Abstract
In acute ischemic stroke (AIS) patients, eligibility for endovascular intervention is commonly determined through computed tomography perfusion (CTP) analysis by quantifying ischemic tissue using perfusion parameter thresholds. However, thresholds are not uniform across all analysis methods due to dependencies on patient demographics and computational algorithms. This study aimed to investigate optimal perfusion thresholds for quantifying infarct and penumbra volumes using two post-processing CTP algorithms: Vitrea Bayesian and singular value decomposition plus (SVD+). We utilized 107 AIS patients (67 non-intervention patients and 40 successful reperfusion of thrombolysis in cerebral infarction (2b/3) patients). Infarct volumes were predicted for both post-processing algorithms through contralateral hemisphere comparisons using absolute time-to-peak (TTP) and relative regional cerebral blood volume (rCBV) thresholds ranging from +2.8 seconds to +9.3 seconds and –0.23 to –0.56 respectively. Optimal thresholds were determined by minimizing differences between predicted CTP and 24-hour fluid-attenuation inversion recovery magnetic resonance imaging infarct. Optimal thresholds were tested on 60 validation patients (30 intervention and 30 non-intervention) and compared using RAPID CTP software. Among the 67 non-intervention and 40 intervention patients, the following optimal thresholds were determined: intervention Bayesian: TTP = +4.8 seconds, rCBV = –0.29; intervention SVD+: TTP = +5.8 seconds, rCBV = –0.29; non-intervention Bayesian: TTP = +5.3 seconds, rCBV = –0.32; non-intervention SVD+: TTP = +6.3 seconds, rCBV = –0.26. When comparing SVD+ and Bayesian post-processing algorithms, optimal thresholds for TTP were significantly different for intervention and non-intervention patients. rCBV optimal thresholds were equal for intervention patients and significantly different for non-intervention patients. Comparison with commercially utilized software indicated similar performance.
Keywords: Bayesian, CT perfusion, fluid-attenuation inversion recovery MRI, ischemic stroke, singular value decomposition plus
Introduction
Computed tomography (CT) perfusion (CTP) is a common imaging modality used to determine acute ischemic stroke (AIS) patients’ eligibility for thrombectomy. This assessment is commonly done by computing amounts of infarct and penumbra tissue from perfusion maps. Infarct is permanently damaged tissue that cannot be salvaged, even in the event of reperfusion procedures. Penumbra is tissue deficient in blood flow but which can be salvaged through reperfusion.1 These tissues are quantified through contralateral hemisphere comparisons of perfusion maps representing hemodynamic parameters using CTP analysis software. Typically, CTP post-processing software uses one or a combination of perfusion parameters such as regional cerebral blood volume (rCBV) and time-to-peak (TTP) for infarct and penumbra segmentation.2 When considering CTP, precise volumetric segmentation is needed to determine penumbra-to-infarct ratios for the purpose of determining endovascular reperfusion procedure eligibility. A penumbra-to-infarct ratio >1 is a minimum requisite for mechanical thrombectomy.3
Although contralateral hemisphere comparisons of hemodynamic features are utilized to quantify infarct and penumbra, it has been proven other parameters can influence the degree of infarct and penumbra currently present. Recent studies have shown that such parameters include: the age of the patient; the patient’s cardiac output; the timing of the contrast bolus injection; the type of contrast media used; whether the lesion presents in the white matter, gray matter, or a combination of both; and the time from onset of stroke symptoms to initial perfusion imaging.4–8 Furthermore, the CTP stroke evaluation software and underlying computational method can influence the amount of ischemic tissue quantified.
Currently, various post-CTP acquisition algorithms are used to generate perfusion maps across different commercially available software, leading to different perfusion thresholds being used to determine infarct and penumbra.9 One common computational method is the singular value decomposition plus (SVD+) algorithm, which is based on deconvolution of the arterial input and focuses on each individual locations within the perfusion scan when computing perfusion maps.10,11 Recently, Bayesian algorithms have been developed, which are based on a probabilistic approach reliant on neighboring regions of perfusion scans and noise reduction to generate perfusion maps.12–15 Calculation differences potentially result in discrepancies in perfusion maps, preventing a standard infarct and penumbra identification protocol from being implemented across all CTP software and vendors.16 This can be dangerous to patients, as overestimating infarct can lead to exclusion from thrombectomy procedures and underestimating infarct can lead to reperfusion injuries.17 Furthermore, this may lead to discrepancies when reporting data from multicenter studies.
The summary above indicates that penumbra-to-infarct ratios in AIS patients may be affected to a certain degree by patient-specific parameters. Applying a software-independent threshold may not be the best approach and may lead to disagreements that limit method application and subgroup analysis. However, it may be possible for accurate CTP data analysis by determining the optimal threshold for AIS patients while taking into account that ischemic volume measurements may fluctuate slightly. This may be included in a quality assurance study of each research protocol. Additionally, it may be possible to assign optimal thresholds to specific subcategories of patients such as those who undergo endovascular intervention and those you undergo no treatment.
This study aimed to establish a work-flow approach to optimize perfusion thresholds for the Bayesian and SVD+ algorithms within Vitrea CTP software (Vital Images, Minnetonka, MN), in predicting final infarct volume (FIV) in intervention and non-intervention AIS patients based on 24-hour fluid-attenuation inversion recovery (FLAIR) magnetic resonance imaging (MRI). Additionally, we aimed to assess performances of the Bayesian and SVD+ algorithms at these optimal thresholds, in comparison with a commercially available RAPID CTP software (iSchemaView, Menlo Park, CA), in determining FIV in intervention patients and infarct and penumbra in non-intervention patients.
Methods
Data collection
For this retrospective study, Institutional Review Board approval was obtained, and informed consent was waived. Inclusion criteria involved AIS patients who underwent CTP evaluation and 24-hour follow-up FLAIR MRI between 2012 and 2016 at our comprehensive stroke center. Patients were initially imaged using non-contrast CT to prevent the inclusion of any patients with hemorrhage. A total of 107 patients were included to optimize the CTP thresholds: 40 mechanical thrombectomy intervention patients, and 67 non-intervention patients who did not receive intravenous thrombolysis or mechanical thrombectomy. The decision for mechanical thrombectomy was made by the attending physician based on several factors, including: time since onset of symptoms, National Institute of Health Stroke Scale (NIHSS) score (NIHSS > 6), and penumbra-to-infarct ratio (ratio > 1). While all patients in the intervention group had a large-vessel occlusion, non-intervention patients had either a large- or small-vessel occlusion. The inclusion of both intervention and non-intervention patients was done to assess infarct and penumbra, respectively. Additionally, data from 30 intervention and 30 non-intervention patients were obtained to validate the optimal thresholds and for comparison with a clinically implemented software, RAPID.
CTP data were acquired through injection of 50 mL of Omnipaque 350 at a rate of 5 mL/second using two Aquilion ONE CT units (Canon Medical Systems, Otawara, Japan). The total dose length product was 2373.9 mGycm, the total CT dose index volume was 148.4 mGy, and 19 scans were obtained. The first volume was obtained seven seconds after contrast injection, the next three volumes 3.2 seconds apart, the following 10 volumes 1.5 seconds apart, and the last five volumes 3.6 seconds apart. Each scan volume contained 320 slices, each at a thickness of 0.5 mm for a total axial coverage of 160 mm. CTP data were available for interpretation within five minutes from the start of the scan and included the scan time, reconstruction time, and data transfer time to the Vitrea workstation. Thrombolysis in cerebral infarction (TICI) scores were obtained for patients who underwent successful reperfusion procedures through consensus between attending physicians and endovascular fellows present during the procedure. Successful reperfusion is defined as TICI 2b or 3, which corresponds to partial perfusion (>50% of distal branch filling) and complete perfusion (100% distal branch filling), respectively.18 FLAIR MRI data were obtained using a Vantage Titan 1.5 Tesla MRI unit (Canon Medical Systems, Otawara, Japan) with the following parameters: inversion time = 2,500 ms, echo time = 120 ms, and repetition time = 10,000 ms.
CTP infarct volume
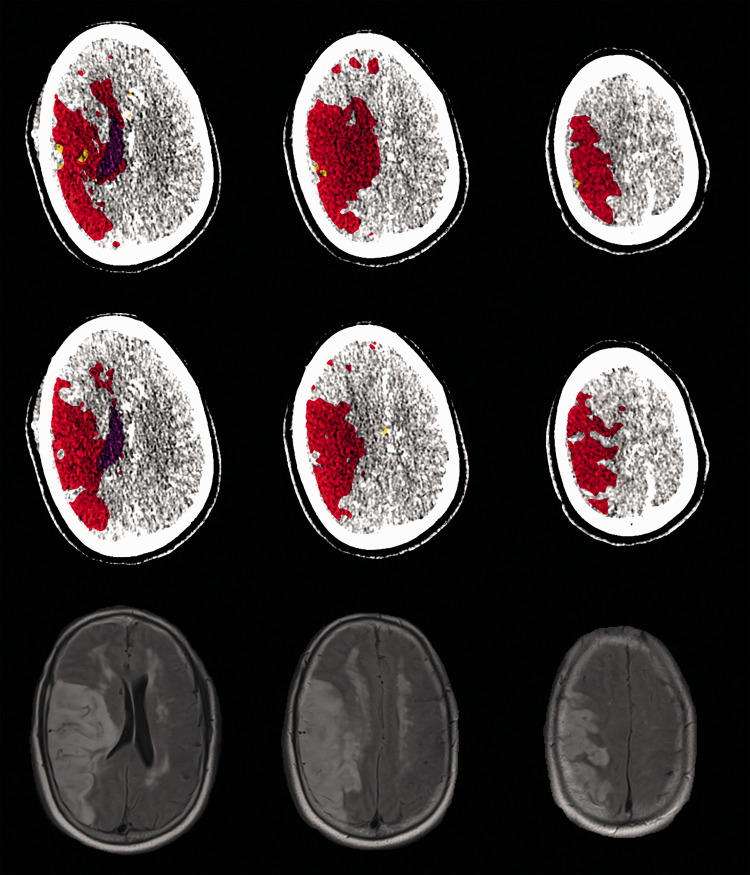
CTP data were loaded into the Vitrea software for perfusion map generation. Regions affected by the stroke were segmented using Vitrea’s segmentation tool through a visual inspection technique. This manual infarct and penumbra segmentation prevented the inclusion of erroneous infarct regions, which can sometimes form at the base and top of the skull. Infarct and penumbra volumes were determined through absolute and relative value contralateral hemisphere comparisons of the TTP and rCBV perfusion maps, respectively. Through use of the following combinations of TTP and rCBV thresholds, voxels exceeding the TTP threshold were labeled as penumbra, and voxels exceeding both the rCBV and TTP thresholds were labeled as infarct. For the Bayesian algorithm, infarct and penumbra volumes were obtained at absolute TTP threshold settings ranging from +2.8 to +7.8 seconds at intervals of +0.5 seconds, along with relative rCBV thresholds ranging from –0.23 to –0.53 at intervals of –0.03. Prior to optimization, default rCBV and TTP thresholds for the Bayesian algorithm were –0.38 and +5.3 seconds, respectively. For the SVD+ algorithm, infarct and penumbra volume measurements were obtained at TTP thresholds ranging from +4.3 to +9.3 seconds at intervals of +0.5 seconds, and rCBV thresholds ranging from –0.26 to –0.56 at intervals of –0.03. Prior to optimization, SVD+ algorithm default rCBV and TTP thresholds were –0.41 and +6.8 seconds, respectively. Starting and ending thresholds values for each parameter were chosen as the minimum and maximum values supplied within the Vitrea software. For each rCBV threshold setting, each TTP threshold setting was tested for a total of 121 perfusion threshold combinations. Segmented volumes of infarct and penumbra for the Bayesian and SVD+ algorithms are shown in Figure 1. Optimal thresholds for predicting FIVs are defined as the instance when average predicted and FIV difference is at its minimum, along with the mean absolute error (MAE) being at its minimum.
Figure 1.
A non-intervention case for a middle cerebral artery occlusion patient. Top and middle rows show Bayesian and SVD+ perfusion analyses, respectively. Red, yellow, and purple regions indicate infarct, penumbra, and ventricles, respectively. The bottom row indicates FLAIR MRI 24 hours post CTP imaging, with hyper-intensified regions indicating infarct. SVD+: singular value decomposition plus; FLAIR: fluid-attenuation inversion recovery; MRI: magnetic resonance imaging; CTP: computed tomography perfusion.
FLAIR MRI infarct volume
FLAIR MRI was used to determine FIVs due to its ability to locate hyper-intensified lesions within the brain such as infarct tissue.19 Diffusion-weighted imaging was not utilized for determining FIVs, since it has shown instances of lesion reversal in the event of rapid reperfusion.20 These instances of lesion reversal have not been documented when using FLAIR MRI. Figure 1 demonstrates a FLAIR MRI with hyper-intensified regions indicating infarct tissue. FLAIR MRI data were loaded into 3D Slicer software (Slicer Community), and segmentation of the infarct was conducted using thresholding, which selected voxels with intensity values >80.21 The segmented infarct was quantified within 3D Slicer software and compared, with Bayesian and SVD+ CTP infarct volumes for intervention cases and a summed infarct and penumbra volume for non-intervention cases. Penumbra and infarct was summed for non-intervention cases, since all penumbra should convert to infarct by the time the FLAIR MRI occurs in untreated patients.22 However, it is worth noting spontaneous recanalization, the dislodging of an embolus through natural vessel flow conditions, is possible, which would prevent all penumbra from converting to infarct. For the purpose of this study, it was assumed spontaneous recanalization did not occur.
Validation set
Following optimization of the Bayesian and SVD+ perfusion thresholds for intervention and non-intervention patients (optimization methods are listed in the Statistical Analysis subsection), we applied these settings to a group of patients not used for the first step of this study. CTP and FLAIR MRI data for an additional 30 intervention and 30 non-intervention patients were obtained. CTP volumes were loaded into the Vitrea software, and the optimal thresholds were utilized to quantify infarct and penumbra. Additionally, RAPID CTP software was utilized to predict FIVs to generalize the results of the Vitrea software to other commercially available CTP software packages. RAPID CTP analysis was conducted offsite by sending the 19 CTP volumes to iSchemaView and receiving the infarct and penumbra predicted view perfusions maps in the hospital’s picture archiving and communications system. RAPID analysis labels regions with a 70% reduction of cerebral blood flow compared to the contralateral hemisphere as infarct and regions with time to reach the peak of the impulse residue function (Tmax) of more than six seconds, as penumbra. FLAIR MRI data were utilized as ground truth infarct labels, and a comparison was conducted between CTP and FLAIR MRI to determine the accuracy of the optimal perfusion thresholds in quantifying infarct and penumbra.
Statistical analysis
Data are represented using summary statistics for continuous variables and frequency distributions for categorical data. Differences in amounts of CTP-predicted infarct and FLAIR MRI infarct were calculated for each Vitrea perfusion threshold combination. MAEs were determined using differences between predicted and FIV for the Bayesian and SVD+ algorithms. Subgroup analysis to calculate mean infarct differences and MAEs was conducted for homogenous cohorts as well to validate optimal thresholds (intervention group N = 40, non-intervention group N = 53). Homogenous cohorts were created by removing all time since onset to CTP imaging outliers from the non-intervention group. Outliers included times exceeding the mean intervention group onset to imaging time plus two standard deviations. Regression analysis and Bland–Altman plots were used to compare predicted infarct for intervention patients at the determined optimal thresholds for each algorithm with FIVs. Additionally, regression analysis and Bland–Altman plots were used to compare summed infarct and penumbra volumes in non-intervention patients with FIVs. Spearman correlation coefficients were determined for all comparisons. Average volume differences, 95% confidence levels, and MAEs were calculated between predicted and FIVs for the validation set. In addition, regression analysis and Bland–Altman plots were generated between predicted and FIVs for each of the CTP software used for the validations set. All plots were created within MATLAB 9.4. Algorithm processing times are represented as 95% confidence intervals. Processing time was defined as the interval from loading CTP volumes into the Vitrea software until perfusion map generation. For RAPID software, processing time was defined as the instance the transfer of CTP scans to iSchemaView until the RAPID perfusion maps were available within the hospitals picture archiving and communications system. Inter-reader variability was assessed for accuracy of manual infarct segmentation across five software users, determining percent difference, average difference, and median absolute deviation in segmentations.
Results
Intervention group
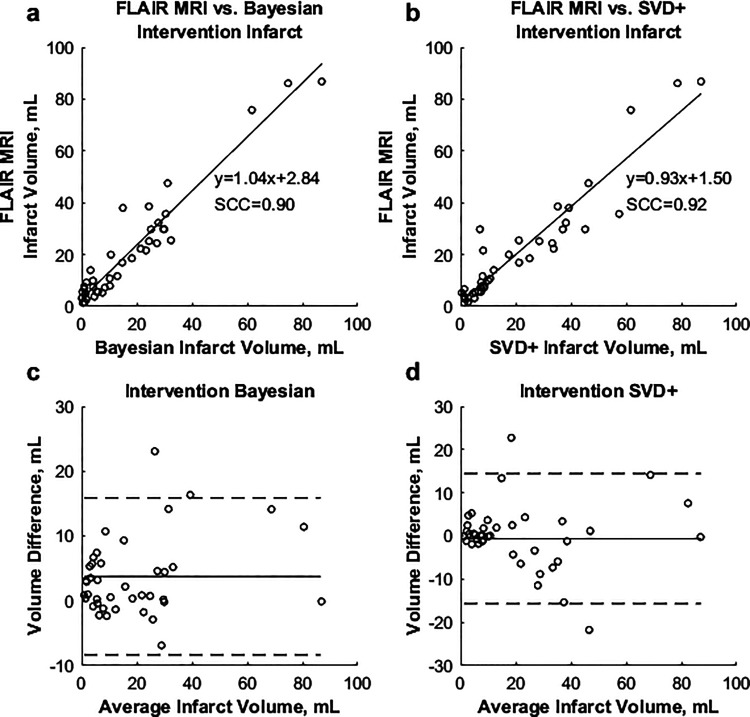
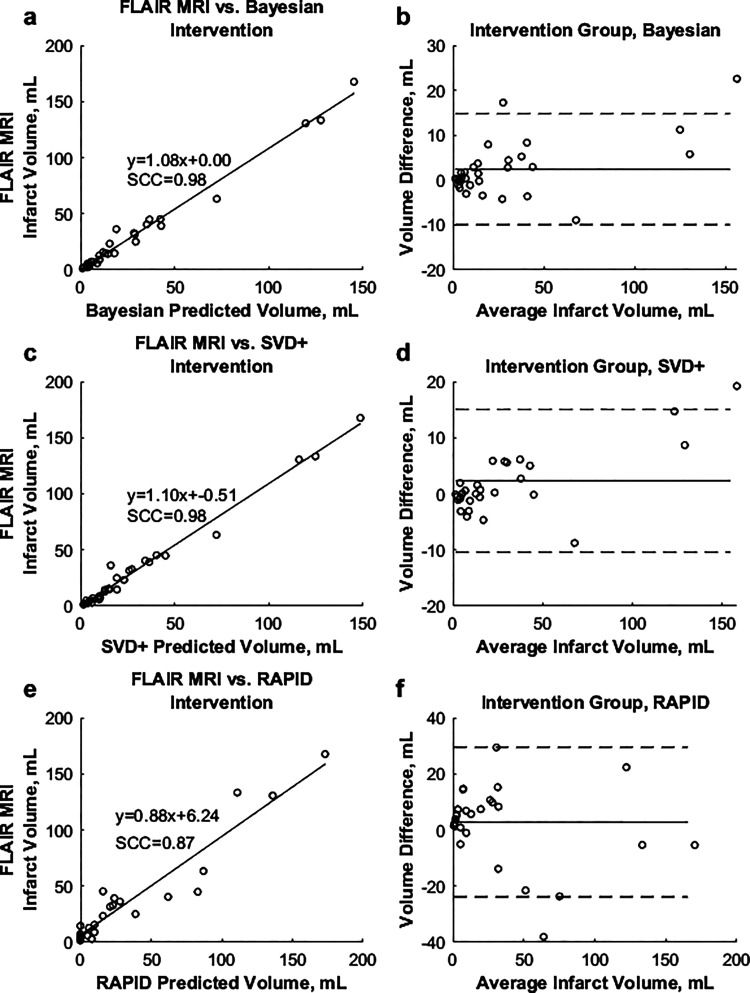
Table 1 shows the demographics for the 107 patients included in the study. Average differences between predicted and FIV, along with MAEs for each perfusion TTP and rCBV threshold combination are shown in Figure 2. The lowest average difference between predicted and FIVs was 3.8 mL (MAE = 5.1 mL) using the +4.8 seconds TTP and –0.29 rCBV parameters for the Bayesian algorithm. For the SVD+ algorithm, the lowest average difference (–0.6 mL) and MAE (4.5 mL) occurred using thresholds of +5.8 seconds and –0.29 for the TTP and rCBV parameters, respectively. Regression analysis found Spearman correlations (SCC) of 0.92 and 0.89 when comparing the Bayesian and SVD+ infarct volumes, respectively, to the FLAIR MRI FIV. Figure 3 demonstrates correlations between final and predicted infarct volumes for both algorithms, along with Bland–Altman plots.
Table 1.
Demographics for AIS patients.
| Characteristic | All (N = 107) | Intervention (N = 40) | Non-intervention (N = 67) |
|---|---|---|---|
| Male sex, n (%) | 43 (40.2%) | 17 (42.5%) | 26 (38.8%) |
| Age (years), M ± SD, median (IQR) | 70.4 ± 15.1, 72 (58–85) | 68.2 ± 13.5, 69 (57–81) | 71.8 ± 15.9, 75 (59–86) |
| National Institutes of Health Stroke Scale score, M ± SD, median (IQR) | 11.1 ± 5.8, 11 (7–15) | 12.2 ± 6.5, 12 (8–16) | 10.4 ± 5.2, 10 (7–15) |
| Middle cerebral artery occlusion, n (%) | 75 (70.1%) | 25 (62.5%) | 50 (74.6%) |
| Posterior cerebral artery occlusion, n (%) | 10 (9.3%) | 5 (12.5%) | 5 (7.5%) |
| Internal carotid artery occlusion, n (%) | 10 (9.3%) | 5 (12.5%) | 5 (7.5%) |
| Anterior cerebral artery occlusion, n (%) | 12 (11.2%) | 5 (12.5%) | 7 (10.4%) |
| Time from onset of stroke to perfusion imaging (minutes), M ± SD, median (IQR) | 250.1 ± 232.9, 176 (134–328) | 168.4 ± 86.8, 131 (120–190) | 298.8 ± 276.1, 195 (141–281) |
| Time from onset of stroke to reperfusion (minutes), M ± SD, median (IQR) | … | 290.6 ± 138.4, 259 (184–330) | … |
| Time from perfusion imaging to recanalization (minutes), M ± SD, median (IQR) | … | 122.2 ± 107.3, 87 (59–158) | … |
| TICI 2b recanalization | … | 12/40 (30.0%) | … |
| TICI 3 recanalization | … | 28/40 (70.0%) | … |
| Bayesian infarct volume (mL), M ± SD, median (IQR) | 29.8 ± 33.0, 5.9 (0.9–32.4) | 17.2 ± 19.9, 10.5 (2.9–25.7) | 37.3 ± 38.9, 8.9 (1.9–42.0) |
| SVD+ infarct volume (mL), M ± SD, median (IQR) | 32.2 ± 37.7, 12.1 (4.6–33.9) | 21.6 ± 22.0, 2.7 (0.1–35.0) | 38.6 ± 44.1, 26.9 (7.78–64.7) |
| FLAIR MRI infarct volume (mL), M ± SD, median (IQR) | 28.4 ± 35.9, 15.4 (5.8–32.6) | 21.0 ± 21.6, 12.8 (5.7–29.8) | 33.4 ± 41.9, 16.5 (7.3–43.1) |
AIS: acute ischemic stroke; SD: standard deviation; IQR: interquartile range; TICI: thrombolysis in cerebral infarction; SVD+: singular value decomposition plus; FLAIR: fluid-attenuation inversion recovery; MRI: magnetic resonance imaging.
Figure 2.
Average differences between predicted and FLAIR FIVs for all available Vitrea Bayesian (a) and SVD+ (b) threshold settings for intervention patients. MAE values for corresponding Bayesian (c) and SVD+ (d) perfusion thresholds. The “x” in each plot corresponds to optimal threshold combinations. FIV: final infarct volume; MAE: mean absolute error.
Figure 3.
(a) and (b) Correlations between predicted Bayesian and SVD+ infarct, respectively, against FLAIR MRI infarct in intervention patients. (c) and (d) Bland–Altman plots for the Bayesian and SVD+ algorithms, respectively.
Non-intervention group
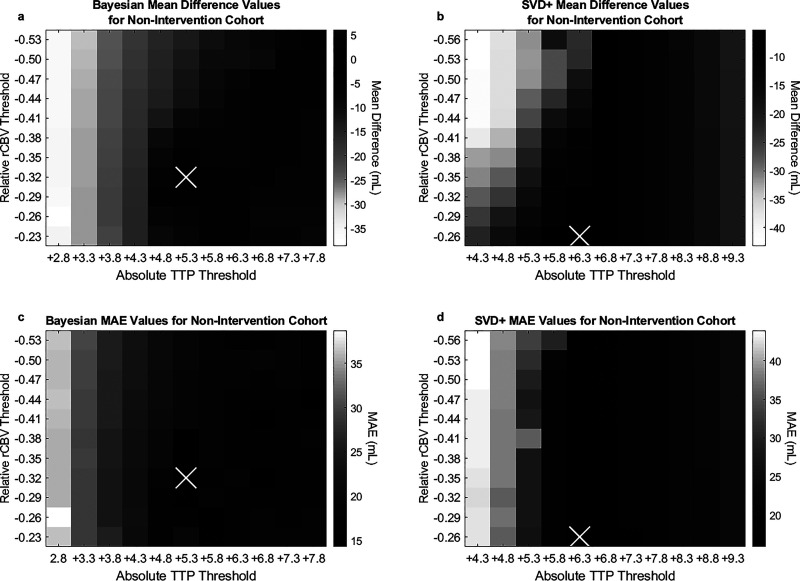
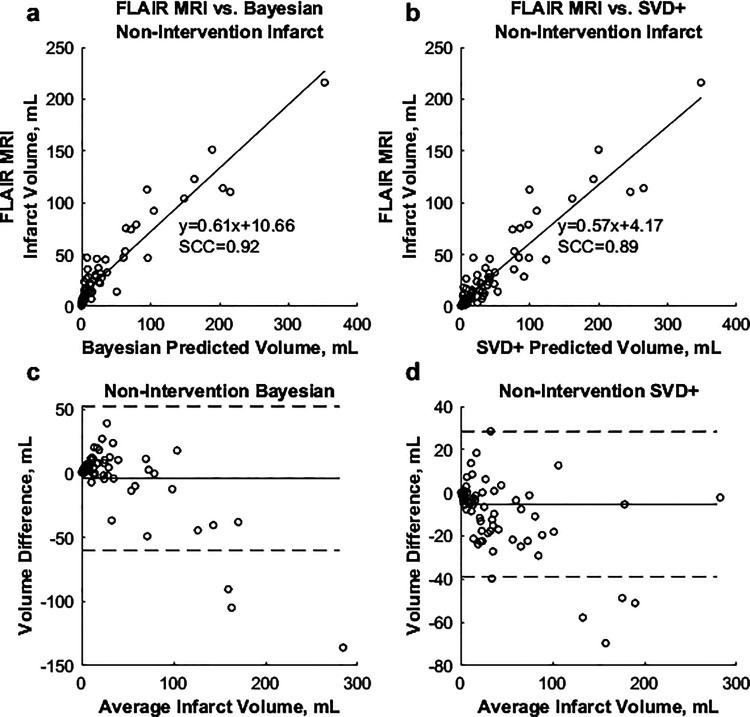
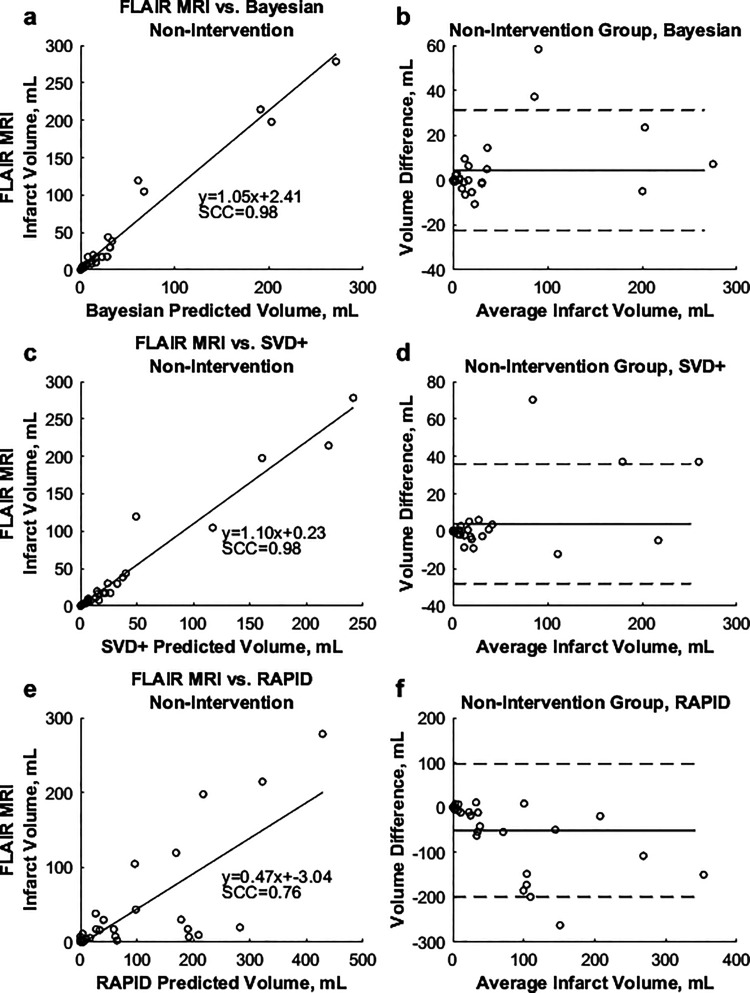
Average differences between predicted and FIV in addition to MAEs for all TTP and rCBV threshold combinations for non-intervention cases utilizing the Bayesian and SVD+ algorithms are indicated in Figure 4. For the Bayesian algorithm, the TTP parameter of +5.3 seconds and rCBV parameter of –0.32 were found to have the lowest difference between FIV and summed predicted infarct and penumbra volume (–3.9 mL) and the lowest MAE (14.3 mL). Using the SVD+ algorithm, the TTP parameter of +6.3 seconds and rCBV parameter of –0.26 were found to minimize differences between predicted penumbra and infarct volumes (FIV = –5.2 mL, MAE = 15.9 mL) in non-intervention patients. Spearman correlations of 0.90 and 0.92 were calculated for respective comparisons of the Bayesian and SVD+ methods against FLAIR MRI. Figure 5 shows correlations between summed predicted infarct and penumbra volumes for non-intervention patients against FLAIR MRI, along with Bland–Altman plots.
Figure 4.
Average difference between predicted and FLAIR FIVs for all available Vitrea Bayesian (a) and SVD+ (b) threshold settings for non-intervention patients. MAE values for the corresponding Bayesian (c) and SVD+ (d) perfusion thresholds. The “x” in each plot corresponds to optimal threshold combinations.
Figure 5.
(a) and (b) Correlations between predicted Bayesian and SVD+ infarct, respectively, against final FLAIR MRI infarct in non-intervention patients. (c) and (d) Bland–Altman plots for the Bayesian and SVD+ algorithms, respectively.
Subgroup analysis
Removal of outlier patients from the non-intervention group resulted in 40 and 53 patients being included in the homogeneous subgroup analysis for intervention and non-intervention cohorts, respectively. Times since onset of symptoms to CTP imaging for intervention and non-intervention cohorts were 168.4 ± 86.8 minutes and 181.7 ± 65.4 minutes, respectively. A p-value of 0.42 was calculated between the onset of symptoms to imaging times between the two cohorts, indicating they are homogeneous. Optimal thresholds remained the same for the intervention category for the Bayesian (rCBV = –0.29, TTP = +4.8 seconds) and SVD+ (rCBV = –0.29, TTP = +5.8 seconds) algorithms, as no patients were excluded for this analysis. Calculation of optimal thresholds for the non-intervention cohort additionally led to the same optimal thresholds for the Bayesian algorithm (rCBV = 0.32, TTP = +5.3 seconds) based on these thresholds having the lowest difference between FIV and predicted infarct volume (–2.8 mL), along with the lowest MAE (13.9 mL). SVD+ optimal thresholds (rCBV = –0.26, TTP = +6.3 seconds) were additionally the same, since these thresholds had the lowest average infarct difference (–4.5 mL) and MAE (14.7 mL).
Validation group
Results for the validation study are shown in Table 2. Results demonstrate average infarct differences, 95% confidence levels, and MAEs for predicted and FIVs for each software and intervention category. Figure 6 shows correlations between predicted and FIVs for all software utilized in the validation set for intervention patients, along with Bland–Altman plots. Figure 7 shows correlations between summed predicted infarct and penumbra volumes against FLAIR MRI for non-intervention patients in the validation set, along with Bland–Altman plots.
Table 2.
Validation results for 30 intervention and 30 non-intervention cases.
| Algorithm/patient group | Average infarct difference (mL) | 95% confidence interval (mL) | Mean absolute error (mL) |
|---|---|---|---|
| Bayesian intervention patients | 2.44 | ±1.65 | 4.31 |
| SVD+ intervention patients | 2.34 | ±1.72 | 4.26 |
| RAPID intervention patients | 2.85 | ±2.87 | 10.44 |
| Bayesian non-intervention patients | 4.36 | ±3.07 | 6.79 |
| SVD+ non-intervention patients | 3.84 | ±3.68 | 7.25 |
| RAPID non-intervention patients | −51.07 | ±17.91 | 58.89 |
Figure 6.
(a), (c), and (e) Correlations between predicted Bayesian, SVD+, and RAPID infarct, respectively, against final FLAIR MRI infarct for intervention patients in the validation set. (b), (d), and (f ) Bland–Altman plots for the Bayesian, SVD+, and RAPID CTP analysis methods, respectively.
Figure 7.
(a), (c), and (e) Correlations between predicted Bayesian, SVD+, and RAPID infarct, respectively, against final FLAIR MRI infarct for non-intervention patients in the validation set. (b), (d), and (f) Bland–Altman plots for the Bayesian, SVD+, and RAPID CTP analysis methods, respectively.
Confidence intervals (95%) representing processing time for the Bayesian, SVD+, and RAPID analysis methods were found to be 148.4 ± 1.7 seconds, 24.7 ± 0.2 seconds, and 847.7 ± 53.1 seconds, respectively. Statistical significance is indicated between all of these processing times (p<0.001). Additionally, inter-reader variability analysis indicated a percentage difference of 16.1%, an average difference of 1.2 mL, and a median absolute deviation of 1.5 mL for infarct segmentation across five users.
Discussion
This study provides insight on software-specific threshold optimization for predicting infarct and penumbra tissue within intervention and non-intervention patients with AIS using Bayesian and SVD+ algorithms within Vitrea. Additionally, this study demonstrates that although thresholds are dependent on both the algorithm and patient type, threshold optimization can be established for determining patient eligibility for endovascular intervention. By determining these optimal thresholds for each algorithm, underestimation of infarct can be prevented, which is essential to prevent hemorrhagic complications due to an intervention procedure.17 These optimal thresholds can also prevent overestimation of infarct, which excludes patients from procedures that could benefit them in regaining lost neurological function.23 Additionally, this study assessed the ability of the SVD+ and Bayesian CTP algorithms in accurately detecting and quantifying infarct and penumbra tissue through comparison with FLAIR MRI and RAPID CTP software. Such assessments of infarct and penumbra are imperative, since the thresholds directly influence the selection of patients eligible for endovascular intervention procedures.23
Within intervention cases, differences between FLAIR MRI FIVs and Bayesian infarct volumes were higher than between SVD+ and FLAIR but not to a statistical significant level due to a greater degree of variability in the SVD+ algorithm. Additionally, near-identical MAE values between the two algorithms and FLAIR MRI indicate the Bayesian algorithm to be non-inferior to the implemented SVD+ method. Regression equations and correlations for each algorithm indicate the Bayesian method to be slightly more consistent in infarct estimation. The Bland–Altman plot in Figure 3 additionally showed variation of volume difference in Bayesian is smaller than the variation of SVD+. This is likely due to the noise reduction and probabilistic Bayesian algorithm leading to more uniformity in perfusion images, resulting in less classification errors within affected stroke regions.24
For the non-intervention group, an inverse trend is indicated, as average differences between predicted infarct and penumbra volumes against FIVs are lower for the Bayesian method than the SVD+ method. This difference is deemed to be insignificant based on statistical comparisons of means. The MAE, however, is higher for the SVD+ algorithm compared to the Bayesian algorithm, indicating the precision of FIV estimation is lower using SVD+. Processing time for the algorithms was found to be different by 123.7 seconds on average and was deemed to be statistically significant, resulting in a maximum of 0.34 mL of infarct forming in this time interval.25
Results for the validation study indicate that optimized thresholds may provide more accurate assessment of infarct and penumbra tissue, with differences between the predicted infarct and FIV being <5 mL. The MAE values remain within 8 mL due to the time-sensitive manner of infarct converting to penumbra. The 95% confidence levels and MAEs are higher for non-intervention cases, possibly due to introduction of error from summing infarct and penumbra to estimate the infarct volume. This error could be introduced by the impact of spontaneous recanalization when a patient essentially reperfuses themselves. Self-reperfusion due to the dislodging of an embolus based on vessel flow conditions would then lead to penumbra potentially not converting to infarct in its entirety. This further leads to the estimation of summed penumbra and infarct being inaccurate as a FIV prediction.
In addition, the limitation regarding spontaneous reperfusion is likely the reason behind differences in the optimal threshold for predicting FIVs between intervention and non-intervention patients. Due to penumbra estimations potentially being influenced by spontaneous reperfusion, FIV measurements could be skewed in non-intervention patients, which further leads to skewing of the optimal perfusion map thresholds. The fact there is no specific method for verifying penumbra volumes, however, leads to the assumption of complete conversion of infarct to penumbra in FLAIR MRI for this study. The difference in optimal thresholds between the intervention and non-intervention categories may have been influenced by the significant differences (p<0.05) seen between the time since onset of symptoms and the average FLAIR FIV for the intervention and non-intervention categories in Table 1. This significant difference implies non-intervention patients would have greater progression of infarct formation compared to intervention patients. Although subgroup analysis indicates time since onset of symptoms to perfusion imaging does not influence the optimal parameters for the non-intervention group, the larger amount of infarct still may. Alternatively, non-intervention patients may have had larger infarct volumes due to overestimation of infarct on FLAIR MRI from the presence of mass effect or edema. Within non-intervention patients, thrombectomy was not conducted, meaning tissue insult was not alleviated in the same manner as intervention patients, which could lead to the presence of edema or mass effect. Although an analysis of infarct quantification after one week could remove errors introduced by edema, it could not be performed, as very few patients in this study had FLAIR imaging at one week. Such analysis, however, should be considered for future studies. This greater progression or overestimation of infarct could then influence the rCBV and TTP parameters generated and potentially the optimal thresholds. Even with different optimal thresholds for intervention and non-intervention patients, however, clinicians can still make decisions based on the predicted infarct volumes using both thresholds settings by observing predicted FIVs in the event an intervention is performed or in the event patients are untreated.
When comparing the optimal thresholds validation set results for the Bayesian and SVD+ algorithms with the commercially available software RAPID, it can be seen that the optimized algorithms perform just as well as RAPID based on average infarct difference values for the intervention cases. RAPID software, however, has a slightly higher MAE and 95% confidence level. For the non-intervention cases, however, the Bayesian and SVD+ optimized software performed superior to RAPID based on RAPID’s average overestimation of infarct by 51.07 mL and MAE value of 58.89 mL. This overestimation of the FIV by RAPID is likely due to an overestimation of penumbra, since RAPID performs well in estimating infarct for intervention patients. This overestimation of penumbra could be due to use of the Tmax parameter to estimate penumbra in RAPID as opposed to use of the TTP parameter in Vitrea. It has been shown that the Tmax parameter is very sensitive to quantum noise, since quantum noise affects the shape of the arterial input function that is used to calculate the Tmax parameter.26 In previously conducted studies, lesion volumes detected using the Tmax parameter differed by 13% in some instances based on the degree of quantum noise present.27
The limitations of this study include use of only TTP and rCBV perfusion parameters in quantifying infarct and penumbra tissue. Specific thresholds set for delay time, mean transit time, and cerebral blood flow parameters could aid in a more accurate assessment of ischemic tissue and could be investigated in a future study. Additionally, this study only utilized rCBV and TTP thresholds between the minimum and maximum values provided within the Vitrea software. Another limitation of this study is for the cases in which recanalization occurred, the reperfusion process and follow-up imaging did not occur much sooner after initial perfusion imaging. Studies have been conducted showing that the faster a patient achieves reperfusion of TICI 2b or 3, the lower the median infarct growth found in follow-up imaging. This is particularly evident in patients who achieve complete reperfusion in <100 minutes following initial CTP imaging.28
Furthermore, voxel-wise analysis of infarct location is not conducted to ensure significant overlap of infarct regions between CTP and FLAIR. In addition, Alberta Stroke Program Early CT Scores were not taken into consideration when conducting this study. Inclusion of these scores should be considered in future studies to verify regions of definitively infarcted brain tissue. Taking these regions into consideration can further aid in verification of regions detected as infarct through utilization of CTP. Lastly, this study only included 12 patients with infarct volumes >50 mL.
Conclusion
In conclusion, Bayesian CTP analysis performed similar to both SVD+ and RAPID in assessing infarct and penumbra in AIS patients. However, when using the SVD+ and Bayesian algorithms for infarct assessment, optimal thresholds for TTP are significantly different for both interventional and non-interventional cohorts. rCBV optimal thresholds were equal for the interventional cohort and significantly different for the non-intervention cohort. This study suggests it is of great importance to optimize any utilized CTP algorithm prior to conducting studies impacting enrollment in endovascular procedures.
Conflict of interest
K.V.S. is consulting for Canon Medical Systems Corporation, Penumbra, Inc., Medtronic, and Jacobs Institute, and is a co-founder of Neurovascular Diagnostics, Inc. M.M. is a consultant for Canon Medical Systems, Cerebrotech, and Imperative Care, and has National Institutes of Health grant support (R21NS109575). A.H.S. has financial interest/investor/stock options/ownership in Amnis Therapeutics, Apama Medical, Blink TBI, Inc., Buffalo Technology Partners, Inc., Cardinal Consultants, Cerebrotech Medical Systems, Inc., Cognition Medical, Endostream Medical Ltd., Imperative Care, International Medical Distribution Partners, Neurovascular Diagnostics, Inc., Q’Apel Medical, Inc., Rebound Therapeutics Corp., Rist Neurovascular, Inc., Serenity Medical, Inc., Silk Road Medical, StimMed, Synchron, Three Rivers Medical, Inc., and Viseon Spine, Inc., and is a consultant or on the advisory board for Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Inc., Cerebrotech Medical Systems, Inc., Cerenovus, Corindus, Inc., Endostream Medical Ltd., Guidepoint Global Consulting, Imperative Care, Integra LifeSciences Corp., Medtronic, MicroVention, Northwest University–DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical, Inc., Rapid Medical, Rebound Therapeutics Corp., Serenity Medical, Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, and W.L. Gore & Associates. He is also a principal investigator/steering comment on the following trials: Cerenovus NAPA and ARISE II, Medtronic SWIFT PRIME and SWIFT DIRECT, MicroVention FRED & CONFIDENCE, MUSC POSITIVE, Penumbra 3D Separator, COMPASS, and INVEST. J.M.D. has received research grants from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001413 to the University at Buffalo and is a shareholder of RIST Neurovascular. E.I.L. owns stock in NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care (formerly the Stroke Project), Rebound Therapeutics, StimMed, and Three Rivers Medical. He is a national principal investigator/on steering committees for Medtronic (merged with Covidien Neurovascular) SWIFT Prime and SWIFT Direct Trials. He is also a consultant for Claret Medical, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, Rebound, and StimMed, and on the advisory board for Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical, and Endostream Medical. He is also a site principal investigator for the CONFIDENCE study (MicroVention) and STRATIS Study—Sub I (Medtronic). C.N.I. has received an equipment grant from Canon Medical Systems and from the Cummings Foundation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially funded by Canon Medical Systems USA, Inc.
ORCID iDs
Ryan A Rava https://orcid.org/0000-0001-6456-8445
Ciprian N Ionita https://orcid.org/0000-0001-7049-0592
References
- 1.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology 2013; 266: 16–21. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979–985. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017; 12: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Bivard A, Lin L, et al. Thresholds for infarction vary between gray matter and white matter in acute ischemic stroke: a CT perfusion study. J Cereb Blood Flow Metab 2019; 39: 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamalian S, Kamalian S, Konstas A, et al. CT perfusion mean transit time maps optimally distinguish benign oligemia from true “at-risk” ischemic penumbra, but thresholds vary by postprocessing technique. Am J Neuroradiol 2012; 33: 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol 2017; 82: 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Kalladka D, Cheripelli BK, et al. The impact of CT perfusion threshold on predicted viable and nonviable tissue volumes in acute ischemic stroke. J Neuroimaging 2017; 27: 602–606. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Scoffings DJ, Jones PS, et al. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acute stroke: a pilot study. J Neurol Neurosurg Psychiatry 2013; 84: 271–276. [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, Christensen S, Kemp S, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol 2017; 81: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles K, Eastwood JD, Konig M. Multidetector computed tomography in cerebrovascular disease: CT perfusion imaging. Abingdon, UK: CRC Press, 2007. [Google Scholar]
- 11.Kim YJ, Jeong CB, Hwang J-M, et al. New parametric imaging method with fluorescein angiograms for detecting areas of capillary nonperfusion. Healthc Inform Res 2014; 20: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nael K, Mossadeghi B, Boutelier T, et al. Bayesian estimation of cerebral perfusion using reduced-contrast-dose dynamic susceptibility contrast perfusion at 3T. Am J Neuroradiol 2015; 36: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutelier T, Kudo K, Pautot F, et al. Bayesian hemodynamic parameter estimation by bolus tracking perfusion weighted imaging. IEEE Trans Med Imaging 2012; 31: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 14.Rava R, Snyder K, Mokin M, et al. Assessment of a Bayesian Vitrea CT perfusion analysis to predict final infarct and penumbra volumes in patients with acute ischemic stroke: a comparison with RAPID. Am J Neuroradiol 2020; 41: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rava R, Snyder KV, Mokin M, et al. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: a comparison of Sphere, Vitrea, and RAPID. J Neurointerv Surg. Epub ahead of print 26 May 2020. DOI: 10.1136/neurintsurg-2020-015966. [DOI] [PubMed] [Google Scholar]
- 16.Bathla G, Limaye K, Policeni B, et al. Achieving comparable perfusion results across vendors. The next step in standardizing stroke care: a technical report. J Neurointerv Surg 2019; 11: 1257–1260. [DOI] [PubMed] [Google Scholar]
- 17.Mokin M, Levy EI, Saver JL, et al. Predictive value of RAPID assessed perfusion thresholds on final infarct volume in SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke 2017; 48: 932–938. [DOI] [PubMed] [Google Scholar]
- 18.Mokin M, Khalessi AA, Mocco J, et al. Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg Focus 2014; 36: E5. [DOI] [PubMed] [Google Scholar]
- 19.Kamran S, Bates V, Bakshi R, et al. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology 2000; 55: 265–269. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Mlynash M, Christensen S, et al. Early DWI reversal following endovascular reperfusion is typically transient in patients imaged 3-6 hours after onset. Stroke 2014; 45: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isa I, Sulaiman S, Abdullah M, et al. Assessing intensity of white matter hyperintensity and normal appearing white matter in healthy adults. In: 2016 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur. New York: IEEE, 2016, pp.503–506.
- 22.Chavez JC, Hurko O, Barone FC, et al. Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke 2009; 40: 558–563. [DOI] [PubMed] [Google Scholar]
- 23.König M. Brain perfusion CT in acute stroke: current status. Eur J Radiol 2003; 45: 11–22. [DOI] [PubMed] [Google Scholar]
- 24.Case JA, Hsu BL, Bateman TM, et al. A Bayesian iterative transmission gradient reconstruction algorithm for cardiac SPECT attenuation correction. J Nucl Cardiol 2007; 14: 324. [DOI] [PubMed] [Google Scholar]
- 25.Guenego A, Mlynash M, Christensen S, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol 2018; 84: 616–620. [DOI] [PubMed] [Google Scholar]
- 26.Wouters A, Christensen S, Straka M, et al. A comparison of relative time to peak and Tmax for mismatch-based patient selection. Front Neurol 2017; 8: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen S, Mishra N, Straka M, et al. Abstract W P28: precision of Tmax and CBF lesion volume estimates in CT perfusion imaging. Stroke 2014; 45: AWP28–AWP28. [Google Scholar]
- 28.Albers GW, Goyal M, Jahan R, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 2016; 79: 76–89. [DOI] [PubMed] [Google Scholar]