Abstract
Purpose
The current COVID-19 pandemic is transforming our urologic practice and most urologic societies recommend to defer any surgical treatment for prostate cancer (PCa) patients. It is unclear whether a delay between diagnosis and surgical management (i.e., surgical delay) may have a detrimental effect on oncologic outcomes of PCa patients. The aim of the study was to assess the impact of surgical delay on oncologic outcomes.
Methods
Data of 926 men undergoing radical prostatectomy across Europe for intermediate and high-risk PCa according to EAU classification were identified. Multivariable analysis using binary logistic regression and Cox proportional hazard model tested association between surgical delay and upgrading on final pathology, lymph-node invasion (LNI), pathological locally advanced disease (pT3–4 and/or pN1), need for adjuvant therapy, and biochemical recurrence. Kaplan–Meier analysis was used to estimate BCR-free survival after surgery as a function of surgical delay using a 3 month cut-off.
Results
Median follow-up and surgical delay were 26 months (IQR 10–40) and 3 months (IQR 2–5), respectively. We did not find any significant association between surgical delay and oncologic outcomes when adjusted to pre- and post-operative variables. The lack of such association was observed across EAU risk categories.
Conclusion
Delay of several months did not appear to adversely impact oncologic results for intermediate and high-risk PCa, and support an attitude of deferring surgery in line with the current recommendation of urologic societies.
Keywords: Prostate cancer, Delay, COVID-19, Biochemical recurrence, Oncologic outcomes
Introduction
The COVID-19 pandemic is raging worldwide, with a consequent major modification of our clinical practice as urologists [1]. This is an unprecedented scenario to which our society was not prepared. While the majority of oncologic surgeries have maintained priority worldwide, most urologic associations have recommended to defer prostate cancer (PCa) surgeries [2–4]. This is based on several retrospective studies reporting the absence of poor oncologic results for PCa no matter the delay between diagnosis and surgery, although high-risk patients could be potentially at risk of higher risk of biochemical recurrence when the surgery was delayed [5–12].
A safe and efficacious timing of surgery for PCa is relevant also outside the context of the current pandemic: it is within the patient’s right to be fully informed of the repercussions of delaying definitive surgical treatment. In fact, many patients seek a second opinion, make own research, and need family reconciliation before taking a decision on treatment [13–15]. Moreover, the diagnostic pathway has evolved with time-consuming supplementary exams and imaging modalities (i.e., genetic assessment, multiparametric magnetic resonance imaging, and PET/CT) which are not readily accessible and could impact treatment delay [16].
We herein analyze a large contemporary series of patients harboring intermediate and high-risk PCa according to EAU classification diagnosed on MRI-targeted biopsy and operated across Europe with various delays, exploring the impact of time to surgery on oncologic results.
Materials and methods
After obtaining institutional review board approval, data were retrospectively gathered between March 2012 and September 2019 on 1139 patients undergoing radical prostatectomy for localized intermediate- and high-risk PCa according to EAU classification across Europeans centers (Belgium, France, Switzerland, and Italy) [17]. All patients had prebiopsy positive mp-MRI (PI-RADS score ≥ 3) followed by MRI/US fusion targeted and systematic biopsies using the KOELIS system (KOELIS, La Tronche, France). No patient received neoadjuvant therapy in the 12 months preceding surgery. Pelvic lymph-node dissection was conducted at the surgeon discretion according to preoperative assessment of lymph-node invasion (LNI)-risk (generally with Briganti 2012 or MSKCC nomograms), including the same lymph-node template in all patients. Adjuvant therapy was administered to patients with adverse pathologic features within 6 months from prostatectomy after multi-disciplinary oncologic meeting. Biochemical recurrence (BCR) was defined as two consecutive PSA of ≥ 0.2 ng/ml after undetectable values. Patients were categorized according to EAU risk categories and time between diagnosis on biopsy and surgery (i.e., surgical delay) was tested as a continuous variable [18]. Multivariable logistic regressions were performed to explore potential associations between surgical delay and upgrading on final pathology, LNI, pathological locally advanced disease (pT3–4 and/or pN1), and need for adjuvant therapy. Similarly, multivariable analysis using Cox proportional hazards model was performed to test association between surgical delay and risk of BCR. A 3 month delay was previously described as a potential cut-off for conducting surgery with a higher risk of BCR rates described [5, 9]. Therefore, Kaplan–Meier curves and log-rank test were conducted to estimate BCR-free survival as a function of surgical delay using this cut-off.
Results
Overall, 926 patients with complete data were included in the final analysis. General characteristics of the overall cohort and among EAU risk categories are available in Table 1. Median time between diagnosis and surgery was 3 months (2–5) and distribution of this delay is shown in Fig. 1. Median preoperative PSA was 8.2 ng/ml (5–12) and pathologic analysis revealed pT2, pT3a, and pT3b-4 stages in 53.9% (499/926), 30.8% (285/926), and 15.2% (141/926) respectively. Upgrading at final pathology was present in 22.7% (210/926), LNI in 9.9% (92/926), and pathological locally advanced disease in 46.9% (434/926), and BCR was detected in 8.7% (81/926) of patients.
Table 1.
Baseline demographic and pathological characteristics
| Variable | Overall | Intermediate risk | High risk | p* |
|---|---|---|---|---|
| (n = 926) | (n = 623) | (n = 303) | ||
| Median age at surgery, year (IQR) | 66 (61–70) | 65 (60–70) | 62 (57–70) | 0.001 |
| Median preoperative PSA, ng/ml (IQR) | 8.2 (5–12) | 7.7 (5.6–10.9) | 10 (7–20) | < 0.001 |
| Clinical stage at DRE, n (%) | ||||
| T1 | 527 (56.9) | 386 (62) | 141 (46.5) | < 0.001 |
| T2 | 351 (37.9) | 213 (34.2) | 138 (45.5) | |
| T3 | 18 (1.9) | 0 (0) | 18 (5.9) | |
| Unknown | 30 (3.2) | 24 (3.8) | 6 (2) | |
| PI-RADS score of index lesion, n (%) | ||||
| 3 | 117 (12.6) | 98 (15.7) | 19 (6.3) | < 0.001 |
| 4 | 440 (47.5) | 323 (51.8) | 117 (38.6) | |
| 5 | 345 (37.3) | 186 (29.8) | 159 (52.5) | |
| Unknown | 24 (2.6) | 16 (2.6) | 8 (2.6) | |
| Median maximum lesion diameter, mm (IQR) | 12 (10–16) | 11 (9–15) | 14 (11–20) | < 0.001 |
| Clinical stage on MRI, n (%) | 6 | |||
| T2 | 750 (81) | 611 (98.1) | 139 (45.9) | < 0.001 |
| T3a | 116 (12.5) | 0 (0) | 116 (38.3) | |
| T3b | 37 (4) | 0 (0) | 37 (12.2) | |
| Unknown | 23 (2.5) | 12 (1.9) | 11 (3.6) | |
| Median no. of systematic cores taken, n (IQR) | 12 (9–13) | 11 (8–12) | 12 (10–14) | 0.1 |
| Median no. of positive systematic cores taken, n (IQR) | 3 (1–5) | 2 (1–4) | 4 (1–6) | < 0.001 |
| ISUP grade group on systematic biopsy, n (%) | ||||
| 0 | 127 (13.7) | 83 (13.3) | 44 (14.5) | < 0.001 |
| 1 | 166 (17.9) | 125 (20.1) | 41 (13.5) | |
| 2 | 359 (38.8) | 292 (46.9) | 67 (22.1) | |
| 3 | 148 (16) | 100 (16) | 48 (15.8) | |
| 4 | 78 (8.4) | 8 (1.3) | 70 (23.1) | |
| 5 | 30 (3.2) | 0 (0) | 30 (10) | |
| Unknown | 18 (1.9) | 15 (2.4) | 3 (1) | |
| Median no. of targeted cores taken, n (IQR) | 4 (2–6) | 4 (2–6) | 4 (2–5) | 0.03 |
| Median no. of positive targeted cores taken, n (IQR) | 2 (2–4) | 2 (1–4) | 2 (2–4) | < 0.001 |
| ISUP grade group on targeted biopsy, n (%) | ||||
| 0 | 83 (9) | 67 (10.7) | 16 (5.3) | < 0.001 |
| 1 | 82 (8.8) | 63 (10.1) | 19 (6.3) | |
| 2 | 402 (43.4) | 333 (53.4) | 69 (22.8) | |
| 3 | 215 (23.1) | 154 (24.7) | 61 (20.1) | |
| 4 | 104 (11.2) | 6 (1) | 98 (32.3) | |
| 5 | 40 (4.3) | 0 (0) | 40 (13.2) | |
| ISUP grade group on final specimen, n (%) | ||||
| 1 | 29 (3.1) | 25 (4) | 4 (1.3) | < 0.001 |
| 2 | 432 (46.6) | 352 (56.5) | 80 (26.4) | |
| 3 | 347 (37.5) | 225 (36.1) | 122 (40.3) | |
| 4 | 56 (6) | 14 (2.2) | 42 (13.9) | |
| 5 | 62 (6.7) | 7 (1.1) | 55 (18.1) | |
| Median time between diagnosis and surgery, mo (IQR) | 3.3 (2.3–4.7) | 3.5 (2.4–5) | 2.8 (1.9–4.1) | < 0.001 |
| Pathologic stage, n (%) | ||||
| pT2 | 499 (53.9) | 402 (64.5) | 97 (32) | < 0.001 |
| pT3a | 285 (30.8) | 166 (26.6) | 119 (39.3) | |
| pT3b | 141 (15.2) | 55 (0.9) | 86 (28.4) | |
| pT4 | 1 (0) | 0 (0) | 1 (0.3) | |
| Positive surgical margin, n (%) | 230 (24.8) | 128 (20.5) | 102 (33.7) | < 0.001 |
| Median no. of lymph node removed, n (IQR) | 13 (8–18) | 13 (8–17) | 14 (9–19) | 0.006 |
| Lymph-node status, n (%) | ||||
| N0/Nx | 834 (90.1) | 595 (95.5) | 239 (78.9) | < 0.001 |
| N1 | 92 (9.9) | 28 (4.5) | 64 (21.1) | |
| Upgrading at final pathology, n (%) | 210 (22.7) | 150 (24.1) | 60 (19.8) | < 0.001 |
| Locally advanced disease at final pathologya, n (%) | 434 (46.9) | 226 (36.3) | 208 (68.6) | < 0.001 |
| Biochemical recurrenceb, n (%) | 81 (8.7) | 42 (6.7) | 39 (12.9) | < 0.001 |
| Median follow-up from RP, mo (IQR) | 26.4 (10–39.7) | 26.2 (10–39) | 26 (10–41.1) | 0.8 |
Pathological locally advanced = ≥ pT3 and/or pN1
Upgrading = higher ISUP GG at final pathology
BCR = two consecutive PSA > 0.2 ng/ml
IQR interquartile range, PSA prostate-specific antigen, DRE digital rectal examination, MRI magnetic resonance imaging, data PI-RADS prostate imaging-reporting and system, RP radical prostatectomy, ISUP International Society of Urological Pathology, BCR biochemical recurrence
aDefined as ≥ pT3 and/or pN1
bDefined as two consecutive PSA ≥ 0.2 ng/ml after undetectable values
*Kruskal–Wallis and Chi-square tests for continuous and categorical data, respectively
Fig. 1.
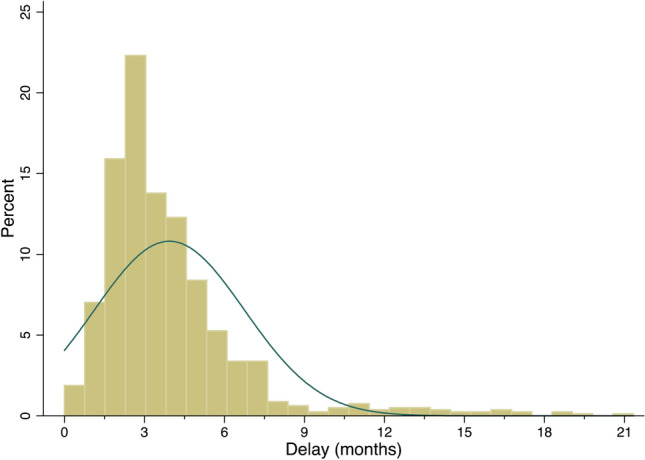
Histogram of the interval of time from diagnosis to surgery
On multivariable analysis (Table 2), surgical delay was not significantly associated with upgrading on final specimen (OR 0.98, 95% CI 0.94–1.02, p = 0.3), nor to LNI (OR 0.88, 95% CI 0.77–1.01, p = 0.07), pathological locally advanced disease (OR 1, 95% CI 0.97–1.03, p = 0.8), or need for adjuvant therapy (OR 0.96, 95%CI 0.84–1.11, p = 0.6). The absence of impact of surgical delay on such pathologic outcomes was maintained in EAU risk categories. When exploring the impact of surgical delay on BCR (HR 0.97, 95% CI 0.91–1.04, p = 0.6), we did not detect any significant association across both the overall population and within the risk groups. No significant different in terms of BCR-free survival was found when a 3 month cut-off was used (all log-rank test p > 0.5) (Fig. 2).
Table 2.
Multivariable analysis using binary logistic regression and Cox proportional hazard model tested surgical delay in predicting the risk of upgrading, lymph-node invasion, locally advance disease, adjuvant therapy, or biochemical-failure
| Variables | Overall population | Intermediate-risk | High-risk | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Upgrading | ||||||
| Time from diagnosis to surgery, months | 0.98 (0.94–1.02) | 0.3 | 0.98 (0.94–1.02) | 0.4 | 0.94 (0.8–1.09) | 0.4 |
| PSA at biopsy, ng/ml | 1.04 (1–1.08) | 0.04 | 1.07 (1.01–1.14) | 0.02 | 1.03 (0.97–1.1) | 0.3 |
| Clinical stage on MRI | ||||||
| T2 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| T3a | 0.77 (0.37–1.59) | 0.48 | – | – | 1.08 (0.34–3.44) | 0.9 |
| T3b | 2.59 (0.66–10.22) | 0.17 | – | – | 3.72 (0.76–18.25) | 0.1 |
| ISUP grade group on biopsy | ||||||
| 1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| 2 | 0.17 (0.08–0.39) | < 0.001 | 0.16 (0.06–0.45) | < 0.001 | 0.24 (0.06–1.03) | 0.055 |
| 3 | 0.03 (0.01–0.07) | < 0.001 | 0.02 (0.01–0.08) | < 0.001 | 0.03 (0.01–0.24) | 0.001 |
| 4 | 0.03 (0.01–0.11) | < 0.001 | – | – | 0.06 (0.01–0.31) | 0.001 |
| 5 | – | – | – | – | – | – |
| Lymph-node invasion | ||||||
| Time from diagnosis to surgery, months | 0.88 (0.77–1.01) | 0.07 | 0.88 (0.71–1.09) | 0.3 | 0.9 (0.77–1.06) | 0.2 |
| PSA at biopsy, ng/ml | 1.09 (1.04–1.3) | < 0.001 | 1.16 (1.05–1.27) | 0.003 | 1.04 (0.99–1.09) | 0.1 |
| Clinical stage on MRI | ||||||
| T2 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| T3a | 0.83 (0.33–2.07) | 0.7 | – | – | 0.45 (0.16–1.22) | 0.1 |
| T3b | 3.15 (0.91–10.85) | 0.07 | – | – | 1.82 (0.53–6.26) | 0.3 |
| ISUP grade group on biopsy | ||||||
| 1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| 2 | 1.74 (0.21–14.24) | 0.6 | 0.28 (0.11–0.67) | 0.005 | 0.94 (0.09–9.92) | 0.9 |
| 3 | 7.03 (0.9–55.1) | 0.06 | – | – | 3.87 (0.41–36.72) | 0.2 |
| 4 | 2.74 (0.31–24.25) | 0.4 | – | – | 0.71 (0.07–7.22) | 0.8 |
| 5 | 30.89 (3.52–270.78) | 0.002 | – | – | 8.08 (0.84–77.82) | 0.07 |
| Locally advance diseasea | ||||||
| Time from diagnosis to surgery, months | 1 (0.97–1.03) | 0.8 | 1 (0.96–1.03) | 0.8 | 1.07 (0.96–1.21) | 0.2 |
| PSA at biopsy, ng/ml | 1.06 (1.03–1.1) | < 0.001 | 1.07 (1.02–1.12) | 0.008 | 1.04 (0.99–1.09) | 0.1 |
| Clinical stage on MRI | ||||||
| T2 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| T3a | 2.55 (1.52–4.28) | < 0.001 | – | – | 1.63 (0.77–3.45) | 0.2 |
| T3b | 8.47 (1.83–39.21) | 0.006 | – | – | 5.33 (1.09–26.09) | 0.04 |
| ISUP grade group on biopsy | ||||||
| 1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| 2 | 2.9 (1.21–6.95) | 0.02 | 4.4 (1.23–15.66) | 0.02 | 2.29 (0.56–9.41) | 0.2 |
| 3 | 4.43 (1.82–10.79) | 0.001 | 7.39 (2.05–26.58) | 0.002 | 1.94 (0.45–8.32) | 0.4 |
| 4 | 5.46 (2.11–14.16) | < 0.001 | – | – | 2.31 (0.55–9.63) | 0.2 |
| 5 | 22.61 (5.71–89.43) | < 0.001 | – | – | 9.36 (1.65–53.05) | 0.01 |
| Adjuvant therapyb | ||||||
| Time from diagnosis to surgery, months | 0.96 (0.84–1.11) | 0.6 | 0.79 (0.57–1.1) | 0.2 | 1.01 (0.9–1.14) | 0.9 |
| ISUP grade group on final specimen | ||||||
| 1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| 2 | 1.37 (0.13–13.82) | 0.8 | 0.13 (0.02–0.81) | 0.03 | 0.49 (0.02–9.94) | 0.6 |
| 3 | 2.93 (0.3–28.78) | 0.4 | 0.36 (0.06–2.13) | 0.3 | 0.71 (0.04–12.88) | 0.8 |
| 4 | 3.52 (0.32–38.2) | 0.3 | – | – | 0.79 (0.04–15.04) | 0.9 |
| 5 | 2.16 (0.17–27.04) | 0.5 | – | – | 0.74 (0.04–14.77) | 0.8 |
| Pathologic stage | ||||||
| pT2 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| pT3a/b | 2.52 (1.27–5.03) | 0.008 | 2.2 (0.84–5.71) | 0.1 | 2.91 (0.96–8.81) | 0.06 |
| Lymph-node involvement | ||||||
| pN0 and/or pNx | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| pN1 | 12.75 (4.88–33.3) | < 0.001 | 26.57 (5.34–132.25) | < 0.001 | 7.57 (2.27–25.25) | 0.001 |
| Positive margin | ||||||
| R0 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| R1 | 5.42 (2.8–10.49) | < 0.001 | 6.97 (2.63–18.42) | < 0.001 | 5.09 (1.96–13.20) | 0.001 |
| Biochemical failurec | ||||||
| Time from diagnosis to surgery, months | 0.97 (0.91–1.04) | 0.6 | 0.95 (0.83–1.08) | 0.4 | 1.06 (0.9–1.2) | 0.4 |
| ISUP grade group on final specimen | ||||||
| 1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| 2 | 2.48 (0.33–18.41) | 0.4 | 1.48 (0.19–11.29) | 0.7 | 2.15e + 8 (4.74e + 7–9.78e + 8) | < 0.001 |
| 3 | 5.18 (0.71–37.84) | 0.1 | 2.56 (0.33–19.58) | 0.4 | 4.13e + 8 (1.15e + 8–1.49e + 9) | < 0.001 |
| 4 | 6.51 (0.82–51.38) | 0.08 | 6.91 (0.67–71.62) | 0.1 | – | – |
| 5 | 9.19 (1.15–73.36) | 0.04 | – | – | 4.2e + 8 (8.6e + 7–2.05e + 9) | < 0.001 |
| Pathologic stage | ||||||
| pT2 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| pT3a/b | 1.15 (0.77–1.71) | 0.5 | 1.27 (0.61–2.63) | 0.5 | 0.84 (0.38–1.89) | 0.7 |
| Lymph-node involvement | ||||||
| pN0 and/or pNx | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| pN1 | 2.77 (1.6–4.82) | < 0.001 | 2.15 (0.74–6.3) | 0.2 | 2.69 (0.94–7.68) | 0.06 |
| Positive margin | ||||||
| R0 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| R1 | 1.55 (1–2.42) | 0.05 | 1.82 (0.88–3.76) | 0.1 | 1.56 (0.66–3.66) | 0.3 |
HR hazard ratio, OR odds ratio, CI confidence interval;
aDefined as ≥ pT3 and/or pN1 at final pathology
bData available for 436 patients
cDefined as two consecutive PSA ≥ 0.2 ng/ml after undetectable values
Fig. 2.
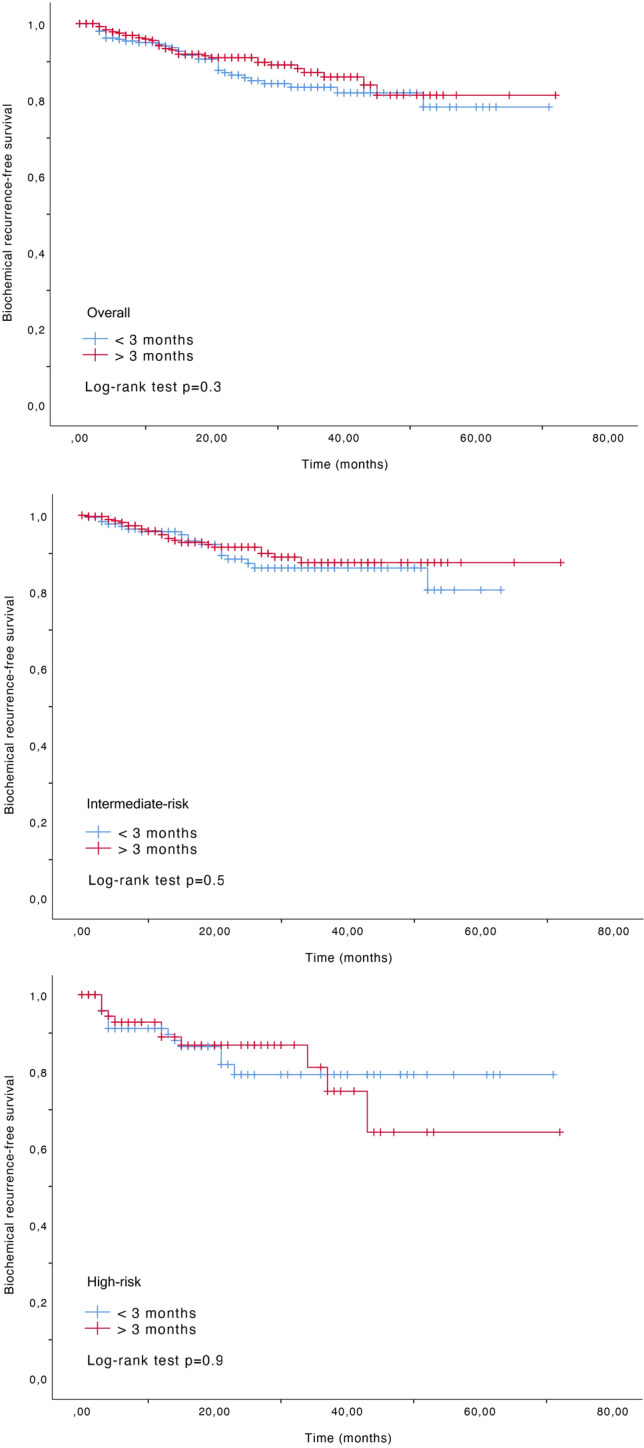
Biochemical recurrence-free survival according to 3 month cut-off for a overall population, b intermediate-risk, and c high-risk patients
Discussion
We herein report results from a contemporary cohort of PCa patients, finding no evidence of a negative association between delay and oncologic outcomes of radical prostatectomy. Our results confirm the findings from John’s Hopkins analyzing 2303 patients who were operated within 6 months from diagnosis [6]. The authors detected no difference in BCR and metastatic-free survival rates as a function of time to surgery, which has supported the current NCCN statement regarding PCa management and deferral during COVID-19 pandemic [4]. Other investigators have reported contrasting results in particular for high-risk patients. A review published by van den Bergh et al. evaluated 17 retrospectives study and concluded that only limited data support an adversely impact of surgical delay for patient harboring intermediate and high-risk PCa [5]. More recently, Zanaty et al. retrospectively analyzed 619 men undergoing robotic-assisted prostatectomy, with mean surgical delays of 5 months. While surgical delay did not impact BCR in low-risk (HR 0.96, 95% CI 0.91–1.01, p = 0.086) and intermediate-risk (HR 1.00, 95% CI 1.00–1.01, p = 0.99) groups, and in high-risk patients (HR 1.02, 95% CI 1.01–1.03, p = 0.001), a significant association between surgical delay and BCR was detected on Cox multivariable analysis [11]. Similarly, Fossati et al. published results from San Raffaele hospital in Milan in 2653 men [12]. In high-risk patients, time to surgery was significantly associated with BCR (HR 1.02, 95% CI 1.01–1.03, p = 0.0005) and clinical recurrence (HR 1.03, 95% CI 1.01–1.04, p = 0.0002), in particular for patients waiting 12 months before undergoing radical prostatectomy. Given those results, the EAU Guidelines Office Rapid Reaction Group (GORRG) recommended to postpone all deferrable surgery until after pandemic, which included most PCa patients [3].
Some factors could influence the absence of impact of surgical delay in high-risk patient when compared to previously published study. As an example, cohort analyzed by Fossati et al. apparently harbored more cT3 (56% vs. 5.9%) and lymph-node invasion (31% vs. 21.1%) but relatively close values regarding pre- and post-operative data [12]. Zanaty et al. described more aggressive PCa with higher ISUP grade on biopsy (84.5% vs. 33.1% for systematic biopsy and 45.4% for MRI-targeted biopsy for ISUP grade ≥ 4), while preoperative PSA and clinical stage seemed quite similar [11]. Consequently, these differences may have an influence on the inconsistent results regarding this subgroup of patient. Moreover, our high-risk population presented relatively low features characteristic when regarding median PSA value and clinical stage which could impact the absence of association between surgical delay and oncologic outcomes in comparison with the other studies. To note, while only 5.9% of cT3 at digital rectal examination in the subgroup of high-risk patients was observed, 50.5% of them have an MRI showing a suspicion of extracapsular extension with or without vesicle seminal invasion. In comparison with the final pathology, a total of 68% patients harboring pT3-4 were described highlighting the importance of this preoperative imaging modality that must be read by specialized radiologists [19]. Regarding median delay between diagnosis and surgical treatment, we noted a significant difference between intermediate and high-risk patients (3.5 vs. 2.8 months, p < 0.001) which illustrates the influence of tumor aggressiveness on faster surgical planning [13].
Strength of the present study in comparison with the previous published works is the description of a contemporary cohort using a modern diagnostic pathway (i.e., preoperative mp-MRI and MRI/US fusion system for targeted and systematic biopsies). This leads to an improved PCa evaluation with higher histopathology concordance and preoperative classification compared to historical series based on transrectal ultrasound biopsy only [20]. In other words, our set of patients was probability more precisely defined thanks to these news preoperative tools leading to fewer unexpected adverse diseases on the final specimen. We acknowledge the retrospective nature of the present analysis introducing a potential selection and confounding bias with unknown factors that led the physician to choose the most appropriate timing for surgery. However, one must bear in mind that designing a prospective trial postponing the treatment of oncologic patients is technically and ethically impossible. Using time from biopsy to surgery as a continuous variable was an arbitrary decision, although we found similar result using 3–6–9–12 months periods. In the present study, most of the men were operated within a period close to 3 months; therefore, care must be taken to the interpretation of our results for longer treatment delays. However, with regard to the results of similar studies, it seems safe to propose a delay up to 6 months for high-risk diseases since diagnosis, while surgery for low- and intermediate-risk diseases could be further delayed. Follow-up period was relatively short and could impact the number of events for biochemical recurrence especially in the intermediate-risk subgroup.
Conclusions
In conclusion, in this large series of European men undergoing radical prostatectomy for intermediate and high-risk prostate cancer, we did not observe any significant association between surgical delay and adverse oncologic outcomes, including upgrading, pathological locally advanced disease, need for adjuvant therapy or BCR. Results were confirmed across EAU risk categories. Our data support a safely deferred approach for PCa patients awaiting surgery in this time of COVID-19 pandemic.
Acknowledgements
Gaelle Fiard, M.D., Ph.D., received funding from the Fondation de France and the European Urology Scholarship Program.
Author contributions
RD and SA: protocol/project development. RD data analysis. RD, SA, and TR: manuscript writing/editing. RD, GP, MR, MO, DB, GF, AP, GS, JVD, BM, CI, JLD, JBR, TQ, TR, and SA: data collection or management:
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Puliatti S, Eissa A, Eissa R, et al. COVID-19 and urology: a comprehensive review of the literature. BJU Int. 2020 doi: 10.1111/bju.15071. [DOI] [PubMed] [Google Scholar]
- 2.Stensland KD, Morgan TM, Moinzadeh A, et al. Considerations in the triage of urologic surgeries during the COVID-19 pandemic. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribal MJ, Cornford P, Briganti A, Knoll T, Gravas S, Babjuk M, Harding C, Breda A, Bex A, on behalf of the GORRG Group, Rassweiler JJ, Gözen AS, Pini G, Liatsikos E, Gianluc JN and on behalf of the ESO and the EGP. EAU Guidelines Office Rapid Reaction Group: An organisation-wide collaborative effort to adapt the EAU guidelines recommendations to the COVID-19 era. Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/HealthAdvance/journals/eururo/EURUROL-D-20–00649–1587127530920.pdf. Published 2020 [DOI] [PMC free article] [PubMed]
- 4.Abramson Cancer Center at the University of Pennsylvania, City of Hope National Cancer Center, Dana-Farber/Brigham and Women’s Cancer Center, Duke Cancer Institute, Fox Chase Cancer Center, Fred Hutchinson Cancer Research Center/Seattle Cancer Care Allian and V-ICC. Management of prostate cancer during the COVID-19 pandemic. NCCN Guidel. (Coronavirus Disease 2019 (COVID-19) Resources for the Cancer Care Community). https://www.nccn.org/covid-19/pdf/NCCN_PCa_COVID_guidelines.pdf.
- 5.van den Bergh RCN, Albertsen PC, Bangma CH, et al. Timing of curative treatment for prostate cancer: a systematic review. Eur Urol. 2013;64(2):204–215. doi: 10.1016/j.eururo.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Bivalacqua TJ, Han M, et al. Evaluating the impact of length of time from diagnosis to surgery in patients with unfavourable intermediate-risk to very-high-risk clinically localised prostate cancer. BJU Int. 2019;124(2):268–274. doi: 10.1111/bju.14659. [DOI] [PubMed] [Google Scholar]
- 7.Loeb S, Folkvaljon Y, Robinson D, et al. Immediate versus delayed prostatectomy: Nationwide population-based study*. Scand J Urol. 2016;50(4):246–254. doi: 10.3109/21681805.2016.1166153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg KB, Curtis GL, Timar RE, George AK, Cher ML. Delayed radical prostatectomy is not associated with adverse oncological outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. 2020 doi: 10.1097/JU.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 9.Meunier ME, Neuzillet Y, Radulescu C, et al. Le delai entre biopsies de prostate et prostatectomie radicale influence-t-il le risque de recidive ? Progrès Urol. 2018;28(10):475–481. doi: 10.1016/j.purol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Westerman ME, Sharma V, Bailey GC, et al. Impact of time from biopsy to surgery on complications, functional and oncologic outcomes following radical prostatectomy. Int Braz J Urol. 2019;45(3):468–477. doi: 10.1590/s1677-5538.ibju.2018.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanaty M, Alnazari M, Ajib K, et al. Does surgical delay for radical prostatectomy affect biochemical recurrence? A retrospective analysis from a Canadian cohort. World J Urol. 2018;36(1):1–6. doi: 10.1007/s00345-017-2105-6. [DOI] [PubMed] [Google Scholar]
- 12.Fossati N, Rossi MS, Cucchiara V, et al. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: can surgery be postponed safely? Urol Oncol Semin Orig Investig. 2017;35(4):150.e9–150.e15. doi: 10.1016/j.urolonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Chen RC, Bensen JT, et al. Who makes the decision regarding the treatment of clinically localized prostate cancer-the patient or physician? Cancer. 2013;119(2):421–428. doi: 10.1002/cncr.27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol Semin Orig Investig. 2003;21(2):93–100. doi: 10.1016/S1078-1439(02)00209-0. [DOI] [PubMed] [Google Scholar]
- 15.Berry DL, Ellis WJ, Russell KJ, et al. Factors That Predict Treatment Choice and Satisfaction with the Decision in Men with Localized Prostate Cancer. Clin Genitourin Cancer. 2006;5(3):219–226. doi: 10.3816/CGC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- 16.Geethanath S, Vaughan JT. Accessible magnetic resonance imaging: a review. J Magn Reson Imaging. 2019;49(7):e65–e77. doi: 10.1002/jmri.26638. [DOI] [PubMed] [Google Scholar]
- 17.Mottet N, Cornford P, van den Bergh RCN, Briers E, De Santis M, Fanti S, Gillessen S, Grummet J, Henry AM, Lam TB, Mason MD, van der Kwast TH, van der Poel HG, Rouvière O, Schoots IG, Tilki TW. EAU guidelines: prostate cancer 2020. Eur Urol. https://uroweb.org/guideline/prostate-cancer/. Published 2020
- 18.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Tay KJ, Gupta RT, Brown AF, Silverman RK, Polascik TJ. Defining the incremental utility of prostate multiparametric magnetic resonance imaging at standard and specialized read in predicting extracapsular extension of prostate cancer. Eur Urol. 2016;70(2):211–213. doi: 10.1016/j.eururo.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Diamand R, Oderda M, Al-Hajj-Obeid W, et al. A multicentric study on accurate grading of prostate cancer with systematic and MRI/US fusion targeted biopsies: comparison with final histopathology after radical prostatectomy. World J Urol. 2019;37(10):2109–2117. doi: 10.1007/s00345-019-02634-9. [DOI] [PubMed] [Google Scholar]


