Abstract
Primary liver cancer (PLC) is a fatal disease that affects millions of lives worldwide. PLC is the leading cause of cancer-related deaths and the incidence rate is predicted to rise in the coming decades. PLC can be categorized into three major histological subtypes: hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined HCC-ICC. These subtypes are distinct with respect to epidemiology, clinicopathological features, genetic alterations, and clinical managements, which are thoroughly summarized in this review. The state of treatment strategies for each subtype, including the currently approved drugs and the potential novel therapies, are also discussed.
Keywords: primary liver cancer, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, combined HCC-ICC, PLC therapy
Graphical Abstract
Public Summary
-
•
Primary liver cancer comprises HCC, ICC, cHCC-ICC, which are markedly distinct in their epidemiology, clinical features and response to therapy
-
•
HCC is viral infection-related malignancy with specific histological features, whereas ICC is associated with chronic liver inflammation
-
•
HCC is prone to respond to targeted therapy, immunotherapy, and antiviral agents, whereas ICCs are benefit from chemotherapy, targeted therapy, and immunotherapy
-
•
Combined cHCC-ICC subclass shows strong ICC-like features and is considered to be treated like ICC, whereas mixed cHCC-ICC subclass resembles HCC and is treated like HCC
Main Text
Introduction
Primary liver cancer (PLC) is a deadly malignancy with significant histological and biological heterogeneity, and ranks as the fourth leading cause of cancer-related death worldwide.1,2 Therefore, it has become a major public healthy challenge. Over the past decades, the morbidity and mortality associated with PLC have steadily risen. According to Globocan's latest Global Cancer Statistics Report, 841,080 cases of liver cancer were reported worldwide in 2018, accounting for 4.7% of the total cancer cases in the same period, while deaths totaled 781,631, accounting for 8.2% of total cancer deaths.3 On the basis of annual projections, the World Health Organization estimates that 1,276,679 patients will die from liver cancer in 2040. Incidence and mortality of PLC differ widely between regions. The highest incidence of PLC was observed in East Asia and in sub-Saharan Africa.4 In particular, China experiences the highest number of cases of PLC, with a high incidence rate (18.3 cases/100,000 inhabitants).5
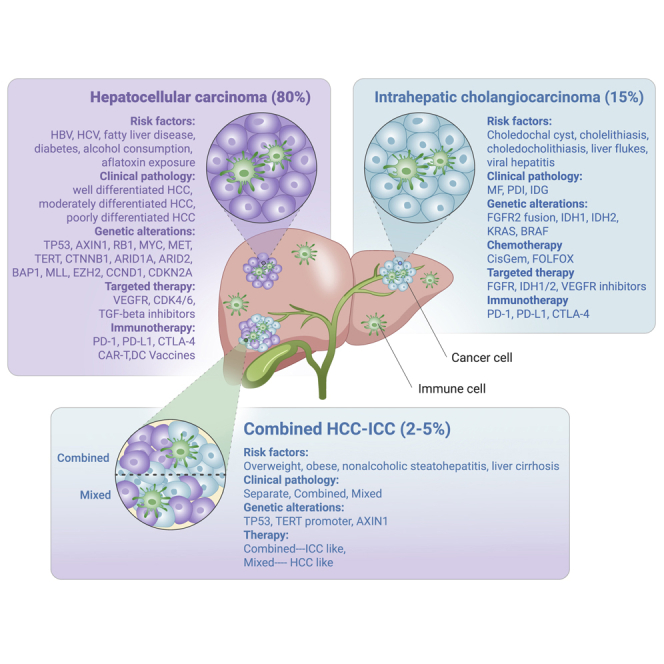
PLC manifests as three subtypes: hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined HCC-ICC (cHCC-ICC), which differ notably in epidemiology, clinicopathological morphology, genetic alteration, and appropriate therapeutic responses. HCCs are primarily related to viral infection, alcohol abuse, and metabolic syndrome,6 whereas ICCs are mainly associated with chronic liver inflammation and biliary tract diseases.7,8 Risk factors for development of cHCC-ICC include overweight, obsess, nonalcoholic steatohepatitis, and liver cirrhosis.9,10 HCCs show a solid and trabecular pattern with local invasion restricted to the liver,11, 12, 13 whereas ICCs are ductular, papillary, or solid tumor structures with high metastasis to distal organs.14, 15, 16 cHCC-ICCs are the combination of the HCC and ICC phenotypes present in liver tissue, and are classified into separate, combined, and mixed cHCC-ICC subclasses, which are more aggressive and have a poorer prognosis.2,17, 18, 19, 20, 21
The three PLC subtypes have distinct genetic alterations and molecular patterns. HCCs are associated with genetic alterations in specific chromosomal regions and genes, including TERT promoter mutation, TP53 deletion, and WNT signaling (CTNNB1 and AXIN1) activation.22, 23, 24, 25, 26, 27, 28, 29 ICCs show a unique mutational landscape with recurrent mutations, with the genetic alterations in TP53, KRAS, isocitrate dehydrogenase (IDH) 1/2, and fibroblast growth factor receptor (FGFR) gene fusions.30, 31, 32, 33, 34, 35 Combined cHCC-ICCs show strong ICC-like features, whereas mixed cHCC-ICCs show HCC-like features.36,37 Understanding the molecular alterations that initiate various PLC subtypes is of great importance for us to decipher the mechanisms of tumorigenesis. Genetic alterations can be transformed into biomarkers that may represent new therapeutic targets, affect the treatment decisions, and ultimately improve the treatment of liver cancer patients. HCCs mainly respond to targeted therapy, immunotherapy, and antiviral agents, while ICC patients benefit from classical chemotherapy, targeted therapy, and immunotherapy. Based on the pathological classification and the molecular features of cHCC-ICCs, combined cHCC-ICCs should be treated with similar therapies to ICCs, whereas mixed cHCC-ICCs are treated more like HCCs. In this review, we systematically summarize the epidemiology, pathogenesis, genetic alteration, and treatment for each subtype and comprehensively describe current therapy drugs and the potential novel therapies for PLC.
Epidemiology and Risk Factors
HCC
HCC represents the major histologic subtype, accounting for approximately 80% of all cases of PLC. The risk factors for HCC includes hepatitis B virus (HBV)/hepatitis C virus (HCV) infection; aflatoxin B1; alcoholic abuse; and nonalcoholic, metabolic symptoms, such as diabetes and obesity.6 According to the Global Burden of Disease from 1990 to 2015, HBV and HCV accounted for 432,000 liver cancer deaths (54%), alcohol for 245,000 (30%), and other causes for 133,000 (16%) deaths. In particular, 55% of all HCC cases worldwide are reported from China38 due to the locally high prevalence of HBV infection.
ICC
As the second most common liver carcinoma following HCC, ICC accounts for around 15% of PLC cases, with a high incidence of 2 per 100,000 population worldwide annually.39 The most common risk factors for ICC are biliary tract diseases, including choledochal cysts; cholelithiasis; choledocholithiasis; liver flukes; viral hepatitis; metabolic syndrome; and other risk factors, including tobacco and alcohol use, and cirrhosis.7 Recently, the incidence of ICC has been increasing more rapidly owing to risk factors8 including increasing chronic liver disease and environmental toxins, and is found more often due to improved diagnostic tools and imaging.
cHCC-ICC
cHCC-ICC presents as a heterogeneous tumor showing both hepatocyte and cholangiocyte differentiation, and has a poor prognosis.40 cHCC-ICC is a rather rare tumor with an incidence rate less than 5%.1 The poor prognosis associated with cHCC-ICC is due to the limited treatment options and difficulty of diagnosis. To date, the largest cohort analysis, which included 529 patients diagnosed with cHCC-ICC between 2004 and 2014 across 18 registries,41 reported that the incidence of cHCC-ICC in men and women was 0.08 and 0.03 per 100,000 per year respectively, with the average age of 63 years at diagnosis. One- and 5-year cause-specific survival rates for cHCC-ICC were 41.9% and 17.7%, respectively, with the median survival of 8 months. Among racial groups, cHCC-ICCs are most common in Asian races and Pacific Islanders. Obesity, nonalcoholic steatohepatitis, and liver cirrhosis were observed in some cHCC-ICC cohorts9,10 and are potential risk factors for cHCC-ICC.
Clinicopathological Features
HCC
HCC shows a solid, trabecular, and pseudoglandular pattern with a high density of tumor cells. It has three subtypes: well-differentiated HCC, moderately differentiated HCC, and poorly differentiated HCC.11, 12, 13 Well-differentiated HCCs are often small (less than 2 cm in diameter) and are composed of cells with a higher nuclear to cytoplasmic ratio, arranged in a thin trabecular pattern with rare pseudoglandular structures. Moderately differentiated HCCs are usually larger tumors (larger than 3 cm) showing polygonal tumor cells in a thick trabecular arrangement with a frequent pseudoglandular pattern. Poorly differentiated HCCs are composed of pleomorphic tumor cells in a solid or compact growth pattern.
ICC
ICC can be divided into two subtypes: a small duct type that originates from small intrahepatic ductules with no or minimal mucin production, and a large bile duct type that arises from large intrahepatic ducts proximal to the bifurcation of the right and left hepatic ducts, with high mucin production ability.14, 15, 16 Further, ICC shows three different growth patterns: mass forming (MF), periductal infiltrating (PI), and intraductal growth (IG).42 MF ICC is a firm, multilobulated, unencapsulated, white-gray tumor, owing to its extensive desmoplastic stroma. The PI subtype shows extensive infiltration along the intrahepatic hilum structure, and the IG subtype is usually restricted to tubes with papillary structures. MF ICC is the most common type associated with a poor prognosis, while IG type is rare but has a favorable prognosis.17
cHCC-ICC
Though the phenomenon of HCC and ICC being present in the same liver was first described in 1903,17 cHCC-ICC was not systematically described until 1949, when it was classified into three subtypes depending on the location of HCC and ICC: type A (separate type) has separate nodules of hepatocellular and bile duct carcinoma; type B (combined type) shows contiguity with intermingling but with clearly defined areas; type C (mixed type) presents as intimate association without clear boundaries.18 In 1985, another classification system with three subtypes was established: type I (collision tumors) is the simultaneous occurrence of both HCC and ICC in the same patient; type II (transitional tumors) is an identifiable intermediate transition between HCC and ICC; type III (fibrolamellar tumors) resembles the fibrolamellar variant of HCC but also contains mucin-producing pseudoglands.19 Presently, the World Health Organization (WHO) 2010 classification is commonly used, in which cHCC-ICC is classified into two main types, the classic type and the stem cell (SC) type (subtype with SC features), with the SC type subdivided into three subtypes, including the typical subtype, intermediate subtype (INT), and cholangiocellular type43.
The lack of a unified classification system greatly adds to the difficulty for cHCC-ICC research, and the clinicopathological characteristics of cHCC-ICC remain ill-defined. cHCC-ICC can exhibit stem/progenitor cell phenotypes consisting of small cells with scant cytoplasm; hyperchromatic nuclei embedded within a thick, desmoplastic stroma; a high nuclear/cytoplasmic ratio; and increased mitotic activity.1 In addition, the immunohistochemistry has identified stemness-related markers (KRT19, CD56, EpCAM, CD117, CD113, OV6).1,20 cHCC-ICC clinicopathologic characteristics include more frequent multifocal lesions, more microvascular emboli, and portal vein and lymph node invasion, all of which indicate a poor prognosis.21
Genetic Alterations
HCC
Wide-scale genomic studies have revealed that hundreds of somatic DNA alterations accrue in HCC, including chromosome aberrations and mutations. High-level DNA amplifications are enriched in chromosome locations 6p21 and 11q13 in HCC,44 which occur in 5%–10% of cases. Recently, some oncogenic genes were identified in the regions of frequent DNA gain. For example, LINC01138 is an oncogenic long intergenic non-coding RNA located in this region, which has been identified as a driver of HCC.45 VEGFA and CCND1/FGF19 have also identified in these regions and are potential therapeutic targets.46 Loss of heterozygosity on chromosome 8p is a frequent event in HCC.47 These DNA alterations are often associated with cancer progression due to the deletion of tumor suppressor genes. Intriguingly, in these regions, a variety of vulnerability genes have recently been identified. For example, TSLNC8 was characterized as a tumor suppressor gene on chromosome 8p12, the region that shows allelic loss in HCC, and was shown to inhibit the proliferation and metastasis of HCC.48 The genetic mutations of HCC have been well studied. Mutations in the TERT promoter occur in approximately 60% of cases and cause recurrent viral insertion of HBV.49 Deletion mutations in TP53 are the most frequent genetic alterations, accounting for about 30% of cases,22, 23, 24, 25, 26, 27, 28, 29 and are thought to be the initiating event driving the formation of precursor lesions. Mutated genes in WNT signaling (CTNNB1 and AXIN1) and chromatin remodeling (ARID1A) account for approximately 27%–40% of cases.22, 23, 24, 25, 26, 27, 28, 29 Accumulation of activating mutations in oncogenes, including activation of AKT or mTOR and of the oxidative stress pathway activation, occurs throughout tumor progression, and could be potentially targeted with molecular therapies in the future.
ICC
ICC shows a unique mutational landscape with recurrent mutations, compared with HCC. It harbors the genetic alterations in TP53, KRAS, ARID1A, BAP1, IDH1, IDH2, PIK3CA, SMARCB1, EPHA2, SMAD4, GNAS, and PBRM1, as well as FGFR gene fusions.30, 31, 32, 33, 34, 35 Gain of function of IDH1 and IDH2 mutation on R132 and R172 two hotpot codons was observed in 10%–28% of ICC cases.32 Fusions, amplifications, translocations, and rearrangements of FGFR genes are found in ICC and are closely related to the initiation and progression of ICC.50 The activating mutation of KRAS (15%–20%) is another frequent genomic alteration in ICC.31,51,52 The KRAS mutation often exists concurrently with FGFR2 fusions and IDH mutations, suggesting a possible cooperative role in ICC pathogenesis.53,54 In addition, recent studies have shown that BRAF and Notch are considerably more prevalent in ICC and function in ICC pathogenesis.55
cHCC-ICC
cHCC-ICCs are genetically complex tumors. The combined subtype of cHCC-ICC shows strong ICC-like features, with the high expression of EPCAM, KRT19, PRDM5, and KRAS. The mixed subtype of cHCC-ICC shows HCC-like features with the high expression levels of AFP, GPC3, APOE, SALL4, and AFP81.36
The most frequent mutation observed in cHCC-ICCs is TP53, with a strikingly high 49.2% mutation frequency, much higher than that in HCC (20%–35%) and ICC (18%–38%).36,56 Interestingly, several studies have found that the disruption of Trp53 alone in livers of mice can induce the formation of cHCC-ICC,37,57 which further implies that TP53 may be the driver gene in cHCC-ICC. It is notable that Nestin, a type VI intermediate filament (IF) protein that is commonly used as a neuroectodermal SC marker, is highly expressed in cHCC-ICC and is strongly associated with poorer prognosis.36 Hence, Nestin may be a promising biomarker for cHCC-ICC.
Challenges and Limitations of Current Treatment Strategies
Resection, Transplantation, Local and Regional Therapies
HCC
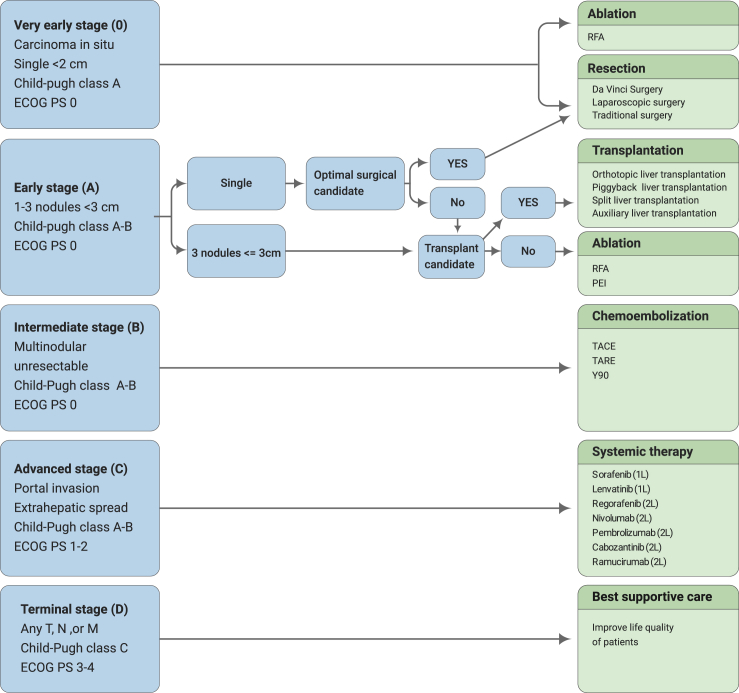
The commonly used staging system for HCC is the Barcelona Clinic Liver Cancer staging system (Figure 1). HCCs in the very early stage or intermediate stage can be treated with local regional therapies, which include radiofrequency ablation (RFA), resection (da Vinci surgery, laparoscopic surgery, or traditional surgery), transplantation (orthotopic liver transplantation, piggyback transplantation, split liver transplantation, auxiliary liver transplantation), percutaneous ethanol injections (PEI), or transcatheter arterial chemoembolization (TACE).58
Figure 1.
Barcelona Clinic Liver Cancer Staging System and Corresponding Treatment Options.
The schematic diagram illustrates therapeutic choice by which a treatment theoretically recommended for a different stage is the best treatment option. 1L, first-line; 2L, second-line; ECOG, Eastern Cooperative Oncology Group; M, metastasis stage; N, nodal stage; PEI, percutaneous ethanol injection; PS, performance status; T, tumor stage; TACE, transarterial chemoembolization; TARE, transarterialradioembolization; Y-90, Y-90 radioembolization
ICC
Surgery is currently the only curative treatment for ICCs but only a minority of patients in early stages are considered candidates for resection. In surgery, ICC is usually treated with hepatic resection to achieve negative resection margins.59 For patients with locally unresectable ICC, tumor ablation such as RFA, or hepatic artery-based therapies like yttrium-90 radioembolization, appear promising.59, 60, 61, 62, 63, 64
cHCC-ICC
An accurate diagnosis is of paramount importance for the treatment of cHCC-ICC. Currently, major hepatectomy is the optimal management for cHCC-ICC.65 The rarity of this cancer as well as the lack of biomarkers have made this cancer difficult to diagnosis and manage. Surgical resection remains the only curative option for patients with cHCC-ICC.
The treatment options for cHCC-ICC are similar to those for HCC and ICC and include surgery, radiation, yttrium-90 radioembolization, chemotherapy, combined radiation and chemotherapy, combined surgery and chemotherapy, and triple therapy (surgery, radiation, and chemotherapy).41,66, 67, 68, 69 A recently retrospective analysis from 2001 to 2015 of 623 PLC patients, including 47 cHCC-ICC, 468 HCC, and 108 ICC patients, who underwent resection found that although cHCC-ICC is more poorly differentiated than HCC and ICC, it had a similar 5-year survival rate (49.7%, 54.8%, and 68.7%, respectively) and 3-year recurrence rate (57.9%, 61.5%, 56%, respectively).70
Systemic Chemotherapy
HCC
Systemic chemotherapy has limited efficacy on HCC: several clinical trials of chemotherapy have shown low response rates and worse toxicity without a significant improvement in the overall survival (OS), including gemcitabine- and doxorubicin-based treatment, FOLFOX (5-fluorouracil [5-FU], leucovorin, oxaliplatin) and PIAF (cisplatin/interferon alpha-2b/doxorubicin/5-FU).71, 72, 73, 74 This suggests a limited role for traditional chemotherapy in the treatment of advanced HCC.
ICC
Current first-line standard of treatment for ICC is the combination of gemcitabine and platinum-derived chemotherapy (Figure 2B). With the poor prognosis, the median survival of advanced ICC patients is less than one year. Very limited effective treatments are available for patients who progress on first-line chemotherapy, so there is a high medical demand.
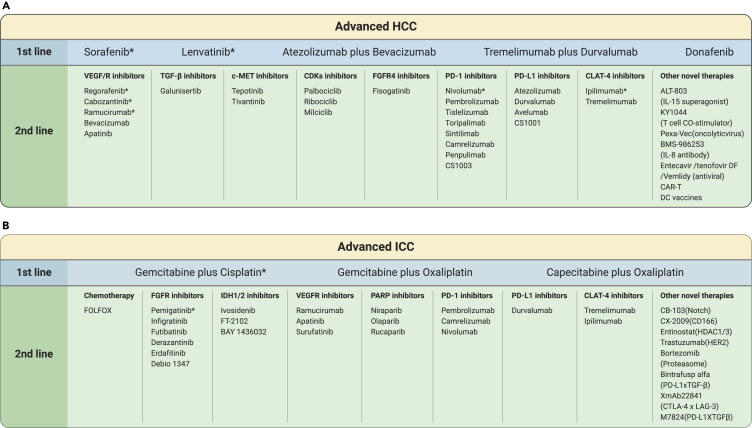
Figure 2.
Treatment Strategy for Advanced HCC and ICC.
The schematic illustration represents FDA-approved drugs for treatment of advanced HCC and ICC. First-line drugs for HCC include sorafenib, lenvatinib, atezolizumab plus bevacizumab, tremelimumab plus durvalumab and donafenib, whereas, for ICC, the combination of gemcitabine and cisplatin is currently proposed as first line. The bottom row represents corresponding second-line therapies that come in when patients are not suitable for their first-line therapy.
First-Line Treatment
Effective molecular targeted therapy and immunotherapy is lacking, so chemotherapy, with gemcitabine, platinum compounds, and fluoropyrimidines, is still the mainstream of standard treatment for unresectable ICC.
The primary chemotherapy for ICC is gemcitabine, which was established as the first-line therapy for advanced biliary tract cancer (BTC) in 1999. In 2010, the randomized, controlled, ABC-02 phase III clinical trial compared the benefit of gemcitabine plus cisplatin (CisGem) chemotherapy with the single agent gemcitabine.75 This study showed an advantage for CisGem in OS (11.7 months versus 8.1 months; hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.52–0.80) and progression-free survival (PFS) (8.0 months versus 5.0 months, p < 0.001). This effectiveness was confirmed in a Japanese randomized phase II study, BT22 (median OS, 11.2 months versus 7.7 months; HR, 0.69).76 Based on these promising results, CisGem is currently regarded as the standard of care in the first-line treatment for advanced cholangiocarcinoma.
Other than cisplatin, gemcitabine plus other agents such as oxaliplatin, S-1, capecitabine, bevacizumab, and Nab-paclitaxel have also been considered as the first-line choices for advanced cholangiocarcinoma based on the promising outcomes from several phase II or III trials.77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91
A recent multicenter, randomized, phase III clinical trial (NCT01470443) showed that XELOX has the comparable efficacious effect to GEMOX in terms of tumor response, survival rate (OS and PFS), and safety. Also, XELOX has an advantage of low hospital visits, compared with GEMOX. Thus, XELOX could be an alternative for cholangiocarcinoma therapies.
Second-Line Treatment
There is no established standard second-line chemotherapy for advanced cholangiocarcinoma, and all regimens have shown limited efficacy, with a median PFS of around 3 months and median OS of about 7 months.92
FOLFOX (L-folinic acid, 5-FU, and oxaliplatin) is an optional second-line treatment option based on the randomized, phase III, multicenter, open-label ABC-06 study (NCT01926236). FOLFOX showed increased benefit for median OS, 6 months and 12 months, and OS rate (%): 6.2 months, 50.6% and 25.9% compared with 5.3 months, 35.5% and 11.4% for the control group (active symptom control [ASC] arm).92
Currently several phase II and III chemotherapy clinical trials are under way (Table 3). Combined therapy with chemotherapy shows promise in the treatment of cholangiocarcinoma: selective internal radiotherapy (SIRT) plus chemotherapy or hepatic arterial infusion plus systemic chemotherapy both had antitumor activity and are promising for the treatment of ICC.93,94
Table 3.
Selected Ongoing Systemic Therapy Clinical Trials for Advanced Cholangiocarcinoma
| Drug | Target | Sponsor | Status | Condition or Disease | Phase | Enrollment | Trial Identifier |
|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||
| FOLFIRINOX versus GEMOX | chemotherapy | Shi Ming, Sim Yat-sen University | recruiting | intrahepatic cholangiocarcinoma | phase 3 | 188 | NCT03771846 |
| Anlotinib versus anlotinib + levamisole | chemotherapy | Zhengzhou University First Hospital | recruiting | intrahepatic cholangiocarcinoma | phase 3 | 152 | NCT03940378 |
| Melphalan/PHP versus CisGem | chemotherapy | Delcath Systems Inc | recruiting | intrahepatic cholangiocarcinoma | phase 2 phase 3 | 295 | NCT03086993 |
| Gemcitabine + capecitabine versus capecitabine | chemotherapy | Tianjin Medical University | recruiting | cholangiocarcinoma | phase 3 | 460 | NCT03779035 |
| Nab-paclitaxel, cisplatin, gemcitabine versus CisGem | chemotherapy | Southwest Oncology Group | recruiting | intrahepatic cholangiocarcinoma | phase 3 | 268 | NCT03768414 |
| CisGem versus capecitabine | chemotherapy | Universitatsklinikum Hamburg-Eppendorf | recruiting | cholangiocarcinoma, gall bladder carcinoma | phase 3 | 781 | NCT02170090 |
| Targeted Therapy | |||||||
| BGJ398 (infigratinib) | FGFR2 | QED Therapeutics, Inc | recruiting | cholangiocarcinoma FGFR2 gene mutation | phase 2 | 160 | NCT02150967 |
| Infigratinib versus CisGem | FGFR2 | QED Therapeutics, Inc | recruiting | cholangiocarcinoma FGFR2 gene mutation | phase 3 | 384 | NCT03773302 |
| Pemigatinib versus CisGem | FGFR2 | Incyte Corporation | recruiting | unresectable cholangiocarcinoma | phase 3 | 432 | NCT03656536 |
| Derazantinib | FGFR2 | Basilea Pharmaceutica | recruiting | intrahepatic cholangiocarcinoma | phase 2 | 143 | NCT03230318 |
| TAS-120 versus CisGem | FGFR2 | Taiho Oncologa, Inc | not yet recruiting | cholangiocarcinoma | phase 3 | 216 | NCT04093362 |
| Ponatinib | FGFR, VEGFR | Sameek Roychowdhury | recruiting | solid tumor with FGFR mutations | phase 2 | 45 | NCT02272998 |
| AG-120 versus placebo | IDH1 | Agios Pharmaceuticals | active, not recruiting | advanced cholangiocarcinoma | phase 3 | 186 | NCT02989857 |
| FT-2102 | IDH1 | Forma Therapeutics, Inc | recruiting | solid tumors including ICC | phase 1 phase 2 | 200 | NCT03684811 |
| BAY 1436032 | IDH1 | Bayer | active, not recruiting | solid tumors including ICC | phase 1 | 81 | NCT02746081 |
| Ramucinimab | VEGFR2 | MD Anderson Cancer Center | recruiting | cholangiocarcinoma | phase 2 | 50 | NCT02520141 |
| Apatinib | VEGFR2 | Zhengzhou University First Hospital | recruiting | intrahepatic cholangiocarcinoma | phase 2 | 30 | NCT03521219 |
| Surufatinib versus capecitabine | VEGFR | Hutchison Medipharma | recruiting | BTC | phase 2 phase 3 | 298 | NCT03873532 |
| Niraparib | PARP | University of Florida | recruiting | cholangiocarcinoma | phase 2 | 57 | NCT03207347 |
| Olaparib | PARP | Academic and Community Cancer Research United | not yet recruiting | BTC | phase 2 | 36 | NCT04042831 |
| CB-103 | NOTCH | Cellestia Biotech AG | recruiting | cholangiocellular carcinoma | phase I phase 2 | 165 | NCT03422679 |
| CX-2009 | CD166 | CytomX Therapeutics | recruiting | cholangiocarcinoma | phase I phase 2 | 150 | NCT03149549 |
| Bortezomib versus supportive care | proteasome inhibitor | Zhengang Yuan | recruiting | intrahepatic cholangiocarcinoma | phase 3 | 50 | NCT03345303 |
| Immunotherapy | |||||||
| Pembrolizumab + CisGem versus placebo + CisGem | PD-1 | Merck Sharp & Dohme | recruiting | biliary tract carcinoma | phase 3 | 788 | NCT04003636 |
| Durvalumab + CisGem versus placebo + CisGem | PD-L1 | AstraZeneca | recruiting | Biliary tract neoplasms | phase 3 | 474 | NCT03875235 |
| Pembrolizumab | PD-1 | Samsung Medical Center | recruiting | biliary tract cancer | phase 2 | 33 | NCT03110328 |
| Durvalumab + CisGem versus placebo + CisGem | PD-L1 | AstraZeneca | recruiting | biliary tract neoplasms | phase 3 | 474 | NCT03875235 |
| Combined Therapies | |||||||
| Systemic chemotherapy versus chemotherapy and radiation | chemotherapy, radiation | Tata Memorial Hospital | recruiting | cholangiocarcinoma | phase 3 | 155 | NCT02773485 |
| CisGem + pembrolizumab | chemotherapy, PD-1 | EORTC | recruiting | BTC | phase 2 | 50 | NCT03260712 |
| Camrelizumab + apatinib versus camrelizumab + FOLFOX4 or GEMOX | PD-1, VEGF, chemotherapy | Jianssu HengRui Medicine | recruiting | advanced biliary tract carcinoma | phase 2 | 152 | NCT03092895 |
| Lenvatinib + pembrolizumab | PD-1, VEGF | Peking Union Medical College Hospital | recruiting | cholangiocarcinoma | phase 2 | 50 | NCT03895970 |
| Bintrafusp alfa + CisGem versus placebo + CisGem | PD-L1xTGF-ß | EMD Serono Research & Development Institute | recruiting | BTC cholangiocarcinoma | phase 2 phase 3 | 512 | NCT04066491 |
| XmAb22841:XmAb22841 + pembrolizumab | PD-l, CTLA-4 x LAG-3 | Xencor, Inc | recruiting | advanced solid tumors including ICC | phase 1 | 242 | NCT03849469 |
| Rucaparib + nivolumab | PD-l, PARP | University of Michigan Rogel Cancer Center | recruiting | BTC | phase 2 | 35 | NCT03639935 |
| Pembrolizumab + olaparib | PD-l, PARP | Georgetown University | recruiting | cholangiocarcinoma | phase 2 | 29 | NCT04306367 |
| Pembrolizumab + sargramostim | PD-1, GM-CSF | Robin Kate Kelley | active, not recruiting | biliary cancer | phase 2 | 42 | NCT02703714 |
| Entinostat + nivolumab | PD-1, HDAC 1/3 | Sidney Kimmel Comprehensive Cancer Center | recruiting | cholangiocarcinoma | phase 2 | 54 | NCT03250273 |
| Nivolumab + ipilimumab | PD-1, CTLA-4 | National Cancer Institute | recruiting | cholangiocarcinoma | phase 2 | 818 | NCT02834013 |
| Durvalumab + tremelimumab versus durvalumab | PD-L1, CTLA-4 | Institut fur Klinische Krebsforschung IKF GmbH | recruiting | intrahepatic cholangiocarcinoma | phase 2 | 50 | NCT04238637 |
| CisGem + nivolumab versus nivolumab + ipilimumab | chemotherapy, PD-1,CTLA-4 | University of Michigan Rogel Cancer Center | active, not recruiting | biliary tract neoplasms | phase 2 | 64 | NCT03101566 |
| Durvalumab + tremelimumab versus durvalumab + tremelimumab + TACE versus durvalumab + tremelimumab+ RFA versus durvalumab + tremelimumab + cryo | PD-L1, CTLA-4, ablative therapies | National Cancer Institute | recruiting | biliary tract neoplasms | phase 2 | 90 | NCT02821754 |
| Nivolumab + radiotherapy versus nivolumab + ipilimumab + radiotherapy | PD-1, CTLA-4, radiation | Herlev Hospital | recruiting | metastatic BTC | phase 2 | 160 | NCT02866383 |
| M7824 | anti-PD-L × TGF-β fusion protein | EMD Serono Research & Development Institute | recruiting | BTC cholangiocarcinoma | phase 2 | 141 | NCT03833661 |
| Pembrolizumab + oxaliplatin + capecitabine | PD-1, chemotherapy | National Cancer Institute | recruiting | biliary tract neoplasms, cholangiocarcinoma | phase 2 | 19 | NCT03111732 |
| Trastuzumab + CisGem | HER2, chemotherapy | Changhoon Yoo | recruiting | cholangiocarcinoma BTC | phase 2 | 15 | NCT03613168 |
| Immune Cell | |||||||
| TC-210T cells | genetically engineered T cells | TCR2 Therapeutics | recruiting | cholangiocarcinoma | phase 1 phase 2 | 70 | NCT03907852 |
| MUC-1 CAR-T cell | target abnormal glycosylation MUC-1 | Zhejians University Second Hospital | recruiting | intrahepatic cholangiocarcinoma | phase 1 phase 2 | 9 | NCT03633773 |
| Tumor-infiltrating lymphocytes | Tumor-infiltrating lymphocytes | Udai Kammula | recruiting | cholangiocarcinoma | phase 2 | 59 | NCT03801083 |
CisGem, cisplatin + gemcitabine; EORTC, European Organisation for Research and Treatment of Cancer; FOLFIRINOX, irinotecan + oxaliplatin + 5-FU + leucovorin; FOLFOX, leucovorin calcium (folinic acid) + 5-FU + oxaliplatin; GEMOX, gemcitabine + oxaliplatin; GM-CSF, granulocyte-macrophage colony-stimulating factor; cryo, cryoablation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization
cHCC-ICC
In contrast to surgery-based treatments for resectable cHCC-ICC, systemic therapy is the nonstandard option for advanced and unresectable cHCC-ICC, based on the standard treatment strategy for the unresectable HCC or ICC. Chemotherapy for advanced or unresectable cHCC-ICC is largely understudied, with only a few case reports and some retrospective studies having been published.9,10,95, 96, 97, 98, 99, 100, 101 Recently, a multicenter retrospective analysis has been conducted by Kobayashi and colleagues.10
According to divided-group treatment with (1) gemcitabine plus cisplatin (n = 12), (2) 5-FU plus cisplatin (n = 11), (3) sorafenib monotherapy (n = 5), (4) others (n = 8), they found that 36 patients with platinum-containing treatment had longer OS time than those treated by sorafenib monotherapy, showing OS of 11.9 months (95% CI, 4.9–18.8), 10.2 months (95% CI, 3.9–16.6), 3.5 months (95% CI, 0.0–7.6), and 8.1 months (95% CI, 0.9–15.4), respectively.
A similar conclusion was drawn in another retrospective study of 123 cHCC-ICC patients, with 68 receiving gemcitabine-based therapy (gemcitabine + platinum or gemcitabine + 5-FU) or targeted agents (sorafenib).9 Median PFS favored gemcitabine/platinum and gemcitabine/5-FU (8.0 and 6.6 months, respectively) over sorafenib monotherapy (4.8 months).
Molecular Targeted Therapy
HCC
First-Line Drugs
Sorafenib
Sorafenib was the first US Food and Drug Administration (FDA)-approved first-line systemic targeted drug for advanced HCC. It is an oral small-molecule multikinase inhibitor targeting VEGFR1, VEGFR2, VEGFR3, PDGFRβ, and Raf. Two large, international, multicenter clinical trials, SHARP and Asian-Pacific, have proved that sorafenib can suppress tumor progression and prolong OS in patients with advanced HCC.102,103 These trials showed that sorafenib can increase PFS and OS by ~3 months in patients with advanced HCC in Western countries. As the first generation of targeted drugs for HCC, sorafenib has been used for over a decade. During this time, many patients have benefited, though others quickly developed resistance to sorafenib.104
Lenvatinib
Lenvatinib is becoming available for HCC patients who develop sorafenib resistance. Lenvatinib is an oral tyrosine kinase inhibitor, inhibiting VEGFR1–3, FGFR1–4, PDGFR, RET, and KIT. In August 2018, the FDA approved lenvatinib for first-line treatment of patients with unresectable HCC after lenvatinib was proved to be noninferior to sorafenib in the phase 3 REFLECT trial.105
Median OS in the lenvatinib arm and sorafenib arm was 13.6 months and 12.3 months (HR, 0.92; 95% CI, 0.79, 1.06), respectively. The adverse effects were hypertension (42%), diarrhea (39%), and decreased appetite (34%) with lenvatinib, and palmar-plantar erythrodysesthesia (52%), diarrhea (46%), decreased weight (31%), hypertension (30%), and decreased appetite (27%) with sorafenib.
Donafenib
Similar to sorafenib, donafenib is a novel multikinase inhibitor targeting RAF kinase and various receptor tyrosine kinases (RTKs), including VEGF receptor (VEGFR) and BRAF.106 According to the report from 2020 International Conference of the American Society of Clinical Oncology (CSCO), donafenib significantly improves OS over sorafenib (12.1 versus 10.3 months) with fewer side effects and higher patient tolerance for advanced HCC patients in its phase II/III open-label trial.107 The grade 3 and above adverse reaction rates for donafenib and sorafenib were 57.4% and 67.5%, respectively. Thus, donafenib was recommended as the first-line therapy in the CSCO guidelines for HCC.
Second-Line Drugs
Regorafenib
Regorafenib, an oral multikinase inhibitor, inhibits the activity of protein kinases involved in multiple biological processes, such as tumorigenesis, tumor angiogenesis, distant metastasis, and tumor immune escape. These kinases include VEGFR 1–3, TIE2, RAF1, KIT, RET, RAF, BRAF, PDGFR, FGFR, and CSF1R. The randomized, double-blind, multicenter, phase III clinical trial RESORCE showed that regorafenib significantly improves the OS of patients, as compared with the placebo, from 7.8 to 10.6 months (HR, 0.63, p < 0.0001).108 Grade 3–4 adverse events were reported in 40% of patients receiving regorafenib and 11% of patients receiving the placebo. In 2017, regorafenib received FDA approval as the second-line drug for the treatment of patients with advanced HCC who fail to respond to the sorafenib treatment.
Cabozantinib
Cabozantinib is an oral inhibitor and targets multiple kinases, including VEGFR2, c-MET, RET, ROS1, TYRO3, MER, KIT, TRKB, FLT3, TIE-2, as well as the GAS6 receptor (AXL)109,110. It was originally approved for medullary thyroid cancer in 2012 and advanced renal carcinoma in 2016. According to the randomized, double-blind, multicenter, phase 2 clinical trial conducted across 95 centers in 19 countries, median OS was 10.2 months for patients receiving cabozantinib, and 8 months for patients treated with placebo (HR, 0.76; p = 0.005).111 Median PFS was 5.2 months and 1.9 months, respectively. Grade 3 or 4 adverse events occurred in 68% of patients in the cabozantinib arm and 36% in the placebo arm. The observed hepatotoxicity can be mostly controlled through dose modifications. Based on the encouraging results of prolonged OS and PFS, cabozantinib received its FDA approval for HCC in 2018.
Ramucirumab
Ramucirumab is a completely human monoclonal antibody that can specifically inhibit VEGFR-2.112 For patients with alpha-fetoprotein ≥400 ng/mL and who have been previously treated with sorafenib, ramucirumab was approved as a monotherapy by the FDA on May 10, 2019.
Approval was based on REACH 2 (NCT02435433), a randomized, double-blind, multicenter phase III study of 292 patients with AFP ≥400 ng/mL who had disease progression after sorafenib or were intolerant to sorafenib.113 More recently, a study further confirmed the efficacy of ramucirumab in elderly patients with HCC and elevated AFP after sorafenib in REACH and REACH-2, with a survival benefit observed across all age subgroups and a tolerable safety profile, supporting its value irrespective of age, including for patients ≥75 years.114
Apatinib
Apatinib, a tyrosine kinase inhibitor targeting VEGFR-2, significantly prolonged OS and PFS in Chinese patients with advanced HCC who had previously been treated with sorafenib and/or chemotherapy, according to the results of a randomized, placebo-controlled, phase III trial conducted in 31 sites in China.115 Median OS was almost 2 months longer for patients who received apatinib compared with patients receiving the placebo (8.7 months versus 6.8 months), and median PFS was more than 2 months longer (4.5 months versus 1.9 months).115 The most common grade 3 or worse adverse events occurred at a rate of 69.2% in the apatinib arm and 3.1% in the placebo arm. With the significantly prolonged OS and PFS and a manageable safety profile, apatinib has potential to become a new second-line therapy for liver cancer.
Novel Therapeutic Targets
Even with all these available treatments (Table 1), the median PFS for HCC patients remains less than a year. Thus, novel treatment is still a critical unmet need for treatment of HCC. Based on the genomic profile and biomarkers reported in HCC, several clinical trials targeting various pathways are currently ongoing (Table 2). Recently, a first-in-human phase I study (NCT02508467) of fisogatinib (BLU-554), an orally bioavailable inhibitor of human FGFR4, demonstrated its antitumor activity in HCC, and further validated that the aberrant FGF19-FGFR4 signaling pathway may be a driver event.116 In addition, the TGF-β1 receptor type I inhibitor galunisertib also showed an acceptable safety and prolonged OS outcome in combination with sorafenib in a phase II trial (NCT01246986).117,118 Other potential candidates, including the cyclin-dependent kinase (CDK) inhibitors regulating the cell cycle pathways (ribociclib, palbociclib,119,120 abemaciclib, and milciclib) as well as the c-MET inhibitors tepotinib121 and tivantinib122 are being evaluated in HCC clinical trials.
Table 1.
Systemic Therapies Currently or Promising Approved for Advanced HCC and ICC
| Drugs | Target | Therapy Line | Approved Year | Trial |
|---|---|---|---|---|
| HCC | ||||
| Sorafenib (Nexavar) | VEGFR-2, VEGFR-3, PDGFR-β, RAF kinases | 1 | 2007 | SHARP Asian-Pacific |
| Lenvatinib (Lenvima) | FGFR, VEGFR, PDGFR-α, RET, KIT | 1 | 2018 | REFLECT |
| Regorafenib (Stivarga) | Tie2, VEGFR, PDGFR, FGFR | 2 | 2017 | RESORCE |
| Nivolumab (Opdivo) | PD-1 | 2 | 2017 | CHECKMATE-040 |
| Cabozantinib (Cabometyx) | c-Met, VEGFR-2, AXL, RET | 2 | 2018 | CELESTIAL |
| Pembrolizumab (Keytruda) | PD-1 | 2 | 2018 | KEYNOTE-224 |
| Ramucirumab (CYRAMZA) | VEGFR-2 | 2 | 2019 | REACH-2 |
| Nivolumab + ipilimumab (Opdivo + Yervoy) | PD-1, CTLA-4 | 2 | 2020 | Cohort 4 of CHECKMATE-040 |
| Atezolizumab + bevacizumab | PD-L1/VEGF | 1 | promising | IMbravel50 |
| Tremelimumab + durvalumab | PD-1, CTLA-4 | 1 | promising | NCT02519348 |
| Donafenib | VEGFR, BRAF | 1 | promising | NCT02645981 |
| Apatinib | VEGFR-2 | 2 | promising | NCT02329860 |
| ICC | ||||
| Gemcitabine + cisplatin | chemotherapy | 1 | 2010 | ABC-02 |
| Pemigatinib (Pemazyre) | FGFR1–3 | 2 | 2020 | FIGHT-202 |
| Ivosidenib | IDH-1/2 | 2 | promising | ClarlDHy |
Table 2.
Selected Ongoing Systemic Therapy Clinical Trials for Advanced HCC
| Drug | Target | Sponsor | Status | Phase | Enrollment | Trial Identifier |
|---|---|---|---|---|---|---|
| Targeted Therapy | ||||||
| Cabozantinib | VEGFR | Hospices Civils de Lyon | recruiting | phase 4 | 170 | NCT03963206 |
| Lenvatinib | VEGFR | Eisai Pharmaceuticals India Pvt Ltd | not yet recruiting | phase 4 | 50 | NCT04297254 |
| Donafenib | VEGFR | Suzhou Zelgen Biopharmaceuticals | completed | phase 2 phase 3 | 668 | NCT02645981 |
| Milciclib | CDK2 | Tiziana LifeSciences | active, not recruiting | phase 2 | 31 | NCT03109886 |
| Palbociclib | CDK46 | Pfizer | active, not recruiting | phase 2 | 23 | NCT01356628 |
| Ribociclib | CDK46 | Texas University | recruiting | phase 2 | 40 | NCT02524119 |
| Galunisertib versus LY2157299 + sorafenib versus placebo + sorafenib | TGF-β | Eli Lilly | active, not recruiting | phase 2 | 120 | NCT02178358 |
| Immunotherapy | ||||||
| Tislelizumab versus sorafenib | PD-1 | BeiGene | active, not recruiting | phase 3 | 674 | NCT03412773 |
| Toripalimab versus placebo | PD-1 | Shanghai Junshi Bioscience | recruiting | phase 2 phase 3 | 402 | NCT03859128 |
| Nivolumab versus placebo | PD-1 | Bristol-Myers Squibb | recruiting | phase 3 | 530 | NCT03383458 |
| Nivolumab versus sorafenib | PD-1 | Bristol-Myers Squibb | active, not recruiting | phase 3 | 1,723 | NCT02576509 |
| Pembrolizumab versus placebo | PD-1 | Merck Sharp & Dohme | recruiting | phase 3 | 950 | NCT03867084 |
| Avelumab | PD-L1 | Seoul National University Hospital | active, not recruiting | phase 2 | 30 | NCT03389126 |
| Combined Therapy | ||||||
| Lenvatinib + pembrolizumab versus lenvatinib + placebo | VGFR, PD-1 | Merck Sharp & Dohme | active, not recruiting | phase 3 | 750 | NCT03713593 |
| CS1003 + lenvatinib versus placebo + lenvatinib | VGFR, PD-1 | CStone Pharmaceuticals | recruiting | phase 3 | 525 | NCT04194775 |
| Tislelizumab + regorafenib versus placebo + regorafenib | VEGF, PD-1 | National Taiwan University Hospital | not yet recruiting | phase 2 | 125 | NCT04183088 |
| Toripalimab + lenvatinib | VGFR, PD-1 | Peking Union Medical College Hospital | not yet recruiting | phase 2 | 76 | NCT04368078 |
| Durvalumab + bevacizumab versus placebo | VEGF, PD-L1 | AstraZeneca | recruiting | phase 3 | 888 | NCT03847428 |
| Atezolizumab + bevacizumab versus sorafenib | VEGF, PD-L1 | Hoffmann-La Roche | recruiting | phase 3 | 480 | NCT03434379 |
| Atezolizumab + bevacizumab | VEGF, PD-L1 | National Health Research Institutes, Taiwan | not yet recruiting | phase 2 | 48 | NCT04180072 |
| Cabozantinib + atezolizumab versus sorafenib | VEGF, PD-L1 | Exelixis | recruiting | phase 3 | 740 | NCT03755791 |
| Atezolizumab + bevacizumab versus active surveillance | VEGF, PD-L1 | Hoffmann-La Roche | recruiting | phase 3 | 662 | NCT04102098 |
| Regorafenib + nivolumab | VEGF, PD-1 | Fundación Clinic per a la Recerca Biomédica | not yet recruiting | phase 1 phase 2 | 60 | NCT04170556 |
| Lenvatinib + pembrolizumab versus lenvatinib + placebo | VEGFR, PD-1 | Merck Sharp & Dohme | recruiting | phase 3 | 750 | NCT03713593 |
| Camrelizumab + apatinib | VEGFR, PD-1 | Zhejiang University | recruiting | phase 1 phase 2 | 120 | NCT04035876 |
| Camrelizumab + apatinib versus sorafenib | VEGFR, PD-1 | Jiangsu HengRui | recruiting | phase 3 | 510 | NCT03764293 |
| Sintilimab + lenvatinib | VEGFR, PD-1 | Beijing Cancer Hospital | not yet recruiting | phase 2 | 56 | NCT04042805 |
| Sintilimab + IBI305 versus sorafenib | VEGF, PD-1 | Innovent Biologics | recruiting | phase 2 phase 3 | 566 | NCT03794440 |
| Regorafenib + avelumab | VEGF, PD-L1 | Institut Bergonié | recruiting | phase 1 phase 2 | 362 | NCT03475953 |
| Sorafenib + toripalimab | VEGF, PD-1 | Sichuan University | not yet recruiting | phase 1 phase 2 | 39 | NCT04069949 |
| Galunisertib + nivolumab | TGF-β, PD-1 | Eli Lilly | active, not recruiting | phase 2 | 75 | NCT02423343 |
| Fisogatinib + CS1001 | FGFR4, PD-L1 | CStone Pharmaceuticals | recruiting | phase 1 phase 2 | 52 | NCT04194801 |
| AK105 + anlotinib versus sorafenib | RTK, PD-1 | Chia Tai Tianqing | not yet recruiting | phase 3 | 648 | NCT04344158 |
| Anlotinib + sintilimab | RTK, PD-1 | Nanjing Medical University First Hospital | recruiting | phase 2 | 20 | NCT04052152 |
| Abemaciclib + nivolumab | CDK4/6, PD-1 | Abramson Cancer Center, Pennsylvania University | Suspended (COVID-19) | phase 2 | 27 | NCT03781960 |
| Durvalumab + tremelimumab versus durvalumab versus sorafenib | PD-L1, CTLA-4 | AstraZeneca | active, not recruiting | phase 3 | 1,310 | NCT03298451 |
| Nivolumab + ipilimumab versus sorafenib/lenvatinib | PD-1, CTLA-4 | Bristol-Myers Squibb | recruiting | phase 3 | 1,084 | NCT04039607 |
| Durvalumab + tremelimumab versus durvalumab monotherapy versus tremelimumab monotherapy versus durvalumab + bevacizumab | VEGF, PD-L1, CTLA-4 | Med Immune LLC | active, not recruiting | phase 2 | 433 | NCT02519348 |
| Galunisertib versus galunisertib + sorafenib ramucirumab | TGF-β,VEGF, VEGFR | Eli Lilly | active, not recruiting | phase 2 | 193 | NCT01246986 |
| Lenvatinib + pembrolizumab + TACE versus placebo + TACE | VEGFR, PD-1, TACE | Merck Sharp & Dohme | not yet recruiting | phase 3 | 950 | NCT04246177 |
| TAI + lenvatinib versus lenvatinib | VEGFR, chemoinfusion | Sim Yat-sen University | recruiting | phase 3 | 206 | NCT04053985 |
| SBRT + sintilimab versus SBRT | PD-1, radiation | Mian XI, Sim Yat-sen University | recruiting | phase 2 phase 3 | 116 | NCT04167293 |
| Donafenib + anti-PD-1 antibody | VEGFR, PD-L1 | Zhejiang University | recruiting | phase 1 | 30 | NCT04418401 |
| Others | ||||||
| ALT-803 + avelumab | PD-L1, IL-15 superagonist | Altor Bioscience | recruiting | phase 2 | 611 | NCT03228667 |
| KYI044 monotherapy versus KY1044 + atezolizumab | PD-LLT cell CO- stimulator | Kymab Limited | recruiting | phase 1 phase 2 | 412 | NCT03829501 |
| Pexa-Vec + nivolumab | PD-1, oncolytic virus | Transgene | active, not recruiting | phase 1 phase 2 | 30 | NCT03071094 |
| Nivolumab + BMS-986253 versus nivolumab + cabiralizumab versus nivolumab monotherapy | VEGFR2, PD-1, interleukin-8 | NYU Langone Health | not yet recruiting | phase 2 | 74 | NCT04050462 |
| Entecavir/tenofovir disoproxil monotherapy | Antiviral therapy (HBV) | West China Hospital | recruiting | phase 4 | 450 | NCT04032860 |
| Vemlidy versus placebo | Antiviral therapy (HBV) | Taipei Veterans General Hospital, Taiwan | not yet recruiting | phase 4 | 402 | NCT04290936 |
| Pexastimogene devacirepvec versus sorafenib | Vaccinia virus-based oncolytic immunotherapy | SillaJen, Inc | active, not recruiting | phase 3 | 600 | NCT02562755 |
| Immune Cell | ||||||
| CD147-CAR-T | CAR-T therapy | Xijing Hospital | recruiting | phase 1 | 34 | NCT03993743 |
| Anti-DR5 CAR-T/TCR-T cells immunotherapy | CAR-T therapy | Shenzhen BinDeBio Ltd | recruiting | phase 1 phase 2 | 50 | NCT03941626 |
| CAR-GPC3 T cells | CAR-T therapy | Zhejiang University | recruiting | phase 1 | 36 | NCT03980288 |
| GPC3 or TGF-β targeting CAR-T cell therapy | CAR-T therapy | Guangzhou Medical University Second Hospital | recruiting | phase 1 | 30 | NCT03198546 |
| GPC3-CAR (GLYCAR T cells) + fludarabine and cytoxan | CAR-T therapy | Baylor College of Medicine | recruiting | phase 1 | 14 | NCT02905188 |
| c-Met/PD-L1 CAR-T cell injection | CAR-T therapy | Second Hospital Nanjing Medical University | not yet recruiting | early phase 1 | 50 | NCT03672305 |
| IMA202 product | TCR-T therapy | Immatics US, Inc | recruiting | phase 1 | 16 | NCT03441100 |
| Microwave ablation + neoantigen vaccines | Neoantigen DC Vaccines | Chinese PLA General Hospital | recruiting | phase 1 | 24 | NCT03674073 |
| DC vaccines | Neoantigen DC Vaccines | Sichuan University | recruiting | phase 1 | 80 | NCT04147078 |
| Autologous DC + conjugate vaccine | Vaccine | Mayo Clinic | recruiting | early phase 1 | 26 | NCT03942328 |
CAR-T, chimeric antigen receptor T cell; SBRT, stereotactic body radiotherapy; TAI, transarteria1 chemoinfusion.
ICC
Molecular targeted therapy controls tumor cell proliferation, apoptosis, adhesion, and movement by inhibiting the surface molecules of tumor cell membranes and thereby inhibiting intracellular signaling pathways. ICC genetic alterations primarily include FGFR, IDH, epidermal growth factor (EGFR), and breast cancer type 1 susceptible protein associated protein-1 (BAP1).123, 124, 125 Genetic alterations of these genes all have implications for therapy. At present, a variety of molecular targeted drugs are in the clinical research stage (Table 3), some of which have made progress in the treatment of ICC (Table 1).
FGFR Inhibitors
The most promising target therapy for cholangiocarcinoma identified in recent years is the inhibitor of the fibroblast growth factor (FGF) signaling pathway, which consists of 22 members labeled FGF1–23 (FGF15 = FGF19, called FGF15/19) and four interacting transmembrane receptors (FGFR1–4).126 FGF signals regulate cell proliferation, in which FGFR2 fusions occurred in 10%–20% of ICC patients and are considered as a promising therapeutic target.33,51,127,128 Currently, several FGFR inhibitors are being evaluated in clinical trials for cholangiocarcinomas with FGFR genetic aberrations.
Pemigatinib (INCB054828)
Pemigatinib is the first and only targeted therapy so far approved (in 2020) by the FDA for the treatment of this rare cancer. It is a selective, potent oral inhibitor of FGFR 1, 2, and 3.129 Approval was based on findings from the phase II FIGHT-202 trial (NCT02924376), which enrolled 107 patients with locally advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements (cohort A), other FGF/FGFR genetic alterations (cohort B), or no FGF/FGFR genetic alterations (cohort C). For those in cohort A, treatment with pemigatinib resulted in a median OS of 21.1 months and median PFS of 6.9 months. The FIGHT-202 study suggests that locally advanced or metastatic cholangiocarcinoma patients with FGFR2 fusions or rearrangements may benefit from potent oral FGFR1, 2, and 3 inhibitor treatment. Median PFS was 6.9 months for patients with FGFR2 alterations, 2.1 months for patients with other FGF/FGFR alterations, and 1.7 months for those with no alterations in these genes. Median OS was 21.1 months, 6.7 months, and 4.0 months for the respective cohorts.130 With the promising results of phase II, the phase III clinical trial of pemigatinib is currently underway (NCT03656536).
Infigratinib (BGJ-398)
Infigratinib (BGJ-398) was the first FGFR inhibitor investigated for treatment of cholangiocarcinoma. It is an oral drug that selectively binds to FGFR2 and shows impressive antitumor efficiency and a manageable safety profile in participants with advanced FGFR-altered cholangiocarcinoma (NCT02150967).131 The FDA granted fast-track designation to infigratinib early in 2020 for first-line treatment of patients with unresectable advanced or metastatic cholangiocarcinoma who harbor FGFR2 gene fusions or translocations. It is currently undergoing a phase III trial (NCT03773302) to assess the efficacy and safety of infigratinib versus standard treatment chemotherapy with CisGem in first-line treatment of cholangiocarcinoma patients. Patients will be randomized 2:1 to receive infigratinib or CisGem.
Futibatinib (TAS-120)
Futibatinib (TAS-120) is a highly potent and selective irreversible pan-FGFR inhibitor for all four FGFR subtypes (FGFR1–4).132 Futibatinib demonstrated a clinically promising benefit with a manageable toxicity profile in patients with cholangiocarcinoma harboring FGFR2 gene fusions in phase I/II (NCT02052778).133,134 Furthermore, futibatinib can overcome acquired resistance to the ATP-competitive FGFR inhibitors BGJ398 and Debio 1347 and still show promise to patients who had previously progressed on FGFR inhibitors.135 A phase III, open-label, randomized study of futibatinib versus CisGem chemotherapy as first-line therapy of patients with advanced cholangiocarcinoma harboring the FGFR2 gene rearrangement (FOENIX-CCA3) has been initiated (NCT04093362).
Derazantinib (BAL087, Formerly ARQ 087)
Derazantinib is an orally administered small-molecule pan-FGFR kinase inhibitor with potent activity against FGFR1–3. Derazantinib has demonstrated antitumor activity and a manageable safety profile in phase I study in ICC patients,136, 137, 138 and has received US and EU orphan drug designation for ICC. Phase II clinical trials are currently ongoing. Basilea announced positive interim results of a phase II trial for derazantinib in ICC patients in 2019. The interim analysis was conducted after 42 patients were included in the study, in which 29 patients received at least one post-baseline imaging assessment. The objective response rate (ORR) was 21% and the disease control rate for patients with partial remission or stable disease was 83%. To date, the safety data obtained from all 42 patients are consistent with the results of previous clinical studies. So far, the results are encouraging.
Erdafitinib (JNJ-42756493)
Erdafitinib (JNJ-42756493) is a potent pan-FGFR1–4 inhibitor with demonstrated antitumor activity in patients with metastatic urothelial cancer and cholangiocarcinoma with FGFR alterations.139 Asian advanced cholangiocarcinoma patients with FGFR alterations treated with erdafitinib in a phase IIa study (NCT02699606) showed promising efficacy and manageable safety profiles similar to those with other tumor types.140
Debio 1347
Debio 1347 is a novel orally administered small molecule that is a highly selective FGFR1–3 ATP-competitive inhibitor. The preliminary phase I clinical trial result showed an encouraging clinical activity and manageable treatment-emergent adverse events in solid tumors harboring a fusion of FGFR1, FGFR2, or FGFR3 (FUZE clinical trial), including 9 cholangiocarcinomas (NCT1948297).141 The phase II FUZE trial of Debio 1347 (NCT03834220) for patients with advanced solid tumors harboring FGFR fusions, including a cohort for patients with cholangiocarcinoma, is currently being assessed. Recent studies have reported that secondary single nucleotide variants, including p.E565A and p.L617M, appear in cells after FGFR inhibition, resulting in acquired resistance to these FGFR inhibitor therapies.142 The study confirmed the upregulation of the PI3K/AKT/mTOR signaling pathway in drug-resistant cells, and proved that the combination of FGFR and mTOR inhibitor can desensitize cells to FGFR drug resistance.142
IDH-1/2 Inhibitors
IDH catalyzes the conversion of isocitrate to α-ketoglutarate. The mutant forms of IDH1 and IDH2 catalyze the non-reversible accumulation of 2-hydroxyglutarate (2HG), an oncometabolite of α-ketoglutarate that is related to DNA methylation and can promote tumor cell proliferation, invasion, and tumor angiogenesis.124 Because IDH1 and IDH2 mutates in about 10%–28% of ICCs, small-molecule targeted inhibitors of mutant IDH1 and IDH2 have been developed and are undergoing pre-clinical and clinical trials.
Ivosidenib (AG120)
Ivosidenib is an oral IDH1 inhibitor developed by Agios and is currently approved to treat relapsed or refractory acute myeloid leukemia with an IDH1 mutation. It is now being evaluated for treatment of ICC. In the phase I trial, AG120 showed good tolerance and clinical benefit, with 40% PFS rate at 6 months in patients with advanced cholangiocarcinoma (NCT02073994). The phase III clinical trial of AG120, ClarIDHy, is a global, multicenter, double-blind study randomizing 186 participants with IDH1 mutations in a 2:1 ratio to AG-120 or placebo (NCT02989857).143 According to the report at the European Society for Medical Oncology (ESMO), AG120 (ivosidenib) improved PFS from 1.4 months to 2.7 months compared with placebo (HR, 0.37; p < 0.001). 32% and 22% of ivosidenib-treated patients were progression free at 6 months and 12 months respectively, while all patients receiving the placebo had disease progression at data cutoff. The risk of disease progression or death was reduced by 63% with AG120 in ClarIDHy.144 Overall, ivosidenib provides a significant improvement in PFS and OS and Agios plans to submit a supplemental approval application for the drug very soon. The company’s another IDH targeting drug enasidenib (AG-221; an IDH2 inhibitor), which has been approved for IDH2 mutation-positive acute myeloid leukemia, has only been examined in one cholangiocarcinoma clinical trial (NCT02273739). Although the trial began in October 2014 and was completed in June 2016, no literature has yet been published about the results of this trial.
FT-2102, BAY 1436032
FT-2102 and BAY 1436032, which target IDH1 mutations, are currently undergoing clinical trials in solid tumors with IDH1 R132 mutations (NCT03684811, NCT02746081).
Other Novel Targets
Vascular endothelial growth factors (VEGFs) were found to be overexpressed in 53.8% if ICCs.145 Currently, several phase II or III clinical trials for treatments of BTCs targeting VEGFRs are ongoing, including ramucirumab (NCT02520141), apatinib (NCT03521219), and surufatinib (NCT03873532). For those with BRCA1/2 mutations (3%–5%) or BAP1 mutations (10%), poly(ADP-ribose) polymerase (PARP) inhibitors rucaparib (NCT03639935), olaparib (NCT04042831), and niraparib (NCT03207347) may provide some options. PARP inhibitors can compromise the DNA repair process, but this DNA single-strand damage can be converted into a double-strand break (DSB) and be repaired by homologous recombination. If the tumor cells have defects in homologous recombination repair (including the BRCA1/2 or BAP1 mutations), making DSB damage unable to be repaired, this can lead to the lethal effect of PARP inhibitors. For the approximately 5% of ICC patients who harbor PIK3CA mutation, the pan-class I PI3K inhibitor copanlisib plus CisGem is in a phase II clinical trial (NCT02631590).146 In addition, binimetinib (MEK162), a potent inhibitor of MEK1/2, in combination with capecitabine was shown in a phase Ib clinical trial (NCT02773459) in RAS/RAF/MEK/ERK pathway activated BTC patients to have acceptable tolerability and encouraging antitumor efficacy, with response rate and disease control rate of 17.6% and 76.5%, and median PFS and OS 3.9 months and 8.0 months.147
Other clinical trials assessing the efficiency of CD166 inhibitor CX-2009 (NCT03149549), NOTCH transcription complex inhibitor CB-103 (NCT03422679), and proteasome inhibitor bortezomib (NCT03345303) are ongoing.
cHCC-ICC
To date, no standard molecular targeted therapy has been determined for cHCC-ICC. Sorafenib has been a standard of care for unresectable HCC. Because cHCC-ICC contains both the HCC and ICC elements, sorafenib has been used in some cHCC-ICC patients. Some studies suggests outcomes with sorafenib were poor compared with those with platinum-containing regimens.9,10 However, in 2018, here was a clinical case of a patient with advanced cHCC-ICC who achieved complete remission after long-term sorafenib treatment and remained in remission after sorafenib was withdrawn.148 The efficacy of sorafenib in cHCC-ICC needs to be further investigated in a large group of samples.
Immunotherapy
PD-1/PD-L1 antibodies are immune checkpoint inhibitors that help T cells to uncover the hypocritical veil of tumor cells and restore their recognition and killing of tumor cells.149 PD-1 is a negative costimulatory molecule on T cells, and PD-L1 is the ligand of PD-1 and is expressed on tumor cells. After binding, the inhibitory signals are generated, which induces T cell apoptosis, inhibits T cell activation, and prevents T cells from attacking the “invaders” with full force, acting like a brake.150 Blocking PD-1 or PD-L1, the restraints on T cells will be lifted, ensuring that T cells can fully fight cancer cells.151
HCC
PD-1 Antibodies
Nivolumab
The PD-1 antibody nivolumab is the first FDA-approved checkpoint inhibitor for HCC. On September 22, 2017, the FDA granted accelerated approval to nivolumab for HCC patients who have been previously treated with sorafenib. The confirmed overall response rate assessed by RECIST 1.1, was 14.3% (95% CI, 9.2, 20.8).152 Currently, some phase III clinical trials of nivolumab are underway, both nivolumab monotherapy (NCT03383458, NCT02576509) and in combination with others (e.g., NCT04170556, NCT02423343, NCT03781960, NCT03510871).
Pembrolizumab
Pembrolizumab is another PD-1 antibody that was granted accelerated approval for second-line therapy of advanced HCC in 2018 based on KEYNOTE 224 (NCT02702414), a single-arm, multicenter trial enrolling 104 patients with HCC.153 Based on the excellent data of phase II keynote 224, Merck went on to conduct the phase III keynote 240 trial. The trial included patients with HCC who had failed previous sorafenib treatment. The control group was treated with a placebo. The results showed that, compared with the control group, the OS results of the Keytruda group showed improvement, but did not reach a statistically significant difference (HR, 0.781; 95% CI, 0.611–0.998; p = 0.0238); PFS results also had an advantage, but did not reach statistically significant difference either (HR, 0.775; 95% CI, 0.609–0.987; p = 0.0186).154 As the OS and PFS failed to achieve superiority, no formal evaluation of the key secondary endpoint ORR was performed.
Although the phase III trial of pembrolizumab is not satisfying, the researchers conducted an early trial (NCT03006926) of pembrolizumab combined with lenvatinib. The results are promising, with all patients except one showing tumor reduction.155 Based on that, in 2019, the FDA granted the breakthrough therapy designation to pembrolizumab in combination with lenvatinib for the potential first-line treatment of patients with advanced unresectable HCC who do not respond to locoregional treatment. The phase III clinical trial of pembrolizumab plus lenvatinib is ongoing (NCT03713593, NCT04246177).
Camrelizumab, Sintilimab, Tislelizumab, and Toripalimab
Camrelizumab, sintilimab, tislelizumab, and toripalimab are four PD-1 inhibitors developed by Chinese pharmaceutical companies that all show great promise in recent clinical trials. According to the recent result published in Lancet Oncology, camrelizumab showed antitumor activity in pretreated Chinese patients with advanced HCC in an open-label multicenter phase II trial (NCT02989922) and displayed manageable toxicities.156 An objective response was displayed by 14.7% of patients (n = 32 of 217; 14.7%) and, among all patients, 6-month OS rate achieved 74.4% (95% CI, 68.0–79.7). On March 4, 2020, camrelizumab was officially approved by the National Medical Products Administration for patients with advanced HCC who have received sorafenib treatment and/or oxaliplatin-containing chemotherapy. This is the first PD-1 inhibitor approved for liver cancer indications in China.
Sintilimab received its first approval for the treatment of classical Hodgkin lymphoma in China in 2018.157 Currently, sintilimab in combination with anlotinib (NCT04042805), lenvatinib (NCT04042805), and IBI305 (NCT03794440) are undergoing clinical trials for the treatment of various solid tumors, including HCC. Recently, Meihua Lin and colleagues reported an ICC patient who, after the first-line chemotherapy failed, achieved complete remission after three cycles of sintilimab treatment with only mild adverse reactions.158 Unlike other PD-1 antibodies, tislelizumab is specifically designed to minimize binding to FcγR on macrophages159 and to escape FcγR1-mediated effector function, because FcγR on macrophages impairs the antitumor activity of PD-1 antibodies by activating antibody-dependent macrophage-mediated T effector cell killing.160 Phase Ia/Ib trials have shown promise for HCC.161 A global, phase III clinical trial (NCT03412773) designed to evaluate the efficacy and safety of tislelizumab compared with sorafenib as a potential first-line treatment of unresectable HCC has been initiated.162 Toripalimab, being developed by Shanghai Junshi Bioscience Co., Ltd, has received approval for the treatment of unresectable melanoma patients who failed previous systemic therapy in China.163 Several clinical studies are currently being conducted to test the safety and efficiency of toripalimab in the treatment of HCC (NCT03412773, NCT04368078). These studies showed that the four domestic drugs showed great antitumor activity and efficiency and could be a first- or second-line treatment option for advanced HCC patients, even for a population with a high proportion of patients with HBV infection.
PD-L1 Antibodies
Atezolizumab, Bevacizumab
In July 2018, the PD-L1 monoclonal antibody atezolizumab in combination with bevacizumab was awarded the FDA designation of a breakthrough therapy in the treatment of advanced HCC based on a phase Ib clinical study (NCT02715531). In this study, patients with advanced unresectable or metastatic HCC were included. Atezolizumab 1200mg + bevacizumab 15 mg/kg was given once every 3 weeks. The median OS was 17.1 months, 6-month OS was 82%, and 12-month OS was 63%.164 Just recently, the NEJM published an exciting phase III trial result,165 in which the combination of atezolizumab and bevacizumab significantly improved OS and PFS (6.8 months versus 4.3 months) in patients with unresectable HCC with similar toxicity to that of sorafenib (grade 3 or 4 adverse events, 56.5% versus 55.1%). The 12-month OS rate of the patients increased to 67.2% compared with 54.6% with sorafenib, breaking the long-standing bottleneck in liver cancer treatment. The combination is currently under review by the FDA, and it is possible that it will become the new standard of care later in 2020.
Durvalumab
Durvalumab is an FDA-approved immunotherapy first used for locally advanced or metastatic urothelial carcinoma and non-small cell lung cancer developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa monoclonal antibody that blocks the interaction of PD-L1 with PD-1. Based on the promising results in other solid tumors, several phase II/III clinical trials are being conducted for HCC. Durvalumab in combination with bevacizumab (NCT03847428) and with tremelimumab (NCT03298451) are currently under phase III evaluation.
CTLA-4 Antibodies
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is another a co-inhibitory molecule that functions to regulate immune responses.166 Antibodies that block the interaction of CTLA-4 with its ligands B7-1/B7-2 can enhance T cell activation as well as antitumor efficacy.167 Two CTLA-4 antibodies that are currently under clinical investigation are tremelimumab and ipilimumab.
Tremelimumab
Tremelimumab is a human monoclonal CTLA-4 antibody. In 2020, the FDA granted an orphan drug designation to tremelimumab plus durvalumab for the treatment of patients with HCC. The combination is being tested in a phase III clinical trial (NCT03298451) to evaluate durvalumab alone and in combination with tremelimumab compared with standard sorafenib in 1,310 patients with unresectable, advanced HCC who have not received prior systemic treatment and are ineligible for locoregional therapy.168 Results from an early phase II trial combining tremelimumab and durvalumab demonstrated a safety and a promising antitumor activity both in HCC and BTC.169 The tremelimumab and durvalumab combination holds great promise in becoming a new first-line treatment for liver cancer.
Ipilimumab
Ipilimumab is another CTLA-4 monoclonal antibody intended to activate the immune system. On 10 March 2020, the FDA granted the first combination therapy accelerated approval for treatment of HCC to the combination of ipilimumab and nivolumab for HCC patients intolerant to sorafenib. The approval was based on the favorable ORR and duration of response (DoR) from cohort 4 of the CHECKMATE-040 (NCT01658878) trial, which included a total of 49 patients who received nivolumab in combination with ipilimumab. The ORR was 33% (n = 16; 95% CI, 20, 48) with four complete responses and 12 partial responses, and DoR ranged from 4.6 to 30.5+ months, with 31% of responses lasting at least 24 months.
Neoantigen-Based Therapy
New and rapidly growing cancer immunotherapy treatments include the development of personalized tumor vaccines that target neoantigens. In most tumor patients, there are certain specific T cells that can recognize short peptide antigens presented by major histocompatibility complex (MHC) on the surface of cancer cells. This short peptide antigen, which can induce specific T cells to eliminate cancer cells, does not exist in normal tissues, and is thus called a tumor-specific antigen or neoantigen.170 Unlike traditional vaccines, which are limited by the dual restrictions of human leukocyte antigen (HLA) diversity and expression, personalized neoantigen vaccines target each patient's tumor tissue mutation antigen, combining precise gene detection and tumor immunotherapy. This approach uses specific tumor gene mutations to design vaccines that stimulate patients' autoimmunity to eventually kill tumor cells.171,172 Neoantigens are mostly caused by errors in the DNA replication process of cancer cells, and some are caused by environmental factors such as viruses, radiation, and chemicals.173 Although personalized tumor vaccines are still in the exploratory stage, the currently reported clinical trials of individualized neoantigen vaccines have shown encouraging results, especially in the treatment of melanoma, with high accuracy and low side effects.174, 175, 176, 177, 178, 179, 180
Dendritic cells are the most effective antigen-presenting cells in the body. After recognizing the antigen, dendritic cells are activated and enhance the antitumor immune response through T cells and natural killer (NK) cells.181 Currently, several neoantigens based on personalized dendritic cells vaccines for HCC patients are under investigation in multiple ongoing clinical trials (NCT03674073, NCT04147078, NCT03942328) (summarized in Table 2). We expect that follow-up trials can achieve good results and realize its potential to bring patients an efficient, safe, and truly personalized tumor vaccine as soon as possible.
ICC
Tumors with mismatch repair (MMR) pathway deficiency have been demonstrated to have favorable responses to PD-1 blockade immunotherapy.182 MMR-deficient (dMMR) tumors cause high levels of microsatellite instability (MSI) and can generate neoantigens that make the cancer cells susceptible to inhibition of the PD-L1/PD-1 interaction and sensitive to immunotherapy.183 MSI is most commonly seen in colorectal and endometrial cancers; however, cholangiocarcinoma has also been reported to exhibit MSI with a frequency above 10%.184,185 Several clinical immunotherapies for ICC are currently in use, including the PD-1 antibodies pembrolizumab and nivolumab, the PD-L1 antibody durvalumab, and the CTLA-4 antibodies ipilimumab and tremelimumab (Figure 2).186
Pembrolizumab
On May 23, 2017, pembrolizumab was granted accelerated approval by the FDA for the treatment of patients with microsatellite instability-high (MSI-H), or dMMR solid tumors. This was the first time that the FDA approved a drug based on the genetic profile instead of the primary tumor site.187 The phase II, multicohort KEYNOTE-158 study (NCT02628067) evaluated the antitumor activity and safety of pembrolizumab in patients with advanced solid tumors, including 104 cholangiocarcinoma patients.188 Median PFS was 1.9 months versus 2.1 months, median OS was 7.2 months versus 9.6 months, and ORR was 6.6% versus 2.9% in patients with PD-L1 combined positive score CPS ≥1 versus CPS < 1. All responders were not MSI-H. Pembrolizumab in another phase Ib study (NCT02054806) with 24 BTCs also showed durable antitumor activity regardless of PD-L1 CPS and had manageable toxicity.188 Currently, several pembrolizumab clinical trials are ongoing, both monotherapy (NCT03110328) and in combination with others therapies, which include the standard first-line care drug, CisGem (NCT03260712); the PARP inhibitor, olaparib (NCT04306367); the bispecific antibody that simultaneously targets immune checkpoint receptors CTLA-4 and LAG-3, XmAb22841 (NCT03849469); and immune cell therapy (NCT03937895).
Nivolumab
In a Japanese multicenter, open-label, phase I trial, researchers found nivolumab showed activity against BTCs that have progressed on prior systemic therapies, with a manageable safety profile in patients with unresectable or recurrent BTC.189 The median patient age was 64.5 years old. Two-thirds of the patients (64.7%) had intrahepatic cholangiocarcinoma, 2.9% had extrahepatic cholangiocarcinoma, and 32.4% had tumors of the gallbladder. The median OS was 5.2 months in nivolumab monotherapy and 15.4 months in nivolumab plus CisGem, with the median PFS 1.4 months and 4.2 months, respectively. A phase II study (NCT02829918) also found nivolumab to have promising efficacy with tolerated toxicity, including durable responses lasting 2 years in BTC.190 These initial assessments of nivolumab for the treatment of advanced BTC provide supportive evidence for future larger randomized studies of nivolumab in this refractory cancer.
Durvalumab and Tremelimumab
A phase I, open-label, multicenter study (NCT01938612) evaluated the safety, tolerability, and pharmacokinetics of durvalumab and tremelimumab in Asian patients with advanced solid tumors including BTC.191 Patients were enrolled in durvalumab (n = 42) and durvalumab plus tremelimumab cohorts (n = 65). Promising clinical efficacy was observed in both groups with no unexpected toxicities. Currently, a phase II trial of durvalumab and tremelimumab (NCT04238637) is ongoing.
The first randomized, double-blind, international phase III clinical trial to evaluate immunotherapy plus chemotherapy in patients with BTC in the first-line setting is also in progress, testing durvalumab in combination with CisGem (NCT03875235).192
cHCC-ICC
Currently, few studies in the literature or clinical trials have focused on the use of immunotherapy for treatment of cHCC-ICC. Therefore, it will not be discussed in this review.
Currently, the drug development field for liver cancer is mainly dominated by antibody drugs, of which PD-1/PD-L1and CTLA-4 are the main targets, and VEGFR and BRAF are the main small-molecule inhibitor targets. Among the first-line treatment research and development drugs, the combination of Roche's atezolizumab and bevacizumab is the most promising first-line treatment for HCC globally, while Suzhou Zelgen Biopharmaceuticals' donafenib is expected to become the first-line treatment in China. Among the drugs developed for second-line treatment, Hengrui Medicine's apatinib can significantly improve the OS of HCC patients, and is expected to become a new second-line therapy for HCC.
Future Perspective
Although many clinical drugs have been approved or tested in advanced HCC and ICC, the median PFS and OS remain dismal. One of the reasons is the acquired drug resistance due to the intra-tumor heterogeneity or the continuous diversification during treatment, which allows certain tumor cells to survive and eventually develop a drug-resistant phenotype. This remains a huge hurdle for the long-term use of targeted therapies for PLC.193,194 It is therefore necessary to further explore the mechanism of drug resistance. Recently, Tang et al.195 reported a novel somatic mutation in OCT4 (c.G52C) associated with sorafenib resistance. Further work in this vein will allow us to understand the mechanism and the exact gene mutation responsible for the drug resistance, allowing for targeting of specific mutation sites, thereby hopefully overcoming drug resistance.
Another challenge for targeted therapies in PLC is lack of precise targets and biomarkers. Unlike breast cancer, which has the precise biomarker HER2, PLC has a high degree of heterogeneity and genomic diversity, with no accurate biomarkers. Although many high-frequency mutant genes such as TERT, TP53, CTNNB1, and KRAS have been confirmed in PLC, it is still not clear whether they play the role of “driver gene” or “passenger gene” in the progression of liver cancer, which limits the development of targeted drugs. There is therefore an unmet need to comprehensively understand the genomic architecture, define the mutation landscape, and identify novel biomarkers and driver genes in order to develop new therapeutic interventions. With this information, future clinical trials could employ precision medicine to treat patients based on specific genetic mutation and drivers. Another point of concern is that PLC has a high recurrence rate; more than 70% of patients will relapse within 5 years after surgery.196 Thus, whether the genetic features remain the same in the primary and recurrent tumors is also worth exploring.
In recent years, immune checkpoint inhibitors (ICIs) have been of increased research interest. The 2018 Nobel Prize in Physiology or Medicine was awarded to American immunologist James Allison and Japanese immunologist Tasuku Honjo for their contributions to the tumor immunity field, leading to the development and progression of PD-1/CTLA-4 inhibitors and other immunotherapy drugs. However, the overall response rate for ICIs has not been very high (10%–20% in PLCs),152,153 which means the majority of patients cannot benefit from ICIs. It has been the main issue for ICIs. Fortunately, recent studies suggest that ICIs combined with other treatments, especially VEGF/VEGFR inhibitors, can significantly improve the overall response rate, with prolonged median PFS and OS. For example, the overall response rate of the atezolizumab (a PD-L1 inhibitor) and bevacizumab (a VEGF inhibitor) combination was 62% in the phase Ib clinical trial and 27% in the phase III trial. Combined therapies are therefore under more study currently. Combination therapies (Table 2) including pembrolizumab (PD-1 inhibitor) plus lenvatinib (VEGFR inhibitor) (NCT03713593), atezolizumab (PD-L1 inhibitor) plus cabozantinib (VEGFR inhibitor) (NCT03755791), durvalumab (PD-L1 inhibitor) plus bevacizumab (VEGF inhibitor) (NCT03847428), CS1003 (PD-1 inhibitor) plus lenvatinib (VEGFR inhibitor) (NCT04194775), and camrelizumab (PD-1 inhibitor) plus apatinib (VEGFR inhibitor) (NCT03764293) are all in phase III clinical trials for HCC, and we are eagerly waiting for the results.
The immunotolerance of the liver protects it from autoimmune damage caused by foreign antigens,197 but also helps liver cancer cells to escape immune cells hunting. A decrease in NK cell number or impairment of function, accumulation of regulatory T cells, and exhausted CD8+ T cells have all been seen in HCC tumors, implicating an immunosuppressive microenvironment.198 Many patients cannot respond to immunotherapy with a low response rate due to an insufficient immune activation. Thus, how to turn a “cold tumor” (immune tolerant) into a “hot tumor” (immunogenic) remains a major challenge for current tumor immunotherapy research and development. Future efforts in immunotherapy should be made in two directions: boosting the existing immune response and stimulating a de novo immune response.
For the first, more combination strategies such as ICIs combined with VEGFR, CTLA-4, or CDKs should be developed. In addition, immunoregulating the function of regulatory T cell (Treg) and CD8+ T cell function is also of great importance. For example, previous studies have shown that TGF-β promotes tumor immune escape by inducing Treg cell differentiation. The mouse model confirmed that TGF-β inhibitor SM-16 administration reduces Treg cell frequency, resulting in a reduced development of HCC,199 providing a mechanistic rationale for the combination of TGF-β inhibitor and ICI in liver cancer.
Cyclic peptide C25 targeting human LAG-3 protein is reported to be able to significantly stimulate CD8+ T cell activation in human peripheral blood mononuclear cells, resulting in inhibition of tumor growth in CT26-, B16-, and B16-OVA-bearing mice.200 The use of C25 and blockade of the LAG-3/HLA-DR interaction may also provide an alternative method for cancer immunotherapy. The bispecific anti-PD-1/LAG-3 antibodies are also promising in future cancer treatment.
For stimulating the de novo immune response, cell-based immunotherapies such as adoptive cell therapies, including CAR-T cell therapies (e.g., NCT03993743, NCT04121273, and NCT03941626), T cell receptor T cell therapies (e.g., NCT03441100), and the vaccine-based therapies such as neoantigen-based vaccines (e.g., NCT03674073), peptide vaccines, and oncolytic virus drugs (NCT03071094, etc.), are currently being evaluated in HCC. We look forward to seeing the evaluation. Also, more research and studies are expected to be conducted in the future.
Recent studies have begun to unveil the complex hepatic immune microenvironment.
Further work is required to decipher the intricate immune microenvironment of liver cancer, such as the function and subtype of diverse immune cell subsets in liver, including T cells, B cells, macrophages, neutrophils, DCs, myeloid-derived suppressor cells, NK cells, and cancer-associated fibroblasts, as well as the dynamic interaction between the immune cells and the tumor ecosystem. Solving these will help us take a deep look inside the tumor microenvironment and understand patients’ responses to immune therapy, and develop more immunotherapy options.
Aside from these mainstream treatments, some novel therapies have also been proposed in the management of PLC. For example, René Bernards and his team recently elaborated some new ideas about combined therapies by devising a “one-two punch” method (named after the effective combination of two rapid consecutive moves in boxing).201 The first punch makes use of specific mutations (like TP53) in tumor cells to specifically induce it to a certain state, like cell senescence, and then the second punch precisely removes aging tumor cells. Therefore, although these two drugs are not used at the same time, they have synergistic effect with reduced toxicity and high precision.
The treatment of PLC is gradually shifting away from traditional chemotherapy and toward targeted therapy, including immunotherapy and especially the combination therapy. These new approaches have shown great potential in clinical trials, and there is a need to develop more combination strategies or try novel combinations of the previously studied drugs.
Despite these past and ongoing trials investigating PLC treatments, publications and clinical trials regarding systematic treatments of the rare cHCC-ICC are still extremely limited. Further study is undoubtedly required to further improve current diagnosis, as well as to better understand the genomic profile and pathogenesis of cHCC-ICC in order to develop novel therapeutics.
Acknowledgments
We thank Zichen Xu for assistance in the preparation of the figures. This work was supported by the National Natural Science Foundation of China (81972735, 31671452, 81970459) and Clinical Medicine Plus X-Young Scholars Project of Peking University (71006Y2435).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ruirui Kong, Email: ruiruikong@pkufh.com.
Shaokun Shu, Email: shaokun_shu@bjmu.edu.cn.
References
- 1.Brunt E., Aishima S., Clavien P.-A., Fowler K., Goodman Z., Gores G., Gouw A., Kagen A., Klimstra D., Komuta M., et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113–126. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng R., Qu C., Zhang S., Zeng H., Sun K., Gu X., Xia C., Yang Z., Li H., Wei W., et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin. J. Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2020 doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements O., Eliahoo J., Kim J.U., Taylor-Robinson S.D., Khan S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J. Hepatol. 2020;72:95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Valle J.W., Borbath I., Khan S.A., Huguet F., Gruenberger T., Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 9.Trikalinos N.A., Zhou A., Doyle M.B.M., Fowler K.J., Morton A., Vachharajani N., Amin M., Keller J.W., Chapman W.C., Brunt E.M., Tan B.R. Systemic therapy for combined hepatocellular-cholangiocarcinoma: a single-institution experience. J. Natl. Compr. Canc. Netw. 2018;16:1193–1199. doi: 10.6004/jnccn.2018.7053. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S., Terashima T., Shiba S., Yoshida Y., Yamada I., Iwadou S., Horiguchi S., Takahashi H., Suzuki E., Moriguchi M., et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549–2557. doi: 10.1111/cas.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman Z.D. Neoplasms of the liver. Mod. Pathol. 2007;20(Suppl 1):S49–S60. doi: 10.1038/modpathol.3800682. [DOI] [PubMed] [Google Scholar]
- 12.Jiang K., Al-Diffhala S., Centeno B.A. Primary liver cancers–Part 1: histopathology, differential diagnoses, and risk stratification. Cancer Control. 2018;25 doi: 10.1177/1073274817744625. 1073274817744625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain D. Tissue diagnosis of hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014;4(Suppl 3):S67–S73. doi: 10.1016/j.jceh.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komuta M., Govaere O., Vandecaveye V., Akiba J., Van Steenbergen W., Verslype C., Laleman W., Pirenne J., Aerts R., Yano H., et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 15.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall T., Verheij J., Gaudio E., Evert M., Guido M., Goeppert B., Carpino G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 17.Lee A.J., Chun Y.S. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin. Oncol. 2018;7:52. doi: 10.21037/cco.2018.07.03. [DOI] [PubMed] [Google Scholar]
- 18.Allen R.A., Lisa J.R. Combined liver cell and bile duct carcinoma. Am. J. Pathol. 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman Z.D., Ishak K.G., Langloss J.M., Sesterhenn I.A., Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F., Chen X.P., Zhang W., Dong H.H., Xiang S., Zhang W.G., Zhang B.X. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 21.Vilchez V., Shah M.B., Daily M.F., Pena L., Tzeng C.W., Davenport D., Hosein P.J., Gedaly R., Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford) 2016;18:29–34. doi: 10.1016/j.hpb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Totoki Y., Tatsuno K., Yamamoto S., Arai Y., Hosoda F., Ishikawa S., Tsutsumi S., Sonoda K., Totsuka H., Shirakihara T., et al. High-resolution characterization of a hepatocellular carcinoma genome. Nat. Genet. 2011;43:464–469. doi: 10.1038/ng.804. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto A., Totoki Y., Abe T., Boroevich K.A., Hosoda F., Nguyen H.H., Aoki M., Hosono N., Kubo M., Miya F., et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 24.Guichard C., Amaddeo G., Imbeaud S., Ladeiro Y., Pelletier L., Maad I.B., Calderaro J., Bioulac-Sage P., Letexier M., Degos F., et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleary S.P., Jeck W.R., Zhao X., Chen K., Selitsky S.R., Savich G.L., Tan T.X., Wu M.C., Getz G., Lawrence M.S., et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology. 2013;58:1693–1702. doi: 10.1002/hep.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totoki Y., Tatsuno K., Covington K.R., Ueda H., Creighton C.J., Kato M., Tsuji S., Donehower L.A., Slagle B.L., Nakamura H., et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014;46:1267–1273. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 27.Amaddeo G., Cao Q., Ladeiro Y., Imbeaud S., Nault J.C., Jaoui D., Gaston Mathe Y., Laurent C., Laurent A., Bioulac-Sage P., et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. 2015;64:820–829. doi: 10.1136/gutjnl-2013-306228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romualdo G.R., Grassi T.F., Goto R.L., Tablas M.B., Bidinotto L.T., Fernandes A.A.H., Cogliati B., Barbisan L.F. An integrative analysis of chemically-induced cirrhosis-associated hepatocarcinogenesis: histological, biochemical and molecular features. Toxicol. Lett. 2017;281:84–94. doi: 10.1016/j.toxlet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Calderaro J., Couchy G., Imbeaud S., Amaddeo G., Letouzé E., Blanc J.F., Laurent C., Hajji Y., Azoulay D., Bioulac-Sage P., et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017;67:727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Ong C.K., Subimerb C., Pairojkul C., Wongkham S., Cutcutache I., Yu W., McPherson J.R., Allen G.E., Ng C.C., Wong B.H., et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012;44:690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 31.Chan-On W., Nairismagi M.L., Ong C.K., Lim W.K., Dima S., Pairojkul C., Lim K.H., McPherson J.R., Cutcutache I., Heng H.L., et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 32.Jiao Y., Pawlik T.M., Anders R.A., Selaru F.M., Streppel M.M., Lucas D.J., Niknafs N., Guthrie V.B., Maitra A., Argani P., et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sia D., Losic B., Moeini A., Cabellos L., Hao K., Revill K., Bonal D., Miltiadous O., Zhang Z., Hoshida Y., et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat. Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 34.Shiao M.-S., Chiablaem K., Charoensawan V., Ngamphaiboon N., Jinawath N. Emergence of intrahepatic cholangiocarcinoma: how high-throughput technologies expedite the solutions for a rare cancer type. Front. Genet. 2018;9:309. doi: 10.3389/fgene.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z.H., Lian B.F., Dong Q.Z., Sun H., Wei J.W., Sheng Y.Y., Li W., Li Y.X., Xie L., Liu L., Qin L.X. Whole-exome mutational and transcriptional landscapes of combined hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma reveal molecular diversity. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(6 Pt B):2360–2368. doi: 10.1016/j.bbadis.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Xue R., Chen L., Zhang C., Fujita M., Li R., Yan S.M., Ong C.K., Liao X., Gao Q., Sasagawa S., et al. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell. 2019;35:932–947.e8. doi: 10.1016/j.ccell.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz S.F., Lechel A., Obenauf A.C., Begus-Nahrmann Y., Kraus J.M., Hoffmann E.M., Duda J., Eshraghi P., Hartmann D., Liss B., et al. Disruption of Trp53 in livers of mice induces formation of carcinomas with bilineal differentiation. Gastroenterology. 2012;142:1229–1239.e3. doi: 10.1053/j.gastro.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhu R.X., Seto W.-K., Lai C.-L., Yuen M.F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valery P.C., Laversanne M., Clark P.J., Petrick J.L., McGlynn K.A., Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600–611. doi: 10.1002/hep.29498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cazals-Hatem D., Rebouissou S., Bioulac-Sage P., Bluteau O., Blanché H., Franco D., Monges G., Belghiti J., Sa Cunha A., Laurent-Puig P., et al. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J. Hepatol. 2004;41:292–298. doi: 10.1016/j.jhep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Ramai D., Ofosu A., Lai J.K., Reddy M., Adler D.G. Combined hepatocellular cholangiocarcinoma: a population-based retrospective study. Am. J. Gastroenterol. 2019;114:1496–1501. doi: 10.14309/ajg.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 42.Tang R., Latchana N., Rahnemai-Azar A.A., Pawlik T.M. In: Primary and Metastatic Liver Tumors: Treatment Strategy and Evolving Therapies. Cardona K., Maithel S.K., editors. Springer International Publishing; 2018. Guidelines for resection of intrahepatic cholangiocarcinoma; pp. 99–110. [Google Scholar]
- 43.Bosman FT C.F., Hruban R., Theise N.D. IARC Press; 2010. WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 44.Schulze K., Nault J.C., Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J. Hepatol. 2016;65:1031–1042. doi: 10.1016/j.jhep.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 45.Li Z., Zhang J., Liu X., Li S., Wang Q., Di Chen, Hu Z., Yu T., Ding J., Li J., et al. The LINC01138 drives malignancies via activating arginine methyltransferase 5 in hepatocellular carcinoma. Nat. Commun. 2018;9:1572. doi: 10.1038/s41467-018-04006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llovet J.M. Focal gains of VEGFA: candidate predictors of sorafenib response in hepatocellular carcinoma. Cancer Cell. 2014;25:560–562. doi: 10.1016/j.ccr.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai Y., Crowther J., Pastor T., Abbasi Asbagh L., Baietti M.F., De Troyer M., Vazquez I., Talebi A., Renzi F., Dehairs J., et al. Loss of chromosome 8p governs tumor progression and drug response by altering lipid metabolism. Cancer Cell. 2016;29:751–766. doi: 10.1016/j.ccell.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Li Z., Liu L., Wang Q., Li S., Chen D., Hu Z., Yu T., Ding J., Li J., et al. Long noncoding RNA TSLNC8 is a tumor suppressor that inactivates the interleukin-6/STAT3 signaling pathway. Hepatology. 2018;67:171–187. doi: 10.1002/hep.29405. [DOI] [PubMed] [Google Scholar]
- 49.Nault J.C., Calderaro J., Di Tommaso L., Balabaud C., Zafrani E.S., Bioulac-Sage P., Roncalli M., Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983–1992. doi: 10.1002/hep.27372. [DOI] [PubMed] [Google Scholar]
- 50.Javle M., Bekaii-Saab T., Jain A., Wang Y., Kelley R.K., Wang K., Kang H.C., Catenacci D., Ali S., Krishnan S., et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122:3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura H., Arai Y., Totoki Y., Shirota T., Elzawahry A., Kato M., Hama N., Hosoda F., Urushidate T., Ohashi S., et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 52.Jusakul A., Cutcutache I., Yong C.H., Lim J.Q., Huang M.N., Padmanabhan N., Nellore V., Kongpetch S., Ng A.W.T., Ng L.M., et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7:1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Dell M.R., Huang J.L., Whitney-Miller C.L., Deshpande V., Rothberg P., Grose V., Rossi R.M., Zhu A.X., Land H., Bardeesy N., Hezel A.F. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012;72:1557–1567. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha S.K., Parachoniak C.A., Ghanta K.S., Fitamant J., Ross K.N., Najem M.S., Gurumurthy S., Akbay E.A., Sia D., Cornella H., et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller J.W., Doyle M.M., Wang-Gillam A., Brunt E., Vachhajarani N., Chapman W., Tan B.R. Analysis of the genomic profile of biphenotypic tumors compared to cholangiocarcinoma and hepatocellular carcinoma. J. Clin. Oncol. 2014;32(3_suppl):226. [Google Scholar]
- 56.Sasaki M., Sato Y., Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70:423–434. doi: 10.1111/his.13084. [DOI] [PubMed] [Google Scholar]
- 57.Cai X., Li H., Kaplan D.E. Murine hepatoblast-derived liver tumors resembling human combined hepatocellular-cholangiocarcinoma with stem cell features. Cell Biosci. 2020;10:38. doi: 10.1186/s13578-020-00395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T., Nault J.-C., Neumann U., Ricke J., Sangro B., et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 59.Wang K., Zhang H., Xia Y., Liu J., Shen F. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017;6:79–90. doi: 10.21037/hbsn.2017.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim S.M., Mulcahy M.F., Lewandowski R.J., Sato K.T., Ryu R.K., Masterson E.J., Newman S.B., Benson A., Omary R.A., Salem R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- 61.Hyder O., Marsh J.W., Salem R., Petre E.N., Kalva S., Liapi E., Cosgrove D., Neal D., Kamel I., Zhu A.X., et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann. Surg. Oncol. 2013;20:3779–3786. doi: 10.1245/s10434-013-3127-y. [DOI] [PubMed] [Google Scholar]
- 62.Boehm L.M., Jayakrishnan T.T., Miura J.T., Zacharias A.J., Johnston F.M., Turaga K.K., Gamblin T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015;111:213–220. doi: 10.1002/jso.23781. [DOI] [PubMed] [Google Scholar]
- 63.Han K., Ko H.K., Kim K.W., Won H.J., Shin Y.M., Kim P.N. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J. Vasc. Interv. Radiol. 2015;26:943–948. doi: 10.1016/j.jvir.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Edeline J., Touchefeu Y., Guiu B., Farge O., Tougeron D., Baumgaertner I., Ayav A., Campillo-Gimenez B., Beuzit L., Pracht M., et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma. JAMA Oncol. 2020;6:51–59. doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garancini M., Goffredo P., Pagni F., Romano F., Roman S., Sosa J.A., Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952–959. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 66.Cai X., Zhai J., Kaplan D.E., Zhang Y., Zhou L., Chen X., Qian G., Zhao Q., Li Y., Gao L., et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012;56:1804–1816. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song P., Midorikawa Y., Nakayama H., Higaki T., Moriguchi M., Aramaki O., Yamazaki S., Aoki M., Teramoto K., Takayama T. Patients' prognosis of intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma after resection. Cancer Med. 2019;8:5862–5871. doi: 10.1002/cam4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Martin E., Rayar M., Golse N., Dupeux M., Gelli M., Gnemmi V., Allard M.A., Cherqui D., Sa Cunha A., Adam R., et al. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl. 2020;26:785–798. doi: 10.1002/lt.25737. [DOI] [PubMed] [Google Scholar]
- 69.Malone C.D., Gibby W., Tsai R., Kim S.K., Lancia S., Akinwande O., Ramaswamy R.S. Outcomes of yttrium-90 radioembolization for unresectable combined biphenotypic hepatocellular-cholangiocarcinoma. J. Vasc. Interv. Radiol. 2020;31:701–709. doi: 10.1016/j.jvir.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Holzner M.L., Tabrizian P., Parvin-Nejad F.P., Fei K., Gunasekaran G., Rocha C., Facciuto M.E., Florman S., Schwartz M.E. Resection of mixed hepatocellular-cholangiocarcinoma, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma. Liver Transpl. 2020;26:888–898. doi: 10.1002/lt.25786. [DOI] [PubMed] [Google Scholar]
- 71.Louafi S., Boige V., Ducreux M., Bonyhay L., Mansourbakht T., de Baere T., Asnacios A., Hannoun L., Poynard T., Taïeb J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109:1384–1390. doi: 10.1002/cncr.22532. [DOI] [PubMed] [Google Scholar]
- 72.Qin S., Bai Y., Lim H.Y., Thongprasert S., Chao Y., Fan J., Yang T.S., Bhudhisawasdi V., Kang W.K., Zhou Y., et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 73.Abou-Alfa G.K., Niedzwieski D., Knox J.J., Kaubisch A., Posey J., Tan B.R., Kavan P., Goel R., Murray J.J., Bekaii-Saab T.S., et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance) J. Clin. Oncol. 2016;34(4_suppl):192. [Google Scholar]
- 74.Liu L., Zheng Y.H., Han L., Qin S.K. Efficacy and safety of the oxaliplatin-based chemotherapy in the treatment of advanced primary hepatocellular carcinoma: a meta-analysis of prospective studies. Medicine (Baltimore) 2016;95:e4993. doi: 10.1097/MD.0000000000004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A., Madhusudan S., Iveson T., Hughes S., Pereira S.P., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 76.Okusaka T., Nakachi K., Fukutomi A., Mizuno N., Ohkawa S., Funakoshi A., Nagino M., Kondo S., Nagaoka S., Funai J., et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br. J. Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raderer M., Hejna M.H., Valencak J.B., Kornek G.V., Weinländer G.S., Bareck E., Lenauer J., Brodowicz T., Lang F., Scheithauer W. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology. 1999;56:177–180. doi: 10.1159/000011961. [DOI] [PubMed] [Google Scholar]
- 78.Andre T., Tournigand C., Rosmorduc O., Provent S., Maindrault-Goebel F., Avenin D., Selle F., Paye F., Hannoun L., Houry S., et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann. Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 79.Cho J.Y., Paik Y.H., Chang Y.S., Lee S.J., Lee D.K., Song S.Y., Chung J.B., Park M.S., Yu J.S., Yoon D.S. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104:2753–2758. doi: 10.1002/cncr.21591. [DOI] [PubMed] [Google Scholar]
- 80.Knox J.J., Hedley D., Oza A., Feld R., Siu L.L., Chen E., Nematollahi M., Pond G.R., Zhang J., Moore M.J. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J. Clin. Oncol. 2005;23:2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 81.Harder J., Riecken B., Kummer O., Lohrmann C., Otto F., Usadel H., Geissler M., Opitz O., Henss H. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br. J. Cancer. 2006;95:848–852. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manzione L., Romano R., Germano D. Chemotherapy with gemcitabine and oxaliplatin in patients with advanced biliary tract cancer: a single-institution experience. Oncology. 2007;73:311–315. doi: 10.1159/000134239. [DOI] [PubMed] [Google Scholar]
- 83.Koeberle D., Saletti P., Borner M., Gerber D., Dietrich D., Caspar C.B., Mingrone W., Beretta K., Strasser F., Ruhstaller T., et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J. Clin. Oncol. 2008;26:3702–3708. doi: 10.1200/JCO.2008.16.5704. [DOI] [PubMed] [Google Scholar]
- 84.Nehls O., Oettle H., Hartmann J.T., Hofheinz R.D., Hass H.G., Horger M.S., Koppenhöfer U., Hochhaus A., Stieler J., Trojan J., et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br. J. Cancer. 2008;98:309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andre T., Reyes-Vidal J.M., Fartoux L., Ross P., Leslie M., Rosmorduc O., Clemens M.R., Louvet C., Perez N., Mehmud F., Scheithauer W. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br. J. Cancer. 2008;99:862–867. doi: 10.1038/sj.bjc.6604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner A.D., Buechner-Steudel P., Moehler M., Schmalenberg H., Behrens R., Fahlke J., Wein A., Behl S., Kuss O., Kleber G., Fleig W.E. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase-II trials. Br. J. Cancer. 2009;101:1846–1852. doi: 10.1038/sj.bjc.6605377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu A.X., Meyerhardt J.A., Blaszkowsky L.S., Kambadakone A.R., Muzikansky A., Zheng H., Clark J.W., Abrams T.A., Chan J.A., Enzinger P.C., et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54. doi: 10.1016/S1470-2045(09)70333-X. [DOI] [PubMed] [Google Scholar]
- 88.Mizusawa J., Morizane C., Okusaka T., Katayama H., Ishii H., Fukuda H., Furuse J. Randomized Phase III study of gemcitabine plus S-1 versus gemcitabine plus cisplatin in advanced biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1113, FUGA-BT) Jpn. J. Clin. Oncol. 2016;46:385–388. doi: 10.1093/jjco/hyv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahai V., Catalano P.J., Zalupski M.M., Lubner S.J., Menge M.R., Nimeiri H.S., Munshi H.G., Benson A.B., O'Dwyer P.J. Nab-paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:1707–1712. doi: 10.1001/jamaoncol.2018.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim S.T., Kang J.H., Lee J., Lee H.W., Oh S.Y., Jang J.S., Lee M.A., Sohn B.S., Yoon S.Y., Choi H.J., et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 2019;30:788–795. doi: 10.1093/annonc/mdz058. [DOI] [PubMed] [Google Scholar]
- 91.Shroff R.T., Javle M.M., Xiao L., Kaseb A.O., Varadhachary G.R., Wolff R.A., Raghav K.P.S., Iwasaki M., Masci P., Ramanathan R.K., et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lamarca A., Palmer D.H., Wasan H.S., Ross P.J., Ma Y.T., Arora A., Falk S., Gillmore R., Wadsley J., Patel K., et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019;37(15_suppl):4003. [Google Scholar]
- 93.Edeline J., Touchefeu Y., Guiu B., Farge O., Tougeron D., Baumgaertner I., Ayav A., Campillo-Gimenez B., Beuzit L., Pracht M., et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6:51–59. doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cercek A., Boerner T., Tan B.R., Chou J.F., Gönen M., Boucher T.M., Hauser H.F., Do R.K.G., Lowery M.A., Harding J.J., et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6:60–67. doi: 10.1001/jamaoncol.2019.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayashi H., Beppu T., Ishiko T., Mizumoto T., Masuda T., Okabe K., Baba Y., Okabe H., Takamori H., Kanemitsu K., et al. A 42-month disease free survival case of combined hepatocellular-cholangiocarcinoma with lymph node metastases treated with multimodal therapy. Gan To Kagaku Ryoho. 2006;33:1941–1943. [PubMed] [Google Scholar]
- 96.Kim G.M., Jeung H.C., Kim D., Kim J.H., Yoon S.H., Jung E.S., Shin S.J. A case of combined hepatocellular-cholangiocarcinoma with favorable response to systemic chemotherapy. Cancer Res. Treat. 2010;42:235–238. doi: 10.4143/crt.2010.42.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tani S., Murata S., Tamura M., Fukunaga K., Morita M., Hirata Y., Iida H., Kakuno A., Nishigami T., Yamanaka N. Effectiveness of systemic chemotherapy of GEM+CBDCA+5-FU/LV and hepatic arterial infusion of CDDP in a case of advanced, combined hepatocellular-cholangiocarcinoma with multiple lung metastases. Nihon Shokakibyo Gakkai Zasshi. 2011;108:1892–1901. [PubMed] [Google Scholar]
- 98.Chi M., Mikhitarian K., Shi C., Goff L.W. Management of combined hepatocellular-cholangiocarcinoma: a case report and literature review. Gastrointest. Cancer Res. 2012;5:199–202. [PMC free article] [PubMed] [Google Scholar]
- 99.Fowler K., Saad N.E., Brunt E., Doyle M.B., Amin M., Vachharajani N., Tan B., Chapman W.C. Biphenotypic primary liver carcinomas: assessing outcomes of hepatic directed therapy. Ann. Surg. Oncol. 2015;22:4130–4137. doi: 10.1245/s10434-015-4774-y. [DOI] [PubMed] [Google Scholar]
- 100.Rogers J.E., Bolonesi R.M., Rashid A., Elsayes K.M., Elbanan M.G., Law L., Kaseb A., Shroff R.T. Systemic therapy for unresectable, mixed hepatocellular-cholangiocarcinoma: treatment of a rare malignancy. J. Gastrointest. Oncol. 2017;8:347–351. doi: 10.21037/jgo.2017.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sano T., Ishikawa T., Imai M., Owaki T., Sato H., Nozawa Y., Iwanaga A., Seki K., Honma T., Yoshida T., et al. Unresectable combined hepatocellular-cholangiocellular carcinoma treated with transcatheter arterial chemoembolization and gemcitabine: a case study. Nihon Shokakibyo Gakkai Zasshi. 2018;115:1069–1077. doi: 10.11405/nisshoshi.115.1069. [DOI] [PubMed] [Google Scholar]
- 102.Cheng A.-L., Kang Y.-K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 103.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 104.Wei L., Lee D., Law C.-T., Zhang M.S., Shen J., Chin D.W., Zhang A., Tsang F.H., Wong C.L., Ng I.O., et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat. Commun. 2019;10:4681. doi: 10.1038/s41467-019-12606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 106.Liu J., Li X., Zhang H., Chen G., Chen H., Hu Y., Niu J., Ding Y. Safety, pharmacokinetics and efficacy of donafenib in treating advanced hepatocellular carcinoma: report from a phase 1b trial. Pharmazie. 2019;74:688–693. doi: 10.1691/ph.2019.9626. [DOI] [PubMed] [Google Scholar]
- 107.Bi F., Qin S.K., Gu S.Z., Bai Y.X., Chen Z.D., Wang Z.S., Ying J., Lu Y.Y., Meng Z.Q., Pan H.M., et al. Donafenib versus sorafenib as first-line therapy in advanced hepatocellular carcinoma: An open-label, randomized, multicenter phase II/III trial. Presented at: ASCO20 Virtual Scientific Program. J. Clin. Oncol. 2020;38(suppl) abstr 4506. [Google Scholar]
- 108.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 109.Yakes F.M., Chen J., Tan J., Yamaguchi K., Shi Y., Yu P., Qian F., Chu F., Bentzien F., Cancilla B., et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 110.Xiang Q., Chen W., Ren M., Wang J., Zhang H., Deng D.Y., Zhang L., Shang C., Chen Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin. Cancer Res. 2014;20:2959–2970. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 111.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., Cicin I., Merle P., Chen Y., Park J.W., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spratlin J.L., Cohen R.B., Eadens M., Gore L., Camidge D.R., Diab S., Leong S., O'Bryant C., Chow L.Q., Serkova N.J., et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kudo M., Okusaka T., Motomura K., Ohno I., Morimoto M., Seo S., Wada Y., Sato S., Yamashita T., Furukawa M., et al. Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial. J. Gastroenterol. 2020;55:627–639. doi: 10.1007/s00535-020-01668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kudo M., Galle P.R., Llovet J.M., Finn R.S., Vogel A., Motomura K., Assenat E., Merle P., Brandi G., Daniele B., et al. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2. Liver Int. 2020 doi: 10.1111/liv.14462. [DOI] [PubMed] [Google Scholar]
- 115.Li Q., Qin S., Gu S., Chen X., Lin L., Wang Z., Xu A., Chen X., Zhou C., Ren Z., et al. Apatinib as second-line therapy in Chinese patients with advanced hepatocellular carcinoma: a randomized, placebo-controlled, double-blind, phase III study. J. Clin. Oncol. 2020;38(15_suppl):4507. [Google Scholar]
- 116.Kim R.D., Sarker D., Meyer T., Yau T., Macarulla T., Park J.W., Choo S.P., Hollebecque A., Sung M.W., Lim H.Y., et al. First-in-human phase I study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov. 2019;9:1696–1707. doi: 10.1158/2159-8290.CD-19-0555. [DOI] [PubMed] [Google Scholar]
- 117.Kelley R.K., Gane E., Assenat E., Siebler J., Galle P.R., Merle P., Hourmand I.O., Cleverly A., Zhao Y., Gueorguieva I., et al. A phase 2 study of galunisertib (TGF-β1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin. Transl. Gastroenterol. 2019;10:e00056. doi: 10.14309/ctg.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faivre S., Santoro A., Kelley R.K., Gane E., Costentin C.E., Gueorguieva I., Smith C., Cleverly A., Lahn M.M., Raymond E., et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468–1477. doi: 10.1111/liv.14113. [DOI] [PubMed] [Google Scholar]
- 119.Huang C.-Y., Hsieh F.-S., Wang C.-Y., Chen L.J., Chang S.S., Tsai M.H., Hung M.H., Kuo C.W., Shih C.T., Chao T.I., Chen K.F. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia–mutated kinase–mediated DNA damage response. Eur. J. Cancer. 2018;102:10–22. doi: 10.1016/j.ejca.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 120.Reiter F.P., Denk G., Ziesch A., Ofner A., Wimmer R., Hohenester S., Schiergens T.S., Spampatti M., Ye L., Itzel T., et al. Predictors of ribociclib-mediated antitumour effects in native and sorafenib-resistant human hepatocellular carcinoma cells. Cell. Oncol. (Dordr.) 2019;42:705–715. doi: 10.1007/s13402-019-00458-8. [DOI] [PubMed] [Google Scholar]
- 121.Bouattour M., Raymond E., Qin S., Cheng A.L., Stammberger U., Locatelli G., Faivre S. Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology. 2018;67:1132–1149. doi: 10.1002/hep.29496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rimassa L., Assenat E., Peck-Radosavljevic M., Pracht M., Zagonel V., Mathurin P., Rota Caremoli E., Porta C., Daniele B., Bolondi L., et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–693. doi: 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- 123.Farshidfar F., Zheng S., Gingras M.C., Newton Y., Shih J., Robertson A.G., Hinoue T., Hoadley K.A., Gibb E.A., Roszik J., et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Waitkus M.S., Diplas B.H., Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34:186–195. doi: 10.1016/j.ccell.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang T., Liang L., Wang M.D., Shen F. FGFR inhibitors for advanced cholangiocarcinoma. Lancet Oncol. 2020;21:610–612. doi: 10.1016/S1470-2045(20)30152-2. [DOI] [PubMed] [Google Scholar]
- 126.Roskoski R. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol. Res. 2020;151:104567. doi: 10.1016/j.phrs.2019.104567. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y.M., Su F., Kalyana-Sundaram S., Khazanov N., Ateeq B., Cao X., Lonigro R.J., Vats P., Wang R., Lin S.F., et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arai Y., Totoki Y., Hosoda F., Shirota T., Hama N., Nakamura H., Ojima H., Furuta K., Shimada K., Okusaka T., et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 129.Liu P.C.C., Wu L.X., Koblish H., Bowman K., Zhang Y., Klabe R., et al. Preclinical characterization of the selective FGFR inhibitor INCB054828. Cancer Res. 2015;75(15 Suppl) [Google Scholar]
- 130.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Javle M.M., Shroff R.T., Zhu A., Sadeghi S., Choo S., Borad M.J., Lowery M.A., El-Khoueiry A., Macarulla T., Philip P.A., et al. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. J. Clin. Oncol. 2016;34(4_suppl):335. [Google Scholar]
- 132.Ochiiwa H., Fujita H., Itoh K., Sootome H., Hashimoto A., Fujioka Y., et al. Abstract A270: TAS-120, a highly potent and selective irreversible FGFR inhibitor, is effective in tumors harboring various FGFR gene abnormalities. Mol. Cancer Ther. 2013;12:A270. [Google Scholar]
- 133.Meric-Bernstam F., Arkenau H., Tran B., Bahleda R., Kelley R., Hierro C., Ahn D., Zhu A., Javle M., Winkler R., et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor (FGFR) inhibitor, in cholangiocarcinoma patients with FGFR pathway alterations who were previously treated with chemotherapy and other FGFR inhibitors. Ann. Oncol. 2018;29(Suppl 5):v100. [Google Scholar]
- 134.Goyal L., Bahleda R., Furuse J., Valle J.W., Moehler M.H., Oh D.-Y., Chang H.-M., Kelley R.K., Javle M.M., Borad M.J., et al. FOENIX-101: a phase II trial of TAS-120 in patients with intrahepatic cholangiocarcinoma harboring FGFR2 gene rearrangements. J. Clin. Oncol. 2019;37(4_suppl):TPS468. [Google Scholar]
- 135.Goyal L., Shi L., Liu L.Y., Fece de la Cruz F., Lennerz J.K., Raghavan S., Leschiner I., Elagina L., Siravegna G., Ng R.W.S., et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9:1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Papadopoulos K.P., El-Rayes B.F., Tolcher A.W., Patnaik A., Rasco D.W., Harvey R.D., LoRusso P.M., Sachdev J.C., Abbadessa G., Savage R.E., et al. A Phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br. J. Cancer. 2017;117:1592–1599. doi: 10.1038/bjc.2017.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gourd E. Derazantinib for intrahepatic cholangiocarcinoma. Lancet Oncol. 2019;20:e11. doi: 10.1016/S1470-2045(18)30891-X. [DOI] [PubMed] [Google Scholar]
- 138.Mazzaferro V., El-Rayes B.F., Droz Dit Busset M., Cotsoglou C., Harris W.P., Damjanov N., Masi G., Rimassa L., Personeni N., Braiteh F., et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bahleda R., Italiano A., Hierro C., Mita A., Cervantes A., Chan N., Awad M., Calvo E., Moreno V., Govindan R., et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin. Cancer Res. 2019;25:4888–4897. doi: 10.1158/1078-0432.CCR-18-3334. [DOI] [PubMed] [Google Scholar]
- 140.Park J.O., Feng Y.-H., Chen Y.-Y., Su W.-C., Oh D.-Y., Shen L., Kim K.-P., Liu X., Bai Y., Liao H., et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J. Clin. Oncol. 2019;37(15_suppl):4117. [Google Scholar]
- 141.Moeini A., Sia D., Bardeesy N., Mazzaferro V., Llovet J.M. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2016;22:291–300. doi: 10.1158/1078-0432.CCR-14-3296. [DOI] [PubMed] [Google Scholar]
- 142.Krook M.A., Lenyo A., Wilberding M., Barker H., Dantuono M., Bailey K.M., Chen H.-Z., Reeser J.W., Wing M.R., Miya J., et al. Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Mol. Cancer Ther. 2020;19:847–857. doi: 10.1158/1535-7163.MCT-19-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lowery M.A., Abou-Alfa G.K., Valle J.W., Kelley R.K., Goya L., Shroff R.T., et al. ClarIDHy: a phase 3, multicenter, randomized, double-blind study of AG-120 vs placebo in patients with an advanced cholangiocarcinoma with an IDH1 mutation. J. Clin. Oncol. 2017;35(15 suppl):TPS4142. [Google Scholar]
- 144.Abou-Alfa G.K., Mercade T.M., Javle M., Kelley R.K., Lubner S., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J.A., et al. ClarIDHy: A global, phase 3, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann. Oncol. 2019 [Google Scholar]
- 145.Simone V., Brunetti O., Lupo L., Testini M., Maiorano E., Simone M., Longo V., Rolfo C., Peeters M., Scarpa A., et al. Targeting angiogenesis in biliary tract cancers: an open option. Int. J. Mol. Sci. 2017;18:418. doi: 10.3390/ijms18020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mehta R.J., Kim D.W., Soares H.P., Kim J., Kim R.D. Phase II study of copanlisib (BAY 80-6946) in combination with gemcitabine and cisplatin in advanced cholangiocarcinoma. J. Clin. Oncol. 2018;36(4_suppl):TPS525. [Google Scholar]
- 147.Kim J.W., Lee K.-H., Kim J.-W., Suh K.J., Bang J.-H., Bang Y.-J., Oh D.-Y. Phase Ib study of binimetinib (MEK162) in combination with capecitabine in gemcitabine-pretreated advanced biliary tract cancer. J. Clin. Oncol. 2018;36:4079. [Google Scholar]
- 148.Futsukaichi Y., Tajiri K., Kobayashi S., Nagata K., Yasumura S., Takahara T., Minemura M., Yasuda I. Combined hepatocellular-cholangiocarcinoma successfully treated with sorafenib: case report and review of the literature. Clin. J. Gastroenterol. 2019;12:128–134. doi: 10.1007/s12328-018-0918-5. [DOI] [PubMed] [Google Scholar]
- 149.He X., Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gandini S., Massi D., Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu A.X., Finn R.S., Edeline J., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 154.Finn R.S., Ryoo B.-Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 155.Ikeda M., Sung M.W., Kudo M., Kobayashi M., Baron A.D., Finn R.S., Kaneko S., Zhu A.X., Kubota T., Kraljevic S., et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2018;36(15_suppl):4076. [Google Scholar]
- 156.Qin S., Ren Z., Meng Z., Chen Z., Chai X., Xiong J., Bai Y., Yang L., Zhu H., Fang W., et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 157.Hoy S.M. Sintilimab: first global approval. Drugs. 2019;79:341–346. doi: 10.1007/s40265-019-1066-z. [DOI] [PubMed] [Google Scholar]
- 158.Zhang J., Wu L., Liu J., Lin M. A metastatic intrahepatic cholangiocarcinoma treated with programmed cell death 1 inhibitor: a case report and literature review. Immunotherapy. 2020;12:555–561. doi: 10.2217/imt-2019-0100. [DOI] [PubMed] [Google Scholar]
- 159.Zhang T., Song X., Xu L., Ma J., Zhang Y., Gong W., Zhang Y., Zhou X., Wang Z., Wang Y., et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol. Immunother. 2018;67:1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dahan R., Sega E., Engelhardt J., Selby M., Korman A.J., Ravetch J.V. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28:543. doi: 10.1016/j.ccell.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 161.Deva S., Lee J.-S., Lin C.-C., Yen C.J., Millward M.J., Chao Y., et al. 70O A phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors. Ann. Oncol. 2018;29 mdy487.042. [Google Scholar]
- 162.Qin S., Finn R.S., Kudo M., Meyer T., Vogel A., Ducreux M., Macarulla T.M., Tomasello G., Boisserie F., Hou J., et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811–1822. doi: 10.2217/fon-2019-0097. [DOI] [PubMed] [Google Scholar]
- 163.Keam S.J. Toripalimab: first global approval. Drugs. 2019;79:573–578. doi: 10.1007/s40265-019-01076-2. [DOI] [PubMed] [Google Scholar]
- 164.Stein S., Pishvaian M.J., Lee M.S., Lee K.-H., Hernandez S., Kwan A., Liu B., Grossman W., Iizuka K., Ryoo B.-Y. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC) J. Clin. Oncol. 2018;36(15_suppl):4074. [Google Scholar]
- 165.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 166.Korman A.J., Peggs K.S., Allison J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 168.Furuse J., Chan S.L., Sangro B., Galle P.R., Kelley R.K., Qin S., et al. A phase 3 study of durvalumab tremelimumab as first-line treatment in patients with unresectable hepatocellular carcinoma: Himalaya study. Liver Cancer. 2018;7:171. [Google Scholar]
- 169.Floudas C.S., Xie C., Brar G., Morelli M.P., Fioravanti S., Walker M., Mabry-Hrones D., Wood B.J., Levy E.B., Krishnasamy V.P., Greten T.F. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC) J. Clin. Oncol. 2019;37(4_suppl):336. [Google Scholar]
- 170.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 171.Yarchoan M., Johnson B.A., 3rd, Lutz E.R., Laheru D.A., Jaffee E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Desrichard A., Snyder A., Chan T.A. Cancer neoantigens and applications for immunotherapy. Clin. Cancer Res. 2016;22:807–812. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- 173.Mardis E.R. Neoantigens and genome instability: impact on immunogenomic phenotypes and immunotherapy response. Genome Med. 2019;11:71. doi: 10.1186/s13073-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu L., Jiang J., Zhan M., Zhang H., Wang Q.T., Sun S.N., Guo X.K., Yin H., Wei Y., Liu J.O., et al. Targeting neoantigens in hepatocellular carcinoma for immunotherapy: a futile strategy? Hepatology. 2020 doi: 10.1002/hep.31279. [DOI] [PubMed] [Google Scholar]
- 175.Shi D., Shi Y., Kaseb A.O., Qi X., Zhang Y., Chi J., Lu Q., Gao H., Jiang H., Wang H., et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase 1 trials. Clin. Cancer Res. 2020;26:3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. [DOI] [PubMed] [Google Scholar]
- 176.Vormehr M., Tureci O., Sahin U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 2019;70:395–407. doi: 10.1146/annurev-med-042617-101816. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y., Yang X., Yu Y., Xu Z., Sun Y., Liu H., Cheng J., Liu M., Sha B., Li L., et al. Immunotherapy of patient with hepatocellular carcinoma using cytotoxic T lymphocytes ex vivo activated with tumor antigen-pulsed dendritic cells. J. Cancer. 2018;9:275–287. doi: 10.7150/jca.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen C., Ma Y.H., Zhang Y.T., Zhang F., Zhou N., Wang X., Liu T., Li Y.M. Effect of dendritic cell-based immunotherapy on hepatocellular carcinoma: a systematic review and meta-analysis. Cytotherapy. 2018;20:975–989. doi: 10.1016/j.jcyt.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 179.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 180.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 182.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sidaway P. MSI-H: a truly agnostic biomarker? Nat. Rev. Clin. Oncol. 2020;17:68. doi: 10.1038/s41571-019-0310-5. [DOI] [PubMed] [Google Scholar]
- 184.Rashid A., Ueki T., Gao Y.T., Houlihan P.S., Wallace C., Wang B.S., Shen M.C., Deng J., Hsing A.W. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin. Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 185.Silva V.W., Askan G., Daniel T.D., Lowery M., Klimstra D.S., Abou-Alfa G.K., Shia J. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin. Oncol. 2016;5:62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 186.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Marcus L., Lemery S.J., Keegan P., Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 188.Bang Y.-J., Ueno M., Malka D., Chung H.C., Nagrial A., Kelley R.K., Piha-Paul S.A., Ros W., Italiano A., Nakagawa K., et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019;37(15_suppl):4079. [Google Scholar]
- 189.Ueno M., Ikeda M., Morizane C., Kobayashi S., Ohno I., Kondo S., Okano N., Kimura K., Asada S., Namba Y., et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019;4:611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 190.Kim R.D., Kim D.W., Alese O.B., Li D., Shah N., Schell M.J., Zhou J.M., Chung V. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers (BTC) J. Clin. Oncol. 2019;37(15_suppl):4097. [Google Scholar]
- 191.Ioka T., Ueno M., Oh D.-Y., Fujiwara Y., Chen J.-S., Doki Y., Mizuno N., Park K., Asagi A., Hayama M., et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC) J. Clin. Oncol. 2019;37(4_suppl):387. [Google Scholar]
- 192.Oh D.Y., Chen L.T., He A.R., Okusaka T., Qin S., Chin S., Rokutanda N., Uchinda H., Vogel A., Valle J.W., Kim H. A phase III, randomized, double-blind, placebo-controlled, international study of durvalumab in combination with gemcitabine plus cisplatin for patients with advanced biliary tract cancers: TOPAZ-1. Ann. Oncol. 2019;30:v319. [Google Scholar]
- 193.Liu J., Dang H., Wang X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018;50:e416. doi: 10.1038/emm.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Losic B., Craig A.J., Villacorta-Martin C., Martins-Filho S.N., Akers N., Chen X., Ahsen M.E., von Felden J., Labgaa I., DʹAvola D., et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020;11:291. doi: 10.1038/s41467-019-14050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang J., Sui C.J., Wang D.F., Lu X.Y., Luo G.J., Zhao Q., Lian Q.Y., Jeong S., Lin X.M., Zhu Y.J., et al. Targeted sequencing reveals the mutational landscape responsible for sorafenib therapy in advanced hepatocellular carcinoma. Theranostics. 2020;10:5384–5397. doi: 10.7150/thno.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ding X., He M., Chan A.W.H., Song Q.X., Sze S.C., Chen H., Man M.K.H., Man K., Chan S.L., Lai P.B.S., et al. Genomic and epigenomic features of primary and recurrent hepatocellular carcinomas. Gastroenterology. 2019;157:1630–1645.e6. doi: 10.1053/j.gastro.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 197.Ringelhan M., Pfister D., O’Connor T., Pikarsky E., Heikenwalder M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 198.Rizvi S., Wang J., El-Khoueiry A.B. Liver cancer immunity. Hepatology. 2020 doi: 10.1002/hep.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shen Y., Wei Y., Wang Z., Jing Y., He H., Yuan J., Li R., Zhao Q., Wei L., Yang T., Lu J. TGF-β regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell. Physiol. Biochem. 2015;35:1623–1632. doi: 10.1159/000373976. [DOI] [PubMed] [Google Scholar]
- 200.Zhai W., Zhou X., Wang H., Li W., Chen G., Sui X., Li G., Qi Y., Gao Y. A novel cyclic peptide targeting LAG-3 for cancer immunotherapy by activating antigen-specific CD8(+) T cell responses. Acta Pharm. Sin B. 2020;10:1047–1060. doi: 10.1016/j.apsb.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang C., Vegna S., Jin H., Benedict B., Lieftink C., Ramirez C., de Oliveira R.L., Morris B., Gadiot J., Wang W., et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–272. doi: 10.1038/s41586-019-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]