Key Points
S. aureus–infected DCs induce Vδ2+ cell IFN-γ secretion via cell contact and IL-12.
Vδ2+ IFN-γ secretion promotes reciprocal enhancement of DC activation.
Coculturing infected DCs with γδ T cells enhances CD4+ cell responses to S. aureus.
Abstract
Murine studies have shown the potential for γδ T cells to mediate immunity to Staphylococcus aureus in multiple tissue settings by the secretion of diverse cytokines. However, the role played by γδ T cells in human immune responses to S. aureus is almost entirely unknown. In this study, we establish the capacity of human Vδ2+ γδ T cells for rapid activation in response to S. aureus. In coculture with S. aureus–infected monocyte-derived dendritic cells (DCs), Vδ2+ cells derived from peripheral blood rapidly upregulate CD69 and secrete high levels of IFN-γ. DCs mediate this response through direct contact and IL-12 secretion. In turn, IFN-γ released by Vδ2+ cells upregulates IL-12 secretion by DCs in a positive feedback loop. Furthermore, coculture with γδ T cells results in heightened expression of the costimulatory molecule CD86 and the lymph node homing molecule CCR7 on S. aureus–infected DCs. In cocultures of CD4+ T cells with S. aureus–infected DCs, the addition of γδ T cells results in heightened CD4+ T cell activation. Our findings identify γδ T cells as potential key players in the early host response to S. aureus during bloodstream infection, promoting enhanced responses by both innate and adaptive immune cell populations, and support their consideration in the development of host-directed anti–S. aureus treatments.
Introduction
Staphylococcus aureus is a leading cause of skin, soft tissue, and surgical site infections, infective endocarditis, osteomyelitis, septic arthritis, and pneumonia, among other diverse pathologic conditions (1, 2). However, it is in the bloodstream where its greatest public health burden lies; S. aureus is a leading cause of bloodstream infection, with a 30-d mortality rate >20% (3, 4). Prognosis was even worse in preantibiotic days when fatality rates were over 75% (5), a sobering thought when one considers the rising problem of antibiotic resistance. Strains of S. aureus resistant to virtually every known antibiotic have now been identified (6). Methicillin-resistant S. aureus, in particular, has spread beyond the healthcare setting, causing community-acquired infections across the globe, and is disproportionately associated with some of the bacterium’s most devastating clinical infections (6). Thus, a protective anti–S. aureus vaccine has never been a higher priority.
Although numerous candidate vaccines have successfully induced lasting protective immunity in mice, this protection has not been successfully translated to humans (7). In a number of cases, vaccine candidates have induced significant anti-staphylococcal Ab titers but were ineffective at reducing bacteremia or mortality (8). To date, no candidate vaccine has successfully induced robust anti–S. aureus T cell responses in humans (9). Cellular immunity is, however, now recognized as a critical component of the antistaphylococcal response in humans, and conditions with compromised IFN-γ responses, such as HIV, diabetes mellitus, and end-stage renal disease, are associated with heightened susceptibility to S. aureus bacteremia (10, 11). We have previously demonstrated that S. aureus infection induces memory Th1 cells in mice and humans, and in a murine model of systemic S. aureus infection, these IFN-γ–expressing CD4+ T cells promoted bacterial clearance and reduced dissemination to peripheral tissues (12).
γδ T cells are an alternate lineage of IFN-γ–secreting lymphocytes that may also have potential in anti-staphylococcal immune responses. In murine studies, γδ T cells have been strongly associated with protection against S. aureus in models of peritonitis (13), skin infection (14–16), and pneumonia (17). Studies in humans and other primates have shown that γδ T cells produce IFN-γ and are expanded in vivo and in vitro in response to numerous bacterial agents (18–21). In studies of nonhuman primates, Vδ2+ γδ T cells have proven to be protective in models of tuberculosis (22, 23). Moreover, Kaufmann and colleagues (18) were able to show some limited expansion of bloodstream-derived γδ T cells from certain human donors in response to S. aureus in vitro. Bukowski and colleagues (24) have demonstrated, using a humanized mouse model of systemic S. aureus infection, that phosphoantigen-stimulated human γδ T cells from the Vδ2+ lineage are capable of promoting rapid bacterial clearance. It may be, therefore, that IFN-γ–expressing γδ T cells have the capacity to play an early protective role against S. aureus bloodstream infection in humans and, as such, could be an important component of protective immunity to be targeted in future vaccine design.
Although γδ T cells can be activated by cytokines alone (25), they can also respond, through their TCR, to a variety of peptides and phosphoantigens (26, 27), and the importance of molecules of the butyrophilin family as binding partners for the γδTCR is the subject of growing interest (28–30). The phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) is the best characterized bacterial agent capable of stimulating human γδ T cells. HMB-PP is a metabolite produced by diverse microorganisms, but not human cells, and is sensed by the B30.2 domain of butyrophilin 3A1 for stimulation of human Vδ2+ cells (31–34). Notably, HMB-PP is not expressed by S. aureus (13). It is unclear, therefore, if human γδ T cells are activated specifically by S. aureus and, if so, by what biological mechanism. To this end, we wished to determine if any subset of circulating human γδ T cells can respond to stimulation by S. aureus, and if so, how that might impact the overall immune response during bloodstream infection.
We found that S. aureus–infected dendritic cells (DCs) specifically activate the Vδ2+ subset of γδ T cells and induce rapid IFN-γ production. This Vδ2–DC interaction required direct cell–cell contact, whereas IL-12 produced by the DCs appeared to amplify IFN-γ expression by Vδ2+ cells. The interaction between S. aureus–infected DCs and Vδ2+ cells was also reciprocal in that several phenotypic markers of activation and maturation were significantly elevated on cocultured DCs. Crucially, IFN-γ expression by conventional CD4+ T cells was elevated following coculture with γδ T cells and S. aureus–infected DCs, suggesting that the enhanced maturity of DCs resulting from γδ T cell coculture may promote heightened activation of S. aureus–specific CD4+ T cells. Our findings identify a multifactorial mechanism by which human Vδ2+ cells are activated by S. aureus–infected DCs to produce IFN-γ, a key orchestrator of anti-staphylococcal immunity.
Materials and Methods
Isolation of human cells
All human cells were derived from anonymized buffy coats obtained from healthy blood donors at the Irish Blood Transfusion Service (Dublin, Ireland) or, for autologous assays, peripheral blood taken from healthy volunteers after informed consent, as approved by the School of Biochemistry and Immunology Research Ethics Committee of Trinity College Dublin.
PBMCs were isolated by density gradient centrifugation using Ficoll (Lymphoprep). Monocytes were isolated from PBMCs by magnetic separation using a CD14 MACS kit (Miltenyi Biotec) and then cultured for propagation of DCs by incubation for 6 d in RPMI 1640 (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich), 2 mM l-glutamine (Sigma-Aldrich), 100 U/ml penicillin (Sigma-Aldrich), 1 mg/ml streptomycin (Sigma-Aldrich), and 100 ng/ml GM-CSF (PeproTech). On day 3, cells were supplemented with fresh RPMI 1640 formulated as above with an additional 50 ng/ml IL-4 (PeproTech). On day 6, monocyte-derived DCs were collected and resuspended in antibiotic-free media for infection.
γδ T cells and CD4+ T cells were isolated directly from PBMCs by magnetic separation using, respectively, γδTCR and CD4 MACS kits (Miltenyi Biotec). CD4+ T cells were CFSE-stained immediately following MACS isolation. All T cells were subsequently resuspended at 5 × 105 cells/ml in RPMI 1640 (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich) and 2 mM l-glutamine (Sigma-Aldrich) before being added to DC coculture or cultured alone in 96-well plates (1 × 105 γδ T cells or 1 × 104 CD4+ T cells per well) for 4 h–4 d. Cell purity was verified by flow cytometry and was routinely >95%.
Bacteria
S. aureus strains PS80 (35), USA300 LAC::lux (36), Newman (37), SH1000 (38), SA68 (39), and SA279 (39), Escherichia coli strain CFT073 (40), and Streptococcus pneumoniae strain ATCC 6301 (41) have been described previously. S. aureus strains were cultured overnight on Columbia agar supplemented with 2% NaCl or on tryptic soy agar; E. coli was grown overnight on tryptic soy agar supplemented with 4% defibrinated sheep’s blood (Thermo Fisher Scientific), and S. pneumoniae was grown overnight on 4% sheep’s blood agar followed by a further 12-h culturing in Todd Hewitt broth. Bacteria were suspended in sterile PBS and diluted to 1 × 108 CFU/ml, calculated by optical spectrometry. CFU counts were verified by plating on appropriate agar overnight.
In vitro infection assay
DCs were resuspended in antibiotic-free RPMI 1640 (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich) and 2 mM l-glutamine (Sigma-Aldrich). Then, 1 × 105 cells were transferred to each well of 96-well flat-bottom cell culture plates (Corning). DCs were inoculated with 1 × 106 CFU of bacteria per well and incubated for 3 h before centrifugation and media replacement with complete RPMI 1640 supplemented with gentamicin (200 μg/ml; Sigma-Aldrich) to eliminate live extracellular bacteria. At this point, 1 × 105 freshly isolated allogeneic (unless otherwise stated) γδ T cells were added to each well for the indicated lengths of time. For most assays (unless otherwise indicated), four sets of γδ T cells isolated from four independent donors were paired with each individual DC donor. For specific experiments, neutralizing Abs for IFN-γ (clone NIB42, 20 μg/ml; BioLegend), IL-12 p40 (clone C8.6, 50 μg/ml; BioLegend), or the γδTCR (clone B1, 10 μg/ml; BioLegend) were added. For contact-blocking experiments, DCs were cultured and infected in the lower chamber of a 96-well 0.4-μm transmembrane culture system (MilliporeSigma) before gentamicin treatment as described, at which point γδ T cells were placed in the upper chamber.
For direct exposure of γδ T cells to bacteria, freshly isolated γδ T cells were resuspended in antibiotic-free RPMI 1640 (Sigma-Aldrich) supplemented with 2 mM l-glutamine (Sigma-Aldrich) and 1 × 105 cells transferred to each well of 96-well flat-bottom cell culture plates (Corning) before inoculation with 1 × 106 CFU of bacteria per well. Cells were then incubated for 3 h before centrifugation, and media was replaced with RPMI 1640 supplemented with gentamicin (200 μg/ml; Sigma-Aldrich) to eliminate live extracellular bacteria. Supernatants were collected at specific timepoints for analysis of cytokine production by ELISA, and in some instances, cells were resuspended in media supplemented with brefeldin A (BFA) (10 μg/ml; Sigma-Aldrich) for the final 4 h of culture, after which cells were collected and stained for flow cytometry.
For γδ–DC–CD4 triple cultures, following coculturing of γδ T cells with infected DCs for 24 h, CD4+ T cells isolated from autologous donors were stained with CFSE (Life Technologies) and then added both to DC-only wells and to γδ–DC wells at 1 × 104 cells per well. All cultures were then further incubated for the indicated lengths of time. Four hours before culture completion, cells were resuspended in media supplemented with BFA (10 μg/ml), after which cells were collected and stained for flow cytometry.
Flow cytometry
Following BFA treatment for the final 4 h, cells were resuspended in 1:1000 dilution of Fixable Viability Stain (Life Technologies) for LIVE/DEAD cell determination. Cells were subsequently resuspended in 1% BSA (Sigma-Aldrich) and treated with Fcγ block (Life Technologies). For extracellular cell staining, cells were labeled with fluorochrome-conjugated Abs against CCR7 (clone G043H7; BioLegend), CD3 (clone OKT3; Life Technologies), CD4 (clone RPA-T4; Life Technologies), CD45RA (clone REA1047; Miltenyi Biotec), CD45RO (clone UCHL1; Invitrogen), CD69 (clone FN50; BD Biosciences), CD86 (clone IT2.2; BioLegend), DC-SIGN (clone eB-h209; Life Technologies), Vγ9 (clone B3; BioLegend), Vδ1 (clone REA173; Miltenyi Biotec), Vδ2 (clone 123R3; Miltenyi Biotec), and Vδ3 (clone P11.5B, fluorescently conjugated in-house; Beckman Coulter Diagnostics). Cells were then fixed and permeabilized using the FIX and PERM Kit (Life Technologies) before intracellular staining with fluorochrome-conjugated Abs against IFN-γ (clone 4S.B3; BioLegend), IL-17A (clone eBIO64 DEC17; Life Technologies), and IL-12 p40 (clone [11C].5; BioLegend). Flow cytometric data were acquired with a BD FACSCanto II (BD Biosciences) or BD LSRFortessa (BD Biosciences) and analyzed using FlowJo software (Tree Star). Fluorescence minus one controls were used for the setting of gates.
ELISA
Sandwich ELISAs were performed on culture supernatants as per the manufacturer’s instructions for IFN-γ, IL-12 (BioLegend), IL-17A, and TNF (Life Technologies).
Statistical analysis
Statistical analysis was performed with SPSS Statistics 24 (IBM) software. Friedman two-way ANOVA on groups was followed by pairwise Wilcoxon signed-rank posttests. For direct comparisons of DC single cultures to γδ–DC cocultures in which DCs were generated from single donors and cocultured with γδ T cells from up to four separate donors, forward fill imputation was used to correctly match each DC donor to each paired γδ T cell donor. In all situations, a p value <0.05 was considered significant.
Results
Human blood–derived Vδ2+ cells produce IFN-γ but not IL-17A in response to S. aureus–infected DCs
Impaired secretion of both IFN-γ and IL-17A has been correlated with increased susceptibility to invasive S. aureus infection in different tissue settings in humans (10, 11, 42). In experimental murine infections with S. aureus, γδ T cells have been identified as key early contributors to IFN-γ and IL-17A secretion and have been confirmed to mediate protection in a variety of tissue settings (13–17). Consequently, we wished to determine if human γδ T cells can similarly respond to S. aureus and to determine the mechanism by which S. aureus could activate these cells. DCs were exposed to a panel of S. aureus isolates, in addition to the Gram-negative bacterium E. coli, which is a known activator of human γδ T cells (43), and an alternative Gram-positive bacterium S. pneumoniae. Following a 3-h infection, extracellular bacteria were eliminated from all cultures by gentamicin treatment, and allogeneic γδ T cells isolated from human blood added for a further 24 h, after which cell culture supernatants were collected and cytokine secretion analyzed by ELISA. Additionally, γδ T cells were directly exposed to live bacteria for 3 h, followed by gentamicin treatment, and supernatants were collected 24 h post–bacterial exposure.
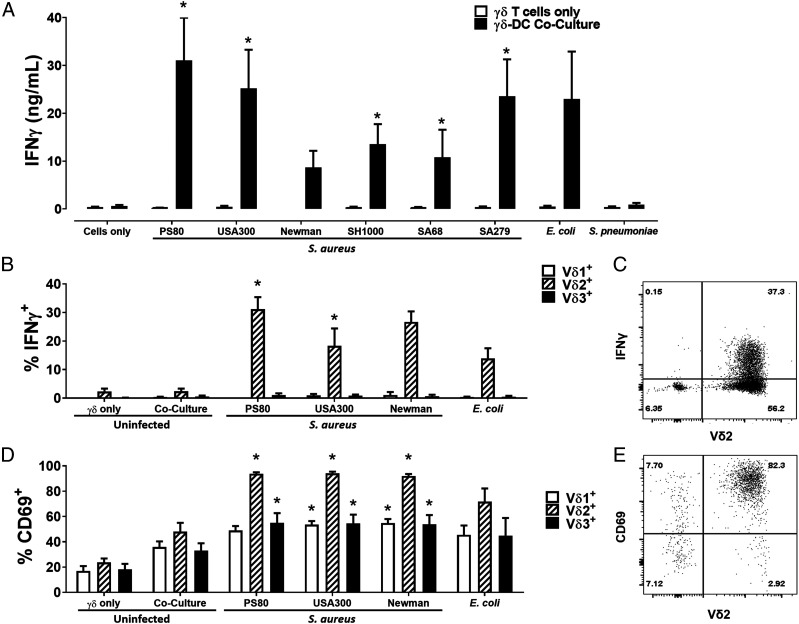
γδ T cells did not secrete IFN-γ following direct exposure to any of the selected strains of S. aureus, E. coli, or S. pneumoniae (Fig. 1A). In contrast, at 24 h, γδ T cells produced high levels of IFN-γ following coculture with DCs infected with all strains of S. aureus tested. The levels of IFN-γ produced were similar to those induced by E. coli–infected DCs, whereas S. pneumoniae induced limited IFN-γ production by γδ T cells in coculture (Fig. 1A). Of note, IL-17A was undetectable in all culture conditions tested. The IFN-γ response to DCs infected with the majority of S. aureus strains was maximal during the first 24 h of culture (Supplemental Fig. 1).
FIGURE 1.
Human blood–derived Vδ2+ cells produce IFN-γ in response to S. aureus–infected DCs. γδ T cells and DCs (5 × 105 cells/ml) were infected with S. aureus (strains PS80, USA300, Newman, SH1000, SA68, and SA279), E. coli (strain CFT073), and S. pneumoniae (strain 6301) at multiplicity of infection 10 for 3 h before elimination of extracellular bacteria by gentamicin treatment. DCs were then cocultured with uninfected γδ T cells (5 × 105/ml) for 24 h. IFN-γ levels in culture supernatants at 20 h were assessed by ELISA (A). Results are expressed as mean IFN-γ concentration in culture supernatants ± SEM. Cells from cocultures with DCs infected with selected S. aureus strains (PS80, USA300, and Newman) and E. coli were treated with BFA for a further 4 h, and IFN-γ expression by Vδ1+, Vδ2+, and Vδ3+ cells was assessed by flow cytometry (B). Results are expressed as mean IFN-γ+ cells within total live singlet cells of each subset ± SEM. Representative FACS plot for PS80 infection is shown (C). CD69 expression on Vδ1+, Vδ2+, and Vδ3+ cells was also assessed in separate experiments (D). Results are expressed as mean CD69+ cells within total live singlet cells of each subset ± SEM. Representative FACS plot for PS80 infection is shown (E). n = 2–3 DC donors per group; n = 7–12 γδ T cell donors per group. Statistical analysis by pairwise Wilcoxon signed-rank test, with all columns compared directly to cells only (A) or uninfected γδ–DC coculture (B and D). *p < 0.05.
To identify the γδ T cell subset responsible for IFN-γ production, γδ T cells were cocultured with DCs infected with selected bacterial strains for 24 h and then analyzed by flow cytometry following BFA treatment for the final 4 h. IFN-γ production was entirely confined to the Vδ2+ subset (Fig. 1B, 1C) with the majority being produced by Vδ2+Vγ9+ cells (Supplemental Fig. 2). Although all subsets of γδ T cells were activated to some extent by S. aureus–infected DCs, almost 100% of Vδ2+ cells had upregulated CD69 expression following 24 h culture (Fig. 1D, 1E). Within the Vδ2+ population, IFN-γ expression was restricted to CD45RO+ memory T cells (Supplemental Fig. 3).
Activation of Vδ2+ cells by S. aureus–infected DCs is dependent on the engagement of cell surface receptors and enhanced by IL-12
The interaction between DCs and conventional T cells during infections comprises distinct contact-mediated and cytokine-mediated components, both of which contribute to T cell activation, polarization, and the resulting effector phenotype (44). To establish the mechanism of activation of γδ T cells by S. aureus–infected DCs, γδ–DC cocultures were modified by the blocking of DC IL-12 secretion, blocking of direct contact, and blocking of the γδTCR.
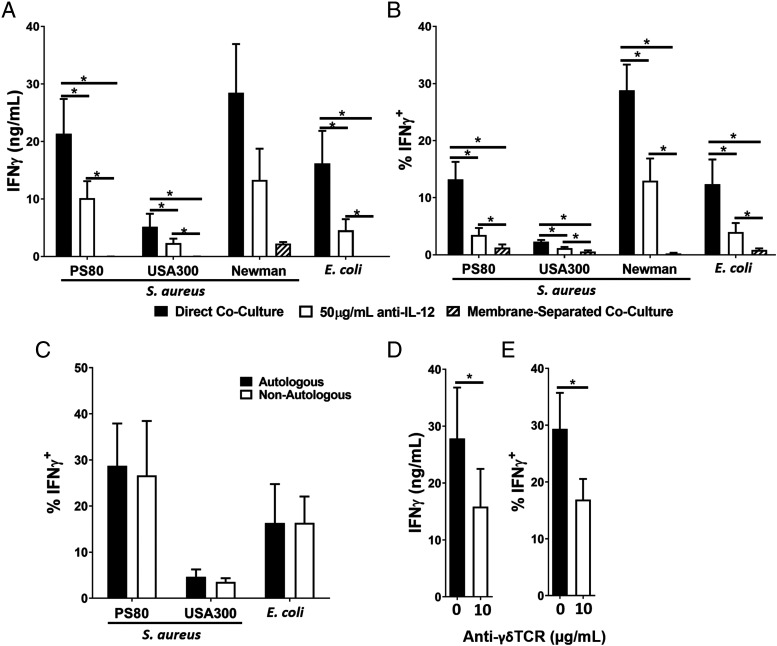
DCs were infected with selected strains of S. aureus or E. coli for 3 h and, following elimination of bacteria by gentamicin treatment, resuspended in media supplemented with IL-12–neutralizing Abs and cocultured with γδ T cells for 24 h. IFN-γ expression by Vδ2+ cells in response to both S. aureus– and E. coli–infected DCs was significantly reduced in the presence of the IL-12–neutralizing Abs, measured by ELISA for IFN-γ accumulation in culture supernatants (Fig. 2A) or by flow cytometry to establish the frequency of IFN-γ–expressing Vδ2+ cells (Fig. 2B). The results indicate that IL-12 plays a significant but not exclusive role in the induction of IFN-γ production by Vδ2+ cells in response to S. aureus–infected DCs.
FIGURE 2.
Activation of Vδ2+ cells by S. aureus–infected DCs is dependent on the engagement of cell surface receptors and enhanced by IL-12. DCs (5 × 105/ml) were infected with S. aureus (strains PS80, USA300, and Newman) or E. coli (strain CFT073) at multiplicity of infection 10 for 3 h before the elimination of extracellular bacteria by gentamicin treatment. DCs were then cocultured with allogeneic (A, B, D, and E) or autologous (C) γδ T cells (5 × 105/ml) for 24 h, with or without treatment with anti–IL-12p40 Abs or in a membrane-separated coculture (A and B) or (for PS80 only) with or without treatment with anti-γδTCR Abs (D and E). IFN-γ levels in culture supernatants at 20 h were assessed by ELISA (A and D). Results are expressed as mean IFN-γ concentration in culture supernatants ± SEM. Cells were treated with BFA for a further 4 h before analysis by flow cytometry (B, C, and E). Results are expressed as mean IFN-γ+ cells within total live singlet Vδ2+ cells ± SEM (B, C, and E). n = 2 DC donors per group, and n = 7–8 γδ T cell donors per group in nonautologous experiments. n = 3–4 paired DC and γδ T cell donors per group in autologous experiments. Statistical analysis by Friedman two-way ANOVA for comparison of multiple groups followed by pairwise Wilcoxon signed-rank test. *p < 0.05.
To determine the importance of direct contact between DCs and γδ T cells during S. aureus infection, γδ T cells were cocultured with infected DCs for 24 h in a multichamber system in which cell–cell contact was blocked by a 0.4-μm membrane, without impeding the exchange of secreted molecules. IFN-γ secretion into culture supernatants (Fig. 2A) and expression by Vδ2+ cells (Fig. 2B) was almost completely ablated. Taken together, although these results indicate a clear role for IL-12 in the activation of Vδ2+ cells in response to S. aureus–infected DCs, they also indicate that this activation is primarily dependent upon direct cell–cell contact, given the total abolition of IFN-γ responses in the membrane-blocking assay, which does not impede cytokine exchange between S. aureus–infected DCs and γδ T cells.
In interactions of conventional T cells with DCs, the interaction of polymorphic MHC molecules with the TCR is critical for activation of the T cell (44). To determine a role for classical MHC in the activation of γδ T cells by S. aureus–infected DCs, autologous γδ–DC coculture infection assays were also performed. For autologous assays, every DC donor was paired with the single donor-matched γδ T cell donor. We observed no difference in IFN-γ production by Vδ2+cells between autologous and allogeneic donor pairings (Fig. 2C), which suggests a largely nonpolymorphic, and thus MHC-independent, mechanism of activation.
However, the γδTCR can also engage DC-expressed MHC-like molecules in nonconventional mechanisms following modification by phosphoantigens such as HMB-PP (45). Therefore, we added γδTCR-blocking Abs to γδ T cells prior to coculture with S. aureus–infected DCs. IFN-γ production by Vδ2+ cells was significantly, but not completely, abrogated by γδTCR blockade, whether measured by ELISA for IFN-γ accumulation in culture supernatants (Fig. 2D) or by flow cytometry for IFN-γ expression by Vδ2+ cells (Fig. 2E), suggesting that the binding of γδTCR to a nonpolymorphic molecule expressed on the surface of S. aureus–infected DCs has a role to play in the activation of γδ T cells.
Activated Vδ2+ cells promote enhanced maturation of S. aureus–infected DCs through IFN-γ secretion and direct contact
The absolute requirement for cell–cell contact in Vδ2+ cell activation in response to S. aureus–infected DCs raises the possibility that γδ T cells may have a similar, reciprocal effect on the infected DCs in coculture to amplify the inflammatory response. To determine the influence of γδ T cells on S. aureus–infected DCs, we measured DC-derived cytokine secretion and expression of phenotypic markers of activation and maturation on cocultured DCs.
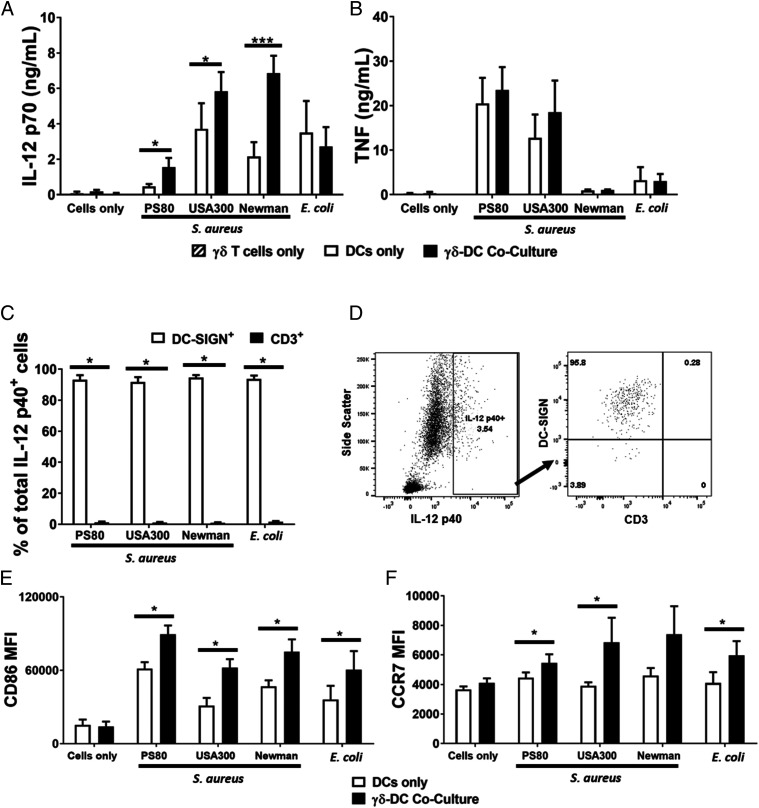
DCs were infected with selected strains of S. aureus or E. coli for 3 h and, following elimination of extracellular bacteria by gentamicin treatment, cocultured with γδ T cells or cultured alone for a further 20 h. The concentration of IL-12 in culture media was significantly elevated in supernatants from cocultures of γδ T cells and S. aureus–infected DCs compared with that observed in supernatants from S. aureus–infected DCs alone (Fig. 3A). By contrast, γδ T cell coculture with E. coli–infected DCs did not alter IL-12 levels (Fig. 3A). Importantly, DC secretion of TNF was unaffected by coculture with γδ T cells (Fig. 3B). To verify that the increased IL-12 in culture supernatants was indeed a result of bona fide upregulation by DCs (rather than a novel contribution by γδ T cells), IL-12 expression in cocultures was measured by flow cytometry and confirmed to be exclusively originating from DCs (Fig. 3C, 3D). This indicates that γδ T cells drive enhanced IL-12 secretion by S. aureus–infected DCs.
FIGURE 3.
Activated Vδ2+ cells promote enhanced maturation of S. aureus–infected DCs. DCs (5 × 105/ml) were infected with S. aureus (strains PS80, USA300, and Newman) or E. coli (strain CFT073) at multiplicity of infection 10 for 3 h before the elimination of extracellular bacteria by gentamicin treatment. DCs were then cultured alone or with γδ T cells (5 × 105/ml) for 24 h. IL-12p70 (A) and TNF (B) levels in culture supernatants at 20 h were assessed by ELISA. Results are expressed as mean concentration in culture supernatants ± SEM. n = 3–14 DC donors per group. n = 6–28 γδ T cell donors per group. Statistical analysis by pairwise Wilcoxon signed-rank test. Cells were treated with BFA for a further 4 h before analysis by flow cytometry. Results are expressed as mean IL-12p40+ cells within total live singlet cells of each subset ± SEM (C). Representative FACS plot for PS80 infection is shown (D). Expression of CD86 (E) and CCR7 (F) was also assessed and expressed as the mean fluorescence intensity of total live singlet DC-SIGN+ cells ± SEM. n = 8–12 DC donors per group. n = 8–12 γδ T cells donors per group. Statistical analysis by pairwise Wilcoxon signed-rank test. *p < 0.05, ***p < 0.001.
To determine whether DC phenotype was affected in the presence of γδ T cells, S. aureus–infected DCs cultured alone or in the presence of γδ T cells were analyzed for the expression of costimulatory molecule CD86 and the chemokine receptor CCR7. DCs from each donor were cultured with γδ T cells from a single allogeneic donor. CD86 expression was significantly upregulated in cocultured DCs (Fig. 3E). This enhancement held true for all S. aureus strains. CCR7 expression by S. aureus–infected DCs was also significantly increased following coculture with γδ T cells (Fig. 3F), potentially indicating enhanced lymphoid homing capacity and the ability to stimulate adaptive immune responses.
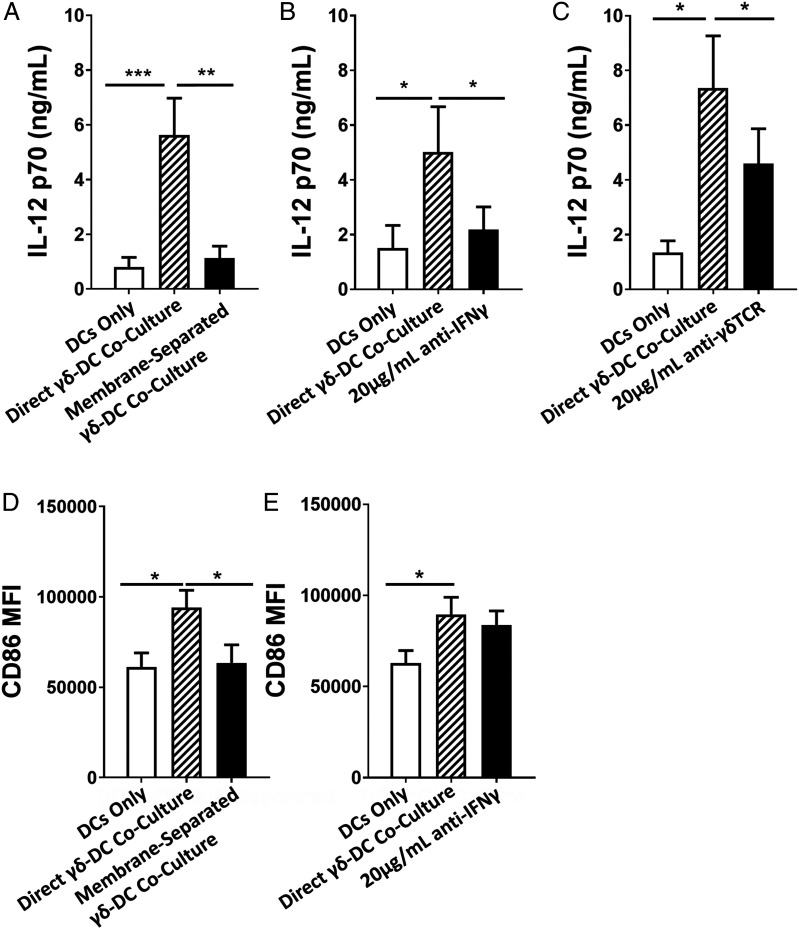
To establish a mechanism by which γδ T cells enhance these hallmarks of DC maturity, γδ T cells and S. aureus–infected DCs were again cultured in a multichamber system in which cell–cell contact was blocked by a 0.4-μm membrane, without impeding the exchange of secreted molecules. DCs from each donor were cultured with γδ T cells from a single allogeneic donor. In the absence of cell–cell contact, the feedback effect of γδ T cells on IL-12 secretion was abolished (Fig. 4A). In parallel assays, S. aureus–infected DCs were cocultured with γδ T cells treated with IFN-γ–neutralizing Abs prior to the addition to cocultures, resulting in a similar reduction in IL-12 levels, again negating the feedback effects of γδ T cells on IL-12 secretion by infected DCs (Fig. 4B). Finally, blocking the γδTCR prior to coculture with DCs led to a significant but incomplete reduction in IL-12 levels in coculture supernatants (Fig. 4C). Given our previous findings that blocking of direct contact abolishes IFN-γ (Fig. 2A, 2B), although γδTCR blocking partially negates the IFN-γ response (Fig. 2D, 2E), these results indicate that IL-12 enhancement in γδ–DC cocultures directly correlates with the level of initial activation of IFN-γ–secreting Vδ2+ cells by S. aureus–infected DCs.
FIGURE 4.
Activated Vδ2+ cells promote enhanced maturation of S. aureus–infected DCs through IFN-γ secretion and direct contact. DCs (5 × 105/ml) were infected with S. aureus (strain PS80) at multiplicity of infection 10 for 3 h before the elimination of extracellular bacteria by gentamicin treatment. DCs were then cultured alone or with γδ T cells (5 × 105/ml) for 24 h, either in a membrane-separated coculture (A and D) or treated with anti–IFN-γ (B and E) or anti-γδTCR (C) Abs. IL-12p70 levels in culture supernatants at 20 h was assessed by ELISA (A–C). Results are expressed as mean IL-12p70 concentration in culture supernatants ± SEM. Expression of CD86 was assessed by flow cytometry (D and E). Results are expressed as the mean fluorescence intensity of total live singlet DC-SIGN+ cells ± SEM. n = 7–15 DC donors per group. n = 7–15 γδ T cell donors per group. Statistical analysis by pairwise Wilcoxon signed-rank test. *p < 0.05, **p < 0.01, ***p < 0.001.
In contrast, γδ T cell–driven upregulation of CD86 expression on S. aureus–infected DCs was not influenced by γδ T cell–derived IFN-γ signaling but was negated in the absence of direct cell–cell contact (Fig. 4D, 4E).
Taken together, these data identify a feedback mechanism active during the first 24 h of γδ T cell exposure to S. aureus–infected DCs. IL-12 produced by S. aureus–infected DCs is necessary for maximal IFN-γ production by Vδ2+ cells (Fig. 2A, 2B). In turn, IFN-γ produced by Vδ2+ cells leads to enhanced production of IL-12 by S. aureus–infected DCs (Fig. 4B), suggesting that S. aureus–infected DCs are primed to respond to IFN-γ with heightened IL-12 production. This mechanism is not observed when DCs are exposed to E. coli, suggesting that this positive feedback loop must also have some other potentially S. aureus–specific element beyond simple IFN-γ production. CD86 expression by infected DCs is also enhanced during γδ T cell coculture but via an IFN-γ–independent mechanism.
Coculture of CD4+ T cells with Vδ2+ cells results in enhanced CD4+ T cell IFN-γ expression in response to S. aureus
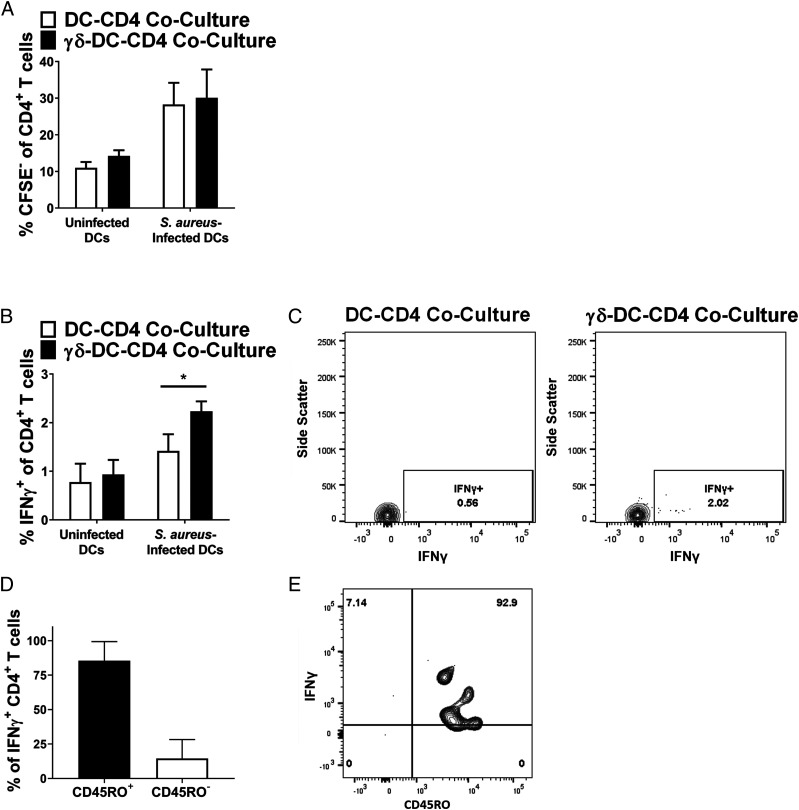
A potential outcome of enhanced DC maturation is that these DCs may in turn amplify the activation of conventional T cells in secondary lymphoid tissues, leading to a stronger and more rapid anti-staphylococcal adaptive immune response. To assess the potential of such an effect, a triple culture system was designed wherein S. aureus–infected DCs were cocultured with autologous γδ T cells for 24 h before the addition of autologous CD4+ T cells to the cultures. The effect of this triple culture on CD4+ T cell proliferation and IFN-γ production was then assessed by flow cytometry. Proliferation of total CD4+ T cells, as measured by CFSE dilution, was not affected following 4 d of coculture with DCs and γδ T cells (Fig. 5A). IFN-γ expression by CD4+ T cells was, however, significantly enhanced after 24 h in triple culture, compared with that seen in cells from DC–CD4 cocultures (Fig. 5B, 5C). The vast majority of these IFN-γ–producing CD4+ cells expressed the marker CD45RO (Fig. 5D, 5E), suggesting a memory phenotype. It has previously been shown that significant numbers of S. aureus Ag-specific conventional T cells exist in the peripheral circulation (46). These results demonstrate that γδ T cells may act to enhance the effector function of conventional CD4+ T cells in an infection setting.
FIGURE 5.
Coculture of CD4+ T cells with Vδ2+ cells results in enhanced CD4+ T cell IFN-γ expression in response to S. aureus. DCs (5 × 105/ml) were infected with S. aureus (strain PS80) at multiplicity of infection 10 for 3 h before the elimination of extracellular bacteria by gentamicin treatment. DCs were then cultured alone or with autologous γδ T cells (5 × 105/ml). After 24 h, autologous CD4+ T cells were added to both DC-only cultures and γδ–DC cocultures, including cultures of uninfected DCs, and cultures were maintained for 4 d. Cells were treated with BFA for the final 4 h of culture before analysis by flow cytometry. Results are expressed as mean CFSE− cells within total live singlet CD4+ T cells at 4 d ± SEM (A), mean IFN-γ+ cells within total live singlet CD4+ T cells at 24 h ± SEM (B), or mean CD45RO+ cells with IFN-γ+ CD4+ T cells at 24 h ± SEM (D). Representative FACS plots for PS80 infection are shown for IFN-γ (C) and CD45RO (E). n = 6 sets of autologous DC, γδ T cell, and CD4+ T cell donors per group. Statistical analysis by pairwise Wilcoxon signed-rank test. *p < 0.05.
Discussion
In this study, we have identified a mechanism in which peripheral γδ T cells, IL-12–secreting DCs, and IFN-γ–secreting CD4+ T cells coordinate a rapid response to S. aureus infection of the bloodstream. We have demonstrated that during S. aureus infection, DCs are capable of promoting rapid IFN-γ release by blood-derived Vδ2+ cells in an interaction that requires both cell–cell contact and the Th1-polarizing cytokine IL-12. This activation also results in enhanced activation of infected DCs and CD4+ T cells. This response does not occur when Vδ2+ cells are directly exposed to S. aureus, indicating that the γδ T cell IFN-γ response is mediated via stimulation of innate immune cells. However, given the rapidity of the IFN-γ response to S. aureus–infected DCs we have observed, we hypothesize that γδ T cell responses to S. aureus in the bloodstream take place sometime before the arrival of large numbers of activated conventional T cells from lymphoid tissues, which can take 7 d or more (47), presenting the possibility that Vδ2+ cells act as a critical stop gap during early infection.
Our study presents conclusive evidence that γδ T cells circulating in human blood have the capacity to respond to S. aureus during infection. We have demonstrated that the most abundant subset of γδ T cells in peripheral human blood, Vδ2+ cells, are activated and secrete IFN-γ, a key correlate of protection against S. aureus bacteremia (10, 11), following exposure to S. aureus–infected DCs. This finding builds on previous studies that demonstrated proliferation of γδ T cells in response to the superantigen staphylococcal enterotoxin A (48) and the production of IFN-γ (49) and TNF (50) in response to supernatants from S. aureus cultures. Among its pleiotropic effects, IFN-γ is an activating cytokine for macrophages (51), mediates efficient killing of intracellular bacteria by S. aureus–infected cells (52), and is a key factor for neutrophil survival (46), all of which may contribute to enhanced clearance of S. aureus bloodstream infection (46). Vδ2+ cells have been shown to be activated by the bacterial phosphoantigen HMB-PP via binding of the TCR (30). Critically, S. aureus does not express HMB-PP (13), but the possibility that S. aureus expresses an agent capable of activating γδ T cells in much the same manner as this phosphoantigen is supported by our demonstration that γδTCR blockade significantly abrogates the IFN-γ response of Vδ2+ cells.
The IFN-γ response was much more comprehensively abolished by complete separation of γδ T cells from DCs by a permeable membrane, indicating the existence of an additional activating interaction between γδ T cells and S. aureus–infected DCs that is contact based but independent of the γδTCR. The DC-expressed binding partner for the γδTCR is likely a member of the butyrophilin family (53, 54); because of the commercial unavailability of an Ab to block butyrophilins, involvement of these molecules could not be fully ruled out in these assays. γδ T cells express a number of other activating molecules, including CD2, CD27, and LFA-1, many of which may contribute to the full γδ T cell IFN-γ response (55). The vast majority of these act as costimulatory receptors, which serve to amplify cellular responses following TCR engagement and are thus unlikely to account for residual γδ T cell activation following γδTCR blockade. Although γδ T cell clones have been identified that bind classical MHC, there is currently no strong evidence that classical MHC plays any role in activating γδ T cells in response to bacterial infection (30), and indeed, mice deficient in either β2 microglobulin (an essential component of MHC class I) or deficient in MHC class II have generally normal γδ T cell compartments (56, 57). Furthermore, our data indicate that there is no donor dependence in interactions between DCs and γδ T cells in this assay. Therefore, the polymorphic molecule MHC was deemed highly unlikely to play any role in this interaction. CD40L, which is expressed by γδ T cells (58), has been shown to trigger high levels of IL-12 production and costimulatory molecule expression by DCs, amplifying a Th1 response by CD4+ T cells (59), and warrants future investigation in the context of the observed γδ T cell response to S. aureus. Additionally, γδ T cells express a number of TLRs, which could sense S. aureus pathogen-associated molecular patterns directly. For example, S. aureus RNA is recognized by TLR8 (60), which has been shown to reverse immunosuppressive activity in γδ T cells (61). Moreover, staphylococcal lipopeptides are recognized by TLR2 (62). Functional studies have shown that Vδ2+ cells can respond to stimulation with the TLR2 agonist Pam3Cys but that ligation of the γδTCR was also required to enhance IFN-γ expression (61). As a result and supported by our finding that direct bacterial exposure to γδ T cells led to no detectable IFN-γ production, it seems likely that TLRs could not functionally account for the residual DC-mediated activation of γδ T cells in the absence of γδTCR signaling. Nevertheless, considering that the study of γδ T cells regularly challenges immunological paradigms, it may be of interest in future work to study the role of these and other stimulatory molecules in the activation of γδ T cells by infected DCs.
Paracrine interactions also play a role in the responses of γδ T cells to S. aureus–infected DCs, indicated by the finding that IFN-γ secretion is minimized, although not abolished, in the context of IL-12 neutralization. The ability of IL-12 to activate γδ T cells is well attested (63–66). The implication of our findings is that in interactions of S. aureus–infected DCs and Vδ2+ cells, IL-12 functions in much the same way as it does in interactions between DCs and conventional T cells, as an additional signal required for fully effective responses (67). Although certain innate cytokines can stimulate murine γδ T cells in the absence of TCR engagement (25), the role of IL-12 in this situation appears ancillary; IL-12, although reduced substantially, was not abolished by the blocking of γδ–DC contact, and given that cytokine exchange is not impeded by the membrane that was used in these assays, γδ T cells in this system will have been exposed to DC-derived IL-12. In light of the absence of IFN-γ expression by γδ T cells in this assay, we may infer that IL-12 exposure alone is not sufficient to rescue the IFN-γ response in the absence of cell contact. Together, these findings demonstrate a multifactorial interaction between γδ T cells and S. aureus–infected DCs, which results in the rapid production of protective IFN-γ by circulating cells.
We have demonstrated that Vδ2+ cells, activated by S. aureus–infected DCs, promote enhanced IL-12 release by DCs in an IFN-γ–dependent fashion and increased surface expression of CD86 through IFN-γ–independent mechanisms. CD86 upregulation by DCs in γδ T cell coculture has previously been demonstrated to be IFN-γ–independent and instead dependent on TNF (68), but our results indicate that upregulation of CD86 on S. aureus–infected DCs is based not on any soluble factor but on a contact-based interaction. The heightened secretion of the Th1-polarizing cytokine IL-12 and heightened expression of CD86 [a potent costimulatory molecule for T cell activation (69)] on γδ-cocultured DCs reinforces the impression of a more proinflammatory DC phenotype, whereas lymph node trafficking is likely enhanced by elevated expression of CCR7 on cocultured DCs (70). There is substantial evidence to support the idea that γδ T cells play a role in enhancing DC activities in vivo. In a murine model of uveitis, there were lower numbers of mature DCs in γδ T cell knockout mice compared with wild type mice (71). Murine γδ T cells have also been demonstrated to promote IL-12 secretion by lymph node cells in vivo in a preclinical model of autoimmunity (72). A number of studies have also highlighted the influence of γδ T cells, and particularly Vδ2+ cells, in the modulation of DC maturity in infection settings, although never before has this been shown in the absence of HMB-PP. DCs infected with HMB-PP–expressing mycobacteria in coculture with γδ T cells were reported to upregulate CD86 surface expression (73) and IL-12 production (74). Vδ2+ cells have been reported to induce enhanced maturation of DCs infected with Brucella suis (also HMB-PP positive), as evidenced by enhanced CD83 and CD86 expression and IL-12 secretion, whereas CD4+ T cell proliferation in coculture with B. suis–infected DCs has been shown to be enhanced by Vδ2+ cell coculture (75). The enhanced maturation of B. suis–infected DCs was attenuated both by neutralization of IFN-γ and by physical separation of γδ T cells and DCs (75). Our study not only adds to these lines of evidence for the power of γδ T cells to effect changes in DC phenotype during infection but enriches the picture; unlike Mycobacterium and B. suis, S. aureus does not express HMB-PP, nor did the Vδ2+ cells in our assay require pretreatment with any phosphoantigen before mediating these effects on DCs. This may suggest that S. aureus expresses some other agent that is equally as capable as HMB-PP in the activation of γδ T cells.
It is possible that the S. aureus–responsive γδ T cells in these assays could be classed as being “precommitted” to this pathway, in light of their rapid IFN-γ response and the fact that they were found within the CD45RO+ population. Similarly, γδ T cells that respond to malaria parasites with IFN-γ production have been shown to derive from the CD45RO+ population, suggesting a memory phenotype (76). It may be that this S. aureus–responsive CD45RO+ population is a product of lifetime exposure to S. aureus during commensal colonization; such S. aureus–specific memory cells have already been identified among circulating conventional T cells (46).
IFN-γ and IL-17A are both key correlates of anti–S. aureus immunity in humans but in different tissue settings. IL-17A is important in soft tissues but does not correlate with protection in the bloodstream (42, 77); conversely, IFN-γ appears to be a key cytokine for tackling S. aureus in the bloodstream (10, 11), where it plays a critical role in the recruitment of inflammatory monocytes (78). We have previously demonstrated that in a murine model of systemic S. aureus infection, IFN-γ–secreting conventional CD4+ T cells mediate enhanced bacterial clearance (12). Furthermore, it has also been demonstrated that IFN-γ–deficient mice are hypersusceptible to i.v. S. aureus infection (79) and that IFN-γ–expressing human Vδ2+ cells, adoptively transferred to immunocompromised SCID mice, positively correlate with reduced S. aureus burden (24). In our assay, blood-derived Vδ2+ cell responses to S. aureus displayed a clear polarization toward an IFN-γ response, with no measurable IL-17A production. It has previously been reported that approximately only 1% of adult Vγ9+Vδ2+ cells produce IL-17A (80). Based on our data, we propose that in the context of bloodstream infection by S. aureus, the relevance of rapid IFN-γ secretion by γδ T cells is both to contribute directly to the killing of S. aureus via its influences on innate immune cells and, by inducing Th1 polarization and IFN-γ expression by conventional T cells, potentiate an even larger IFN-γ response by effector CD4+ T cells recruited to the site of infection. CD4+ T cells, with their potential for long-term memory responses to repeated S. aureus infections of the bloodstream, may be the key population for future vaccines to target. Our results indicate that effective targeting of CD4+ T cells may involve concurrent activation of γδ T cells by vaccine or adjuvant components. In addition, a growing body of literature indicates that γδ T cells may themselves be a potential target for vaccination (21), displaying potential for the development of pathogen-specific memory in response to mycobacteria (81), malaria (76), CMV (82), and EBV (83). Furthermore, murine experiments have revealed the potential for γδ T cells to generate memory responses during infection (13, 15, 84, 85), further supporting the consideration of these cells as vaccine targets.
The idea of targeting γδ T cells in vivo to achieve positive clinical outcomes has gained credence in the field of tumor immunology. Bisphosphonates such as zoledronate have been administered to patients alongside IL-2 to successfully expand tumoricidal Vδ2+ cells in cases of lymphoma (86), prostate cancer (87), and breast cancer (88). Evidence suggests that the effectiveness of γδ T cells in tumor cell killing is highest in the acute phase, during which IFN-γ–secreting γδ T cells are initially recruited to the tumor microenvironment (89). S. aureus bacteremia, like early tumor development, is an acute condition in which IFN-γ correlates with protection (10, 11). Therefore, we suggest that the γδ T cell responses to S. aureus demonstrated in the current study, including the rapid secretion of IFN-γ and the enhancement of both DC maturity and CD4+ T cell activation, make γδ T cells strong candidates for a similar approach in the treatment of S. aureus bacteremia.
Our findings make a powerful case for the consideration of γδ T cells, particularly IFN-γ–secreting Vδ2+ cells, in the future design of vaccines against S. aureus bacteremia. The activation of S. aureus–responsive γδ T cells in peripheral blood may, through their influence on DC function, unlock the successful mobilization of conventional T cells and the generation of a robust anti–S. aureus memory response.
Supplementary Material
Acknowledgments
We thank the Irish Blood Transfusion Service for provision of buffy coats, Barry Moran for flow cytometry expertise, Prof. Derek Doherty for provision of Vδ3 Ab, and Prof. Stephen Smith for provision of E. coli strain CFT073.
This work was supported by a Science Foundation Ireland Investigator Award (15/IA/3041) and a Science Foundation Ireland European Research Council Development Award (17/ERCD/5345) to R.M.M.
The online version of this article contains supplemental material.
- BFA
- brefeldin A
- DC
- dendritic cell
- HMB-PP
- (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Tong S. Y., Davis J. S., Eichenberger E., Holland T. L., Fowler V. G., Jr 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28: 603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert D. M., Ricks P., Edwards J. R., Schneider A., Patel J., Srinivasan A., Kallen A., Limbago B., Fridkin S., National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 34: 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Melzer M., Welch C. 2013. Thirty-day mortality in UK patients with community-onset and hospital-acquired meticillin-susceptible Staphylococcus aureus bacteraemia. J. Hosp. Infect. 84: 143–150. [DOI] [PubMed] [Google Scholar]
- 4.Turnidge J. D., Kotsanas D., Munckhof W., Roberts S., Bennett C. M., Nimmo G. R., Coombs G. W., Murray R. J., Howden B., Johnson P. D. R., Dowling K., Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis 2009. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med. J. Aust. 191: 368–373. [DOI] [PubMed] [Google Scholar]
- 5.Mendell T. H. 1939. Staphylococcic septicemia: a review of thirty-five cases, with six recoveries, twenty-nine deaths and sixteen autopsies. Arch. Intern. Med. (Chic.) 63: 1068–1083. [Google Scholar]
- 6.Chambers H. F., Deleo F. R. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller L. S., Fowler V. G., Shukla S. K., Rose W. E., Proctor R. A. 2020. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 44: 123–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler V. G., Jr., Allen K. B., Moreira E. D., Moustafa M., Isgro F., Boucher H. W., Corey G. R., Carmeli Y., Betts R., Hartzel J. S., et al. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309: 1368–1378. [DOI] [PubMed] [Google Scholar]
- 9.Redi D., Raffaelli C. S., Rossetti B., De Luca A., Montagnani F. 2018. Staphylococcus aureus vaccine preclinical and clinical development: current state of the art. New Microbiol. 41: 208–213. [PubMed] [Google Scholar]
- 10.Wiese L., Mejer N., Schønheyder H. C., Westh H., Jensen A. G., Larsen A. R., Skov R., Benfield T., Danish Staphylococcal Bacteraemia Study Group 2013. A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J. Infect. 67: 199–205. [DOI] [PubMed] [Google Scholar]
- 11.Laupland K. B., Ross T., Gregson D. B. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J. Infect. Dis. 198: 336–343. [DOI] [PubMed] [Google Scholar]
- 12.Brown A. F., Murphy A. G., Lalor S. J., Leech J. M., O’Keeffe K. M., Mac Aogáin M., O’Halloran D. P., Lacey K. A., Tavakol M., Hearnden C. H., et al. 2015. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 11: e1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy A. G., O’Keeffe K. M., Lalor S. J., Maher B. M., Mills K. H., McLoughlin R. M. 2014. Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. [Published erratum appears in 2015 J. Immunol. 194: 4588.] J. Immunol. 192: 3697–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho J., Pietras E., Garcia N., Ramos R. I., Farzam D., Monroe H., Magorien J., Blauvelt A., Kolls J., Cheung A., et al. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection. J. Immunol. 184: 37.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillen C. A., Pinsker B. L., Marusina A. I., Merleev A. A., Farber O. N., Liu H., Archer N. K., Lee D. B., Wang Y., Ortines R. V., et al. 2018. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Invest. 128: 1026–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchitto M. C., Dillen C. A., Liu H., Miller R. J., Archer N. K., Ortines R. V., Alphonse M. P., Marusina A. I., Merleev A. A., Wang Y., et al. 2019. Clonal Vγ6+Vδ4+ T cells promote IL-17–mediated immunity against Staphylococcus aureus skin infection. Proc. Natl. Acad. Sci. USA 116: 10917–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng P., Liu T., Zhou W.-Y., Zhuang Y., Peng L. S., Zhang J. Y., Yin Z.-N., Mao X. H., Guo G., Shi Y., Zou Q. M. 2012. Role of γ-δ T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munk M. E., Gatrill A. J., Kaufmann S. H. 1990. Target cell lysis and IL-2 secretion by γ/δ T lymphocytes after activation with bacteria. J. Immunol. 145: 2434–2439. [PubMed] [Google Scholar]
- 19.Bender A., Heckl-Östreicher B., Grondal E. J., Kabelitz D. 1993. Clonal specificity of human γ δ T cells: V γ 9+ T-cell clones frequently recognize Plasmodium falciparum merozoites, Mycobacterium tuberculosis, and group-A streptococci. Int. Arch. Allergy Immunol. 100: 12–18. [DOI] [PubMed] [Google Scholar]
- 20.Bender A., Kabelitz D. 1992. Preferential activation of peripheral blood V γ 9+ γ/δ T cells by group A, B and C but not group D or F streptococci. Clin. Exp. Immunol. 89: 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalor S. J., McLoughlin R. M. 2016. Memory γδ T cells–Newly appreciated protagonists in infection and immunity. Trends Immunol. 37: 690–702. [DOI] [PubMed] [Google Scholar]
- 22.Qaqish A., Huang D., Chen C. Y., Zhang Z., Wang R., Li S., Yang E., Lu Y., Larsen M. H., Jacobs W. R., Jr, et al. 2017. Adoptive transfer of phosphoantigen-specific γδ T cell subset attenuates Mycobacterium tuberculosis infection in nonhuman primates. J. Immunol. 198: 4753–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L., Frencher J., Huang D., Wang W., Yang E., Chen C. Y., Zhang Z., Wang R., Qaqish A., Larsen M. H., et al. 2019. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. USA 116: 6371–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., Kamath A., Das H., Li L., Bukowski J. F. 2001. Antibacterial effect of human V γ 2V δ 2 T cells in vivo. J. Clin. Invest. 108: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341. [DOI] [PubMed] [Google Scholar]
- 26.Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M. 2009. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31: 321–330. [DOI] [PubMed] [Google Scholar]
- 27.Born W. K., Zhang L., Nakayama M., Jin N., Chain J. L., Huang Y., Aydintug M. K., O’Brien R. L. 2011. Peptide antigens for γ/δ T cells. Cell. Mol. Life Sci. 68: 2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harly C., Guillaume Y., Nedellec S., Peigné C.-M., Mönkkönen H., Mönkkönen J., Li J., Kuball J., Adams E. J., Netzer S., et al. 2012. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Henry O., Distefano M. D., Wang Y.-C., Räikkönen J., Mönkkönen J., Tanaka Y., Morita C. T. 2013. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 191: 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermijlen D., Gatti D., Kouzeli A., Rus T., Eberl M. 2018. γδ T cell responses: how many ligands will it take till we know? Semin. Cell Dev. Biol. 84: 75–86. [DOI] [PubMed] [Google Scholar]
- 31.Sandstrom A., Peigné C.-M., Léger A., Crooks J. E., Konczak F., Gesnel M.-C., Breathnach R., Bonneville M., Scotet E., Adams E. J. 2014. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao C. H., Lin X., Barney R. J., Shippy R. R., Li J., Vinogradova O., Wiemer D. F., Wiemer A. J. 2014. Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes. Chem. Biol. 21: 945–954. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes D. A., Chen H.-C., Price A. J., Keeble A. H., Davey M. S., James L. C., Eberl M., Trowsdale J. 2015. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J. Immunol. 194: 2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H., Morita C. T. 2015. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 195: 4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asheshov E. H. 1969. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J. Gen. Microbiol. 59: 289–301. [DOI] [PubMed] [Google Scholar]
- 36.Diep B. A., Gill S. R., Chang R. F., Phan T. H., Chen J. H., Davidson M. G., Lin F., Lin J., Carleton H. A., Mongodin E. F., et al. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367: 731–739. [DOI] [PubMed] [Google Scholar]
- 37.Duthie E. S., Lorenz L. L. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6: 95–107. [DOI] [PubMed] [Google Scholar]
- 38.Horsburgh M. J., Aish J. L., White I. J., Shaw L., Lithgow J. K., Foster S. J. 2002. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184: 5457–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Keeffe K. M., Wilk M. M., Leech J. M., Murphy A. G., Laabei M., Monk I. R., Massey R. C., Lindsay J. A., Foster T. J., Geoghegan J. A., McLoughlin R. M. 2015. Manipulation of autophagy in phagocytes facilitates Staphylococcus aureus bloodstream infection. Infect. Immun. 83: 3445–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mobley H. L., Green D. M., Trifillis A. L., Johnson D. E., Chippendale G. R., Lockatell C. V., Jones B. D., Warren J. W. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eddy B. F. 1944. Public health weekly reports for APRIL 7, 1944. Public Health Rep. 59: 449–484. [PMC free article] [PubMed] [Google Scholar]
- 42.Holland S. M., DeLeo F. R., Elloumi H. Z., Hsu A. P., Uzel G., Brodsky N., Freeman A. F., Demidowich A., Davis J., Turner M. L., et al. 2007. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357: 1608–1619. [DOI] [PubMed] [Google Scholar]
- 43.Hintz M., Reichenberg A., Altincicek B., Bahr U., Gschwind R. M., Kollas A.-K., Beck E., Wiesner J., Eberl M., Jomaa H. 2001. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 509: 317–322. [DOI] [PubMed] [Google Scholar]
- 44.Kumai T., Fan A., Harabuchi Y., Celis E. 2017. Cancer immunotherapy: moving forward with peptide T cell vaccines. Curr. Opin. Immunol. 47: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu S., Sachleben J. R., Boughter C. T., Nawrocka W. I., Borowska M. T., Tarrasch J. T., Skiniotis G., Roux B., Adams E. J. 2017. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation. Proc. Natl. Acad. Sci. USA 114: E7311–E7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bröker B. M., Mrochen D., Péton V. 2016. The T cell response to Staphylococcus aureus. Pathogens 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godfrey D. I., Uldrich A. P., McCluskey J., Rossjohn J., Moody D. B. 2015. The burgeoning family of unconventional T cells. [Published erratum appears in 2016 Nat. Immunol. 17: 214, 469.] Nat. Immunol. 16: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 48.Morita C. T., Li H., Lamphear J. G., Rich R. R., Fraser J. D., Mariuzza R. A., Lee H. K. 2001. Superantigen recognition by gammadelta T cells: SEA recognition site for human Vgamma2 T cell receptors. Immunity 14: 331–344. [DOI] [PubMed] [Google Scholar]
- 49.Johansson M. A., Björkander S., Mata Forsberg M., Qazi K. R., Salvany Celades M., Bittmann J., Eberl M., Sverremark-Ekström E. 2016. Probiotic lactobacilli modulate Staphylococcus aureus-induced activation of conventional and unconventional T cells and NK cells. Front. Immunol. 7: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liuzzi A. R., Kift-Morgan A., Lopez-Anton M., Friberg I. M., Zhang J., Brook A. C., Roberts G. W., Donovan K. L., Colmont C. S., Toleman M. A., et al. 2016. Unconventional human T cells accumulate at the site of infection in response to microbial ligands and induce local tissue remodeling. J. Immunol. 197: 2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decker T., Stockinger S., Karaghiosoff M., Müller M., Kovarik P. 2002. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 109: 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubica M., Guzik K., Koziel J., Zarebski M., Richter W., Gajkowska B., Golda A., Maciag-Gudowska A., Brix K., Shaw L., et al. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3: e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vavassori S., Kumar A., Wan G. S., Ramanjaneyulu G. S., Cavallari M., El Daker S., Beddoe T., Theodossis A., Williams N. K., Gostick E., et al. 2013. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 14: 908–916. [DOI] [PubMed] [Google Scholar]
- 54.Rigau M., Ostrouska S., Fulford T. S., Johnson D. N., Woods K., Ruan Z., McWilliam H. E. G., Hudson C., Tutuka C., Wheatley A. K., et al. 2020. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367: eaay5516. [DOI] [PubMed] [Google Scholar]
- 55.Ribot J. C., debarros A., Silva-Santos B. 2011. Searching for “signal 2”: costimulation requirements of γδ T cells. Cell. Mol. Life Sci. 68: 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Correa I., Bix M., Liao N. S., Zijlstra M., Jaenisch R., Raulet D. 1992. Most gamma delta T cells develop normally in beta 2-microglobulin-deficient mice. Proc. Natl. Acad. Sci. USA 89: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bigby M., Markowitz J. S., Bleicher P. A., Grusby M. J., Simha S., Siebrecht M., Wagner M., Nagler-Anderson C., Glimcher L. H. 1993. Most γ δ T cells develop normally in the absence of MHC class II molecules. J. Immunol. 151: 4465–4475. [PubMed] [Google Scholar]
- 58.Horner A. A., Jabara H., Ramesh N., Geha R. S. 1995. γ/δ T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J. Exp. Med. 181: 1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergstrøm B., Aune M. H., Awuh J. A., Kojen J. F., Blix K. J., Ryan L., Flo T. H., Mollnes T. E., Espevik T., Stenvik J. 2015. TLR8 senses Staphylococcus aureus RNA in human primary monocytes and macrophages and induces IFN-β production via a TAK1–IKKβ–IRF5 signaling pathway. J. Immunol. 195: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 61.Dar A. A., Patil R. S., Chiplunkar S. V. 2014. Insights into the relationship between toll like receptors and gamma delta T cell responses. Front. Immunol. 5: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietrocola G., Arciola C. R., Rindi S., Di Poto A., Missineo A., Montanaro L., Speziale P. 2011. Toll-like receptors (TLRs) in innate immune defense against Staphylococcus aureus. Int. J. Artif. Organs 34: 799–810. [DOI] [PubMed] [Google Scholar]
- 63.Skeen M. J., Ziegler H. K. 1995. Activation of γ δ T cells for production of IFN-γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 154: 5832–5841. [PubMed] [Google Scholar]
- 64.Klein J. L., Fickenscher H., Holliday J. E., Biesinger B., Fleckenstein B. 1996. Herpesvirus saimiri immortalized γ δ T cell line activated by IL-12. J. Immunol. 156: 2754–2760. [PubMed] [Google Scholar]
- 65.Marx S., Wesch D., Kabelitz D. 1997. Activation of human γ δ T cells by Mycobacterium tuberculosis and Daudi lymphoma cells: differential regulatory effect of IL-10 and IL-12. J. Immunol. 158: 2842–2848. [PubMed] [Google Scholar]
- 66.Yang R., Yao L., Shen L., Sha W., Modlin R. L., Shen H., Chen Z. W. 2019. IL-12 expands and differentiates human Vγ2Vδ2 T effector cells producing antimicrobial cytokines and inhibiting intracellular mycobacterial growth. [Published erratum appears in 2019 Front. Immunol. 10: 1742.] Front. Immunol. 10: 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schurich A., Pallett L. J., Lubowiecki M., Singh H. D., Gill U. S., Kennedy P. T., Nastouli E., Tanwar S., Rosenberg W., Maini M. K. 2013. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 9: e1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ismaili J., Olislagers V., Poupot R., Fournié J.-J., Goldman M. 2002. Human γ δ T cells induce dendritic cell maturation. Clin. Immunol. 103: 296–302. [DOI] [PubMed] [Google Scholar]
- 69.Chen C., Gault A., Shen L., Nabavi N. 1994. Molecular cloning and expression of early T cell costimulatory molecule-1 and its characterization as B7-2 molecule. J. Immunol. 152: 4929–4936. [PubMed] [Google Scholar]
- 70.MartIn-Fontecha A., Sebastiani S., Höpken U. E., Uguccioni M., Lipp M., Lanzavecchia A., Sallusto F. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang B., Tian Q., Guo D., Lin W., Xie X., Bi H. 2020. Activated γδ T cells promote dendritic cell maturation and exacerbate the development of experimental autoimmune uveitis (EAU) in mice. Immunol. Invest. DOI: 10.1080/08820139.2020.1716786. [DOI] [PubMed] [Google Scholar]
- 72.Odyniec A., Szczepanik M., Mycko M. P., Stasiolek M., Raine C. S., Selmaj K. W. 2004. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J. Immunol. 173: 682–694. [DOI] [PubMed] [Google Scholar]
- 73.Devilder M.-C., Maillet S., Bouyge-Moreau I., Donnadieu E., Bonneville M., Scotet E. 2006. Potentiation of antigen-stimulated V γ 9V δ 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J. Immunol. 176: 1386–1393. [DOI] [PubMed] [Google Scholar]
- 74.Meraviglia S., Caccamo N., Salerno A., Sireci G., Dieli F. 2010. Partial and ineffective activation of V γ 9V δ 2 T cells by Mycobacterium tuberculosis-infected dendritic cells. J. Immunol. 185: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 75.Ni M., Martire D., Scotet E., Bonneville M., Sanchez F., Lafont V. 2012. Full restoration of Brucella-infected dendritic cell functionality through Vγ9Vδ2 T helper type 1 crosstalk. PLoS One 7: e43613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teirlinck A. C., McCall M. B., Roestenberg M., Scholzen A., Woestenenk R., de Mast Q., van der Ven A. J., Hermsen C. C., Luty A. J., Sauerwein R. W. 2011. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 7: e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minegishi Y., Saito M., Nagasawa M., Takada H., Hara T., Tsuchiya S., Agematsu K., Yamada M., Kawamura N., Ariga T., et al. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schroder K., Hertzog P. J., Ravasi T., Hume D. A. 2004. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75: 163–189. [DOI] [PubMed] [Google Scholar]
- 79.Lin L., Ibrahim A. S., Xu X., Farber J. M., Avanesian V., Baquir B., Fu Y., French S. W., Edwards J. E., Jr., Spellberg B. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5: e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ness-Schwickerath K. J., Jin C., Morita C. T. 2010. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J. Immunol. 184: 7268–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoft D. F., Brown R. M., Roodman S. T. 1998. Bacille Calmette-Guérin vaccination enhances human γ δ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 161: 1045–1054. [PubMed] [Google Scholar]
- 82.Davey M. S., Willcox C. R., Hunter S., Kasatskaya S. A., Remmerswaal E. B. M., Salim M., Mohammed F., Bemelman F. J., Chudakov D. M., Oo Y. H., Willcox B. E. 2018. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9- subsets. Nat. Commun. 9: 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujishima N., Hirokawa M., Fujishima M., Yamashita J., Saitoh H., Ichikawa Y., Horiuchi T., Kawabata Y., Sawada K. I. 2007. Skewed T cell receptor repertoire of Vdelta1(+) gammadelta T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein-Barr virus-infected B cells in clonal restriction. Clin. Exp. Immunol. 149: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misiak A., Wilk M. M., Raverdeau M., Mills K. H. 2017. IL-17–producing innate and pathogen-specific tissue resident memory γδ T cells expand in the lungs of Bordetella pertussis–infected mice. J. Immunol. 198: 363–374. [DOI] [PubMed] [Google Scholar]
- 85.Romagnoli P. A., Sheridan B. S., Pham Q.-M., Lefrançois L., Khanna K. M. 2016. IL-17A-producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 113: 8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilhelm M., Kunzmann V., Eckstein S., Reimer P., Weissinger F., Ruediger T., Tony H.-P. 2003. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102: 200–206. [DOI] [PubMed] [Google Scholar]
- 87.Dieli F., Vermijlen D., Fulfaro F., Caccamo N., Meraviglia S., Cicero G., Roberts A., Buccheri S., D’Asaro M., Gebbia N., et al. 2007. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67: 7450–7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meraviglia S., Eberl M., Vermijlen D., Todaro M., Buccheri S., Cicero G., La Mendola C., Guggino G., D’Asaro M., Orlando V., et al. 2010. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 161: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo Presti E., Di Mitri R., Pizzolato G., Mocciaro F., Dieli F., Meraviglia S. 2018. γδ cells and tumor microenvironment: a helpful or a dangerous liason? J. Leukoc. Biol. 103: 485–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.