Abstract
Background
Waardenburg syndrome is an uncommon genetic condition characterized by at least some degree of congenital hearing loss and pigmentation deficiencies. However, the genetic pathway affecting the development of stria vascularis is not fully illustrated.
Methods
The transcript profile of stria vascularis of Waardenburg syndrome was studied using Mitf-M mutant pig and mice models. Therefore, GO analysis was performed to identify the differential gene expression caused by Mitf-M mutation.
Results
There were 113 genes in tyrosine metabolism, melanin formation, and ion transportations showed significant changes in pig models and 191 genes in mice models. In addition, there were some spice's specific gene changes in the stria vascularis in the mouse and porcine models. The expression of tight junction-associated genes, including Cadm1, Cldn11, Pcdh1, Pcdh19, and Cdh24 genes, were significantly higher in porcine models compared to mouse models. Vascular-related and ion channel-related genes in the stria vascularis were also shown significantly difference between the two species. The expression of Col2a1, Col3a1, Col11a1, and Col11a2 genes were higher, and the expression of Col8a2, Cd34, and Ncam genes were lower in the porcine models compared to mouse models.
Conclusions
Our data suggests that there is a significant difference on the gene expression and function between these two models.
1. Background
Waardenburg syndrome (WS) is a rare genetic condition characterized by at least some degree of congenital hearing loss and pigmentation deficiencies [1]. WS has more than 20 mutations in the Mitf allele [2, 3], including Mitfmi-vga9, Mitfmi-bw, and Mitfmi-ce, which have been identified to cause hearing loss and changes in pigmentation. Although the mouse model is widely used in disease phenotypes and pathogenic mechanisms of deafness-related research [4–8], many shortcomings have also been found in studying human genetic diseases. As there is a tremendous revolutionary difference between mouse and human, it may cause a huge biological difference in anatomy, energy metabolism, and auditory perception [9, 10]. For example, the developmental patterns of auditory organs are different in mice and humans: human's hearing developed before birth while mouse's hearing did not fully developed until two weeks after birth [11]. Some studies [4, 12] found that human embryonic developmental diseases are difficult to be replicated in some of the mouse models. Therefore, different animal models, such as cattle [13], horses [14], dogs [15, 16], and pigs [17] were also necessary to be used to study genetic diseases. Pigs are precocial species with fully developed auditory system at birth. Recent studies also found the cochlear anatomy is very similar to human [18–21]. As pigs are large-scale animals with high reproductive efficiency and economical convenience, it is a good model for study auditory genetic diseases [22–24].
The stria vascularis plays an important role in maintaining the cochlear endolymphatic potentials (EP) which is essential for the mechanical electrical conduction of the hair cells [25–29]. The stria vascularis is composed with the macrophage-like melanocytes, which also called the intermediate cells [28, 30, 31]. The potassium ions in the Scala media are produced by the intermediate cells and several potassium channels and transporters in the lateral wall, such as KCNQ1/KCNE1, KCNQ4, KCNN2, KCNJ10, and SLC12A2, are also involved in maintaining the endolymphatic potentials [26, 32–34]. When malfunction of stria vascularis will result in hearing loss [35]. For example, Marcus [36] reported that Kcnj10 knockout can decrease EP value from +80 mV to 1 mV, and the K+ concentration decrease from 110 mM to 60 mM in their mouse models. In our previous studies, we found that Mitf-M knockout can decrease the EP to 18 mV in the mouse model [37]. In the Mitf knockout pig model, we found that the Mitf mutation caused the value of EP dropped from +78 mV to +3 mV, which was lower than the mouse model [18]. In the wild type pig, the potassium concentration in the endolymph was 142 mM higher than those in the perilymph. Our previous study found the potassium concentration dropped to 0 mM in the Mitf mutant pigs [18]. We expect that there may be different genes in maintaining the EP in mice and pigs, and the mutation of Mitf gene may cause a different change in potassium channels. To answer these questions, this study attempted to detect changes in the genetic profiles of these two species caused by Mitf-M gene mutation in RNA transcriptome level. As most of the current researches only use mouse models, this paper will further detect the RNA transcriptome difference in the stria vascularis between the large animals and mouse models.
2. Results
2.1. Gene Expression Changes Caused by Mitf-M Mutation
The activation of different genes in the stria vascularis of pigs and mice caused by the Mitf-M mutation compared to their W/T controls were screened using the DESeq package software. The conditions for screening differential genes were corrected p value <0.05. The intersections of the differential genes in the pigs and mice were obtained using the Venn diagrams. The results were shown in Table 1. There were 14 common differential genes between the Mitf mutant animals and the controls. There were 177 specific differential genes in mouse model and 99 specific differential genes in pig models (Figure 1).
Table 1.
The common DEGs in pigs/mice with/without Mitf-M mutation.
| Gene_Name | hom_pig | het_pig | MM_mouse | WW_mouse |
|---|---|---|---|---|
| Tyr | 0.014667 | 9.40833 | 0.00233 | 37.4824 |
| Emilin2 | 65.6574 | 36.3192 | 52.3576 | 20.7289 |
| Gsn | 199.663 | 289.825 | 64.9488 | 223.727 |
| Dct | 1.09829 | 135.912 | 0.00277 | 774.431 |
| Gpnmb | 2.22801 | 47.8019 | 1.14682 | 86.5487 |
| Ednrb | 1.19691 | 12.2565 | 1.66075 | 26.9099 |
| Ucma | 198.465 | 285.077 | 256.207 | 523.427 |
| Slc45a2 | 0.793729 | 24.5716 | 3.9E-07 | 45.1788 |
| Tspan10 | 3.93361 | 18.2708 | 0.036796 | 14.1373 |
| Clca2 | 68.3216 | 50.1806 | 1.59181 | 0.120315 |
| Kcnj10 | 4.4472 | 14.4644 | 16.594 | 111.93 |
| Trpm1 | 0.009755 | 14.7644 | 0.035483 | 6.75993 |
| Plp1 | 0.215656 | 4.79304 | 7.71325 | 34.9961 |
| Kcnj13 | 0.149383 | 14.6624 | 1.753 | 14.403 |
Figure 1.
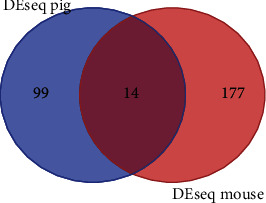
Venn diagram of DEGs in Mitf-M mutant and normal pigs/mice. The left circle represents the DEGs in the Mitf-M mutant and normal pigs; the right circle represents the DEGs in the Mitf-M mutant and normal mice. The middle part represents the DEGs in the Mitf-M mutant and normal pigs/mice.
The GO analysis and the KEGG pathway analysis were performed on the David Database (adjusted p value <0.05). The results were shown in Figure 2. The main pathway caused by Mitf mutation was the KEGG pathway, enriched in the tyrosine metabolism (mmu00350) and the melanogenesis pathway (Melanogenesis, mmu04916). The GO analysis was mainly enriched in the biological process of ion transport (ion transport, GO: 0006811) and the integral component of plasma membrane (GO: 0005887) (Figure 2).
Figure 2.
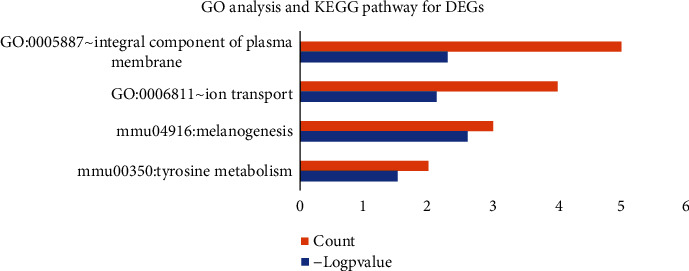
List of GO-terms with significant enrichment of DEGs. From top to bottom, the enrichment value decreases. The red X-axis indicates the number of unigenes in a category; the blue X-axis indicates the value of log2 (p value) in corresponding category. The Y-axis indicates the specific category.
2.2. Stria Vascularis Specific Ion Transport-Related Gene Analysis
Ion transport-related genes were extracted from RNA transcriptome data from the normal and Mitf-m mutant pigs and mice samples for cluster analysis. The results showed many ion transport-related genes were highly expressed in both species through MeV cluster analysis (Figure 3). The Mitf mutation was coaffected with Trpm1, Kcnj13, and Slc45a2 genes in both species. There were significant differences in ion channel regulation between pigs and mice. The expression of Kcnn1, Clcn2, and Trpm4 genes was higher in pigs than those genes in the mice, whereas the expression of Trpm7, Kcnq1, and Kcnj8 genes was found higher in mice compared to the pigs.
Figure 3.
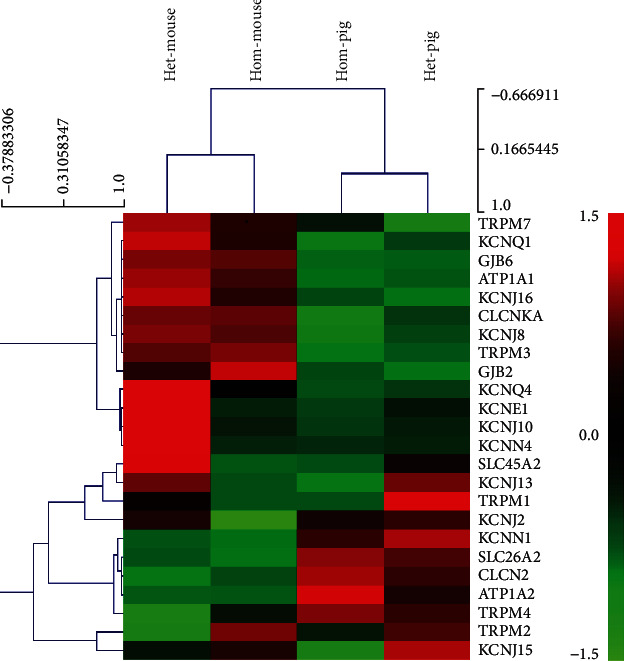
Ion channel relevant genes for cluster analysis heat map. Each column represents an experimental sample. Hom-mouse and het-mouse represent Mitf-M knockout mice and normal control mice. Hom-pig and het-pig represent Mitf-M mutant pigs and normal control pigs. Each row represents a gene. Different expressions are shown in different colors: red represents more expression and green represents less expression.
2.3. The Specific Tight Junction-Associated Genes in the Stria Vascularis
The tight junction-associated genes were extracted from the RNA transcriptome data from the Mitf mutant and normal pigs/mice for cluster analysis. The expression of tight junctions in the stria vascularis of the two species was different (Figure 4). The Cadm1, Cldn11, Pcdh1, Pcdh19, and Cdh24 genes expressed higher in pigs compared to those genes in mice, whereas Ncam, Cldn6, Cldn9, and Cldn14 genes expressed higher in mice compared to pigs. And it was found that both the structures of the stria vascularis in two groups were intact. As the marginal nuclei and the cell connections were intact. The three layers of cells were obvious, and the basal cells were closely connected (Figure 5).
Figure 4.
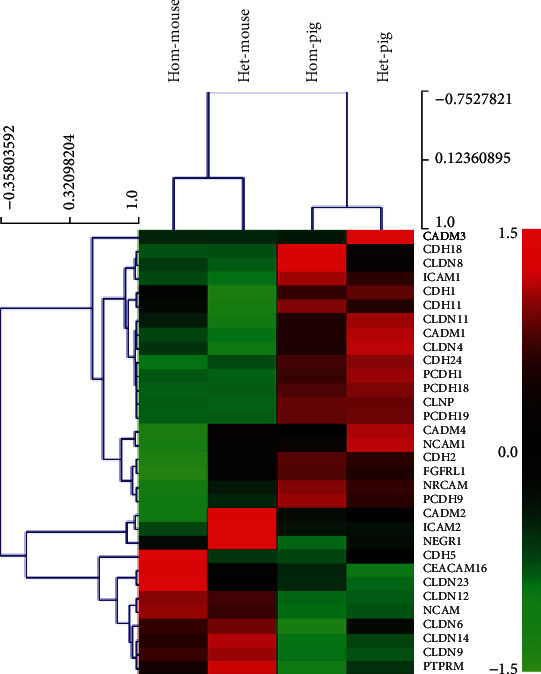
Tight junction relevant genes for cluster analysis heat map. Hom-mouse and het-mouse represent Mitf-M knockout mice and normal control mice. Hom-pig and het-pig represent Mitf-M mutant pigs and normal control pigs.
Figure 5.
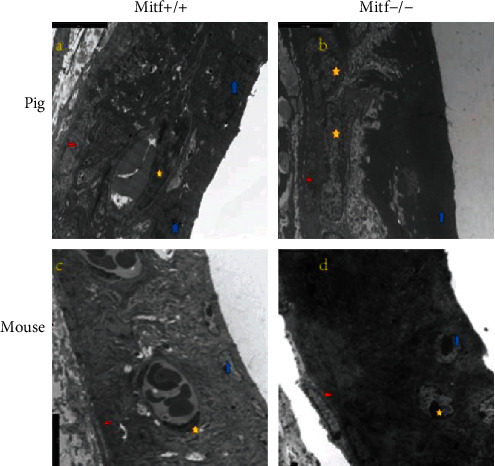
The SEM shows the cochlear stria vascularis of Mitf mutant and normal pigs/mice. (a) A normal pig's stria vascularis. (b) The stria vascularis from a mutant pig. (c) The stria vascularis from a normal mouse. (d) The stria vascularis from a mutant mouse. The red triangle marks the basal cells, the yellow pentagon marks the middle cells, and the blue arrow marks the marginal cells. The scale bar is 5 μm.
2.4. Stria Vascularis Specific Vascular Development-Related Genes
Extracted vascular development-related genes from the RNA transcriptome data of the Mitf mutant and normal pigs/mice were used for cluster analysis. There was a significant difference in the vascular developmental genes in the stria vascularis between these two species (Figure 6). The Col2a1, Col3a1, Col11a1, and Col11a2 genes expressed higher in the pigs than the mice, whereas the Col8a2, Cd34, and Ncam genes expressed higher in the mice compared to the pigs.
Figure 6.
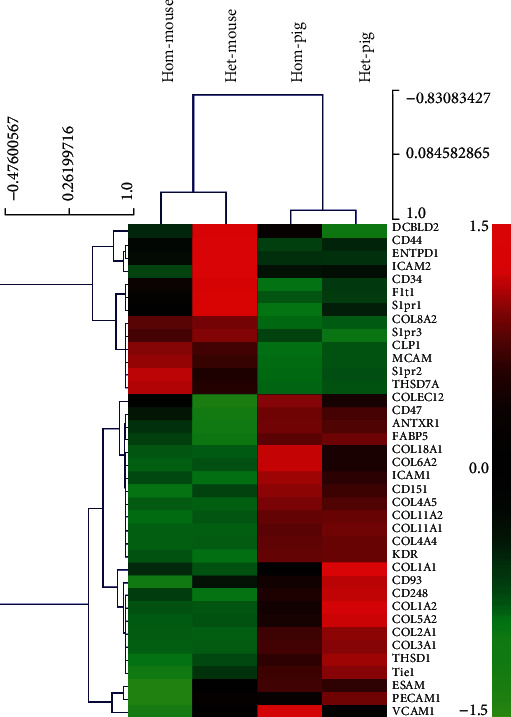
Heat analysis of clustering analysis of genes associated with specific vascular endothelial cell in normal and Mitf mutant pigs/mice (hom-mouse and het-mouse represent Mitf-M knockout mice and normal mice, hom-pig and het-pig represent Mitf-M mutant pigs and normal pigs).
3. Discussion
This study reviewed the changes of Mitf-M mutation on gene expression in the cochlea. Mitf has many subtypes [38], in which type M is specifically expressed in melanocytes [39], by direct association with related pigmentases such as tyrosinase (Tyr), dopachrome tautomerase (Dct), endothelin receptor type B (Ednrb), and solute carrier family 45 member 2 (Slc45a2), regulating the survival, migration, and differentiation of melanocytes [40]. Among them, Mitf-M gene [38, 39] plays a key role in regulating tyrosine metabolic pathway and melanin production, mainly regulating downstream pigment-related enzymes such as Tyr, Dct, and Tyrp1. Mitf-M also controls cytoskeleton and intercellular tight protein to regulate morphology and migration of melanocytes. In this study, we found the main gene pathway caused by the Mitf-M mutation is on ion transport pathway, including the tyrosine, acid metabolism, and melanin formation pathways, in the cochlear stria vascularis of both mice and pigs. Our data are consistent with previous reports [39].
Our previous studies reported that the Mitf-M gene mutation in the Waardenburg 2A pigs and mice through a deletion of the Mitf-M genes, which caused melanocytes failed to migrate to the cochlear stria vascularis. It can cause drops of EP and damages of cochlear hair cells. In this study, we also identified a significant decrease of the K+ channel-associated genes, i.e., Trpm1, Kcnj13, Slc45a2, and Kcnj10. From our RNA-seq sequencing analysis, Clcnka and Kcnj15 genes showed a significant difference in pig models, whereas the Kcnq4, Kcnn4, Kcne1, and Kcnj2 genes are affected mainly in the mouse models. KCNJ13 (KIR7.1) and KCNJ10 (KIR4.1) belong to the inward rectifier potassium channel category. KCNJ10 is known as the key channel of potassium transport. It has been deeply studied in deafness-related diseases, and its deletion can lead to the reduction of EP and potassium ion concentration [36, 41–45]. However, Kcnj13, Trpm1, and Slc45a2 were rarely reported in auditory researches. In addition, TRPM1 is a nonselective voltage-gated cation channel in the transient receptor potential (TRP) family, and the Mitf mutation can lead to the deletion of Trpm1 [46]. SLC45A2 is a cross-mediated melanin synthesis in membrane transporter [47], which is regulated by Mitf via the cAMP pathway through Tyr and Dct genes, the major pigment-related genes [48, 49].
The stria vascularis transcriptome data of the two species indicated that Mitf-M played an important role in regulating the expression of the Trpm1, Kcnj13, Slc45a2, and Kcnj10 genes in the stria vascularis. In both species, Mitf-M may play an important role in the auditory development and maintain the EP in the cochlea. Although there were huge biological differences between pigs and mice, we found that common gene changes in both species caused by Mitf-M. Mitf-M mutation induced a significant change in Clcn2, Kcnn1, and Trpm4 genes in the both models. CLCN2 [34] is an important component of chloride channel, which coordinates potassium and chloride exchanges. The function of KCNN1 has not been reported in the inner ears. KCNN1 belongs to the calcium ion-mediated potassium channels and plays an important role in the regulation of neural inflammation and nerve aging by microglia [50]. In mice, Kcnq1, Trpm7, and Kcnj8 were significantly affected. KCNQ1 is a calcium ion-dependent potassium channel [45]. When Kcnq1 is deleted, it will cause degeneration of the outer hair cells, which is clinically characterized as Jervell and Lange-Nielsen syndrome, one condition that causes profound hearing loss from birth and a disruption of the heart's normal rhythm. KCNE1 and KCNQ1 are important potassium-secreting channels in the stria vascularis marginal cells [26, 45, 51]. KCNE1 regulates KCNQ1 expression and increases ion transport [52].
The tight junctions and vascular endothelial cells are important components of the blood labyrinth barrier as well as ion channels [30, 53–57]. Our cluster analysis of the RNA transcriptome data from both pigs and mice showed that the tight junctions were significantly different in the stria vascularis of these two species. The expression of Cadm1, Cldn11, Pcdh1, Pcdh19, and Cdh24 was found higher in pigs compared to mice, whereas the expression of Ncam, Cldn6, Cldn9, and Cldn14 genes were higher in mice compared to pigs. Cluster analysis of vascular-related genes revealed that it was significantly different in the stria vascularis of the two species. The higher expression in pigs is Col2a1, Col3a1, Col11a1, and Col11a2, whereas the expression of Col8a2, Cd34, and Ncam genes was higher in mice compared to pigs. These results may reveal that the two animals may invoke different genes to regulate the tight junction, just as the ion channels. The differences between the two species' evolutionary relationship, living habits, and anatomy may result in significant differences in these gene expressions [56, 58, 59]. It is more suitable to choose animal model closer to humans to study auditory related diseases.
In summary, we show that by leveraging RNA-seq for the analysis of the stria vascularis of the WS models, it helps to understand the regulatory mechanisms related to the loss of EP and deafness. These data provide insight into ion channel-defining genes and illustrate the possible genes associated with the WS hearing loss. These results may make a fundamental effect on the gene therapy, which used to rescue the elapse of the endocochlear potential in the stria vascularis.
4. Conclusion
Our research reveals that there exists a huge difference on the gene expression and function between these two models. According to the different expression in the genetic profiles of these two species caused by Mitf-M gene mutation in RNA transcriptome level, there may be different genes transcript pathway caused by mitf mutation in regulating the potassium channels in mice and pigs. And this transcriptome data may provide a basis for the gene therapy in treating the Waardenburg syndrome.
5. Material and Methods
5.1. Animals
Both Mitf mutant and normal pigs and mice have been used in this experiment. The generation of the Mitf mutant pigs and mice have been described in our previous publications [17, 37]. The experimental protocols were approved by the ethics committee of the Chinese PLA Medical School.
5.2. RNA Isolation from Stria Vascularis Tissue
Tissues of the stria vascularis of pigs were obtained from four normal pigs and four Mitf mutant pigs at E85 of embryonic stage as previous study described [17]. The tissues of stria vascularis of mice were obtained from ten normal mice and ten Mitf mutant mice at postnatal 30 days as previous studies described [7, 37]. The total RNA of these tissues was extracted separately using Trizol reagent (Invitrogen, CA, USA) following the manufacturer's protocol. The quantity, purity, and integrity of the collected total RNA were analyzed with NanoPhotometer® spectrophotometer (IMPLEN, CA, USA), a Bioanalyzer 2100, and RNA Nano 6000 Assay Kit (Agilent, CA, USA). Approximately, 4 μg of total RNA was used for the RNA sample preparations as previous studies described [60, 61].
5.3. Library Construction and Sequencing
The NEBNext® Ultra TM RNA Library Prep Kit for Illumina® (NEB, USA) was used for the sequencing library preparation, which was conducted with an Illumina HiSeq TM 2000 system following the manufacturer's recommended protocol (Illumina Company Ltd, San Diego, CA, USA) as previous studies described [61, 62].
5.4. RNA-Seq Reads Mapping
The reference genome and gene model annotation files were obtained from Genome Web (http://asia.ensembl.org/index.html). The index of the reference genome was built using Hisat2 software (v2.0.5), and the paired-end clean reads were aligned to the reference genome. A database of potential splice junctions was built and confirmed by comparing the previously unmapped reads against the database of putative junctions. The aligned read files were processed by Cufflinks software, which used the normalized RNA-seq fragment counts to measure the relative abundances of the transcriptome. The unit of measurement was fragmented per kilobase of exons per million fragments mapped (FPKM). Reads were mapped into the mouse NCBIM38 (ensemble release 68) and the Sus scrofa 11.1 (sus scrofa ensembl release 94) using default options.
5.5. Gene Ontology (GO) and Pathway Enrichment Analysis of DEGs
Differential expression analysis of Mitf-M mutant and normal pigs/mice were performed using the DEseq R package [63]. Using the adjusted p values 0.05 and setting the absolute fold change of 2 as the threshold for significantly differential expression. Using Gene Ontology (GO) and KEGG to analyze high-throughput genome and transcriptome data in the DAVID database [64–68], which is an important online tool for these analyses. The DEGs list was uploaded to the DAVID [64] analysis tool, and p < 0.05 was considered statistically significant. The DEGs was uploaded to the MeV software (https://sourceforge.net/projects/mev-tm4/) to get the relevant heat map.
5.6. Selecting Deafness Gene of SV Transcriptomes from RNA-Seq Data
In obtaining our data for known deafness genes, we used a database of known deafness genes from these sources: (1) Hereditary Hearing Loss homepage [69] and (2) Hereditary hearing loss and deafness overview [27, 45].
5.7. The Transmission Electron Microscopy (TEM)
To prepare samples for TEM examination, the stria vascularis were washed with 0.1 M PBS and then postfixed in 1% osmium tetroxide and then dehydrated by a series of ethanol before embedded in plastic Agar 100 resin. After polymerization, the stria vascularis was cut into ultrathin sections (3 μm), stained with toluidine blue, were mounted on 0.7% formvar coated copper grids, contrasted by 0.5% uranyl acetate and lead citrate, then examined under a transmission electron microscopy (Philips Tecnai10) [70].
Acknowledgments
This research was supported by grants from the National Science Foundation of China (81470683, 81770992 to Weiju Han and 81670940 to Shiming Yang). Neither the entire paper nor any part of its contents has been published or has been accepted elsewhere.
Contributor Information
Hui Zhao, Email: huizhao301ent@163.com.
Weiju Han, Email: hanweiju@aliyun.com.
Shiming Yang, Email: shm_yang@163.com.
Data Availability
Readers can access additional experimental data in optional supplementary materials.
Conflicts of Interest
The authors declare no competing financial interests.
Authors' Contributions
Linjun Chen and Yi Wang contributed equally to this work.
Supplementary Materials
Deseq GO Analysis. Pig and mouse DEG.
References
- 1.Song J., Feng Y., Acke F. R., Coucke P., Vleminckx K., Dhooge I. J. Hearing loss in Waardenburg syndrome: a systematic review. Clinical Genetics. 2016;89(4):416–425. doi: 10.1111/cge.12631. [DOI] [PubMed] [Google Scholar]
- 2.Hai T., Guo W., Yao J., et al. Creation of miniature pig model of human Waardenburg syndrome type 2A by ENU mutagenesis. Human Genetics. 2017;136(11-12):1463–1475. doi: 10.1007/s00439-017-1851-2. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe A., Takeda K., Ploplis B., Tachibana M. Epistatic relationship between Waardenburg Syndrome genes MITF and PAX3. Nature Genetics. 1998;18(3):283–286. doi: 10.1038/ng0398-283. [DOI] [PubMed] [Google Scholar]
- 4.Ohlemiller K. K., Jones S. M., Johnson K. R. Application of mouse models to research in hearing and balance. Journal of the Association for Research in Otolaryngology. 2016;17(6):493–523. doi: 10.1007/s10162-016-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omichi R., Shibata S. B., Morton C. C., Smith R. J. H. Gene therapy for hearing loss. Human molecular genetics. 2019;28(R1):R65–R79. doi: 10.1093/hmg/ddz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X., Tao Y., Lamas V., et al. Treatment of autosomal dominant hearing loss by _in vivo_ delivery of genome editing agents. Nature. 2018;553(7687):217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan F., Chu C., Qi J., et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nature Communications. 2019;10(1):p. 3733. doi: 10.1038/s41467-019-11687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan B., Askew C., Galvin A., et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nature Biotechnology. 2017;35(3):264–272. doi: 10.1038/nbt.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D. Evolution and Restoration of Structures and Functions of the Human Central Nervous System—A Review. Journal of Neurorestoratology. 2015;1(1):60–70. doi: 10.18679/cn11-6030_r.2015.007. [DOI] [Google Scholar]
- 10.Müller U., Barr-Gillespie P. G. New treatment options for hearing loss. Nature Reviews Drug Discovery. 2015;14(5):346–365. doi: 10.1038/nrd4533. [DOI] [PubMed] [Google Scholar]
- 11.Tritsch N. X., Bergles D. E. Developmental regulation of spontaneous activity in the mammalian cochlea. The Journal of Neuroscience. 2010;30(4):1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bommakanti K., Iyer J. S., Stankovic K. M. Cochlear histopathology in human genetic hearing loss: State of the science and future prospects. Hearing Research. 2019;382:p. 107785. doi: 10.1016/j.heares.2019.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philipp U., Lupp B., Mömke S., et al. PLoS ONE. 12. Vol. 6. e28857; 2011. A MITF mutation associated with a dominant white phenotype and bilateral deafness in German Fleckvieh cattle; p. p. e28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauswirth R., Haase B., Blatter M., et al. Mutations in MITF and PAX3 cause "splashed white" and other white spotting phenotypes in horses. PLoS Genetics. 2012;8(4):p. e1002653. doi: 10.1371/journal.pgen.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchida S., Takizawa T., Abe K., Okamoto M., Tagawa M. Identification of microphthalmia-associated transcription factor isoforms in dogs. Veterinary Journal. 2009;182(2):283–293. doi: 10.1016/j.tvjl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Körberg I. B., Sundström E., Meadows J. R. S., et al. A simple repeat polymorphism in the MITF-M promoter is a key regulator of white spotting in dogs. PLoS ONE. 2014;9(8):p. e104363. doi: 10.1371/journal.pone.0104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Guo W., Ren L., et al. A de novo silencer causes elimination of MITF-M expression and profound hearing loss in pigs. BMC Biology. 2016;14(1) doi: 10.1186/s12915-016-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W., Yi H., Ren L., et al. The Morphology and Electrophysiology of the Cochlea of the Miniature Pig. The Anatomical Record. 2015;298(3):494–500. doi: 10.1002/ar.23095. [DOI] [PubMed] [Google Scholar]
- 19.Du Y., Ren L.-l., Jiang Q.-q., et al. Degeneration of saccular hair cells caused by MITF gene mutation. Neural Development. 2019;14(1):p. 1. doi: 10.1186/s13064-019-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao Q.-Q., Li L., Chen W., et al. Key Genes and Pathways Associated With Inner Ear Malformation in SOX10 p.R109W Mutation Pigs. Frontiers in Molecular Neuroscience. 2018;11 doi: 10.3389/fnmol.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi H., Guo W., Chen W., Chen L., Ye J., Yang S. Miniature pigs: a large animal model of cochlear implantation. American Journal of Translational Research. 2016;8(12):5494–5502. [PMC free article] [PubMed] [Google Scholar]
- 22.Ji X.-J., Chen W., Wang X., et al. Canalostomy is an ideal surgery route for inner ear gene delivery in big animal model. Acta Oto-Laryngologica. 2019;139(11):939–947. doi: 10.1080/00016489.2019.1654130. [DOI] [PubMed] [Google Scholar]
- 23.An F.‐. W., Yuan H., Guo W., et al. Establishment of a Large Animal Model for Eustachian Tube Functional Study in Miniature Pigs. The Anatomical Record. 2018;302(6):1024–1038. doi: 10.1002/ar.24098. [DOI] [PubMed] [Google Scholar]
- 24.Lovell J. M., Harper G. M. The morphology of the inner ear from the domestic pig Sus scrofa. Journal of Microscopy. 2007;228(3):345–357. doi: 10.1111/j.1365-2818.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 25.Dror A. A., Avraham K. B. Hearing Impairment: A Panoply of Genes and Functions. Neuron. 2010;68(2):293–308. doi: 10.1016/j.neuron.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Korrapati S., Taukulis I., Olszewski R., et al. Single Cell and Single Nucleus RNA-Seq Reveal Cellular Heterogeneity and Homeostatic Regulatory Networks in Adult Mouse Stria Vascularis. Frontiers in molecular neuroscience. 2019;12:p. 316. doi: 10.3389/fnmol.2019.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uetsuka S., Ogata G., Nagamori S., et al. Molecular architecture of the stria vascularis membrane transport system, which is essential for physiological functions of the mammalian cochlea. The European Journal of Neuroscience. 2015;42(3):1984–2002. doi: 10.1111/ejn.12973. [DOI] [PubMed] [Google Scholar]
- 28.Nyberg S., Abbott N. J., Shi X., Steyger P. S., Dabdoub A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Science Translational Medicine. 2019;11(482):p. eaao0935. doi: 10.1126/scitranslmed.aao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Chen L., Giffen K. P., et al. Cell-Specific Transcriptome Analysis Shows That Adult Pillar and Deiters' Cells Express Genes Encoding Machinery for Specializations of Cochlear Hair Cells. Frontiers in Molecular Neuroscience. 2018;11 doi: 10.3389/fnmol.2018.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review) Hearing Research. 2016;338:52–63. doi: 10.1016/j.heares.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen P., Chai Y., Liu H., et al. Postnatal Development of Microglia-Like Cells in Mouse Cochlea. Neural Plasticity. 2018;2018:5. doi: 10.1155/2018/1970150.1970150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zdebik A. A., Wangemann P., Jentsch T. J. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology. 2009;24(5):307–316. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Z., Uhlen I., Wei-Jia K., Mao-li D. Cochlear homeostasis and its role in genetic deafness. Journal of Otology. 2009;4(1):15–22. doi: 10.1016/s1672-2930(09)50003-7. [DOI] [Google Scholar]
- 34.Lang F., Vallon V., Knipper M., Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. American Journal of Physiology-Cell Physiology. 2007;293(4):C1187–C1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- 35.Egilmez O. K., Kalcioglu M. T. Genetics of Nonsyndromic Congenital Hearing Loss. Scientifica. 2016;2016:9. doi: 10.1155/2016/7576064.7576064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus D. C., Wu T., Wangemann P., Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. American Journal of Physiology-Cell Physiology. 2002;282(2):C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 37.Liu H., Li Y., Chen L., et al. Organ of Corti and Stria Vascularis: Is there an Interdependence for Survival? PLoS One. 2016;11(12):p. e0168953. doi: 10.1371/journal.pone.0168953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goding C. R., Arnheiter H. MITF-the first 25 years. Genes & Development. 2019;33(15-16):983–1007. doi: 10.1101/gad.324657.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T., Zhao B., Liu Y., et al. MITF-M regulates melanogenesis in mouse melanocytes. Journal of Dermatological Science. 2018;90(3):253–262. doi: 10.1016/j.jdermsci.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Michael H. T., Graff-Cherry C., Chin S., et al. Partial Rescue of Ocular Pigment Cells and Structure by Inducible Ectopic Expression of Mitf-M in MITF-Deficient Mice. Investigative Opthalmology & Visual Science. 2018;59(15):6067–6073. doi: 10.1167/iovs.18-25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locher H., de Groot J. C. M. J., van Iperen L., Huisman M. A., Frijns J. H. M., Chuva de Sousa Lopes S. M. Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Developmental Neurobiology. 2015;75(11):1219–1240. doi: 10.1002/dneu.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J., Zhao H. B. The role of an inwardly rectifying K+ channel (Kir4.1) in the inner ear and hearing loss. Neuroscience. 2014;265:137–146. doi: 10.1016/j.neuroscience.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H., Xiong H., Huang Q., et al. Compromised potassium recycling in the cochlea contributes to conservation of endocochlear potential in a mouse model of age-related hearing loss. Neuroscience Letters. 2013;555:97–101. doi: 10.1016/j.neulet.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 44.Wangemann P., Itza E. M., Albrecht B., et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Medicine. 2004;2(1) doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittal R., Aranke M., Debs L. H., et al. Indispensable role of ion channels and transporters in the auditory system. Journal of Cellular Physiology. 2017;232(4):743–758. doi: 10.1002/jcp.25631. [DOI] [PubMed] [Google Scholar]
- 46.Miller A. J., Du J., Rowan S., Hershey C. L., Widlund H. R., Fisher D. E. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Research. 2004;64(2):509–516. doi: 10.1158/0008-5472.can-03-2440. [DOI] [PubMed] [Google Scholar]
- 47.Inagaki K., Suzuki T., Ito S., et al. Oculocutaneous albinism type 4: six novel mutations in the membrane-associated transporter protein gene and their phenotypes. Pigment Cell Research. 2006;19(5):451–453. doi: 10.1111/j.1600-0749.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoek K. S., Schlegel N. C., Eichhoff O. M., et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell & Melanoma Research. 2008;21(6):665–676. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 49.Du J., Fisher D. E. Identification ofAim-1as theunderwhiteMouse Mutant and Its Transcriptional Regulation by MITF. Journal of Biological Chemistry. 2001;277(1):402–406. doi: 10.1074/jbc.m110229200. [DOI] [PubMed] [Google Scholar]
- 50.Dolga A. M., Culmsee C. Protective roles for potassium SK/KCa2 channels in microglia and neurons. Frontiers in Pharmacology. 2012;3 doi: 10.3389/fphar.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilms V., Köppl C., Söffgen C., Hartmann A.-M., Nothwang H. G. Molecular bases of K+ secretory cells in the inner ear: shared and distinct features between birds and mammals. Scientific Reports. 2016;6(1) doi: 10.1038/srep34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strutz-Seebohm N., Seebohm G., Fedorenko O., et al. Functional Coassembly of KCNQ4 with KCNE-ß- Subunits in Xenopus Oocytes. Cellular Physiology and Biochemistry. 2006;18(1-3):57–66. doi: 10.1159/000095158. [DOI] [PubMed] [Google Scholar]
- 53.Bartle E. I., Rao T. C., Urner T. M., Mattheyses A. L. Bridging the gap: Super-resolution microscopy of epithelial cell junctions. Tissue Barriers. 2017;6(1):p. e1404189. doi: 10.1080/21688370.2017.1404189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W., Schrott-Fischer A., Glueckert R., Benav H., Rask-Andersen H. The Human "Cochlear Battery" - Claudin-11 Barrier and Ion Transport Proteins in the Lateral Wall of the Cochlea. Frontiers in Molecular Neuroscience. 2017;10 doi: 10.3389/fnmol.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitajiri S., Katsuno T., Sasaki H., Ito J., Furuse M., Tsukita S. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biology Open. 2014;3(8):759–766. doi: 10.1242/bio.20147799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2008;1778(3):794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y., Qi J., Chen X., et al. Critical role of spectrin in hearing development and deafness. Science Advances. 2019;5(4) doi: 10.1126/sciadv.aav7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goncharov N. V., Nadeev A. D., Jenkins R. O., Avdonin P. V. Markers and biomarkers of endothelium: when something is rotten in the state. Oxidative Medicine and Cellular Longevity. 2017;2017:27. doi: 10.1155/2017/9759735.9759735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trune D. R. Ion homeostasis in the ear: mechanisms, maladies, and management. Current Opinion in Otolaryngology & Head and Neck Surgery. 2010;18(5):413–419. doi: 10.1097/MOO.0b013e32833d9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Guo L., Lu X., et al. Characterization of Lgr6+ Cells as an Enriched Population of Hair Cell Progenitors Compared to Lgr5+ Cells for Hair Cell Generation in the Neonatal Mouse Cochlea. Frontiers in Molecular Neuroscience. 2018;11 doi: 10.3389/fnmol.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S., Zhang Y., Yu P., et al. Characterization of Lgr5+ progenitor cell transcriptomes after neomycin injury in the neonatal mouse cochlea. Frontiers in Molecular Neuroscience. 2017;10:p. 213. doi: 10.3389/fnmol.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng C., Guo L., Lu L., et al. Characterization of the transcriptomes of Lgr5+ hair cell progenitors and Lgr5- supporting cells in the mouse cochlea. Frontiers in Molecular Neuroscience. 2017;10 doi: 10.3389/fnmol.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta R., Dewan I., Bharti R., Bhattacharya A. Differential expression analysis for RNA-Seq data. ISRN Bioinformatics. 2012;2012:8. doi: 10.5402/2012/817508.817508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang D. W., Sherman B. T., Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 65.Dennis G., Sherman B. T., Hosack D. A., et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biology. 2003;4(9) doi: 10.1186/gb-2003-4-9-r60. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S., Zhang Y., Dong Y., et al. Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea. Cellular and Molecular Life Sciences. 2020;77(7):1401–1419. doi: 10.1007/s00018-019-03291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang M., Li J., He L., et al. Transcriptomic profiling of neural stem cell differentiation on graphene substrates. Colloids and Surfaces B: Biointerfaces. 2019;182:p. 110324. doi: 10.1016/j.colsurfb.2019.06.054. [DOI] [PubMed] [Google Scholar]
- 68.Cheng C., Wang Y., Guo L., et al. Age-related transcriptome changes in Sox2+ supporting cells in the mouse cochlea. Stem Cell Research & Therapy. 2019;10(1):p. 365. doi: 10.1186/s13287-019-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Camp G., Smith R. 2015;18 Hereditary Hearing Loss Home Page Available at: http://hereditaryhearingloss.org. [Google Scholar]
- 70.He Z., Guo L., Shu Y., et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy. 2017;13(11):1884–1904. doi: 10.1080/15548627.2017.1359449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Deseq GO Analysis. Pig and mouse DEG.
Data Availability Statement
Readers can access additional experimental data in optional supplementary materials.


