Abstract
Pregnant patients with severe acute respiratory syndrome coronavirus 2, the virus responsible for the clinical condition newly described in 2019 as coronavirus disease 2019 (COVID-19) and illness severity to warrant intensive care have a complex disease process that must involve multiple disciplines. Guidelines from various clinical societies, along with direction from local health authorities, must be considered when approaching the care of an obstetric patient with known or suspected COVID-19. With a rapidly changing landscape, a simplified and cohesive perspective using guidance from different clinical society recommendations regarding the critically-ill obstetric patient with COVID-19 is needed. In this article, we synthesize various high-level guidelines of clinical relevance in the management of pregnant patients with severe disease or critical illness due to COVID-19.
Key Points
When caring for severely ill obstetric patients with COVID-19, one must be well versed in the complications that may need to be managed including, but not limited to adult respiratory distress syndrome with need for mechanical ventilation, approach to refractory hypoxemia, hemodynamic shock, and multiorgan system failure.
Prone positioning can be done safely in gravid patients but requires key areas of support to avoid abdominal compression.
For the critically ill obstetric patient with COVID-19, the focus should be on supportive care as a bridge to recovery rather than delivery as a solution to recovery.
Keywords: COVID-19, adult respiratory distress syndrome, pregnancy, critical care, shock
The global emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated clinical syndrome of coronavirus disease 2019 (COVID-19) have pushed the limits of both health care providers and the health care system. Limited data suggest pregnant patients may not be more vulnerable to severe SARS-CoV-2 infection compared with nonpregnant women. 1 However, the large volume of people infected with SARS-CoV-2 means that managing a pregnant patient with severe disease or critical illness is inevitable, particularly if the expected surge materializes in winter.
The physiologic alterations of pregnancy coupled with unique considerations of managing patients who develop severe COVID-19 challenge even the most seasoned clinicians. Guidelines to address the urgent needs of those on the frontlines of clinical care have been published by professional organizations, but they do not fully integrate considerations of the ill obstetric patient that crosses subspecialty expertise. 2 3 4 5 The focus of this commentary is to converge existing guidelines within best practices from the obstetric, anesthesia, and critical care literature to propose a framework for the management of pregnant patients with severe or critical illness due to COVID-19.
Triage and Admission of Pregnant Patients with Known or Suspected COVID-19
The clinical presentation of COVID-19 in pregnancy appears to mirror that of the general population. 1 The American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine (SMFM) guidelines recommend patients seek medical care if they experience fever (>100.4°F), cough, shortness of breath, difficulty breathing, gastrointestinal symptoms, anosmia, or loss of taste. 1 The majority of obstetric patients with this symptomatology can be evaluated on labor and delivery units. The physiologic modifications of pregnancy coupled with a generally young patient population give pregnant women a remarkable ability to mask the severity of their clinical disease until their clinical reserves are exhausted. The potential for rapid clinical decompensation supports a low threshold to admit pregnant patients for observation. 2
A lower threshold for inpatient observation should be also considered for patients with mild symptoms but underlying comorbidities (i.e., underlying pulmonary disease, asthma, autoimmune disease, maternal cardiac disease, or hypertension). Diagnostic considerations are outlined in Table 1 . The decision to admit patients to labor and delivery or a general medical service with an obstetric consultation varies by institution. Hospitals should have a well-defined pathway for the admission of pregnant patients with COVID-19 that accounts for gestational age, disease severity, and the presence of comorbid obstetric diagnoses or complaints.
Table 1. Diagnostic studies for obstetrics patients with condition warranting admission for COVID-19.
| Diagnostic studies | Recommended patients | Clinical rationale |
|---|---|---|
| CBC and differential | All patients | Leukopenia/lymphopenia common |
| Basic metabolic panel | All patients | Assess renal function |
| Transaminases and bilirubin | All patients | Baseline assessment may inform medication choices |
| Ferritin | All patients | Trend may inform prognosis |
| PT-INR, PTT, fibrinogen, D-dimer | All patients | Assess for coagulopathy (low or normal fibrinogen can be first sign of DIC in pregnancy), D-dimer trend in VTE evaluation |
| Troponin | Concern for cardiac disease | Evaluate baseline cardiac status (CK secreted by placenta, not reliable) |
| C-reactive protein | Consider in admitted patients with severe disease | Elevated in pregnancy but trend may inform treatment response in severe disease |
| Urinalysis and urine culture | Admitted febrile patients | Evaluate for alternate explanation for fever or superimposed infection |
| Blood cultures | Admitted febrile patients | Evaluate for alternate explanation for fever or superimposed infection |
| Chest X-ray | Admitted febrile patients or with shortness of breath | Evaluate for pneumonia |
| Electrocardiogram | Patients with tachycardia or severe disease | Obtain to establish baseline, some medications cause QT prolongation |
| Chest computed tomography | Clinical scenario where results may change management (concern for pulmonary embolism) | Chest CT safe in pregnancy but may be technically limited in cases of tachypnea |
| Transthoracic echocardiogram | Concern for cardiac disease or objective evidence of cardiovascular insult (shock refractory tachycardia, elevated troponin) | Evaluate for myocardial damage secondary to COVID or raise concern for cardiogenic pulmonary edema or cardiogenic shock related to COVID, pregnancy, or both |
Abbreviations: CBC, complete blood count; CK, creatine kinase; COVID, coronavirus disease; DIC, disseminated intravascular coagulation; PT-INR, prothrombin time-international normalized ratio; PTT, partial thromboplastin time; VTE, venous thromboembolism.
Management of Respiratory Failure in Obstetrics Patients with COVID-19
The respiratory manifestations of COVID-19 include acute hypoxemic respiratory failure accompanied by tachypnea and increased work of breathing (WOB). The initial 12 to 24 hours after admission are of paramount importance in assessing clinical trajectory. Supplemental oxygen should be delivered to achieve a resting SpO 2 > 95% to support adequate fetal oxygenation, and the patient should be placed on continuous pulse oximetry monitoring. Providers should adopt a low threshold to check an arterial blood gas (ABG) for patients with an increasing oxygen requirement or increased WOB to evaluate for CO 2 retention that may herald respiratory failure.
Acute Respiratory Distress Syndrome in Pregnancy
Patients with critical illness from COVID-19 develop a clinical picture consistent with acute respiratory distress syndrome (ARDS), defined as respiratory failure that develops within a week of a known clinical insult accompanied by bilateral opacities on imaging, in the absence of cardiogenic pulmonary edema. 6 The presence of moderate or severe impairment in oxygenation is required for the diagnosis of ARDS and is defined by the ratio of the arterial oxygen concentration (PaO 2 ) to the fraction of inspired oxygen (FiO 2 ). The PaO 2 :FiO 2 or P:F ratio is calculated by dividing the PaO 2 on the patient's ABG by the FiO 2 delivered via the mode of supplemental oxygenation. Defining the severity of ARDS is of paramount importance to estimate disease prognosis, inform management strategies, and coordinate early mobilization of resources including intubation or extracorporeal membrane oxygenation (ECMO).
There should be a low threshold to notify the airway team of patients with an increasing oxygen requirement because of the risk of rapid decompensation, challenges of intubation during pregnancy, and the time needed to don requisite PPE. Oxygen delivery via nonrebreather mask can provide temporary delivery of a higher FiO 2 than nasal cannula, and is not thought to increase aerosol exposure risk to health care workers. The use of high-flow nasal cannula can provide additional oxygenation as a bridge to recovery and is preferred over noninvasive positive pressure ventilation. 7 10 27 Bi-level positive airway pressure (BiPAP) should be used with caution both because of aerosolization risk to providers in the setting of COVID-19 and due to the chronicity of the ARDS with a goal for controlled ventilation with this diagnosis. 3 8
It is important to recognize the indications for intubation are the same in pregnant and nonpregnant patients. That said, the increased minute ventilation in pregnancy leads to a decreased baseline CO 2 compared with the nonpregnant state. The normal range of PCO 2 in pregnancy is 27 to 32 mm Hg. 9 Therefore, a PCO 2 at or above 40 mm Hg (normal in the nonpregnant state) or steadily increasing value raise concern for CO 2 retention due to hypoventilation and should prompt additional clinical concern and potentially support to improve ventilation.
The pregnant patient should be approached as having a difficult airway until proven otherwise, as there are physiologic changes in pregnancy that increase the risk for oropharyngeal edema. It is important to also recognize the need for preoxygenation in the pregnant patient with acute respiratory failure from COVID-19, which can take several minutes. Guidelines from the Surviving Sepsis Campaign recommend the use of a video laryngoscope when possible to avoid high-risk SARS-CoV-2 exposure due to close proximity using the alternative, direct laryngoscopy. 10 Given the potential for a difficult airway, the most experienced provider should perform the intubation to lessen the risk of multiple attempts or failure. 10 When positioning a pregnant patient for intubation, manual uterine displacement should be performed during the intubation if the uterus is at the level of the umbilicus to avoid aorto–caval compression that can result in supine hypotension and hemodynamic instability during intubation.
Obstetric Concerns in Mechanical Ventilation
The obstetrician tasked with managing an intubated pregnant patient should refamiliarize oneself with the underlying principles of oxygenation and ventilation in the patient with ARDS, modified for pregnancy ( Table 2 ). The goal of mechanical ventilation is to support oxygenation and ventilation for both the patient and her fetus but minimize additional injury while awaiting recovery of the lungs. Low tidal volume ventilation with tidal volumes set at 4 to 8 mL/kg of predicted body weight in pregnant patients, and lower inspiratory pressures should be the guiding principles of mechanical ventilation for these patients, along with appropriate use of positive end-expiratory pressure (PEEP) to support alveolar recruitment, but not overdistension. Additional considerations for the mechanically ventilated pregnant patient include a strategy of adequate sedation and possible paralysis to fully support maternal oxygenation. Commonly used sedatives and paralytics are safe in pregnancy, but the impact on these agents on tests of fetal wellbeing should be appreciated.
Table 2. Mechanical ventilation parameters and recommended management strategies in pregnant patients with adult respiratory distress syndrome.
| Parameter | Description | Target in ARDS |
|---|---|---|
| TV | Volume delivered by ventilator with each breath | 4–8 mL/kg predicted body weight (this is based on the patient's height) |
| RR | Number of breaths per minute delivered by ventilator | Minimal RR required to match baseline MV, which is elevated in pregnant women typically by 30 to 40% and driven mostly by increased TV in pregnancy. MV = TV × RR |
| P plat | Pressure applied to small airways and alveoli measured by an inspiratory pause at end-expiration on the ventilator | P plat ≤ 35 cm H 2 O in pregnancy (accounts for pressure from gravid uterus while reducing volutrauma) |
| PEEP | Pressure applied to mitigate end-expiratory alveolar collapse | PEEP applied in combination with FiO 2 to achieve desired oxygenation of PaO 2 60 to 80 mm Hg or SpO 2 ≥ 95% |
| FiO 2 | Fraction of oxygen delivered by ventilator (room air is 21%) | |
| PCO 2 | Measured carbon dioxide in arterial or venous blood Marker of alveolar ventilation Hypercapnia is a trade-off in low tidal volume lung protective ventilation |
Permissive hypercapnia threshold in pregnancy is not well established; however, ranges of 50 to 60 mm Hg may be safe |
Abbreviations: ARDS, adult respiratory distress syndrome; FiO 2 , fraction of inspired oxygen; MV, minute ventilation; P plat plateau pressure; PEEP, positive end-expiratory pressure; RR, respiratory rate; TV, tidal volume.
Anecdotal experience supports that both intubated and nonintubated patients with COVID-19 have improved oxygenation with early prone positioning. The randomized PROSEVA trial demonstrated lower mortality using early proning in ARDS patients with a PaO 2 :FiO 2 < 150, FiO 2 ≥ 60%, and PEEP ≥ 5 cm H 2 O. 11 Prone positioning was instituted at an earlier time point in this study compared with other studies exploring the use of prone positioning with negative results. Given the data supporting the efficacy of early prone positioning, this life-saving therapy should not be withheld from pregnant patients or reserved as a strategy of last resort. Case reports of proning in the second and third trimesters of pregnancy provide reassurance about its safety, and centers skilled at proning patients should be similarly adept at safely proning a pregnant patient. 12 Prone positioning in pregnant patients should be done with support of the hips and chest, and aimed at reducing abdominal pressure. Fig. 1 demonstrates the key areas of support needed to avoid direct pressure on the gravid uterus.
Fig. 1.
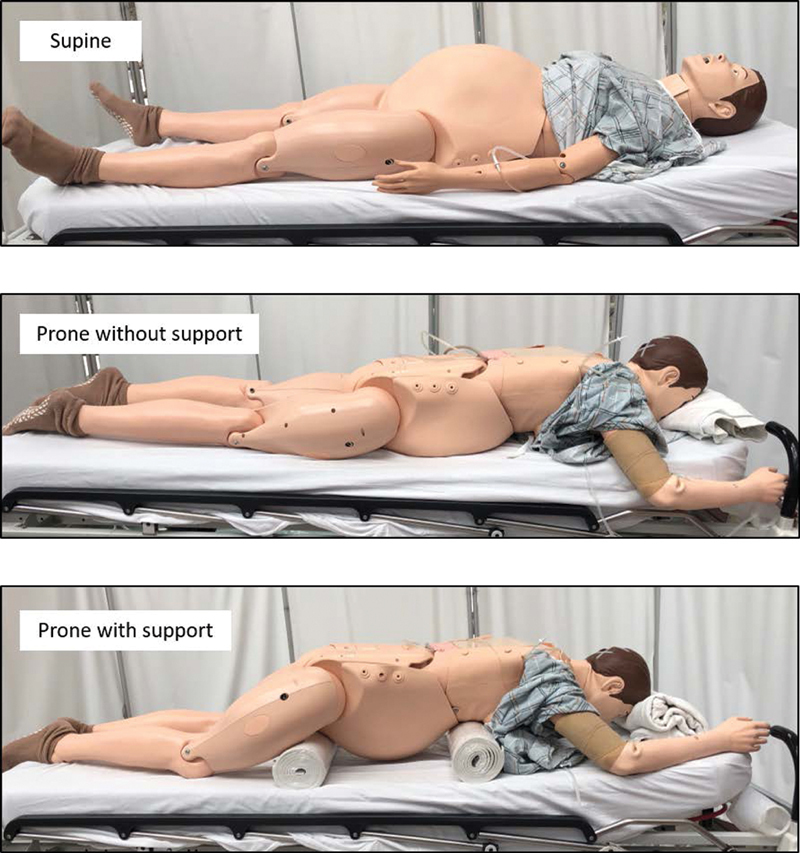
Prone positioning of the pregnant patient with key areas of support.
In cases of severe ARDS from COVID-19 that are refractory to proning or other alveolar recruitment maneuvers using mechanical ventilation, consideration of veno venous-extracorporeal membrane oxygenation (VV-ECMO) may be indicated. 13 The indications and management of VV-ECMO in the pregnant population mirrors the general population with some additional considerations. 14 In pregnancies where the uterine fundus is at the level of the umbilicus, compression of the iliac and other pelvic veins can obscure blood flow, which makes lower extremity use for VV-ECMO a challenge. A single catheter with a dual lumen that can be placed in the right internal jugular vein for VV-ECMO avoids the need for lower extremity cannulation. The potential downside of using a single dual lumen catheter for VV-ECMO, however, is the smaller size of these cannulas limiting the ability to generate adequate flow to match the increased cardiac output of pregnancy. 15 The decision to cannulate and the specific approach will ultimately lie with the ECMO team; however, the obstetrician can contribute immensely by advocating for the patient to receive standard of care and by sharing these pregnancy-specific caveats as part of a collaborative multidisciplinary approach.
Other Intensive Care Considerations for the Critically Ill Pregnant Patient with COVID-19
In obstetric patients with severe manifestations of COVID-19, the obstetrician will be called upon to provide input on hemodynamic targets in pregnancy, comment on medication safety in pregnancy, and make recommendations on obstetric issues including the strategy for fetal monitoring and timing and mode of delivery. Table 3 provides an outline of important organ specific considerations for the obstetrician and intensivist to be mindful of when caring for gravid patients with severe COVID-19.
Table 3. Assessment of volume status and differentiation of shock.
| Parameter a | Septic/inflammatory | Cardiogenic |
|---|---|---|
| Diastolic blood pressure | Low | Normal/high |
| Straight leg raise | Increased MAP | Decreased MAP |
| IVC assessment | Collapsible with respiration | No respiratory variation |
| Left ventricle TTE | Empty/hyperdynamic | Full/low contractility |
| Central venous O 2 | High | Low |
| Central venous pressure | Low | High |
| Stroke volume variation | High/present | Low/absent |
| Cardiac output | High | Low |
Abbreviations: IVC; inferior vena cava; MAP, mean arterial pressure; TTE, transthoracic echocardiogram.
Parameters listed in order of least invasive to most invasive hemodynamic monitoring technique.
Medication Safety
The majority of medications used in the severe or critically ill patient with SARS-CoV-2 infection are safe in pregnancy with nonsteroidal anti-inflammatory drugs, fluoroquinolones, and doxycycline being notable and relevant exceptions. Evidence-based medications for the treatment of COVID-19 are lacking, and most regimens are recommended to be given in the context of a clinical trial. 3 16 Unfortunately, pregnant patients have been excluded from essentially all clinical trials involving investigational drugs for COVID-19 despite long-standing safety data for some agents like hydroxychloroquine.
Hemodynamic Targets and Fluid Management
Balancing intravascular volume resuscitation to support maternal hemodynamics and fetal perfusion with the imperative to avoid pulmonary edema is a clinical challenge of paramount importance in patients with severe manifestations of COVID-19. Early resuscitation with IV fluids for patients with signs of intravascular volume depletion or distributive shock should be prioritized. Crystalloid fluids are favored over colloids, with a preference for lactated ringers or balanced crystalloid solutions to avoid the hyperchloremic metabolic acidosis of normal saline. 17 Routine administration of maintenance fluids should be avoided in favor of frequent volume assessment and targeted fluid boluses in the setting of evidence of hypovolemia.
Existing ARDS literature shows that a conservative fluid management strategy results in improved lung function and shortened duration of mechanical ventilation. 18 We recommend adhering to this strategy in the management of acute hypoxemic respiratory failure secondary to COVID-19. The possible cardiac complications of COVID-19 (including arrhythmia and myocarditis) can precipitate heart failure, a physiologic state that also favors a net-even or negative fluid balance. 19
The complex interplay between septic shock and ARDS compounded by the possibility of cardiogenic shock supports a low threshold to engage an intensivist in cases of persistent hypotension, tachycardia, or oliguria. For patients with hypotension a 30 cc/kg bolus of crystalloid infused over 30 minutes can help treat hypovolemia and assess fluid responsiveness. Physical exam, coupled with point of care ultrasonography, can be helpful in assessing the need for more fluids and elucidating the etiology of shock in the absence of more traditional invasive means of hemodynamic monitoring ( Table 3 ). For patients with persistent or worsened hypotension in the face of a fluid challenge or with hypotension with evidence of volume overload we recommend starting vasopressors instead of ongoing fluid challenges. Common pregnancy-compatible medications for the management of shock and their physiologic characteristics are outlined in Table 4 .
Table 4. Common pregnancy-compatible medications in the management of shock.
| Medication | Mechanism | Indication | Typical dose |
|---|---|---|---|
| Lactated ringers | Temporary increase in intravascular volume and preload | First line resuscitation for shock (preferred over normal saline which causes chloride-associated acidosis) | 250 to 500 cc boluses until no clinical response is seen and patient thought to be euvolemic Avoid maintenance fluids |
| Furosemide (Lasix) | Loop diuretic used to decrease intravascular volume | Treatment of volume overload in cardiogenic shock to obtain euvolemia | Start with 10 mg IV, double until desired effect. For continuous infusion: bolus 0.1 mg/kg followed by 0.1 mg/kg/h, double every 2 hours to a maximum of 0.4 mg/kg/h |
| Norepinephrine (Levophed) | α: ↑ SVR to ↑ BP and ↑ preload β-1: ↑ HR (minor effect) |
First line vasopressor for undifferentiated shock and septic shock | 0.05 to 0.1 mcg/kg/min, not to exceed 2 mcg/kg/min |
| Vasopressin | V1a agonist: peripheral vasoconstriction ↑ SVR (also oxytocin analog) | Addition to norepinephrine in septic shock to reduce risk of arrhythmia; treats sedation-associated vasodilation | 0.03 units/min added to norepinephrine infusion once dose reaches 5 to 10 mcg/min |
| Dobutamine | β-1: ↑ HR, ↑ cardiac contractility; minimal α- and β-2: ↓SVR | Inodilator used for cardiogenic shock to increase cardiac output but decrease afterload | 0.5 to 1 mcg/kg/min, maximum dose 40 mcg/kg/min |
| Epinephrine | α and β: ↑ SVR, ↑ HR, ↑ cardiac contractility | Refractory cardiogenic shock (first line vasopressor in anaphylactic shock) | IV drip: 1 to 10 mcg/min Endotracheal tube: 2 to 2.5 mg epinephrine is diluted in 10cc NS and given directly into the ET tube |
| Phenylephrine | α: ↑ SVR | Second line for septic shock when tachyarrhythmias limit use of norepinephrine (can decrease cardiac contractility) | IV drip: 10 to 35 mcg/min; max dose 200 mcg/min |
Abbreviations: BP, blood pressure; HR, heart rate; IV, intravenous; NS, normal saline; SVR, systemic vascular resistance.
Prophylaxis for Venous Thromboembolism
Given the hypercoagulable state generally seen in pregnant patients as well as the thoughts of microthrombi contributing to the pathophysiology in COVID-19, it stands to reason that all obstetrics patients admitted to the hospital with COVID-19 should be given venous thromboembolism (VTE) prophylaxis. 21 In patients admitted with obstetric complaints, prophylaxis should be balanced with the potential for delivery and SMFM-SOAP guidelines supporting early epidural for labor analgesia to mitigate risks of general anesthesia. 5 For patients who are critically ill, empiric therapeutic anticoagulation with a heparin infusion should be considered, as a VTE in this patient population with limited cardiopulmonary reserve and high risk for clot propagation at time of delivery could be a life-threatening consequence. 22 Reports of lupus-anticoagulant mediated artificial prolongation of the partial thromboplastin time (PTT) in patients with COVID-19 make monitoring of therapeutic anticoagulation using anti-Xa levels ideal. 23 A conservative approach to VTE prophylaxis according to severity of COVID-19 and comorbid disease states is outlined in Table 5 .
Table 5. Strategies for prevention of venous thromboembolism.
| Medication | Clinical indications | Monitoring and delivery |
|---|---|---|
| Heparin 5,000 units SQ TID | Antepartum inpatient without critical illness | Check PTT prior to neuraxial anesthesia |
| Enoxaparin SQ 40 mg daily | Discharged antepartum patients with disease severity: mild (< 2L NC oxygen requirement)-moderate disease (> 2L oxygen requirement but not intubated and severe (> 5L NC oxygen requirement) or critical disease (mechanical ventilation, ECMO) | Neuraxial anesthesia possible 12 hours after last dose of enoxaparin Consider 2 weeks of VTE prophylaxis for mild–moderate disease, potentially longer for severe or critical disease |
| Postpartum on discharge if mild disease with VTE risk factors or moderate or more disease severity without | Consider 2 weeks for those with vaginal birth and 6 weeks for those with cesarean delivery (or severe or critical illness) | |
| Enoxaparin SQ 40 mg BID | Prophylactic dose in nonintubated critical illness with normal renal function | Consider heparin 7,500 units TID if CrCl < 30 mL/min |
| Enoxaparin SQ 0.5 mg/kg BID | Intermediate dose in critical illness with BMI > 40 kg/m 2 or at high risk of thrombosis without active obstetric issues or renal failure | Consider monitoring peak anti-Xa levels with target 0.2 to 0.5 μ/mL 4 to 6 hours after injections |
| Heparin infusion | Therapeutic dose in critical illness at high risk of bleeding or thrombosis with active obstetric issues or renal failure | Monitor anti-Xa levels Q6 hours targeting range 0.3 to 0.7 μ/mL |
Abbreviations: BID, twice daily; BMI, body mass index; CrCl, creatinine clearance; ECMO, extracorporeal membrane oxygenation; NC, nasal cannula; PTT, partial thromboplastin time; TID, three times daily; SQ, subcutaneous; VTE, venous thromboembolism.
Other Obstetric Specific Considerations in the Critically Ill Pregnant Patient with COVID-19
Fetal Surveillance
The frequency of antepartum fetal surveillance should be individualized based on gestational age and maternal status. Daily fetal assessment should be performed after 24 weeks of gestation during inpatient evaluation with a low threshold to monitor the fetus with new subjective complaints or changes in clinical status. The frequency of hemodynamic fluctuations in a critically ill patient may warrant more frequent fetal assessment both for reassurance of fetal well-being, assessment for evidence of labor in the intubated and sedated patient, and to titrate hemodynamic parameters to ensure fetal perfusion. One must keep in mind the potential effects of sedative medications on the fetal assessment in women who are intubated.
Delivery Planning
As a general principle, delivery for patients with COVID-19 should be reserved for routine obstetric indications with rare exception. 3 For the critically ill pregnant patient, the goal is to provide supportive care to optimize the maternal condition and provide additional time for fetal maturation. The decision to deliver a patient for worsening maternal respiratory status alone is controversial. It is unclear that delivery improves maternal respiratory outcomes, and the acute hemodynamic changes accompanying the auto-transfusion of delivery may be enough to destabilize a clinically tenuous patient. Given the anticipated albeit prolonged course of lung recovery in COVID-19, the focus should be on supportive care as a bridge to recovery rather than delivery as a solution to recovery. Notable exceptions to avoiding delivery include scenarios of nonreassuring fetal heart rate monitoring not improved with optimization of maternal hemodynamics and peri-mortem cesarean delivery for maternal cardiac arrest. 24
When delivery is indicated, a vaginal delivery should be prioritized for all patients with COVID-19 who lack an obstetric indication for cesarean delivery. This recommendation extends to women with oxygen requirements and the critically ill, acknowledging this may pose more logistic challenges. Obstetrical interventions such as fetal scalp electrode placement and operative vaginal delivery have not yet been determined problematic. 5 Early labor epidural analgesia is recommended in patients given the impetus to avoid general anesthesia. Rapid preparations for potential cesarean delivery if there is a maternal or fetal indication must take into consideration the time, it takes to safely transport the patient to the operating room and for all health care workers to don PPE.
A Comprehensive Approach to Recovery
More often than not, pregnant patients admitted for symptoms related to COVID-19 will not be delivered. Although there is lack of high-level evidence, concerns about the impact of hypoxia or hypercoagulability on the fetus over time may warrant obstetric ultrasound for growth surveillance as well as antenatal fetal testing in women who have recovered from critical illness related to COVID-19. Outpatient anticoagulation for VTE prophylaxis should be considered. Postdischarge follow-up using tele-health and/or remote pulse oximetry monitoring is encouraged to monitor for recurrence of disease or downstream complications, proactively address questions, and provide psychosocial support for patients who may be experiencing trauma from admission or anxiety from social isolation.
The impact of the pandemic on life and medicine is overwhelming. The management of COVID-19 challenges even the most experienced clinician struggling to make clinical decisions about an emerging disease in the absence of evidence and with comparatively limited resources. As evidence continues to emerge and guidelines are updated, obstetricians should feel empowered to take a leading role in the management of women whose pregnancies are impacted by COVID-19 infection. Whether on labor and delivery or in the ICU, obstetric care providers are well equipped to provide input on the impact of management strategies on the maternal–fetal dyad, make quick clinical decisions as challenges arise, and advocate for the pregnant patient to ensure she receives the standard of care to optimize outcomes for all.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Schwartz D A.An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomesArch Pathol Lab Med2020. Doi: 10.5858/arpa.2020-0901-SA [DOI] [PubMed]
- 2.American College of Obstetricians and Gynecologists.Outpatient assessment and management for pregnant women with suspected or confirmed novel coronavirus (COVID-19)April 10, 2020. Available at:https://www.acog.org/-/media/project/acog/acogorg/files/pdfs/clinical-guidance/practice-advisory/covid-19-algorithm.pdf. Accessed April 16, 2020
- 3.et al. Management considerations for pregnant patients with COVID-19May 8, 2020. Available at:https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf. Accessed May 9, 2020
- 4.et al. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to knowApril 11, 2020. Available at:https://s3.amazonaws.com/cdn.smfm.org/media/2322/COVID19-What_MFMs_need_to_know_revision_4-11-20_(final)_highlighted_changes._PDF.pdf. Accessed April 16, 2020
- 5.et al. Labor and delivery COVID-19 considerationsApril 14, 2020. Available at:https://s3.amazonaws.com/cdn.smfm.org/media/2327/SMFM-SOAP_COVID_LD_Considerations_-_revision_4-14-20_-_changes_highlighted.pdf. Accessed April 16, 2020
- 6.Ranieri V M, Rubenfeld G D, Thompson B T et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco L D, Saad A F, Saade G.Early acute respiratory support for pregnant patients with coronavirus disease 2019 (COVID-19) infectionObstet Gynecol2020; Epub ahead of print [DOI] [PMC free article] [PubMed]
- 8.van Doremalen N, Bushmaker T, Morris D H et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim V S, Katz A I, Lindheimer M D. Acid-base regulation in pregnancy. Am J Physiol. 1976;231(06):1764–1769. doi: 10.1152/ajplegacy.1976.231.6.1764. [DOI] [PubMed] [Google Scholar]
- 10.Alhazzani W, Møller M H, Arabi Y Met al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19)Crit Care Med2020. Doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed]
- 11.Guérin C, Reignier J, Richard J C et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 12.Samanta S, Samanta S, Wig J, Baronia A K. How safe is the prone position in acute respiratory distress syndrome at late pregnancy? Am J Emerg Med. 2014;32(06):6870–687000. doi: 10.1016/j.ajem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Combes A, Hajage D, Capellier G et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 14.Saad A F, Rahman M, Maybauer D M et al. Extracorporeal membrane oxygenation in pregnant and postpartum women with H1N1-related acute respiratory distress syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2016;127(02):241–247. doi: 10.1097/AOG.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 15.Pacheco L D, Saade G R, Hankins G DV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42(01):21–25. doi: 10.1053/j.semperi.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Bhimraj A, Morgan R L, Shumaker A Het al. Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19April 11, 2020. Available at:https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed April 16, 2020 [DOI] [PMC free article] [PubMed]
- 17.Semler M W, Self W H, Wanderer J P et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(09):829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedemann H P, Wheeler A P, Bernard G R et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 19.Madjid M, Safavi-Naeini P, Solomon S D, Vardeny O.Potential effects of coronaviruses on the cardiovascular system: a reviewJAMA Cardiol2020. Doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed]
- 20.Leffert L, Butwick A, Carvalho B et al. The Society for Obstetric Anesthesia and Perinatology Consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126(03):928–944. doi: 10.1213/ANE.0000000000002530. [DOI] [PubMed] [Google Scholar]
- 21.Wichmann D, Sperhake J P, Lütgehetmann Met al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort studyAnn Intern Med2020. Doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed]
- 22.Thachil J, Tang N, Gando S et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(05):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowles L, Platton S, Yartey Net al. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19N Engl J Med2020. Doi: 10.1056/NEJMc2013656 [DOI] [PMC free article] [PubMed]
- 24.Jeejeebhoy F M, Zelop C M, Lipman S et al. Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation. 2015;132(18):1747–1773. doi: 10.1161/CIR.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 25.Hirshberg A, Kern-Goldberger A R, Levine L Det al. Care of critically ill pregnant patients with COVID-19: a case seriesAm J Obstet Gynecol2020. S0002-9378(20)30515-9 Doi: 10.1016/j.ajog.2020.04.029 [DOI] [PMC free article] [PubMed]
- 26.Baud D, Greub G, Favre Get al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infectionJAMA2020. Doi: 10.1001/jama.2020.7233 [DOI] [PMC free article] [PubMed]
- 27.Li J, Fink J, Ehrmann S.High-low nasal cannula for COVID-19 patients: low risk for bio-aerosol dispersionEur Respir J2020. Doi: 10.1183/13993003.00892-2020 [DOI] [PMC free article] [PubMed]


