Abstract
Manganese (Mn) is an essential metal, but excessive exposures have been well-documented to culminate in neurotoxicity. Curiously, the precise mechanisms of Mn neurotoxicity are still unknown. One hypothesis suggests that Mn exerts its toxicity by inhibiting mitochondrial function, which then (if exposure levels are high and long enough) leads to cell death. Here, we used a Huntington’s disease cell model with known differential sensitivities to manganese—STHdhQ7/Q7 and STHdhQ111/Q111 cells—to examine the effects of acute Mn exposure on mitochondrial function. We determined toxicity thresholds for each cell line using both changes in cell number and caspase-3/7 activation. We used a range of acute Mn exposures (0–300 µM), both above and below the cytotoxic threshold, to evaluate mitochondria-associated metabolic balance, mitochondrial respiration, and substrate dependence. In both cell lines, we observed no effect on markers of mitochondrial function at subtoxic Mn exposures (below detectable levels of cell death), yet at supratoxic exposures (above detectable levels of cell death) mitochondrial function significantly declined. We validated these findings in primary striatal neurons. In cell lines, we further observed that subtoxic Mn concentrations do not affect glycolytic function or major intracellular metabolite quantities. These data suggest that in this system, Mn exposure impairs mitochondrial function only at concentrations coincident with or above the initiation of cell death and is not consistent with the hypothesis that mitochondrial dysfunction precedes or induces Mn cytotoxicity.
Keywords: manganese, mitochondria, Huntington’s disease
Metal neurotoxicity, or the impairment of neurological function as a result of exposure to a number of transition metals, is thought to affect millions worldwide and can cause neurodevelopmental and neurodegenerative disorders (Chen et al., 2016; Freire et al., 2018; Gorini et al., 2014; Rehman et al., 2018; Wright and Baccarelli, 2007). Exposure is mainly a result of the anthropogenic introduction of metals into the environment, and subsequent ingestion or inhalation by those affected (Jaishankar et al., 2014; Tchounwou et al., 2012). Manganese (Mn) is one such metal, and toxic exposures occur primarily through environmental pollution or occupational exposures from industrial processes, such as mining, smelting, and battery manufacturing (Horning et al., 2015; Howe et al., 2004; O’Neal and Zheng, 2015; Santamaria and Sulsky, 2010). Exposure to excess Mn can result in manganism, a neurological disorder that symptomatically resembles Parkinson’s disease (PD) (Cersosimo and Koller, 2006; Chen et al., 2016; Neal and Guilarte, 2013; Racette, 2014). Importantly, manganism is distinct from PD as it seems insensitive to L-DOPA therapy, and Mn exposure does not directly cause dopaminergic neurodegeneration. Mn can cause dysregulation of dopaminergic neurotransmission, as well as neuropathology of the globus pallidus and the striatum, which likely contributes to an aberrant functioning of the basal ganglia analogous to PD (Guilarte, 2010; Guilarte et al., 2006; Kwakye et al., 2015). Mn is also an essential micronutrient, as Mn is a cofactor in a number of key metabolic enzymes, including pyruvate carboxylase, glutamine synthetase, and arginase (Ash, 2004; Horning et al., 2015; Mildvan et al., 1966; Wedler et al., 1982).
Despite the extensive characterization of the clinical presentation of manganism, the intracellular mechanisms that mediate Mn neurotoxicity remain unclear. Mitochondria are an attractive target for Mn toxicity, as they act as a sink for Mn. Mn is readily taken up into the mitochondrial matrix through the mitochondrial calcium uniporter (Gavin et al., 2009; Kamer et al., 2018). Mn is exported from mitochondria in an NCLX-independent mechanism (Gavin et al., 2009) which has not been conclusively identified, though a Ca2+/H+ exchanger may be involved (Austin et al., 2017; De Stefani et al., 2016; Tsai et al., 2014). This efflux mechanism appears to operate more slowly than influx, as multiple in vivo studies have demonstrated that systemic Mn administration results in the accumulation of Mn in the mitochondrial matrix (Gavin et al., 1992; Liccione and Maines, 1989; Maynard and Cotzias, 1955; Morello et al., 2008). Furthermore, ultrastructural studies have reported that chronic Mn exposure induces mitochondrial swelling (Mousa and Shehab, 2015; Villalobos et al., 2015). Once inside the matrix, Mn may pathologically interact with multiple mitochondrial functions, including impairing Mn-superoxide dismutase and/or generating reactive oxygen species, inhibiting intramitochondrial metabolic enzymes, and interfering with or accelerating the function of the respiratory chain (in particular, complexes I and II) (Fernandes et al., 2017; Galvani et al., 1995; Gavin et al., 1992; Gunter et al., 2009; Kaur et al., 2017; Malecki, 2001; Neely et al., 2017; Yin et al., 2008; Zheng et al., 1998). However, many of the in vitro studies which have characterized the specific interactions between Mn and various mitochondrial enzymes have used Mn concentrations far in excess of those that would be physiologically relevant to Mn exposures that can cause neurotoxicity (Bowman and Aschner, 2014).
We sought to rigorously examine the effects of acute Mn exposure on mitochondrial and intracellular metabolic function. We used 2 cell lines with known differential abilities to take up Mn—immortalized murine striatal cell lines STHdhQ7/Q7 and STHdhQ111/Q111 (Trettel et al., 2000; Williams et al., 2010). These 2 cell lines express wild-type or mutant forms, respectively, of Huntingtin, the protein underlying Huntington’s disease (HD). HD is an autosomal dominant neurodegenerative disorder, which is caused by the accumulation of CAG repeats in a coding region of the mutant Huntingtin (Bates et al., 2015; Labbadia and Morimoto, 2013; Roos, 2010). HD selectively causes degeneration of the striatum, which is also one of the brain regions that accumulates the most Mn (Morello et al., 2008). Previous studies from our lab have demonstrated that both in vitro and in vivo models of HD have both deficient Mn uptake and reduced activity of Mn-dependent enzymes such as arginase and ataxia-telangiectasia mutated kinase (Bichell et al., 2017; Tidball et al., 2015; Williams et al., 2010). We therefore hypothesized that STHdhQ111/Q111 cells would be more resistant to Mn-induced cytotoxicity due to decreased uptake. Furthermore, using these 2 models, we closely interrogated relationship between Mn-induced cytotoxicity and Mn-induced inhibition of mitochondrial function.
MATERIALS AND METHODS
Immortalized cell lines
Immortalized, wild-type (STHdhQ7/Q7) and HD-type (STHdhQ111/Q111) murine striatal cell lines were obtained from Coriell Cell Repository (Camden, New Jersey). STHdh cells were cultured in Dulbecco’s Modified Eagle Medium, DMEM (D6546, Sigma-Aldrich, St Louis, Missouri) supplemented with 10% FBS (Atlanta Biologicals, Flowery Branch, Georgia), 2 mM GlutaMAX (Life Technologies, Carlsbad, California), Penicillin-Streptomycin, 0.5 mg/ml G418 Sulfate (Life Technologies), MEM nonessential amino acids solution (Life Technologies), and 14 mM HEPES (Life Technologies). They were incubated at 33°C and 5% CO2. Cells were passaged before reaching greater than 90% confluency and were maintained below passage number 14. The cells were split by trypsinization using 0.05% Trypsin-EDTA solution (Life Technologies) incubated for 5 min. For all experiments, cells were split into Matrigel (BD Biosciences, Franklin Lakes, New Jersey)-coated plates 48 h prior to assay at 2.5 × 104 cells/cm2 (STHdhQ7/Q7) or 3.5 × 104 cells/cm2 (STHdhQ111/Q111), unless otherwise indicated.
Primary cell culture
All animal experiments were conducted under institutional guidelines and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Neuronal cultures were established from Sprague Dawley rat embryos at E15 (Charles River). Timed pregnant dams were euthanized by carbon dioxide inhalation, and embryos were removed. Around 12 embryos from different dams were pooled and were used for the preparation of the cultures. The striatum (STR) were quickly dissected from embryos in Hank’s Balanced Salt Solution as described previously (Weinert et al., 2015). For dissociation, the tissue was incubated in DMEM containing papain (20 U/ml) and DNase I (0.01%) (Worthington Biochemical, Lakewood, New Jersey) for 30 min at 37°C following by pipetting and filtered with a 70- and 40-μm nylon cell strainer (Corning, Corning, New York). Neurons were plated in 96-well plates at a density of 1 × 104 cells/well in Neurobasal media containing B27 (2×), GlutaMAX (1×), and penicillin/streptomycin (1×) (Gibco, Waltham, Massachusetts). After 3 days, half the media was replaced with new media. Neuronal culture purity was assessed by immunostaining, which confirmed that > 95% of the cell population was positive for the neuron-specific marker β3-tubulin (Cell Signaling Technologies, Danvers, Massachusetts, No. 4466, 1:1000) (Supplementary Figure 1).
Extracellular metabolic flux analyses
The Seahorse XFe96 bioanalyzer (Agilent, Santa Clara, California) was used to conduct all STHdh experiments. Forty-eight hours prior to experiments, STHdhQ7/Q7 and STHdhQ111/Q111 cells were plated into Matrigel-coated plates at 3.3 × 104 and 4.7 × 104 cells/cm2, respectively. Twenty-four hours prior to experiments, culture media was replaced and indicated concentrations of Mn were added. One hour before the start of the assay, media was replaced with XF Base Media (Agilent). For MitoStress Tests and substrate dependency assays, XF Base Media was supplemented with 25 mM glucose (Sigma-Aldrich), 4 mM glutamine (Life Technologies), and 1 mM pyruvate (Life Technologies). To determine basal, leak, and maximum respiration rates, the MitoStress Test program was used for recording 3 mix and measure cycles following the addition of each drug. In an independent experiment, 2 μM oligomycin, 1 µM FCCP, and 0.5 µM rotenone and antimycin were determined to be the most effective concentrations. For substrate dependency assays, 4 mix and measure cycles were used to establish basal respiration, then a combination of compounds were added to block-specific metabolic pathways (etomoxir—40 µM, BPTES—3 µM, UK5099—2 µM). Five additional mix and measure cycles were used to establish compound-suppressed basal respiration, and subsequently the compound additions and mix and measure cycles of the MitoStress Test program were performed. For GlycoStress Tests, XF Base Media was supplemented with 4 mM glutamine. To determine glutamine-, glucose-, and oligomycin-linked extracellular acidification rates (ECAR), the GlycoStress Test program was used for recording 3 mix and measure cycles following the addition of each drug. Ten mM glucose, 2 µM oligomycin, and 500 mM 2-deoxyglucose were sequentially used for each phase of the experiment. All compounds used for these experiments were obtained from Sigma-Aldrich.
The Seahorse XFe24 bioanalyzer was used for all STR experiments. Primary rat neuron cultures were plated in Seahorse XFe24 plates (Agilent) which were previously coated with poly-d-lysine/laminin solution (Thermo Fisher Scientific, Waltham, Massachusetts). For measurement, neurons were rinsed gently with 100 µl/well assay medium (XF base medium, supplemented with 1 mM sodium pyruvate and 1 mM GlutaMAX), put into 675 µl/well fresh assay medium and incubated in a non-CO2 incubator for 30 min. For the MitoStress Test, 2 mM oligomycin, 1.5 mM FCCP, and 1 mM rotenone/antimycin were used (Agilent). The Wave report generator (Agilent) was used for analysis.
NADH/NAD+ measures
Relative NAD+, NADH, and NADH/NAD+ ratios were quantified using an NADH/NAD+ Assay (NADH-Glo, Promega, Madison, Wisconsin), according to the suggested protocol of separable nucleotide measurements. Relative quantities of NAD(H) were normalized to controls from each assay, but each ratio was calculated independently.
ADP/ATP measures
ADP/ATP ratios were determined with an ADP/ATP assay (Abcam, Cambridge, Massachusetts), according to the manufacturer’s protocol.
MTT assay
The MTT test was used to assess cell viability in STR cultures. After Mn exposure, neurons were washed and 200 µl/well of culture medium containing 1 mg/ml of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich) was replaced. After 4 h of incubation at 37°C, neurons were washed and lysed with 200 µl of DMSO and the absorbance at 570 and 690 nm was determined.
Mitochondrial membrane potential measurement
Mitochondrial membrane potential was measured with a cationic fluorescent dye, tetramethylrhodamine methyl ester (TMRM) (Thermo Fisher Scientific, Cat. I34361). After Mn exposure, neurons were washed and incubated with TMRM at 10 nM in culture medium for 30 min at 37°C. Next, neurons were imaged at 540 and 645 nm, and the fluorescence was determined.
Caspase activity measurement
Caspase activity was measured by quantifying the fluorescence shift of a known caspase-3/7 substrate (Walsh et al., 2008), Ac-Asp-Glu-Val-Asp-7-Amino-4-trifluoromethylcoumarin (Ac-DEVD-AFC, Santa Cruz, Santa Cruz, California, SC-311274). Briefly, Ac-DEVD-AFC assay buffer was prepared (20 mM HEPES pH 7.5, 10% glycerol, 4 mM DTT, 25 µg/ml Ac-DEVD-AFC in DMSO) and loaded into an assay plate. Mn-treated cells were lysed with RIPA (Thermo Fisher Scientific) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich), then cell lysate was transferred to assay buffer plate. Following incubation, AFC released by activated caspases was detected with 400/505 excitation/emission. The arbitrary fluorescence units were normalized to the total protein content of the sample. For each experiment, the fluorescence detected in untreated controls was assumed to be 0% caspase activity, while the highest-reading well was set to 100% caspase activity to control for variation in fluorescence arising from differences in incubation time.
Cell number quantification
Following STHdh extracellular flux analysis experiments, Hoechst (1:2000, Thermo Fisher Scientific) was added to each well to facilitate nuclear staining for cell counting. In other multiwell assay experiments, treatment-matched wells were stained to estimate cell number for each condition. Cells were counted using the MetaXpress imager (Molecular Devices, Sunnyvale, California) with automated software. Assay measurements were normalized to cell number as indicated, and changes in cell number as a measure of cell viability (Figure 1A) were aggregated from these experiments.
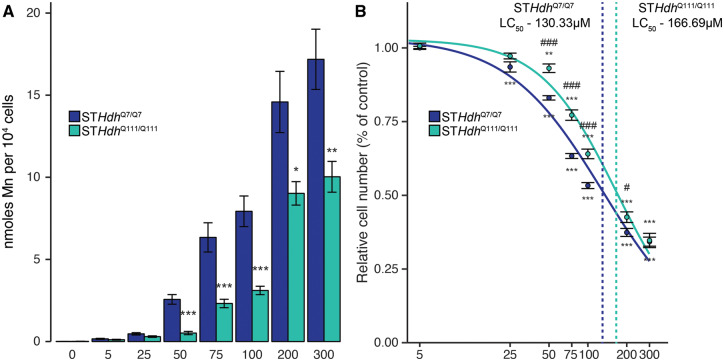
Figure 1.
Effects on Mn uptake and cell number in 2 STHdh cell lines following 24-h exposure. A, µM Mn per 104 cells, calculated by cellular fura-2 manganese extraction assay. Mn concentration (µM) is indicated below respective bars. Combined from 3 independent experiments where n = 3–6, normalized to respective controls; total n per group 13. *p < .05, **p < .01, ***p < .001, by Student’s t test comparing STHdhQ7/Q7 and STHdhQ111/Q111 Mn uptake at each respective dose. B, µM concentrations of Mn reduce cell number in both wild-type (STHdhQ7/Q7) and HD (STHdhQ111/Q111) cell lines, quantified by loss of cell nuclear staining relative to control. Mn dose (µM) is indicated on the log10-transformed x-axis. Results combined from 8 independent experiments, n = 3–6; normalized to respective controls; total n = 32–36. **p < .01, ***p < .001, relative to control by Dunnett’s test. #p < .05, ###p < .001 by Student’s t test comparing STHdhQ7/Q7 and STHdhQ111/Q111 relative number at each respective dose. Error bars reflect SEM. The Benjamini-Hochberg method was used to correct for multiple comparisons.
1H NMR spectroscopy
For these experiments, STHdh cultures were grown in 100-mm plates. At 80% confluence, cells were treated with Mn, and following a 24-h exposure, samples were harvested for analysis. Samples were prepared and analyzed as described (Warren et al., 2017). Briefly, aqueous metabolites were extracted using 1:1:1 MeOH:CHCl3:H2O and desiccated. The samples were reconstituted in 5 mM Na(2)H(2)PO4 at pH 7.0 (Sigma-Aldrich) in D2O (99.9%, Cambridge Isotope Labs, Andover, Massachusetts) containing 0.25 mM sodium trimethylsilyl propionate-d4 (TMSP, 98%, Cambridge Isotope Labs). Sample pH was readjusted and transferred to a 3-mm NMR tube (Bruker BioSpin, Rheinstetten, Germany). 1H NMR spectra were recorded using a Bruker AVANCE III 600 MHz spectrometer equipped with a 5-mm CPQCI cryoprobe (Bruker), at 298 K. A water signal presaturation sequence (zgpr) was used, with the following acquisition parameters: 128 scans, 64k data points, 13 ppm spectral width, 4 s acquisition time, 6 s relaxation delay. Spectra were processed using Topspin software (v3.5, Bruker) with automatic apodization (0.3 Hz) and zero filling, and manual reference, phase, and baseline correction. Peaks were identified and assigned using Chenomx (v8.2, Edmonton, Canada) and cross-referenced against the Human Metabolomic Database (hmdb.ca). Peak fitting and deconvolution were manually performed using ACD/SpecManager (v12, Advanced Chemistry Development, Toronto, Canada). Concentrations were calculated using peak areas by reference to TMSP. Final metabolite concentrations are expressed as nmol/100-mm plate.
Statistical analysis
For all experiments with 2 groups, means were compared with the unpaired 2-tailed Student’s t test. For all experiments comparing multiple groups to control, 1-way ANOVA tests were performed, followed by Dunnett’s post hoc tests. All error bars reflect SEM. All statistical analyses and plotting were conducted in R (cran.r-project.org). A difference in mean was considered statistically significant when p < .05.
RESULTS
The STHdh Cell Lines as Paired Models of Distinct Relationships Between Mn Exposure and Toxicity
The STHdh cell lines, STHdhQ7/Q7 and STHdhQ111/Q111, were immortalized from murine primary striatal cells, and differ in the expression of wild-type (Q7/Q7) or mutant (Q111/Q111) Huntingtin (Trettel et al., 2000). Previously, our lab demonstrated that these 2 cell lines have different capacities for Mn uptake, which leads to differential susceptibility to Mn toxicity. We first confirmed this difference in Mn response by exposing both cell lines to a range of micromolar Mn concentrations for 24 h, and then quantified intracellular Mn using a cellular fura-2 manganese extraction assay (CFMEA), previously developed in our lab (Kwakye et al., 2011). At every exposure concentration, STHdhQ111/Q111 intracellular Mn concentrations were lower than in the wild-type STHdhQ7/Q7s (Figure 1A), and this decrease was statistically significant at every concentration, 50 µM and above. Consequently, we used the 2 cell lines as paired models of the relationship between intracellular Mn uptake and cell toxicity to dissociate intracellular Mn from extracellular Mn exposure levels.
To further our observations of reduced Mn uptake in the STHdhQ111/Q111 cell line, we quantified cell viability by counting the number of cells remaining following Mn exposure in both cell lines (Figure 1B). We observed statistically significant reductions in cell number in both cell lines at nearly every tested concentration (STHdhQ7/Q7—F = 629.6, p < .001; STHdhQ111/Q111—F = 289, p < .001). Supporting the ability of Mn uptake to induce cytotoxicity, we observed that the STHdhQ7/Q7 cell number was greatly reduced by Mn exposure, as at the majority of Mn concentrations used, and that STHdhQ7/Q7 relative cell number was significantly lower than that of STHdhQ111/Q111 at analogous concentrations. In modeling the concentration-response curve for Mn against cell number, we calculated an LC50 (Ritz et al., 2015) of 130.33 µM (±3.74) for the STHdhQ7/Q7 cell line, and an LC50 of 166.69 µM (±5.21) for the STHdhQ111/Q111 cell line (Figure 1B).
Determining Cytotoxicity Thresholds for Mn Exposure in Both STHdh Cell Lines
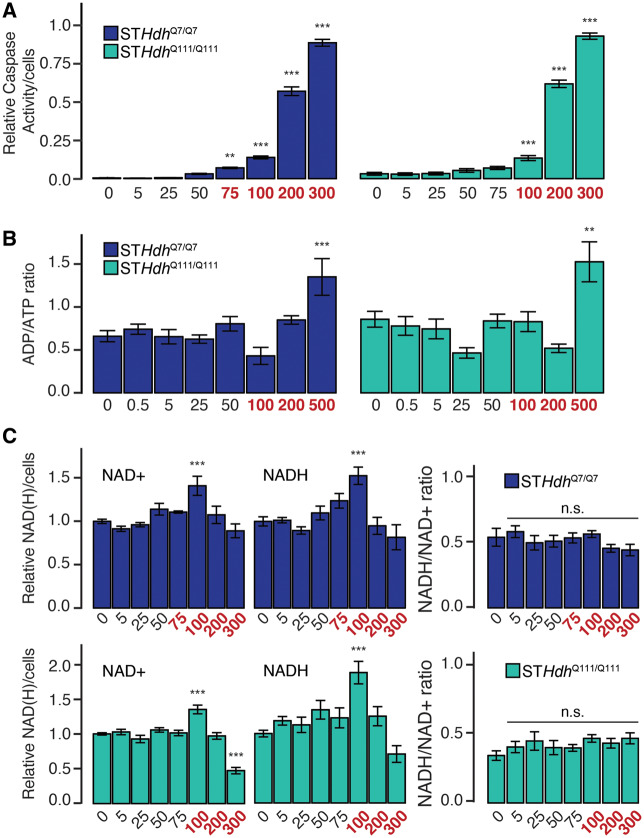
Although we were able to quantify changes in cell number in response to Mn exposure, we observed significant reductions in cell number in both cell lines even in response to low Mn concentrations. We hypothesized that some of the reduction in cell number following 24-h Mn exposure may be merely due to cytostatic effects of Mn. To differentiate between cytostatic and cytotoxic effects of Mn, we used a caspase activity assay to determine an apoptotic threshold for Mn exposure. We observed significant caspase activation following all exposures 75 µM and above in the STHdhQ7/Q7 cell line, and 100 µM and above in the STHdhQ111/Q111 cell line (F = 624.8, p < .001; F = 551.9, p < .001, respectively) (Figure 2A). These 2 concentrations in their respective cell lines had comparable reductions in cell number (approximately 40% cell loss) (Figure 1B). Although these concentrations are lower than the calculated LC50, which is likely determined by a combination of the cytostatic and cytotoxic effects of Mn, for the interpretation of subsequent experiments we determined that these concentrations (75 and 100 µM) represented reasonable cytotoxicity “thresholds” for each cell line; ie, Mn exposures below these concentrations would be considered “subtoxic,” and those at or above would be considered “supratoxic.” We have color-coded subsequent figure legends to reflect this demarcation—ie, subtoxic concentrations are black, whereas supratoxic concentrations are red.
Figure 2.
Subtoxic concentrations of Mn do not affect metabolic balance. A, Relative caspase-3/7 activity following 24-h Mn exposure. Left, STHdhQ7/Q7; Right, STHdhQ111/Q111. Results combined from 3 independent experiments, n = 4–6; total n = 16 (see Materials and Methods section). B, ADP/ATP ratio following 24-h Mn exposure. Left, STHdhQ7/Q7; Right, STHdhQ111/Q111. Results combined from 3 independent experiments, n = 3–4; total n = 6–10. C, Relative quantity NAD+, NADH, and NADH/NAD+ ratio following 24-h Mn exposure. Top, STHdhQ7/Q7; Bottom, STHdhQ111/Q111. Results combined from 3 independent experiments, n = 3; total n = 9. Mn concentration (µM) is indicated below respective bars. Concentrations in red reflect concentrations above which significant cytotoxicity is observed. Error bars reflect SEM. **p < .01, ***p < .001, by Dunnett’s test relative to control.
Subtoxic Concentrations of Mn Do Not Substantially Affect NADH/NAD+ or ADP/ATP Balance
We hypothesized that if Mn induces cell death by first impairing mitochondrial function, subtoxic Mn exposures should impair important readouts of mitochondrial activity below the cytotoxic threshold; ie, that only when the level of mitochondrial dysfunction reached a certain threshold would cell death occur. Two such readouts include the NADH/NAD+ ratio, and ADP/ATP ratio. Though the balance between both of these pairs of metabolites is maintained through both cytosolic and mitochondrial mechanisms, perturbations to mitochondrial function affect these ratios within the whole cell. In the mitochondrion, ATP is synthesized by the activity of the respiratory chain, and reductions in that activity should increase the ADP/ATP ratio. Similarly, NADH is generated through TCA cycle activity and is used as an electron donor for the respiratory chain, regenerating NAD+. Deficits in either part of this pathway should alter the NADH/NAD+ ratio. Interestingly, in both cell types, we observed no significant change in the ADP/ATP ratio (Figure 2B) except at the highest tested Mn concentration (500 µM) (STHdhQ7/Q7—F = 5.887, p < .001; STHdhQ111/Q111—F = 5.929, p < .001). Similarly, Mn exposure did not affect the NADH/NAD+ ratio in either cell line at any tested concentration (Figure 2C) (STHdhQ7/Q7—F = 1.178, p = .328; STHdhQ111/Q111—F = 0.983, p = .452). We observed small perturbations in the relative quantities of both NAD+ and NADH in both cell lines when normalized to cell number at Mn exposures over 100 µM (STHdhQ7/Q7—NAD+ F = 6, p < .001, NADH F = 7.427, p < .001; STHdhQ111/Q111—NAD+ F = 30.06, p < .001, NADH F = 7.427, p < .001). As both NAD+ and NADH quantities decreased, it is possible that the total NAD(H) pool is affected by Mn exposure, though likely not in proportion with cytotoxicity.
Mitochondrial Respiration Is Impaired by Supratoxic Mn Exposures
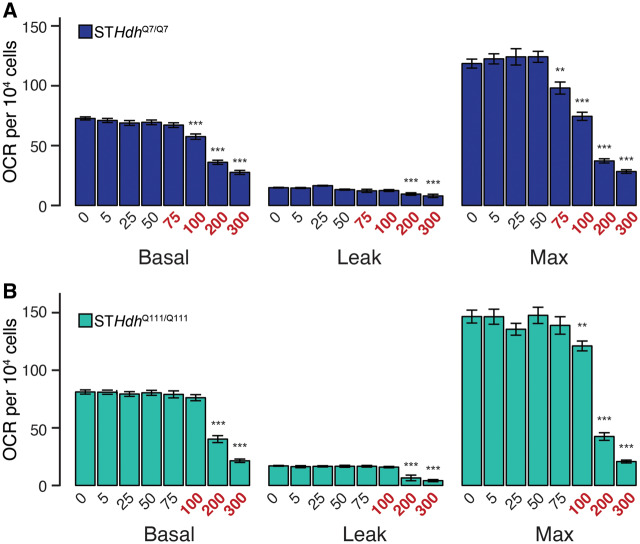
To more directly measure mitochondrial function, we measured oxygen consumption rates (OCR) following Mn exposure. We examined basal, leak, and maximum OCR (Figure 3). After controlling for cell number, we observed that only supratoxic Mn exposures (STHdhQ7/Q7—75µM and above, Figure 3A; STHdhQ111/Q111—100µM and above, Figure 3B) affected any of these parameters. Interestingly, all 3 parameters were similarly affected; ie, high concentrations of Mn reduced basal, leak, and maximum respiration (STHdhQ7/Q7—basal F = 82.82, p < .001, leak F = 10.25, p < .001, max F = 85.97, p < .001; STHdhQ111/Q111—basal F = 89.18, p < .001, leak F = 19.54, p < .001, max F = 79.89, p < .001). These data suggest that Mn does not significantly inhibit mitochondrial function at acute subtoxic concentrations.
Figure 3.
Subtoxic Mn exposures do not inhibit mitochondrial respiration. (A) STHdhQ7/Q7 and (B) STHdhQ111/Q111 basal, leak, and maximum mitochondrial oxygen consumption rates (OCR) (pmol/min) per 104 cells. Mn concentrations (µM), after 24 h exposure, are indicated below the respective bars. Concentrations in red reflect concentrations above which significant cytotoxicity is observed. Results combined from 3 independent experiments, n = 6; total n = 18. Error bars reflect SEM. **p < .01, ***p < .001, by Dunnett’s test relative to control for each measurement.
Subtoxic and Supratoxic Acute Mn Exposures in Primary Striatal Neurons Are Consistent With Observations in the STHdh Cell Lines
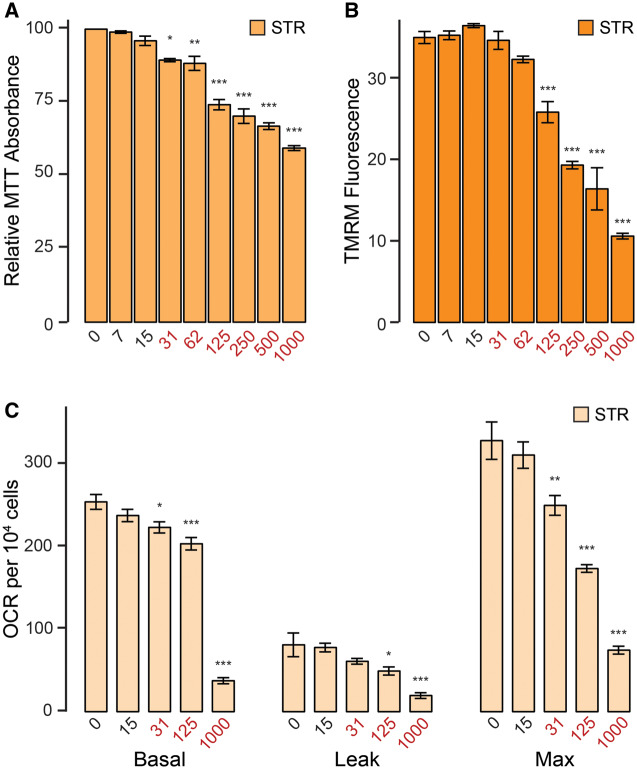
We validated our findings in the STHdh cell lines by performing a similar acute (24 h) Mn exposure paradigm on primary striatal neurons (STR) at days in vitro 7. We determined the cytotoxicity threshold for this cell type with the MTT cytotoxicity assay (Figure 4A). Mn concentrations at or above 31 µM significantly reduced MTT absorbance (F = 51.81, p < .001), whereas lower concentrations did not. We first investigated the effects of Mn on STR mitochondrial function by assessing mitochondrial membrane potential through TMRM fluorescence (Figure 4B). Mn concentrations at or above 125 µM significantly reduced membrane potential (F = 76.6, p < .001). We next evaluated STR mitochondrial respiration (Figure 4C). Basal, leak, and maximum respiration were only inhibited at concentrations at or above the 31-µM cytotoxicity threshold (basal F = 154.2, p < .001, leak F = 10.96, p < .001, max F = 57.63, p < .001), analogous to our findings in the STHdh cell line.
Figure 4.
Mn exposures inhibit mitochondrial respiration in primary striatal cultures only at doses coincident with cytotoxicity. A, Relative MTT absorbance following 24-h Mn exposure. Results combined from 3 independent experiments. B, Tetramethylrhodamine methyl ester (TMRM) fluorescence (in arbitrary fluorescence units) following 24-h Mn exposure. Results combined from 3 independent experiments. C, Basal, leak, and maximum mitochondrial oxygen consumption rates (OCR) (pmol/min) per 104 cells. Results combined from 4 independent experiments. Mn concentration (µM) is indicated below respective bars. Concentrations in red reflect concentrations above which significant cytotoxicity is observed. Error bars reflect SEM. *p < .05, **p < .01, ***p < .001, by Dunnett’s test relative to control for each measurement. Abbreviations: MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); STR, striatum.
Glycolysis, But Not Glycolytic Induction, Is Impaired by Supratoxic Mn Exposures
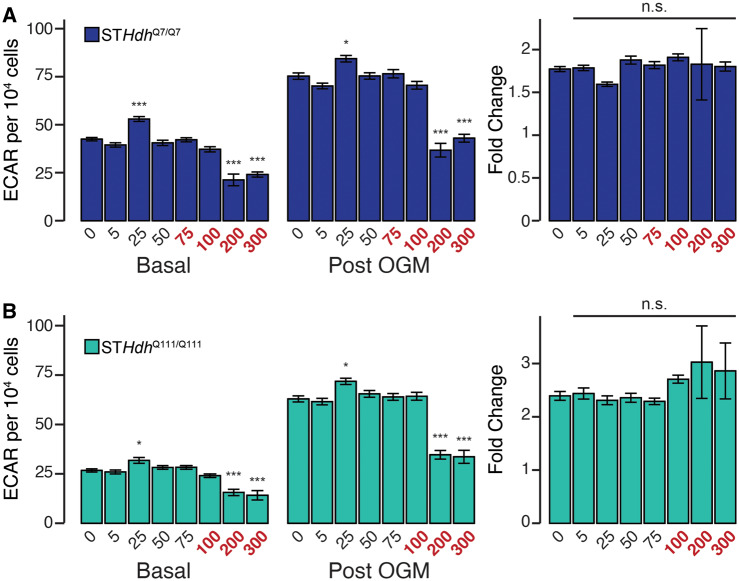
In most proliferating cell lines, the majority of ATP synthesis occurs in the glycolytic branch of glucose metabolism, instead of in the mitochondria. We assessed the impact of Mn exposure on glycolysis by measuring ECAR, simultaneously measured with OCR. We examined basal ECAR, ECAR following treatment with oligomycin (post-OGM), and the fold change between these 2 groups (Figure 5). As treatment with oligomycin inhibits mitochondrial ATP synthesis, this fold change approximates glycolytic induction in response to increased metabolic demand. After controlling for cell number, we observed that only very high concentrations of Mn impaired either basal or induced ECAR in both cell lines (Figure 5A—STHdhQ7/Q7—basal F = 39.77, p < .001, post-OGM F = 60.86, p < .001; Figure 5B—STHdhQ111/Q111—basal F = 22.21, p < .001, post-OGM F = 51.41, p < .001). Strikingly, even these high concentrations of Mn did not affect the fold change between basal and post-OGM ECAR (Figure 5A—STHdhQ7/Q7—F = 0.328, p = .94; Figure 5B—STHdhQ111/Q111—F = 0.823, p = .57).
Figure 5.
Supratoxic Mn exposures reduce glycolytic activity, but not glycolytic induction. A, (left and center) STHdhQ7/Q7 basal and postoligomycin extracellular acidification oligomycin-extracellular acidification rates (OGM-ECAR) (pmol/min) per 104 cells; (right) Percent change between OGM-ECAR and basal ECAR. B, (left and center) STHdhQ111/Q111 basal ECAR and OGM-ECAR per 104 cells, (right) percent change between OGM addition and basal ECAR. Mn concentrations (µM), after 24 h exposure, are indicated below the respective bars. Concentrations in red reflect concentrations above which significant cytotoxicity is observed. Results combined from 3 independent experiments, n = 6; total n = 18. Error bars reflect SEM. *p < .05, ***p < .001, by Dunnett’s test relative to control for each measurement.
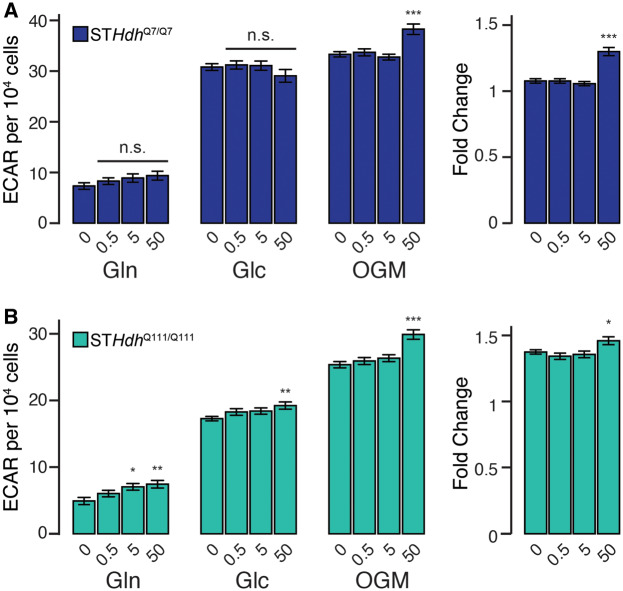
Although our data suggest that only acute supratoxic Mn exposures inhibit glycolytic activity, we additionally observed that subtoxic Mn exposures may increase ECAR. To probe this phenomenon further, we examined glutamine- (Gln), glucose- (Glc), and oligomycin-linked (OGM) ECAR following exposures of subtoxic doses of Mn (0.5, 5, and 50 µM) (Figure 6). In STHdhQ7/Q7 cells, Mn did not affect Gln- or Glc-ECAR (Figure 6A—F = 1.426, p = .24; F = 1.127, p = .34, respectively), but 50 µM Mn did increase OGM-ECAR as well as the fold change between Glc-ECAR and OGM-ECAR (Figure 6A—F = 11.97, p < .001; F = 29.58, p < .001, respectively). In contrast, subtoxic concentrations of Mn slightly increased ECAR in STHdhQ111/Q111 cells. Five and 50 µM Mn increased Gln-ECAR (Figure 6B—F = 4.716, p < .01), whereas 50 µM Mn increased Glc-ECAR (Figure 6B—F = 3.116, p < .05). Similarly to the STHdhQ7/Q7 cells, 50 µM Mn increased OGM-ECAR and the fold change between Glc-ECAR and OGM-ECAR (Figure 5B—F = 13.05, p < .001; F = 4.637, p < .01). Collectively, these data suggest that though supratoxic Mn concentrations may inhibit glycolysis, subtoxic Mn may slightly increase glycolytic activity or glycolytic induction.
Figure 6.
Subtoxic Mn exposures may slightly increase glycolytic activity and induction. (A) STHdhQ7/Q7 and (B) STHdhQ111/Q111 glutamine- (Gln), glucose- (Glc), oligomycin-linked (OGM) extracellular acidification rates (ECAR) (pmol/min) per 104 cells, and fold change between glucose- and oligomycin-linked ECAR. Mn concentrations (µM), after 24-h exposure, are indicated below respective bars. Results combined from 3 independent experiments, n = 10–12; total n = 30–36. Error bars reflect SEM. *p < .05, **p < .01, ***p < .001, by Dunnett’s test relative to control for each measurement.
Mitochondrial Substrate Dependency Is Not Substantially Affected by Subtoxic Mn Exposures
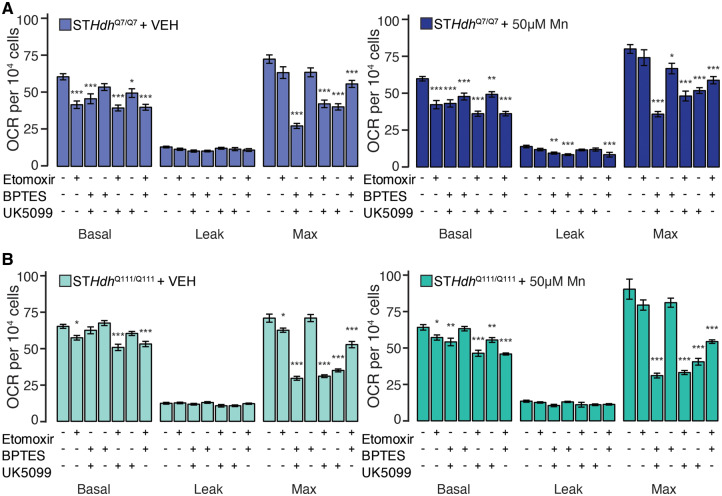
As our previous observations suggested that subtoxic Mn exposures do not affect direct measures of mitochondrial function (ie, ADP/ATP ratio, NADH/NAD+ ratio, and OCR), we next examined mitochondrial function through the lens of substrate dependency. To fuel the respiratory chain, mitochondria can synthesize NADH and FADH2 from a number of fuel sources—namely, glucose, glutamine, and fatty acids. We performed a substrate dependency-sufficiency assay by acutely blocking the major enzymes involved in each of these pathways, and assessing the effects on basal, leak, and maximum OCR (Figure 7). We used etomoxir, which inhibits the carnitine-palmitate transferase (CPT1), to block fatty acid metabolism, BPTES, which inhibits glutaminase, to block glutamine metabolism, and finally UK5099, which inhibits the mitochondrial pyruvate carrier, to block glucose metabolism. In all cell lines, we first confirmed that there were no differences in OCR precompound addition (Supplementary Figure 2). In the unexposed STHdhQ7/Q7 cell line, we observed that etomoxir most potently inhibited basal OCR (Figure 7A) (F = 9.898, p < .001). Alone, BPTES did not significantly reduce OCR, and UK5099 only slightly reduced it, but in combination, both compounds reduced OCR to a similar degree as etomoxir. This suggests that fatty acids are potentially the most significant contributor to sustaining basal OCR, but simultaneously, fatty acid metabolism is not sufficient to maintain vehicle-treated OCR. We observed no differences between groups in leak OCR (F = 1.758, p = .123). In contrast, UK5099 most strongly inhibited maximum respiration, but similarly was insufficient to maintain vehicle-treated levels of maximum OCR (F = 35.47, p < .001). These data suggest that in this cell model basal and maximum respiration are dependent on different fuel pathways. We saw a similar pattern of substrate dependence in the STHdhQ111/Q7111 cell line (Figure 7B), though basal respiration was more subtly impaired by compound addition (basal—F = 11.41, p < .001, leak—F = 2.17, p = .058, max—F = 101, p < .001). In both genotypes, we observed very few effects of Mn exposure. In the STHdhQ7/Q7 cell line (Figure 7A), we observed that again, etomoxir was the strongest inhibitor of basal respiration (F = 35.46, p < .001), and UK5099 was the strongest inhibitor of maximum respiration (F = 23.51, p < .001). Interestingly, compound additions, including BPTES, reduced leak OCR (F = 4.889, p < .001). However, this effect size was small, and not reproduced when the Mn exposure was increased to 100 µM (Supplementary Figure 3). We observed no major differences in substrate dependence in Mn-treated STHdhQ111/Q7111 cell line (Figure 7B) (basal—F = 15.09, p < .001, leak—F = 1.901, p = .094, max—F = 52.65, p < .001). Consequently, we hypothesize that neither subtoxic nor supratoxic Mn exposures impact mitochondrial substrate dependence in STHdh cells.
Figure 7.
Subtoxic Mn exposure does not substantially affect mitochondrial substrate dependence. (A) STHdhQ7/Q7 and (B) STHdhQ111/Q111 basal, leak, and maximum mitochondrial oxygen consumption rates (OCR) (pmol/min). Left, vehicle-treated cultures, right, cultures treated with 50 µM Mn 24 h prior to assay. Compounds added to inhibit substrate utilization during the course of the assay are indicated below respective bars. Results combined from 3 independent experiments, n = 3–5; total n = 10. Error bars reflect SEM. *p < .05, **p < .01, ***p < .001, by Dunnett’s test relative to control for each measurement.
Subtoxic Mn Exposures Do Not Significantly Alter Intracellular Metabolite Quantity
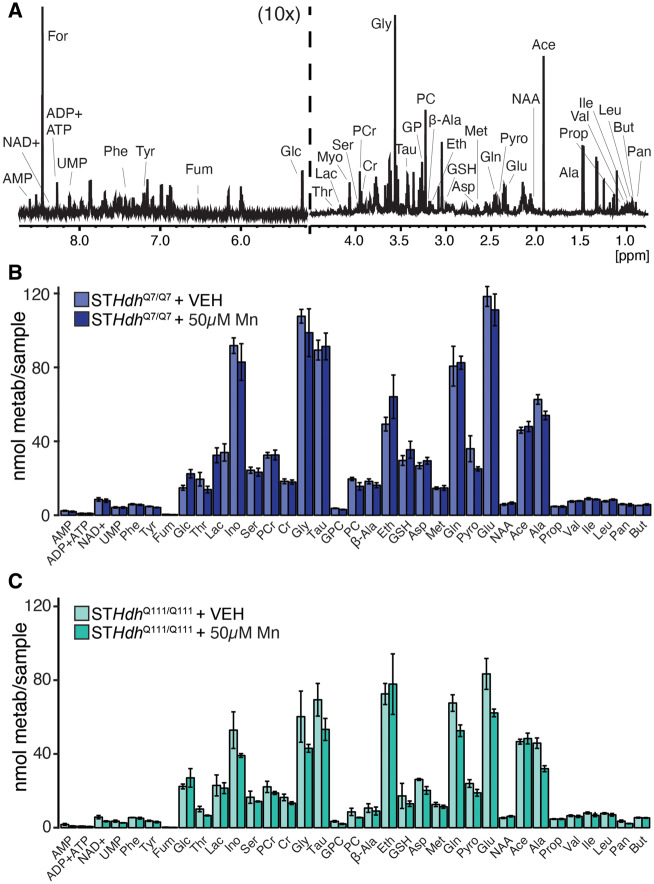
To further probe for metabolic perturbations induced by Mn exposure, we performed 1H NMR spectroscopy to examine intracellular metabolite quantity in both STHdh cell lines. Figure 8A is a representative trace from one of these samples, with the peaks used to quantify each metabolite indicated (see figure legend for abbreviations). We subsequently compared STHdhQ7/Q7 (Figure 8B) and STHdhQ111/Q7111 (Figure 8C) metabolite quantities with and without Mn exposure. Though in some cases we observed a difference in mean between the treated and untreated samples, none of these differences were significant following correction for multiple comparisons. Thus, this further supports our observations that acute subtoxic Mn exposures do not seem to substantially affect mitochondrial or metabolic function in either of our models.
Figure 8.
Threshold toxic Mn exposure does not significantly affect intracellular metabolite quantities. A, Representative 1H NMR spectrum of detectable aqueous metabolites from STHdh cells from 0.8 to 4.5 and 5.2 to 8.6 ppm; 5.2–8.6 ppm is magnified ×10 relative to 0.8–4.5 ppm section. Peaks used to quantify each compound are indicated; additional compound peaks are omitted for clarity. Abbreviations: Ace, acetate; Ala, alanine; β-Ala, β-Alanine; AMP, adenosine monophosphate; Asp, aspartate; But, butyrate; Cr, creatine; Eth, ethanolamine; For, formate; Fum, fumarate; Glc, glucose; Gln, glutamine; Glu, glutamate; Gly, glycine; GPC, glycerophosphocholine; GSH, glutathione; Ile, isoleucine; Ino, myo-inositol; Lac, lactate; Leu, leucine; Met, methionine; NAA, N-acetyl aspartate; NAD+, nicotinaminde adenine dinucleotide; Pan, pantothenate; PC, phosphocholine; PCr, phosphocreatine; Phe, phenylalanine; Prop, propionate; Pyro, pyroglutamate; Ser, serine; Tau, taurine; Thr, threonine; Tyr, tyrosine; UMP, uridine monophosphate; Val, valine. Formate peak is a contaminant from sample preparation. (B) STHdhQ7/Q7 and (C) STHdhQ111/Q111, metabolite quantifications of VEH-treated and 50 µM Mn-treated cultures, n = 5. Error bars reflect SEM. Each metabolite quantity compared by Student’s t test, with the Benjamini-Hochberg method used to correct for multiple comparisons. No comparisons were significant following correction.
DISCUSSION
In this study, we examined the effect of acute Mn exposure on mitochondrial and metabolic function at biologically relevant concentrations (Bowman and Aschner, 2014). Across these experiments, we did not observe any reduction in mitochondrial or metabolic function at subtoxic concentrations of Mn, whereas at supratoxic concentrations, we observed an increase in the ADP/ATP ratio and a reduction in OCR and ECAR.
We calculated an LC50 of media Mn concentration at 130.33 µM for STHdhQ7/Q7 cells, and an LC50 of 166.69 µM for STHdhQ111/Q111 cells (Figure 1). The LC50 of 130.33 µM is within the toxicity-relevant range of 60.1–158.4 µM (Bowman and Aschner, 2014). This concentration is lower than some LC50 values (Castano and Gomez-Lechon, 2005; Rovetta et al., 2007), though comparable to IC50 values reported for inhibition of metabolic activity in other cell lines (Malthankar et al., 2004; Sarkar et al., 2018). The higher LC50 for the STHdhQ111/Q111 line, 166.69 µM, supports the reduction in Mn uptake robustly reported in our lab (Bichell et al., 2017; Tidball et al., 2015; Williams et al., 2010). Extrapolating from our CFMEA results, these doses are equivalent to, in the STHdhQ7/Q7 line, approximately 10.63 nmoles Mn/104 cells, and in the STHdhQ111/Q111 line, approximately 6.74 nmoles Mn/104 cells. At our determined cytotoxicity thresholds, 75 and 100 µM, this corresponds to 5.77 nmoles Mn/104 cells and 3.81 nmoles Mn/104 cells. This supports our previous observations that the mutant Q111/Q111 genotype may protect against Mn exposure by reducing Mn uptake, without conferring additional resistance against Mn toxicity (Tidball et al., 2015; Williams et al., 2010). We did not calculate an LC50 for the STR experiments, as MTT absorbance is an approximation of cell viability that is partially dependent on mitochondrial activity, which precludes the assessment of a “true” cytotoxic threshold, however we observe the same trend between cytotoxicity and mitochondrial toxicity in these cells.
We did not observe any changes in mitochondrial respiration at subtoxic Mn exposures (Figs. 3 and 4). We did observe reduced mitochondrial activity at supratoxic concentrations, in excess of that accountable for by cell death, supporting observations that at high exposures, Mn inhibits mitochondrial function. Similarly, we only observed a reduction in glycolytic activity following supratoxic Mn exposures (Figure 5). We observed that although the STHdhQ7/Q7 line and STHdhQ111/Q111 line have comparable OCR per cell, the STHdhQ111/Q111 line has a much lower basal ECAR, which we observed both when glucose and glutamine are available as substrates (Figure 5B, “Basal”), and additionally when only glutamine is available (Figure 6B, “Gln”).
Glycolytic deficiency has been previously observed in the STHdhQ111/Q111 cell line (Lee et al., 2007). Furthermore, deficits in glycolytic metabolism have been observed in human HD subjects (Powers et al., 2007) and murine models of HD (Dubinsky, 2017; Gouarne et al., 2013; Hamilton et al., 2015). Our data recapitulate these findings, lending further support to the phenotype of deficient glycolytic metabolism in HD. Two observations warrant further investigation. First, we observed an increase in ECAR in both cell lines in response to subtoxic doses of Mn, though slightly inconsistent between the assays used (Figs. 5 and 6), likely due to the different treatment paradigms and assay media in these 2 experiments. Mn regulates the activity of metabolic enzymes (see below) which may increase glycolytic rate, though it is surprising that we see this effect more consistently in the STHdhQ111/Q111 cell line which has a lower Mn uptake. This difference may be attributable to both the low basal ECAR of the STHdhQ111/Q111 cell line, which makes small changes more visible, and the possibility that STHdhQ111/Q111 cells are Mn-deficient, such that even small differences in Mn uptake may alter Mn-regulated metabolic enzymes. Intriguingly, this Mn-induced increase in glycolytic function observed in both cell lines may be related to a cellular mechanism by which Mn enhances insulin receptor and/or insulin growth factor receptor signaling (Bryan et al., 2020). Amplifying insulin signaling increases glucose uptake and likely glycolytic activity. Second, we observed that although the STHdhQ111/Q111 cell line has a lower basal ECAR, the fold change in glycolytic induction in response to OGM was about 1.5× greater than in STHdhQ7/Q7 cells. This suggests that the lower glycolytic rate in STHdhQ111/Q111 cells is inducible. It may be useful for future studies to determine if reduced glycolytic activity is a cause of perturbed metabolism due to mutant Htt.
We hypothesized that Mn may affect mitochondrial function by altering substrate utilization, as Mn regulates the activity of several crucial metabolic enzymes. These include arginase (Ash, 2004; Bichell et al., 2017), which catalyzes a portion of the urea cycle, pyruvate carboxylase and phosphoenolpyruvate carboxykinase, an anaplerotic and a cataplerotic enzyme adjacent to the TCA cycle, respectively (Baly et al., 1985; Horning et al., 2015), glutamine synthetase (Rose et al., 2013; Wedler et al., 1982), an essential astrocytic enzyme that regenerates glutamine from glutamate, and acetyl CoA carboxylase (Scorpio and Masoro, 1970), the rate-limiting step of de novo lipogenesis. Consequently, Mn exposure may affect carbohydrate, nitrogen, or lipid metabolism (Fordahl et al., 2012). We did not observe any effects of either subtoxic (Figure 7) or supratoxic (Supplementary Figure 2) Mn on the pattern of substrate dependency in this assay, nor did we observe any substantial genotype-driven differences in substrate utilization. Broadly, we observed that inhibition of fatty acid utilization inhibited basal respiration, whereas inhibition of carbohydrate utilization inhibited maximum respiration in both cell lines and independently of Mn. Furthermore, we did not observe any significant alterations in 1H NMR-detectable metabolite quantity from subtoxic (50 µM) Mn exposures (Figure 8).
In this study, we observed that acute Mn exposures do not inhibit mitochondrial respiration, glycolysis, or perturb intracellular metabolism at subcytotoxic doses. We put forward a new hypothesis that acute Mn-induced cytotoxicity/cell death is not due to the crossing a cytotoxic threshold of Mn-induced mitochondrial dysfunction from processes initiated at subtoxic doses, which suggests that acute Mn cytotoxicity may be driven by other mechanisms. One potential mechanism may be the Mn-linked activation of p53, which can induce apoptosis by impairing mitochondrial function (Zhao et al., 2005). We have demonstrated that Mn robustly activates p53 at 50 µM in the STHdhQ7/Q7 cell line, a dose which does not impair mitochondrial function (Tidball et al., 2015). Inhibition of p53 improved many indicators of Mn-induced mitotoxicity, including RONS generation and mitochondrial membrane potential reduction (Wan et al., 2014), suggesting that Mn-mediated activation of p53 may contribute to mitochondrial dysfunction. We have also recently demonstrated that Mn inhibits the transcription factor EB, which impairs mitophagic flux (Zhang et al., 2019) implicating autophagy as a potential regulator of Mn-induced mitochondrial dysfunction in astrocytes. Future studies should clarify the link between Mn exposure and mitochondrial dysfunction.
In this study, we provide evidence that Mn-induced cell death precedes inhibition of mitochondrial respiration in murine neuroprogenitors and striatal primary neurons. However, numerous studies have demonstrated the impact of Mn exposures on mitochondrial function. What is causing these observed dichotomies in Mn-induced toxicity between model systems? We propose 5 potential explanations for these differences:
First, as previously suggested, is concentration. At high concentrations, acute Mn affects mitochondrial function and triggers apoptosis in cell lines (Alaimo et al., 2013; Fernandes et al., 2017; Galvani et al., 1995; Prabhakaran et al., 2009) and primary neurons and astrocytes (Malecki, 2001; Sarkar et al., 2018; Stuntz et al., 2017). As many of these studies use concentrations of Mn near or above the calculated LC50 for each system, our data suggest that it may be necessary to determine whether the inhibition of mitochondrial function is a primary or secondary effect to other intracellular cascades. It will be important to distinguish the effects of Mn on mitochondrial respiration and metabolism from other aspects of mitochondrial function that may be disrupted (eg, trafficking, fusion, mitophagy).
Second, an important distinction may come from acute or chronic exposure paradigms. Although we have demonstrated that acute exposures may trigger nonmitochondrial cytotoxic mechanisms, it is likely that chronic, low-dose exposures may impair mitochondrial activity without triggering other cytotoxic pathways (Jiang et al., 2014; Morello et al., 2008; Zhang et al., 2003). Kanthasamy and colleagues recently reported that chronic Mn accelerates neurodegeneration and motor deficits in the MitoPark mouse, a model of PD generated via the elimination of TFAM, a key factor in mitochondrial biogenesis, in dopaminergic neurons (Langley et al., 2018). Gradual Mn mitochondrial accumulation, as mitochondria act as a sink for Mn (Gavin et al., 1990, 1992), will be an important future direction to investigate in vitro. This will facilitate the disaggregation of cytosolic responses from true mitochondrial responses to long-term Mn exposure.
Third, differences in cell type may play an important role. Although we have focused on primary neurons and neuroprogenitors in this study, astrocytes take up more Mn than neurons (Aschner et al., 1992; Hazell, 2002), and may be key elements in the cascade following Mn exposure to cause neurodegeneration. Recent studies suggest microglia may contribute to neurodegeneration through Mn activation of inflammatory responses (Kirkley et al., 2017; Tjalkens et al., 2017). Future studies should elucidate key differences in Mn neurotoxicity among neurons, astrocytes, and microglia, and the mechanistic basis by which this occurs.
Fourth, interactions between mitochondrial Ca2+ and Mn uptake and efflux may impact the mitochondrial toxicity of Mn. In this study, we have used cell lines and unstimulated striatal neurons to the impact of Mn, but in vivo, neuronal intracellular calcium is constantly in flux in response to electrochemical stimulation. Cytosolic calcium concentrations are highly regulated by mitochondrial uptake (Duchen, 2012; Pivovarova and Andrews, 2010); moreover, many intramitochondrial metabolic reactions are calcium-dependent (McCormack et al., 1990), and Mn is a potent inhibitor of Ca2+ efflux from mitochondria (Gavin et al., 2009). Consequently, further studies should use stimulation paradigms to explore the magnitude and toxicity of mitochondrial Mn uptake during neuronal activity.
Finally, Mn accumulation may occur in different subcellular compartments across cell types. A recent study used nanosynchorotron x-ray fluorescence to reveal the Golgi may be a key site of Mn storage in HEK293T cells (Das et al., 2019), confirming previous observations of perinuclear and likely Golgi Mn accumulation (Carmona et al., 2010; Ducic et al., 2013; Robison et al., 2015). We hypothesize that Mn may preferentially accumulate in different organelles to elicit differential toxicities to those cell types.
These data highlight the needs to further study how manganese causes neurotoxicity and neurodegeneration in cell-type and organelle-specific manner. Further investigation into the crosstalk between cell types and specific trafficking into/from organelles may elucidate not only the key pathways involved, but also the order in which these processes occur. Such studies will be imperative to fully understand how Mn causes potent and selective neurodegeneration and can provide multiple therapeutic avenues to target Mn toxicity.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Donald Stec, PhD, Markus Voehler, PhD, Dehui Mi, PhD, and Joshua Bauer, PhD, for instrument training and support.
FUNDING
National Institutes of Health/National Institute of Environmental Health Sciences (RO1 ES010563 to A.B.B. and M.A., RO1 ES016931 to A.B.B., F31 ES028084 to M.R.B.).
DECLARATION OF CONFLICTING INTERESTS
The senior author, Dr Aaron Bowman, is an associate editor for this journal.
REFERENCES
- Alaimo A., Gorojod R. M., Miglietta E. A., Villarreal A., Ramos A. J., Kotler M. L. (2013). Manganese induces mitochondrial dynamics impairment and apoptotic cell death: A study in human Gli36 cells. Neurosci. Lett. 554, 76–81. [DOI] [PubMed] [Google Scholar]
- Aschner M., Gannon M., Kimelberg H. K. (1992). Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 58, 730–735. [DOI] [PubMed] [Google Scholar]
- Ash D. E. (2004). Structure and function of arginases. J. Nutr. 134, 2760S–2764, discussion 2765S–2767. [DOI] [PubMed] [Google Scholar]
- Austin S., Tavakoli M., Pfeiffer C., Seifert J., Mattarei A., De Stefani D., Zoratti M., Nowikovsky K. (2017). LETM1-mediated K(+) and Na(+) homeostasis regulates mitochondrial Ca(2+) efflux. Front. Physiol. 8, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baly D. L., Keen C. L., Hurley L. S. (1985). Pyruvate carboxylase and phosphoenolpyruvate carboxykinase activity in developing rats: Effect of manganese deficiency. J. Nutr. 115, 872–879. [DOI] [PubMed] [Google Scholar]
- Bates G. P., Dorsey R., Gusella J. F., Hayden M. R., Kay C., Leavitt B. R., Nance M., Ross C. A., Scahill R. I., Wetzel R., et al. (2015). Huntington disease. Nat. Rev. Dis. Primers 1, 15005. [DOI] [PubMed] [Google Scholar]
- Bichell T. J. V., Wegrzynowicz M., Tipps K. G., Bradley E. M., Uhouse M. A., Bryan M., Horning K., Fisher N., Dudek K., Halbesma T., et al. (2017). Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington’s disease mouse model. Biochim. Biophys. Acta 1863, 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A. B., Aschner M. (2014). Considerations on manganese (Mn) treatments for in vitro studies. NeuroToxicology 41, 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan M. R., Nordham K. D., Rose D. I. R., O’Brien M. T., Joshi P., Foshage A. M., Goncalves F. M., Nitin R., Uhouse M. A., Aschner M., et al. (2020). Manganese acts upon insulin/IGF receptors to phosphorylate AKT and increase glucose uptake in Huntington’s disease cells. Mol. Neurobiol. 57, 1570–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona A., Deves G., Roudeau S., Cloetens P., Bohic S., Ortega R. (2010). Manganese accumulates within Golgi apparatus in dopaminergic cells as revealed by synchrotron x-ray fluorescence nanoimaging. ACS Chem. Neurosci. 1, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A., Gomez-Lechon M. J. (2005). Comparison of basal cytotoxicity data between mammalian and fish cell lines: A literature survey. Toxicol. In Vitro 19, 695–705. [DOI] [PubMed] [Google Scholar]
- Cersosimo M. G., Koller W. C. (2006). The diagnosis of manganese-induced parkinsonism. NeuroToxicology 27, 340–346. [DOI] [PubMed] [Google Scholar]
- Chen P., Miah M. R., Aschner M. (2016). Metals and neurodegeneration. F1000Res 5, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Carmona A., Khatua K., Porcaro F., Somogyi A., Ortega R., Datta A. (2019). Manganese mapping using a fluorescent Mn(2+) sensor and nanosynchrotron x-ray fluorescence reveals the role of the Golgi apparatus as a manganese storage site. Inorg. Chem. 58, 13724–13732. [DOI] [PubMed] [Google Scholar]
- De Stefani D., Rizzuto R., Pozzan T. (2016). Enjoy the trip: Calcium in mitochondria back and forth. Annu. Rev. Biochem. 85, 161–192. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M. (2017). Towards an understanding of energy impairment in Huntington’s disease brain. J. Huntingtons Dis. 6, 267–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. (2012). Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 464, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducic T., Barski E., Salome M., Koch J. C., Bahr M., Lingor P. (2013). x-Ray fluorescence analysis of iron and manganese distribution in primary dopaminergic neurons. J. Neurochem. 124, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Hao L., Bijli K. M., Chandler J. D., Orr M., Hu X., Jones D. P., Go Y. M. (2017). From the cover: Manganese stimulates mitochondrial H2O2 production in SH-SY5Y human neuroblastoma cells over physiologic as well as toxicologic range. Toxicol. Sci. 155, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordahl S., Cooney P., Qiu Y., Xie G., Jia W., Erikson K. M. (2012). Waterborne manganese exposure alters plasma, brain, and liver metabolites accompanied by changes in stereotypic behaviors. Neurotoxicol. Teratol. 34, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C., Amaya E., Gil F., Fernandez M. F., Murcia M., Llop S., Andiarena A., Aurrekoetxea J., Bustamante M., Guxens M., et al. (2018). Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) project. Sci. Total Environ. 621, 340–351. [DOI] [PubMed] [Google Scholar]
- Galvani P., Fumagalli P., Santagostino A. (1995). Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharmacol. 293, 377–383. [DOI] [PubMed] [Google Scholar]
- Gavin C. E., Gunter K. K., Gunter T. E. (1990). Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem. J. 266, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin C. E., Gunter K. K., Gunter T. E. (1992). Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol. Appl. Pharmacol. 115, 1–5. [DOI] [PubMed] [Google Scholar]
- Gavin C. E., Gunter K. K., Gunter T. E. (2009). Manganese and calcium transport in mitochondria: Implications for manganese toxicity. NeuroToxicology 30, 427–453. [PubMed] [Google Scholar]
- Gorini F., Muratori F., Morales M. A. (2014). The role of heavy metal pollution in neurobehavioral disorders: A focus on autism. Rev. J. Autism Dev. Disord. 1, 354–372. [Google Scholar]
- Gouarne C., Tardif G., Tracz J., Latyszenok V., Michaud M., Clemens L. E., Yu-Taeger L., Nguyen H. P., Bordet T., Pruss R. M. (2013). Early deficits in glycolysis are specific to striatal neurons from a rat model of Huntington disease. PLoS One 8, e81528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. R. (2010). Manganese and Parkinson’s disease: A critical review and new findings. Environ. Health Perspect. 118, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. R., Chen M. K., McGlothan J. L., Verina T., Wong D. F., Zhou Y., Alexander M., Rohde C. A., Syversen T., Decamp E., et al. (2006). Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 202, 381–390. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Gavin C. E., Gunter K. K. (2009). The case for manganese interaction with mitochondria. NeuroToxicology 30, 727–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J., Pellman J. J., Brustovetsky T., Harris R. A., Brustovetsky N. (2015). Oxidative metabolism in YAC128 mouse model of Huntington’s disease. Hum. Mol. Genet. 24, 4862–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell A. S. (2002). Astrocytes and manganese neurotoxicity. Neurochem. Int. 41, 271–277. [DOI] [PubMed] [Google Scholar]
- Horning K. J., Caito S. W., Tipps K. G., Bowman A. B., Aschner M. (2015). Manganese is essential for neuronal health. Annu. Rev. Nutr. 35, 71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, P. D., Malcolm, H. M, Dobson, S., and World Health Organization & International Programme on Chemical Safety. (2004). Manganese and Its Compounds: Environmental Aspects. World Health Organization, Geneva. Available at: https://apps.who.int/iris/handle/10665/42992. Accessed June 12, 2020. [Google Scholar]
- Jaishankar M., Tseten T., Anbalagan N., Mathew B. B., Beeregowda K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. K., Ma X., Wu Q. Y., Qian W. B., Wang N., Shi S. S., Han J. L., Zhao J. Y., Jiang S. Y., Wan C. H. (2014). Upregulation of mitochondrial protease HtrA2/Omi contributes to manganese-induced neuronal apoptosis in rat brain striatum. Neuroscience 268, 169–179. [DOI] [PubMed] [Google Scholar]
- Kamer K. J., Sancak Y., Fomina Y., Meisel J. D., Chaudhuri D., Grabarek Z., Mootha V. K. (2018). MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca(2+) and Mn(2 +) Proc. Natl. Acad. Sci. U.S.A. 115, E7960–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Kumar V., Arora A., Tomar A., Ashish A., Sur R., Dutta D. (2017). Affected energy metabolism under manganese stress governs cellular toxicity. Sci. Rep. 7, 11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley K. S., Popichak K. A., Afzali M. F., Legare M. E., Tjalkens R. B. (2017). Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflamm.14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakye G. F., Li D., Kabobel O. A., Bowman A. B. (2011). Cellular fura-2 manganese extraction assay (CFMEA). Curr. Protoc. Toxicol. Chapter 12, Unit 12.18. [DOI] [PMC free article] [PubMed]
- Kwakye G. F., Paoliello M. M., Mukhopadhyay S., Bowman A. B., Aschner M. (2015). Manganese-induced parkinsonism and Parkinson’s disease: Shared and distinguishable features. Int. J. Environ. Res. Public Health 12, 7519–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J., Morimoto R. I. (2013). Huntington’s disease: Underlying molecular mechanisms and emerging concepts. Trends Biochem. Sci. 38, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley M. R., Ghaisas S., Ay M., Luo J., Palanisamy B. N., Jin H., Anantharam V., Kanthasamy A., Kanthasamy A. G. (2018). Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: Relevance to gene and environment interactions in metal neurotoxicity. NeuroToxicology 64, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Ivanova E. V., Seong I. S., Cashorali T., Kohane I., Gusella J. F., MacDonald M. E. (2007). Unbiased gene expression analysis implicates the Huntingtin polyglutamine tract in extra-mitochondrial energy metabolism. PLoS Genet. 3, e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccione J. J., Maines M. D. (1989). Manganese-mediated increase in the rat brain mitochondrial cytochrome P-450 and drug metabolism activity: Susceptibility of the striatum. J. Pharmacol. Exp. Ther. 248, 222–228. [PubMed] [Google Scholar]
- Malecki E. A. (2001). Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res. Bull. 55, 225–228. [DOI] [PubMed] [Google Scholar]
- Malthankar G. V., White B. K., Bhushan A., Daniels C. K., Rodnick K. J., Lai J. (2004). Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem. Res. 29, 709–717. [DOI] [PubMed] [Google Scholar]
- Maynard L. S., Cotzias G. C. (1955). The partition of manganese among organs and intracellular organelles of the rat. J. Biol. Chem. 214, 489–495. [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. (1990). Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Scrutton M. C., Utter M. F. (1966). Pyruvate carboxylase. VII. A possible role for tightly bound manganese. J. Biol. Chem. 241, 3488–3498. [PubMed] [Google Scholar]
- Morello M., Canini A., Mattioli P., Sorge R. P., Alimonti A., Bocca B., Forte G., Martorana A., Bernardi G., Sancesario G. (2008). Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats an electron spectroscopy imaging and electron energy-loss spectroscopy study. NeuroToxicology 29, 60–72. [DOI] [PubMed] [Google Scholar]
- Mousa A. M., Shehab A. A. (2015). The effect of manganese on the olfactory bulb of adult male albino rat and the role of meloxicam: A histological and immunohistochemical study. J. Microsc. Ultrastruct. 3, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal A. P., Guilarte T. R. (2013). Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. (Camb.) 2, 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely D. M., Davison C., Aschner M., Bowman A. B. (2017). From the cover: Manganese and rotenone-induced oxidative stress signatures differ in iPSC-derived human dopamine neurons. Toxicol. Sci. 159, 366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S. L., Zheng W. (2015). Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovarova N. B., Andrews S. B. (2010). Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 277, 3622–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers W. J., Videen T. O., Markham J., McGee-Minnich L., Antenor-Dorsey J. V., Hershey T., Perlmutter J. S. (2007). Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proc. Natl. Acad. Sci. U.S.A. 104, 2945–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Chapman G. D., Gunasekar P. G. (2009). BNIP3 up-regulation and mitochondrial dysfunction in manganese-induced neurotoxicity. NeuroToxicology 30, 414–422. [DOI] [PubMed] [Google Scholar]
- Racette B. A. (2014). Manganism in the 21st century: The Hanninen lecture. NeuroToxicology 45, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K., Fatima F., Waheed I., Akash M. (2018). Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 119, 157–184. [DOI] [PubMed] [Google Scholar]
- Ritz C., Baty F., Streibig J. C., Gerhard D. (2015). Dose-response analysis using R. PLoS One 10, e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G., Sullivan B., Cannon J. R., Pushkar Y. (2015). Identification of dopaminergic neurons of the substantia nigra pars compacta as a target of manganese accumulation. Metallomics 7, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. A. (2010). Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 5, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C. F., Verkhratsky A., Parpura V. (2013). Astrocyte glutamine synthetase: Pivotal in health and disease. Biochem. Soc. Trans. 41, 1518–1524. [DOI] [PubMed] [Google Scholar]
- Rovetta F., Catalani S., Steimberg N., Boniotti J., Gilberti M. E., Mariggio M. A., Mazzoleni G. (2007). Organ-specific manganese toxicity: A comparative in vitro study on five cellular models exposed to MnCl(2). Toxicol. In Vitro 21, 284–292. [DOI] [PubMed] [Google Scholar]
- Santamaria A. B., Sulsky S. I. (2010). Risk assessment of an essential element: Manganese. J. Toxicol. Environ. Health A 73, 128–155. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Malovic E., Harischandra D. S., Ngwa H. A., Ghosh A., Hogan C., Rokad D., Zenitsky G., Jin H., Anantharam V., et al. (2018). Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. NeuroToxicology 64, 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorpio R. M., Masoro E. J. (1970). Differences between manganese and magnesium ions with regard to fatty acid biosynthesis, acetyl-coenzyme A carboxylase activity and malonyl-coenzyme A decarboxylation. Biochem. J. 118, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuntz E., Gong Y., Sood D., Liaudanskaya V., Pouli D., Quinn K. P., Alonzo C., Liu Z., Kaplan D. L., Georgakoudi I. (2017). Endogenous two-photon excited fluorescence imaging characterizes neuron and astrocyte metabolic responses to manganese toxicity. Sci. Rep. 7, 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. (2012). Heavy metal toxicity and the environment. EXS 101, 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball A. M., Bryan M. R., Uhouse M. A., Kumar K. K., Aboud A. A., Feist J. E., Ess K. C., Neely M. D., Aschner M., Bowman A. B. (2015). A novel manganese-dependent ATM-p53 signaling pathway is selectively impaired in patient-based neuroprogenitor and murine striatal models of Huntington’s disease. Hum. Mol. Genet. 24, 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalkens R. B., Popichak K. A., Kirkley K. A. (2017). Inflammatory activation of microglia and astrocytes in manganese neurotoxicity. Adv. Neurobiol. 18, 159–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel F., Rigamonti D., Hilditch-Maguire P., Wheeler V. C., Sharp A. H., Persichetti F., Cattaneo E., MacDonald M. E. (2000). Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum. Mol. Genet. 9, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Tsai M. F., Jiang D., Zhao L., Clapham D., Miller C. (2014). Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J. Gen. Physiol. 143, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos V., Hernandez-Fonseca J. P., Bonilla E., Medina-Leendertz S., Mora M., Mosquera J. (2015). Ultrastructural changes of caudate nucleus in mice chronically treated with manganese. Ultrastruct. Pathol. 39, 217–225. [DOI] [PubMed] [Google Scholar]
- Walsh J. G., Cullen S. P., Sheridan C., Luthi A. U., Gerner C., Martin S. J. (2008). Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. U.S.A. 105, 12815–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C., Ma X., Shi S., Zhao J., Nie X., Han J., Xiao J., Wang X., Jiang S., Jiang J. (2014). Pivotal roles of p53 transcription-dependent and -independent pathways in manganese-induced mitochondrial dysfunction and neuronal apoptosis. Toxicol. Appl. Pharmacol. 281, 294–302. [DOI] [PubMed] [Google Scholar]
- Warren E. B., Aicher A. E., Fessel J. P., Konradi C. (2017). Mitochondrial DNA depletion by ethidium bromide decreases neuronal mitochondrial creatine kinase: Implications for striatal energy metabolism. PLoS One 12, e0190456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedler F. C., Denman R. B., Roby W. G. (1982). Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry 21, 6389–6396. [DOI] [PubMed] [Google Scholar]
- Weinert M. Selvakumar T. Tierney T. S., Alavian K. N., (2015). Isolation, culture and long-term maintenance of primary mesencephalic dopaminergic neurons from embryonic rodent brains. J. Vis. Exp. 52475. doi: 10.3791/52475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. B., Li D., Wegrzynowicz M., Vadodaria B. K., Anderson J. G., Kwakye G. F., Aschner M., Erikson K. M., Bowman A. B. (2010). Disease-toxicant screen reveals a neuroprotective interaction between Huntington’s disease and manganese exposure. J. Neurochem. 112, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. O., Baccarelli A. (2007). Metals and neurotoxicology. J. Nutr. 137, 2809–2813. [DOI] [PubMed] [Google Scholar]
- Yin Z., Aschner J. L., dos Santos A. P., Aschner M. (2008). Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 1203, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhou Z., Fu J. (2003). Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ. Res. 93, 149–157. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yan J., Bowman A. B., Bryan M. R., Singh R., Aschner M. (2019). Dysregulation of TFEB contributes to manganese-induced autophagic failure and mitochondrial dysfunction in astrocytes. Autophagy doi: 10.1080/15548627.2019.1688488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chaiswing L., Velez J. M., Batinic-Haberle I., Colburn N. H., Oberley T. D., St. Clair D. K. (2005). p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 65, 3745–3750. [DOI] [PubMed] [Google Scholar]
- Zheng W., Ren S., Graziano J. H. (1998). Manganese inhibits mitochondrial aconitase: A mechanism of manganese neurotoxicity1Published on the World Wide Web on 3 June 1998.1. Brain Res. 799, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.