Abstract
Objective The aim of this study is to summarize currently available evidence on vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Study Design A systematic review was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement.
Results A total of 22 studies comprising 83 neonates born to mothers diagnosed with coronavirus disease 2019 were included in the present systematic review. Among these neonates, three were confirmed with SARS-CoV-2 infection at 16, 36, and 72 hours after birth, respectively, by nasopharyngeal swab real-time polymerase chain reaction (RT-PCR) tests; another six had elevated virus-specific antibody levels in serum samples collected after birth, but negative RT-PCR test results. However, without positive RT-PCR tests of amniotic fluid, placenta, or cord blood, there is a lack of virologic evidence for intrauterine vertical transmission.
Conclusion There is currently no direct evidence to support intrauterine vertical transmission of SARS-CoV-2. Additional RT-PCR tests on amniotic fluid, placenta, and cord blood are needed to ascertain the possibility of intrauterine vertical transmission. For pregnant women infected during their first and second trimesters, further studies focusing on long-term outcomes are needed.
Key Points
We review neonates of mothers diagnosed with coronavirus disease 2019 detected by RT-PCR.
No direct virologic evidence of vertical transmission has been reported.
No evidence that cesarean delivery is safer than vaginal delivery.
More RT-PCR tests on amniotic fluid, placenta, and cord blood are recommended.
Keywords: severe acute respiratory syndrome coronavirus 2, coronavirus disease 2019, vertical transmission, neonate, systematic review
Coronavirus disease 2019 (COVID-19) is an emerging disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with a rapid increase in cases and deaths since it was first reported in Wuhan, Hubei Province, China. 1 2 Pneumonia caused by SARS-CoV-2 is highly infectious and the World Health Organization declared a global pandemic on March 11, 2020. As of April 22, 2020, over 2,500,000 confirmed cases and 173,000 confirmed deaths have been reported globally. The evidence base for person-to-person transmission of SARS-CoV-2 is solid, and there has been a rapid increase in knowledge of the genetic, virologic, epidemiologic, and clinical aspects of this disease. 3 Due to the physiological changes during pregnancy, expectant mothers may face greater risk of infection. 4 However, existing data about its vertical transmission from an expectant mother to her fetus and neonate remain inconclusive. Answers to this question are essential for formulating the principles of obstetric care for pregnant women with SARS-CoV-2 infection. Therefore, we conducted this systematic review to summarize the currently available evidence on vertical transmission of SARS-CoV-2.
Materials and Methods
We conducted a systematic review following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement. The protocol of this systematic review is registered with Open Science Framework (DOI: 10.17605/OSF.IO/Y5SR6). A comprehensive literature search of the following databases was performed on April 20, 2020: PubMed, the China National Knowledge Infrastructure, CBMdisc, and Wanfang Data. Two authors independently and systematically searched these databases using the following MeSH terms: “pregnancy,” “infant, newborn,” “COVID-19,” and “severe acute respiratory syndrome coronavirus 2.” We also performed a manual search in Google Scholar and the websites of key journals in the related field. Reference lists of included studies were also hand searched for potential eligible studies. Studies were considered for inclusion if they were observational studies or case report/series reporting neonates of mothers diagnosed with COVID-19 by real-time polymerase chain reaction (RT-PCR). There was no restriction on language, but the date of publication was limited to the period from January 1, 2020 to April 20, 2020.
The Newcastle-Ottawa scale was used to assess the quality (risk of bias) of cohort and case–control studies. For case report/series, a modified tool for quality appraisal was used; details can be found in the protocol. All methodological procedures were conducted by two authors independently. Disagreements were resolved by discussion with an expert in the related field.
Results
In the initial search, 197 unique records were identified and the full text of 25 was assessed. In total, 22 studies comprising 83 neonates were included in this review. The flow diagram of the study selection process and the characteristics of included studies are shown in Fig. 1 and Table 1 , respectively. All 22 studies were rated as low quality. Among the 83 neonates, 9 had evidence of SARS-CoV-2 infection (positive RT-PCR results or elevated level of virus-specific antibodies in serum samples). Timelines illustrating the evolution of the results of RT-PCR and antibody tests are shown in Fig. 2 .
Fig. 1.

Flow diagram of study selection process.
Table 1. Characteristics of included studies.
| Study (year) | Country | Delivery mode | Number of neonates tested | Samples (time of sample collection) | Sample test results |
|---|---|---|---|---|---|
| Alzamora et al 5 (2020) | Peru | Cesarean delivery | 1 | Serum sample IgG, IgM (at birth); neonatal nasopharyngeal swab (16 hours after delivery and repeated 48 hours later) | IgG, IgM negative; nasopharyngeal swab positive |
| Chen et al 4 (2020) | China | Cesarean delivery | 6 | Amniotic fluid (at the time of delivery); cord blood (immediately after delivery); neonatal throat swab (immediately after delivery); breastmilk (after first lactation) | Negative |
| Chen et al 18 (2020) | China | Cesarean delivery | 1 | Amniotic fluid (at the time of delivery); cord blood (immediately after delivery); placenta (immediately after delivery); breastmilk (day 1–5 postpartum); neonatal throat swab (day 1–7 after birth); stool (day 1–7 after birth) | Negative |
| Chen et al 14 (2020) | China | Cesarean delivery | 3 | Placenta (immediately after delivery); neonatal throat swab (specific time not reported) | Negative |
| Dong et al 9 (2020) | China | Cesarean delivery | 1 | Breastmilk (6 days after delivery); serum sample IgG, IgM (at 2 hours of age and 15 days of age); nasopharyngeal swab (5 tests from 2 hours to 16 days of age) | IgG 140.32 AU/mL, IgM 45.83 AU/mL (2 hours of age); IgG 69.94 AU/mL, IgM 11.75 AU/mL (15 days of age). All RT-PCR tests results were negative |
| Khan et al 19 | China | Vaginal delivery | 3 | Nasopharyngeal swab (time of collection not reported) | Negative |
| Lee et al 20 | Korea | Cesarean delivery | 1 | Placenta, amniotic fluid, and cord blood (at delivery); two consecutive neonatal nasopharyngeal swab (time of collection not reported) | Negative |
| Lei et al 21 | China | Vaginal delivery (1), cesarean delivery (3) | 4 | Vaginal secretion (time of collection not reported); amniotic fluid (at the time of delivery); cord blood (at the time of delivery); breastmilk (time of collection not reported); neonatal nasal and throat swab (time of collection not reported) | Negative |
| Li et al 6 (2020) | China | Cesarean delivery | 1 | Neonatal throat swab (3 days after birth) | Positive (neonatal throat swab) |
| Li et al 22 (2020) | China | Cesarean delivery | 1 | Amniotic fluid, cord blood, placenta, and breastmilk (on the delivery day); infant's oropharyngeal swab, blood, feces, and urine samples (seven different times at day 1 and day 2 after birth) | Negative |
| Liu et al 23 (2020) | China | Cesarean delivery | 9 | Not reported | Negative |
| Liu et al 24 (2020) | China | Vaginal delivery (1), cesarean delivery (10) | 11 | Not reported | Negative |
| González et al 25 | Spain | Cesarean delivery | 1 | Not reported | Negative |
| Wang et al 26 | China | Cesarean delivery | 1 | Amniotic fluid (during delivery); placenta (during delivery); cord blood (during delivery); gastric juice (during delivery); neonatal throat swab (during delivery, day 3, 7, and 9 after cesarean delivery); neonatal stool samples (day 3 after cesarean delivery) | Negative |
| Xiong et al 27 | China | Vaginal delivery | 1 | Amniotic fluid, neonatal throat swab, and rectal swab (time of collection not reported); neonatal IgG and IgM antibodies (time of collection not reported) | Negative |
| Yao et al 28 | China | Cesarean delivery | 1 | Neonatal blood sample (after delivery, day 1 after birth); neonatal nasal and throat swab (after delivery, day 1 and 9 after birth); anal swab (day 9 after birth) | Negative |
| Yu et al 7 (2020) | China | Cesarean delivery | 9 | Neonatal throat swab (at 36 hours after birth) | Negative (8), positive (1) |
| Zeng et al 8 | China | Cesarean delivery | 6 | Neonatal throat swab and blood sample (at birth); serum sample IgG, IgM (at birth) | Two had IgG and IgM higher than normal; three had elevated IgG but normal IgM levels. All RT-PCR test results were negative. |
| Zhang et al 29 | China | Cesarean delivery | 10 | Neonatal throat swab (after birth) | Negative |
| Zhou et al 30 | China | Cesarean delivery | 1 | Neonatal blood sample and throat swab (on the day of birth, four and seven days after birth) | Negative |
| Zhu et al 31 | China | Vaginal delivery (2), cesarean delivery (8) | 10 | Neonatal throat swab (day 1–9 after birth) | Negative (9), no data (1) |
| Zhuang et al 32 | China | Cesarean delivery | 1 | Breastmilk (day 5 after cesarean delivery); neonatal throat swab (day 5 after cesarean delivery) | Negative |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M.
Note: Unless specified otherwise, all tests were real-time polymerase chain reaction.
Fig. 2.
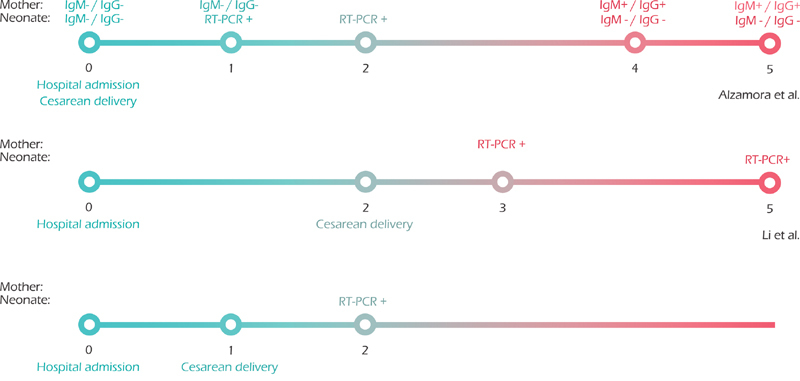
Timelines illustrating the evolution of laboratory tests from the three cases with neonatal infection confirmed by real-time polymerase chain reaction.
Alzamora et al 5 reported a pregnant woman who developed respiratory failure and underwent a cesarean delivery at 33 weeks of gestation. Neonatal isolation was implemented immediately after birth, without any physical contact with the mother. A nasopharyngeal swab collected from the neonate at 16 hours after birth tested positive for SARS-CoV-2 by RT-PCR, although serum samples were negative for virus-specific immunoglobulin G (IgG) and immunoglobulin M (IgM) using automated chemiluminescence immunoassays. The RT-PCR test repeated 48 hours later for confirmation was also positive. Similarly, Li et al 6 and Yu et al 7 also reported neonatal infection and the implementation of neonatal isolation measures immediately after birth. In these two cases, the mothers wore an N95 mask during delivery and the newborns were cared for under isolation in a separate pediatric room. RT-PCR test results at 36 hours and 3 days after birth were positive. In these three cases, no amniotic fluid, placenta, or cord blood were collected for RT-PCR tests.
Zeng et al 8 and Dong et al 9 both reported positive detection of virus-specific antibodies in serum samples drawn from the neonates following birth, although serum and throat swab samples tested negative by RT-PCR. Three of seven infants had elevated IgG and IgM levels; another three had elevated IgG levels, while IgM levels were normal. The mothers' vaginal secretions and breastmilk also tested negative by RT-PCR.
Discussion
In terms of virus infection during pregnancy, obstetricians are most concerned about the possibility of vertical transmission from the mother to her fetus. Vertical transmission has been confirmed for many viruses and can lead to adverse perinatal outcomes including miscarriage, fetal growth restriction, preterm birth, and even stillbirth. 10 11 The routes of vertical transmission include intrauterine transmission, transmission during delivery, breast milk transmission, and contact after delivery. 12 Of these, intrauterine vertical transmission is the least likely to be controlled and managed, and is the most important route of mother-to-child transmission that affects the fetus and neonates. Based on the results of the present systematic review, there is currently no direct evidence to suggest that the development of COVID-19 pneumonia in pregnancy can lead to fetal infection by intrauterine vertical transmission. Although evidence of neonatal infection has been reported, no positive RT-PCR results of the tests of amniotic fluid, placenta, cord blood, vaginal secretions, or breast milk have been reported. Thus, whether these neonates are infected during delivery or after birth is a concern.
The latest research suggests that angiotensin-converting enzyme 2 (ACE2) is the SARS-CoV-2 receptor required for cell entry, but the low level of ACE2 expression in cells at the maternal–fetal interface suggest that there are no susceptible cell subsets in these tissues. 13 Moreover, pathological analysis suggests that there are no morphological changes related to the infection in placenta tissues. 14 Therefore, these evidences do not support the possibility of intrauterine vertical transmission.
We noted that currently available data included only expectant mothers infected in their third trimester and due to the uncertainty of the impact of COVID-19 on maternal–fetal and neonatal outcomes, most babies were delivered by cesarean delivery as soon as possible after admission to minimize the risk. However, patients diagnosed in their first and second trimesters were still pregnant when these studies were published. The risk of adverse perinatal outcomes and vertical transmission is unclear, and this issue remains to be clarified by longer follow-up. Compared with the third trimester, maternal virus infection in the first or second trimesters of pregnancy may have different effects. For example, rubella virus infection before 12 weeks of pregnancy causes congenital rubella syndrome in 90% of cases, whereas the incidence is 50% in cases of infection at 13 to 14 weeks of pregnancy and 25% at the end of the second trimester; infection at the third trimester has little influence on the fetus. 15
In terms of breastfeeding, although no positive RT-PCR results have been reported in tests of breast milk, this finding is limited by relatively small sample sizes and short follow-up time. Expert consensus 16 suggests that infants should not be breastfed by mothers with confirmed or suspected SARS-CoV-2 infection. Indeed, breastfeeding may not be safe until COVID-19 is ruled out or until both mother and neonate clear the virus. Further analyses are needed to guide clinical practice in this setting.
As there is no evidence to support the possibility of intrauterine vertical transmission, the timing of delivery should not be based solely on the condition that a pregnant patient is infected but should be individualized in each case; that is, obstetricians may consider maternal and fetal well-being, gestational age, and other concomitant conditions to determine the time of delivery. 17 In terms of delivery mode, there is a lack of convincing evidence that cesarean delivery is safer. The mode of delivery should be based on routine obstetrical indications, allowing vaginal delivery when possible and reserving cesarean delivery for when obstetrically necessary. 16
Conclusion
The currently available evidence does not support the possibility of intrauterine vertical transmission of SARS-CoV-2 infection during the third trimester of pregnancy. No positive RT-PCR results of tests of amniotic fluid, placenta, cord blood, or breast milk have been reported. For pregnant women infected during their first and second trimesters, further studies focusing on long-term outcomes are needed. We recommend additional RT-PCR testing of amniotic fluid, placenta, and cord blood to confirm these findings.
Footnotes
Conflict of Interest None declared.
References
- 1.Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(08):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li Xet al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China Lancet 2020395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv M, Luo X, Estill J et al. Coronavirus disease (COVID-19): a scoping review. Euro Surveill. 2020;25(15):25. doi: 10.2807/1560-7917.ES.2020.25.15.2000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Guo J, Wang Cet al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records Lancet 2020395(10226):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzamora M C, Paredes T, Caceres D, Webb C M, Valdez L M, La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(08):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Xu M, Zhan W, Han T, Zhang G, Lu Y. Report of the first cases of mother and infant infections with 2019 novel coronavirus in Xinyang City Henan Province. Chin J Infect Dis. 2020 doi: 10.3760/cma.j.issn.1000-6680.2020.0007. [DOI] [Google Scholar]
- 7.Yu N, Fang Z, Wu J. Novel coronavirus pnuemonia in pregnancy: perinatal outcomes. Progress Obstet Gynecol. 2020 doi: 10.1002/uog.22006. [DOI] [Google Scholar]
- 8.Zeng H, Xu C, Fan Jet al. Antibodies in infants born to mothers with COVID-19 pneumoniaJAMA2020323181848–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Tian J, He Set al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newbornJAMA2020 [DOI] [PMC free article] [PubMed]
- 10.Arora N, Sadovsky Y, Dermody T S, Coyne C B. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21(05):561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silasi M, Cardenas I, Kwon J Y, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(03):199–213. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih Y F, Liu C J. Mother-to-infant transmission of hepatitis B virus: challenges and perspectives. Hepatol Int. 2017;11(06):481–484. doi: 10.1007/s12072-017-9831-0. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Q, Duan T, Jin L.Single-cell RNA expression profiling of ACE2 and AXL in the human maternal–fetal interfaceReprod Dev Med2020. Available at:http://www.repdevmed.org/article.asp?issn=2096-2924;year=2020;volume=4;issue=1;spage=7;epage=10;aulast=Zheng. Accessed April 30, 2020
- 14.Chen S, Huang B, Luo D. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Chin J Patho. 2020 doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 15.De Santis M, Cavaliere A F, Straface G, Caruso A. ubella infection in pregnancy. Reprod Toxicol. 2006;21(04):390–398. doi: 10.1016/j.reprotox.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Yang H, Cao Y et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;149(02):130–136. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi H, Luo X, Zheng Y et al. Safe delivery for COVID-19 infected pregnancies. BJOG. 2020 doi: 10.1111/1471-0528.16231. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. Pregnant women complicated with corona virus disease 2019 (COVID-19): a clinical analysis of 3 cases. J Zhejiang Univ 2020. DOI: 10.3785/j.issn.1008-9292.2020.03.08 [DOI] [PMC free article] [PubMed]
- 19.
- 20.Lee DH, Lee J, Kim E, Woo K, Park HY, An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol 2020. DOI: 10.4097/kja.20116 [DOI] [PMC free article] [PubMed]
- 21.
- 22.
- 23.Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect 2020. DOI: 10.1016/j.jinf.2020.02.028 [DOI] [PMC free article] [PubMed]
- 24.
- 25.González RD, Ocampo PJ, González BL, Santana-Cabrera L. Pregnancy and perinatal outcome of a woman with COVID-19 infection. Revista Clínica Española 2020. DOI: 10.1016/j.rce.2020.04.006 [DOI] [PMC free article] [PubMed]
- 26.Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis 2020. DOI: 10.1093/cid/ciaa200 [DOI] [PMC free article] [PubMed]
- 27.
- 28.Yao L, Wang J, Zhao J, Cui J, Hu Z. Asymptomatic COVID-19 infection in pregnant woman in the third trimester: a case report. Chin J Perinat Med 2020. DOI: 10.3760/cma.j.cn113903-20200221-00143
- 29.
- 30.
- 31.
- 32.


