Abstract
Background
Treatment of individuals with asymptomatic carotid artery stenosis is still handled controversially. Recommendations for treatment of asymptomatic carotid stenosis with carotid endarterectomy (CEA) are based on trials having recruited patients more than 15 years ago. Registry data indicate that advances in best medical treatment (BMT) may lead to a markedly decreasing risk of stroke in asymptomatic carotid stenosis. The aim of the SPACE-2 trial (ISRCTN78592017) was to compare the stroke preventive effects of BMT alone with that of BMT in combination with CEA or carotid artery stenting (CAS), respectively, in patients with asymptomatic carotid artery stenosis of ≥70% European Carotid Surgery Trial (ECST) criteria.
Methods
SPACE-2 is a randomized, controlled, multicenter, open study. A major secondary endpoint was the cumulative rate of any stroke (ischemic or hemorrhagic) or death from any cause within 30 days plus an ipsilateral ischemic stroke within one year of follow-up. Safety was assessed as the rate of any stroke and death from any cause within 30 days after CEA or CAS. Protocol changes had to be implemented. The results on the one-year period after treatment are reported.
Findings
It was planned to enroll 3550 patients. Due to low recruitment, the enrollment of patients was stopped prematurely after randomization of 513 patients in 36 centers to CEA (n = 203), CAS (n = 197), or BMT (n = 113). The one-year rate of the major secondary endpoint did not significantly differ between groups (CEA 2.5%, CAS 3.0%, BMT 0.9%; p = 0.530) as well as rates of any stroke (CEA 3.9%, CAS 4.1%, BMT 0.9%; p = 0.256) and all-cause mortality (CEA 2.5%, CAS 1.0%, BMT 3.5%; p = 0.304). About half of all strokes occurred in the peri-interventional period. Higher albeit statistically non-significant rates of restenosis occurred in the stenting group (CEA 2.0% vs. CAS 5.6%; p = 0.068) without evidence of increased stroke rates.
Interpretation
The low sample size of this prematurely stopped trial of 513 patients implies that its power is not sufficient to show that CEA or CAS is superior to a modern medical therapy (BMT) in the primary prevention of ischemic stroke in patients with an asymptomatic carotid stenosis up to one year after treatment. Also, no evidence for differences in safety between CAS and CEA during the first year after treatment could be derived. Follow-up will be performed up to five years. Data may be used for pooled analysis with ongoing trials.
Keywords: Asymptomatic carotid artery stenosis, stroke, primary prevention, carotid endarterectomy, carotid artery stenting, best medical treatment, disease-free survival, epidemiology, prospective study
Introduction
The prevalence of asymptomatic carotid artery stenosis (ACS) >50% is about 1%–2% in the general population1,2 (US/European or Korean population) and increases with age ≥70 years up to 12.5% in men and 6.9% in women.3 Choice of treatment for patients with ACS still varies considerably among and within countries4 and is a matter of debate.5,6 Two interventional methods such as carotid endarterectomy (CEA) and carotid artery stenting (CAS) compete with best medical treatment (BMT) alone. Guidelines recommending interventional treatment of ACS refer to data of two large randomized trials (Asymptomatic Carotid Atherosclerosis Study (ACAS) and Asymptomatic Carotid Surgery Trial 1 (ACST-1)) having compared CEA with conservatively treated control groups more than 15 years ago. ACAS7 showed in 1995 an annual risk of ipsilateral stroke in medically treated patients of 2.2% and ACST-18 in 2004 a risk of about 1%.
Since then a decline in stroke risk has been shown9,10 and an increasing number of retrospective analyses indicates quite low annual stroke rates of about 1% or less under modern intensive BMT.11–15 On the other hand, publications with low stroke rates of ACS under BMT have been criticized for mixing patients with low-grade (50%–69%) and high-grade (≥70%) stenosis, having small patient numbers, not reporting adherence to BMT, neglecting transient ischemic attack (TIA)/high-risk plaque morphology and consecutive carotid revascularization (therefore preventing stroke), or discriminating insufficiently between ipsilateral and contralateral events.16 Furthermore, two independent studies showed much higher annual rates of ipsilateral ischemic events in BMT (2.9% in a group of 1121 patients17 and 2.4% in a group of 794 patients18). However, in ACST-1, lipid-lowering therapy showed lower rates of long-term stroke in both groups,19 indicating that stroke risk decreases with intensive medical therapy including statin medication9 by plaque stabilization and consecutive reduction of microembolization.20–23
To establish any benefit of interventional therapies over BMT alone, peri-interventional complications such as stroke, myocardial infarction, and death have to be taken into account. The peri-interventional risk of stroke within the first 30 days in the asymptomatic cohort of the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) was reported with 1.4% for CEA and 2.5% for CAS.24,25 In the Asymptomatic Carotid Trial (ACT-I), it was 1.4% for CEA and 2.8% for CAS.26 Data collected outside randomized controlled trials indicate even higher stroke rates27,28 Regarding composite endpoints including stroke, death, and myocardial infarction, the peri-interventional risk for patients with ACS partially exceeded 3%: Within the first 30 days from intervention, it amounted to 3.6% for CEA and 3.5% in CAS in CREST24,25 and to 2.6% for CEA and to 3.3% for CAS in ACT-I.26 Considering the low risk rate under modern pharmacotherapy, interventional therapies have to prove at least non-inferiority to BMT alone.29
In order to attain a higher evidence level for treatment of ACS based upon optimal current treatment options, randomized controlled trials comparing CEA with CAS with a third medical arm were requested.30–33 Up to now, no data of randomized controlled trials with a BMT arm have been published. The SPACE-2 trial was initiated in 2009 comparing both interventional methods to BMT alone. Due to low recruitment rates, further enrollment had to be stopped prematurely.34 Data from the one-year follow-up examination are presented in this publication.
Methods
In a randomized, controlled, multicenter, open trial, we recruited patients with asymptomatic stenosis of the common and/or internal carotid artery of at least 70% European Carotid Surgery Trial (ECST) criteria equivalent to at least 50% North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (ISRCTN78592017). Stenoses were considered asymptomatic if having caused no ipsilateral neurological deficits (amaurosis fugax (AF), TIA, or stroke) within the last 180 days. We enrolled patients in 36 study centers in Germany, Switzerland, and Austria. Risks of stroke, death, myocardial infarction, and restenosis were analyzed for three treatment modalities: BMT alone vs. CEA + BMT vs. CAS+BMT. BMT was defined as up-to-date medication following national and international guidelines (see Supplementary Table S11). Details about trial rationale, design, sample size, selection of study patients, and qualification of interventionalists have been published previously.34,35 SPACE-2 started in 2009. Due to insufficient recruitment rates, a change in study design was implemented in 2013.36 However, the continuing low recruitment rates led to the premature termination of enrollment of the SPACE-2 study in 2014. Vascular risk factors were controlled regularly at screening visit, intervention, and one day (one month and six months) after intervention and every year after randomization.
This paper reports results on efficacy and safety for the one-year period after treatment. The primary efficacy endpoint of SPACE-2 (cumulative rate of any stroke or death from any cause within 30 days plus an ipsilateral ischemic stroke within five years of follow-up) will be reported later.
Endpoints
The composite major secondary endpoint is defined as cumulative rates of any stroke or death from any cause within 30 days plus ipsilateral ischemic stroke within one year of follow-up. Secondary and tertiary endpoints were single components of the one-year endpoint, cardiac events, restenosis (recurrent stenosis of at least 70%ECST following ultrasound criteria37), technical failures, and observations at different time points. In the interventional groups, safety was assessed as the rate of any stroke within 30 days from intervention and death from any cause within 30 days. TIA was defined as temporary neurologic deficits lasting less than 24 h. A relevant disabling stroke was defined having a modified Rankin score scale of more than two 30 days after stroke. Vascular death was defined as any death due to stroke, myocardial infarction, or hemorrhage as well as any death that were not clearly non-vascular.
Statistical analysis
Details on the sample size calculation are given in the Supplement. The primary analysis is intention to treat (ITT). Also, a per-protocol analysis (PPA) is performed after excluding patients who did not finish therapy or showed other serious protocol violations. Time-to-event analyses were performed by Kaplan–Meier estimators, and Cox proportional hazards regression. Event rates within a fixed time period (for example, during first 30 days or during the first year) were analyzed with chi-square test, Fisher's exact test, or logistic regression. Several subgroup analyses were predefined (age, sex, and number of vascular risk factors including hypertension, diabetes, coronary heart disease, hyperlipidemia, and smoking). Technical aspects (e.g. use of a protection system, stent design, use of shunts, eversion technique vs. endarterectomy) were analyzed for the respective groups. Analyses were performed with STATA/IC 13.0 (College Station, TX, USA).
The study was approved by the ethics committee of the University of Heidelberg, Germany (S-311/2008).
Results
Study recruitment
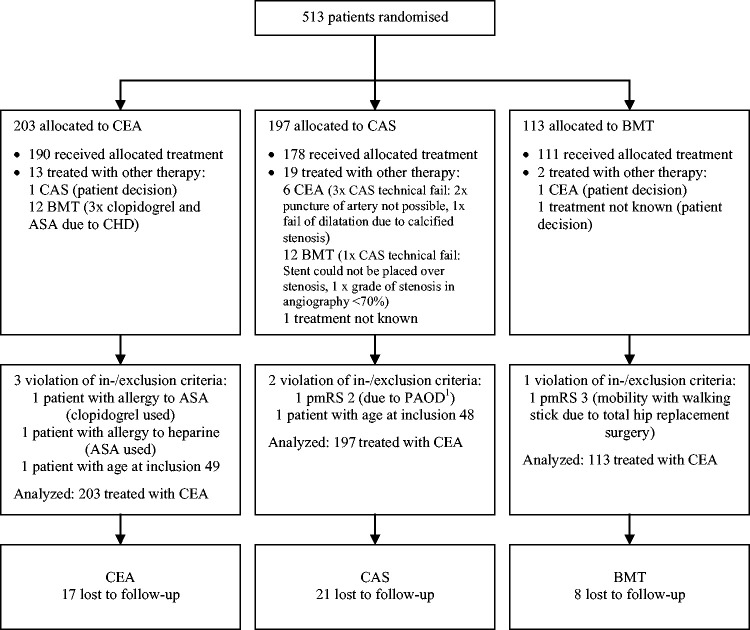
A total of 513 patients were recruited in 36 centers. Of these, 203 patients were randomized into the CEA group (39.6%), 197 into the CAS group (38.4%), and 113 into the BMT group (22.0%). For details concerning applied treatment, protocol violation and loss to follow-up see Figure 1.
Figure 1.
Randomization and follow-up of the study patients within one year. All patients included in intention-to-treat analysis. PAOD: Peripheral arterial occlusive disease; CHD: coronary heart disease; ASA: acetylsalicylic acid; CAS: carotid artery stenting; BMT: best medical treatment; CEA: carotid endarterectomy.
Baseline characteristics
Baseline characteristics did not differ substantially between the three study arms except for occlusion of the contralateral carotid artery, mainly in CAS group (Table 1). Medication at baseline did not differ between groups except for the use of diuretics. Over 80% of patients were treated with statins prior to intervention. In CAS, embolic protection devices (EPDs) were used in 36.0%; in addition, 62.7% of CEA were performed in general anesthesia. For details of medication and intervention procedures, see Supplementary Table S1, peri- and postprocedural complications other than stroke, death, or myocardial infarction in the interventional groups are described in Supplementary Table S2.
Table 1.
Baseline characteristics of the study population, according to treatment group.
| CEA (n = 203) | CAS (n = 197) | BMT (n = 113) | Total (n = 513) | |
|---|---|---|---|---|
| Male sex (% of patients) | 151 (74.4%) | 143 (72.6%) | 87 (77.0%) | 381 (74.3%) |
| Age (years, median (IQR)) | 70 (64, 75) | 70 (63, 75) | 68 (64, 74) | 70 (64, 75) |
| Side randomized: left | 105 (51.7%) | 105 (53.3%) | 49 (43.4%) | 259 (50.5%) |
| Ipsilateral symptomsa | 16 (7.9%) | 8 (4.1%) | 5 (4.4%) | 29 (5.7%) |
| Grade of stenosisb (median (IQR)) | 80 (75, 85) | 80 (75, 85) | 80 (75, 85) | 80 (75, 85) |
| Grade of contralateral stenosisb | 20 (0, 50) | 30 (0, 60) | 10 (0, 55) | 20 (0, 55) |
| Contralateral occlusion | 3 (1.5%) | 12 (6.1%) | 3 (2.7%) | 18 (3.5%) |
| Systolic BPc (median (IQR)) | 147 (131, 160) | 145 (130, 162) | 150 (133, 160) | 146 (130, 160) |
| Diastolic BPc (median (IQR)) | 80 (72, 88) | 80 (74, 90) | 80 (75, 89) | 80 (73, 89) |
| Hypertension (% of patients) | 180 (88.7%) | 177 (89.8%) | 102 (90.3%) | 459 (89.5%) |
| Diabetes | 52 (25.6%) | 59 (29.9%) | 40 (35.4%) | 151 (29.4%) |
| Coronary heart disease | 70 (34.5%) | 72 (36.5%) | 40 (35.4%) | 182 (35.5%) |
| Hypercholesterolemia | 158 (77.8%) | 158 (80.2%) | 91 (80.5%) | 407 (79.3%) |
| Current smoker | 45 (22.2%) | 31 (15.7%) | 24 (21.2%) | 100 (19.5%) |
| Past smoker | 91 (44.8%) | 99 (50.3%) | 67 (59.3%) | 257 (50.1%) |
| Cholesterold (median (IQR)) | 181 (153, 209) | 173.5 (152, 200) | 169.5 (146.5, 192.5) | 174 (151, 203) |
| Glucosed (median (IQR)) | 103.5 (92, 121) | 104 (93, 125) | 104 (92, 119) | 104 (92.5, 122) |
| HbA1c in % (median (IQR)) | 6 (5.7, 6.45) | 6 (5.7, 6.6) | 5.9 (5.55, 6.75) | 6 (5.7, 6.5) |
| Number of vascular risk factors | 2 (2, 3) | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) |
| HDLd (median (IQR)) | 48 (40, 62) | 49 (40, 56.5) | 49 (41.5, 57.5) | 49 (41, 58) |
| LDLd (median (IQR)) | 105 (81, 130) | 98.5 (80, 126) | 97 (80, 117) | 99 (80, 123.5) |
| Triglycerided (median (IQR)) | 131 (98, 182.5) | 128.5 (92, 184) | 120 (91, 181) | 126.5 (94, 182) |
| BMI (median (IQR)) | 27 (25, 30) | 27 (25, 30) | 27 (24, 29) | 27 (25, 30) |
| Time delaye (median (IQR)) | 14 (8, 21) | 14 (8, 21) | – | 14 (8, 21) |
Note: Continuous variables are given as medians with interquartile range (lower and upper quartiles), and as n (%) for categorical variables. Dashes indicate not applicable; IQR: interquartile range; CAS: carotid artery stenting; BMT: best medical treatment; CEA: carotid endarterectomy; BP: blood pressure; BMI: body mass index; HDL: High-densitiy lipoprotein; LDL: Low-density lipoprotein.
>180 days on side of randomized carotid artery.
% ECST.
BP in mm Hg.
mg/dl.
Time from randomization to treatment (days).
Endpoint events within 30 days
For the interventional approaches, the safety endpoint 30-day complication rate (any stroke or death) did not exceed 3%: 2.5% in CEA and 2.5% in CAS. At the day of intervention, four strokes occurred after CEA (2.0%, all ipsilateral) and three strokes after CAS (1.5%, all ipsilateral). Further strokes occurred in the CEA group on day 2 (contralateral) and in the CAS group on days 11 and 22 (both ipsilateral) after intervention. No stroke occurred in the BMT group within the first 30 days; no patient died or had a myocardial infarction. Stroke risk did not differ significantly between CEA, CAS and BMT (p = 0.240; Table 2). In the CEA and CAS groups, there were no pre-interventional endpoint events after randomization and before intervention.
Table 2.
Periprocedural period (d0–d30): Incidence of stroke, death, and myocardial infarction (95% confidence interval; n = 513; intention to treat).
| CEA (n = 203) | CAS (n = 197) | BMT (n = 113) | p | |
|---|---|---|---|---|
| Any stroke or death until d30a | 5 (2.5%; 0.8%–5.7%) | 5 (2.5%; 0.8%–5.8%) | Not applicable | 0.962b |
| Any stroke at d0 (only CEA/CAS)c | 4 (2.0%; 0.5%–5.0%) | 3 (1.5%; 0.3%–4.4%) | Not applicable | 1.000± |
| Any stroke until d30d | 5 (2.5%; 0.8%–5.7%) | 5 (2.5%; 0.8%–5.8%) | 0 (0.0%; 0.0%–3.2%) | 0.240± |
| Ipsilateral stroke until d30d | 4 (2.0%; 0.5%–5.0%) | 5 (2.5%; 0.8%–5.8%) | 0 (0.0%; 0.0%–3.2%) | 0.238± |
| Myocardial infarctione | 0 (0.0%; 0.0%–1.8%) | 0 (0.0%; 0.0%–1.9%) | 0 (0.0%; 0.0%–3.2%) | |
| Any deathe | 0 (0.0%; 0.0%–1.8%) | 0 (0.0%; 0.0%–1.9%) | 0 (0.0%; 0.0%–3.2%) |
Note: CAS: carotid artery stenting; BMT: best medical treatment; CEA: carotid endarterectomy; ± : Fisher's exact test; if 0%: Confidence interval one-sided 97.5%.
Safety endpoint, evaluated after intervention only in patients randomized to CEA or CAS.
χ2 test.
Day of intervention.
After intervention for CEA/CAS and after randomization for BMT.
After randomization.
One-year major secondary endpoint
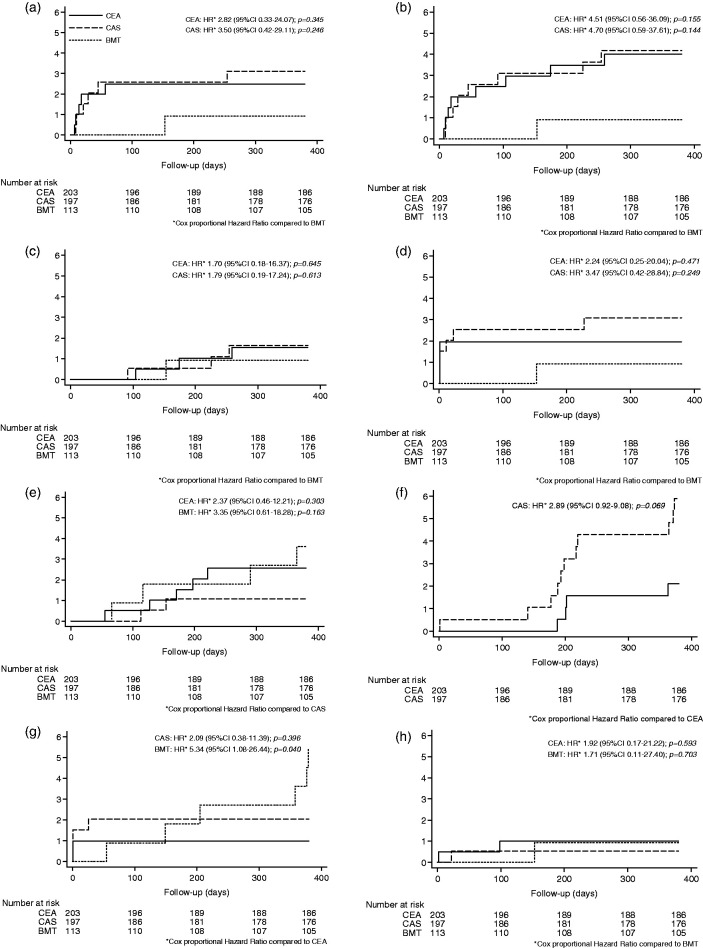
Altogether 12 patients (2.3%) suffered a one-year major secondary endpoint event. The endpoint occurred five times in the CEA group (2.5%), six times in the CAS group (3.0%), and once in the BMT group (0.9%; Table 3). Compared to BMT, the hazard ratio for the one-year major secondary endpoint was 2.82 in CEA (p = 0.345, 95% confidence interval (CI) 0.33–24.07) and 3.50 in CAS (p = 0.246; 95% CI 0.42–29.11). Details are shown in Figure 2 and described in Table 3.
Table 3.
Endpoint event rates (95% confidence interval) within the first year; n = 513.
| CEA (n = 203) | CAS (n = 197) | BMT (n = 113) | p | |
|---|---|---|---|---|
| One-year major secondary endpoint (n = 12) | 5 (2.5%; 0.8%–5.7%) | 6 (3.0%; 1.1%–6.5%) | 1 (0.9%; 0.0%–4.8%) | 0.530± |
| Any stroke (n = 17) | 8 (3.9%; 1.7%–7.6%) | 8 (4.1%; 1.8%–7.8%) | 1 (0.9%; 0.0%–4.8%) | 0.256± |
| Any stroke after d30a (n = 7) | 3 (1.5%; 0.3%–4.3%) | 3 (1.5%; 0.3%–4.4%) | 1 (0.9%; 0.0%–4.8%) | 1.000± |
| Ipsilateral stroke (n = 11) | 4 (2.0%; 0.5%–5.0%) | 6 (3.0%; 1.1%–6.5%) | 1 (0.9%; 0.0%–4.8%) | 0.497± |
| Ipsilateral stroke after d30a (n = 2) | 0 (0.0%; 0.0%–1.8%) | 1 (0.5%; 0.0%–2.8%) | 1 (0.9%; 0.0%–4.8%) | 0.521± |
| Disabling strokeb (n = 4) | 2 (1.0%; 0.1%–3.5%) | 1 (0.5%; 0.0%–2.8%) | 1 (0.9%; 0.0%–4.8%) | 1.000± |
| Any death (n = 11) | 5 (2.5%; 0.8%–5.7%) | 2 (1.0%; 0.1%–3.6%) | 4 (3.5%; 1.0%–8.8%) | 0.304± |
| Myocardial infarction (n = 1) | 1 (0.5%; 0.0%–2.7%) | 0 (0.0%; 0.0%–1.9%) | 0 (0.0%; 0.0%–3.2%) | 1.000± |
| Restenosisc (n = 15) | 4 (2.0%; 0.5%–5.0%) | 11 (5.6%; 2.8%–9.8%) | – | 0.068± |
| Rec- or progressived stenosis (n = 20) | 4 (2.0%; 0.5%–5.0%) | 11 (5.6%; 2.8%–9.8%) | 5 (4.4%; 1.5%–10.0%) | 0.158± |
| TIA (n = 15) | 4 (2.0%; 0.5%–5.0%) | 5 (2.5%; 0.8%–5.8%) | 6 (5.3%; 2.0%–11.2%) | 0.230± |
| Ipsilateral TIA (n = 12) | 2 (1.0%; 0.1%–3.5%) | 4 (2.0%; 0.6%–5.1%) | 6 (5.3%; 2.0%–11.2%) | 0.063± |
Note: Dashes indicate not applicable. CAS: carotid artery stenting; BMT: best medical treatment; CEA: carotid endarterectomy; ± : Fisher's exact test; TIA: transient ischemic attack; if 0%: Confidence interval one-sided 97.5%.
After intervention for CEA/CAS and after randomization for BMT.
mRS 30 days after stroke > 2.
Only in CEA and CAS.
Only in BMT.
Figure 2.
Kaplan–Meier estimates of cumulative incidence (%) for major outcomes within one year. (a) Major secondary endpoint. (b) Any stroke. (c) Any stroke after day 30 up to one year.** (d) Ipsilateral stroke.** (e) Any death. (f) Restenosis ≥ 70%ECST in CEA and CAS. (g) Ipsilateral TIA.** (h) Disabling stroke.** CAS: carotid artery stenting; BMT: best medical treatment; CEA: carotid endarterectomy; CI: confidence interval; HR: hazard ratio.
** After intervention for CEA/CAS and after randomization for BMT.
Centers did not differ evidently regarding incidence of major secondary endpoint. The volume of patients treated per center did not show evident influence on major secondary endpoint either.
Risk factors for the one-year major secondary endpoint
The following analyses are exploratory. General anesthesia, use of a shunt, or clamping duration in CEA were not related with higher risk of major secondary outcome events. In CEA and CAS, the side of intervention was associated with major secondary endpoint events (4.8% of left-treated CEA; 0% of right-treated CEA, p = 0.060; 6.5% of right-treated CAS; and 0% of left-treated CAS, p = 0.009). In all CAS patients with major secondary outcome events, no embolic protection system was used. For further details of risk factors for major secondary endpoint, see Supplementary Table S3. The type of stent used for CAS did not relate with major secondary outcome event (Supplementary Table S4).
Secondary one-year results
Within one year, 17 patients (3.3%) had either an ipsilateral or contralateral stroke. This endpoint occurred eight times in the CEA group (3.9%, four ipsilateral and four contralateral), eight times in the CAS group (4.1%, six ipsilateral and two contralateral) and once in the BMT group (0.9%, ipsilateral). Compared to BMT, the hazard ratio for any stroke was 4.51 in CEA (p = 0.155, 95% CI 0.56–36.09) and 4.70 in CAS (p = 0.144, 95% CI 0.59–37.61). The risk of ipsilateral stroke was higher in the early phase (81.8% of all ipsilateral strokes occurred in first 30 days). The risk of isolated ipsilateral stroke beyond 30 days from intervention dropped down to 0% in CEA and 0.5% in CAS. For more details, also on further endpoints, see Figure 2 and Table 3. Within the first year, four patients (0.8%) had a (relevant) disabling stroke: two times in the CEA group (1.0%), once in the CAS group (0.5%), and once in the BMT group (0.9%). Two disabling strokes occurred within 30 days after intervention (one in CEA and one in CAS). Compared to CAS, the hazard ratio for disabling stroke was 1.92 in CEA (p = 0.593, 95% CI 0.17–21.22) and 1.71 in BMT (p = 0.703, 95% CI 0.11–27.40) Figure 2 and Table 3 present corresponding details. Non-disabling strokes (n = 13) and disabling strokes did not differ between study groups (p = 0.246; Supplementary Table S5).
Within one year, 11 patients (2.1%) died (of vascular or other reasons). This endpoint occurred five times in the CEA group (2.5%), two times in the CAS group (1.0%), and four times in the BMT group (3.5%). Compared to CAS, the hazard ratio for any death was 2.37 in CEA (p = 0.303, 95% CI 0.46–12.21) and 3.35 in BMT (p = 0.163, 95% CI 0.61–18.28). For details, see Figure 2 and Table 3. Regarding reasons of death, one cardiac death occurred in the CEA and CAS group, respectively (0.5%). No cardiac death occurred in the BMT group (for details, see Supplementary Table S6).
Within one year, 15 (3.8%) patients treated within the CEA or CAS group, respectively, had a restenosis of ≥70%ECST. This endpoint occurred 4 times in the CEA group (2.0%) and 11 times in the CAS group (5.6%). None of the patients with restenosis had a major secondary endpoint event, a stroke (ipsilateral or contralateral), or TIA. Compared to CEA, the hazard ratio for restenosis was 2.89 in CAS (p = 0.069, 95% CI 0.92–9.08). For details, see Figure 2 and Table 3. The median time to detection of restenosis did not differ between CEA and CAS (CEA 201.5 days (p25: 194, p75: 282.5) vs. CAS 198 days (p25: 177, p75: 364); p = 0.896). Five patients in the BMT group (4.4%) showed a progressive stenosis (increasing grade of stenosis, above 70%ECST) that lead to neurological symptoms <24 h and in four cases to intervention. None of these patients suffered a major secondary outcome event or any stroke. For details, see Supplement Figure S1.
Within one year, 15 patients (2.9%) suffered a neurologically proven TIA localized ipsilateral or contralateral to the randomized stenosis. TIA occurred four times in the CEA group (2.0%), five times in the CAS group (2.5%), and six times in the BMT group (5.3%). Within these TIA patients, three had an AF (2 CEA, 1 BMT). No patient within the CEA/CAS group with a restenosis suffered a TIA. Among TIA patients, cerebral imaging showed infarction in three patients with CAS (not all patients with TIA received cerebral imaging). Compared to CEA, the hazard ratio for any TIA was 1.30 in CAS (p = 0.693, 95% CI 0.35–4.86) and 2.66 in BMT (p = 0.129, 95% CI 0.75–9.43; Supplement Figure S2). In four of the five patients with TIA and cerebral imaging, clinical silent infarction was detected. Seven patients of the BMT group received CEA or CAS after TIA and/or progressive stenosis. Ipsilateral TIA occurred two times in the CEA group (1.0%), four times in the CAS group (2.0%), and 6 times in the BMT group (5.3%). Compared to CEA, the hazard ratio for ipsilateral TIA was 5.34 in BMT (p = 0.040, 95% CI 1.08–26.44) and 2.09 in CAS (p = 0.396, 95% CI 0.38–11.39, Figure 2 and Table 3). Most TIAs (any or only ipsilateral) in CEA/CAS patients occurred peri-interventionally, whereas TIA in the BMT group occurred in the follow-up period. Two of the six patients with ipsilateral TIA in the BMT group (33.3%) had a progressive stenosis to 75% or 100%, respectively (odds ratio 17.33; p = 0.006).
Per-protocol analysis
The PPA did not reveal relevant differences to the ITT analysis. Regarding outcome events, none of the patients excluded in the PPA or with different therapy from randomization had a major secondary outcome event, any stroke, TIA, restenosis, or myocardial infarction. One patient randomized to CEA and treated with BMT died. For details, see Supplement Figures S4 to S6 and Supplementary Tables S8 to S10.
Discussion
It was planned to enroll 3550 patients into the randomized, controlled, multicenter, open SPACE-2 trial. Due to low recruitment, the enrollment of patients was stopped prematurely after randomization of 513 patients in 36 centers to CEA (n = 203), CAS (n = 197) or BMT (n = 113). Here, we report the one-year results of these 513 patients. One year after enrollment patients in the interventional groups had more major secondary endpoint events than the BMT group (CEA 2.5%, CAS 3.0% vs. BMT 0.9%). Within these 513 patients, no stroke was prevented with interventional therapies compared to BMT alone within one year.
Relating to safety concerns, the 30-day stroke/death rate remained under the guideline recommendation of 3% for interventional therapies,38 and we found no difference between CEA and CAS. Also within one year, no differences for stroke or death were found between CEA and CAS. In a pooled meta-analysis of 3019 patients (including data of CREST and ACT-1),39 CAS trended toward an increased risk of periprocedural stroke/death compared to CEA. In comparison to previously published data of CREST (CEA 1.4%, CAS 2.5%)25 and ACT-1 (CEA 1.7%, CAS 2.9%),26 the CEA group in SPACE-2 showed slightly higher 30-day stroke/death rates; for CAS, our 30-day stroke/death rates accorded with the previously published data of CREST and ACT-1. In both interventional groups, stroke risk primarily increased due to peri-interventional ipsilateral strokes and decreased considerably afterwards. Thirty days after CEA or CAS, a stroke risk lower than in only BMT-treated patients should be expected. However, within the first year after intervention, the overall risk of stroke in CEA and CAS exceeded the stroke risk in the BMT group.
Although 64% of patients in the SPACE-2 CAS group were treated without EPDs, the risk of any stroke within the first 30 days was comparable to data of patients treated with distal EPD.25,26 Also, other data indicate that an EPD might be waived with asymptomatic plaques.40
With only one (however disabling) stroke in the BMT group and a risk rate of 0.9%, it could not be proven that patients have a benefit from intervention within the first year after the procedure. Thus, for interventional treatment, a selection of patients with elevated stroke risk is important and also strongly recommended by recent guidelines.41–43
The annual stroke risk of our BMT treated patients was below the 2.2% shown in ACAS7 but still higher than reported risks below 0.5% in a well-controlled cohort in Oxford.12 The low stroke risk in our BMT group may be related to inclusion of patients with lower grade of stenosis (≥70%ECST) with a median of 80%ECST. However, the rate of ipsilateral TIA—partially with proof of cerebral infarction—was with 5.3% in BMT more than twice as high as in the interventional groups and showed a significant difference. Concerning relevant disabling strokes (mRS > 2), stroke rates were quite low and not significantly different between all groups. Regarding death, no differences between groups were found, and reasons of death were predominantly non-vascular. The death rate in SPACE-2 did not exceed death rates for patients with ACS.44 Concerning our data, carotid revascularization does not seem to be indicated for prevention of vascular death. Less patients than presumed suffered a myocardial infarction. This may be related to the strict definition (see definition of myocardial infarction in Supplementary Material).
CAS was not significantly associated with higher rates of restenosis than CEA and restenosis did not lead to any TIA, stroke, or major secondary outcome events in both interventional groups. In contrast, progressive stenosis in BMT group was significantly correlated with a high risk for ipsilateral TIA. However, none of the BMT-patients with a progressive stenosis suffered a major secondary outcome or any stroke events. In a retrospective study of 214 patients with a median follow-up of 13 years, progression of stenosis was not correlated with ipsilateral ischemic events45 either, but the majority of published data contradicts this findings and report an increased risk in progressing stenosis.17,46–48 The five-year results of the SPACE-2 collective may get clearer results on this issue. Considerably lower restenosis rates especially in CAS were found in ACT-I (at one year 0.6% in CAS and 2.6% in CEA).26
The age modifying effect preferring CEA in patients older than 70 years has been described in symptomatic carotid stenosis.25,49,50 With its limited sample size, SPACE-2 failed to validly demonstrate this effect (Supplement Figure S3).
In patients with ACS, first and foremost the focus should be placed on intensive conservative treatment not only including statin medication but also strict management of hypertension, diabetes, smoking cessation, and lifestyle modification as health diet and physical activity. These factors have shown to reduce risk of ischemic events or progression of stenosis.14,41 In specific cases, selected patients with high-risk stenosis (e.g. cerebral microembolization, intraplaque hemorrhage, echolucent plaque formation, silent embolic infarcts on brain imaging, or reduced cerebral blood flow reserve) should be considered for treatment with interventional methods.41,51–53 Assessing the benefit after CEA or CAS, patient age and life expectancy have to be taken into account.54
The most relevant limitation of our study is the low sample size as a consequence of the premature recruitment stop because of insufficient patient enrollment. Thus, the analyses presented have to be interpreted with caution. A further limitation was due to the fact that routine cerebral magnetic resonance imaging was not part of the study protocol and thereby silent infarction could not be detected in all cases. Within one year, four patients with clinically silent cerebral infarction could be identified. However, one important aim has been achieved: Providing data for pooled analysis with ongoing trials comparing CEA/CAS with a BMT arm in ACS as CREST-2,55 ECST-2,56 or ACTRIS.57
Conclusions
Due to a reduced sample size and low power, interpretation of SPACE-2 results is difficult. The follow-up data of one year did not show a better preventive effect for stroke by CEA or CAS over BMT alone in patients with ACS. However, patients with BMT alone had more TIA. Regarding interventional therapies, CAS did not differ from CEA in terms of safety and efficacy. Higher rates of restenosis with CAS did not differ significantly and were not associated with elevated stroke rates. A further follow-up of SPACE-2 patients will be performed for the five years period after treatment. Data are for pooled analysis and may support the planning of new trials and allow individual data meta-analyses when combined with the data of ongoing trials.
Supplemental Material
Supplemental Material for Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: One-year interim results of SPACE-2 by T Reiff, HH Eckstein, U Mansmann, O Jansen, G Fraedrich, H Mudra, D Böckler, M Böhm, H Brückmann, ES Debus, J Fiehler, W Lang, K Mathias, EB Ringelstein, J Schmidli, R Stingele, R Zahn, T Zeller, A Hetzel, U Bodechtel, A Binder, J Glahn, W Hacke and PA Ringleb in International Journal of Stroke
Collaborators
Principal investigators and further study personnel
Weinbeck M, Schonhardt S, Winker T, Berger H, Poppert H, Barlinn K, von Kummer R, Weiss N, Bergert H, Groß J, Glahn J, Gerdes B, Reinbold WD, Wuttig H, Maier-Hasselmann A, Segerer M, Fuchs H-H, Gass S, Schultz H, Groden C, Niedergethman M, Griebe M, Rosenkranz M, Zeumer H, Jauß M, Kneist W, Kneist M, Staudacher T, Prey N, Knippschild J, Kastrup O, Bongers V, Hoffmann J, Kniemeyer H-W, Liman J, Knauth M, Stojanovic T, Emmert H, Tacke J, Schwalbe B, Nam E-M, van Lengerich U, Lowens S, Gröschel K, Boor S, Dorweiler B, Schmid E, Henkes H, Hupp T, Singer O, Wagner-Heck M, Kilic M, Huppert P, Niederkorn K, Fruhwirth J, Klein G, Pulkowski U, Wacks J-H, Kloppmann E, Vatankhah B, Stolze H, Müller-Hülsbeck S, Walluscheck KP, Schmitt H-M, Seemann J, Tilahun B, Dichgans M, Gäbel G, Hedtmann G, Petermann C, Kirsch S, Bosnjak B, Heiß J, Mühling H, Sabisch PN, Heiß J, Gahn G, Storck M, Arnold S, von Mering M, Dißmann R, Kirsch D, Schmidauer C, Waldenberger P, Furtner M, Kazarians H, Breuer P, Schmidt G, Arnold M, Schroth G, Weise J, Zanow J, Mayer T, Töpper R, Gross-Fengels W, Daum H, Dittrich R, Ritter M, Kasprzak B, Torsello G, Pohlmann C, Brüning R, Breuer P, Amiri H, Ludwig I, Blessing E, Möhlenbruch M, Crispin A, Hofman M, and Müller T.
Contributor Information
Collaborators: M Weinbeck, S Schonhardt, T Winker, H Berger, H Poppert, K Barlinn, R von Kummer, N Weiss, H Bergert, J Groß, J Glahn, B Gerdes, WD Reinbold, H Wuttig, A Maier-Hasselmann, M Segerer, H-H Fuchs, S Gass, H Schultz, C Groden, M Niedergethman, M Griebe, M Rosenkranz, H Zeumer, M Jauß, W Kneist, M Kneist, T Staudacher, N Prey, J Knippschild, O Kastrup, V Bongers, J Hoffmann, H-W Kniemeyer, J Liman, M Knauth, T Stojanovic, H Emmert, J Tacke, B Schwalbe, E-M Nam, U van Lengerich, S Lowens, K Gröschel, S Boor, B Dorweiler, E Schmid, H Henkes, T Hupp, O Singer, M Wagner-Heck, M Kilic, P Huppert, K Niederkorn, J Fruhwirth, G Klein, U Pulkowski, J-H Wacks, E Kloppmann, B Vatankhah, H Stolze, S Müller-Hülsbeck, KP Walluscheck, H-M Schmitt, J Seemann, B Tilahun, M Dichgans, G Gäbel, G Hedtmann, C Petermann, S Kirsch, B Bosnjak, J Heiß, H Mühling, PN Sabisch, J Heiß, G Gahn, M Storck, S Arnold, M von Mering, R Dißmann, D Kirsch, C Schmidauer, P Waldenberger, M Furtner, H Kazarians, P Breuer, G Schmidt, M Arnold, G Schroth, J Weise, J Zanow, T Mayer, R Töpper, W Gross-Fengels, H Daum, R Dittrich, M Ritter, B Kasprzak, G Torsello, C Pohlmann, R Brüning, P Breuer, H Amiri, I Ludwig, E Blessing, M Möhlenbruch, A Crispin, M Hofman, and T Müller
Authors' Contribution
WH: Coordinating investigator, conception and design of the study, final approval of the version to be published, member of Executive Committee (EC), and member of Steering Committee (SC).
PAR: Study coordinator, conception and design of the study, cleaning and interpretation of data, manuscript writing, final approval of the version to be published, member of EC, and member of SC.
HHE: Principal co-investigator, conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published, member of EC, and member of SC.
UM: Designing data collection tools, monitoring data collection, conception and design of the study, cleaning and statistical analysis of data, interpretation of data, manuscript writing, final approval of the version to be published, member of EC, and member of SC.
OJ: Principal co-investigator, conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published, member of EC and member of SC.
GF: Conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published, member of EC, and member of SC.
HM: Conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published, member of EC, and member of SC.
RS: Conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published, and member of SC.
TR: Study coordinator, monitoring data collection, conception and design of the study, cleaning and statistical analysis of data, interpretation of data, manuscript writing, submitting manuscript, and final approval of the version to be published.
DB, MB, HB, ESD, JF, WL, KM, EBR, JS, RZ: Interpretation of data, final approval of the version to be published, and member of SC.
TZ, AZ, UB, AB, JG: Final approval of the version to be published.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Bundesministerium fuer Bildung und Forschung (BMBF) and Deutsche Forschungsgemeinschaft (DFG) (HA 1394/5-1).
References
- 1.de Weerd M, Greving JP, Hedblad B, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke 2010; 41: 1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SY, Joh JH, Han SA, Park HC. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: a population-based screening study. Medicine (Baltimore) 2017; 96: e5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke 2009; 40: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 4.Venermo M, Wang G, Sedrakyan A, et al. Editor's choice – carotid stenosis treatment: variation in international practice patterns. Eur J Vasc Endovasc Surg 2017; 53: 511–519. [DOI] [PubMed] [Google Scholar]
- 5.Schneider PA, Naylor AR. Asymptomatic carotid artery stenosis – medical therapy alone versus medical therapy plus carotid endarterectomy or stenting. J Vasc Surg 2010; 52: 499–507. [DOI] [PubMed] [Google Scholar]
- 6.Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke 2015; 46: 3288–3301. [DOI] [PubMed] [Google Scholar]
- 7.Endarterectomy for Amptomatic Carotid Artery Stenosis. Executive committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273: 1421–1428. [PubMed] [Google Scholar]
- 8.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 9.Hadar N, Raman G, Moorthy D, et al. Asymptomatic carotid artery stenosis treated with medical therapy alone: temporal trends and implications for risk assessment and the design of future studies. Cerebrovasc Dis 2014; 38: 163–173. [DOI] [PubMed] [Google Scholar]
- 10.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40: e573–e583. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Bogiatzi C, Spence JD. Risk of stroke at the time of carotid occlusion. JAMA Neurol 2015; 72: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 12.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41: e11–e17. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM. Carotid stenting: more risky than endarterectomy and often no better than medical treatment alone. Lancet 2010; 375: 957–959. [DOI] [PubMed] [Google Scholar]
- 14.Shah Z, Masoomi R, Thapa R, et al. Optimal medical management reduces risk of disease progression and ischemic events in asymptomatic carotid stenosis patients: a long-term follow-up study. Cerebrovasc Dis 2017; 44: 150–159. [DOI] [PubMed] [Google Scholar]
- 15.den Hartog AG, Achterberg S, Moll FL, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke 2013; 44: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 16.Pini R, Faggioli G, Vacirca A, et al. The fate of asymptomatic severe carotid stenosis in the era of best medical therapy. Brain Inj 2017; 31: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 17.Kakkos SK, Nicolaides AN, Charalambous I, et al. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg 2014; 59: 956–967.e1. [DOI] [PubMed] [Google Scholar]
- 18.Conrad MF, Baloum V, Mukhopadhyay S, Garg A, Patel VI, Cambria RP. Progression of asymptomatic carotid stenosis despite optimal medical therapy. J Vasc Surg 2013; 58: 128–135.e1. [DOI] [PubMed] [Google Scholar]
- 19.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376: 1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 2005; 36: 2373–2378. [DOI] [PubMed] [Google Scholar]
- 21.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67: 180–186. [DOI] [PubMed] [Google Scholar]
- 22.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yla-Herttuala S, Bentzon JF, Daemen M, et al. Stabilization of atherosclerotic plaques: an update. Eur Heart J 2013; 34: 3251–3258. [DOI] [PubMed] [Google Scholar]
- 24.Silver FL, Mackey A, Clark WM, et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke 2011; 42: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brott TG, Hobson RW, II, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016; 374: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 27.Wang FW, Esterbrooks D, Kuo YF, Mooss A, Mohiuddin SM, Uretsky BF. Outcomes after carotid artery stenting and endarterectomy in the Medicare population. Stroke 2011; 42: 2019–2025. [DOI] [PubMed] [Google Scholar]
- 28.Paraskevas KI, Kalmykov EL, Naylor AR. Stroke/death rates following carotid artery stenting and carotid endarterectomy in contemporary administrative dataset registries: a systematic review. Eur J Vasc Endovasc Surg 2016; 51: 3–12. [DOI] [PubMed] [Google Scholar]
- 29.Naylor AR. Why is the management of asymptomatic carotid disease so controversial?. Surgeon 2015; 13: 34–43. [DOI] [PubMed] [Google Scholar]
- 30.Brott T. Asymptomatic carotid disease: where do we go from here? Vasc News 2010; Jan(45): 4–6. https://vascularnews.com/vascular-news-45-january-2010/ (1 February 2010).
- 31.Naylor AR, Gaines PA, Rothwell PM. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals?. Eur J Vasc Endovasc Surg 2009; 37: 625–632. [DOI] [PubMed] [Google Scholar]
- 32.Spence JD. Asymptomatic carotid stenosis: why a moratorium is needed on intervention outside clinical trials. Neurology 2017; 88: 1990–1991. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi S, Chimowitz M, Brown RD, Jr, Lal BK, Meschia JF. The urgent need for contemporary clinical trials in patients with asymptomatic carotid stenosis. Neurology 2016; 87: 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckstein HH, Reiff T, Ringleb P, et al. SPACE-2: a missed opportunity to compare carotid endarterectomy, carotid stenting, and best medical treatment in patients with asymptomatic carotid stenoses. Eur J Vasc Endovasc Surg 2016; 51: 761–765. [DOI] [PubMed] [Google Scholar]
- 35.Reiff T, Stingele R, Eckstein HH, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2 – a three-arm randomised-controlled clinical trial. Int J Stroke 2009; 4: 294–299. [DOI] [PubMed] [Google Scholar]
- 36.Reiff T, Amiri H, Eckstein HH, et al. Modification of SPACE-2 study design. Int J Stroke 2014; 9: E12–E13. [DOI] [PubMed] [Google Scholar]
- 37.Arning C, Widder B, von Reutern GM, Stiegler H, Gortler M. [Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement]. Ultraschall Med 2010; 31: 251–257. [DOI] [PubMed] [Google Scholar]
- 38.Eckstein HH. Evidence-based management of carotid stenosis: recommendations from international guidelines. J Cardiovasc Surg (Torino) 2012; 53: 3–13. [PubMed] [Google Scholar]
- 39.Moresoli P, Habib B, Reynier P, Secrest MH, Eisenberg MJ, Filion KB. Carotid stenting versus endarterectomy for asymptomatic carotid artery stenosis: a systematic review and meta-analysis. Stroke 2017; 48: 2150–2157. [DOI] [PubMed] [Google Scholar]
- 40.Wodarg F, Turner EL, Dobson J, et al. Influence of stent design and use of protection devices on outcome of carotid artery stenting: a pooled analysis of individual patient data. J Neurointerv Surg 2018; 10: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 41.Naylor AR, Ricco JB, de Borst GJ, et al. Editor's choice – management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 3–81. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 517–584. [DOI] [PubMed] [Google Scholar]
- 43.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Stroke 2011; 42: e420–e463. [DOI] [PubMed] [Google Scholar]
- 44.Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38: 1470–1475. [DOI] [PubMed] [Google Scholar]
- 45.Singh TD, Kramer CL, Mandrekar J, Lanzino G, Rabinstein AA. Asymptomatic carotid stenosis: risk of progression and development of symptoms. Cerebrovasc Dis 2015; 40: 236–243. [DOI] [PubMed] [Google Scholar]
- 46.Balestrini S, Lupidi F, Balucani C, et al. One-year progression of moderate asymptomatic carotid stenosis predicts the risk of vascular events. Stroke 2013; 44: 792–794. [DOI] [PubMed] [Google Scholar]
- 47.Sabeti S, Schlager O, Exner M, et al. Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high-risk patients. Stroke 2007; 38: 2887–2894. [DOI] [PubMed] [Google Scholar]
- 48.Hirt LS. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke 2014; 45: 702–706. [DOI] [PubMed] [Google Scholar]
- 49.Stingele R, Berger J, Alfke K, et al. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol 2008; 7: 216–222. [DOI] [PubMed] [Google Scholar]
- 50.Bonati LH, Fraedrich G. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis – a pooled analysis of EVA-3S, SPACE and ICSS. Eur J Vasc Endovasc Surg 2011; 41: 153–158. [DOI] [PubMed] [Google Scholar]
- 51.Paraskevas KI, Veith FJ, Mikhailidis DP, Liapis CD. Appropriate patient selection for carotid revascularization procedures is urgently needed. Angiology 2018; 69: 12–16. [DOI] [PubMed] [Google Scholar]
- 52.Naylor AR. Which patients with asymptomatic carotid stenosis benefit from revascularization?. Curr Opin Neurol 2017; 30: 15–21. [DOI] [PubMed] [Google Scholar]
- 53.Spence JD, Pelz D, Veith FJ. Asymptomatic carotid stenosis: identifying patients at high enough risk to warrant endarterectomy or stenting. Stroke 2014; 45: 655–657. [DOI] [PubMed] [Google Scholar]
- 54.Wallaert JB, Cronenwett JL, Bertges DJ, et al. Optimal selection of asymptomatic patients for carotid endarterectomy based on predicted 5-year survival. J Vasc Surg 2013; 58: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard VJ, Meschia JF, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke 2017; 12: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaturvedi S, Sacco RL. How recent data have impacted the treatment of internal carotid artery stenosis. J Am Coll Cardiol 2015; 65: 1134–1143. [DOI] [PubMed] [Google Scholar]
- 57.Endarterectomy combined with optimal medical therapy (OMT) vs. OMT alone in patients with asymptomatic severe atherosclerotic carotid artery stenosis at higher-than-average risk of ipsilateral stroke (ACTRIS), https://clinicaltrials.gov/ct2/show/study/NCT02841098 (2016, accessed 13 February 2019)..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: One-year interim results of SPACE-2 by T Reiff, HH Eckstein, U Mansmann, O Jansen, G Fraedrich, H Mudra, D Böckler, M Böhm, H Brückmann, ES Debus, J Fiehler, W Lang, K Mathias, EB Ringelstein, J Schmidli, R Stingele, R Zahn, T Zeller, A Hetzel, U Bodechtel, A Binder, J Glahn, W Hacke and PA Ringleb in International Journal of Stroke