Abstract
Introduction
The treatment of aneurysms in the internal carotid bifurcation region (ICABR), including aneurysms of the true internal carotid artery (ICA) terminus, those inclined on the proximal A1 or M1 segments or at the most distal pre-bifurcation (ICA) segment, is often challenging in microsurgical clipping and endovascular surgery. Few reports had discussed flow diversion as a therapeutic option for this group.
Methods
This was a retrospective study analysing flow diversion in treating ICABR aneurysms. Seven patients harbouring eight aneurysms in the ICABR were treated with flow diversion. Five aneurysms were inclined on the proximal A1 segment, and three were located at the most distal pre-bifurcation segment. Patients’ demographics, presentation, procedure technical description, angiographic and clinical follow-up were recorded. PubMed and Ovid MEDLINE were also reviewed for articles published in English, including case series or case reports, for ICABR aneurysms treated with flow diverters.
Results
All patients except one underwent angiographic follow-up. The Karman–Byrne occlusion scale was used to determine the occlusion rate. All six patients with documented angiographic follow-up had a class IV occlusion score. No permanent or transient neurological or non-neurological complications were encountered in this study.
Conclusion
Treating ICABR aneurysms using flow diversion is feasible, with a promising angiographic occlusion rate. Further studies are needed to analyse long-term clinical and angiographic results.
Keywords: Flow diverter, ICA terminus, ICA termination, proximal A1 segment, bifurcation aneurysms
Introduction
Aneurysms located in the internal carotid artery (ICA) bifurcation region (ICABR) account for about 5% of all intracranial aneurysms and 15% of all ICA aneurysms.1,2 They usually present with bleeding at a younger age and are smaller than aneurysms in other locations.2,3 Treatment of such aneurysms is challenging for both microsurgical clipping and endovascular surgery. The ICABR is considered the highest point of the circle of Willis, with multiple perforators coming off the A1 and M1 segments of the anterior cerebral artery (ACA) and middle cerebral artery (MCA), respectively, as well as perforators also coming off the bifurcation itself.2 Perforators in such locations together with the presence of the recurrent artery of Heubner and anterior choroidal artery in close proximity to this aneurysm group represent a considerable challenge during surgical dissection.4 The results obtained from flow diversion in proximal internal carotid aneurysms encouraged interventionists to expand its use to treat complex aneurysms in other locations, such as posterior circulation, MCA bifurcation and distal ICA. Conventional endovascular treatment of some aneurysms in those locations might be challenging and carries relatively higher risks of complications and a higher incidence of re-canalisation. Few case series have reported flow diversion as a therapeutic option for ICABR aneurysms.1,5–12
In this study, we present our experience in treating aneurysms located in the ICABR, including those inclined on the proximal A1 segment, true ICA bifurcation and distal prebifurcation ICA segment (choroid segment) using flow diversion as a sole therapeutic tool for either previously coiled re-canalised aneurysms or non-previously treated aneurysms. An English literature review is also included.
Methods
Seven patients were identified in a retrospective review of our database of patients with ICABR aneurysms treated with flow diversion as the sole therapeutic option. ICABR aneurysms included in this study were located at the distal ICA (choroid segment), ICA bifurcation and proximal A1 segment. Patients’ demographics and aneurysm characteristics are summarised in Table 1. Institutional Ethics Board approval was obtained for this retrospective cohort study. Informed written consent was obtained from all patients.
Table 1.
Clinical data and demographics of patients treated by flow diversion for ICABR aneurysms.
| Age | Sex | Presentation | No. of aneurysms/patient | Site | Size (max. diameter) | Ruptured/non-Ruptured | Angiography result at 6 months | EVD inserted | FD model | Site of deployment | No. of FD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Male | Headache | 1 | Proximal A1 | Re-canalised part 8 mm | Non-ruptured | Complete cure | No | Pipeline | MCA to ICA | 1 |
| 2 | 58 | Male | Family history of SAH | 2 | 1 in proximal A1 and 1 in distal ICA | Both are 2.5 mm | Non-ruptured | (Pending) | No | Pipeline | ACA to ICA | 1 |
| 3 | 43 | Female | Headache | 1 | Proximal A1 | 5 mm | Non-ruptured | Complete cure | No | Pipeline | MCA to ICA | 1 |
| 4 | 62 | Male | SAH, 10 days | 1 | Distal ICA | 1.8 mm | Ruptured | Complete cure | Yes | Surpass | MCA to ICA | 1 |
| 5 | 27 | Female | SAH | 1 | Proximal A1 | 1 mm | Ruptured | Complete cure | No | Pipeline | MCA to ICA | 2 |
| 6 | 53 | Female | 10-year follow-up after previous SAH | 1 | Proximal A1 | 2 mm | Non-ruptured | Complete cure | No | Pipeline | MCA to ICA | 1 |
| 7 | 62 | Female | Headache | 1 | Distal ICA | 20 mm | Non-ruptured | Complete cure | No | Pipeline | MCA to ICA | 1 |
EVD: external ventricular drain; FD: flow diverter; SAH: subarachnoid haemorrhage; ICA: internal carotid artery; ACA: anterior cerebral artery; MCA: middle cerebral artery.
Patient selection
The decision to use a flow diverter (FD) was based on the aneurysm morphology and size, risk of perforation with coils and the potential risk of re-canalisation with conventional coiling. The presence of a well-developed contralateral A1 segment and anterior communicating artery was an important factor in determining the direction of stent deployment. All cases except one were discussed in neuroscience multidisciplinary departmental meetings.
Devices used
All cases were treated with a pipeline embolisation device (PED; Covidien, Irvine, CA) except for one case which was treated with a Surpass Streamline FD (Stryker, Fremont, CA).
Dual antiplatelet treatment
For the unruptured group, we used ticagrelor 90 mg b.i.d. and acetyl salicylic acid (ASA) 150 mg two days prior to the procedure. For the ruptured cases, when FD was initially planned, 180 mg ticagrelor and 300 mg ASA were administered two hours before the procedure. There was one ruptured case where FD use was not initially planned. We referred to FD in this case after failed balloon-assisted coiling using a HyperForm 4 mm×7 mm balloon (Ev3/Covidien). In this case, ticagrelor (180 mg) and ASA (300 mg) were administered via nasogastric tube two hours before proceeding to FD deployment. Ticagrelor was used routinely, as the VerifyNow test is not available in Egypt.
Procedure technique
Systemic heparinisation to reach a target activated clotting time of 250–300 seconds was achieved after the insertion of the femoral introducer in all cases. Cerebral angiography in the standard anteroposterior, lateral and both oblique views was performed followed by a rotational angiography and 3D reconstruction. Careful study of the size of the ICA and both A1 segments, as well as the landing zones, was carried out. We used an 8F guider soft-tip 90 cm catheter (Stryker) placed in the cervical segment of the involved ICA.
A 5F or 6F Navien (ev3/Covidien) or Catalyst (Cat5; Stryker) intermediate catheter was introduced further into the ICA, reaching the cavernous segment. A 2.7F Marksman (Medtronic, Minneapolis, MN) or XT 27 (Stryker) microcatheter was navigated to reach the targeted zone of deployment in the M1 segment in six cases and in the A1 segment in one case. Single-shot non-subtracted acquisitions were used for better visualisation of the stent during deployment.
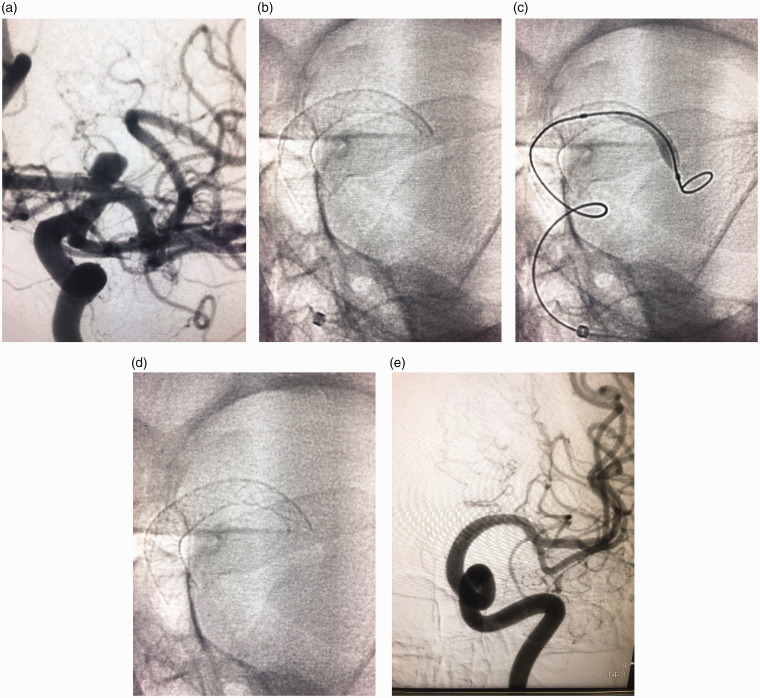
Following full microcatheter deployment, re-catheterisation was performed to ensure full FD apposition. Wall apposition was then checked by cone-beam computed tomography. All stents were deployed to cover the ACA origin, except one with hypoplastic contralateral A1 segment where the stent was deployed to cover the MCA origin. Malapposition at the distal end of a FD was detected in one case, requiring dilatation using a 4 mm × 10 mm HyperGlide balloon (Ev3/Covidien; Figure 1).
Figure 1.
(a) Left internal carotid digital subtraction angiography anteroposterior projection showing an internal carotid termination aneurysm inclined on the A1 segment. (b) Native image showing inadequately opened proximal end of the flow diverter. (c) Native image showing balloon inflation of the proximal end of the flow diverter. (d) Native image post balloon dilatation showing fully opened proximal end of the pipeline device. (e) Follow-up 12 months digital subtraction angiography anteroposterior projection showing non-filling of the aneurysm as well as the A1 segment.
All patients were treated in a single-stage procedure. We used the Karman–Byrne occlusion scale to evaluate the aneurysm occlusion rate in the angiographic follow-up. The Karman–Byrne occlusion scale consists of five points evaluating the degree of aneurysm occlusion post flow diversion: 0 = no change in the endo aneurysmal flow, 1 = residual contrast filling >50% of the pretreatment aneurysm volume, 2 = residual contrast filling <50% of the pretreatment aneurysm volume, 3 = residual filling confined to the neck region and not extending beyond the width of the neck and 4 = no residual filling (i.e. complete obliteration).13
Literature review
Ovid MEDLINE and PubMed were reviewed for articles published in English using the keywords FD, ICA terminus, ICA termination, proximal A1 segment and bifurcation aneurysms, which were used in AND/OR combinations. Case reports and case series for aneurysms located in the ICA bifurcation or inclined on the proximal A1 segment treated with FD solely were included.
Exclusion criteria
Exclusion criteria were ICA aneurysm series treated with FD with no precise identification of those located in the choroid or pre-bifurcation region, A1 aneurysms studies with no clear identification of those located at the proximal A1 segment and studies using other endovascular therapeutic tools in the same setting of flow diversion.
Results
Patients and population
Our series included seven patients harbouring eight aneurysms. The sample included four females and three males. The age range was as 27–62 years, with an average age of 49 years.
Aneurysm characteristics
The maximum aneurysm diameter ranged from 1.5 mm to 20 mm, with an average diameter of 5.3 mm. One blister aneurysm was included in this study. Five aneurysms were inclined on the A1 segment and three at the most distal pre-bifurcation choroid segment.
Ruptured cases
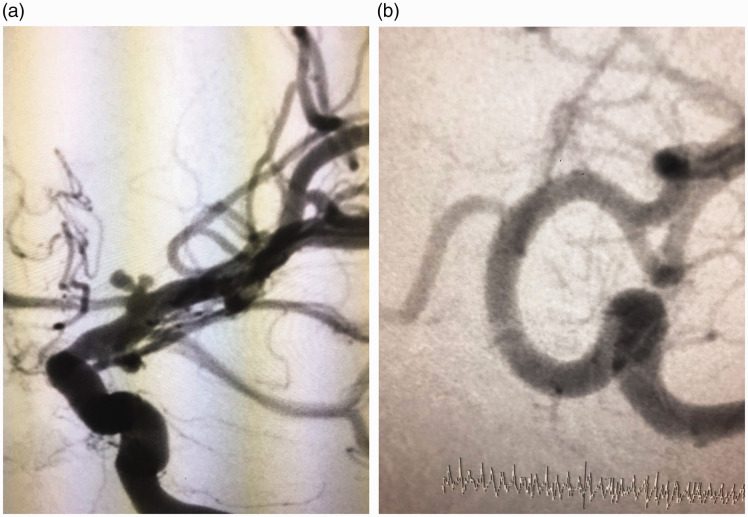
Two patients presented with subarachnoid haemorrhage (SAH). Balloon remodelling was attempted in one. This failed due to coil instability, despite maximum balloon inflation, and a PED was inserted (Figure 2). The other case harboured a 1.7 mm aneurysm at the distal pre-bifurcation (choroid) ICA segment and was considered at high risk of perforation with coil use. They were treated on days 12 and 15 of SAH onset. One patient had an external ventricular drain inserted on day 1 of bleeding.
Figure 2.
(a) Digital subtraction angiography showing a left internal carotid termination aneurysm inclined on the A1 segment. (b) Follow-up digital subtraction angiography showing aneurysm disappearance, despite the presence of flow in the A1 segment.
Unruptured cases
The chief complaint was headache in four patients. Their aneurysms were diagnosed during magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) work-up. One patient had a strong family history of SAH and was diagnosed with two aneurysms in a MRA examination.
We did not encounter any neurological or non-neurological complications in this series. No MRI examination was performed to check for silent perforator infarctions.
Angiographic follow-up
Conventional angiographic follow-up was performed at six months in five cases and at 12 months in one case. One patient was lost from angiographic follow-up. All cases showed a complete angiographic disappearance class IV occlusion score on the Karman–Byrne scale.13
Ten studies including ours were found in the literature review. A total of 38 patients were identified harbouring 42 aneurysms (Table 2).1,5–12 Of the 38 patients, four (10.5%) presented with SAH.
Table 2.
Clinical data and demographics of patients included in the literature review.
| Author | No. of cases | Sex | Age | Site | SAH cases | Months for DSA follow-up | Occlusion rate | Flow diverter used | No. of devices | Number of procedures | Direction of deployment | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morales-Valero 20141 | 2 | NI | NI | ICA term. | 1 | 12 and 36 | NI | Pipeline | 1 and 3 | One had two procedures; the other had one | MCA-ICA | One had non-disabling stroke one week after the first treatment |
| Lin 20185 | 7 | NI | NI | 5 A1 seg.2 ICA term. | 0 | Average 15 | 6 Class IV1 dead | Pipeline | 1 | All had one | ACA-ICA | One major stroke and retroperitoneal haematoma leading to deathOne minor stroke |
| Pujari 20186 | 10 | 6F | 46.9 | ICA term. | 1 | Median 7.5 | 3 Class IV4 class III1 Class II1 Class 01 FD thrombosed | Pipeline | Eight had one, one had two and one had three | All had one | MCA-ICA | One had a groin pseudo-aneurysm and FD thrombosisOne had distal SAH |
| Trivelato 20177 | 1 | F | 64 | ICA term. | 0 | Not spec. | Not spec. | Pipeline | 1 | 1 | Horizontal ACA-ACA | None |
| Van Rooij 20108 | 1 | F | 68 | Prox A1 | 0 | Not spec. | Not spec. | Pipeline | 2 | 1 | ACA-ICA | Ganglionic infarctionTransient RT hemiparesisPersistent cognitive and memory dysfunction |
| Akgul 20169 | 3 | 2F, 1M | 55, 58, 49 | 3 ICAb2 A ch. | – | 3 | Karman 4 in two aneurysms | Derivo | 1 | 1 | Not spec. | One mortality, aneurysm rupture after six months |
| Mattingly 201310 | 1 | F | 42 | Prox A1 | – | 3 | Karman 1 | PED | 1 | 1 | ICA/MCA | – |
| Lubicz 201011 | 4 | 3F, 1M | 50, 29, 50, 60 | 2 ICA b3 A ch. | – | 3–6 | Karman 4 in one aneurysm | Silk | 1 | 1 | Not spec. | – |
| Briganti 201512 | 2 | F | 67, 68 | Ach, ICAB | – | Not spec. | – | P64 | 1 | 1 | Not spec. | – |
| Mahmoud 2020 (present study) | 7 | 4F | 49 | 6 A1 seg. 3 ICA term. | 1 | Five at six months, one at 13 months | 6 Class IVOne had no follow-up | 6 Pipeline1 Surpass | One had two, six had one | 1 | Six had MCA-ICA stentsOne had an ACA-ICA stent | None |
F: female; M: male; term.: termination; Ach: anterior choroidal artery; Prox: proximal; Spec.: specified.
Aneurysms locations
Thirteen aneurysms were inclined on the proximal A1 segment, while 29 were located at the ICA bifurcation or the distal pre-bifurcation ICA.
Devices used and direction of deployment
PED (Medtronic Neurovascular, Irvine, CA) was the most-used device in 28 patients. A Silk stent (Balt Extrusion, Montmorency, France) was used in four patients, a Derivo stent (Acandis GmbH & Co. KG, Pforzheim, Germany) was used in three patients, a P64 (Phenox, Bochum, Germany) was used in two patients and a Surpass Streamline FD (Stryker) was used in only one patient.
The direction of deployment was identified in 29 patients. The FD device was deployed from the MCA to the ICA in 19 patients, and from the ACA to the ICA in nine patients. Horizontal deployment between both A1 segments was achieved in one patient. Two patients had two telescopic devices, and one patient had three telescopic devices.
Complications
Peri-procedure neurological morbidity occurred in 3/38 (7.9%) cases. Two patients had a minor stroke, and one patient had ganglionic infarction, leading to permanent cognition affection and memory disturbance. One patient had distal SAH with no neurological consequences. One non-neurological morbidity was identified in a patient who developed groin pseudo-aneurysm and stent thrombosis (Table 2). Two (5.8%) mortalities were identified in this review: one from major stroke consequent on MCA occlusion and retroperitoneal haematoma,5 and the other following aneurysm rupture six months post flow diversion.9
Occlusion rate
Of 42 patients, 31 (73%) had an angiographic follow-up. Of the 31 who had an angiographic follow-up, 19 (61%) had class 4 complete occlusion on the Karman–Byrne scale.
Discussion
Treatment of ICABR aneurysms is challenging for either microsurgical clipping or endovascular techniques. Aneurysms in this location involving the distal ICA segment, the true ICA bifurcation or those more inclined on A1 or M1 are subject to remarkable haemodynamic stress.1 Many aneurysms at the ICA terminus are not located at the true termination or bifurcation. Many are deviated to the A1 segment or, less likely, to the M1 segment.2
Sakamoto et al. analysed the angiographic anatomy of 10 (ICA bifurcation) aneurysms. They found all of them arose from the A1 segment. They found that the A1 segment calibre is smaller than that of the M1 segment, and the ICA–ACA angle was smaller than the ICA–MCA angle, which may expose the A1 segment to higher haemodynamic stress and hence a greater risk of aneurysm development.14 According to Brown et al., repeated haemodynamic stress may lead to wall element disruption in a way similar to a wire breaking with repeated bending.15 Rossitti et al. stated that the apex of a bifurcation is the site of maximum haemodynamic stress as a result of deflection and the separation of the blood-flow streamlines and vortex formation at the lateral angles.16 As they are subject to the same stress and turbulent factors, in this study, we looked at aneurysms arising from the ICABR as one group.
Beside the known challenges for surgical dissection of this aneurysm group, such as the presence of multiple perforators arising from the A1, M1 or distal ICA segments17 or the presence of the anterior choroidal artery and the recurrent artery of Heubner in close proximity to some aneurysms,4 another point of surgical challenge is the direction of some aneurysm domes towards the frontal lobe or in close relation to the Sylvian fissure apex.3 Post-surgery morbidity/mortality is another concern. Studies by Gupta et al. on the microsurgical treatment of ruptured ICA bifurcation aneurysms revealed good outcomes in 68.6% of cases, with a 6.3% mortality rate mainly due to intraoperative aneurysm rupture and thrombo-embolic complications.18 Another study by Miyazawa et al. revealed good outcomes after surgery in only 58.8% of the ruptured group.17
Other endovascular therapeutic options for internal carotid bifurcation aneurysms carry a non-negligible morbidity/mortality risk, and some have considerable rates of re-canalisation and recurrence. In a meta-analysis study conducted by the Morales-Valero team that included 158 patients with ICA terminus aneurysms, it was found that endovascular treatment of this aneurysm group carries 4% perioperative morbidity and 3% mortality risks. Retreatment was carried out in 14% of the treated aneurysms.1 This was similar to a study by Van Rooij et al., where 14% of the coiled aneurysms required retreatment.19 Oishi et al. found a 13.6% recurrence rate in their series of 25 ICA termination aneurysms treated with coils.20
As haemodynamic stress and turbulence plays an important role in aneurysm development in this location, it will probably be responsible for recurrence and re-canalisation of this aneurysm group treated with coiling. Shapiro et al. analysed 1517 patients with non-flow-diverting stent-assisted coiling. They found a 19% overall procedure complication rate and 2.1% peri-procedure mortality. Complete occlusion was achieved in only 61% of their cases.21 Clajus et al. studied 108 patients with 114 aneurysms, including 10 ICA bifurcation aneurysms treated with a Woven EndoBridge (WEB) device, and demonstrated an adequate occlusion rate of 76%.22 Another study conducted by Pierot et al. with 169 aneurysms treated with a WEB device, including 17 ICA bifurcation aneurysms, revealed a complete or near-complete occlusion rate of 79% with 3% procedure-related morbidity.23
The treatment challenge of ICABR aneurysms by either the microsurgical clipping or various endovascular techniques and the success gained from the use of FD in treating proximally located ICA aneurysms encouraged the use of FD to treat aneurysms located in more distal ICA locations, including those inclined on the proximal A1 segment. A FD is a device of low porosity, aiming to divert the flow and promote healing of the aneurysm via arterial reconstruction and neo-intimal growth.24 Application of FD in treating aneurysms in such a location will require lenticulostriate perforators and the ostium of large arteries (ACA or MCA) to be covered, with the subsequent potential risk of ischaemic events. Covering a main branch or perforators has been discussed in many studies. Raz et al. studied the patency of the anterior choroidal artery after coverage with PED. They stated that the covered artery would probably not be occluded as long as it was an end artery. They pointed to the collateral circulation which may determine the patency of the covered artery post FD placement. The artery will remain patent if the collateral circulation is poor or will be occluded in the case of the presence of efficient collateral circulation.25
Many reports studied the long-term patency of the anterior circulation branches following flow diversion. Rangel-Castilla et al. evaluated the patency of 127 arterial branches covered by a PED at a mean angiographic follow-up of 10 months in 82 aneurysms. Arterial side branches were occluded in 13 patients, accounting for 15.8%, including two anterior cerebral arteries. Despite the arterial occlusion, none of them experienced neurological manifestations.26 The results were similar to those in the study by Wu et al. evaluating the patency of arterial branches covered by a PED in 126 patients. From 173 branches covered by a PED, 24 (13.9%) were occluded, and 29 (16.8%) showed diminished flow by angiography. None of their patients had any permanent neurological complications.27 Lin et al. reported MCA angiographic flow delay in all cases treated by a FD covering the origin of M1. They reported one death from major MCA stroke and transient MCA minor stroke secondary to platelet plug treated by intra-arterial abciximab.6
Perforator patency following FD insertion has been studied in many reports. Perforators are considered end arteries. Following FD insertion, a pressure gradient is maintained through its orifice, hence maintaining and securing the flow. This is in contrast to larger arteries, which when covered by a FD will undergo a significant decrease in the pressure gradient. The pressure gradient across their ostia will not be enough to maintain an antegrade flow. The opposing effect of the retrograde flow or the competition of flow from the collateral side will lead to a further decrease of flow in the covered arteries or even lead to their occlusion, usually with no clinical consequences.28,29
Post-flow diversion perforator occlusion is not always without neurological risk. Philips et al. reported 9.4% permanent neurological complications following perforator territory infarctions.30 Van Rooij reported a ganglionic infarction due to the compromise of lenticulostriate perforators following placement of FD to treat a proximal A1 unruptured aneurysm.8
Healing of an aneurysm located in the proximal A1 segment after placing a FD covering the ACA origin with no direct contact between the FD struts and the aneurysm neck might be explained by two mechanisms. Covering the ACA origin may result in A1 segment occlusion as long as the contralateral A1 segment is of normal calibre and the anterior communicating artery is functioning adequately.
Alteration of dynamic fluid exchange across the aneurysm/parent vessel interface may create an intra-aneurysmal environment that is conducive to thrombosis, as was stated by Nelson et al.24 An aneurysm in the proximal A1 segment will disappear when the flow stops in the parent artery. However, the aneurysm may disappear, even with slowing down of the flow in the A1 segment parent artery. This was obvious in our experience in cases 1, 5 and 6, which showed considerable slowing down of the flow compared to the pre-stent-deployment angiography. Aneurysm occlusion may therefore be a consequence of the hypothesised decreased turbulence after flow diversion allowing healing of the neck, or of the progressive slowing of flow within the parent artery promoting aneurysm thrombosis. A systematic review conducted by Ravindran et al. pointed to the potential presence of a biomarker that may activate the process of endothelial cell growth following haemodynamic alteration post FD insertion. Identification of this biomarker may predict successful aneurysm occlusion.31
Two ruptured aneurysms were included in our series. As the use of FDs will usually require administration of dual antiplatelet therapy, its use in acute SAH cases may carry a risk of aneurysm rebleeding, since aneurysm occlusion following flow diversion is a progressive process. In a meta-analysis conducted by Cognazzo et al. that included 20 studies with 223 ruptured aneurysms, rebleeding occurred in 4% of patients post flow diversion.32 A surface-modified FD stent (Pipeline Flex with Shield Technology; Medtronic Neurovascular) is now available. It has 3 nm phosphorylcholine covalently bound to the device wires. Phosphorylcholine reduces platelet adhesion and activation, and hence the use of a Pipeline Flex shield has a theoretically lower thrombo-embolic risk.33 Manning et al. reported the use of Pipeline Flex with Shield Technology in 14 patients with acutely ruptured aneurysms using a single antiplatelet agent. Two patients in their group had aneurysm rebleeding in the acute phase following treatment, leading to death of one of them.34 The use of FDs should probably be reserved as a last resort in acutely ruptured aneurysms (even with the use of the Pipeline Flex with Shield technology). FD should also be used cautiously in cases when covering of the M1 origin is considered.
We are aware that our series lacks large cohorts as well as long-term angiographic and clinical follow-up. Long-term angiographic follow-up of ICABR aneurysms treated with flow diversion, notably those inclined on the proximal A1 segment, is required for a proper understanding of the mechanism of aneurysm occlusion and the flow pattern in the ipsilateral A1 segment. Long-term clinical follow-up will be important in the analysis of long-term complications, notably those of a thrombo-embolic nature.
Conclusion
Treating ICABR aneurysms using FDs is feasible, with a promising angiographic occlusion rate. Further studies are needed to analyse long-term clinical and angiographic results.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Mostafa Mahmoud https://orcid.org/0000-0003-2247-5843
References
- 1.Morales-Valero SF, Brinjikji W, Murad MH, et al. Endovascular treatment of internal carotid artery bifurcation aneurysms: a single-center experience and a systematic review and meta-analysis. AJNR Am J Neuroradiol 2014; 35: 1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Pira B, Lanzino G. Section II aneurysms-anterior circulation. Internal carotid artery bifurcation aneurysms In: Rangel-Castilla L, Nakaji P, Siddiqui AH, et al. (eds) Decision making in neurovascular diseases. Stuttgart, Germany: Thieme, 2018, pp.163–170. [Google Scholar]
- 3.Savardekar AR, Patra DP, Narayan V, et al. Internal carotid artery bifurcation aneurysms: microsurgical strategies and operative nuances for different aneurysmal directions. Oper Neurosurg (Hagerstown) 2018; 15: 386–394. [DOI] [PubMed] [Google Scholar]
- 4.Nossek E, Chalif D, Levine M, et al. Modifying flow in the ACA–ACoA complex: endovascular treatment option for wide-neck internal carotid artery bifurcation aneurysms. J Neurointerv Surg 2015; 7: 351–356. [DOI] [PubMed] [Google Scholar]
- 5.Pujari A, Howard B, Madaelil T, et al. Pipeline embolization device treatment of internal carotid artery terminus aneurysms. J Neurointerv Surg 2019; 11: 485–488. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Bender M, Colby G, et al. Flow diversion covering the M1 origin as a last resort. Stroke Vasc Neurol 2018; 4: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivelato FP, Araújo JF, Salles Rezende MT, et al. A novel configuration of pipeline embolization device for internal carotid bifurcation region aneurysms: horizontal deployment. Clin Neuroradiol 2017; 27: 57–60. [DOI] [PubMed] [Google Scholar]
- 8.Van Rooij WJ, Sluzewski M. Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured A1 aneurysm. Am J Neuroradiol 2010; 31: E43–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akgul E, Bilen Onan H, Akpinar S, et al. The DERIVO embolization device in the treatment of intracranial aneurysms: short- and midterm results. World Neurosurg 2016; 95: 229–240. [DOI] [PubMed] [Google Scholar]
- 10.Mattingly T, Van Adel B, Dyer E, et al. Failure of aneurysm occlusion by flow diverter: a role for surgical bypass and parent artery occlusion. J Neurointerv Surg 2015; 7: e13. [DOI] [PubMed] [Google Scholar]
- 11.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 12.Briganti F, Leone G, Marseglia M, et al. P64 Flow Modulation Device in the treatment of intracranial aneurysms: initial experience and technical aspects. J Neurointerv Surg 2016; 8: 173–180. [DOI] [PubMed] [Google Scholar]
- 13.Kamran M, Yarnold J, Grunwald IQ, et al. Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology 2011; 53: 501–508. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto S, Ohba S, Shibukawa M, et al. Characteristics of aneurysms of the internal carotid artery bifurcation. Acta Neurochir (Wien) 2006; 148: 139–143. [DOI] [PubMed] [Google Scholar]
- 15.Brown N. A mathematical model for the formation of cerebral aneurysms. Stroke 1991; 22: 619–625. [DOI] [PubMed] [Google Scholar]
- 16.Rossitti S, Löfgren J. Optimality principles and flow orderliness at the branching points of cerebral arteries. Stroke 1993; 24: 1029–1032. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa N, Nukui H, Horikoshi T, et al. Surgical management of aneurysms of the bifurcation of the internal carotid artery. Clin Neurol Neurosurg 2002; 104: 103–114. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Khosla V, Chhabra R, et al. Internal carotid artery bifurcation aneurysms: surgical experience. Neurol Med Chir (Tokyo) 2007; 47: 153–158. [DOI] [PubMed] [Google Scholar]
- 19.Van Rooij W, Sluzewski M, Beute G. Internal carotid bifurcation aneurysms: frequency, angiographic anatomy and results of coiling in 50 aneurysms. Neuroradiology 2008; 50: 583–587. [DOI] [PubMed] [Google Scholar]
- 20.Oishi H, Yamamoto M, Nonaka S, et al. Endovascular therapy of internal carotid artery bifurcation aneurysms. J Neurointerv Surg 2013; 5: 400–404. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro M, Becske T, Sahlein D, et al. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol 2012; 33: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clajus C, Strasilla C, Fiebig T, et al. Initial and mid-term results from 108 consecutive patients with cerebral aneurysms treated with the WEB device. J Neurointerv Surg 2017; 9: 411–417. [DOI] [PubMed] [Google Scholar]
- 23.Pierot L, Moret J, Barreau X, et al. Safety and efficacy of aneurysm treatment with WEB in the cumulative population of three prospective, multicenter series. J Neurointerv Surg 2018; 10: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz E, Shapiro M, Becske T, et al. Anterior choroidal artery patency and clinical follow-up after coverage with the pipeline embolization device. Am J Neuroradiol 2015; 36: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangel-Castilla L, Munich S, Jaleel N, et al. Patency of anterior circulation branch vessels after Pipeline embolization: longer-term results from 82 aneurysm cases. J Neurosurg 2017; 126: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Tian Z, Li W, et al. Patency of branch vessels after pipeline embolization: comparison of various branches. Front Neurol 2019; 10: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 29.Puffer RC, Kallmes DF, Cloft HJ, et al. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg 2012; 116: 892–896. [DOI] [PubMed] [Google Scholar]
- 30.Phillips TJ, Wenderoth JD, Phatouros CC, et al. Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol 2012; 33: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravindran XK, Salem XMM, Alturki XAY, et al. Endothelialization following flow diversion for intracranial aneurysms: a systematic review. AJNR Am J Neuroradiol 2019; 40: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagnazzo F, Di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. Am J Neuroradiol 2018; 39: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell EJ, O’Byrne V, Stratford PW, et al. Biocompatible surfaces using methacryloylphosphorylcholine laurylmethacrylate copolymer. Asaio J 1994; 40: M853–857. [DOI] [PubMed] [Google Scholar]
- 34.Manning N, Cheung A, Phillips T, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]