Abstract
Objective
This study compared the efficacy and safety of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) with conventional taxanes as neoadjuvant chemotherapy for breast cancer.
Methods
We searched the literature using PubMed, the Cochrane Library, and Web of Science from their inception to December 15, 2019 based on predetermined inclusion and exclusion criteria. The relevant studies compared pathologic complete response (pCR) and adverse event rates.
Results
The meta-analysis included five studies and 2335 patients. Compared with conventional taxanes, neoadjuvant chemotherapy with nab-paclitaxel was associated with a higher pCR rate (odds ratio [OR] = 1.39, 95% confidence interval [CI] = 1.16–1.67), especially among patients with triple-negative breast cancer or Ki67 indices of >20%. Pooled outcomes also revealed better event-free survival in the nab-paclitaxel group (hazard ratio = 0.69, 95% CI = 0.57–0.85). However, all-grade (OR = 2.17, 95% CI = 1.38–3.40) and grade ≥3 peripheral sensory neuropathy (OR = 3.92, 95% CI = 2.44–6.28) were more frequent in the nab-paclitaxel group.
Conclusions
This meta-analysis implied that nab-paclitaxel more effectively improved pCR than conventional taxanes. Nab-paclitaxel may have greater benefits in patients with triple-negative breast cancer. However, additional attention is required for the early diagnosis and management of peripheral sensory neuropathy.
Keywords: Breast cancer, neoadjuvant chemotherapy, nab-paclitaxel, taxanes, pathologic complete response, peripheral sensory neuropathy
Introduction
Neoadjuvant chemotherapy (NAC) is an important treatment option for invasive breast cancer. Some studies observed similar efficacy between adjuvant and neoadjuvant therapy.1–3 Taxanes and anthracyclines are two of the most of effective chemotherapeutic classes used as NAC in breast cancer. The conventional taxanes include docetaxel and paclitaxel. The NSABP B27 study found that the addition of docetaxel notably increased the pathologic complete response (pCR) rate from 13.7 to 26.1%.4,5
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a novel taxane that was first approved by the FDA for its superior efficacy compared with solvent-based paclitaxel in the treatment of metastatic breast cancer. Nab-paclitaxel allows the use of larger drug doses without premedication, e.g., 260 mg/m2 versus 175 mg/m2 for paclitaxel.6 However, another phase III trial found that nab-paclitaxel was not superior with a trend toward inferiority, and toxicity was increased in the metastatic setting.7 There is no clear consensus concerning whether nab-paclitaxel is more effective than conventional taxanes in the treatment of metastatic breast cancer. The same is also true for the application of nab-paclitaxel as NAC in breast cancer. Two randomized clinical trials reported different results.8,9 Early single-arm studies on the efficacy of nab-paclitaxel in NAC for breast cancer were summarized in a meta-analysis.10 However, because of the small number of comparative studies, the meta-analysis extracted data from only three studies and pooled the results from two studies to evaluate the safety of these two generations of taxanes. In addition, the study did not explore which individuals can benefit from nab-paclitaxel.
More recently, the results of some comparative studies have been updated. Therefore, we conducted this meta-analysis to compare efficacy and safety between nab-paclitaxel and conventional taxanes.
Materials and methods
We conducted this meta-analysis following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.11
Literature search strategy
We performed a literature search using PubMed, the Cochrane Library, and Web of Science from inception to December 15, 2019 using predetermined inclusion and exclusion criteria without language limitations. We searched the terms ((“albumin-bound paclitaxel” or “nab-paclitaxel” or “ABI-007” or “Abraxane”) and (“Taxanes” or “paclitaxel” or “docetaxel”) and (“neoadjuvant” or “preoperative”) and (“breast cancer”)) using the Boolean ‘AND’ operator. Two reviewers (W. Li and Lu) independently analyzed all potentially relevant studies. In the case of any disagreements, consensus was reached by discussion. The same method was also used for quality assessment of the selected studies.
Inclusion and exclusion criteria
The included studies compared nab-paclitaxel with conventional taxanes (paclitaxel or docetaxel) as NAC for patients with non-metastatic breast cancer. The two arms in the studies had the same chemotherapy regimens excluding the taxanes used. Studies had clear definitions of pCR. Editorials, case reports, letters to the editor, review articles, and studies with insufficient information for data extraction were excluded.
Data extraction
Data from the included studies were extracted and summarized independently by two of the authors (Lu and Lin). Any disagreement was resolved by a third author (Y. Li).
The primary outcomes were the pCR and adverse event rates. If sufficient data were available, then the pCR rate for invasive subtypes of breast cancer was extracted, such as triple-negative breast cancer (TNBC; negative for estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [HER2]), HER2-positive breast cancer (HER2-positive regardless of the ER or PR status), and tumors with high Ki67 expression, defined as a Ki67 index exceeding 20%.
There are two common definitions of pCR in breast cancer, namely ypT0 ypN0 (no invasive or non-invasive residual tumor in the breast and axillary lymph nodes) and ypT0/is ypN0 (no invasive residual tumor in the breast and axillary lymph nodes). However, if we adopted either of the two definitions, some studies had to be abandoned, which would weaken the stability of the results because of the fewer numbers of studies and samples included. Therefore, we extracted all pCR data using ypT0 ypN0 or ypT0/is ypN0 as the definition. This ensured the availability of more studies for each pooled analysis to choose pCR according to a definition employed in our included studies. Adverse events included hematologic toxicity, peripheral sensory neuropathy, fatigue, rash, nausea, allergic reactions, and elevated liver enzymes. Adverse events were categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events. Severe adverse events were defined as Grade 3 or higher.
The secondary outcomes were the clinical overall response rate (ORR), overall survival (OS), and event-free survival (EFS). The events for EFS were disease progression during neoadjuvant therapy resulting in inoperability, any invasive locoregional recurrence of disease after neoadjuvant therapy, any invasive contralateral breast cancer, any distant recurrence of disease, or all-cause mortality, whichever occurred first.
Quality assessment and statistical analysis
The quality of randomized controlled trials (RCTs) was assessed using the Cochrane risk of bias tool.12 The quality of retrospective studies was checked using the modified Newcastle–Ottawa Scale (NOS).13,14 RCTs and retrospective studies achieving eight or more stars on the NOS were considered to be of higher quality. Sensitivity analysis was performed for high-quality studies, and only outcomes reported in more than three studies were included in this analysis.
Because the definition of pCR differed among the studies, the adopted definition should include more studies or samples for data analysis. Odds ratios (ORs) were used to compare dichotomous variables. Hazard ratios (HRs) were used as summary statistics for OS and EFS. All results were reported with 95% confidence intervals (CIs). The random-effects model was used after exploring the causes of heterogeneity. Otherwise, the fixed-effects model was used.15 Two-tailed P < 0.05 with a 95% CI that did not include 1 was considered statistically significant. Statistical heterogeneity was calculated using Cochrane’s Q statistic and the I2 statistic, and significance was indicated by P < 0.1. Funnel plot asymmetry was assessed using Begg’s and Egger’s tests to evaluate publication bias, and two-tailed P < 0.05 was considered statistically significant.
Statistical analyses were performed using Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) and Stata 12.0 (Stata Corporation, College Station, TX, USA).
Results
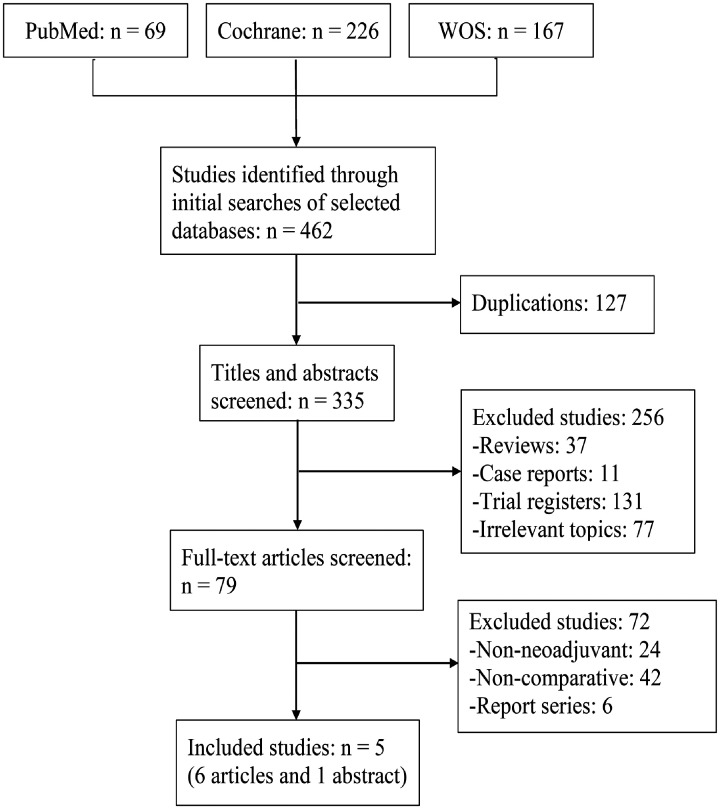
Five studies8,9,16–18 were identified using the predefined search strategy in the final analysis. Two19,20 studies reported survival after short follow-up periods. The sample sizes ranged from 120 to 1206. In total, 2335 patients were eligible for the analysis, with 1140, 1118, and 77 patients receiving nab-paclitaxel, solvent-based paclitaxel, and docetaxel, respectively (Figure 1). The study characteristics, patient demographics, regimens, dosages, and quality score for each study are shown in Table 1. The analyzed studies included three RCTs,8,9,17 one retrospective study,18 and one matched case-control study.16 The details of the quality assessment are shown in Tables 2 and 3.
Figure 1.
Flow diagram of the included studies.
WOS, Web of Science
Table 1.
Characteristics of the included studies.
| Study | Population | Stage | Neoadjuvant regimens | No. of patients | Taxane dosage | pCR (%) | ORR (%) | EFS HR (95% CI) |
Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Gianni 2018 (8, 19) | HER2-negative | T2-4, N0-3 | nab-P→AC/EC/FEC | 346 | 125 mg, days 1, 8, and 15, q4w × 4 | 22.5 | 77.2 | 0.83 (0.60–1.14) | RCT |
| P→AC/EC/FEC | 349 | 90 mg, days 1, 8, and 15, q4w × 4 | 18.6 | 74.5 | |||||
| Huang 2015 (16) | All | II–III | nab-P + Cb (H) | 30 | 125 mg, qw × 12 | 26.7 | 90 | NA | ★★★★ ★★★★ |
| P + Cb (H) | 90 | 80 mg, qw × 12 | 25.6 | 80 | |||||
| Kuwayama 2018 (17) | HER2-negative | I–III | nab-P→FEC | 75 | 100 mg, days 1, 8, and 15, q4w × 4 | 17.3 | 57 | NA | RCT |
| T→FEC | 77 | 75 mg, day 1, q3w × 4 | 11.7 | 53 | |||||
| Untch 2016 (9, 20) | All | T > 2 cm or 1–2 cm with high risk | nab-P (H + Per)→ EC (H + Per) |
606 | 150 mg→125 mg, qw × 12 | 48.7 | 81.7 | 0.62 (0.48–0.80) | RCT |
| P (H + Per)→ EC (H + Per) |
600 | 80 mg, qw × 12 | 39.7 | 79.2 | |||||
| Xie 2019 (18) | All | I–III | EC→nab-P (H) | 83 | 260 mg, day 1, q2w × 4 | 24.1 | NA | NA | ★★★★ ★★ |
| EC→P (H) | 79 | 175 mg, day 1, q2w × 4 | 13.9 | NA |
NA, not available; RCT, randomized controlled trial; pCR, pathologic complete response; ORR, overall response rate; EFS, event-free survival; HER2, human epidermal growth factor receptor 2; qw, once weekly; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; nab-P, nab-paclitaxel; H, trastuzumab; F, fluorouracil; E, epirubicin; A, doxorubicin; C, cyclophosphamide; Cb, carboplatin; T, docetaxel; Per, pertuzumab; P, paclitaxel.
Table 2.
Risk of bias in the prospective randomized controlled trials.
| Study | Adequate random sequence generation | Allocation concealment | Blinding of participants, personnel and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Gianni 2018 | Yes | No | No | No | No | No |
| Kuwayama 2018 | Unclear | Unclear | Unclear | No | No | No |
| Untch 2016 | Yes | No | No | No | No | No |
Table 3.
Assessment of the risk of bias in retrospective studies using the modified Newcastle–Ottawa scale.
| Selection |
Comparability |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|
| Assignment for treatment# | Representative treatment group | Representative reference group | Comparable for 1, 2, and 3* | Comparable for 4, 5, 6, 7, and 8* | Assessment of outcome | Adequate follow-up | Quality score | |
| Huang 2015 | No | Yes | Yes | 1, 2, 3 | 4, 5, 6, 7, 8 | Yes | Yes | ★★★★★★★★ |
| Xie 2019 | No | Yes | Yes | 1, 3 | 4, 5, 6, 7, 8 | Yes | No | ★★★★★★ |
Comparability variables: 1 = age; 2 = Eastern Cooperative Oncology Group; 3 = Menopausal status ; 4 = Tumor stage; 5 = Clinical nodal stage; 6 = Estrogen receptor/progesterone receptor status; 7 = human epidermal growth factor receptor 2 status; 8 = Ki67 index. *: Two stars, all characteristics were comparable; one star, two or three characteristics were comparable; no star, zero or one characteristic was comparable. #: Details of the criteria for the adequate random assignment of patients to treatments were provided.
Primary outcomes
pCR
All studies reported pCR using the definition of ypT0/is ypN0 in the whole population, whereas ypT0 ypN0 was more frequently used in subtype analyses. Therefore, to obtain more stable results, we pooled the pCR data using the ypT0/is ypN0 definition in the global analysis and the ypT0 ypN0 definition in the subtypes analyses.
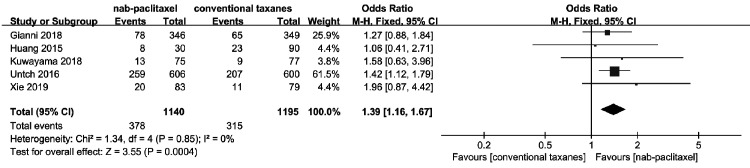
Pooling the data of all five studies8,9,16–18 that assessed pCR (ypT0/is ypN0) in 2335 patients, a significant difference favoring nab-paclitaxel was identified (OR = 1.39, 95% CI = 1.16–1.67; P < 0.001; Figure 2) with no heterogeneity found among the studies (χ2 = 1.34, I2 = 0%).
Figure 2.
Forest plots of the odds ratios for pathologic complete response between nab-paclitaxel and conventional taxanes.
CI, confidence interval; M-H, Mantel–Haenszel method.
Pooling the data from three studies9,17,18 that assessed pCR (ypT0 ypN0) in 1029 patients with Ki67 > 20%, a significant difference favoring nab-paclitaxel was noted (OR = 1.64, 95% CI = 1.26–2.13, P < 0.001) with no significant heterogeneity observed among the studies (χ2 = 0.58, I2 = 0). Pooling the data from three studies9,17,18 that assessed pCR (ypT0 ypN0) among 364 patients with TNBC, a significant difference favoring nab-paclitaxel was observed (OR = 2.45, 95% CI = 1.57–3.82, P < 0.001) with moderate heterogeneity noted among the studies (χ2 = 3.24, I2 = 38%). Pooling the data of two studies9,18 that assessed pCR (ypT0 ypN0) in 449 patients with HER2-positive breast cancer, comparable efficacy was noted between the two groups (OR = 1.31, 95% CI = 0.89–1.92) with low heterogeneity found among the studies (χ2 = 1.05, I2 = 5%).
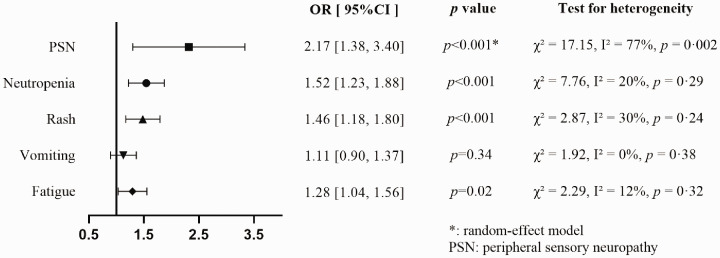
Incidence of adverse events
We gathered adverse event data for neutropenia, peripheral sensory neuropathy, fatigue, rash, and vomiting at all grades. The frequency of all-grade vomiting was similar between the groups (OR = 1.11, 95% CI = 0.90–1.37), but neutropenia (OR = 1.52, 95% CI = 1.23–1.88), peripheral sensory neuropathy (OR = 2.17, 95% CI = 1.38–3.40), rash (OR = 1.46, 95% CI = 1.18–1.80), and fatigue (OR = 1.28, 95% CI = 1.04–1.56) were significantly more frequent in the nab-paclitaxel arm (Figure 3). However, there was significant between-study heterogeneity regarding the incidence of peripheral sensory neuropathy (χ2 = 17.15, I2 = 77%, P = 0.002). We found that the major source of heterogeneity originated from two studies that used a higher cumulative dose of nab-paclitaxel.
Figure 3.
Forest plot for incidence of each specific all-grade adverse event.
OR, odds ratio; CI, confidence interval.
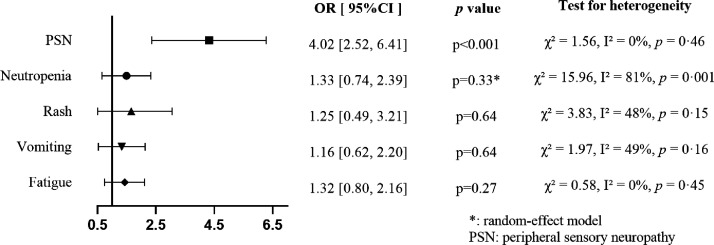
Comparing nab-paclitaxel to conventional taxanes in terms of severe adverse events, there were no significant differences in the rates of neutropenia (OR = 1.33, 95% CI = 0.74–2.39), rash (OR = 1.25, 95% CI = 0.49–3.21), vomiting (OR = 1.16, 95% CI = 0.62–2.20), and fatigue (OR = 1.32, 95% CI = 0.80–2.16), whereas peripheral sensory neuropathy was significantly more common in the nab-paclitaxel arm (OR = 4.01, 95% CI = 2.52–6.41; Figure 4). There was a significant between-study heterogeneity concerning the incidence of severe neutropenia (χ2 = 15.96, I2 = 81%, p = 0.001); however, we failed to identify the source of heterogeneity.
Figure 4.
Forest plot for incidence of each specific adverse event at grade ≥3.
OR, odds ratio; CI, confidence interval.
Secondary outcomes
ORR
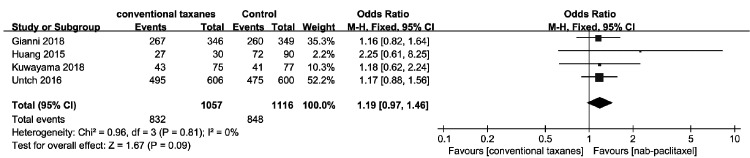
Four studies8,9,16,17 assessed the clinical ORR in 2173 patients. The clinical ORR was not significantly different between the nab-paclitaxel and conventional taxane groups (OR = 1.19, 95% CI = 0.97–1.46), and no significant heterogeneity was noted among the studies (χ2 = 0.96, I2 = 0%; Figure 5).
Figure 5.
Forest plots of the odds ratios for clinical overall response rate between nab-paclitaxel and conventional taxanes.
CI, confidence interval; M-H, Mantel–Haenszel method.
EFS
Among the included studies, two studies reported EFS data,19,20 and only one study reported OS.20 The meta-analysis illustrated that nab-paclitaxel was associated with significantly better EFS than conventional treatment (HR = 0.69, 95% CI = 0.57–0.85, P < 0.001), and moderate between-study heterogeneity was observed (χ2 = 1.91, I2 = 48%).
Sensitivity analysis and publication bias
Three RCTs8,9,17 and one retrospective study16 with NOS scores of at least eight stars were included in the sensitivity analysis, which revealed no change in the significance of any of the outcomes (Table 4).
Table 4.
Sensitivity analysis comparing nab-paclitaxel to conventional taxanes.
| Test for heterogeneity |
|||||
|---|---|---|---|---|---|
| OR (95% CI) | p value | χ² | I2 | P | |
| pCR | 1.37 (1.13–1.65) | 0.001 | 0.62 | 0% | 0.89 |
| All-grade PSN | 1.88 (1.18–2.99) | 0.008* | 13.03 | 77% | 0.005 |
| Grade ≥ 3 PSN | 3.77 (2.32–6.10) | <0.001 | 0.81 | 0% | 0.37 |
*: random-effects model.
pCR, pathologic complete response; PSN, peripheral sensory neuropathy; OR, odds ratio; CI, confidence interval.
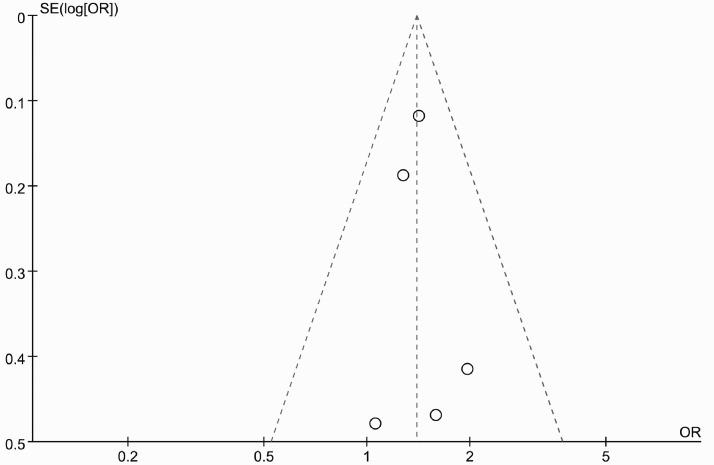
Figure 6 presents a funnel plot of the studies included in this meta-analysis that reported pCR. All studies lied inside the 95% CIs with an even distribution around the vertical, indicating no obvious publication bias. Neither Begg’s funnel plot nor Egger’s test indicated the existence of publication bias.
Figure 6.
Funnel plot no evidence of publication bias for pathologic complete response.
Discussion
This meta-analysis comparing nab-paclitaxel and conventional taxanes as NAC for breast cancer revealed that nab-paclitaxel was linked to better pCR. Both severe and all-grade peripheral sensory neuropathy were more common in the nab-paclitaxel group. Because no change was found in the sensitivity analysis of high-quality studies, the stability of the results was confirmed.
Among the included studies, two large RCTs recently reported short-term follow-up results.19,20 The pooled EFS data favored nab-paclitaxel. A meta-analysis by CTNeoBC demonstrated that patients with pCR had better EFS.21 We therefore speculated that the better EFS in the nab-paclitaxel group could be attributable to the higher pCR rate. Nonetheless, we also noted that the clinical ORR was comparable between the two groups, in line with the results of another meta-analysis focusing on metastatic breast cancer.22
This study demonstrated that patients with TNBC or Ki67 > 20% could more greatly benefit from nab-paclitaxel. This finding has considerable clinical implications for the clinical treatment of TNBC given that chemotherapy is the main adjuvant treatment for TNBC. However, whether nab-paclitaxel has benefits for the treatment of other types of breast cancer remains obscure. Most breast cancers treated with NAC in clinical practice have Ki67 > 20%. In addition, several clinicopathological parameters are significantly associated with the Ki67 index, such as the molecular subtype, lymph node status, tumor size, tumor grade, and TNM stage.23 Concerning HER2-positive breast cancer, the result must be interpreted with caution because of the small number of studies of HER2-positive breast cancer. However, there will be few opportunities to draw valid conclusions regarding this issue in further research. In this era of precision medicine, targeted therapy will attract more attention than chemotherapy for HER2-positive breast cancer. As indicated by the design of the TEAL study, an RCT of early-stage HER2-positive breast cancer, although nab-paclitaxel and solvent-based paclitaxel were examined in different study arms, the researchers focused on comparisons of efficacy between two dual anti-HER2 therapeutic strategies (T-DM1 and lapatinib vs. trastuzumab and pertuzumab).24
Peripheral sensory neuropathy, rash, neutropenia, and fatigue were more frequent in the nab-paclitaxel arm than in the conventional taxane arm, but regarding severe events, only the rate of severe peripheral sensory neuropathy differed between the groups. Taxanes are microtubule-stabilizing agents, and the major toxicity of these agents is peripheral sensory neuropathy.25 The mechanism of taxane-induced peripheral sensory neuropathy remains unclear, but some risk factors may explain the higher risk of taxane-induced peripheral sensory neuropathy. In a randomized phase III study, the mean cumulative dose to the onset of grade ≥2 peripheral sensory neuropathy was 371 mg/m2 for docetaxel, versus 715 mg/m2 for paclitaxel.26 The severity of peripheral sensory neuropathy is also related to the dose. In the CALGB 9342 trial, grade ≥3 peripheral sensory neuropathy was observed in 33% of patients who received paclitaxel at a dose of 250 mg/m2, 19% of patients treated at a dose of 210 mg/m2, and 7% of patients treated at a dose of 175 mg/m2.27 Nab-paclitaxel uses a nanoparticle drug delivery system, thereby permitting higher dose delivery and intensity.28 This may explain why nab-paclitaxel is associated with a higher incidence of peripheral sensory neuropathy than conventional taxanes. Currently, there is no agent for preventing peripheral sensory neuropathy. Thereby, early detection and timely dose reduction can alleviate symptoms in patients with peripheral sensory neuropathy. This is consistent with the finding from the GeparSepto trial that proper dose reduction reduced the incidence and severity of peripheral sensory neuropathy without compromising treatment efficacy.29
This study had some potential limitations. First, only five studies were included, and some pooled analyses were performed with two or three studies. Second, the follow-up period was short; therefore, the evidence supporting the survival benefits of nab-paclitaxel is inadequate. Third, the patient cohorts, treatment strategies (concurrent or sequential administration), drug dosages, and administration interval varied among the studies, which may have led to bias. Finally, no language restrictions were applied in the literature search, but all available studies were published in English. Thus, clinical data reported in other languages may have been missed.
Conclusions
Although further studies are needed to identify patients who might benefit from nab-paclitaxel, our meta-analysis clearly indicated that nab-paclitaxel is more effective than conventional taxanes as NAC in terms of pCR in breast cancer. Moreover, early diagnosis and intervention are needed to control peripheral sensory neuropathy during the administration of nab-paclitaxel.
Author contributions
W. Li and Y. Li conceived and designed the study. W. Li and Lu conducted the literature selection and quality assessment. Lu, Lin, and Y. Li performed the data acquisition. Lu, Y. Li performed the statistical analysis. Y. Li provided the manuscript editing. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Van Der Hage JH, Van De Velde CC, Mieog SJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007; 16: CD005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001; 30: 96–102. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 4.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006; 24: 2019–2027. [DOI] [PubMed] [Google Scholar]
- 5.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003; 21: 4165–4174. [DOI] [PubMed] [Google Scholar]
- 6.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23: 7794–7803. [DOI] [PubMed] [Google Scholar]
- 7.Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol 2015; 33: 2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer-the evaluating treatment with neoadjuvant abraxane (ETNA) trial: a randomized phase 3 clinical trial. JAMA Oncol 2018; 4: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto—GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016; 17: 345–356. [DOI] [PubMed] [Google Scholar]
- 10.Zong Y, Wu J, Shen K. Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: a systematic review and meta-analysis. Oncotarget 2017; 8: 17360–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Tugwell P, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2015, accessed 30 December 2019)
- 14.Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001; 358: 870–875. [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Chen S, Yao L, et al. Phase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancer. Int J Nanomedicine 2015; 10: 1969–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwayama T, Nakamura S, Hayashi N, et al. Randomized multicenter phase II trial of neoadjuvant therapy comparing weekly nab-paclitaxel followed by FEC with docetaxel followed by FEC in HER2(-) early-stage breast cancer. Clin Breast Cancer 2018; 18: 474–480. [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Chen R, Zhang L, et al. Efficacy of two-weekly nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy for breast cancer. Nanomedicine (Lond) 2019; 14: 1595–1603. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Mansutti M, Anton A, et al. Event-free survival analysis of the prospectively randomized phase III ETNA study with neoadjuvant nab-paclitaxel (nab-P) versus paclitaxel (P) followed by anthracycline regimens in women with HER2-negative high-risk breast cancer. J Clin Oncol 2019; 37: 515. [Google Scholar]
- 20.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel improves disease-free survival in early breast cancer: GBG 69–GeparSepto. J Clin Oncol 2019; 37: 2226–2234. [DOI] [PubMed] [Google Scholar]
- 21.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Ye G, Yan D, et al. Role of nab-paclitaxel in metastatic breast cancer: a meta-analysis of randomized clinical trials. Oncotarget 2017; 8: 72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Chen C, Wei W, et al. Associations and indications of Ki67 expression with clinicopathological parameters and molecular subtypes in invasive breast cancer: a population-based study. Oncol Lett 2015; 10: 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel TA, Ensor JE, Creamer SL, et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res 2019; 21: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 2006; 24: 1633–1642. [DOI] [PubMed] [Google Scholar]
- 26.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005; 23: 5542–5551. [DOI] [PubMed] [Google Scholar]
- 27.Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol 2004; 22: 2061–2068. [DOI] [PubMed] [Google Scholar]
- 28.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012; 30: 2055–2062. [DOI] [PubMed] [Google Scholar]
- 29.Furlanetto J, Jackisch C, Untch M, et al. Efficacy and safety of nab-paclitaxel 125 mg/m2 and nab-paclitaxel 150 mg/m2 compared to paclitaxel in early high-risk breast cancer. Results from the neoadjuvant randomized GeparSepto study (GBG 69). Breast Cancer Res Treat 2017; 163: 495–506. [DOI] [PubMed] [Google Scholar]