds.2014-9065 [DOI] [PubMed] [Google Scholar]
- 20.Zamberi NR, Mohamad NE, Yeap SK, Ky H, Beh BK, Liew WC, et al. 16S metagenomic microbial composition analysis of kefir grain using MEGAN and BaseSpace. Food Biotechnol. 2016;30(3):219–30. 10.1080/08905436.2016.1200987 [DOI] [Google Scholar]
- 21.Walsh AM, Crispie F, Kilcawley K, O’Sullivan O, O’Sullivan MG, Claesson MJ, et al. Microbial succession and flavor production in the fermented dairy beverage kefir. mSystems. 2016;1(5):e00052–16. 10.1128/mSystems.00052-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalamaki MS, Angelidis AS. Isolation and molecular identification of yeasts in Greek kefir. Int J Dairy Technol. 2017;70(2):261–8. 10.1111/1471-0307.12329 [DOI] [Google Scholar]
- 23.Yang J, Summanen PH, Henning SM, Hsu M, Lam H, Huang J, et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study. Front Physiol. 2015;6:216. 10.3389/fphys.2015.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7(1-2):203–14. 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- 25.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCBI Resource Coordinators Database resources if the National Center for Biotechnology Information. Nucleic Acids Res. 2017;45 D1:D12–7. 10.1093/nar/gkw1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daly AJ, Baetens JM, De Baets B. Ecological diversity: Measuring the unmeasurable. Mathematics. 2018;6(7):119 10.3390/math6070119 [DOI] [Google Scholar]
- 29.Jost L. Entropy and diversity. Oikos. 2006;113(2):363–75. 10.1111/j.2006.0030-1299.14714.x [DOI] [Google Scholar]
- 30.Chao A, Chiu CH, Jost L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu Rev Ecol Evol Syst. 2014;45:297–324. 10.1146/annurev-ecolsys-120213-091540 [DOI] [Google Scholar]
- 31.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience. 2013;2(1):16. 10.1186/2047-217X-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W, Zhang L. Comparative analysis of the microbial community composition between Tibetan kefir grains and milks. Food Res Int. 2019;116:137–44. 10.1016/j.foodres.2018.11.056 [DOI] [PubMed] [Google Scholar]
- 34.Fujisawa T, Adachi S, Toba T, Arihara K, Mitsuoka T. Lactobacillus kefiranofaciens sp. nov. isolated from kefir grains. Int J Syst Bacteriol. 1988;38(1):12–4. 10.1099/00207713-38-1-12 [DOI] [Google Scholar]
- 35.Kesmen Z, Kacmaz N. Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods. J Food Sci. 2011;76(5):M276–83. 10.1111/j.1750-3841.2011.02191.x [DOI] [PubMed] [Google Scholar]
- 36.Moradi Z, Kalanpour N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr Polym. 2019;223:115100. 10.1016/j.carbpol.2019.115100 [DOI] [PubMed] [Google Scholar]
- 37.Wang SY, Chen KN, Lo YM, Chiang ML, Chen HC, Liu JR, et al. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012;32(2):274–85. 10.1016/j.fm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Savin KW, Zawadzki J, Auldist MJ, Wang J, Ram D, Rochfort S, et al. Faecalibacterium diversity in dairy cow milk. PLoS One. 2019;14(8):e0221055. 10.1371/journal.pone.0221055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan H, Zhai Q, Chen W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res Int. 2019;116:637–44. 10.1016/j.foodres.2018.08.088 [DOI] [PubMed] [Google Scholar]
- 40.Grilli DJ, Cerón ME, Paez S, Egea V, Schnittger L, Cravero S, et al. Isolation of Pseudobutyrivibrio ruminis and Pseudobutyrivibrio xylanivorans from rumen of Creole goats fed native forage diet. Folia Microbiol (Praha). 2013;58:367–73. 10.1007/s12223-012-0219-1 [DOI] [PubMed] [Google Scholar]
- 41.De Angelis M, Montemurno E, Vannini L, Cosola C, Cavallo N, Gozzi G, et al. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl Environ Microbiol. 2015;81(22):7945–56. 10.1128/AEM.02507-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Wielen PWJJ, Rovers GMLL, Scheepens JMA, Biesterveld S. Clostridium lactatifermentans sp. nov., a lactate-fermenting anaerobe isolated from the caeca of a chicken. Int J Syst Evol Microbiol. 2002;52(Pt 3):921–5. 10.1099/ijs.0.02048-0 [DOI] [PubMed] [Google Scholar]
- 43.Ueki A, Goto K, Ohtaki Y, Kaku N, Ueki K. Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int J Syst Evol Microbiol. 2017;67(10):4146–53. 10.1099/ijsem.0.002268 [DOI] [PubMed] [Google Scholar]
- 44.Bonnet M, Ricaboni D, Mailhe M, Vitton V, Benezech A, Delerce J, et al. Genome sequence and description of Coprococcus phoceensis gen. nov., sp. nov., a new bacterial genus isolated from the human left colon. New Microbes New Infect. 2019;30:100548. 10.1016/j.nmni.2019.100548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes. 2019;5:28. 10.1038/s41522-019-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmstrøm K, Collins MD, Møller T, Falsen E, Lawson PA. Subdoligranulum variabile gen. nov., sp. nov. from human feces. Anaerobe. 2004;10(3):197–203. 10.1016/j.anaerobe.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 47.Seifert H, Strate A, Schulze A, Pulverer G. Vascular catheter-related bloodstream infection due to Acinetobacter johnsonii (formerly Acinetobacter calcoaceticus var. lwoffii): Report of 13 cases. Clin Infect Dis. 1993;17(4):632–6. 10.1093/clinids/17.4.632 [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y, Qi H, Zhang XM. Co-biodegradation of naphthalene and phenanthrene by Acinetobacter johnsonii. Polycycl Aromat Compd. 2020;40(2):422–31. 10.1080/10406638.2018.1441881 [DOI] [Google Scholar]
- 49.Konikoff T, Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24(7):523–4. 10.1016/j.tim.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 50.Frost F, Storck LJ, Kacprowski T, Gärtner S, Rühlemann M, Bang C, et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS One. 2019;14(7):e0219489. 10.1371/journal.pone.0219489 [DOI] [PMC free article] [PubMed] [Google Scholar]


