Abstract
Background
We describe use of a canine model to evaluate physiological effects and neuroprotective strategies in the setting of cerebral ischemia and endovascular neurosurgery training.
Methods
We performed transfemoral digital subtraction cerebral and cervical angiography on eight anesthetized dogs. Angiographic images of cerebral arteries were obtained following cannulation of the femoral artery. Cerebral ischemia models were made after angiography.
Results
The canine cerebral vasculature exhibited extensive tortuosity of the carotid and vertebral arteries. Conversely, the bilateral anterior spinal arteries were easily catheterized using microcatheters and microguidewires. The basilar artery and its branches were facilely cannulable. Circle of Willis continuity sans hypoplasia or aplasia of its constitutive segments was appreciated in all animals. The middle cerebral arteries could be easily accessed via the posterior communicating arteries. We generated an empirically evaluable therapeutically interventional experimental animal model of cerebral ischemia by occluding the middle cerebral artery using small coils for a duration between 15 and 60 min.
Conclusion
Unique amenability of the canine intracranial vasculature to selective and microcatheter cannulation renders experimentally induced cerebral, cerebellar, and brainstem via occlusion of the supratentorial and infratentorial arteries a simple matter. The neural vasculature irrigating the canine cerebrum, brainstem, and cerebellum may consequently prove useful in helping young and nascent endovascular neurosurgeons in developing and refining their skills of microcatheter navigation and manipulation and deployment of therapeutic devices to achieve effective occlusion of aneurysms, arteriovenous malformations, arteriovenous fistulas, and neoplasms of the intracranial cavity.
Keywords: Canine, cerebrovascular, neurovascular, cerebral, brain circulation, training, cerebral ischemia
Background
The development of, and innovation in, endovascular techniques has placed endovascular neurosurgery at the forefront of primary, adjunctive, or multimodal treatment of the majority of neurovascular anomalies.1 So-called “neuroendovascular surgery” (i.e. endovascular neurosurgery) significantly facilitates the non-invasive preadjunctive flow reduction or monotherapeutic obliteration of intracranial aneurysms, arteriovenous malformations, arteriovenous fistulas, and richly vascularized neoplasms via transarterial (e.g. transfemoral, transradial) and or transvenous (e.g. transjugular, transtorcular) routes. These procedures carry a significant non-negligible risk of precipitating significant neurologic morbidity and mortality consequent to embolic ischemia, iatrogenic rupture and hemorrhage, vessel dissection, and coil migration, as well as medical complications including femoral hematoma, pseudoaneurysm, arteriovenous fistula, aortic tears, retroperitoneal hematoma, iodinated contrast–mediated constriction of the renal arterioles, acute necrosis of the proximal convoluted tubules of the nephron, and type I IgE hypersensitivity-mediated anaphylaxis. Steepness of the learning curve in acquiring these skills varies across young resident and fellow endovascular neurosurgeons and the most appropriate training method remains to be established with finer precision and algorithmic utility. We would like to take this opportunity to indicate that many neurosurgeons receive their initial clinical experience to acquire the procedural skill necessary to independently perform neuroendovascular interventions upon vascular lesions of the intracranium on relatively healthy and profoundly sick patients; thus these neurosurgeons putatively incrementally compound the cumulative procedural risk. We assert this practice is not only outdated, but also dangerous; we indicate the elementary neurocognitive and neuromotor heuristics comprising any given neuroendovascular surgeon’s neocortical and striatal visuospatial memory, oculomotor coordination, and sequential maneuvers collectively necessary to faithfully execute these procedures with therapeutic precision should be most appropriately trained and refined in animal models.1–3 Authors have previously reported in vitro and swine models to represent effective practice for young trainees prior to intervening upon sick patients or stable patients who could suffer disastrous neurological consequences from readily preventable naïve errors.4,5 Other types of canine stroke models, including vertebral artery occlusion and basilar artery occlusion, can also be accomplished by endovascular thrombi injection.6–9 In the present study, we generate a canine model of neurovascular ischemia, which may prove useful in evaluating the molecular and physiological effects of hypoxia upon the cerebrum and salvific effects of neuroprotective agents and helping young endovascular neurosurgery trainees acquire the skill set necessary to faithfully treat neurovascular lesions of the intracranial cavity.
Methods
From 2017 to 2018, we evaluated the intracranial vasculature of eight male dogs weighing 11–17 kg (mean weight 15 kg) utilizing digital subtraction cerebral angiography under general anesthesia–induced surgical coma. Ethical approval was obtain from an Institutional Animal Care Committee for animal experimentation following international guidelines. We generated a working animal model of cerebral ischemia by occluding the middle cerebral artery for 15–60 min.
Technique
Under general anesthesia, eight canines underwent digital subtraction cerebral angiography. We initially introduced two 5-French diagnostic guiding catheters separately via bilateral femoral artery punctures. Following femoral artery cannulation and the administration of a small amount of iodinated contrast agent to visualize the aortoiliac anatomy, the 5-F guiding catheter was advanced towards the subclavian artery. Another 5-F catheter was advanced into the contralateral internal carotid artery used for angiography to confirm occlusion. Under roadmap visualization, an SL-10 microcatheter was navigated by a Synchro-14 guidewire into the middle cerebral artery of the posterior communicating artery–internal carotid artery junction through the posterior communicating artery, via the precommunicating posterior cerebral artery, through the basilar artery, via the anterior spinal artery. Coils sized 1.5 mm × 4 cm, 2 mm × 4 cm, or 2 mm × 6 cm were used to endovascularly fashion complete thrombotic occlusion of the middle cerebral artery. The coil was left non-detached and in place, effectively occluding the middle cerebral artery for 30–60 min, contrasted with immediate coil deployment when treating aneurysms or arteriovenous malformations of the intracranium. Complete restitution of blood flow to the parenchyma irrigated by the middle cerebral artery immediately followed coil retrieval at the conclusion of the procedure. The layers constituting the soft tissue and skin of the inguinal femoral puncture site were reapproximated using sutures and the canine was awakened from general anesthesia. On postoperative day 1 (the day following the procedure), a magnetic resonance imaging examination was scheduled in order to neuroradiologically evaluate and confirm the cerebral infarction. Following magnetic resonance imaging examination, the canine was euthanized and the cerebrum gross anatomically and microanatomically evaluated.
Results
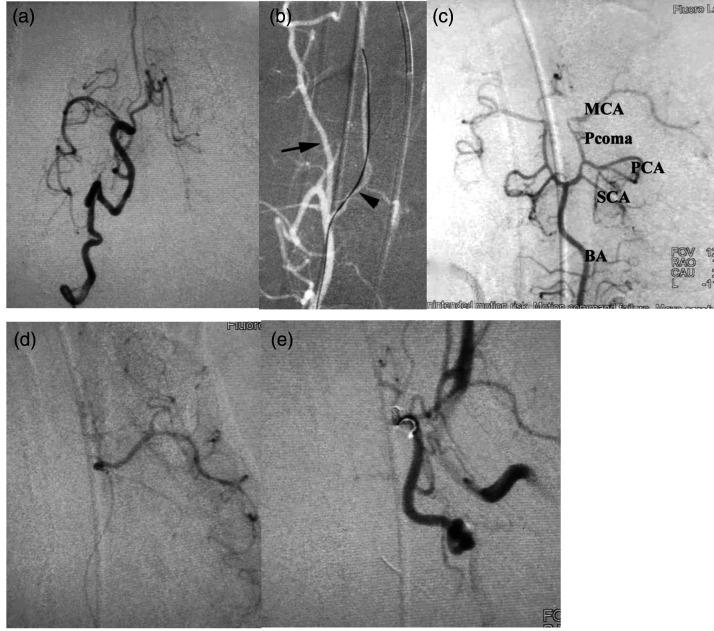
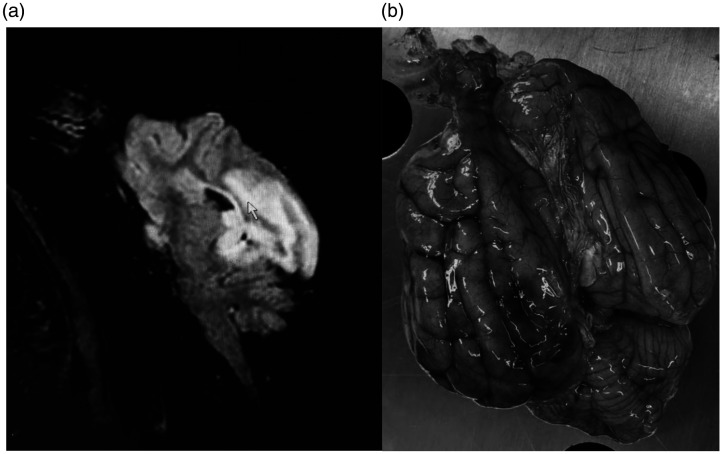
Embolization procedures were successfully performed according to the planned endovascular approach in eight canines. Common technical failures which may occur in these procedures, including vascular perforation, vessel spasm, incapacity to navigate or negotiate microcatheter into the middle cerebral artery, or inability to deploy coil into the middle cerebral artery, were not appreciated in any of the experiments. Procedural duration varied between 50 and 200 min (mean 130 min). Our endovascular interrogation of the canine intracranial vasculature revealed a consistent feature of carotid and vertebral arterial tortuosity (Figure 1(a)). The bilateral anterior spinal arteries were proved to be easily cannulable using microcatheter and microguidewires (Figure 1(b)). Universal accessibility of the basilar artery and its derivative branches facilitated access to distally related vasculature of the intracranium. Continuity of the arterial circle of Willis was appreciated in all animals, and the middle cerebral arteries were proved to be amenable to access through the posterior communicating arteries (Figure 1(c)). The right middle cerebral artery was successfully coil occluded for 60 min in six canines and the left middle cerebral artery was successfully coil occluded for 30 min in two canines (Figure 1(d) and (e)). We were only able to perform neuroradiological evaluation of two canines via magnetic resonance imaging surviving to postoperative day 1 (day following the middle cerebral artery occlusion). The remaining six dogs expired 5–19 h (mean 14 h) following the procedure, likely consequent to malignant cerebral edema, intracranial hypertension, and herniation of the cerebrum across dural enclosures and bony orifices. The two canines surviving sufficiently to permit neuroradiological examination recovered from sub-complete occlusion of the middle cerebral artery and developed parietal lobe preferential cerebral edema ipsilateral to ischemia (Figure 2(a)). The autopsy findings of these animals revealed a deeply dark red hue to infarcted brain tissue, ipsilateral swelling of the brain tissue (Figure 2(b)), and grooved impressions of cerebral herniation. The procedures and outcomes of the eight canines are shown in Table 1.
Figure 1.
(a) Frontal view of the right internal carotid artery showing the canine carotid artery is tortuous. (b) Showing the vertebral artery was tortuous (arrow) and the right anterior spinal artery was chosen easily with a guidewire under roadmap (arrowhead). (c) Canine circle of Willis is intact. (d) Selective angiography of the left MCA. (e) Frontal review of the left carotid angiogram showing the left MCA was occluded.
BA: basilar artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; Pcoma: posterior communicating artery; SCA: superior cerebellar artery.
Figure 2.
(a) Sagittal view of canine magnetic resonance imaging examination, T2-weighted, showing the brain edema caused by infarction (arrow). (b) Showing the obvious swelling of the left hemisphere and dark infarction area.
Table 1.
Procedures and outcomes in eight canines.
| No. | Weight (kg) | Duration of procedure (min) | Occlusion vessel | Duration of occlusion (min) | MRI | Outcome | Time to death after procedure (h) |
|---|---|---|---|---|---|---|---|
| 1 | 11.6 | 180 | RMCA | 60 | No | Died | 14 |
| 2 | 15 | 180 | RMCA | 60 | No | Died | 11 |
| 3 | 17 | 200 | RMCA | 60 | Yes | Survived | – |
| 4 | 14.7 | 180 | RMCA | 60 | No | Died | 5 |
| 5 | 15 | 80 | RMCA | 60 | Yes | Survived | – |
| 6 | 17 | 100 | RMCA | 60 | No | Died | 18 |
| 7 | 14.5 | 50 | LMCA | 30 | No | Died | 18 |
| 8 | 15 | 80 | LMCA | 30 | No | Died | 19 |
LMCA: left middle cerebral artery; MRI: magnetic resonance imaging; RMCA: right middle cerebral artery.
Discussion
Our study chiefly validates a novel canine model of cerebral ischemia for empirically interrogating the molecular and physiological effects of transient occlusions of the major cerebral vessels and for helping aspiring and young endovascular neurosurgeons acquire the skill set necessary to faithfully treat neurovascular lesions of the intracranium. The findings of our study collectively indicate and demonstrate that the canine cerebrum experiences catastrophic infarction following occlusion of the middle cerebral artery blood flow despite restitution of blood flow.
The molecular and physiological effects of ischemia upon the cerebrum represent widely investigated features of aberrant cerebral hemodynamics and guide our optimization of the most widely used therapeutic modalities of mechanical thrombectomy and pharmacological thrombolysis using molecular analogues and congeners of tissue plasminogen activator (e.g. alteplase, anistreplase, reteplase, tenecteplase, streptokinase).
Canines possess a gyrencephalically impressioned cerebrum bearing striking resemblance to the human cerebrum and an endovascularly accessible and interrogable neurovascular tree useful in generating experimental animal models of cerebral ischemia.5 Canine stroke models may be widely utilized in order to experimentally dissect and conceptually elucidate the pathogenesis and pathophysiology of cerebrovascular ischemia.6 Pauci-invasive endovascular embolization of vessels supplying cerebral tissue delivered via injections through the internal carotid artery and its branches may thus be used to generate empirical models of transient or permanent middle cerebral artery occlusion, ontogenically recapitulating the pathodynamics noted in human patients. The extensive collateral arterial circulation irrigating the canine brain may effectively save penumbral transformation to liquefactive necrosis and thus limit the extent and volume of cerebral infarcts. Contemporaneous transient experimental occlusion of the internal carotid artery may reduce collateral flow supplying compromised cerebral parenchyma and increase volume of the cerebral infarct to some degree. Canine stroke models developed by other investigators, including occlusion of the vertebral and basilar arteries, may also be accomplished by endovascular injection of emboli.
Rapid development and burgeoning use of endovascular neurosurgical intervention have significantly enhanced the demand for a comprehensive training program for young trainees, in order to safely and efficiently perform these procedures.10 Vascular silicone model training represents a dynamic way to foster the acquisition of a uniquely distinct oculomotor and neuromotor skill set for the emerging generation of endovascular neurosurgeons.11 These skills include material handling, tool manipulation, navigation, and negotiation through variably forgiving or tortuous e vasculature.2 A paucity of resemblance of the physical properties of silicone models to natural friction, hemodynamics, and hemostasis natively present biologically represents the principal disadvantage of these models. Lack of fragility and reflexive contraction reactions of the in silico arteries also fail to ontogenically recapitulate the biological condition, rendering the empirical replication of complication management (e.g. dissection or rupture of the vessel wall) infeasible, causing the specific features of the skill set acquired through the use of these models to be somewhat wanting.7 The cerebrovascular anatomy of swine models precludes utility and their use is deemed inappropriate in training intracranial manipulations.8 A fine network of vessels in the skull base of pigs precludes facile accessibility of the swine intracranial vasculature.9,12,13
Canine intracranial anatomy somewhat resembles human cerebrovascular anatomy. Authors have described the use of experimental canine models of intracranial arteriovenous malformations to evaluate the utility of embolic materials in the treatment of these lesions and newly developed catheters in achieving superselective navigation using microcatheters.4 Although we deem the model unsuitable for evaluating the pathophysiology of intracranial vascular lesions, its use may provide training in the handling of endovascular devices and acquisition of a skill set constituted by catheter, guidewire, and device manipulation, navigation, and negotiation necessary to effectively treat vascular lesions of the intracranium via endovascular transarterial or transvenous routes, given similar hemodynamic and hemostatic conditions noted to occur in humans.4,5
Vessel sizes of the intracranial vasculature of the canine are comparable with that of humans, permitting training using standard-sized devices easily obtainable from the supply room in any operating theater. The autologous blood thrombus, enriched by a contrast agent, enables visualization of the interaction with devices, and possible dislocation or fragmentation during angiography. The intracranial vasculature of canines recapitulates a set of neurovascular pathways reflecting the human conditions, permits endovascular neurosurgery trainees to practice complication management, and allows the “fine-tuning” of skills and competencies in clinically relevant settings.
Conclusion
Future studies using this model should be conducive to enhancing our capacity to empirically and experimentally interrogate mechanisms underlying the pathogenesis and pathophysiologic consequences of cerebral ischemia and enhance the capacity of aspiring and young endovascular neurosurgeons to acquire the skill set necessary to gain familiarity with transarterial and transvenous approaches to the vasculature of the intracranium and intervene upon neurovascular lesions.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (PX2020039) and National Natural Science Program (81471767), Beijing, China.
ORCID iD
Xianli Lv https://orcid.org/0000-0001-8270-8464
References
- 1.Day AL, Siddiqui AH, Meyers PM, et al. Training standards in neuroendovascular surgery: program accreditation and practitioner certification. Stroke 2017; 48: 2318–2325. [DOI] [PubMed] [Google Scholar]
- 2.Paramasivam S, Baltsavias G, Psatha E, et al. Silicone models as basic training and research aid in endovascular neurointervention—a single-center experience and review of the literature. Neurosurg Rev 2014; 37: 331–337. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Fujitsuka M, Chaloupka JC. Simulation of endovascular neurointervention using silicone models: imaging and manipulation. Neurol Med Chir (Tokyo) 2005; 45: 567–573. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher M, Schellhammer F. Experimental pseudo arteriovenous malformation. A model for training and research. Interv Neuroradiol 1999; 5: 213–217. [DOI] [PubMed] [Google Scholar]
- 5.Atchaneeyasakul K, Guada L, Ramdas K, et al. Large animal canine endovascular ischemic stroke models: a review. Brain Res Bull 2016; 127: 134–140. [DOI] [PubMed] [Google Scholar]
- 6.Shaibani A, Khawar S, Shin W, et al. First results in an MR imaging–compatible canine model of acute stroke. AJNR Am J Neuroradiol 2006; 27: 1788–1793. [PMC free article] [PubMed] [Google Scholar]
- 7.Rink C, Christoforidis G, Abduljalil A, et al. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A 2008; 105: 14100–14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christoforidis GA, Rink C, Kontzialis MS, et al. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol 2011; 46: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rink C, Christoforidis G, Khanna S, et al. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab 2011; 31: 2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu JH, Younan D, Pandalai S, et al. Use of computer simulation for determining endovascular skill levels in a carotid stenting model. J Vasc Surg 2004; 40: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 11.Sandmann J, Müschenich FS, Riabikin A, et al. Can silicone models replace animal models in hands-on training for endovascular stroke therapy? Interv Neuroradiol 2019; 25: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namba K, Mashio K, Kawamura Y, et al. Swine hybrid aneurysm model for endovascular surgery training. Interv Neuroradiol 2013; 19: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Liang S, Zhang W, et al. Liquid embolic agent of Fe3O4-EVOH for endovascular AVM embolization: preliminary evaluation in an in vivo swine rete mirabile model. Neuroradiol J 2020; doi:10.1177/1971400920917130. [DOI] [PMC free article] [PubMed]